Antioxidant Treatment Ameliorates Respiratory Syncytial Virus–induced Disease and Lung Inflammation (original) (raw)
Abstract
Rationale: Respiratory syncytial virus (RSV) is a major cause of lower respiratory tract infection in children. No treatment has been shown to significantly improve the clinical outcome of patients with this infection. Recent evidence suggests that oxidative stress could play an important role in the pathogenesis of acute and chronic lung inflammatory diseases. We do not known whether RSV induces pulmonary oxidative stress and whether antioxidant treatment can modulate RSV-induced lung disease.
Objectives: To investigate the effect of antioxidant administration on RSV-induced lung inflammation, clinical disease, and airway hyperreactivity (AHR).
Methods: BALB/c mice were infected with 107 plaque-forming units of RSV, in the presence or absence of orally administered butylated hydroxyanisole (BHA), an antioxidant. Malondialdehyde and 4-hydroxynonenal were measured in bronchoalveoar lavage (BAL) by colorimetric assay. Cytokines and chemokines were measured in BAL by Bio-Plex and leukotrienes were measured by enzyme-linked immunosorbent assay. AHR to methacholine challenge was measured by whole-body plethysmography.
Results: BHA treatment significantly attenuated RSV-induced lung oxidative stress, as indicated by the decrease of malondialdehyde and 4-hydroxynonenal content in BAL of RSV-infected mice. RSV-induced clinical illness and body weight loss were also reduced by BHA treatment, which inhibited neutrophil recruitment to the lung and significantly reduced pulmonary cytokine and chemokine production after RSV infection. Similarly, antioxidant treatment attenuated RSV-induced AHR.
Conclusion: Modulation of oxidative stress represents a potential novel pharmacologic approach to ameliorate RSV-induced acute lung inflammation and potentially prevent long-term consequences associated with RSV infection, such as bronchial asthma.
Keywords: antioxidant, chemokines, lung inflammation, oxidative stress, respiratory syncytial virus
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Oxidative stress has been shown to play an important role in the pathogenesis of acute and chronic lung inflammatory diseases, such as asthma, lung fibrosis, and chronic obstructive pulmonary disease.
What This Study Adds to the Field
Respiratory syncytial virus induces oxidative stress in vivo. Antioxidant administration significantly reduces lung pulmonary inflammation and ameliorates clinical disease due to respiratory syncytial virus.
Respiratory syncytial virus (RSV) is a major cause of respiratory tract infection in children worldwide and is the leading cause of virally induced bronchiolitis (1). Each year, approximately 100,000 children are hospitalized with RSV disease, with an estimated annual cost close to $300 million in the United States alone (2, 3). There is no safe and efficacious vaccine for RSV, and immunity is incomplete, resulting in repeat attacks of acute illness throughout adulthood. The long-term consequences of RSV infection, which include increased airway resistance and recurrent wheezing (1), are associated risk factors for the development of asthma (4). Although the mechanisms of RSV-induced airway disease and the associated long-term consequences have yet to be clearly defined, the local inflammatory response is believed to play a fundamental role. Airway epithelial cells are the target of RSV infection, and they respond to the infection by producing a variety of mediators involved in lung immune/inflammatory responses, like cytokines, chemokines, and interferons, and by up-regulating adhesion molecules and major histocompatibility complex antigens on the cell surface (reviewed in Reference 5).
Reactive oxygen species (ROS) are highly reactive molecules implicated in cellular damage. In the past few years, there has been increased recognition of their role as redox regulators of cellular signaling (reviewed in References 6 and 7). We have previously shown that RSV-infected airway epithelial cells generate ROS and that antioxidant treatment with butylated hydroxyanisole (BHA), as well as a panel of chemically unrelated antioxidants, blocks RSV-induced signal transduction cascades, leading to chemokine expression in vitro through inhibition of transcription factors belonging to interferon regulatory factor (IRF) and signal transducers and activators of transcription (STAT) families (8, 9). Recent studies have indicated, directly or indirectly, an important role of ROS produced by epithelial and inflammatory cells in the pathogenesis of acute and chronic lung inflammatory diseases, such as acute respiratory distress, asthma, and chronic obstructive pulmonary disease (reviewed in References 10–12). Surprisingly, little is known regarding the oxidative stress response in patients with virally induced lung inflammation. In an animal model of influenza infection, inhibition of oxygen radicals through administration of antioxidants or increased lung superoxide dismutase levels significantly reduced lung injury and improved the survival rate of infected animals, suggesting that oxidative stress can play a significant role in the pathogenesis of virally induced pneumonia (13–17). We have previously shown that RSV induces reactive nitrogen species and nitric oxide synthase (NOS) in the lungs of infected mice, and that inhibition of NOS expression significantly reduces RSV-induced lung inflammation (18). However, it is not known whether RSV infection induces significant lung oxidative stress damage and whether inhibiting ROS production by antioxidants modifies RSV-induced lung disease. Therefore, the aim of this study was to investigate the effect of antioxidant administration on RSV-induced lung inflammation, clinical disease, and airway hyperreactivity (AHR).
METHODS
RSV Preparation
The RSV A2 strain was grown in Hep-2 cells and purified by centrifugation on discontinuous sucrose gradients as described elsewhere (19). The virus titer of the purified RSV pools was 8 to 9 log10 plaque-forming units (PFU) per milliliter using a methylcellulose plaque assay. No contaminating cytokines were found in these sucrose-purified viral preparations (20). LPS, assayed using the limulus hemocyanin agglutination assay, was not detected. Virus pools were aliquoted, quick-frozen on dry ice/alcohol, and stored at −70°C until used. Sucrose-purified extracts from uninfected Hep-2 cells were also generated under the same conditions.
Inoculation Procedure and Mice
Female BALB/c mice were purchased from Harlan (Houston, TX) and were housed in pathogen-free conditions in the animal research facility of the University Texas Medical Branch (UTMB), Galveston, Texas, in accordance with the National Institutes of Health and UTMB institutional guidelines for animal care. Under light anesthesia, mice were inoculated intranasally with either sucrose-purified Hep-2 extracts (sham-infected) or sucrose-purified RSV at 1 × 107 PFU, diluted in sterile phosphate-buffered saline (PBS) for a total inoculation volume of 50 μl, as previously described (21). BHA was prepared by dissolving the right amount of compound in a 100-μl volume of corn oil, which was administered by gavage, once a day, without anesthesia. A total of four experimental groups consisting of two treatment groups, vehicle (corn oil) and BHA, for each infection group, sham and RSV, were used for all experiments. There was no effect of the vehicle alone; therefore, the groups sham + vehicle and RSV + vehicle are usually identified in the figures as sham and RSV. In AHR experiments, mice were infected with RSV at 1 × 105 PFU.
Clinical Illness
We used a well-established clinical illness grading scale for mice to establish the severity of infection (0 = healthy; 1 = barely ruffled fur; 2 = ruffled fur but active; 3 = ruffled fur and inactive; 4 = ruffled fur, inactive, and hunched; 5 = dead) (21). In addition, daily determination of body weight was used to monitor the progression of disease over the experimental period. These parameters have been shown to closely correlate with lung pathology in experimental paramyxovirus infection of BALB/c mice (22). Visual differences were observed and captured by digital photography.
AHR
The effect of BHA on RSV-induced AHR was measured in mice using a whole-body plethysmograph (Buxco, Troy, NY) as previously described (18, 23). Measurement of airway responses was performed on individual, unrestrained, and nonanesthetized mice within a two-chamber plethysmograph. AHR was expressed as an enhanced pause (Penh). Penh, a dimensionless parameter used to measure pulmonary resistance, is calculated by changes in chamber pressure induced by methacholine challenge during inspiration and expiration. After a brief acclimatization to the chamber, the mice received an initial baseline challenge of saline followed by increasing doses of nebulized methacholine (3, 6, 12, 24, and 50 mg/ml). Recordings were taken for 3 min after each nebulization. The respiratory rate in breaths per minute was extrapolated from readings of every 10 breaths. The box pressure waveforms generated from the respiratory cycle were used to calculate peak expiratory pressure (PEP), peak inspiratory pressure (PIP), and the time of expiration. Penh was then calculated using the following formula: Penh = Pause × PEP/PIP. Penh values were averaged and reported as a percentage of baseline saline values.
Pulmonary Inflammation
Bronchoalveolar lavage (BAL) was obtained from the lungs of mice at various time points postinfection. In brief, anesthetized and tracheotomized mice were cannulated with a 1-ml syringe, and the lungs flushed three times with 1 ml of sterile, cold PBS. Total cellular influx and differential cell counts were measured in the BAL of all experimental groups. Total cell counts were determined by staining 50 μl of BAL with trypan blue and counting viable cells using a hemocytometer. For differentials, 100 μl of BAL was used to generate cytospin preparations. Slides were dried, fixed, and stained with Protocol Hema3 (Fisher Diagnostics, Middletown, VA). A total of 300 cells were counted per sample using light microscopy.
Lung Pathology
Selected mice in each group were killed at various time points, and the entire lung was perfused, removed, and fixed in 10% buffered formalin and embedded in paraffin. Multiple 4-μm longitudinal cross-sections were stained with hematoxylin and eosin. The slides were analyzed and scored for cellular inflammation under light microscopy by a board-certified pathologist, as previously described (21). The pathology score reported includes the number of abnormal perivascular and peribronchial regions divided by the total number of perivascular and peribronchial regions and are reported as the percentage of inflamed tissue.
Effect of Antioxidants on Viral Replication
Lungs were excised on Days 3, 5, and 7 postinfection, snap-frozen in liquid nitrogen, and stored at −70°C. Viral titers were determined by plaque assay using confluent Hep-2 cells grown in 24-well plates, as previously described (24).
Lipid Peroxidation in BAL
BAL was centrifuged at 15,000 rpm for 1 min at 4°C. BHT was added to the supernatant to prevent further oxidation, and the samples were immediately frozen in liquid nitrogen. Measurement of lipid peroxidation markers malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) was carried out using a lipid peroxidation kit from Calbiochem (EMD Biosciences, Inc., San Diego, CA). To detect MDA and 4-HNE, 200 μl of BAL was added to 650 μl of a 3:1 solution of N-methyl-2-phenylindole in acetonitrile:ferric ions in methanol and quickly vortexed. A total of 150 μl of methanesulfonic acid were added and incubated at 45°C for 1 h. The samples were cooled and measured at 586 nm using a spectrophometer.
Measurements of 8-isoprostane were performed using a competitive enzyme immunoassay from Cayman Chemical (Ann Arbor, MI), according to the manufacturer's instructions.
Chemokine and Cytokine Protein Profile
To assess the production of chemokines and cytokines, BAL was obtained at 12, 24, 48, and 72 h postinfection. Samples were tested for multiple cytokines using the Bio-Plex Cytokine Mouse 18-Plex panel (Bio-Rad Laboratories, Hercules, CA), according to the manufacturer's instructions, as previously described (25). The panel includes the following cytokines: interleukin (IL)-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12 (p40), IL-12 (p70), IL-17, granulocyte colony–stimulating factor, granulocyte-macrophage colony–stimulating factor, IFN-γ, KC, MIP-1α, MCP-1, RANTES, and tumor necrosis factor (TNF)-α. The broad assay range was from 0.2 to 5,000 pg/ml.
Cysteinyl Leukotriene Determination
BAL was used to measure leukotriene C4 (LTC4) by a competitive ACE enzyme immunoassay (Cayman Chemical). The sensitivity of the assay was 7.8 pg/ml. The specificity of the assay for traditionally classified cysteinyl leukotrienes (cys-LTs) LTC4 and LTC5 was 100%. The cross-reactivities of the assay with LTC4 metabolites are 48% with LTD5, 46% with LTD4, 28% with LTE4, 7% with LTE5, and 2% with LTE4.
Statistical Analysis
When two groups are compared, the values are analyzed using an unpaired, two-tailed, Student's t test (GraphPad Prism; GraphPad Software, Inc., San Diego, CA). Results are expressed as mean ± SD unless otherwise stated. One-way analysis of variance with Dunnett's multiple comparison tests were performed to analyze body weight loss in RSV versus RSV + BHA treatment groups.
RESULTS
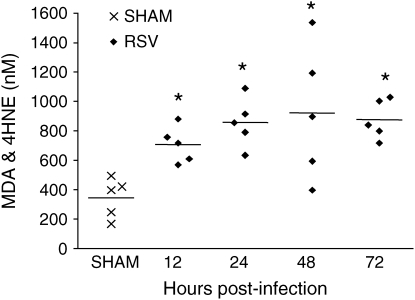
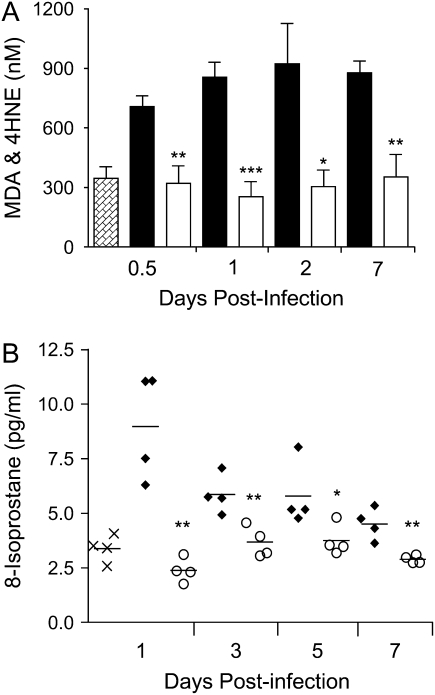
RSV infection induces oxidative stress in the lung. Lipid peroxidation products, such as MDA, 4-HNE, and isoprostanes, are useful markers of pulmonary oxidative stress and by themselves have been shown to mimic all the pathophysiologic features of asthma (bronchoconstriction, AHR, mucus hypersecretion, enhanced arachidonic acid cascade, increased synthesis of chemoattractants, epithelial damage, and microvascular leakage; reviewed in Reference 26). To determine whether RSV infection induced oxidative damage of the lung, MDA and 4-HNE were measured in the BAL of RSV-infected mice at different times postinfection. As shown in Figure 1, RSV infection was associated with a significant increase of MDA and 4-HNE levels in BAL samples at all time points tested, when compared with sham mice, indicating that lung oxidative stress damage indeed occurs in RSV infection. MDA and 4-HNE values from the sham group did not change over time (data not shown).
Figure 1.
Lipid peroxidation products in the bronchoalveolar lavage (BAL). malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) were detected by a colorimetric assay in BAL of respiratory syncytial virus (RSV)– and sham-infected mice at various times postinfection. The bar in the scatter plot represents the mean of five different animals. The figure is representative of two different experiments. *p < 0.01 relative to sham-infected mice.
BHA Attenuates RSV-induced Clinical Illness
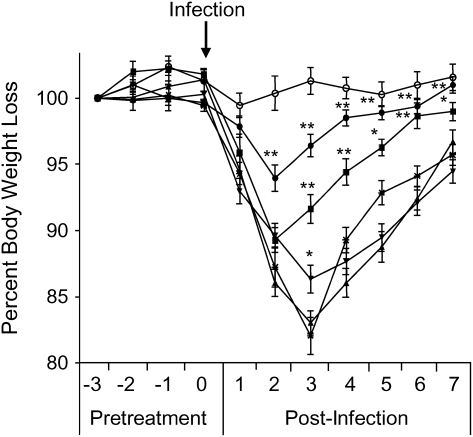
To determine whether antioxidant administration was capable of altering RSV-induced disease, we initially assessed the effect of three different BHA treatment protocols on body weight loss. In protocol 1, animals were pretreated with various doses of BHA, ranging from 50 to 250 mg/kg, 3 d before and during the first 5 d of infection. As shown in Figure 2, mice inoculated with RSV alone progressively lost weight during the first 3 d of infection, with a peak of 15 to 20% loss at Day 3 postinfection. The 250-mg/kg dose of BHA consistently attenuated RSV-induced body weight loss, as the mice experienced a weight loss of 6% and regained their original body weight earlier. A statistically significant difference in body weight loss was also observed with the 150-mg/kg dose, whereas the 100-mg/kg had some effect only at Day 3 postinfection, which represents the peak of body weight loss. We then determined whether the 250-mg/kg dose was still effective if treatment was started the same day of RSV infection (protocol 2) or at 1 d postinfection (protocol 3). Protocol 3, or post-treatment with BHA, failed to attenuate RSV-induced body weight loss and was not further investigated (data not shown). There was no statistically significant difference in body weight loss between protocols 1 and 2 (data not shown); therefore, all subsequent experiments were performed using the dose of BHA 250 mg/kg given during the first 5 d of RSV infection.
Figure 2.
Effect of butylated hydroxyanisole (BHA) administration on RSV-induced weight loss. Mice were treated with increasing concentrations of BHA for 3 d before RSV infection and during the first 7 d of infection. The following is a representative diagram of three different experiments. All data points represent the mean of at least four animals. Open circles, sham; cross marks, RSV; triangles, RSV + BHA 50 mg/kg; inverted triangles, RSV + BHA 100 mg/kg; solid squares, RSV + BHA 150 mg/kg; solid circles, RSV + BHA 250 mg/kg. *p < 0.05 relative to sham-infected mice; **p < 0.01 relative to sham-infected mice.
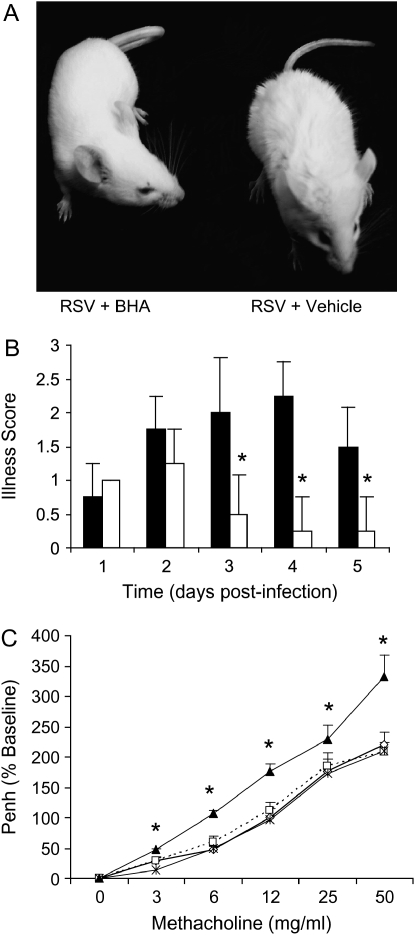
A positive effect of antioxidant treatment was also observed on other clinical parameters of RSV infection that constitute the viral-induced illness score (see Methods for details). Typically, the peak of illness severity coincides with the peak of RSV-induced body weight loss and occurs between Day 3 and 4 of infection (27). We observed a significant difference in appearance of BHA-treated versus untreated RSV-infected mice (Figure 3A), as well as in the total illness score, starting at Day 3 postinfection (Figure 3B), indicating that antioxidant treatment is effective in modulating RSV-induced clinical disease.
Figure 3.
Effect of BHA on clinical illness and airway hyperreactivity (AHR). Mice were infected with RSV and treated with BHA 250 mg/kg for the following 7 d. (A) Differences in the appearance of fur in RSV + BHA– versus RSV + vehicle–treated mice at Day 3 postinfection. (B) Clinical illness scores of RSV + BHA (open squares) and RSV + vehicle (solid squares) were measured from Day 1 to Day 5 postinfection. Sham-infected mice treated with either vehicle or BHA received a healthy illness score = 0 throughout the course of the experiment (data not shown). Data are expressed as mean ± SD of five different animals and are representative of three different experiments. *p < 0.01 relative to RSV-infected mice. (C) The effect of BHA treatment on RSV-induced AHR during methacholine challenge was measured at Day 4 postinfection by whole-body plethysmography. All treatment groups were given an initial dose of saline and subsequently challenged with increasing concentrations of methacholine (mg/ml). Penh is reported as a percentage increase from baseline saline challenge. Data are expressed as mean ± SD of eight different animals. Open squares, sham; RSV, solid triangles; cross marks, RSV + BHA. *p < 0.01 relative to RSV-infected mice.
We and others have previously shown that RSV infection induces AHR in response to methacholine challenge (18, 23, 28). As shown in Figure 3C, we observed a significant difference between RSV- and RSV + BHA–treated animals, because administration of BHA strongly attenuated RSV-induced AHR at all methacholine doses. BHA treatment did not alter baseline Penh values or airway response to methacholine in sham-infected animals.
Effect of BHA on RSV-induced Pulmonary Inflammation
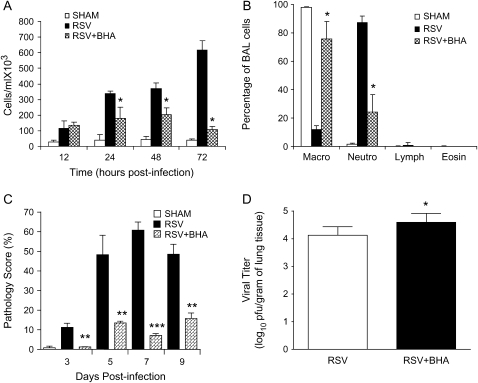
To determine whether BHA altered RSV-induced lung inflammation, total and differential cell counts in the BAL were measured. We observed a significant attenuation of cellular influx by BHA treatment in RSV-infected mice, starting at Day 1 postinfection and continuing throughout the infection, with the most significant difference observed at Day 3 postinfection (Figure 4A), which, in our mouse model, corresponds to the peak of inflammatory cell recruitment in the BAL after RSV infection. Macrophages are the normal resident cell type found within the airways in uninfected mice. RSV infection induces a significant lung recruitment of neutrophils, which become the predominant inflammatory cell observed in the BAL in the first few days of infection (29). As shown in Figure 4B, BHA administration significantly blocked neutrophil recruitment into the airways, with little difference observed among the different experimental groups in lymphocyte and eosinophil populations.
Figure 4.
Effect of BHA on airway inflammation and viral replication. (A) Total number of cells was measured in BAL of sham and RSV-infected mice, either untreated or treated with BHA, at various times postinfection. Data are expressed as mean ± SD of five different animals and representative of three different experiments. *p < 0.01 relative to RSV-infected mice. (B) Differential cell count was measured in BAL of sham and RSV-infected mice, either untreated or treated with BHA, at Day 3 postinfection. *p < 0.01 relative to RSV-infected mice. (C) Mice were infected with RSV or sham-infected and treated with either vehicle or BHA. At various days postinfection, mice were killed and lungs were excised, fixed in 10% buffered formalin, and embedded in paraffin. Lung sections were stained with hemotoxylin and eosin, and peribronchial, perivascular inflammation was scored. Data are expressed as mean ± SD of four animals/group. Data shown are representative of three independent experiments. **p < 0.01 relative to RSV-infected mice; ***p < 0.001 relative to sham-infected mice. (D) Mice were infected with RSV and either treated with vehicle or BHA. At Day 5 postinfection, lungs were excised and viral load was determined by plaque assay. Data shown are representative of three independent experiments (n = 10, mean ± SD). *p < 0.05 relative to RSV-infected mice.
To further examine the effect of BHA treatment on RSV-induced inflammation, lungs of infected mice were analyzed for histopathology. The perivascular and peribronchial mononuclear cell recruitment in the lung was examined at Days 3, 5, 7, and 9 postinfection. A dramatic decrease in lung inflammation between untreated and BHA-treated infected mice was observed as early as Day 3 postinfection, bringing the histopathology score of the treated RSV-infected mice to levels similar to that of uninfected control animals, with a similar, although less striking, effect at Days 5, 7, and 9 postinfection, as shown in Figure 4C.
Because inflammatory cells, especially neutrophils, can play an important role against viral replication, at the early stage of infection (30, 30), we examined whether BHA altered RSV replication. In our mouse model of RSV infection, increased viral titer can be detected as early as Day 3 postinfection, with peak viral titer occurring at Day 5 postinfection, and viral clearance by Day 7 postinfection (27). We found a consistent increase of approximately a half log in viral titer in BHA-treated animals at Days 4 and 5 postinfection, as shown in Figure 4D, where antioxidant administration increased peak viral load from 4.12 ± 0.31 to 4.59 ± 0.32 log10. RSV replication was usually no longer detectable at Day 7 postinfection in both untreated and BHA-treated infected mice, although occasionally we could recover virus at a very low titer (101) in the treated animals.
BHA Attenuates RSV-induced Oxidative Stress in the Lung
To determine whether antioxidant treatment affected RSV-induced oxidative damage of the lung, MDA and 4-HNE, as well as 8-isoprostanes, were measured in the BAL of RSV-infected mice, with or without BHA treatment. As shown in Figure 5, the average levels of MDA and 4-HNE were between 2- and 2.5-fold higher in RSV-infected BAL samples in comparison to control mice at all time points of RSV infection. BHA administration clearly lowered RSV-induced lipid peroxidation to values equivalent to control at all time points. Similarly, RSV-induced 8-isoprostane production was significantly reduced at all time points tested.
Figure 5.
Effect of BHA on lipid peroxidation. (A) MDA and 4-HNE or (B) 8-isoprostane were detected by either colorimetric assay or ELISA in BAL of mice infected with RSV and treated with either vehicle (solid bars) or BHA (open bars) at various times postinfection. Hatched bars, sham-treated mice. The bar in the scatter plot represents the mean of five different animals. ×, sham; solid diamonds, RSV; open circles, RSV + BHA. Data shown are representative of two independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001 relative to RSV-infected mice.
Effect of BHA on Chemokine and Cytokine Induction
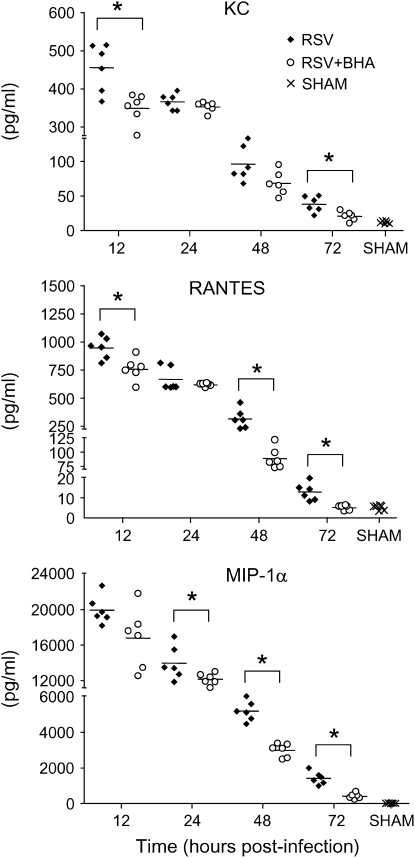
RSV infection is a potent inducer of chemokine production, and increased chemokine release has been shown to play an important role in RSV-induced lung inflammation and to correlate with disease severity (reviewed in Reference 5). In our model, the peak of chemokine production occurs during the first 3 d of infection (25), after which chemokines are no longer measurable. To determine whether BHA treatment was able to modulate RSV-induced chemokine secretion, we analyzed chemokine levels in BAL samples collected during the first 3 d of infection. As shown in Figure 6, RANTES, KC, and MIP-1α were strongly up-regulated in the lungs of infected animals, starting as early as 12 h postinfection. BHA treatment consistently reduced all three chemokine protein levels, especially at 48 and 72 h postinfection.
Figure 6.
Effect of BHA on chemokine release in the BAL. Chemokine production was measured by Bio-Plex bead suspension array in the BAL of mice infected with RSV and treated with either vehicle or BHA at various times postinfection. The bars in the scatter plot represent the mean of six different animals. Data shown are representative of three independent experiments. *p < 0.01 relative to RSV-infected mice.
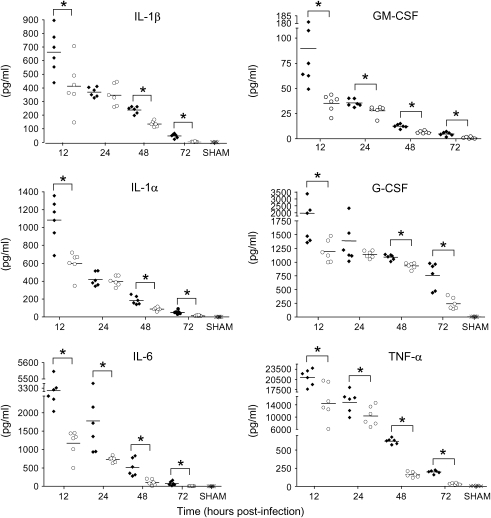
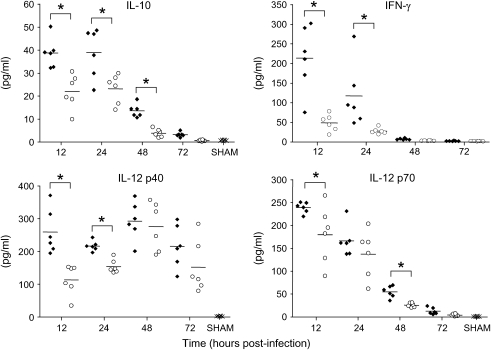
Proinflammatory cytokines, such as IL-1, IL-6, and TNF, as well as immunomodulatory cytokines, such as IL-10, IL-12, and IFN-γ, have been implicated in the pathogenesis of RSV-induced lung disease (reviewed in References 5 and 31). As with chemokine secretion, significant cytokine production after RSV infection can be measured mainly during the first 3 d of infection, with the exception of IL-12 and IFN-γ (25). Similar to what we observed for chemokine production, BHA treatment effectively reduced the secretion of all tested RSV-induced cytokines at Days 1, 2, and 3 postinfection (Figures 7 and 8). IFN-γ secretion was also significantly reduced at later time points (Day 5: RSV, 128.7 ± 63.3, vs. RSV + BHA, 22.1 ± 5.3 pg/ml; Day 7: RSV, 294.8 ± 69, vs. RSV + BHA, 5.8 ± 1.7), indicating that antioxidant treatment exerts a general antiinflammatory activity in the context of RSV infection of the lung.
Figure 7.
Effect of BHA on proinflammatory cytokines release in BAL. Cytokine production was measured by Bio-Plex bead suspension array in the BAL of mice infected with RSV and treated with either vehicle or BHA at various times postinfection. The bars in the scatter plot represent the mean of six different animals. Data shown are representative of three independent experiments. ×, sham; solid diamonds, RSV; open circles, RSV + BHA.*p < 0.01 relative to RSV-infected mice.
Figure 8.
Effect of BHA on immunomodulatory cytokines in BAL. Cytokine production was measured by Bio-Plex bead suspension array in the BAL of mice infected with RSV and treated with either vehicle or control at various times postinfection. The bars in the scatter plot represent the mean of six different animals. Data shown are representative of three independent experiments.×, sham; solid diamonds, RSV; open circles, RSV + BHA. *p < 0.01 relative to RSV-infected mice.
Effect of BHA on RSV-induced LT Production
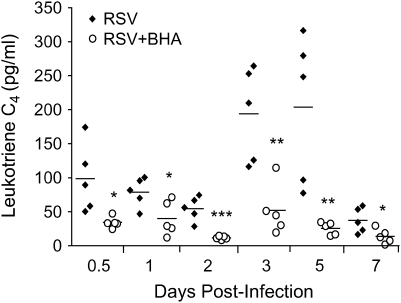
Cys-LTs, which include LTC4, LTD4, and LTE4, are important mediators of airway inflammation and bronchoconstriction (reviewed in Reference 32). They have been shown to increase in the respiratory secretion of children infected with RSV (33) and are associated with RSV-induced wheezing (34). We and others have shown that inhibition of LT synthesis (35) or treatment with LT receptor antagonist (36) inhibits lung inflammation and AHR in a mouse model of RSV infection. To determine whether the antioxidant BHA had an effect on RSV-induced cys-LT release, LTC4 was measured in BAL at various times postinfection. As shown in Figure 9, BHA-treated mice had significantly lower levels of LTC4, which was particularly evident at Days 3 and 5 postinfection.
Figure 9.
Effect of BHA on RSV-induced leukotriene release. Leukotriene C4 production was measured by ELISA in BAL of RSV-infected mice treated with either vehicle or BHA at various time points after infection. The bars in the scatter plot represent the mean of five different animals. Data shown are representative of three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001 relative to RSV-infected mice.
DISCUSSION
Oxidative stress has been implicated in the pathogenesis of several acute and chronic airway diseases, such as asthma and chronic obstructive pulmonary disease (reviewed in Reference 37). ROS generation, due to the respiratory burst of activated phagocytic cells recruited to the airways during viral infections, is an important antiviral defense, necessary for viral clearance. However, a robust production of ROS can lead to depletion of antioxidants and cause oxidative stress. Surprisingly, little is known regarding the role of ROS in respiratory virus–induced lung diseases, with the exception of influenza. It was not known whether modulation of ROS production with antioxidants would be of clinical benefit in the contest of RSV infection, for which no effective therapeutic treatment is currently available. In this study, we took advantage of an in vivo model of RSV infection to test the effect of antioxidant treatment on RSV-induced lung disease. We show for the first time that RSV infection induces lung oxidative stress, as evidenced by increase in the lipid peroxidation products. This is an important finding, because this information was available only for a mouse model of influenza infection (15). Remarkably, there are no studies investigating lipid peroxidation products in children or adults infected with respiratory viruses. These biomarkers of oxidative stress have been shown to be elevated in plasma and breath condensate of patients, both children and adults, with various acute and chronic inflammatory lung diseases, and to correlate with severity of illness in certain disease conditions (reviewed in Reference 38). In our study, antioxidant treatment with BHA significantly decreased the content of MDA and 4-HNE in BAL of RSV-infected mice and ameliorated both body weight loss and clinical illness, indicating that modulation of oxidative stress in the context of RSV infection can lead to improvement in lung disease. It is important to note that these findings were reproduced by the use of another antioxidant agent, dimethyl sulfoxide (DMSO). Oral administration of DMSO at the time of infection significantly reduced RSV-induced body weight loss and clinical disease (data not shown). However, given the systemic toxicity of DMSO, we did not use it for further experiments.
Although it is difficult to determine the precise mechanisms by which antioxidants provided protection in the context of RSV infection, attenuation of inflammation is likely a key one. We and others have shown that RSV-induced chemokine and cytokine release and subsequent pulmonary inflammation play a fundamental role in the pathogenesis of RSV-induced disease (reviewed in References 5, 39, and 40). Lack of production of specific chemokines, such as MIP-1α (21), or modulation of chemokine secretion, for example by perflubron treatment (24), has been shown to significantly reduce RSV-induced pulmonary inflammation. BHA treatment significantly reduced pulmonary cytokine and chemokine production after RSV infection, resulting in inhibition of lung recruitment of inflammatory cells, especially neutrophils, which are the major cell type responsible for oxidative burst in response to infectious stimuli. BHA treatment was effective if given before or at the moment of RSV infection, but did not result in significant improvement of clinical disease administered 1 d after infection, when clinical disease and lung inflammation are already present. This finding suggests that early therapeutic intervention may be necessary to improve clinical outcome in RSV infection. In fact, treatment of children with RSV-induced lower respiratory tract infection with antiinflammatory drugs, such as steroids, has been shown to be mostly ineffective in improving clinical disease, as these drugs are usually administered when children are hospitalized, days after manifestation of initial symptoms (reviewed in Reference 41).
Although BHA administration slightly increased RSV replication in the lung, there is no documented correlation between RSV viral titer and severity of illness. Antiviral treatment with ribavirin alone or immunotherapy with specific immunoglobulin does not provide significant clinical improvement, indicating that RSV-induced lung disease is most likely the result of the inflammatory response more than a direct viral cytopathic effect (reviewed in Reference 42). On the other hand, inhibition of NO production, which is antiviral against RSV in vitro and in vivo (18, 43), significantly reduces pulmonary inflammation and airway hyperresponsiveness, although it increases viral replication (18), suggesting again that modulation of inflammation is fundamental to controlling RSV disease.
RSV infection early in life has been associated with AHR and recurrent wheezing, and possibly long-term pulmonary abnormalities (4, 44, 45). In the mouse model of RSV infection, whole-body plethysmography has been used to measure airway resistance, and we have found that AHR to inhaled methacholine correlates with pulmonary resistance when measured with more invasive techniques (18, 36, 46). In our study, we found that antioxidant treatment attenuated RSV-induced AHR, possibly due to a reduction in cys-LT production. Cys-LTs are potent bronchoconstrictors, as well as proinflammatory mediators, and their role in the pathogenesis of airway inflammation and obstruction has been recently recognized as a new target for therapeutic intervention (reviewed in Reference 32). They have been shown to increase in the respiratory secretion of children infected with RSV (33, 47) and are associated with RSV-induced wheezing (34). In a mouse model of RSV infection, increased levels of cys-LTs correlate with increased airway responsiveness (35), and either inhibition of LT production (35) or treatment with LT receptor antagonist (36) inhibits AHR. In children, treatment with a cys-LT receptor antagonist decreased exacerbations of reactive airway disease after RSV bronchiolitis (48). In our study, BHA administration significantly decreased LTC4 secretion, with parallel reduction of AHR after methacholine challenge, suggesting a causal relationship of the two phenomena, although we cannot exclude that inhibition of other important mediators could be responsible for AHR reduction after BHA treatment.
Although little information is available regarding respiratory infections, there is a body of literature showing that antioxidants can afford protection against virally induced morbidity and mortality and are important in host immune responses (reviewed in References 49 and 50). For example, butylated hydroxytoluene, an analog of BHA, provided increased survival of chickens infected with Newcastle disease virus (51). In conclusion, the ability of antioxidants to attenuate symptoms and pathology in RSV infection warrants further investigation of these agents as a novel therapeutic approach to virally induced pulmonary disease.
Supported by grants NIEHS 06676 (National Institute of Environmental Health Sciences) and NIAID 053785 and 062885 (National Institute of Allergy and Infectious Diseases). S.M.C. was supported by NIEHS T32 ES07254 and NRSA F31 GM072231 (Ruth L. Kirchstein National Research Service Award).
Originally Published in Press as DOI: 10.1164/rccm.200603-319OC on September 28, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hall CB. Respiratory syncytial virus: a continuing culprit and conundrum. J Pediatr 1999;135:2–7. [PubMed] [Google Scholar]
- 2.Glezen WP, Taber LH, Frank AL. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986;140:543–546. [DOI] [PubMed] [Google Scholar]
- 3.Groothuis JR, Gutierre KM, Lauer BA. Respiratory syncytial virus infection in children with bronchopulmonary dysplasia. Pediatrics 1988;82:199–203. [PubMed] [Google Scholar]
- 4.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics 1995;95:500–505. [PubMed] [Google Scholar]
- 5.Garofalo RP, Haeberle H. Epithelial regulation of innate immunity to respiratory syncytial virus. Am J Respir Cell Mol Biol 2000;23:581–585. [DOI] [PubMed] [Google Scholar]
- 6.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med 2000;28:463–499. [DOI] [PubMed] [Google Scholar]
- 7.Prasad Gabbita S, Robinson KA, Stewart CA, Floyd RA, Hensley K. Redox regulatory mechanisms of cellular signal transduction. Arch Biochem Biophys 2000;376:1–13. [DOI] [PubMed] [Google Scholar]
- 8.Casola A, Burger N, Liu T, Jamaluddin M, Brasier AR, Garofalo RP. Oxidant tone regulates RANTES gene transcription in airway epithelial cells infected with respiratory syncytial virus: role in viral-induced interferon regulatory factor activation. J Biol Chem 2001;276:19715–19722. [DOI] [PubMed] [Google Scholar]
- 9.Liu T, Castro S, Brasier AR, Jamaluddin M, Garofalo RP, Casola A. Reactive oxygen species mediate virus-induced STAT activation: role of tyrosine phosphatases. J Biol Chem 2004;279:2461–2469. [DOI] [PubMed] [Google Scholar]
- 10.MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol 2001;429:195–207. [DOI] [PubMed] [Google Scholar]
- 11.Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 1996;154:1055–1060. [DOI] [PubMed] [Google Scholar]
- 12.Morcillo EJ, Estrela J, Cortijo J. Oxidative stress and pulmonary inflammation: pharmacological intervention with antioxidants. Pharmacol Res 1999;40:393–404. [DOI] [PubMed] [Google Scholar]
- 13.Akaike T, Ando M, Oda T, Doi T, Ljiri S, Araki S, Maeda H. Dependence on O2 generation by xanthine oxidase of pathogenesis of influenza virus infection in mice. J Clin Invest 1990;85:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akaike T, Noguchi Y, Ijiri S, Setoguchi K, Suga M, Zheng YM, Dietzschold B, Maeda H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA 1996;93:2448–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suliman HB, Ryan LK, Bishop L, Folz RJ. Prevention of influenza-induced lung injury in mice overexpressing extracellular superoxide dismutase. Am J Physiol Lung Cell Mol Physiol 2001;280:L69–L78. [DOI] [PubMed] [Google Scholar]
- 16.Ghezzi P, Ungheri D. Synergistic combination of N-acetylcysteine and ribavirin to protect from lethal influenza viral infection in a mouse model. Int J Immunopathol Pharmacol 2004;17:99–102. [DOI] [PubMed] [Google Scholar]
- 17.Kumar P, Sharma S, Khanna M, Raj HG. Effect of Quercetin on lipid peroxidation and changes in lung morphology in experimental influenza virus infection. Int J Exp Pathol 2003;84:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stark JM, Khan AM, Chiappetta CL, Xue H, Alcorn JL, Colasurdo GN. Immune and functional role of nitric oxide in a mouse model of respiratory syncytial virus infection. J Infect Dis 2005;191:387–395. [DOI] [PubMed] [Google Scholar]
- 19.Ueba O. Respiratory syncytial virus: I. Concentration and purification of the infectious virus. Acta Med Okayama 1978;32:265–272. [PubMed] [Google Scholar]
- 20.Patel JA, Kunimoto M, Sim TC, Garofalo R, Eliott T, Baron S, Ruuskanen O, Chonmaitree T, Ogra PL, Schmalstieg F. Interleukin-1 alpha mediates the enhanced expression of intercellular adhesion molecule-1 in pulmonary epithelial cells infected with respiratory syncytial virus. Am J Respir Cell Mol Biol 1995;13:602–609. [DOI] [PubMed] [Google Scholar]
- 21.Haeberle HA, Kuziel WA, Dieterich HJ, Casola A, Gatalica Z, Garofalo RP. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1alpha in lung pathology. J Virol 2001;75:878–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piedra P, Camussi G, Ogra PL. Immune response to experimentally induced infection with respiratory syncytial virus: possible role in the development of pulmonary disease. J Gen Virol 1989;70:325–333. [DOI] [PubMed] [Google Scholar]
- 23.Colasurdo GN, Hemming VG, Prince GA, Gelfand AS, Loader JE, Larsen GL. Human respiratory syncytial virus produces prolonged alterations of neural control in airways of developing ferrets. Am J Respir Crit Care Med 1998;157:1506–1511. [DOI] [PubMed] [Google Scholar]
- 24.Haeberle HA, Nesti F, Dieterich HJ, Gatalica Z, Garofalo RP. Perflubron reduces lung inflammation in respiratory syncytial virus infection by inhibiting chemokine expression and nuclear factor-κB activation. Am J Respir Crit Care Med 2002;165:1433–1438. [DOI] [PubMed] [Google Scholar]
- 25.Guerrero-Plata A, Casola A, Garofalo RP. Human metapneumovirus induces a profile of lung cytokines distinct from that of respiratory syncytial virus. J Virol 2005;79:14992–14997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kharitonov SA, Barnes PJ. Biomarkers of some pulmonary diseases in exhaled breath. Biomarkers 2002;7:1–32. [DOI] [PubMed] [Google Scholar]
- 27.Guerrero-Plata A, Baron S, Poast JS, Adegboyega PA, Casola A, Garofalo RP. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J Virol 2005;79:10190–10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jafri HS, Chavez-Bueno S, Mejias A, Gomez AM, Rios AM, Nassi SS, Yusuf M, Kapur P, Hardy RD, Hatfield J, et al. Respiratory syncytial virus induces pneumonia, cytokine response, airway obstruction, and chronic inflammatory infiltrates associated with long-term airway hyperresponsiveness in mice. J Infect Dis 2004;189:1856–1865. [DOI] [PubMed] [Google Scholar]
- 29.Graham BS, Perkins MD, Wright PF, Karzon DT. Primary respiratory syncytial virus infection in mice. J Med Virol 1988;26:153–162. [DOI] [PubMed] [Google Scholar]
- 30.Tsuru S, Fujisawa H, Taniguchi M, Zinnaka Y, Nomoto K. Mechanism of protection during the early phase of a generalized viral infection. II. Contribution of polymorphonuclear leukocytes to protection against intravenous infection with influenza virus. J Gen Virol 1987;68:419–424. [DOI] [PubMed] [Google Scholar]
- 31.Graham BS, Johnson TR, Peebles RS. Immune-mediated disease pathogenesis in respiratory syncytial virus infection. Immunopharmacology 2000;48:237–247. [DOI] [PubMed] [Google Scholar]
- 32.Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol 2004;173:1503–1510. [DOI] [PubMed] [Google Scholar]
- 33.Dimova-Yaneva D, Russell D, Main M, Brooker RJ, Helms PJ. Eosinophil activation and cysteinyl leukotriene production in infants with respiratory syncytial virus bronchiolitis. Clin Exp Allergy 2004;34:555–558. [DOI] [PubMed] [Google Scholar]
- 34.van Schaik SM, Tristram DA, Nagpal IS, Hintz KM, Welliver RC Jr, Welliver RC. Increased production of IFN-gamma and cysteinyl leukotrienes in virus-induced wheezing. J Allergy Clin Immunol 1999;103:630–636. [DOI] [PubMed] [Google Scholar]
- 35.Welliver RC, Hintz KH, Glori M, Welliver RC Sr. Zileuton reduces respiratory illness and lung inflammation, during respiratory syncytial virus infection, in mice. J Infect Dis 2003;187:1773–1779. [DOI] [PubMed] [Google Scholar]
- 36.Fullmer JJ, Khan AM, Elidemir O, Chiappetta C, Stark JM, Colasurdo GN. Role of cysteinyl leukotrienes in airway inflammation and responsiveness following RSV infection in BALB/c mice. Pediatr Allergy Immunol 2005;16:593–601. [DOI] [PubMed] [Google Scholar]
- 37.Folkerts G, Kloek J, Muijsers RB, Nijkamp FP. Reactive nitrogen and oxygen species in airway inflammation. Eur J Pharmacol 2001;429:251–262. [DOI] [PubMed] [Google Scholar]
- 38.Wood LG, Gibson PG, Garg ML. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur Respir J 2003;21:177–186. [DOI] [PubMed] [Google Scholar]
- 39.Mejias A, Chavez-Bueno S, Ramilo O. Respiratory syncytial virus pneumonia: mechanisms of inflammation and prolonged airway hyperresponsiveness. Curr Opin Infect Dis 2005;18:199–204. [DOI] [PubMed] [Google Scholar]
- 40.Tripp RA. Pathogenesis of respiratory syncytial virus infection. Viral Immunol 2004;17:165–181. [DOI] [PubMed] [Google Scholar]
- 41.Broughton S, Greenough A. Drugs for the management of respiratory syncytial virus infection. Curr Opin Investig Drugs 2004;5:862–865. [PubMed] [Google Scholar]
- 42.Blanco JC, Boukhvalova MS, Hemming P, Ottolini MG, Prince GA. Prospects of antiviral and anti-inflammatory therapy for respiratory syncytial virus infection. Expert Rev Anti Infect Ther 2005;3:945–955. [DOI] [PubMed] [Google Scholar]
- 43.Ali-Ahmad D, Bonville CA, Rosenberg HF, Domachowske JB. Replication of respiratory syncytial virus is inhibited in target cells generating nitric oxide in situ. Front Biosci 2003;8:a48–a53. [DOI] [PubMed] [Google Scholar]
- 44.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med 1997;156:766–775. [DOI] [PubMed] [Google Scholar]
- 45.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354:541–545. [DOI] [PubMed] [Google Scholar]
- 46.Collins RA, Gualano RC, Zosky GR, Atkins CL, Turner DJ, Colasurdo GN, Sly PD. Hyperresponsiveness to inhaled but not intravenous methacholine during acute respiratory syncytial virus infection in mice. Respir Res 2005;6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garofalo RP, Welliver RC, Ogra PL. Concentrations of LTB4,LTC4,LTD4 and LTE4 in bronchiolitis due to respiratory syncytial virus. Pediatr Allergy Immunol 1991;2:30–37. [Google Scholar]
- 48.Bisgaard H. Montelukast in RSV-bronchiolitis. Am J Respir Crit Care Med 2004;169:542–543. [DOI] [PubMed] [Google Scholar]
- 49.Beck MA, Handy J, Levander OA. The role of oxidative stress in viral infections. Ann N Y Acad Sci 2000;917:906–912. [DOI] [PubMed] [Google Scholar]
- 50.Beck MA. Antioxidants and viral infections: host immune response and viral pathogenicity. J Am Coll Nutr 2001;20:384S–388S. [DOI] [PubMed] [Google Scholar]
- 51.Brugh M Jr. Butylated hydroxytoluene protects chickens exposed to Newcastle disease virus. Science 1977;197:1291–1292. [DOI] [PubMed] [Google Scholar]