Aldosterone Antagonists for Preventing the Progression of Chronic Kidney Disease: A Systematic Review and Meta-analysis (original) (raw)
Abstract
Background and objectives: Addition of aldosterone antagonists (AA) might provide renal benefits to proteinuric chronic kidney disease (CKD) patients over and above the inhibition of renin-angiotensin system blockers (RAS). We evaluated the benefits and harms of adding selective and nonselective AA in CKD patients already on RAS.
Design, setting, participants, & measurements: MEDLINE, EMBASE, and Renal Health Library were searched for relevant randomized clinical trials in adult CKD patients. Results were summarized using the random-effects model.
Results: Eleven trials (991 patients) were included. In comparison to angiotensin- converting enzyme inhibitors (ACEi) and/or angiotensin receptor blockers (ARB) plus placebo, nonselective AA along with ACEi and/or ARB significantly reduced 24 h proteinuria (seven trials, 372 patients, weighted mean difference [WMD] −0.80 g, 95% CI −1.27, −0.33) and BP. This did not translate into an improvement in GFR (WMD −0.70 ml/min/1.73m2, 95% CI −4.73, 3.34). There was a significant increase in the risk of hyperkalemia with the addition of nonselective AA to ACEi and/or ARB (relative risk 3.06, 95% CI 1.26, 7.41). In two trials, addition of selective AA to ACEi resulted in an additional reduction in 24 h proteinuria, without any impact on BP and renal function. Data on cardiovascular outcomes, long-term renal outcomes and mortality were not available in any of the trials.
Conclusions: Aldosterone antagonists reduce proteinuria in CKD patients already on ACEis and ARBs but increase the risk of hyperkalemia. Long-term effects of these agents on renal outcomes, mortality, and safety need to be established.
Chronic kidney disease (CKD) affects 25 to 30 million Americans and several million people across the globe in both developed and developing nations (1,2). A number of treatment options have been shown to delay the progression of kidney damage (3,4). To date, the major impact on delaying the progression of CKD and the risk of end-stage kidney disease (ESKD) has been provided by the use of renin-angiotensin system (RAS) blockers such as angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor-blocking agents (ARB). These agents have become standard of care in proteinuric CKD patients (5–8). Both ACEi and ARB significantly reduce proteinuria and the risk of ESKD by about 20 to 30% (9). But this is suboptimal given the higher costs and burden of ESKD (10). Thus, studies exploring interventions for traditional and nontraditional risk factors for CKD are advocated (11).
Animal studies have shown that aldosterone has an independent role in the development of hypertensive nephropathy and vascular injury resulting in myocardial and renal fibrosis, and its blockade reduces proteinuria (12–16). In humans, RAS blockade with ACEi or ARB results in an incomplete suppression of serum aldosterone levels, a phenomenon known as “aldosterone escape” (17). When patients are treated with ACEi or ARB, aldosterone levels decrease during the earlier part of the treatment period but subsequently increase within a few months, despite continued treatment with ACEi or ARB therapy. This aldosterone escape is associated with enhanced excretion of urinary albumin and decline in GFR.
Commercially available aldosterone antagonists include selective (eplerenone) and nonselective antagonists (spirinolactone). Both selective and nonselective aldosterone antagonists reduce the risk of cardiovascular mortality and hospitalization in patients with congestive heart failure (18–20). These beneficial effects may be counterbalanced by harms. In the general population, gynecomastia occurs in about 10% of patients with spirinolactone but not with the selective aldosterone blocker, eplerenone (21). Hyperkalemia is a concern with both these agents, and the combined use of aldosterone blockade with ACEi and ARB in patients with CKD could further affect it (22).
Both these agents have been tested in randomized controlled trials (RCTs) to analyze their role in reducing the albuminuria or proteinuria and retarding the progression of CKD (23–27). We analyzed the benefits and harms of adding selective and nonselective aldosterone antagonists to patients with CKD and proteinuria who were already on ACEi or ARB or both and the effects of aldosterone antagonist alone on proteinuria, renal function, and other major patient level end-points in comparison to placebo in these same populations.
Materials and Methods
We searched MEDLINE (1966 to August 2008); EMBASE (1980 to August 2008); the Renal Health Library (Issue 2, 2008); and hand-searched reference lists of textbooks, articles, and scientific proceedings for relevant articles (Search strategy-appendix 1) (28,29).
Type of Studies
All RCTs and quasi-RCTs of aldosterone antagonists (both selective and nonselective antagonists) used alone or in combination with ACEi alone, ARB alone, or both for preventing the progression of CKD in patients with proteinuria or albuminuria and CKD were included. The first period of randomized cross-over studies was also considered for inclusion. There was no language restriction.
Types of Participants
Studies enrolling any patient with CKD stages 1 to 4 (as defined by the Kidney-Disease Outcomes and Quality Initiative [K-DOQI] guidelines: stage 1 = GFR ≥90 ml/min/1.73 m2; stage 2 = GFR 60 to 89 ml/min/1.73 m2; stage 3 = GFR 30 to 59 ml/1.73 m2; stage 4 = GFR 15 to 29 ml/min/1.73 m2) with albuminuria or proteinuria secondary to both diabetic and nondiabetic CKD were included. We excluded studies enrolling patients with CKD stage 5 (GFR <15 ml/min/1.73 m2) and/or receiving hemodialysis or other forms of renal replacement therapy.
Types of Interventions
We included studies of aldosterone antagonists (both selective and nonselective antagonists) with ACEi or ARB or both given for at least four weeks.
Types of Outcome Measures
Data on the effects of aldosterone antagonists over and above that of renin-angiotensin blockade on the following outcome measures were planned to be analyzed: a) end of treatment urinary albumin/protein excretion (24 h proteinuria or 24 h albuminuria in g/d); b) renal function measured as end of treatment GFR (ml/min or ml/min/1.73 m2); c) end of treatment BP –systolic BP and diastolic BP (mmHg); d) doubling of serum creatinine; e) progression of micro- to macroalbuminuria; f) regression of macro- to microalbuminuria; g) regression of micro- to normoalbuminuria; h) need for renal replacement therapy; i) mortality rates; j) hospitalization rates; and k) adverse events –hyperkalemia (defined as serum potassium >5.5 mEq/L or mmol/L), and gynecomastia or breast pain.
Data Collection
The search strategy was used to obtain titles and abstracts of studies that may be relevant to the review. Titles and abstracts were screened independently by two reviewers (SDN, SUN), who discarded studies that did not meet the inclusion criteria. Studies and reviews that might include relevant data or information on trials were retained initially. The reviewers independently assessed retrieved abstracts and, if necessary, the full text of these studies to determine if they satisfied the inclusion criteria.
Data extraction was carried out by the reviewers independently, using standard data extraction forms. It was planned that studies reported in non-English language journals would be translated before assessment. It was also planned that where more than one publication of one trial existed, reports would be grouped together and the most recent or complete dataset would be used. Further information required from the original authors was requested by written correspondence whenever needed, and any relevant information obtained was included in the review. Disagreements between reviewers were resolved in consultation with Giovanni FM Strippoli.
Study Quality
The quality of included studies was assessed independently by (SDN) and (SUN) without blinding to authorship or journal using the checklist developed by the Cochrane Renal Group. The quality items assessed were allocation concealment, intention-to-treat analysis, completeness to follow-up and blinding of investigators, participants, and outcome assessors.
Statistical Assessment
For dichotomous outcomes (hyperkalemia, gynecomastia), results were expressed as relative risks (RR) with 95% confidence intervals (CI). Data were pooled using a random-effects model (30) but the fixed-effects model was also analyzed to ensure robustness of the model chosen and susceptibility to outliers. Where continuous scales of measurement were used to assess the effects of treatment (24 h proteinuria or albuminuria, GFR, BP) the WMD and its 95% CI were used. Heterogeneity was analyzed with a chi-squared test on N-1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I2 test (31). Analyses were performed using Revman 5 (©2007, The Cochrane Collaboration, UK).
Separate analyses were conducted for selective and nonselective aldosterone antagonists. An I2 value of >50% indicates statistically significant heterogeneity among the included studies. If substantial statistical heterogeneity were noted (I2 > 50%), we planned to explore individual study characteristics and those of subgroups of the main body of evidence. Plausible reasons for variations in treatment effect (heterogeneity) were explored using subgroup analysis specifically for length of follow-up, and baseline kidney disease. We also conducted a post hoc subgroup analysis to compare the incidence of hyperkalemia in patients who received spirinolactone plus two RAS inhibitors versus two RAS inhibitors alone, and in patients who received spirinolactone plus one RAS inhibitor, either ACEi or ARB versus ACEi or ARB alone.
Results
Search Results
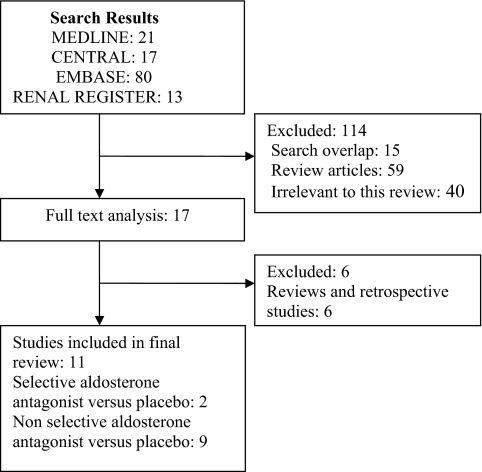
The combined search of MEDLINE, EMBASE, CENTRAL, and the Renal Health Library identified 133 citations, of which 116 were excluded (non-RCTs, trials that evaluated other interventions not relevant to this review). Full-text assessment of 17 potentially relevant articles resulted in the identification of 11 eligible trials (ten published studies {n = 776} and one abstract {n = 215}) enrolling a total of 991 patients (23–27,32–37) (Figure 1). Nine trials (482 patients) compared non-selective aldosterone antagonists plus ACEi and/or ARB to ACEi and/or ARB alone (23,24,26,27,32–36), and two trials (509 patients) compared selective aldosterone antagonists plus ACEi and/or ARB to ACEi and/or ARB alone (25,37). We did not find any studies that compared aldosterone antagonists alone to placebo (without ACEi and/or ARB). Authors of all included trials were contacted for additional information and clarification relating to study methods and any unreported data, with four responding to our queries (23,24,26,27).
Figure 1.
Flow chart showing the number of citations retrieved by individual searches and number of trials included in the systematic review.
Trial Characteristics
Eight studies included diabetic patients; the large study of Bianchi et al. included patients with various forms of glomerulonephritis (23); the remaining two studies included patients with nondiabetic renal disease encompassing IgA nephropathy, benign nephrosclerosis, and membranous nephropathy (35,36). All studies excluded patients with GFR <30 ml/mim/1.73m2. Baseline albuminuria/proteinuria ranged from 0.8 g/d to 3.6 g/d. Of nine studies analyzing the efficacy of nonselective aldosterone antagonists, three compared spirinolactone plus ACEi to ACEi alone (24,32,33) and six compared spirinolactone plus ACEi and/or ARB to ACEi and/or ARB alone (23,26,27,34–36).
In all studies 25 mg/d of spirinolactone was used throughout the study period except for the study of Meiracker et al., which used spirinolactone 25 to 50 mg/d (32). Study duration varied from two to 20 mo. Sample size of all studies was small (n = 18 to 268), and none were powered to detect hard primary outcomes including mortality or long-term renal outcomes. All studies reported change in 24 h proteinuria or albuminuria as the primary end-point. Studies that analyzed the role of selective aldosterone antagonist included patients with type 2 diabetes and the change in urinary albumin-creatinine ratio was the primary outcome measure. Other characteristics of the participants and the interventions of the included trials are detailed in Table 1.
Table 1.
Characteristics of the populations and interventions in the included trials of aldosterone antagonistsa
| Study | Baseline kidney disease | Baseline proteinuria (in g/24 h) or UACR (mean +/− SD) | Baseline renal function (ml/min/1.73 m2) | No. of patients | Intervention | Co-intervention in treatment arm(n) (ACEI and/or ARB) | Study duration (months) | Study end points |
|---|---|---|---|---|---|---|---|---|
| Bianchi 2006 (23) | Idiopathic GN | Treatment: 2.1+/−0.08 Control: 2.0+/−0.07 | 62.3 +/− 1.6 | 165 | Spirinolactone 25 mg daily | ACEI alone: 29 ARB alone: 17 ACEI + ARB: 37 | 12 | 24 h proteinuria, BP, serum creatinine, eGFR, hyperkalemia, gynecomastia |
| Chrysostomou 2006b (24) | Diabetic and non-diabetic nephropathy | ACEI alone: 2.6+/− 1.6 ACEI + ARB: 2.5 +/− 1.8 ACEI + Spirinolactone: 2.2+/−1.4 ACEI + ARB + Spirinolactone: 3.1+/− 1.9 | ACEI alone: 81.6 ACEI + ARB: 68.0 ACEI + Spirinolactone: 59.4 ACEI + ARB + Spirinolactone: 57.6 | 41 | Spirinolactone 25 mg daily | ACEI alone: 10 ACEI + ARB: 10 ACEI + Spirinolactone: 10 ACEI + ARB + Spiriniloactone: 11 | 6 | Primary: 24 h proteinuria Secondary: BP, serum creatinine, Cockraft-Gault creatinine clearance hyperkalemia |
| Epstein 2002 (37) | Diabetic nephropathy (Type 2) | NA | NA | 215 | EPL 200 mg/d vs. ACEI 40 mg/d vs. EPL 200 mg/d + ACEI 10 mg | NA | 6 | 24 h proteinuria, BP, serum creatinine, eGFR, hyperkalemia, gynaecomastia |
| Epstein 2006 (25) | Diabetic nephropathy (Type 2) | UACR Treatment 50 mg: 422 100 mg: 240 Control: 280 | Eplerenone 50 mg: 91 100 mg: 86 Control: 91 | 268 | EPL 50 mg and 100 mg daily | ACEI: 268 | 12 | Primary: Percentage change in UACR, incidence of hyperkalemia Secondary: change in BP, eGFR, adverse events, gynaecomastia |
| Furamatsu 2008 (35) | Non-diabetic nephropathy | > 0.5 g/day (both groups) u-Prot/u-Cr (g/g·Cr) Treatment: 1.42 ±0.28 Control: 1.44 ±0.28 | Treatment: 91.8 ±11.8 Control: 68.9 ±7.8 | 30 | Spirinolactone 25 mg daily | ACEI + ARB: 30 | 12 | Primary: Reduction in proteinuria Secondary: BP, Cockraft-Gault creatinine clearance, hyperkalemia, gynaecomastia |
| Rachmani 2004 (33) | Diabetic nephropathy (Type 2) | UACR Treatment: 456 mg/g Control: 451 mg/g | Serum creatinine >160 mmol/l | 46 | Spirinolactone 100 mg daily and decreased to 50 mg daily | NA | 20 | UACR, BP, serum creatinine, hyperkalemia |
| Rossing 2005c (26) | Diabetic nephropathy (Type 2) | >300 mg/24 h | GFR > 30 | 21 | Spirinolactone 25 mg daily | NA | 2 | 24 h albuminuria, BP, GFR (based on EDTA), hyperkalemia, |
| Schjoedt 2005c (27) | Diabetic nephropathy (Type 1) | >300 mg/24 h | GFR >30 | 20 | Spirinolactone 25 mg daily | NA | 2 | Primary: 24 h albuminuria Secondary: BP, serum creatinine, GFR (based on EDTA), hyperkalemia, gynaecomastia |
| Schjoedt 2006c (34) | Diabetic nephropathy (Type 1 and 2) | >2.5 g/24 h | GFR >30 | 20 | Spirinolactone 25 mg daily | 2 | 24 h albuminuria, BP, serum creatinine, GFR (based on EDTA), hyperkalemia | |
| Tylicki 2008 (36) | Non-diabetic nephropathy | 0.97 ±0.18 at the randomization point | Mean GFR 107.8 (93-140.9) | 18 | Spirinolactone 25 mg daily | ACEI + ARB: 18 | 2 | Primary: 24 h proteinuria Secondary: BP, Cockraft-Gault creatinine clearance, hyperkalemia |
| Van den Meiracker 2006 (32) | Diabetic nephropathy (Type 2) | Treatment: 0.7 Control: 1.0 | Treatment: 87 Control: 64 | 53 | Spirinolactone 50 mg daily | ACEI: 17 ARB: 7 | 12 | 24 h proteinuria, BP, serum creatinine, eGFR, hyperkalemia, gynecomastia |
Trial Quality
By current methodological standards, trial quality was variable. Allocation concealment was adequate in five of 11 trials (45%), and unclear in six out of 11 trials (55%). Participants, investigators, and outcome assessors were not blinded in any of the trials. Only one of the 11 trials (9%) was analyzed on an intention-to-treat basis. The drop-out rate ranged from 0 to 15% and did not differ between the treatment and control groups.
Study Outcomes
Nonselective aldosterone antagonists (Spirinolactone) plus ACEi and/or ARB versus ACEi and/or ARB alone
End of Treatment 24 h Proteinuria
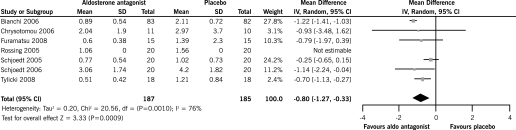
There was a significant reduction in 24 h proteinuria with spirinolactone plus ACEi and/or ARB (seven RCTs, 372 patients, WMD −0.80 g, 95% CI −1.27, −0.33) compared with ACEi and/or ARB alone. There was a significant heterogeneity between the studies included in this analysis (chi square = 20.56, P = 0.001, I2 = 76%) (Figure 2).
Figure 2.
Effect of aldosterone antagonists plus ACEi and/or ARB compared with ACEI and/or ARB alone on the end of treatment proteinuria.
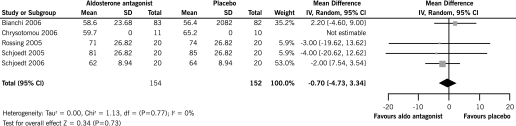
End of Treatment GFR
There was no significant difference in GFR with spirinolactone plus ACEi and/or ARB compared with ACEi and/or ARB alone (five RCTs, 306 patients, WMD −0.70 ml/min, 95% CI −4.73 to 3.34) and there was no significant heterogeneity between the studies included in the analysis (chi square = 1.13, P = 0.77, I2 = 0%) (Figure 3).
Figure 3.
Effect of aldosterone antagonists plus ACEi and/or ARB compared with ACEI and/or ARB alone on the end of treatment GFR.
End of Treatment Systolic BP
There was a significant reduction in systolic BP with spirinolactone along with ACEi and/or ARB compared with ACEi and/or ARB alone (seven RCTs, 372 patients, WMD −3.40 mmHg, 95% CI −5.13, −1.68) with no significant heterogeneity in this analysis (chi square = 3.67, P = 0.72, I2 = 0%).
End of Treatment Diastolic BP
There was a significant reduction in diastolic BP with spirinolactone along with ACEi and/or ARB compared with ACEi and/or ARB alone (six RCTs, 336 patients, WMD −1.79 mmHg, 95% CI −2.99, −0.59) and with no significant heterogeneity between the included studies (chi square = 2.87, P = 0.72, I2 = 0%).
Adverse Effects
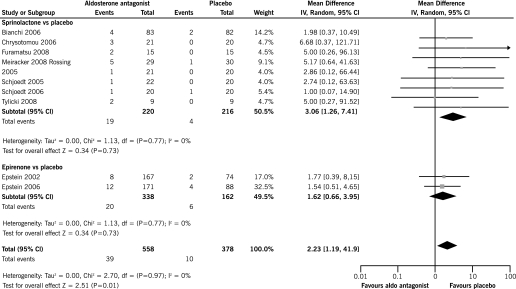
Hyperkalemia
There was a significant increase in the risk of hyperkalemia with spirinolactone along with ACEi and/or ARB compared with ACEi and/or ARB alone (eight trials, 436 patients, RR 3.06, 95% CI 1.26, 7.41) with no heterogeneity in this analysis (chi square = 1.69, P = 0.98, I2 = 0%) (Figure 4). In subgroup analyses, there was a significant increase in the risk of hyperkalemia with spirinolactone plus two RAS inhibitors (ACEi and ARB) in comparison to ACEi and ARB alone (four RCTs, 149 patients, RR 4.30, 95% CI 1.12, 16.51) while there was a nonsignificant increase in the risk of hyperkalemia when spirinolactone in addition to one RAS inhibitor only (ACEi or ARB) was compared with ACEi or ARB alone (five RCTs, 277 patients, RR 2.41, 95% CI 0.79, 7.31). A formal test of interaction found no significant differences across the estimates (P = 0.31).
Figure 4.
Incidence of hyperkalemia with aldosterone antagonists plus ACEi and/or ARB in comparison to ACEI and/or ARB alone.
Gynecomastia
In six included studies, there was no incidence of gynecomastia reported in both the treatment and control groups. Bianchi et al. reported that six out of 83 patients developed gynecomastia (only one patient warranting discontinuation of medication and five patients with mild gynecomastia) in the spirinolactone group but none (out of 82 patients) in the placebo arm (23). Furamatsu et al. reported one of 15 patients who developed gynecomastia in the spironolactone group that did not require discontinuation of therapy (35).
Analysis of Heterogeneity
Heterogeneity in the effects of nonselective aldosterone antagonists on 24 h proteinuria was explored through subgroup analyses by type of kidney disease and study duration. We could not explore the influence of baseline kidney function on the heterogeneity, as relevant data were not available from the studies.
Type of Baseline Kidney Disease
There was a significant reduction of 24 h proteinuria at the end of treatment period in studies that included patients with nondiabetic kidney disease (three RCTs, 231 patients, WMD −0.99 g/d, 95% CI −1.40, −0.57), but not in studies that included patients with diabetic kidney disease (four RCTs, 141 patients, WMD −0.46 g/d, 95% CI −1.00, 0.08). However, a formal test of interaction showed that the type of baseline kidney disease was not an effect modifier (P = 0.13).
Study Duration
There was a significant reduction of 24 h proteinuria at the end of the treatment period in both studies that had follow-up <6 mo (five RCTs, 177 patients, WMD −0.53 g/d, 95% CI −0.88, −0.18) and in studies that had follow-up >6 mo (two RCTs, 195 patients, WMD −1.21 g/d, 95% CI −1.40, −1.02), with no significant interaction (P value = 0.001).
Other Outcomes
We did not have outcome data for a) doubling of serum creatinine, b) progression of micro to macroalbuminuria, c) regression of macro to microalbuminuria, d) regression of micro to normoalbuminuria, e) need for renal replacement therapy, f) mortality rates, or g) hospitalization rates in the studies. Thus, a meta-analysis could not be conducted for these outcomes.
Selective Aldosterone Antagonists (Eplerenone) Plus ACEi and/or ARB versus ACEi and/or ARB alone
End of Treatment Proteinuria
At 12 wk, there was a significant reduction in proteinuria with the use of eplerenone plus ACEi in comparison to ACEi plus placebo.
End of Treatment BP
At 12 wk, there was a significant reduction in end of treatment systolic and diastolic BP with eplerenone plus ACEi in comparison to ACEi plus placebo arm.
End of Treatment GFR
There was no significant difference in end of treatment GFR with eplerenone plus ACEi compared with ACEi plus placebo.
We could not conduct a meta-analysis for the above outcomes as only two studies (one published study and one abstract) were included and additional data could not be obtained from the investigators.
Adverse Events
Hyperkalemia
There was no significant increase in the risk of hyperkalemia with eplerenone plus ACEi compared with ACEi plus placebo (two trials, 509 patients, RR 1.62, 95% CI 0.66, 3.95). There was no significant heterogeneity between the included studies (chi square = 0.02, P = 0.89, I2 = 0%) (Figure 4).
Gynecomastia
In one study, there was no incidence of gynecomastia, or female breast pain, reported in the eplerenone arm in comparison to ACEi (25).
Discussion
Key Findings
Our analysis of existing trials of selective and nonselective aldosterone antagonists administered over RAS inhibition in patients with CKD found that both reduced end-of-treatment 24 h proteinuria and BP but have not been found to have significant impact on end-of-treatment GFR. There was, however, a significantly higher incidence of hyperkalemia (>5.5 mEq/L) with nonselective antagonists compared with RAS inhibition only. This was not found with selective antagonists compared with RAS inhibition only, but direct comparison trials of selective versus nonselective aldosterone antagonists are not available. The impact of adding aldosterone blocking agents to RAS blockers on mortality and long-term renal outcomes has not been evaluated in existing studies.
Comparison to Other Studies
Introduction of RAS inhibition and the widespread use of this approach in both diabetic and nondiabetic CKD patients has determined a significant reduction, by about 20 to 30%, in the risk of progression of CKD to ESKD requiring renal replacement therapy (9,38,39). In addition, these agents have been found to have cardioprotective effects (9,38,39). Some patients exhibit aldosterone escape, a phenomenon resulting in blunting of the effect of ACEi or ARB (17). Addition of both selective and nonselective aldosterone antagonists has been purported to avoid this phenomenon. The beneficial effects noted with this add-on therapy are attributed to the profibrotic and BP lowering effects of aldosterone antagonists both in animal and human studies (40,41). Our data show that these agents contribute to a significant decrease in end-of-treatment 24 h urine protein excretion, independent of effects on BP but without any impact on the decline of GFR.
Bomback et al. recently analyzed the effects of adding mineralocorticoid receptor blockade to RAS blockers in patients with CKD in a systematic review (42). They included both observational and RCTs and concluded that this regimen decreases proteinuria without causing any hyperkalemia or impaired renal function. Authors did not conduct a meta-analysis due to the lack of relevant data despite their attempts to contact the investigators, and thus only reported a qualitative data. They did not find an increase in the risk of hyperkalemia in individual studies possibly due to the smaller sample size and because studies excluded patients with baseline potassium >5.0 mEq/L.
Our pooled analysis of these studies did show a threefold increase in the risk of hyperkalemia. In a subgroup analysis, restricting to triple blockade resulted in a fourfold increase in hyperkalemia when compared with dual blockade alone (with either ACEi or ARB alone). We also found that adding spirinolactone to ACEi or ARB alone in comparison to ACEi or ARB alone resulted in a nonsignificant increase in the risk of hyperkalemia. Thus, it appears that triple blockade might further increase the risk for hyperkalemia in comparison to ACEi or ARB plus spirinolactone. This is under the assumption (which is unique to good quality randomized controlled trials) that patients included in the studies had received similar doses of ACEi or ARB in both arms of the study, as varying doses of ACEi or ARB would alter the risk in treatment and control groups. However, we could not exclude a potential confounding due to imbalanced dosing of RAS inhibitors across the treatment and control group, as these data were not consistently reported and trials were small. Most patients who developed hyperkalemia in these studies had GFR between 30 to 60 ml/min/1.73m2. A separate analysis based on the severity of kidney disease could not be conducted as data stratified by GFR were unavailable from all the studies.
After publication of the Randomized Aldactone Study (RALES study), a higher incidence of hyperkalemia was reported as spirinolactone use increased in patients with congestive heart failure (43). Phillips et al. showed that combination therapy with ACEi and ARB in participants with symptomatic left ventricular dysfunction was accompanied by a fourfold increase in the risk of hyperkalemia (RR, 4.87, 95% CI, 2.39 to 9.94) (44). The recently published Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial found a higher incidence of hyperkalemia in patients who were on ACEi and ARB in comparison to ACEi or ARB alone (45). Thus, physicians should be aware of this risk of hyperkalemia whenever RAS blockade is being used and that addition of aldosterone antagonist may further increase this risk. Close monitoring of potassium levels is warranted when spirinolactone is added to treat proteinuria in patients on ARBs and/or ACEi in all patients and especially in patients with GFR <30 ml/min/1.73m2.
Strengths and Limitations
Our review has a number of strengths and limitations. Strengths include a systematic search of medical databases, data extraction and analysis, and trial quality assessment by two independent reviewers based on a prespecified protocol (46). The major limitation is the lack of long-term studies analyzing the efficacy of aldosterone antagonists on mortality, progression of CKD, and development of ESKD. The majority of the included studies enrolled few patients and were powered to observe differences in surrogate end-points rather than patient-focused outcomes. Five studies had a cross-over design (26,27,33,34,36) and most did not adequately report study methods to assess methods and trial quality.
There was a significant heterogeneity noted in our analysis of effects of adding an aldosterone antagonist to RAS inhibition on proteinuria. Our subgroup analyses, although limited by a very small number of trials, show that heterogeneity can be partly explained by the differences in the study duration of included studies. This may be interpreted as if duration of treatment seems to influence the reduction in proteinuria, i.e., longer the treatment duration, better the treatment effect (based on the WND). Although tempting, it would not be appropriate to conclude that proteinuria stabilizes after an initial favorable response with aldosterone antagonists, or longer treatment duration would result in better outcomes, as these results are from a subgroup analyses that included only a small number of studies/patients. We used the mean and SD of proteinuria (rather than geometric mean) from the included studies (either reported or obtained from the authors by request). Albuminuria/proteinuria has, however, a skewed distribution, which further limits the interpretation of these analyses.
Implications for Practice
In CKD patients with GFR >30 ml/min/1.73m2 who have persistent proteinuria despite being on maximal doses of ACEi and/or ARB, aldosterone antagonists could be added to reduce proteinuria. These agents may, however, have to be added at the lowest dose (spirinolactone should be initiated at 25 mg daily and eplerenone 50 mg daily) with close monitoring for hyperkalemia during the treatment period.
Implications for Research
Long-term studies analyzing the effect of aldosterone antagonists on surrogate end- points such as decline in GFR, doubling of serum creatinine, progression of micro- to macroalbuminuria, regression of micro- to normoalbuminuria, and hard end points such as development of ESKD and mortality are warranted. Studies could also compare the addition of aldosterone antagonists to ACEi and ARB separately and to their combination. These studies may analyze the efficacy of aldosterone antagonists in patients who exhibit aldosterone escape versus those who do not, as the beneficial effects and risk of hyperkalemia might be different between these two groups.
Disclosures
None.
Acknowledgments
We thank Narelle Willis, of Cochrane Renal Group, for her help in coordinating and editing this review and Ruth Mitchell and Gail Higgins, of Cochrane Renal Group, for assistance in the development of search strategies. We also thank Drs. K.J. Schjoedt, K. Rossing, A. Chrysostomou, and S. Bianchi for providing additional details about their studies that were included in this review. This systematic review was presented in abstract form at the American Society of Nephrology Annual Meeting, San Francisco, California; October 31 through November 5, 2007.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Grassmann A, Gioberge S, Moeller S, Brown G: ESRD patients in 2004: Global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant 20: 2587–2593, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Brenner B: Retarding the progression of renal disease. Kidney Int 64: 370–378, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G: The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 6.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia): Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 7.Mathiesen ER, Hommel E, Hansen HP, Smidt UM, Parving HH: Randomised controlled trial of long term efficacy of captopril on preservation of kidney function in normotensive patients with insulin dependent diabetes and microalbuminuria. BMJ 319: 24–35, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jafar TH, Schmid CH, Landa M, Giatras I, Toto R, Remuzzi G, Maschio G, Brenner BM, Kamper A, Zucchelli P, Becker G, Himmelmann A, Bannister K, Landais P, Shahinfar S, de Jong PE, de Zeeuw D, Lau J, Levey AS: Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease: A meta-analysis of patient-level data. Ann Intern Med 135: 73–87, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Strippoli GF, Bonifati C, Craig M, Navaneethan SD, Craig JC: Angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists for preventing the progression of diabetic kidney disease. Cochrane Database of Systematic Reviews Issue 4. Art. No.: CD006257. DOI: 10.1002/14651858.CD006257. 2006 [DOI] [PMC free article] [PubMed]
- 10.USRDS. http://www.usrds.org/2008/pdf/V2_11_2008.pdf. Accessed September 17, 2008.
- 11.Griffin KA, Bidani AK: Progression of renal disease: Renoprotective specificity of renin-angiotensin system blockade. Clin J Am Soc Nephrol 1: 1054–1065, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Schieppati A, Remuzzi G: The June 2003 Barry M. Brenner Comgan lecture. The future of renoprotection: Frustration and promises. Kidney Int 64: 1947–1955, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Aldigier JC, Kanjanbuch T, Ma LJ, Brown NJ, Fogo AB: Regression of existing glomerulosclerosis by inhibition of aldosterone. J Am Soc Nephrol 16: 3306–3314, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Greene EL, Kren S, Hostetter TH: Role of aldosterone in the remnant kidney model in the rat. J Clin Invest 98: 1063–1068, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocha R, Chander PN, Khanna K, Zuckerman A, Stier CT Jr: Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension 31: 451–458, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Silvestre JS, Robert V, Heymes C, Aupetit-Faisant B, Mouas C, Moalic JM, Swynghedauw B, Delcayre C: Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J Biol Chem 273: 4883–4891, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Staessen J, Lijnen P, Fagard R, Verschueren LJ, Amery A: Rise in plasma concentration of aldosterone during long-term angiotensin II suppression. J Endocrinol 91: 457–465, 1981 [DOI] [PubMed] [Google Scholar]
- 18.Hostetter TH, Ibrahim HN: Aldosterone in chronic kidney and cardiac disease. J Am Soc Nephrol 14: 2395–2401, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J: The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341: 709–717, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators: Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 348: 1309–1321, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Thompson DF, Carter JR: Drug-induced gynecomastia. Pharmacotherapy 13: 37–45, 1993 [PubMed] [Google Scholar]
- 22.Sica DA: The risks and benefits of therapy with aldosterone receptor antagonist therapy. Curr Drug Saf 2: 71–77, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Bianchi S, Bigazzi R, Campese VM: Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int 70: 2116–2123, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Chrysostomou A, Pedagogos E, MacGregor L, Becker GJ: Double-blind, placebo-controlled study on the effect of the aldosterone receptor antagonist spironolactone in patients who have persistent proteinuria and are on long-term angiotensin-converting enzyme inhibitor therapy, with or without an angiotensin II receptor blocker. Clin J Am Soc Nephrol 1: 256–262, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Epstein M, Williams GH, Weinberger M, Lewin A, Krause S, Mukherjee R, Patni R, Beckerman B: Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol 1: 940–951, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH: Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: A randomized, double-masked, cross-over study. Diabetes Care 28: 2106–2112, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Schjoedt KJ, Rossing K, Juhl TR, Boomsma F, Rossing P, Parving HH: Beneficial impact of spironolactone in diabetic nephropathy. Kidney Int 68: 2829–2836, 2005 [DOI] [PubMed] [Google Scholar]
- 28.United States Cochrane Center. Master list of journals being searched. http://apps1.jhsph.edu/cochrane/masterlist.asp. Accessed May 2007.
- 29.Lefebvre C, McDonald S: Development of a sensitive search strategy for reports of randomized controlled trials in EMBASE. Fourth International Cochrane Colloquium; 1996 Oct 20–24; Adelaide (Australia). 1996
- 30.DerSimonian R, Laird N: Meta-analysis in clinical trials. Controlled Clinical Trials 7: 177–188, 1986 [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ 327: 557–560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Meiracker AH, Baggen RG, Pauli S: Spironolactone in type 2 diabetic nephropathy: Effects on proteinuria, blood pressure, and renal function. J Hypertens 24: 2285–2292, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Rachmani R, Slavachevsky I, Amit M: The effect of spironolactone, cilazapril, and their combination on albuminuria in patients with hypertension and diabetic nephropathy is independent of blood pressure reduction: A randomized controlled study. Diabet Med 21: 471–475, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Schjoedt KJ, Rossing K, Juhl TR: Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy. Kidney Int 70: 536–542, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Furumatsu Y, Nagasawa Y, Tomida K, Mikami S, Kaneko T, Okada N, Tsubakihara Y, Imai E, Shoji T: Effect of renin-angiotensin-aldosterone system triple blockade on non-diabetic renal disease: Addition of an aldosterone blocker, spironolactone, to combination treatment with an angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker. Hypertens Res 31: 59–67, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Tylicki L, Rutkowski P, Renke M, Larczyński W, Aleksandrowicz E, Lysiak-Szydlowska W, Rutkowski B: Triple pharmacological blockade of the renin-angiotensin-aldosterone system in nondiabetic CKD: An open-label crossover randomized controlled trial. Am J Kidney Dis 52: 486–493, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Epstein M, Buckalew V, Martinez F: Antiproteinuric efficacy of eplerenone, enalapril, and eplerenone/enalapril combination therapy in diabetic hypertensives with microalbuminuria [Abstract]. Am J Hypertens 15: 24A, 2002. 11824855 [Google Scholar]
- 38.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Strippoli GF, Craig M, Schena FP, Craig JC: Antihypertensive agents for primary prevention of diabetic nephropathy. J Am Soc Nephrol 16: 3081–3091, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Tylicki L, Rutkowski P, Renke M, Rutkowski B: Addition of aldosterone receptor blocker to dual renin-angiotensin-aldosterone blockade leads to limitation of tubulointerstitial injury of kidney. Kidney Int 72: 1164–1165, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Nakhoul F, Khankin E, Yaccob A, Kawachi H, Karram T, Awaad H, Nakhoul N, Hoffman A, Abassi Z: Eplerenone potentiates the antiproteinuric effects of enalapril in experimental nephrotic syndrome. Am J Physiol Renal Physiol 294: F628–37, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ: Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: A systematic review. Am J Kidney Dis 51: 199–211, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA: Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 351: 543–551, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Phillips CO, Kashani A, Ko DK, Francis G, Krumholz HM: Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction: A quantitative review of data from randomized clinical trials. Arch Intern Med 167: 1930–1936, 2007 [DOI] [PubMed] [Google Scholar]
- 45.ONTARGET Investigators, Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C: Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358: 1547–1559, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Navaneethan SD, Nigwekar SU, Strippoli GFM: Aldosterone antagonists for preventing the progression of chronic kidney disease. (Protocol) Cochrane Database of Systematic Reviews Issue 1. Art. No.: CD007004. DOI: 10.1002/14651858.CD007004, 2008 [DOI] [PubMed]