Identification of targets of the Wnt pathway destruction complex in addition to β-catenin (original) (raw)
Abstract
The proteasomal degradation of β-catenin mediated by the glycogen synthase kinase 3β (GSK3β) and destruction complex is the central step in the canonical Wnt signaling pathway. However, that there are branches of Wnt signaling pathways that do not depend on β-catenin/Tcf-mediated transcription activation has long been understood. In this study, we hypothesized that there are many more GSK3 and destruction complex-dependent proteolytic target proteins that mediate Wnt signaling in the cell. To test this hypothesis, we have developed and carried out a screen for such candidate proteins using an in vitro expression cloning technique and biochemical reconstitution of Wnt signaling in Xenopus egg cytoplasmic extracts. Forty-two proteins have been identified as potential candidates for GSK3-regulated phosphorylation, proteasomal degradation, or both, of which 12 are strong candidates for Wnt-pathway-regulated degradation. Some of them have been reported to interact with β-catenin and implicated in the canonical Wnt signaling pathway, and other targets identified include proteins with various cellular functions such as RNA processing, cytoskeletal dynamics, and cell metabolism. Thus, we propose that Wnt/GSK3/destruction complex signaling regulates multiple target proteins to control a broad range of cellular activities in addition to β-catenin-mediated transcription activation.
Keywords: glycogen synthase kinase 3β, proteolysis, screen, Axin, expression cloning
The canonical Wnt signaling pathway plays critical roles in cell proliferation and cell fate determination during embryogenesis and adult tissue homeostasis (1). Regulation of the canonical Wnt pathway depends on the posttranslational regulation of the key mediator, β-catenin (2, 3). In the absence of Wnt signaling, cytoplasmic and nuclear β-catenin levels are normally maintained at low levels by ubiquitination and subsequent degradation by the 26S proteasome. The targeting of β-catenin for degradation is mediated by phosphorylation of its N-terminal serine residues by glycogen synthase kinase 3 (GSK3). The destruction complex composed of GSK3, Axin/Conductin, adenomatous polyposis coli (APC), and other proteins mediates these biochemical reactions. Binding of Wnt proteins to the extracellular domain of Frizzled and low-density-lipoprotein-receptor-related protein 5 or 6 (Lrp5/6) receptors facilitates the phosphorylation of Lrp5/6 cytoplasmic tail by GSK3 (4). This triggers the interaction of the Frizzled–Lrp5/6 complex with Dishevelled (Dsh) and Axin, which leads to the inactivation of destruction complex through an unknown mechanism (presumably dissociation of the complex), allowing β-catenin levels to increase in the cytosol. The accumulated β-catenin then translocates to the nucleus and binds to Tcf/Lef transcription factors to regulate downstream target genes. Misregulation of β-catenin phosphorylation and degradation is the hallmark of several cancers, especially colon cancer.
That not all functions of Wnt signaling can be attributed to canonical β-catenin-mediated transcription regulation has long been understood. A number of β-catenin-independent “noncanonical” Wnt signaling pathways have been identified, including the planar cell polarity pathway that involves Rho A and Jun kinase activation, and a Wnt/Ca2+ pathway that involves an increase in intracellular Ca2+ upon Wnt stimulation (5). As the central regulator of the canonical Wnt signaling pathway, GSK3 and destruction complex activity is not implicated in these noncanonical Wnt signaling pathways.
In additional to β-catenin, there is an increasing list of proteins whose stability is regulated by GSK3-phosphorylation-dependent proteasomal degradation (6–11). In most cases, the upstream signaling pathways that regulate GSK3 remain to be determined. However, the regulation of Snail and Smad1 has been reported to be controlled by the Wnt signaling pathway. The zinc finger transcription factor Snail has a series of β-catenin-like destruction motifs that mediate GSK3β-dependent phosphorylation, β-TrCP-dependent ubiquitination, and proteasomal degradation (10). In the case of Smad1, mitogen-activated protein kinase phosphorylates and activates Smad1 in the presence of bone morphogenetic protein (BMP) signals. GSK3 phosphorylates the activated Smad1 and triggers its degradation. Activation of Wnt signaling or inhibition of GSK3 can block the degradation of Smad1, thereby enhancing BMP signaling (11).
The existence of these 2 Wnt/GSK3-regulated proteins besides β-catenin suggests that there may be more proteolytic targets that are controlled by Wnt/GSK3-mediated signaling pathways. Therefore, we hypothesized that there are multiple mediators of the Wnt signaling pathway other than β-catenin that are regulated by Wnt/GSK3/destruction complex signaling at the level of protein stability similar to β-catenin. Identification of such target proteins would have significant potential impact on our understanding of the Wnt/GSK3 signaling pathway. In this study, we have developed and carried out a screen for potential Wnt/GSK3-regulated protein degradation substrates using an in vitro expression cloning (IVEC) technique and a Xenopus egg cytoplasmic extract system (12, 13), and we report on the discovery of numerous such proteins.
Results
Validation of the Xenopus Egg Extract as an in Vitro System for the Identification of Wnt/GSK3/Destruction Complex Target Proteins.
The Xenopus egg extract provides a powerful in vitro system to recapitulate many intracellular processes and signaling pathways, including the cell cycle and apoptotic pathways (14, 15). Moreover, the Xenopus egg extract system was reported to be able to accurately reproduce the cytosolic part of the canonical Wnt signaling pathway: the destruction-complex-mediated β-catenin degradation and the inhibition of this degradation by various Wnt pathway activators (12). When added into the reaction, the GSK3 inhibitor LiCl and other Wnt pathway activators can inhibit the degradation of β-catenin (12). This provides an in vitro system that is ideal for our purpose to screen for proteolytic target proteins of the Wnt/GSK3 signaling pathway.
Before screening, we verified the robustness of the Xenopus egg extract system by using β-catenin as a positive control. In vitro translated Xenopus β-catenin protein was degraded in the Xenopus egg extract within 3 h, and the GSK3 inhibitor LiCl blocked this degradation in a concentration-dependent manner (Fig. S1_A_). β-Catenin degradation was mediated specifically by GSK3-dependent phosphorylation, because a phosphorylation-deficient mutant β-catenin (S33D) was stable in the extract (Fig. S1_A_).
We also tested the specificity of the reaction using additional Wnt-signaling-pathway-activating proteins. Among them, GSK3β interaction domain of Axin (GID), kinase-dead GSK3β (dnGSK3β), and an Axin mutant that lacks the APC binding domain (AxinΔRGS) all work as dominant-negative proteins that disrupt the destruction complex, whereas Dsh is a potent upstream Wnt pathway mediator that inhibits the destruction complex (16–19). Purified recombinant proteins GID, dnGSK3β, AxinΔRGS, and Dsh efficiently blocked the degradation of β-catenin by the Xenopus egg extract (Fig. S1_B_), indicating that degradation of β-catenin is specifically mediated by the destruction complex, as reported in ref. 12.
Screening of Proteolytic Targets of Wnt/GSK3 Signaling in Xenopus Egg Extracts by IVEC.
IVEC screening using the Xenopus egg extract has been used to identify numerous proteins that are mitotically phosphorylated or degraded and participate in the regulation of mitotic events (14, 20, 21). To identify proteolytic target proteins of the Wnt/GSK3 pathway, this method was used with modification (Fig. S1_C_). Small pools containing 50–100 clones of cDNAs from a gastrula-stage Xenopus cDNA library were in vitro translated to generate [35S]methionine-labeled proteins. These radiolabeled protein pools were incubated with freshly prepared Xenopus egg extracts at room temperature for 3 h. The 3-h limit favors finding proteolytic target proteins with relatively short half-lives. Incubation times greater than 3 h were avoided because the apoptotic activities of the Xenopus egg extract become significant after 3 h. In vitro translated β-catenin protein was used as a positive control, and pro-caspase 3 protein was used to monitor the apoptotic activity of Xenopus egg extracts in every assay. Xenopus extracts that cleaved pro-caspase 3 or did not degrade β-catenin were discarded.
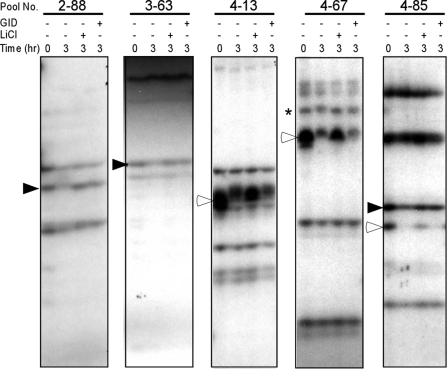
In the initial screens, we used LiCl and the recombinant GID protein to inhibit GSK3 activity and the Wnt-regulated destruction complex, respectively. The selectivity of GID for Wnt-regulated destruction complex targets is described later in Fig. 4A along with the characterization of the identified candidate target proteins. LiCl was used to more broadly inhibit the degradation or modification of GSK3 targets in general, as demonstrated for several identified candidate proteins in Fig. S2. Protein bands that exhibited either mobility shift or degradation after the reaction and were rescued by LiCl or GID were regarded as putative phosphorylation targets, proteolytic targets, or both of the Wnt/GSK3 pathway. For the identification of proteolytic targets, we included only bands with >50% signal reduction after 3 h of incubation compared with the starting material in the autoradiograph. Positive protein bands were validated in at least 3 independent experiments using different Xenopus egg extracts. Representative examples of pools having at least one positive band are shown in Fig. 1.
Fig. 4.
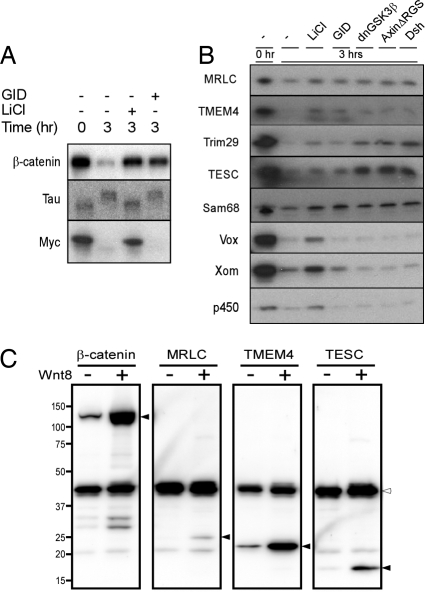
Specificity of the degradation of selected candidates for the Wnt/GSK3 signaling pathway destruction complex. (A) GID does not inhibit the activity of GSK3β toward all protein targets in Xenopus egg extracts. GID blocked the degradation of β-catenin but not the mobility shift of Tau or the degradation of Myc protein. Lanes: −, 1× XB buffer added in the reaction as a negative control; Li, 25 mM LiCl; GID, 0.8 μM GID. (B) Selected proteolytic candidates of the Wnt/GSK3 signaling pathway were incubated in the Xenopus egg extract with various inhibitors of the destruction complex. The degradation of Li/GID-positive proteins was rescued by all of the Wnt pathway activators, whereas Li-Only-positive genes were not. Lanes: −, 1× XB buffer added in the reaction as a negative control; LiCl, 25 mM LiCl; GID, 0.8 μM GID; dnGSK3β, 0.3 μM dominant-negative GSK3β (kinase dead); AxΔRGS, 0.2 μM Axin protein missing the RGS domain (which binds to APC); Dsh, 0.2 μM recombinant Dishevelled protein. (C) Wnt-dependent protein stabilization in Xenopus embryos. mRNA encoding one of the target proteins indicated was injected into one of the ventral blastomeres of the 4-cell-stage embryo along with Myc-EGFP mRNA as a nontarget control. Cells were also coinjected with either Wnt8 mRNA or a control mRNA. Filled arrowheads: β-catenin and target proteins; open arrowhead: Myc-EGFP.
Fig. 1.
Examples of the degradation assay of in vitro translated Xenopus cDNA pools used in the screen. Small pools containing 50–100 clones of gastrula stage Xenopus cDNA library were in vitro translated with [35S]methionine. Radiolabeled protein pools were incubated with Xenopus egg extract for 3 h at room temperature and then analyzed by SDS/PAGE and autoradiography. To inhibit GSK3 activity and the destruction complex, 25 mM LiCl or 0.8 μM recombinant GID protein was used, respectively. Filled arrowheads indicate the protein bands that are degraded in the Xenopus egg extract and rescued by LiCl and GID (Li/GID). Open arrowheads indicate protein bands that are degraded in the Xenopus egg extract and rescued by LiCl but not GID (Li-Only). Asterisk indicates protein bands that showed mobility shifts in SDS/PAGE after incubation in the Xenopus egg extract and LiCl blocked the mobility shift.
We screened 549 Xenopus cDNA pools encoding close to 10,000 distinguishable protein bands. Most protein bands exhibited no change in mobility or stability after incubation in the extract and thus served as internal controls. There were also a small number of protein bands that were shifted or degraded by the Xenopus egg extract but not rescued by LiCl or GID, indicating Wnt/GSK3-independent phosphorylation and degradation. They were not pursued further. Curiously, there were also 3 proteins whose degradation was stimulated by LiCl, and they turned out to be ribosomal proteins (Fig. S3). We also identified 2 other ribosomal proteins whose degradation was rescued by LiCl in the Xenopus egg extract (Figs. 2 and 3), suggesting that GSK3 may have both positive and negative roles in ribosomal protein turnover. LiCl, GID, or both blocked the degradation or mobility shift of 57 protein bands from 45 different cDNA pools. This represents ≈0.6% of all bands detected in the screen. Of these 57 protein bands, 9 exhibited mobility shifts that were inhibited by LiCl. LiCl inhibited the degradation of 32 protein bands, and 16 protein bands were degraded and rescued by both LiCl and GID. There were no protein bands rescued by GID alone, indicating that the regulation of protein phosphorylation and degradation by GID depends on GSK3 activity.
Fig. 2.
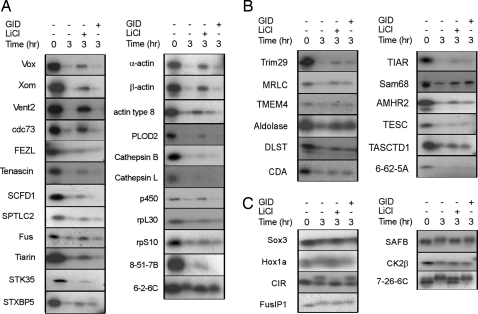
Degradation assay of isolated putative Wnt/GSK3 target proteins identified in the screen. The genes encoding positive protein bands were identified by progressive subselection (see Methods). Positive individual clones were sequenced and verified with the degradation assay. (A and B) Of 42 identified proteins, the degradation of 23 proteins by the Xenopus egg extract was inhibited by LiCl but not GID (A), and 12 proteins were positive for both LiCl and GID (B). (C) Seven proteins showed mobility shift after incubation in the Xenopus egg extract that was inhibited by LiCl. Lanes: −, 1× XB buffer added in the reactive as a negative control; Li, 25 mM LiCl; GID, 0.8 μM GID (GSK3β interaction domain of Axin).
Fig. 3.
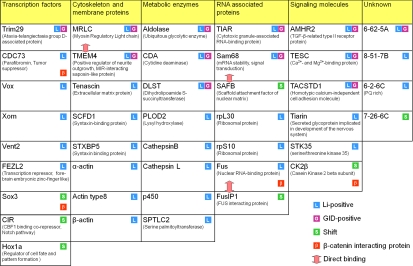
Proteins identified in the screen arranged in functional groups. Forty-two phosphorylation or proteolytic targets of Wnt, GSK3, or both are identified by whether their degradation is inhibited by LiCl or GID or their mobility shift is inhibited by LiCl. Also indicated are proteins reported to interact with β-catenin. Known interactions between candidate proteins are indicated by double-headed arrows.
Cloning and Identification of Specific Target Proteins Detected in the Screen.
The genes encoding the positive protein bands were identified by subsequent subselection (see Methods), cloning, and sequencing. Once identified, the LiCl- or GID-dependent degradation or mobility shift of proteins from positive cDNA single clones was confirmed in the degradation assay with Xenopus egg extract. Of the 57 positive protein bands, 42 unique genes were identified. Twenty-nine of these are known genes in Xenopus, whereas 9 could not be found in the Xenopus EST database (Table S1) but exhibited high sequence similarity to human genes. Four genes were completely unknown and could not be found in any database. Eight genes were identified in more than 2 independent pools. (Table S1).
Of these 42 unique genes, 7 encoded proteins that exhibited a mobility shift after incubation in the Xenopus egg extract and LiCl blocked the mobility shift, indicating a potential GSK3-mediated phosphorylation. GID did not block their mobility shifts. The degradation of 23 proteins by the Xenopus egg extract was inhibited by LiCl alone (Li-Only), and 12 proteins were positive for both LiCl and GID (Li/GID) (Fig. 2). The latter 12 proteins are strong candidates for regulation by the Wnt signaling pathway and the destruction complex.
On the basis of their cellular functions, positive proteins can be categorized in 7 different groups: transcription factors, cytoskeleton and membrane proteins, metabolic enzymes, RNA-associated proteins, signaling molecules, and unknown functions (Fig. 3), indicating that Wnt, GSK3, or both can specifically regulate a broad range of intracellular mediators other than the well-studied β-catenin in the canonical Wnt signaling pathway.
Further Characterization of Positive Proteins Identified in the Screen.
We used GID to screen for destruction-complex-dependent targets, because it should interfere with the binding of GSK3β to Axin in the complex. Indeed, we were able to clearly distinguish the Li/GID from LiCl-Only candidates. However, GID has been claimed to act as a more general GSK3 inhibitor (18). Therefore, we tested the specificity of GID in the Xenopus egg extract system using 2 known GSK3 targets not thought to be Wnt pathway regulated. The microtubule-associated protein Tau is phosphorylated by GSK3 in the Xenopus extract (22), as detected by a mobility shift (Fig. 4A). Phosphorylation of Tau was inhibited by LiCl but not at all by GID (Fig. 4A). c-Myc is also known to be a GSK3β substrate that targets it for degradation (6, 7). Although the degradation of c-Myc by the extract is inhibited by LiCl, it is unaffected by GID (Fig. 4A). Therefore, GID does not block all GSK3β-mediated activities in the extract and is probably more specific for destruction complex targets.
We further characterized the candidate proteins with other destruction complex inhibitors dnGSK, AxinΔRGS, and Dsh. The Li/GID-positive candidates were stabilized by all of the inhibitors, whereas the LiCl-Only-positive candidates were not (Fig. 4B), confirming the specificity of GID as a Wnt destruction complex inhibitor. The stability of the Li/GID-positive proteins thus seems to be specifically regulated by the destruction complex and therefore by the Wnt signaling pathway.
We wished to test whether the stability of some of these Li/GID-positive proteins is regulated by Wnt signaling in vivo. In vitro transcribed mRNAs of individual selected Li/GID-positive candidates were injected along with myc-EGFP as a control into one of the ventral blastomeres of 4-cell-stage Xenopus embryos. Either Wnt8 mRNA or a control mRNA (GFP) was coinjected, and at stage 12–12.5 levels of the expressed target proteins in dissected animal caps [which have no endogenous Wnt activity (23)] were analyzed by Western blotting. As expected, Wnt8 mRNA coinjection promoted the stabilization of β-catenin in animal caps without affecting the level of myc-EGFP protein. The levels of the candidate target proteins, MRLC, TMEM4, and TESC, were also increased by Wnt8 coinjection (Fig. 4C), indicating that their stabilities are similarly regulated by Wnt signaling.
The LiCl-Only-positive candidates may be substrates whose degradation is regulated by GSK3 but independent of the destruction complex. It is also possible that LiCl can affect some other unknown kinases or enzymes in the Xenopus egg extract that could potentially control the stability of these proteins. Interestingly, at a high concentration (1.3 μM), dnGSK3β blocked the GSK3-mediated phosphorylation of Tau protein in the Xenopus egg extract, indicating that it acts as a general GSK3β inhibitor at this concentration (Fig. S2_A_). Therefore, we asked whether a high concentration of dnGSK3β affects the stability of some representative Li-Only candidates in the Xenopus egg extract. Although low concentrations of dnGSK3β selectively stabilized β-catenin and Li/GID-positive targets (Fig. S2_A_ and Fig. 4B), high concentrations of dnGSK3β effectively blocked the degradation of the Li-Only targets (Fig. S2_A_). The cytoplasmic domain of the Wnt coreceptor protein Lrp6 (Lrp6ICD) has also been shown to regulate β-catenin stability in Xenopus egg extracts due to its interaction with GSK3β (24). We found that Lrp6ICD selectively stabilized β-catenin and Li/GID-positive target proteins in the extract when used at low concentrations (Fig. S2_B_). However, similar to dnGSK3β, at higher concentrations Lrp6ICD also stabilized several Li-Only targets (Fig. S2_C_). The stabilization of these Li-Only targets by the 2 GSK3-selective inhibitors, dnGSK3β and Lrp6ICD, and LiCl, suggests that they are regulated by GSK3-dependent phosphorylation.
Interestingly, we identified 3 members of the Vent2B/Vox family (Vox1, Vent2, and Xom) as putative proteolytic target proteins regulated by GSK3 (Li-Only) from different cDNA pools. Xom is already known to be degraded in an early gastrula Xenopus embryo extract and rescued by treatment with LiCl (25). The putative GSK3 phosphorylation sites that are important for the proteolytic regulation of Xom in Xenopus embryos are well conserved among the Vent2B/Vox subfamily members but not the closely related Vent1B subfamily, which has a different response to upstream BMP signaling (25) (Fig. S4_A_). To determine whether this proteolytic event is specific and applicable to the whole Vent/Vox subfamily, we cloned another Vent2B/Vox subfamily member, Vent2B, and Vent1 of the distinct Vent1B subfamily. The degradation assay showed that Vent2B protein is degraded by the Xenopus egg extract and rescued by LiCl like the other Vent2B/Vox subfamily members, whereas Vent1 is stable in the Xenopus egg extract (Fig. S4_B_). Mutation of the conserved GSK3 consensus phosphorylation sites completely blocked the degradation of Vox1, Vent2, and Xom (Fig. S4_C_), and dnGSK3β and Lrp6ICD effectively inhibited their degradation (Fig. S2 A and C). This finding suggests that protein half-lives of all of the Vent2B/Vox subfamily members are specifically regulated by GSK3β and that the conserved GSK3 consensus sites are important for this regulation.
Discussion
In this study, we successfully identified 42 potential target proteins of the Wnt signaling pathway, GSK3 regulation, or both in a screen using IVEC and the Xenopus egg extract degradation assay, including 35 proteolytic and 7 phosphorylation targets. They fall into several categories, including transcriptional regulators, RNA-associated proteins, cytoskeletal proteins and regulators, signaling proteins, and metabolic enzymes. The identification of this very selective but diverse number of target proteins in this screen suggests that Wnt- or GSK3-dependent signaling can regulate a diversity of cellular processes.
Of particular interest are 12 proteins whose degradation is inhibited by the GID protein, a potent inhibitor of Axin-dependent GSK3 phosphorylation, making them strong candidates for targets of the Wnt signaling pathway and the destruction complex. The GID-sensitive candidates were a small subset of all of the LiCl-sensitive targets. Also, GID was not able to inhibit the phosphorylation or degradation of known GSK3β targets in the Xenopus egg extract, indicating that GID works specifically on the destruction complex, whereas LiCl directly inhibits GSK3 kinase activity.
Further analysis of the specificity of the degradation of the 12 Li/GID candidate proteins using additional Wnt pathway activators confirmed that their stability was regulated, similar to β-catenin, by Axin, APC, and Dsh, and therefore the destruction complex. Furthermore, we verified that Wnt signaling regulates the levels of at least 3 of these proteins (MRLC, TMEM4, and TESC) in vivo, as a result of Wnt8 expression in Xenopus embryos. At least some of the LiCl-Only candidates are likely to be targets of different, non-Wnt- but GSK3-dependent signaling pathways, because dnGSK3β and Lrp6ICD inhibit their degradation.
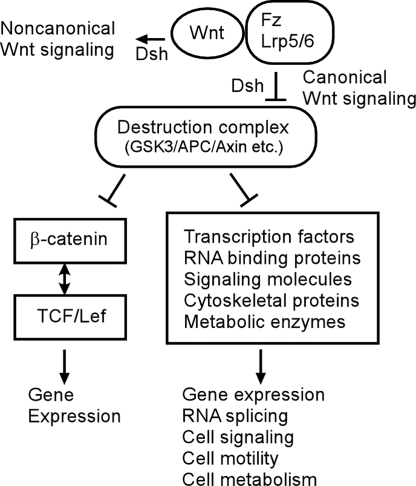
By identifying these Wnt/GSK3-regulated target proteins, we provide direct evidence in support of our hypothesis that there are multiple signaling mediators besides β-catenin whose degradation is specifically regulated by the Wnt/GSK3 pathway. Instead of the traditional linear view of the canonical Wnt signaling pathway through regulation of β-catenin-dependent transcription activation, we propose that the Wnt signaling pathway is better understood as a broad signaling network in which Wnt signaling can regulate the activity, stability, or both of a diversity of proteins, including transcription factors, RNA-binding proteins, and cytoskeletal proteins, that in turn control gene expression, cell physiology, and cell behavior (Fig. 5).
Fig. 5.
A model for the expanded canonical Wnt/GSK3 signaling pathway. Binding of Wnt proteins to receptors triggers canonical (β-catenin and destruction complex dependent) or noncanonical (β-catenin and the destruction complex independent) Wnt signaling. In the canonical Wnt signaling pathway, the stability of β-catenin is controlled by destruction complex composed by GSK3, APC, and Axin, etc. In addition to β-catenin, the degradation of many different proteins in various functional classes is regulated by the destruction complex and Wnt receptor signaling.
Some proteins identified in the screen are known to interact with β-catenin and play roles in the canonical Wnt signaling pathway. Parafibromin is a tumor suppressor gene that was reported to directly associate with β-catenin in the nucleus together with Pygopus and mediate β-catenin/Tcf transcriptional activity (26). Casein kinase 2 is also a positive regulator of the canonical Wnt signaling pathway (27, 28). However, Sox3 and Fus, an RNA- and DNA-binding protein, both negatively regulate β-catenin/Tcf transcription activity when they bind to β-catenin (29–31). Curiously, however, none of these proteins appears to be specific targets of the destruction complex by the criteria of inhibition by GID and other Wnt pathway activators. Nonetheless, GSK3 regulation of the stability, phosphorylation, or both of these proteins could suggest the existence of layers of regulation in the canonical Wnt signaling pathway or provide possible cross-talk between other signaling pathways and the Wnt pathway.
Many of the proteins identified in the screen are known to interact with each other or belong to functional groups (Fig. 3), raising the possibility of coordinated regulation of certain cellular processes. One exciting group consists of the cytoskeletal regulatory proteins myosin regulatory light chain (MRLC) and transmembrane protein 4 (TMEM4), which are known to interact with each other (32). Both appear to be specifically regulated by the Wnt signaling pathway destruction complex, raising the possibility that cell motility can be developmentally controlled by canonical Wnt signaling. Another good example is a group of RNA-binding proteins including Fus, Fus interacting protein 1 (FusIP1), and Sam68. Sam68, which is degraded specifically by the Wnt signaling pathway destruction complex, has been shown to regulate mRNA splicing in a phosphorylation-dependent manner (33). Fus degradation was regulated by GSK3 only, but it has been suggested to work together with β-catenin to regulate pre-mRNA splicing (31). FusIP1, a splicing factor that binds to Fus (34) and regulates RNA splicing in response to cellular signals (35), was identified in the screen by GSK3-dependent mobility shift. Taken together, these results raise the exciting possibility that Wnt/GSK3 signaling may affect gene expression via regulation of multiple mRNA splicing and processing proteins.
An interesting family of proteins identified in the screen as candidates for GSK3-regulated degradation is the Vent/Vox protein family. The Vent/Vox homeobox-containing transcriptional repressor is known to play critical roles in early patterning of Xenopus and zebrafish embryos by antagonizing the dorsalization effect of the Spemann organizer, and the transcription of the Vent/Vox family genes is regulated by the BMP and Wnt signaling pathways (25, 36–38). Consistent with our findings, one of the family members, Xom, was reported to be degraded during early gastrulation and regulated by a LiCl-sensitive activity via a GSK3-like consensus sequence (25). The GSK3 consensus sequence of Xom protein is well conserved in the other member of the family, which in Xenopus includes Vent2B, Vent2, and Vox. We found that all Vent2B/Vox family members were similarly degraded in the Xenopus egg extract and rescued by LiCl, dnGSK3β, and Lrp6ICD. Mutation of the conserved GSK3 consensus phosphorylation sites stabilized the proteins in the Xenopus egg extract. Thus, our findings indicate that rapid proteolytic regulation of Vent2B/Vox family proteins during embryonic development is likely to be universal, and this regulation might be dependent on the GSK3 activity but not Wnt signaling.
Although degradation of Xom in the previous study was found to be mediated by a GSK3 consensus sequence, immune depletion of GSK3 from the Xenopus egg extract did not prevent degradation (25). However, our findings that 2 additional GSK3 inhibitors, dnGSK3β and Lrp6ICD, blocked the degradation of these family members suggests that GSK3 might be involved in their regulation. We cannot completely exclude that Xom degradation is regulated by another kinase that is highly similar to GSK3β. Further study of these Wnt/GSK3 and destruction complex target proteins should provide valuable insights into the mechanism and regulation of Wnt and GSK3 signaling pathways during embryonic development and tumorigenesis.
Methods
In Vitro Expression Cloning.
A gastrula stage Xenopus embryo cDNA library [a gift from Todd Stukenberg, University of Virginia, Charlottesville (14)] was used to transform Escherichia coli and titrated to grow ≈50–100 colonies per plate. The colonies were pooled, and plasmid DNA was isolated. 35S-labeled in vitro translated proteins were made from the resulting plasmid pools using the TNT kit (Promega) and used in the screen. The bacteria containing positive pools were grown again, and plasmids were isolated from 96 individual colonies picked from each pool. The plasmids of each row of the 96-well plate were pooled as a “subpool,” from which in vitro translated proteins were made and subjected to degradation assay. Positive single clones were then identified from the positive subpools by protein band size, verified again by the degradation assay, and sequenced to identify the genes encoded.
Degradation Assay.
The degradation assay was performed as described in ref. 13 with minor modifications. The degradation reactions contained 6.5 μL of Xenopus egg extract, 0.2 μL of 14 mg/mL bovine ubiquitin in 1× extraction buffer (XB), and 0.1–0.3 μL of 35S-labeled in vitro translated proteins. Reactions were carried out at 22 °C for 3 h and then analyzed by SDS/PAGE and autoradiography. The quality of the Xenopus egg extracts was evaluated by degradation of β-catenin as a positive control and cleavage of pro-caspase-3 as an indication of apoptotic activity, as described in the text. During the screen, 25 mM LiCl and 0.8 μM recombinant GID protein were used as GSK3 and destruction complex inhibitors.
Others.
The preparation of DNA constructs, recombinant proteins, and Xenopus egg extracts and Xenopus embryo RNA injection are described in the SI Methods.
Supplementary Material
Supporting Information
Acknowledgments.
We thank Todd Stukenberg for providing the Xenopus cDNA library, Ethan Lee for generous technical advice on preparation of the Xenopus extract and the degradation assay, Marissa Nadeau for technical support in DNA cloning, Doug Desimone (University of Virginia, Charlottesville) for the Xenopus cDNAs, and Fred Simon for help with Xenopus. The work described in this article was supported by National Institutes of Health Grant R37 GM37432 (to B.M.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Kimelman D, Xu W. β-Catenin destruction complex: Insights and questions from a structural perspective. Oncogene. 2006;25:7482–7491. doi: 10.1038/sj.onc.1210055. [DOI] [PubMed] [Google Scholar]
- 3.Huang H, He X. Wnt/β-catenin signaling: New (and old) players and new insights. Curr Opin Cell Biol. 2008;20:119–125. doi: 10.1016/j.ceb.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeng X, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of β-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 6.Sears R, et al. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14:2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gregory MA, Qi Y, Hann SR. Phosphorylation by glycogen synthase kinase-3 controls c-myc proteolysis and subnuclear localization. J Biol Chem. 2003;278:51606–51612. doi: 10.1074/jbc.M310722200. [DOI] [PubMed] [Google Scholar]
- 8.Zhou BP, et al. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 9.Kang T, et al. GSK-3β targets Cdc25A for ubiquitin-mediated proteolysis, and GSK-3β inactivation correlates with Cdc25A overproduction in human cancers. Cancer Cell. 2008;13:36–47. doi: 10.1016/j.ccr.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yook JI, Li XY, Ota I, Fearon ER, Weiss SJ. Wnt-dependent regulation of the E-cadherin repressor snail. J Biol Chem. 2005;280:11740–11748. doi: 10.1074/jbc.M413878200. [DOI] [PubMed] [Google Scholar]
- 11.Fuentealba LC, et al. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salic A, Lee E, Mayer L, Kirschner MW. Control of β-catenin stability: Reconstitution of the cytoplasmic steps of the Wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- 13.Salic A, King RW. Identifying small molecule inhibitors of the ubiquitin-proteasome pathway in Xenopus egg extracts. Methods Enzymol. 2005;399:567–585. doi: 10.1016/S0076-6879(05)99038-1. [DOI] [PubMed] [Google Scholar]
- 14.Stukenberg PT, et al. Systematic identification of mitotic phosphoproteins. Curr Biol. 1997;7:338–348. doi: 10.1016/s0960-9822(06)00157-6. [DOI] [PubMed] [Google Scholar]
- 15.Deming P, Kornbluth S. Study of apoptosis in vitro using the Xenopus egg extract reconstitution system. Methods Mol Biol. 2006;322:379–393. doi: 10.1007/978-1-59745-000-3_27. [DOI] [PubMed] [Google Scholar]
- 16.Pierce SB, Kimelman D. Regulation of Spemann organizer formation by the intracellular kinase Xgsk-3. Development. 1995;121:755–765. doi: 10.1242/dev.121.3.755. [DOI] [PubMed] [Google Scholar]
- 17.Zeng L, et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 18.Hedgepeth CM, Deardorff MA, Rankin K, Klein PS. Regulation of glycogen synthase kinase 3β and downstream Wnt signaling by axin. Mol Cell Biol. 1999;19:7147–7157. doi: 10.1128/mcb.19.10.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sokol SY, Klingensmith J, Perrimon N, Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development. 1995;121:3487. doi: 10.1242/dev.121.10.3487. [DOI] [PubMed] [Google Scholar]
- 20.Lustig KD, et al. Small pool expression screening: Identification of genes involved in cell cycle control, apoptosis, and early development. Methods Enzymol. 1997;283:83–99. doi: 10.1016/s0076-6879(97)83009-1. [DOI] [PubMed] [Google Scholar]
- 21.McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 22.Hong M, Chen DC, Klein PS, Lee VM. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J Biol Chem. 1997;272:25326–25332. doi: 10.1074/jbc.272.40.25326. [DOI] [PubMed] [Google Scholar]
- 23.Schohl A, Fagotto F. β-Catenin, MAPK and Smad signaling during early Xenopus development. Development. 2002;129:37–52. doi: 10.1242/dev.129.1.37. [DOI] [PubMed] [Google Scholar]
- 24.Cselenyi CS, et al. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of β-catenin. Proc Natl Acad Sci USA. 2008;105:8032–8037. doi: 10.1073/pnas.0803025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Z, Kirschner M. Regulated proteolysis of Xom mediates dorsoventral pattern formation during early Xenopus development. Dev Cell. 2002;3:557–568. doi: 10.1016/s1534-5807(02)00270-8. [DOI] [PubMed] [Google Scholar]
- 26.Mosimann C, Hausmann G, Basler K. Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with β-catenin/Armadillo. Cell. 2006;125:327–341. doi: 10.1016/j.cell.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 27.Dominguez I, et al. Protein kinase CK2 is required for dorsal axis formation in Xenopus embryos. Dev Biol. 2004;274:110–124. doi: 10.1016/j.ydbio.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Wang S, Jones KA. CK2 controls the recruitment of Wnt regulators to target genes in vivo. Curr Biol. 2006;16:2239–2244. doi: 10.1016/j.cub.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 29.Zorn AM, et al. Regulation of Wnt signaling by Sox proteins: XSox17 α/β and XSox3 physically interact with β-catenin. Mol Cell. 1999;4:487–498. doi: 10.1016/s1097-2765(00)80200-2. [DOI] [PubMed] [Google Scholar]
- 30.Sinner D, et al. Sox17 and Sox4 differentially regulate β-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol. 2007;27:7802–7815. doi: 10.1128/MCB.02179-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato S, et al. β-Catenin interacts with the FUS proto-oncogene product and regulates pre-mRNA splicing. Gastroenterology. 2005;129:1225–1236. doi: 10.1053/j.gastro.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 32.Bornhauser BC, Olsson PA, Lindholm D. MSAP is a novel MIR-interacting protein that enhances neurite outgrowth and increases myosin regulatory light chain. J Biol Chem. 2003;278:35412–35420. doi: 10.1074/jbc.M306271200. [DOI] [PubMed] [Google Scholar]
- 33.Matter N, Herrlich P, Konig H. Signal-dependent regulation of splicing via phosphorylation of Sam68. Nature. 2002;420:691–695. doi: 10.1038/nature01153. [DOI] [PubMed] [Google Scholar]
- 34.Yang L, Embree LJ, Tsai S, Hickstein DD. Oncoprotein TLS interacts with serine-arginine proteins involved in RNA splicing. J Biol Chem. 1998;273:27761–27764. doi: 10.1074/jbc.273.43.27761. [DOI] [PubMed] [Google Scholar]
- 35.Shi Y, Manley JL. A complex signaling pathway regulates SRp38 phosphorylation and pre-mRNA splicing in response to heat shock. Mol Cell. 2007;28:79–90. doi: 10.1016/j.molcel.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt JE, von Dassow G, Kimelman D. Regulation of dorsal-ventral patterning: The ventralizing effects of the novel Xenopus homeobox gene Vox. Development. 1996;122:1711–1721. doi: 10.1242/dev.122.6.1711. [DOI] [PubMed] [Google Scholar]
- 37.Onichtchouk D, Glinka A, Niehrs C. Requirement for Xvent-1 and Xvent-2 gene function in dorsoventral patterning of Xenopus mesoderm. Development. 1998;125:1447–1456. doi: 10.1242/dev.125.8.1447. [DOI] [PubMed] [Google Scholar]
- 38.Ramel MC, Lekven AC. Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development. 2004;131:3991–4000. doi: 10.1242/dev.01277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information