Pharmacokinetics and Pharmacodynamics of Oral Testosterone Enanthate Plus Dutasteride for 4 Weeks in Normal Men: Implications for Male Hormonal Contraception (original) (raw)
. Author manuscript; available in PMC: 2009 Apr 1.
Abstract
Oral administration of testosterone enanthate (TE) and dutasteride increases serum testosterone and might be useful for male hormonal contraception. To ascertain the contraceptive potential of oral TE and dutasteride by determining the degree of gonadotropin suppression mediated by 4 weeks of oral TE plus dutasteride, 20 healthy young men were randomly assigned to 4 weeks of either 400 mg oral TE twice daily or 800 mg oral TE once daily in a double-blinded, controlled fashion at a single site. All men received 0.5 mg dutasteride daily. Blood for measurement of serum luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, dihydrotesterone (DHT), and estradiol was obtained prior to treatment, weekly during treatment, and 1, 2, 4, 8, 12, 13, 14, 16, 20, and 24 hours after the morning dose on the last day of treatment. FSH was significantly suppressed throughout treatment with 800 mg TE once daily and after 4 weeks of treatment with 400 mg TE twice daily. LH was significantly suppressed after 2 weeks of treatment with 800 mg TE, but not with 400 mg TE. Serum DHT was suppressed and serum estradiol increased during treatment in both groups. High-density lipoprotein cholesterol was suppresed during treatment, but liver function tests, hematocrit, creatinine, mood, and sexual function were unaffected. The administration of 800 mg oral TE daily combined with dutasteride for 28 days significantly suppresses gonadotropins without untoward side effects and might have utility as part of a male hormonal contraceptive regimen.
Keywords: Androgens, FSH, LH, 5α reductase, male fertility
It was first demonstrated in 1939 that the chronic administration of testosterone reversibly inhibits sperm production in men (Heckel, 1939). Testosterone exerts its contraceptive effect by suppressing the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary. Low levels of LH and FSH deprive the testis of the stimulatory signals required for spermatogenesis, leading to markedly decreased sperm concentrations in all men and infertility in most, but not all, cases (Matthiesson and McLachlan, 2006).
One of the main challenges in the development of a male hormonal contraceptive is the need for parenteral administration of testosterone, which has been administered by intramuscular injections or transdermally in experimental contraceptive trials (Brady et al, 2006; Page et al, 2006). When surveyed, however, a majority of men state a preference for an oral form of contraceptive delivery (Martin et al, 2000; Weston et al, 2002; Heinemann et al, 2005). Unfortunately, in the United States there is currently no safe form of oral androgen therapy that could be incorporated in a trial of male hormonal contraception. To address this issue, our group has recently studied oral testosterone enanthate (TE) combined with the 5α-reductase inhibitors dutasteride and finasteride, which inhibit the metabolism of testosterone to dihydrotestosterone (DHT). This combination of agents results in elevations of serum testosterone levels to the normal range for 8–12 hours in medically castrated men (Amory and Bremner, 2005; Amory et al, 2006). In addition, serum DHT levels are significantly suppressed. Such long-term DHT suppression could enhance the efficacy, safety, and appeal of a male hormonal contraceptive regimen, since the inhibition of DHT production might reduce the risk of diseases, such as prostate hyperplasia and/or prostate cancer, acne, and male pattern baldness (Thompson et al, 2003; Marberger, 2006; Olsen et al, 2006: Otberg et al, 2007). Moreover, chronic administration of dutasteride to normal men significantly decreases sperm count and could therefore improve suppression of spermatogenesis in a contraceptive regimen (Amory et al, 2007).
While our initial studies have demonstrated the plausibility of using oral TE plus dutasteride for male hormonal contraception, the degree of gonadotropin suppression mediated by oral testosterone as well as the safety of extended oral administration of TE plus dutasteride are unknown. We hypothesized that 1 month of oral TE plus dutasteride would effectively suppress FSH and LH below the lower limit of the normal range. This degree of gonadotropin suppression has been shown to suppress spermatogenesis when administered to men for at least 12 weeks (Matthiesson and McLachlan, 2006). In addition, we hypothesized that once-daily dosing of oral TE would be as effective as twice-daily dosing in terms of gonadotropin suppression, and that 4 weeks of oral TE would be safe and well tolerated without any significant impact on health, mood, or sexual function. Therefore, to determine the safety and efficacy of chronic oral TE plus dutasteride for male hormonal contraception, we conducted a randomized, double-blinded trial of 4 weeks of oral TE plus dutasteride in healthy men.
Subjects and Methods
Subjects
A total of 20 men ages 18–55 years and in good health were recruited through local newspapers and campus fliers. After informed consent was obtained, subjects underwent screening procedures consisting of a medical history, a physical examination, and measurements of serum hormone levels and routine laboratory tests (complete blood count, serum chemistry), and prostate specific antigen (PSA). Specific exclusion criteria included: body mass index greater than 35, history or current use of testosterone, infertility, poor general health, significantly abnormal laboratory results, history of testicular disease or severe testicular trauma, a history of sleep apnea and/or major psychiatric disorders, use of illicit drugs, or the use of more than 3 alcoholic beverages daily. The University of Washington Investigational Review Board approved all aspects of this study before study initiation. This study was registered in advance at clinicaltrials.gov as study NCT00399165.
Study Design
We conducted a randomized, double-blinded, 2-arm study of 4 weeks of oral TE plus dutasteride in healthy men. For the study, TE in sesame oil (Delatestryl; Indevus Pharmaceuticals, Lexington, Massachusetts) and placebo sesame oil were prepared in syringes by the hospital pharmacist at the University of Washington. Subjects self-administered oral TE once or twice daily during treatment. There were no restrictions placed on concomitant food intake. For inhibition of 5α-reductase, a loading dose of 24.5 mg dutasteride (GlaxoSmithKline, Research Triangle Park, North Carolina) was administered on the first day of the study to achieve rapid inhibition of both isozymes of 5α-reductase (Clark et al, 2004). Thereafter, subjects self-administered 0.5 mg daily for the remaining 27 days with their morning TE syringe. Subjects were randomly assigned to either 1) 400 mg TE in sesame oil by mouth twice daily or 2) 800 mg TE in sesame oil by mouth every morning plus placebo sesame oil every evening. Dose assignment/randomization was performed by the hospital pharmacist using a random number sequence. Subjects and study investigators were blinded to study group so that subjective assessments of mood and sexual function would not be biased. At the first study visit, the subjects were instructed on self-administration of the study drug and given a study medication record to record both the timing of each subsequent dose of study medication and any illnesses or adverse reactions to study medications. During treatment, subjects had weekly physical examinations by study personnel and blood tests for measurement of serum LH, FSH, testosterone, DHT, and estradiol as well as blood counts and serum chemistries, including liver function tests. Fasting serum lipids (total cholesterol, high-density lipoprotein [HDL] cholesterol, low-density lipoprotein [LDL] cholesterol, triglycerides) and serum PSA were measured at baseline and at after 4 weeks of treatment. In addition, subjects completed validated questionnaires assessing mood and sexual function (McNair et al, 1971; O’Leary et al, 1995) at baseline and after 4 weeks of treatment.
On the last day of dosing of the 28-day study period, subjects were admitted to the clinical research center at the University of Washington and underwent 24-hour sampling of their serum concentrations of testosterone, DHT, and estradiol. Blood was drawn before dosing and 1, 2, 4, 8, 12, 13, 14, 16, 20, and 24 hours after their morning dose to allow for analysis of the steady-state pharmacokinetics of oral TE. Three to four weeks after the last dose of oral TE, subjects returned to clinic for a follow-up physical examination and blood tests. The study was designed with the primary endpoint as the suppression of LH and FSH after 28 days of therapy by oral testosterone plus dutasteride. Before the study, it was determined that a sample size of 10 subjects per group conferred an 80% power to detect a difference of greater than 0.4 IU/L in serum LH between the 2 doses of oral TE.
Measurements
FSH and LH levels were measured by immunofluorometric assay (Delfia; Wallac Oy, Turku, Finland). The sensitivity of the assay for FSH and LH was 0.016 IU/L and 0.019 IU/L, respectively. For low-, mid-, and high-pooled values of 0.05, 1.0, and 21 IU/L of FSH, the intra-assay coefficients of variation were 5.9%, 3.0%, and 3.0%, and the interassay coefficients of variation were 20.7%, 5.0%, and 6.2%, respectively. For low-, mid-, and high-pooled values of 0.06, 1, and 16 IU/L of LH, the intra-assay coefficients of variation were 12.6%, 5.6%, and 4.1%, and the interassay coefficients of variation were 16.5%, 13.9%, and 10.5%, respectively. The normal ranges were 1.2–7.3 IU/L for LH and 1.1–6.7 IU/L for FSH. Serum total testosterone was measured by a radioimmunoassay (Diagnostic Products Corp, Webster, Texas). The assay had a sensitivity of 0.35 nmol/L and interassay variations for low, mid, and high pools of 13.6%, 6.1%, and 6.8%, and intra-assay variations of 10.0%, 5.3%, and 6.6%. The normal range was 8.7–33 nmol/L. Serum estradiol was measured using a radioimmunoassay (Diagnostic Products). The assay had a sensitivity of 20 pmol/L and interassay and intra-assay coefficients of variation of 8.1% and 7.1%, respectively. The normal value for estradiol in men was <220 pmol/L. DHT was measured using high-performance liquid chromatography–mass spectroscopy as described previously (Kalhorn et al, 2007). Intra-assay coefficients of variation generated using human serum for low-, mid-, and high-range samples were 15.8%, 4.3%, and 6.3% for DHT. The normal range for serum DHT was 1.0–3.1 nmol/L.
Statistical Analysis
Serum testosterone, DHT, and estradiol were natural log-transformed prior to analysis. Due to nonnormal distributions, even after transformation, serum LH and FSH were analyzed in a nonparametric fashion. Baseline characteristics and laboratory values were compared using a 2-sample t test. Changes from baseline in serum hormones were analyzed using a paired t test (for serum testosterone, DHT, and estradiol) or a Wilcoxon sign-rank test (for LH and FSH) with a Bonferroni correction for multiple comparisons. Differences between dose groups were compared with a 2-sample t test with unequal variances (for serum testosterone, DHT, and estradiol) or a Wilcoxon rank-sum test (for LH and FSH). Responses on the sexual function and mood questionnaires from baseline and between groups were compared using an extended χ2 test. For the analysis of the serum hormone concentrations during the last 24 hours of dosing, maximum concentration after dosing (Cmax), time to maximum concentration (Tmax), area under the curve (AUC), and elimination phase half-life (T1/2) were calculated for each subject using a computer program (PK Solutions, Golden, Colorado) after correction for baseline values. Pharmacokinetic parameters between doses were compared using the Wilcoxon rank-sum test. Correlations between the degree of gonadotropin suppression and pharmacokinetic parameters were performed using the Spearman technique. All statistical analyses were performed using STATA (College Park, Texas). For all comparisons, an α of .05 was considered significant.
Results
Subjects
A total of 21 subjects were screened for participation in this study; 20 met all inclusion criteria and were randomized to treatment. The subject who failed to meet entry criteria had a serum testosterone level below the normal range on 2 occasions. Of the 20 men enrolled in the study, 10 received 400 mg TE twice daily, and 10 received 800 mg TE once daily. There were no significant hormonal or anthropomorphic differences between the treatment groups (Table 1). All subjects completed the study, and none were lost to follow-up. Compliance with the dosing regimen was greater than 95% on average, and no subject missed more than 20% of his medication doses. Therefore, results from all 20 subjects were analyzed.
Table 1.
Baseline characteristics of study subjects (mean ± SD)
| 400 mg Twice Daily (n = 10) | 800 mg Once Daily (n = 10) | P | |
|---|---|---|---|
| Age, y | 36 ± 6.1 | 35 ± 11 | .78 |
| Weight, kg | 95 ± 11 | 84 ± 11 | .05 |
| Height, cm | 181 ± 7 | 179 ± 8 | .60 |
| Body mass index, kg/m2 | 29 ± 4.6 | 26 ± 2.5 | .10 |
| Total testosterone, nmol/L | 12.3 ± 4.0 | 12.6 ± 3.3 | .75 |
| Dihydrotestosterone, nmol/L | 1.0 ± 0.7 | 1.4 ± 0.6 | .21 |
| Estradiol, pmol/L | 87 ± 26 | 103 ± 27 | .22 |
| Luteinizing hormone, IU/L | 3.0 ± 0.7 | 4.6 ± 4.3 | .26 |
| Follicle-stimulating hormone, IU/L | 2.7 ± 1.6 | 3.7 ± 4.0 | .47 |
A total of 16 subjects reported 29 adverse events during the study. The most common adverse events were headache (7 subjects), upper respiratory infections (5 subjects), stomach pain/reflux (4 subjects), and back pain (2 subjects). Side effects of particular interest thought to be related to the study medication in included acne (2 subjects) and a transient decrease in libido (1 subject). In addition, 1 subject complained of unusually vivid dreams. There were no serious adverse effects.
Serum Steroid Hormones
During treatment, predose (nadir) serum testosterone remained in the normal range and did not significantly differ from baseline (Figure 1A); however, serum DHT was significantly suppressed (Figure 1B), and serum estradiol was significantly increased (Figure 1C). There were no significant differences between groups in predose (nadir) serum testosterone, DHT, or estradiol. Serum testosterone and estradiol concentrations returned to baseline values after treatment; however, serum DHT remained significantly suppressed 14 days after the completion of TE and dutasteride dosing (Figure 1B).
Figure 1.
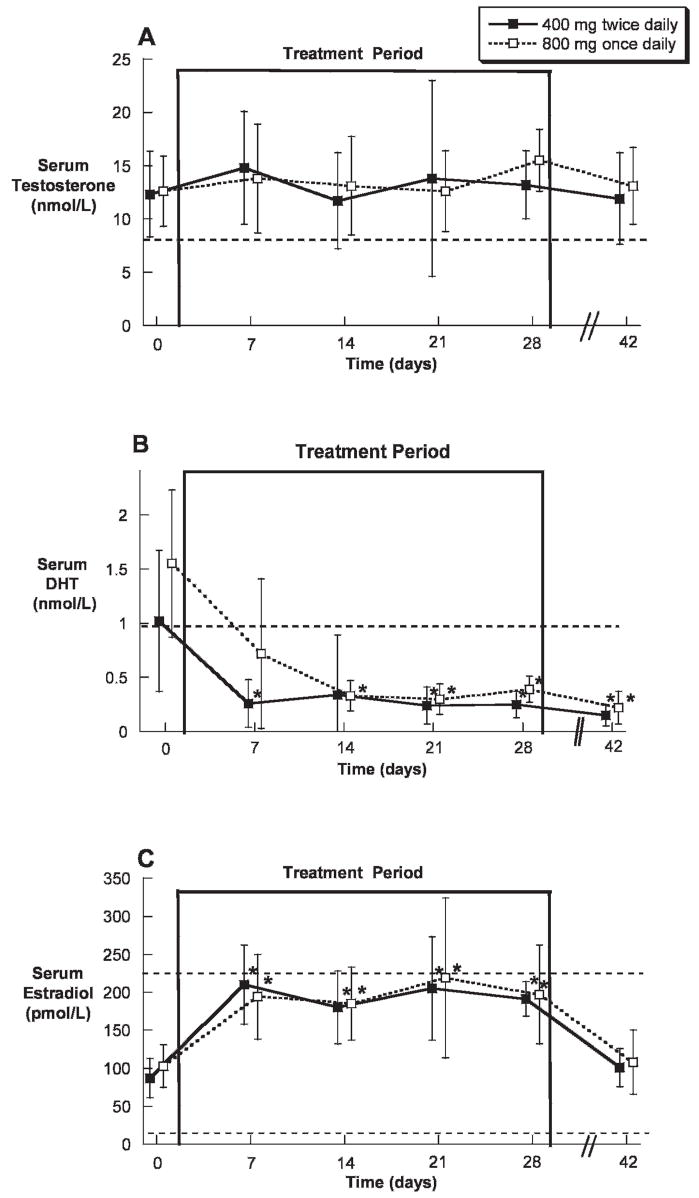
Nadir serum testosterone (A), dihydrotestosterone (DHT) (B), and estradiol (C) concentrations (mean ± SD) prior to, during, and after oral administration of 400 mg twice daily or 800 mg once daily of testosterone enanthate (TE) in oil with dutasteride for 28 days in healthy men. The dotted lines represent the lower limits of the normal range for testosterone and DHT and the upper and lower limits of the normal range for estradiol. * indicates P < .05 compared with baseline.
Steroid Hormone Pharmacokinetics
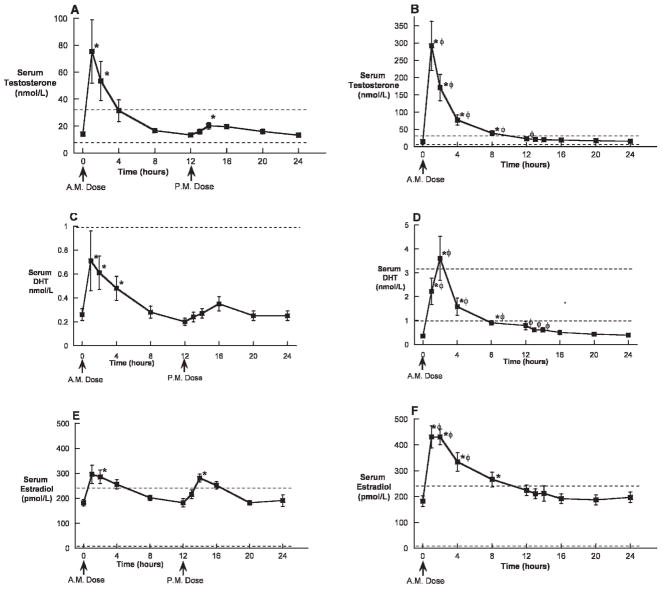
During the 28th day of treatment, mean serum testosterone was significantly increased above baseline by both twice-daily 400 mg TE and once-daily 800 mg TE within 1 hour of dosing (Figure 2A and B). Mean serum testosterone remained significantly elevated above baseline for 8 hours in the once-daily 800 mg TE group and for 2 hours in the twice-daily 400 mg TE group after the morning dose. In addition, the serum testosterone concentrations in the 800 mg TE group were significantly greater than those in the twice-daily 400 mg TE group for 12 hours after dosing. Interestingly, the evening dose of TE in the twice-daily 400 mg TE group did not result in nearly the postdose elevation of serum testosterone observed after the morning dose. The mean Cmax of serum testosterone was significantly greater in the once-daily 800 mg TE group compared with the twice daily 400 mg TE group. In addition, the AUC over 24 hours for testosterone was significantly greater with 800 mg once daily compared with the 400 mg twice daily (906 ± 612 nmol-h/L vs 249 ± 154 nmol-h/L; P < .01; Table 2).
Figure 2.
Serum testosterone, DHT, and estradiol concentrations (mean ± SEM) over 24 hours on the 28th day of oral administration of 400 mg twice daily (A, C, E) or 800 mg once daily (B, D, F) of TE in oil with dutasteride. The dotted lines represent the lower limits of the normal range. * indicates P < .05 compared with baseline; φ P < .05 between dose groups at a given time point.
Table 2.
Serum testosterone, dihydrotestosterone (DHT), and estradiol pharmacokinetic parameters (mean ± SEM) over 24 hours on the 28th day of oral administration of 400 mg twice daily or 800 mg once daily of testosterone enanthate (TE) in oil with dutasteride in healthy men
| Dose | Cmax, nmol/L | Tmax, h | AUC, nmol-h/L | T1/2, h |
|---|---|---|---|---|
| Testosterone | ||||
| 400 twice daily | 102 ± 62 | 2.6 ± 3.4 | 249 ± 154 | 7.5 ± 1.5 |
| 800 once daily | 316 ± 212a | 1.3 ± 0.5 | 906 ± 612a | 6.9 ± 2.7 |
| DHT | ||||
| 400 twice daily | 0.9 ± 0.2 | 2.5 ± 0.4 | 7.9 ± 1.3 | 7.0 ± 1.1 |
| 800 once daily | 4.1 ± 0.9a | 1.6 ± 0.2 | 23 ± 3.5a | 6.3 ± 1.0 |
| Estradiol | ||||
| 400 twice daily | 372 ± 59 | 2.7 ± 3.4 | 3268 ± 457 | 11 ± 3.8 |
| 800 once daily | 481 ± 120 | 1.5 ± 0.5 | 3630 ± 1380 | 15 ± 3.7 |
During the 28th day of treatment, mean serum DHT was significantly increased above baseline by both twice-daily 400 mg TE and once-daily 800 mg TE immediately within 1 hour of dosing (Figure 2C and D), but it remained below the lower limit of normal in the 400 mg twice-daily group. Mean serum DHT remained significantly elevated compared with baseline and within the normal range for 8 hours in the once-daily 800 mg TE group and for 4 hours in the twice-daily 400 mg TE group only after the morning dose. The serum DHT concentrations in the 800 mg TE group were significantly greater than those in the twice-daily 400 mg TE group for 14 hours after the morning dose. As was observed with the serum testosterone concentrations, the evening dose of TE in the twice-daily 400 mg TE group did not result in nearly the postdose elevation of serum DHT observed after the morning dose. The mean Cmax of serum DHT was significantly greater in the once-daily 800 mg TE group compared with the twice daily 400 mg TE group. In addition, the AUC over 24 hours for DHT was significantly greater with 800 mg once daily compared with the 400 mg twice daily (23 ± 3.5 nmol-h/L vs 7.9 ± 1.3 nmol-h/L; P < .01; Table 2).
Mean serum estradiol was significantly increased above baseline by both twice-daily 400 mg TE and once-daily 800 mg TE within 1 hour of dosing (Figure 2E and F). Mean serum estradiol remained significantly elevated for 8 hours in the once-daily 800 mg TE group and for 2 hours in the twice-daily 400 mg TE group after the morning dose. In addition, the serum estradiol concentrations in the 800 mg TE group were significantly greater than those in the twice-daily 400 mg TE group for 4 hours after dosing. In contrast to the marked difference observed for serum testosterone between the morning and evening doses, the serum estradiol concentrations observed after the evening dose of TE in the twice-daily 400 mg TE group were very similar to those observed after the morning dose, and the Cmax and AUC did not differ between treatment groups (Table 2).
Gonadotropin Suppression
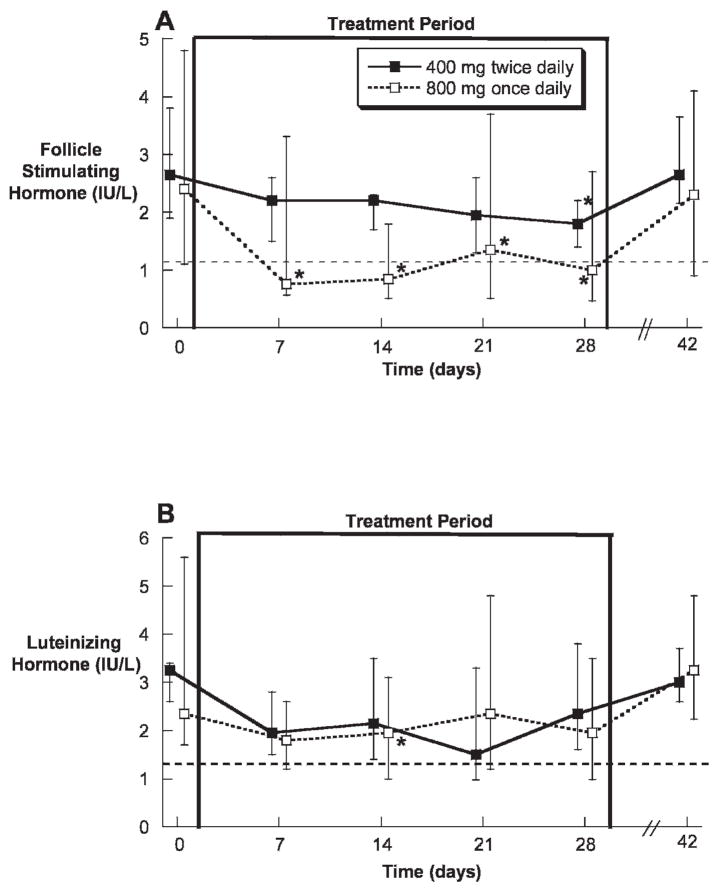
There were no significant differences in serum FSH or LH at baseline between the dose groups (Table 1). During treatment, median serum FSH was significantly decreased in the once-daily 800 mg TE group throughout treatment, whereas median serum FSH was significantly decreased only after 4 weeks of treatment with 400 mg TE twice daily (Figure 3A). In contrast, median serum LH was significantly suppressed only at 2 weeks of treatment in the once-daily 800 mg TE group (Figure 3B). Both FSH and LH had returned to normal 2 weeks after treatment.
Figure 3.
Serum FSH (A) and LH (B) concentrations (medians and interquartile ranges) prior to, during, and after oral administration of 400 mg twice daily or 800 mg once daily of TE in oil with dutasteride for 28 days in healthy men. The dotted lines represent the lower limits of the normal range. * indicates P < .05 compared with baseline.
After 28 days of treatment in the 400 mg twice-daily TE group, only 1 subject was suppressed below the lower limit of the normal range for serum FSH compared with 6 of 10 subjects in the 800 mg once-daily group (P = .057). Similarly, only 1 subject in the 400 mg twice-daily TE group was suppressed below the lower limit of the normal range for serum LH, compared with 3 of 10 subjects in the 800 mg once-daily group (P = .58). Interestingly, 1 subject in the 800 mg TE once-daily group had his gonadotropins suppressed to below the lower limit of quantitation for both the FSH and the LH assay after 3 weeks of treatment. Intriguingly, this subject exhibited the highest serum concentrations of estradiol but not testosterone during treatment. When all subjects were considered together, the percentage suppression of FSH was significantly correlated with the AUC for estradiol (r = 0.53, P = .01), but not with any other individual pharmacokinetic, hormonal, or anthropomorphic parameter.
Laboratory Monitoring
During treatment, there was a significant decrease in serum PSA observed in the twice-daily TE group (Table 3). In addition, a significant decrease in serum HDL cholesterol was noted in both treatment groups (Table 3), but no significant change in total cholesterol, LDL cholesterol, or triglycerides was noted. Weekly measurements of serum markers of liver function, liver inflammation, hematocrit, and kidney function did not change during treatment (Table 3). One subject had an increase in serum alanine aminotransferase (ALT) of greater than threefold after 2 weeks of treatment, but admitted to imbibing approximately 10 alcoholic beverages prior to his visit. This subject’s serum ALT normalized despite continued TE treatment.
Table 3.
Liver tests, hematocrit, prostate specific antigen (PSA), creatinine, and serum lipoproteins before and after 4 weeks of treatment with oral testosterone plus dutasteride
| Measurement and Group | Baseline | Treatment Day 28 |
|---|---|---|
| Bilirubin, mg/dL | ||
| 400 mg twice daily | 0.8 ± 0.3 | 0.8 ± 0.3 |
| 800 mg once daily | 0.9 ± 0.2 | 0.9 ± 0.3 |
| AST, U/L | ||
| 400 mg twice daily | 25 ± 2 | 24 ± 5 |
| 800 mg once daily | 27 ± 9 | 31 ± 20 |
| ALT, U/L | ||
| 400 mg twice daily | 28 ± 10 | 26 ± 8 |
| 800 mg once daily | 28 ± 7 | 23 ± 6 |
| Hematocrit, % | ||
| 400 mg twice daily | 45 ± 2 | 44 ± 3 |
| 800 mg once daily | 43 ± 5 | 41 ± 4 |
| Creatinine, mg/dL | ||
| 400 mg twice daily | 1.0 ± 0.1 | 1.0 ± 0.1 |
| 800 mg once daily | 1.1 ± 0.1 | 1.0 ± 0.1 |
| PSA, ng/dL | ||
| 400 mg twice daily | 0.9 ± 0.7 | 0.6 ± 0.5a |
| 800 mg once daily | 0.7 ± 0.3 | 0.6 ± 0.4 |
| Total cholesterol, mg/dL | ||
| 400 mg twice daily | 205 ± 41 | 205 ± 48 |
| 800 mg once daily | 182 ± 20 | 172 ± 29 |
| HDL cholesterol, mg/dL | ||
| 400 mg twice daily | 39 ± 12 | 34 ± 9b |
| 800 mg once daily | 48 ± 8 | 38 ± 7b |
| LDL cholesterol, mg/dL | ||
| 400 mg twice daily | 138 ± 34 | 142 ± 41 |
| 800 mg once daily | 120 ± 20 | 121 ± 24 |
| Triglycerides, mg/dL | ||
| 400 mg twice daily | 139 ± 98 | 142 ± 88 |
| 800 mg once daily | 70 ± 28 | 70 ± 24 |
Mood and Sexual Function
There was no significant difference in any parameter of mood (Table 4) or sexual function at baseline (Table 5). During treatment, there was no significant change in mood as determined by the questionnaire (Table 4). In addition, no subject complained of worrisome symptoms of depression, anxiety, or mania. Similarly, there were no significant changes in sexual function as measured by self-administered questionnaire, excepting a slight increase in overall satisfaction with sexual activity noted in the once-daily 800 mg TE group (Table 5). One subject complained of decreased libido after 2 weeks of treatment; however, this symptom abated despite continued treatment. At this time, the subject did not complain of erectile, ejaculatory, or orgasmic dysfunction.
Table 4.
Responses to selected questions on the Profile of Mood States mood questionnaire (mean ± SD) prior to and after oral administration of 400 mg twice daily or 800 mg once daily of TE in oil with dutasteride for 28 days in healthy men (n = 10 per group)a
| Question and Group | Baseline | Treatment Day 28 |
|---|---|---|
| Have you been a very nervous person? | ||
| 400 mg twice daily | 4.8 ± 0.9 | 4.8 ± 0.8 |
| 800 mg once daily | 4.2 ± 1.8 | 4.7 ± 1.8 |
| Did you have a lot of energy? | ||
| 400 mg twice daily | 3.0 ± 0.5 | 3.2 ± 1.1 |
| 800 mg once daily | 3.2 ± 1.1 | 3.4 ± 1.1 |
| Did you feel tired? | ||
| 400 mg twice daily | 4.5 ± 0.7 | 4.4 ± 0.8 |
| 800 mg once daily | 3.5 ± 1.1 | 3.9 ± 0.7 |
| Did you feel irritable? | ||
| 400 mg twice daily | 4.7 ± 0.5 | 4.9 ± 1.0 |
| 800 mg once daily | 4.4 ± 0.8 | 4.5 ± 1.0 |
| Were you able to concentrate well? | ||
| 400 mg twice daily | 2.7 ± 0.7 | 2.3 ± 0.5 |
| 800 mg once daily | 2.7 ± 1.1 | 3.0 ± 1.4 |
| Have you felt aggressive? | ||
| 400 mg twice daily | 5.0 ± 0.7 | 4.8 ± 1.0 |
| 800 mg once daily | 5.2 ± 0.9 | 5.2 ± 1.0 |
| Did you feel good/well? | ||
| 400 mg twice daily | 2.3 ± 0.8 | 2.1 ± 0.6 |
| 800 mg once daily | 2.6 ± 1.0 | 2.2 ± 0.6 |
Table 5.
Self-reported sexual function during treatment a
| Domain | Baseline | Treatment Day 28 |
|---|---|---|
| Sex drive (0–8)b | ||
| 400 mg twice daily | 5.5 ± 0.7 | 5.2 ± 0.9 |
| 800 mg once daily | 5.0 ± 0.8 | 5.5 ± 0.9 |
| Erectile function (0–12)c | ||
| 400 mg twice daily | 10.6 ± 0.7 | 9.9 ± 0.7 |
| 800 mg once daily | 10.6 ± 0.7 | 9.9 ± 0.6 |
| Ejaculatory function (0–8)d | ||
| 400 mg twice daily | 7.7 ± 0.4 | 7.4 ± 0.5 |
| 800 mg once daily | 7.1 ± 0.4 | 7.5 ± 0.8 |
| Freedom from problems with sexual activity (0–12)e | ||
| 400 mg twice daily | 11.0 ± 0.6 | 10.5 ± 0.5 |
| 800 mg once daily | 10.5 ± 0.4 | 10.6 ± 0.5 |
| Overall satisfaction with sexual activity (0–4)f | ||
| 400 mg twice daily | 3.2 ± 0.6 | 2.9 ± 0.7 |
| 800 mg once daily | 2.4 ± 1.1 | 3.1 ± 0.8g |
Discussion
In this work, we have shown that the administration of oral TE plus dutasteride over 4 weeks may be effective at suppressing serum gonadotropin concentrations to the extent that oral TE might be useful as part of a long-term male contraceptive regimen. Suppression of gonadotropins by oral TE is similar, if slightly inferior, to that seen after 1 month of weekly injections of 100 mg TE, which leads to azoospermia in roughly one half of men (Bebb et al, 1996). Therefore, as is the case with injectable TE, the coadministration of a second compound, such as a progestogen or a gonadotropin-releasing hormone (GnRH) analog, will be required to suppress serum gonadotropins to the point where effective suppression of spermatogenesis could be expected.
The 800 mg once-daily oral dose of TE suppressed FSH and LH to a much greater degree than the 400 mg twice-daily dose of oral TE. This difference between groups was likely due to either the significantly greater serum concentrations of testosterone, DHT, or estradiol during treatment. Which of these hormones is most important for gonadotropin suppression after oral administration is unclear. On one hand, the AUC and Cmax of testosterone and DHT were significantly greater in the 800 mg once-daily group, whereas the estradiol pharmacokinetics were similar between groups. On the other hand, only the AUC for estradiol was the only parameter significantly correlated with the degree of gonadotropin suppression during treatment. Clearly, whether the serum concentration of testosterone, DHT, or estradiol is more important in gonadotropin suppression will require additional study.
A second notable finding of this study was that the suppression of serum FSH by oral TE was greater than the suppression of serum LH. The reason for this is likely due to the increases in serum estradiol observed with oral TE. Estradiol acts to inhibit the hypothalamic-pituitary-gonadal axis at both the hypothalamus and the pituitary and is thought to provide much of the negative feedback regulation of FSH secretion in men (Hayes et al, 2000, 2001). In contrast, DHT likely plays a lesser role, since men with idiopathic hypogonadotropic hypogonadism who are stimulated with pulsatile GnRH do not exhibit feedback inhibition of gonadotropins when administered an infusion of DHT over 72 hours (Bagatell et al, 1994). More likely, DHT exerts its negative feedback effect at the level of the hypothalamus by inhibiting GnRH pulse amplitude and pulse frequency, since these are increased in men with congenital 5_α_-reductase deficiency (Canovatchel et al, 1994). Therefore, in our study the marked suppression of DHT by dutasteride may be allowing for the relative preservation of LH compared with FSH, which is well suppressed by the increased serum levels of estradiol.
As in our prior work, oral TE plus dutasteride results in transient supraphysiologic elevations in serum testosterone. Indeed, in this study, the serum testosterone concentrations were significantly higher than those observed in our previous work with the same doses of oral TE (Amory and Bremner, 2005). Since the predose concentrations of serum testosterone on day 27 were almost identical to the baseline testosterone concentrations, it is unlikely that this is due to accumulation of testosterone. In previous studies, we intentionally inhibited gonadotropin secretion with GnRH analogs prior to treatment, resulting in castrate baseline testosterone concentrations. Since gonadotropin suppression was the primary endpoint in this study, no such suppression was used, and it is likely that the remaining LH, although suppressed, still stimulated some endogenous production of testosterone. Additional studies using oral TE in combination with second agents intended to synergistically suppress gonadotropins will be required to fully determine the pharmacokinetics of oral TE in such a contraceptive setting.
One unanticipated feature of the long-term pharmacokinetics of oral TE was the marked diminution of the postdose testosterone peak with the evening administration of oral TE in the 400 mg twice-daily group. The reason for this is unclear but is not likely due to increased testosterone metabolism at night, since previous studies of metabolism have determined that testosterone metabolism is actually reduced at night (Wang et al, 2004). Our earlier work has demonstrated the concomitant food intake only slightly reduces serum testosterone concentrations achieved after oral dosing of TE in oil (Amory et al, 2006). Since the evening dose was administered after dinner, delayed postprandial absorption may play some role in this difference; however, in general, differences in gastrointestinal transit appear to explain little of the variability in bioavailability with most drugs (Riley et al, 1992). Also, this is not due to the effect of dutasteride “wearing off” by the evening, since the serum DHT peak was similarly attenuated. Obviously, further study of this intriguing phenomenon is required. This observation also suggests the possibility that dosing the oral TE at night might reduce the supraphysiologic Cmax observed with morning doses. Whether this is possible will be the subject of future studies of oral TE plus dutasteride. Importantly, there was no evidence of either liver or kidney toxicity associated with the doses of oral TE administered in this study. While no liver inflammation or other serious adverse events were reported during the 4 weeks of oral testosterone plus dutasteride, it is possible that such effects could be observed with more prolonged treatment. Additional studies administering oral testosterone plus dutasteride for longer periods of time will be required to determine whether this combination is safe long term. In theory, the ability to selectively increase serum testosterone without increasing serum DHT may be attractive in minimizing the risk for DHT-dependent disease, such as benign prostatic hyperplasia, acne, and alopecia, which are associated with testosterone therapy. It is notable that the serum DHT concentration remained suppressed during recovery. This is likely due to the long half-life of dutasteride (Clark et al, 2004).
Notably, no significant changes in mood or sexual function were noted in these healthy volunteers over 1 month. However, since these subjects were still likely producing endogenous testosterone, it is difficult to conclude that oral TE was able to maintain these endpoints. Nevertheless, it can be said that the transient supraphysiologic hormone concentrations observed in this study did not confer apparent psychologic or physiologic alterations, as assessed by self-reported questionnaire.
In conclusion, we have demonstrated that 1 month of oral TE combined with dutasteride can result in suppression of FSH and LH in healthy men. Such gonadotropin suppression would presumably be effective as part of a male contraceptive regimen in combination with either a progestogen or a GnRH analog when administered for periods of at least 12 weeks. Additional, longer-term studies of oral TE plus dutasteride in combination with one of these agents are warranted to determine whether oral TE can be a component of an effective oral form of male contraception.
Acknowledgments
Supported by the National Institute of Child Health and Human Development, a division of the National Institutes of Health (NIH), through cooperative agreements U54-HD-12629 and U54 HD42454 as part of the specialized Cooperative Centers Program in Reproductive Research and the Cooperative Contraceptive Research Centers Program. J.K.A. is supported in part by the National Institute of Child Health and Human Development, a division of the NIH, by grant 1K23 HD45386-10A1. A portion of this work was conducted through the Clinical Research Center facility at the University of Washington and supported by NIH grant M01-RR-00037.
The authors would like to thank Ms Kathy Winter, Ms Marilyn Busher, and Ms Kymberly Anable for assistance with the clinical aspects of the studies, and Ms Dorothy McGuinness for performance of hormone assays. In addition, we would like to thank Dr William J. Bremner, Dr Richard Clark, and Dr David W. Amory Sr for critical review of the manuscript, and Indevus Pharmaceuticals for the donation of the Delatestryl (testosterone enanthate).
References
- Amory JK, Bremner WJ. Oral testosterone in oil plus dutasteride: a pharmacokinetic study in men. J Clin Endocrinol Metab. 2005;90:2610–2617. doi: 10.1210/jc.2004-1221. [DOI] [PubMed] [Google Scholar]
- Amory JK, Page ST, Bremner WJ. Oral testosterone in oil: pharmacokinetic effects of 5α reduction with finasteride or dutasteride and food intake in men. J Androl. 2006;27:72–78. doi: 10.2164/jandrol.05058. [DOI] [PubMed] [Google Scholar]
- Amory JK, Wang C, Swerdloff R, Anawalt BD, Matsumoto AM, Bremner WJ, Walker SE, Haberer LJ, Clark RV. The effect of 5α-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J Clin Endocrinol Metab. 2007;92:1659–1665. doi: 10.1210/jc.2006-2203. [DOI] [PubMed] [Google Scholar]
- Bagatell CJ, Bahl KD, Bremner WJ. The direct pituitary effect of testosterone to inhibit gonadotropin secretion in men is partially mediated by aromatization to estradiol. J Androl. 1994;15:15–21. [PubMed] [Google Scholar]
- Bebb RA, Anawalt BD, Christensen RB, Paulsen CA, Bremner WJ, Matsumoto AM. Combined administration of levonorgestrel and testosterone induces more rapid and effective suppression of spermatogenesis than testosterone alone: a promising male contraceptive approach. J Clin Endocrinol Metab. 1996;81:757–762. doi: 10.1210/jcem.81.2.8636300. [DOI] [PubMed] [Google Scholar]
- Brady DM, Amory JK, Perheentupa A, Zitzmann M, Hay C, Apter D, Anderson RA, Bremner WJ, Huhtaniemi I, Nieshclag E, Wu FCW, Kersemaekers WM. A multi-centre study investigating subcutaneous etonogestrel implants with injectable testosterone decanoate as a potential long-acting male contraceptive. Hum Reprod. 2006;21:285–294. doi: 10.1093/humrep/dei300. [DOI] [PubMed] [Google Scholar]
- Canovatchel WJ, Volquez D, Huang S, Wood E, Lesser Ml, Gautier T, Imperato-McGinley J. Luteinizing hormone pulsatility in subjects with 5-alpha-reductase deficiency and decreased dihydrotestosterone production. J Clin Endocrinol Metab. 1994;78:916–921. doi: 10.1210/jcem.78.4.8157721. [DOI] [PubMed] [Google Scholar]
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004;89:2179–2184. doi: 10.1210/jc.2003-030330. [DOI] [PubMed] [Google Scholar]
- Hayes FJ, Decruz S, Seminara SB, Boepple PA, Crowley WF. Differential regulation of gonadotorpin secretion by testosterone in the human male: absence of a negative feedback effect of testosterone on follicle-stimulating hormone secretion. J Clin Endocrinol Metab. 2001;86:53–58. doi: 10.1210/jcem.86.1.7101. [DOI] [PubMed] [Google Scholar]
- Hayes FJ, Seminara SB, Decruz S, Boepple PA, Crowley WF. Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. J Clin Endocrinol Metab. 2000;85:3027–3035. doi: 10.1210/jcem.85.9.6795. [DOI] [PubMed] [Google Scholar]
- Heckel MJ. Production of oligospermia in a man by the use of testosterone propionate. Proc Soc Exp Biol Med. 1939;40:658–659. [Google Scholar]
- Heinemann K, Saad F, Wiesemes M, White S, Heinemann L. Attitudes toward male fertility control: results of a multinational survey on four continents. Hum Reprod. 2005;20:549–556. doi: 10.1093/humrep/deh574. [DOI] [PubMed] [Google Scholar]
- Kalhorn TF, Page ST, Howald WN, Mostaghel EA, Nelson PS. Analysis of testosterone and dihydrotestosterone from biological fluids as the oxime derivatives using high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21:3200–3206. doi: 10.1002/rcm.3205. [DOI] [PubMed] [Google Scholar]
- Marberger M. Drug insight: 5alpha-reductase inhibitors for the treatment of benign prostatic hyperplasia. Nat Clin Pract Urol. 2006;3:495–503. doi: 10.1038/ncpuro0577. [DOI] [PubMed] [Google Scholar]
- Martin CW, Anderson RA, Chang L, Ho PC, van der Spuy Z, Smith KB, Glasier AF, Everington D, Baird DT. Potential impact of hormonal male contraception: cross-cultural implications for development of novel male preparations. Hum Reprod. 2000;15:637–645. doi: 10.1093/humrep/15.3.637. [DOI] [PubMed] [Google Scholar]
- Matthiesson KL, McLachlan RI. Male hormonal contraception: concept proven, product in sight? Hum Reprod Update. 2006;12:463–482. doi: 10.1093/humupd/dml010. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States: Manual. San Diego, CA: Education and Industrial Testing Service; 1971. [Google Scholar]
- O’Leary MP, Fowler FJ, Lenderking WR, Barber B, Sagnier PP, Guess HA, Barry MJ. Brief male sexual function inventory for urology. Urology. 1995;46:697–706. doi: 10.1016/S0090-4295(99)80304-5. [DOI] [PubMed] [Google Scholar]
- Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, Wilson T, Rittmaster RS. The importance of dual 5alpha-reductastse inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006;55:1014–1023. doi: 10.1016/j.jaad.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Otberg N, Finner WM, Shapiro J. Androgenetic alopecia. Endocrinol Metab Clin North Am. 2007;36:379–398. doi: 10.1016/j.ecl.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Page ST, Amory JK, Anawalt BD, Irwig M, Brockenbrough A, Matsumoto AM, Bremner WJ. Testosterone gel combined with depomedroxyprogesterone acetate (DMPA) is an effective male hormonal contraceptive regimen but is not enhanced by the addition of the GnRH antagonist acyline. J Clin Endocrinol Metab. 2006;91:4374–4380. doi: 10.1210/jc.2006-1411. [DOI] [PubMed] [Google Scholar]
- Riley SA, Sutcliffe F, Kim M, Kapas M, Rowland M, Turnberg LA. The influence of gastrointestinal transit on drug absoprtion in healthy volunteers. Br J Clin Pharmacol. 1992;34:32–39. doi: 10.1111/j.1365-2125.1992.tb04104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–224. doi: 10.1056/NEJMoa030660. [DOI] [PubMed] [Google Scholar]
- Wang C, Catlin DH, Starcevic B, Leung A, DiStefano E, Lucas G, Hull L, Swerdoff RS. Testosterone metabolic clearance and production rates determined by stable isotope dilution/tandem mass spectrometry in normal men: influence of ethnicity and age. J Clin Endocrinol Metab. 2004;89:2936–2941. doi: 10.1210/jc.2003-031802. [DOI] [PubMed] [Google Scholar]
- Weston GC, Schlipalious ML, Bhuinneasin MN, Vollenhoven BJ. Will Australian men use male hormonal contraception? Med J Aust. 2002;175:204–205. doi: 10.5694/j.1326-5377.2002.tb04374.x. [DOI] [PubMed] [Google Scholar]