A Protein Complex Containing Mdm10p, Mdm12p, and Mmm1p Links Mitochondrial Membranes and DNA to the Cytoskeleton-based Segregation Machinery (original) (raw)
Abstract
Previous studies indicate that two proteins, Mmm1p and Mdm10p, are required to link mitochondria to the actin cytoskeleton of yeast and for actin-based control of mitochondrial movement, inheritance and morphology. Both proteins are integral mitochondrial outer membrane proteins. Mmm1p localizes to punctate structures in close proximity to mitochondrial DNA (mtDNA) nucleoids. We found that Mmm1p and Mdm10p exist in a complex with Mdm12p, another integral mitochondrial outer membrane protein required for mitochondrial morphology and inheritance. This interpretation is based on observations that 1) Mdm10p and Mdm12p showed the same localization as Mmm1p; 2) Mdm12p, like Mdm10p and Mmm1p, was required for mitochondrial motility; and 3) all three proteins coimmunoprecipitated with each other. Moreover, Mdm10p localized to mitochondria in the absence of the other subunits. In contrast, deletion of MMM1 resulted in mislocalization of Mdm12p, and deletion of MDM12 caused mislocalization of Mmm1p. Finally, we observed a reciprocal relationship between the Mdm10p/Mdm12p/Mmm1p complex and mtDNA. Deletion of any one of the subunits resulted in loss of mtDNA or defects in mtDNA nucleoid maintenance. Conversely, deletion of mtDNA affected mitochondrial motility: mitochondria in cells without mtDNA move 2–3 times faster than mitochondria in cells with mtDNA. These observations support a model in which the Mdm10p/Mdm12p/Mmm1p complex links the minimum heritable unit of mitochondria (mtDNA and mitochondrial outer and inner membranes) to the cytoskeletal system that drives transfer of that unit from mother to daughter cells.
INTRODUCTION
Mitochondria are indispensable organelles for normal eukaryotic cell function. Because mitochondria cannot be synthesized de novo, these organelles are inherited, i.e., transferred from mother to daughter cell during cell division. In the budding yeast, this process is mediated by cell cycle linked mitochondrial motility events. At G1 phase, subsequent to selection of a bud site, mitochondria align along the presumptive mother-bud axis. During S, G2, and M phases, mitochondria display two types of motility: some mitochondria undergo linear, polarized movement from mother to daughter cells, and some mitochondria are immobilized in the bud tip or in the tip of the mother cell distal to the site of bud emergence. These motility events result in equal distribution of mitochondria between mother and daughter cells (Simon et al., 1997; Yang et al., 1999).
Previous studies indicate that mitochondrial movement in dividing yeast is mediated by the actin cytoskeleton. Mitochondria show reversible, saturable, ATP-sensitive binding to actin filaments in vitro. In addition, mitochondria colocalize with actin cables (bundles of actin filaments that align along the mother-bud axis) and use these as tracks for movement in vivo. The force generator for this actin-based movement does not seem to be a myosin protein. Instead, we find that Arp2/3 complex is required for movement of mitochondria along actin cables (Boldogh et al., 2001).
The Arp2/3 complex, a seven-subunit complex that stimulates actin nucleation and cross-linking, is required for actin polymerization-driven processes, including movements of bacterial pathogens such as Listeria monocytogenes in infected host cells, internalization of extracellular material by endocytosis or phagocytosis and extension of the leading edge of motile cells (Taunton et al., 2000; Goldberg, 2001; Pollard and Borisy, 2003). Several lines of evidence support a role for Arp2/3 complex as the force generator for mitochondrial movement in budding yeast. First, subunits of the Arp2/3 complex are recovered with mitochondria upon subcellular fractionation and colocalize with mitochondria in intact yeast. Second, Arp2/3 complex-dependent actin nucleation activity is present on mitochondria in living yeast. Finally, incubation of yeast bearing a conditional mutation of an Arp2/3 complex subunit at restrictive temperature results in inhibition of mitochondrial motility but has no significant effect on mitochondrial morphology or association with actin cables (Boldogh et al., 2001)
Other studies indicate that association of mitochondria with actin cables requires at least two integral mitochondrial outer membrane (OM) proteins: Mdm10p and Mmm1p. These proteins were originally identified in yeast genetic screens designed to reveal genes required for mitochondrial inheritance. Yeast bearing mutations in MDM10 or MMM1 show similar phenotypes: an accumulation of abnormal, spherical mitochondria and defects in mitochondrial activities, including actin association, motility, inheritance, mitochondrial DNA (mtDNA) maintenance and respiration (Burgess et al., 1994; Sogo and Yaffe, 1994; Boldogh et al., 1998). Consistent with this, suppressors that restore mitochondrial morphology, growth rate and mtDNA stability to MMM1 mutants also suppress MDM10 mutants (Hanekamp et al., 2002).
Mmm1p was localized to punctate structures in proximity to mtDNA nucleoids, punctate structures containing mtDNA and proteins, which localize to the matrix surface of the mitochondrial inner membrane (IM). The localization of Mmm1p, together with the finding that deletion of MMM1 results in rapid loss of mtDNA and mtDNA nucleiod instability led to the proposition of a link between Mmm1p and mtDNA nucleoids at sites of close contact between mitochondrial OM and IM (Aiken Hobbs et al., 2001).
Here, we studied the relation of the protein Mdm12p to Mmm1p and Mdm10p, and its possible role in inheritance of mitochondria and mtDNA. Like Mmm1p and Mdm10p, Mdm12p is an integral mitochondrial OM protein first identified in a yeast genetic screen for genes required for mitochondrial inheritance (Berger et al., 1997). Yeast carrying mutations in MDM12 show phenotypes similar to those observed in MMM1 and MDM10 deletion mutations: all three mutants show defects in mitochondrial morphology, inheritance, and respiratory activity. Our studies support the model that Mmm1p, Mdm10p, and Mdm12p are part of a mitochondrial OM complex that links mitochondrial membranes and mtDNA to the actin-based force generator that drives transfer of mitochondrial membranes and DNA from mother to daughter cells.
METHODS AND MATERIALS
Yeast Strains and Tagging of MDM10, MDM12, and MMM1 Genes
Table 1 lists yeast strains used for this study. Yeast cell growth and manipulations were carried out according to Sherman (1991). The COOH termini of Mdm10p, Mdm12p, and Mmm1p were tagged with 13 tandem copies of the Myc or three copies of the hemagglutinin (HA) epitope by using polymerase chain reaction (PCR)-based insertion into the chromosomal copy of each gene (Longtine et al., 1998; Nowakowski et al., 2001). PCR fragments for integration by homologous recombination were first amplified from plasmids pFA6a-13Myc-TRP1, or pFA6a-13Myc-_HIS3_MX6. The primers used for tagging are as follows (underlined sequences correspond to the plasmid sequence): MDM10 forward, 5′-TTTCCCGGCAAAGTTTGGCATACAATTCCAGTACTCCACACGGATCCCCGGGTTAATTAA-3′; reverse, 5′-TGTATATTAAAACCTTTATTTTATTTCACATTACTCATCAGAATTCGAGCTCGTTTAAAC-3′; MDM12 forward, 5′-GCATGGCCAAGTTGGATTAATCTGGATTTCAACGATGGTGATGAGCGGATCCCCGGGTTAATTAA-3′; reverse, 5′-TTTATGTAGACACTATTTTCAAACTATCTTTGTTAAATTAGAATTCGAGCTCGTTTAAAC-3′; and MMM1 forward, 5′-ACGTAGTAAAAATACGAGAGAAGAAAAGCCTACAGAGTTACGGATCCCCGGGTTAATTAA-3′; reverse, 5′-AGGAAAAAGATAGAACAAAAAATTTGTACATAAATATTTAGAATTCGAGCTCGTTTAAAC-3′.
Table 1.
Yeast strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| YPH252 | MATα, ade2, his3, leu2, trp1, lys2, ura3 | R. Jensen (Johns Hopkins University, Baltimore, MD) |
| IBY113 | MATα, ade2, his3, leu2, trp1, lys2, ura3, MDM10:13Myc-HIS3MX6 | This study |
| IBY118 | MATα, ade2, his3, leu2, trp1, lys2, ura3, MDM12:13Myc-HIS3MX6 | This study |
| MYY291 | MATα, ura3, leu2, his3 | M. Yaffe (Berger et al., 1997) |
| MYY624 | MATα, ura3, leu2, his3, mdm12::URA3 | M. Yaffe (Berger et al., 1997) |
| D273-10b | MATα | American Type Culture Collection |
| DNY108 | MATα, leu2, trp1, ura3, MDM10:3HA-kanMX6 | This study |
| DNY364 | MATα, leu2, trp1, ura3, MDM10:3HA-kanMX6, MDM12:13Myc-TRP1 | This study |
| DNY365 | MATα, leu2, trp1, ura3, MDM10:3HA-kanMX6, MMM1:13Myc-TRP1 | This study |
| DNY422 | MATa/α, ade2/ADE2, leu2/leu2, ura3/URA3, MMM1:3HA-kanMX6/MMM1, MDM12/MDM12:13 Myc-TRP1 | This study |
| DNY366 | MATa/α, ade2/ADE2, leu2/leu2, trp1/TRP1 ura3/ura3, MMM1/MMM1:3HA-kanMX6 | This study |
| DNY401(YRJ484) | MATa,ade2, his3, leu2, lys2, trp1, ura3, mmm1::URA3 | R. Jensen |
| HCY330 | MATa,ade2, his3, leu2, lys2, trp1, ura3, mmm1::URA3, MDM10:13Myc-HIS3MX6 | This study |
| HCY340 | MATa,ade2, his3, leu2, lys2, trp1, ura3, mmm1::URA3, MDM12:13Myc-HIS3MX6 | This study |
| HCY351 | MATα, ura3, leu2, his3, mdm12::URA3, MMM1:13Myc-HIS3MX6 | This study |
| HCY361 | MATα, ura3, leu2, his3, mdm12::URA3, MDM10:13Myc-HIS3MX6 | This study |
| HCY372 | MATa,ade2, ura3, leu2, trp1, MDM12:13Myc-TRP1, rho° | This study |
| HCY371 | MATα, leu2, trp1, ura3, MDM10:13Myc-TRP1, rho° | This study |
| KAY40: | MATa/α, ura3-52/ura3-52 | K. Ayscough (Warren et al., 2002) |
| BY4743: | MATa/α, his3Δ1/his3Δ1, leu2Δ/leu2Δ, MET15/met15Δ, LYS2/lys2Δ, ura3Δ/ura3Δ | Research Genetics |
| RG24813: | MATa/α, his3Δ1/his3Δ1, leu2Δ/leu2Δ, MET15/met15Δ, LYS2/lys2Δ, ura3Δ/ura3Δ, qcr9::kanMX4/qcr9::kanMX4 | Research Genetics |
Yeast cells were transformed with the PCR products by the lithium acetate method (Gietz and Schiestl, 1995). Transformants that were positive for integration at the target locus were validated by PCR, analyzed for protein expression via Western blot, and the tagged construct were visualized in cells by immunofluorescence staining (see below). Deletion of the open reading frame of the MDM10, MDM12, or MMM1 gene results in yeast that accumulate large spherical mitochondria and are unable to grow on nonfermentable carbon sources. Therefore, mitochondrial respiratory activity was evaluated by testing for growth defects on glycerol-based media (YP-glycerol) at 30° and 37°C. Mitochondrial morphology was tested by visualization of mitochondria by indirect immunofluorescence using an antibody raised against mitochondrial outer membrane proteins (see below). None of the tags used for these studies had any obvious effect on mitochondrial structure, respiration or motility. The rho0 strains were created by treating cells with ethidium bromide as described by Fox et al. (1991). The absence of mtDNA in rho0 cells was confirmed by 4,6-diamidino-2-phenylindole (DAPI) staining of selected clones for detection of mtDNA and by the lack of growth on nonfermentable carbon sources.
Immunoprecipitation
Yeast mitochondria were isolated as described previously (Lazzarino et al., 1994). Isolated mitochondria were solubilized as described by Kerscher et al. (1997). Mitochondria were solubilized to 1 mg/ml in a buffer containing 0.5% digitonin, 50 mM NaCl, 30 mM HEPES, pH 7.4, 1 mM phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor cocktail (Lazzarino et al., 1994), incubated for 45 min at 4°C with gentle agitation, and centrifuged at 12,500 × g for 10 min at 4°C. The supernatant from 750 μg of mitochondria was mixed with 15 μl of protein G-Sepharose beads (Amersham Pharmacia AB, Uppsala, Sweden) coupled to a monoclonal anti-myc antibody (Evan et al., 1985) and incubated for 2 h at 4°C with gentle rotation. Thereafter, the beads were washed twice with solubilization buffer and once with the solubilization buffer without digitonin. Proteins bound to protein G-Sepharose beads were eluted with 1× SDS-PAGE sample buffer. After separation by SDS-PAGE, proteins were immunoblotted with an antibody to the myc epitope and polyclonal antibodies raised against HA (Roche Diagnostics, Indianapolis, IN) and the mitochondrial marker protein, OM45 (gift from G. Schatz, Swiss Science and Technology Council, Bern, Switzerland).
Visualization of Mitochondria and the Actin Cytoskeleton
In living cells mitochondria were visualized using a fusion protein consisting of the mitochondrial signal sequence of citrate synthase 1 fused to green fluorescent protein (CS1-GFP). CS1-GFP was expressed using a centromere-based plasmid under the endogenous citrate synthase promoter (Okamoto et al., 2001). Yeast cells were transformed using the lithium acetate method (Ito et al., 1983). Cells expressing CS1-GFP were grown to mid-log phase in a synthetic, glucose-based liquid media or in a rich, raffinose-based media at 30°C. Samples were mounted on microscope slides and visualized by fluorescence microscopy as described below. CS1-GFP labeling of mitochondria is specific and has no detectable effect on mitochondrial morphology, respiration, or movement under our experimental conditions.
The actin cytoskeleton was visualized using rhodamine-phalloidin (Molecular Probes, Eugene, OR), a ligand that binds specifically to actin polymers (Cooper, 1987). Rhodamine-phalloidin was added to fixed samples to a final concentration of 2.5 mM in a solution consisting of a 4:1 ratio of NS (20 mM Tris-HCl, pH 7.6, 0.25 M sucrose, 1 mM EDTA, 1 mM MgCl2, 0.1 mM ZnCl2, 0.1 mM CaCl2, 0.8 mM PMSF, 0.05% [vol/vol] 2-mercaptoethanol) to methanol, and samples were allowed to stand in the dark at 4°C for 16 h. Stained cells were mounted onto microscope slides and visualized by fluorescence microscopy.
The method used for indirect immunofluorescence is a modification of published methods (Pringle et al., 1989). All samples were fixed by addition of paraformaldehyde solution (Electron Microscopy Sciences, Ft. Washington, PA) to the cell culture medium to a final concentration of 3.7% and incubation for 1 h under growth conditions. Cells were collected by centrifugation and fixative was removed by three washes with wash solution (25 mM KPO4, pH 7.5, 0.4M KCl). Cell walls were removed from fixed cells by incubation with zymolyase (Smith et al., 1995). Zymolyase-treated cells were washed three times with NS. Fixed spheroplasts were applied to polylysine-coated coverslips and allowed to adhere to the coverslips for 40 min.
Immobilized spheroplasts were then gently washed in phosphate-buffered saline (PBS) and incubated in PBT (1× PBS, 0.1% [vol/vol] Triton X-100, 0.02% [vol/vol] sodium azide, 1% [wt/vol] bovine serum albumin) for 5 min at room temperature (RT). This was followed by incubation with primary antibody for 2 h at RT. Mitochondria were visualized with a rabbit polyclonal antiserum raised against total mitochondrial outer membrane proteins (Smith et al., 1995). Myc-tagged proteins were visualized using a monoclonal anti-myc antibody (see above). Subsequent to incubation with primary antibodies, spheroplasts were washed with PBT, and incubated with fluorescently labeled secondary antibody for 60 min at RT. The secondary antibodies used for these studies, fluorescein isothiocyanate (FITC)-coupled goat anti-mouse IgG and rhodamine-coupled goat anti-rabbit IgG (Kirkegaard and Perry Laboratories, Gaithersburg, MD), were reconstituted, stored, and used according to the manufacturer's instructions. The spheroplasts were washed with PBS to remove unbound secondary antibody and mounted on microscope slides by using mounting solution (1 mg/ml _p_-phenylenediamine, 90% [wt/vol] glycerol, 1× PBS) with 0.5 μg/ml DAPI to detect DNA.
Light Microscopy
Images were collected with an Axioplan II microscope (Carl Zeiss, Oberkochen, Germany) by using a Plan-Apochromat 100×, 1.4 numerical aperture objective lens, and a cooled charge-coupled device camera (Orca-100; Hamamatsu, Bridgewater, NJ). Illumination with a 100-W mercury arc lamp was controlled with a shutter (Uniblitz D122; Vincent Associates, Rochester, NY). Camera control and image enhancement were performed using Open Lab software (Improvision, Coventry, UK).
For analysis of localization of Mdm10p, Mdm12p, and Mmm1p relative to DAPI-stained mtDNA, 25 z-sections were obtained at 0.2-μm intervals through the entire cell. Z sectioning for three-dimensional (3-D) imaging was carried out using a piezoelectric focus motor mounted on the objective lens of the microscope (Polytech PI, Auburn, MA). Out-of-focus light was removed by deconvolution of each image section, and each series of deconvolved images was projected and rendered with Volocity software (Improvision).
Quantitation of Mitochondrial Movement In Vivo
Mitochondria were defined as motile if they displayed linear movement for three consecutive frames. In all cases, the only portion of the organelle that was evaluated for movement was the tip of the organelle. Moreover, for any given cell, mitochondrial movement was evaluated only in a single optical plane. The velocities of motile mitochondria were determined by measuring the change in position of the tip of each moving mitochondrion as a function of time in time-lapse series recorded at 20-s intervals >10 min of real time. In wild-type cells (MDM12), only velocities of organelles undergoing linear movement for at least 3 consecutive frames (1 min of real time) were measured. In the _mdm12_Δ mutant, linear movements were not observed. For all velocity measurements, NIH Image version 1.60 was used to determine the change in position (x-y coordinates) of mitochondria per unit time, and these were averaged to obtain a mean velocity. Polarized movement is defined as that which achieves a net displacement toward the bud in budding cells and is expressed as the percentage of all motile organelles exhibiting polarized movement over the time-lapse course.
Other Methods
To detect levels of epitope-tagged Mmm1p, Mdm10p, and Mdm12p proteins in yeast, whole cell extract was prepared by vortexing mid-log phase yeast cells with 0.5-mm glass beads in a solution consisting of 10% glycerol, 10 mM EGTA, 1% Triton X-100, 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 2 mM PMSF, and protease inhibitor cocktail. Protein concentration of the cell lysate was determined using the bicinchoninic acid assay following the vendor's protocol (Pierce Chemical, Rockford, IL). Gel electrophoresis and Western blot analysis were performed as described above.
RESULTS
Mdm10p and Mdm12p Are Localized to Mitochondria, as Patches Adjacent to mtDNA Nucleoids
Previous studies showed that Mmm1p localizes to the mitochondrial surface in discrete patches adjacent to mtDNA (Aiken Hobbs et al., 2001). Because mutations in MDM10 and MDM12 genes produce phenotypes similar to those observed upon mutation of MMM1, we investigated whether Mdm10p and Mdm12p have comparable distribution patterns. These localization studies were carried out in strains in which multiple copies of the Myc epitope were inserted at the carboxy terminus of either Mdm10p or Mdm12p and the proteins were expressed from their chromosomal loci. In both cases cell growth on fermentable and nonfermentable carbon sources was indistinguishable from that observed in strains expressing untagged, endogenous genes (Figure 1A). This suggests that the fusion proteins function normally. Recently Hanekamp et al. (2002) reported that tagging of the MDM10 gene with three copies of the HA epitope resulted in abnormal mitochondrial morphology upon growth on a nonfermentable carbon source. Our cells expressing MDM10-13Myc, however, exhibited normal mitochondrial morphology under similar conditions (our unpublished data). The reverse primer used by Hanekamp et al. (2002) to insert HA tag produced an ∼80 base pairs deletion immediately downstream of the tagged MDM10 gene. This difference in approach may account for the phenotypic differences.
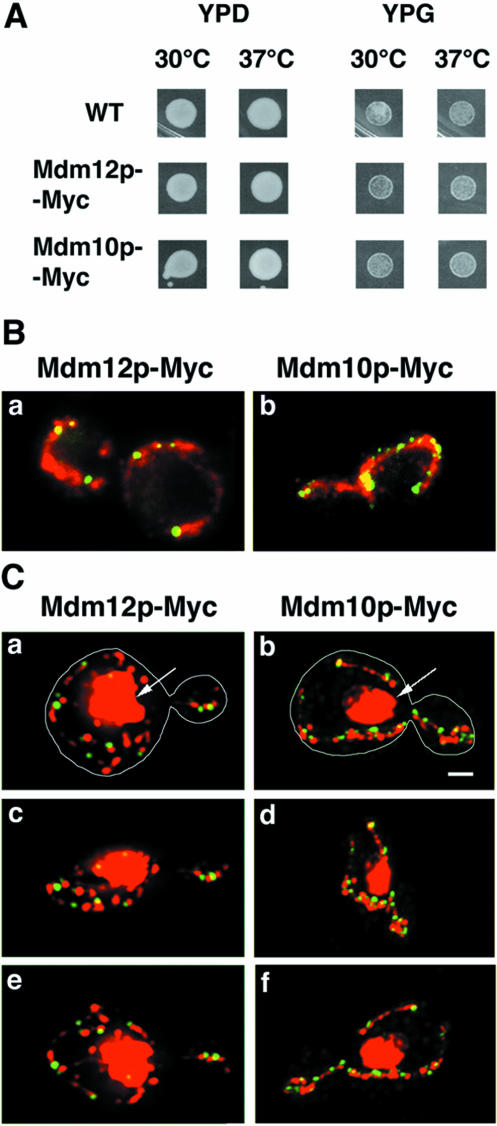
Figure 1.
Mdm12p-Myc– and Mdm10p-Myc–containing structures are adjacent to mtDNA nucleoids. (A) Growth on glucose-(YPD) and glycerol (YPG)-containing media of wild-type parent (YPH252), Mdm12p-Myc–(IBY118), and Mdm10p-Myc (IBY113)–expressing cells. Equal amounts of cells were spotted onto plates and grown for 3 d. (B) Mdm12p-Myc– and Mdm10p-Myc–expressing cells were grown at 30°C to early mid-log phase. Fixed cells were stained for Mdm12p-Myc (a) or Mdm10p-Myc (b) by indirect immunofluorescence with anti-Myc antibody (green) and for mitochondria by an antibody raised against mitochondrial OM proteins (red). Z-sections through the cell were collected and projected into a single image. (C) Mdm12p-Myc– (a, c, and e) and Mdm10p-Myc (b, d, and f)–expressing cells were grown and fixed as for B. Tagged proteins were visualized using anti-Myc antibody (green). mtDNA was visualized using DAPI (red). Z-sections through the cell were collected, deconvolved, and projected with the Volocity 3-D rendering tool. DAPI signal is projected at maximum opacity, whereas Myc fluorescence is projected at 25–30% of maximum opacity, resulting in more solid fluorescent signal and removal of background. Three views of the 3-D models rotated 0° (a and b), 70° (c and d), and 150° (e and f) are shown. Nuclear DNA was resolved as large objects (arrows) and mtDNA resolved as small dots. Bar, 1 μm.
By fluorescence microscopy, both Mdm12-Myc and Mdm10p-Myc were resolved as punctate structures along mitochondria. We detected four to nine (on average, six) Mdm12p-Myc–containing structures per cell, all of which colocalized with mitochondria (Figure 1B, a). Under these conditions we observed an average of six Mmm1p-Myc–containing structures per cell (our unpublished data). In contrast, Mdm10-Myc–containing structures were more numerous. We observed an average of 13 of these structures per cell, with numbers ranging from 6 to 21 (Figure 1B, b). Like the punctae containing Mdm12p, Mdm10p-Myc structures were distributed evenly along the whole length of mitochondria.
In haploid yeast cells mtDNA is organized into 10–20 separate DNA–protein complexes, called nucleoids (MacAlpine et al., 2000). Each nucleoid contains two to eight mtDNA molecules (Stevens, 1981). We investigated whether the Mdm12p-Myc– or Mdm10p-Myc–containing structures are associated with mtDNA. To visualize mtDNA, cells were stained with DAPI, a DNA binding dye. Deconvolved and 3-D projected fluorescent images of Mdm12p-Myc–expressing cells (n = 40) (Figure 1C, a, c, and e) and Mdm10p-Myc–expressing cells (n = 26) (Figure 1C, b, d, and f) showed that 75% of Mdm12-Myc–containing structures and 78% of Mdm10p-Myc–containing structures were in proximity to mtDNA. Under these experimental conditions, 77% of Mmm1p-Myc–containing punctate structures were in proximity to mtDNA nucleoids (our unpublished data). This percentage is somewhat lower than was previously published for Mmm1p-GFP in living cells, where the nucleus is poorly stained by DAPI and nuclear staining therefore does not obscure mtDNA (Aiken Hobbs et al., 2001). However, in fixed cells, where DAPI stains nuclear and mtDNA well, we were unable to determine whether some Mmm1p-Myc structures were adjacent to mtDNA. Therefore, although we underestimate the number of Myc structures associated with mtDNA, these experiments suggest that Mdm10p and Mdm12p proteins, like Mmm1p, are also in proximity to mtDNA.
MDM12 Mutants Show Defects in Mitochondrial Movement and mtDNA Nucleoid Stability
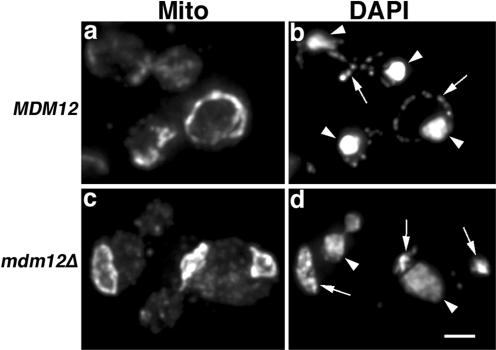
We confirmed that mitochondrial morphology is defective in MDM12 mutant cells. Mitochondria in _mdm12_Δ mutant cells, like those in _mmm1_Δ and _mdm10_Δ cells, form abnormal, large spherical structures (Figures 2 and 3). Moreover, we found that defects in mtDNA maintenance in _mdm12_Δ mutants may result from defects in mtDNA nucleoid stability. In our haploid wild-type strains, there are on average 20 nucleoid structures per cell. Unlike the _mmm1_Δ mutants that rapidly lose all mtDNA, ∼70% of _mdm12_Δ cells still contained mtDNA (Figure 2). However, in contrast to wild-type cells that contain an average of 20 mtDNA nucleoids per cell, _mdm12_Δ cells contained only two to four mtDNA nucleoids per cell (n = 27). Thus, the number of nucleoids in _mdm12_Δ cells decreased markedly compared with wild-type cells. This suggests that Mdm12p, like Mmm1p, contributes to mtDNA organization. Although some punctate mtDNA-containing structures were detected in _mdm12_Δ cells, we also observed diffuse DAPI staining within mitochondria in this mutant (Figure 2). Thus, we observed defects in mtDNA nucleoid stability or formation upon deletion of MDM12.
Figure 2.
Defects in mtDNA nucleoid stability in _mdm12_Δ mutants. MDM12 (MYY291) (a and b) and _mdm12_Δ cells (MYY624) (c and d) were grown at 24°C to mid-log phase in synthetic, glucose-based liquid media (SC). Fixed cells were stained for mitochondrial OM proteins by indirect immunofluorescence by using anti-mitochondrial OM antibody (a and c). mtDNA was visualized using DAPI (b and d). Z-sections throughout the cells were collected, deconvolved, and projected. Arrowheads mark nuclear DNA. Arrows point to mtDNA. Bar, 2 μm.
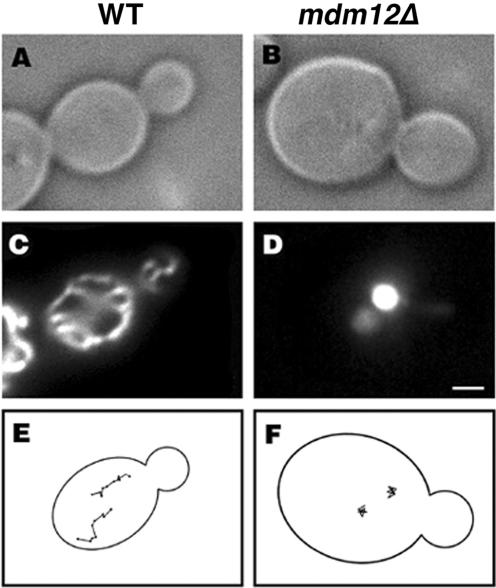
Figure 3.
Mitochondria in _mdm12_Δ cells do not exhibit long-distance movement. Wild-type (MYY291) (A and C) and _mdm12_Δ mutant cells (MYY624) (B and D) expressing a fusion protein consisting of the mitochondrial signal sequence of CS1-GFP were grown at 23°C in YP-raffinose media. Cells were viewed by differential interference contrast (A and B), and GFP-labeled mitochondria were visualized by fluorescence microscopy (C and D). Mitochondrial movements in CS1-GFP cells were monitored by time-lapse imaging. Images were acquired at 20-s intervals over a period of 10 min. The points denote the position of the tips of organelles at 20-s intervals in wild-type cells (E) and in _mdm12_Δ mutant cells (F). Bar, 1 μm.
Moreover, we found that Mdm12p is required for normal mitochondrial motility. By time-lapse fluorescence microscopy, mitochondrial movement in MDM12 wild-type cells was similar to that in other wild-type strains: mitochondria exhibit linear, long distance movement that is directed toward the bud with an average velocity of 27 nm/s. In contrast, mitochondria in _mdm12_Δ mutants undergo short oscillatory movements and <1% of these mitochondria exhibit bud-directed movements (Figure 3 and Table 2). Thus, _mdm12_Δ mutants, like _mmm1_Δ and _mdm10_Δ mutant cells, have severe defects in mitochondrial motility.
Table 2.
Characteristics of mitochondrial motility in the _mdm12_Δ mutant
| Velocity (nm/sec) | |||
|---|---|---|---|
| Strain | Linear movements | Nonlinear movement | % polarized movements |
| MDM12 | 27.6 ± 5.9 | ND | 51.7 (n = 63) |
| _mdm12_Δ | 0 | 6.3 ± 3.3 | 0.9 (n = 68) |
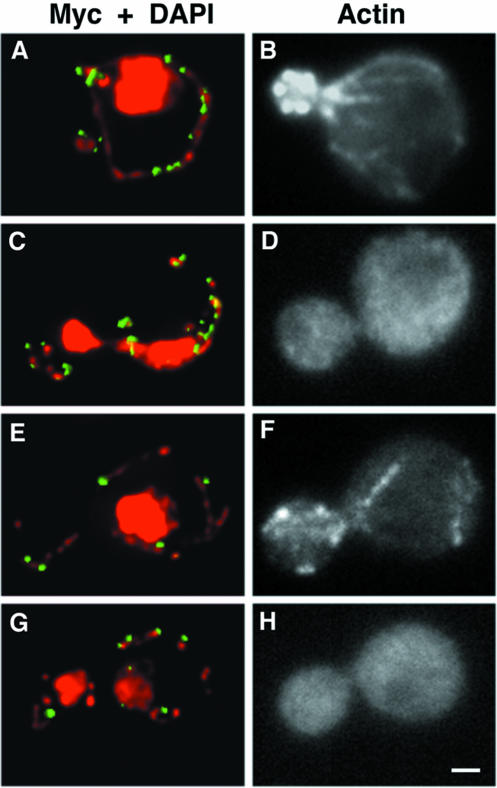
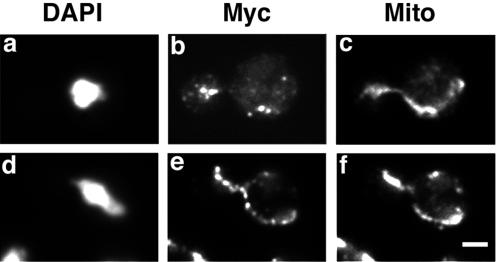
These results support the idea that Mdm12p, like Mmm1p and Mdm10p, is important for linear, long distance movement of mitochondria. Because mitochondrial movement in yeast requires the actin cytoskeleton, we tested whether the actin cytoskeleton contributes to the localization of Mdm12p or Mdm10p proteins. Cells expressing Mdm12p-Myc or Mdm10p-Myc were treated with latrunculin-A (Lat-A), a drug that promotes rapid and quantitative depolymerization of actin structures (Ayscough et al., 1997). After 10 min of Lat-A treatment, we could not detect any F-actin structures by using fluorochrome-coupled phalloidin. Under these conditions, mtDNA nucleoid organization was maintained and both Mdm10p-Myc (Figure 4, A–D) and Mdm12p-Myc (Figure 4, E–H) persisted as punctate structures in proximity to mtDNA. Thus, localization of these proteins to mitochondria is independent of the actin cytoskeleton.
Figure 4.
Mdm10p-Myc and Mdm12p-Myc localize to mtDNA in the absence of actin structures. Yeast cells expressing Mdm10p-Myc– (IBY113) (A–D) or Mdm12-Myc (IBY118) (E–H)–expressing cells were grown at 30°C to mid-log phase. Then 0.4 mM Lat-A dissolved in dimethyl sulfoxide (C, D, G, and H) or an equal volume of dimethyl sulfoxide alone (A, B, E, and F) was added to each culture. After 10-min incubation under growth conditions, cells were fixed and stained for the Myc tag by using anti-Myc antibody (green) and for mtDNA by using DAPI (red) (A, C, E, and G). F-actin–containing structures were visualized with rhodamine phalloidin (B, D, F, and H). Bar, 1 μm.
Mmm1p, Mdm10p, and Mdm12p Form a Complex in Mitochondrial Membranes
We investigated physical and functional interactions among Mmm1p, Mdm10p, and Mdm12p. First, we tested whether these proteins coimmunoprecipitate when tagged with Myc or HA epitopes. Second, we studied the effect of deletion of MMM1 on localization of Mdm10p and Mdm12p, and of deletion of MDM12 on localization of Mmm1p and Mdm10p.
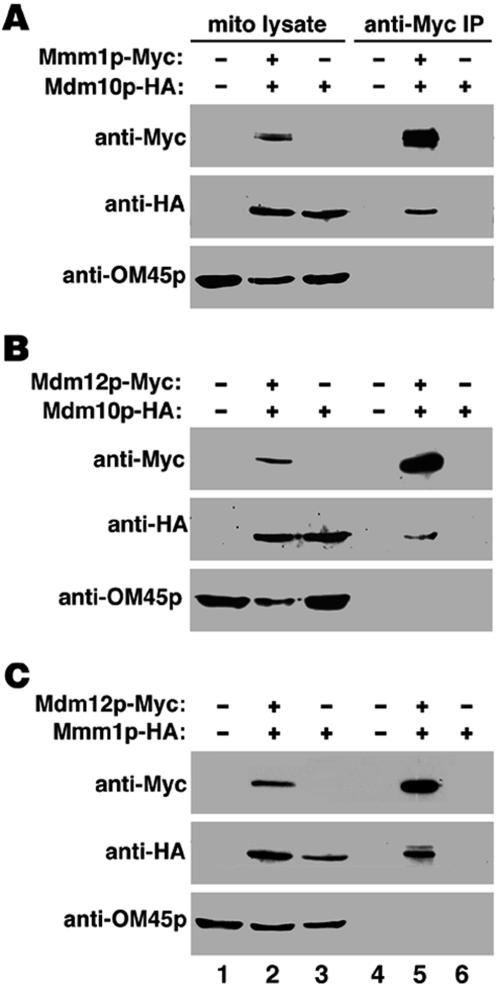
Using Myc antibody for immunoprecipitation and probing for the presence of HA-tagged proteins in the immunoprecipitated samples, we observed coimmunoprecipitation in all three pairwise combinations, Mmm1p-Myc/Mdm10-HA, Mdm12-Myc/Mdm10-HA, and Mdm12-Myc/Mmm1p-HA (Figure 5 A–C, lane 5). Tagging any two of the three proteins did not produce changes in mitochondrial morphology and distribution. Also, cells expressing two tagged constructs displayed normal growth rates on a nonfermentable carbon source (glycerol), indicating normal mitochondrial function (our unpublished data). To test the specificity of these associations, immunoprecipitated samples were probed for the presence of the most abundant integral mitochondrial OM protein, OM45p (Figure 5, A–C, lane 6). The absence of OM45p among the immunoprecipitated proteins suggests that the associations between Mmm1p, Mdm10p, and Mdm12p proteins were specific. These results support physical associations between Mmm1p, Mdm10p, and Mdm12p.
Figure 5.
Mmm1p, Mdm10p, and Mdm12p coimmunoprecipitate with each other. Mitochondria were purified from an untagged strain (D273-10b) (lanes 1 and 4), strains expressing only HA-tagged construct (DNY108 in A and B; DNY366 in C) (lanes 3 and 6) or strains coexpressing either Mmm1p-Myc with Mdm10p-HA (DNY365 in A), Mdm12p-Myc with Mdm10p-HA (DNY364 in B), or Mdm12p-Myc with Mmm1p-HA (DNY422 in C) (lanes 2 and 5). Mitochondria were solubilized in a 0.5% digitonin buffer (lanes 1–3) and from this lysate, Myc-tagged proteins were immunoprecipitated (see MATERIALS AND METHODS) using a monoclonal anti-Myc antibody (lanes 4–6). Immunoprecipitated proteins were probed using anti-Myc, anti-HA, and control, anti-OM45 antibodies. OM45 is an abundant integral OM protein that does not immunoprecipitate with Mmm1p, Mdm10p, or Mdm12p.
To further investigate the relationship among these three proteins, we expressed Myc-tagged Mdm10p and Mdm12p proteins in _mmm1_Δ mutant cells, and Mmm1p-Myc and Mdm10p-Myc in _mdm12_Δ mutant cells (Figure 6). Tagging did not change the growth rate compared with untagged mutant cells. Moreover, analysis of the level of Myc-tagged proteins by immunoblotting or immunofluorescence with Myc antibody showed that the levels of expression of the Myc tagged proteins in the deletion mutant strains were comparable with those in wild-type strains (our unpublished data).
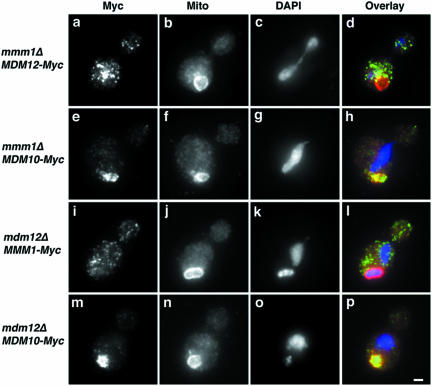
Figure 6.
Localization of proteins in deletion mutants. _mmm1_Δ mutant cells expressing Mdm12p-Myc (HCY340; a–d) or Mdm10p-Myc (HCY330; e–h), and _mdm12_Δ mutant cells expressing Mmm1p-Myc (HCY351; i–l) or Mdm10p-Myc (HCY361; m–p) were grown at 24°C to early mid-log phase in synthetic, glucose-based liquid media (SC-his, -ura). Fixed cells were stained for the Myc epitope and mitochondrial OM proteins by indirect immunofluorescence by using anti-Myc antibody (a, e, i, and m) and anti-OM antibody (b, f, j, and n), respectively. mtDNA was visualized using DAPI (c, g, k, and o). d, h, l, and p show the overlays of Myc epitope (green), mitochondrial OM (red), and DNA (blue) staining. Bar, 1 μm.
Mmm1p-Myc localizes to punctate structures in _mdm12_Δ cells (Figure 6, i–l). However, none of the Mmm1p-Myc-containing punctate structures localize to mitochondria. Similarly, Mdm12p-Myc localization to mitochondria was dependent upon the presence of Mmm1p: Mdm12p-Myc in the _mmm1_Δ strain localized to punctate structures that were distributed throughout the mother cell and bud (Figure 6, a–d). Our studies indicate that Mmm1 and Mdm12p depend upon each other for their localization to mitochondria, suggesting functionally significant interactions between Mmm1p and Mdm12p. In contrast, the localization of Mdm10p-Myc to mitochondria is independent of the presence of Mdm12p or Mmm1p. Mdm10p-Myc–containing structures localize to punctate structures that colocalize with mitochondria in both _mdm12_Δ and _mmm1_Δ cells (Figure 6, e–h and m–p).
Motility of rho0 Mitochondria
Because Mdm10p and Mdm12p contribute to mtDNA nucleoid stability and maintenance, we tested whether the absence of mtDNA has an effect on the assembly of these proteins. We found that mtDNA nucleoids are not required for Mdm10p or Mdm12p assembly. Mdm10p-Myc and Mdm12p-Myc, like Mmm1p, localized to punctate structures on mitochondria in rho0 cells, which lack mtDNA (Figure 7).
Figure 7.
Mdm10p-Myc and Mdm12p-Myc show punctate localization to mitochondria in the absence of mtDNA. Mdm12p-Myc–(HCY372) (a–c) and Mdm10p-Myc (HCY371) (d–f)–expressing cells were grown at 30°C to early mid-log phase. Fixed cells were stained for the Myc epitope and mitochondrial OM proteins by indirect immunofluorescence using anti-Myc antibody (b and e) and anti-OM antibody (c and f), respectively. mtDNA was visualized using DAPI (a and b). Bar, 2 μm.
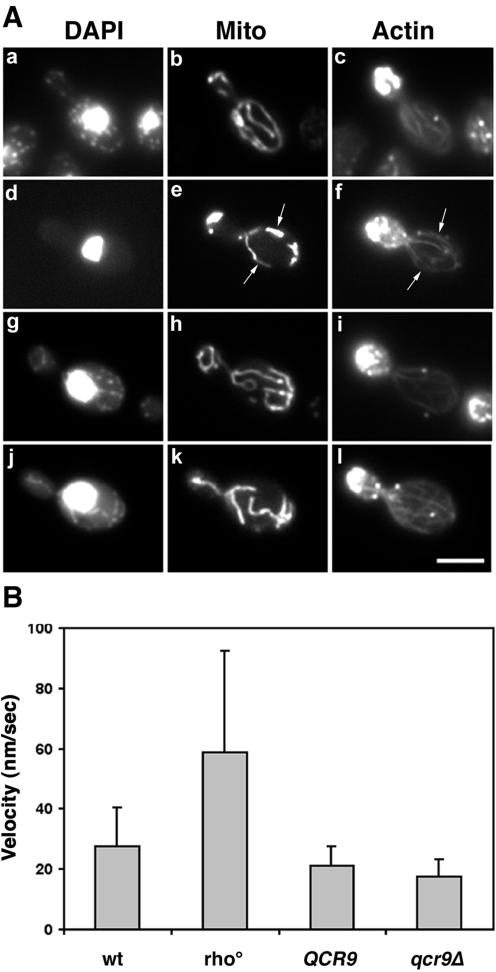
In contrast, we find that mtDNA does affect mitochondrial motility, a process that also depends on Mmm1p, Mdm10p, and Mdm12p. Deletion of mtDNA has no significant effect on mitochondrial morphology or colocalization with actin cables (Figure 8A, e and f). However, rho0 mitochondria, which have no mtDNA, move twice as fast as wild-type, rho+ mitochondria (Figure 8B). To verify that this change in mitochondrial motility was not due to respiratory deficiency of the rho0 strain, we investigated mitochondrial movement in cells bearing a deletion in the nuclear gene QCR9, a subunit of the ubiquinol cytochrome c oxidoreductase complex. These cells, which have wild-type mtDNA but no respiratory activity (Graham et al., 1992), show normal mitochondrial morphology and normal velocity of mitochondrial movement (Figure 8A, j–l; and B).
Figure 8.
Mitochondrial velocities in rho0 cells are higher than wild-type cells. (A) Mitochondrial morphology in rho0 and respiratory deficient mutants is normal: wild-type (KAY40; a–c), rho0 cells derived from KAY40 (d–f), QCR9 parent (BY4743; g–i), and _qcr9_Δ mutant (RG24813; j–l) cells expressing CS1-GFP were grown to mid-log phase at 23°C in synthetic, glucose-based liquid media (SC-ura). After fixation, nuclear structures (a, d, g, and j) and actin cytoskeleton (c, f, i, and l) were visualized by staining with DAPI and rhodamine-phalloidin, respectively. CS1-GFP was used to detect mitochondria (b, e, h, and k). Arrows point to examples of colocalization of mitochondria with actin cables in rho0 cells. Bar, 5 μm. (B) To measure velocities of mitochondrial movement, cells grown as described above were harvested and immediately visualized by fluorescence microscopy. Time-lapse images of mitochondria in living cells were acquired at 20-s intervals over a period of 10 min. All velocities are averages of 50 measurements. Error bars represent SD. Mitochondria in KAY40 rho0 cells moved significantly faster than in KAY40 rho+ cells (p <0.001, t test).
DISCUSSION
Mutations in any of three mitochondrial outer membrane proteins, Mmm1p, Mdm10p, and Mdm12p, produce strikingly similar defects in mitochondrial morphology and inheritance (Burgess et al., 1994; Sogo and Yaffe, 1994). In addition, cells carrying mutations in any two of these proteins show no synthetic phenotypes (Berger et al., 1997; Hanekamp et al., 2002). These observations support the model that these proteins function together as a complex. Here, we provide evidence that this is indeed the case. Moreover, we show functionally significant, reciprocal interactions between this complex and mtDNA. Mmm1p, Mdm10p, and Mdm12p, proteins essential for actin-dependent mitochondrial motility, are required for mtDNA nucleoid maintenance and/or stability. Conversely, mtDNA affects mitochondrial motility.
Previous studies indicated that GFP-tagged Mmm1p localizes to discrete, punctate structures (Aiken Hobbs et al., 2001). Our studies with Myc-tagged Mmm1p confirmed these observations (our unpublished data). Moreover, we found that Mmm1p, Mdm10p, and Mdm12p localize to similar structures. Using Mdm12p-Myc and Mdm10p-Myc fusion constructs, we found that Mdm10p and Mdm12p localize to punctate structures on mitochondria. Additionally, we found that there are equal numbers of Mmm1p- and Mdm12p-containing structures per cell and roughly twice as many Mdm10p-containing structures as Mmm1p- or Mdm12p-containing structures.
Two lines of evidence indicate that these proteins reside in the same complex. First, we found that all pairwise combination of these tagged proteins coimmunoprecipitate. The amount of Mmm1p, Mdm10p, and Mdm12p proteins in the immunoprecipitated complex is a small percentage of their total cellular levels. One explanation for this may be that the associations in the complex are weak or the proteins spend only limited time in the complex. Second, we found that Mmm1p is required for association of Mdm12p with mitochondria and that Mdm12p is required for association of Mmm1p with mitochondria. Our studies do not reveal the precise composition of the complex or distinguish whether the interactions among Mmm1, Mdm10p, and Mdm12p in the complex are direct or indirect. Nonetheless, taken together with previous studies, our data support the model that Mmm1p, Mdm10p, and Mdm12p assemble into a complex within the mitochondrial OM.
Our studies also support a mechanism for assembly of the Mmm1/Mdm10p/Mdm12p complex. We found that deletion of either MMM1 or MDM12 has no effect on Mdm10p localization: Mdm10p-Myc localizes to punctate structures on mitochondria in _mdm12_Δ and _mmm1_Δ mutant cells. Moreover, as described above, Mdm10p-containing structures are more numerous than either Mmm1p- or Mdm12p-containing structures. Finally, we observed that Mmm1p mislocalizes in _mdm12_Δ cells, and Mdm12p mislocalizes in _mmm1_Δ cells. These observations support a model for assembly of the complex in which Mdm10p is the core component of the complex that can assemble in mitochondrial membranes in the absence of Mdm12p or Mmm1p. Because Mmm1p is required for localization of Mdm12p to mitochondria and Mdm12p is required for localization of Mmm1p to mitochondria, we favor the model that Mmm1p together with Mdm12p binds to the Mdm10p-containing core of the complex.
What is the function of this complex? Previous studies indicate that defects in mitochondrial morphology and inheritance occur in mmm1 and mdm10 mutants because these proteins are required for reversible binding of mitochondria to F-actin and for actin-dependent mitochondrial motility. Here, we find that Mdm12p is also required for mitochondrial motility.
Although the most prominent mitochondria in cells bearing deletions in MMM1, MDM10, or MDM12 are large and spherical, there are also small spherical mitochondria in these cells. We monitored the movement of mitochondria of all sizes and did not detect any directed movement in any mitochondria. Thus, the defects in mitochondrial movement observed in the _mdm12_Δ strain do not seem to be secondary consequences of altered mitochondrial size. Rather, our interpretation of these observations is that Mmm1p, Mdm10p, and Mdm12p form a complex that is required to link mitochondria to actin cables, the tracks that direct mitochondrial movement from mother cells to developing daughter cells.
Our data also support a link between Mmm1p, Mdm10p, Mdm12p, and mtDNA. Yeast mitochondria contain multiple copies of mtDNA that are organized as nucleoids, protein-DNA complexes that seem punctate by light microscopy (Stevens, 1981; Miyakawa et al., 1987; Kaufman et al., 2000). Inheritance of mitochondrial respiratory activity requires the maintenance of mtDNA and transmission of the full mitochondrial genome into the daughter cell. This inheritance depends on a wide array of proteins that function in mtDNA metabolism, recombination, and mitochondrial gene expression (reviewed in Berger and Yaffe, 2000). Previous studies revealed that Mdm10p and Mdm12p also contribute to mtDNA stability and transmission because deletion of either of these genes results in increased petite formation (Berger et al., 1997). Recently, it was shown that mmm1 mutations lead to loss of mtDNA, and that a suppressor that promotes mtDNA maintenance in mmm1 also suppresses mdm10 or yme4 mutants (Aiken Hobbs et al., 2001; Hanekamp et al., 2002). Here, we find that mtDNA is absent in 30% of _mdm12_Δ cells. Mutant cells that maintain mtDNA contain fewer mtDNA nucleoids. Moreover, in contrast to wild-type cells, in which all mtDNA is incorporated into nucleoids, in _mdm12_Δ cells mtDNA is localized to punctate structures and as diffuse material throughout mitochondria. Thus, it is possible that loss of mtDNA in mmm1, mdm10, and mdm12 mutants results from defects in mtDNA nucleoid stability and/or assembly.
These observations suggest a functional link between Mmm1p, Mdm10p, and Mdm12p and mtDNA in spite of the fact these proteins reside in the mitochondrial OM and mtDNA is associated with the matrix side of the mitochondrial IM. Previous subcellular fractionation studies, however, indicated that Mmm1p is located at contact sites, regions where mitochondrial OM and IM are closely apposed (Aiken Hobbs et al., 2001). These observations raised the possibility that there is a physical connection between the complex and mtDNA.
Consistent with this, we observe a functional link between mtDNA and Mmm1p, Mdm10p, and Mdm12p. We find that mtDNA influences actin-dependent mitochondrial movement: deletion of mtDNA results in an increase in the velocity of mitochondrial motility without affecting organelle morphology or colocalization with actin cables. It is formally possible that the motility defects observed in rho0 cells is due to loss of mtDNA, and not due to defects in interactions between mtDNA and the Mmm1p/Mdm10p/Mdm12p complex. Nonetheless, this increase in mitochondrial movement velocities is not a consequence of loss of mitochondrial respiratory activity: ubiquinol cytochrome c oxidoreductase mutants, cells that contain mtDNA but are respiratory deficient as a result of deletion in a nuclear gene, show normal rates of mitochondrial movement. Our explanation for this surprising finding is that mtDNA, interacting with the Mmm1p/Mdm10p/Mdm12p complex, can modulate the mitochondria–actin interaction. These interactions in vivo involve at least two activities. The first, a reversible binding of mitochondria to actin cables, is mediated by proteins in the Mmm1p/Mdm10p/Mdm12p complex. This binding is required to link mitochondria to actin cable tracks for linear, polarized movement from mother cells to buds. The second activity is the force generator that drives this linear polarized movement. We suggest that in the absence of mtDNA Mmm1p/Mdm10p/Mdm12p-mediated, reversible binding of mitochondria to actin cables is weakened. This, in turn, would allow the organelle to move faster along its cytoskeletal track.
Recently, Itoh et al., (2002) demonstrated a role for a Rab-like GTPase (Ypt11p) and a type V myosin (Myo2p) in mitochondrial inheritance in budding yeast. Although the authors suggest that these proteins contribute to movement of mitochondria from mother cells to buds, they never looked directly at mitochondrial movement in living yeast. In contrast, we showed that the velocity of mitochondrial movement in the temperature-sensitive myo2-66 mutant incubated at restrictive temperatures was indistinguishable from that of wild-type cells incubated under similar temperature conditions (Simon et al., 1995). Consistent with this, we find that deletion of YPT11 has no effect on the velocity of mitochondrial movement (our unpublished data). Therefore, although Ypt11p and Myo2p may contribute to mitochondria inheritance, neither of these proteins has any obvious role in directing movement of mitochondria from mother cells to buds. Rather, our studies indicate that the force generator for mitochondrial movement in budding yeast is the Arp2/3p complex (Boldogh et al., 2001).
Taken together, our studies suggest the existence of a mtDNA segregation machinery that consists of a complex that mediates interaction between the cytoskeleton and mtDNA, and an actin-dependent force generator that drives movement of mitochondrial membranes and mtDNA from mother to daughter cell during cell division. Indeed, mitochondrial nucleoids distribute independently of mitochondrial matrix proteins: mtDNA displays more limited mixing than other mitochondrial constituents (Azpiroz and Butow, 1993; Nunnari et al., 1997; Okamoto et al., 1998). We propose that the Mmm1p/Mdm10p/Mdm12p complex is part of this segregation machinery.
Mitochondrial membranes contain the machinery for import of proteins into mitochondria, which is encoded in the nucleus. Therefore, mitochondrial membranes can be produced only from preexisting mitochondrial membranes. Because mitochondrial membranes and mtDNA are both reproduced by template-dependent process, they must be transferred from mother to daughter cells to ensure that the cells contain fully functional mitochondria. Thus, mitochondrial membranes and mtDNA comprise the minimum heritable unit of mitochondria. Our studies support the model that the Mmm1p/Mdm10p/Mdm12p complex may be functionally similar to the kinetochore. That is, it links the minimum heritable unit in mitochondria to the cytoskeleton-based force-generating machinery that drives movement of that unit from mother cells to developing daughter cells during cell division. Our results are consistent with the idea that a complex containing Mmm1p, Mdm10p and Mdm12p is a “mitochore.”
Acknowledgments
We thank members of the Pon laboratory for critical evaluation of the manuscript; Drs. M. Yaffe, R. Jensen, and K. Ayscough for yeast strains; Dr. P. Crews for latrunculin-A; and Drs. M. Longtine and J. Pringle for tagging cassette DNA. This work was supported by research grants to L.P. from the National Institutes of Health (GM-45735 and GM-66037).
Abbreviations used: DAPI, 4,6-diamidino-2-phenylindole; HA, hemagglutinin; IM, inner membrane; Lat-A, latrunculin-A; mtDNA, mitochondrial DNA; OM, outer membrane.
References
- Aiken Hobbs, E., Srinivasan, M., McCaffery, J.M., and Jensen, R.E. (2001). Mmm1p, a mitochondrial outer membrane protein, is connected to mitochondrial DNA (mtDNA) nucleoids and required for mtDNA stability. J. Cell Biol. 152, 401-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough, K.R., Stryker, J., Pokala, N., Sanders, M., Crews, P., and Drubin, D.G. (1997). High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137, 399-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiroz, R., and Butow, R.A. (1993). Patterns of mitochondrial sorting in yeast zygotes. Mol. Biol. Cell 4, 21-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, K.H., Sogo, L.F., and Yaffe, M.P. (1997). Mdm12p, a component required for mitochondrial inheritance that is conserved between budding and fission yeast. J. Cell Biol. 136, 545-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, K.H., and Yaffe, M.P. (2000). Mitochondrial DNA inheritance in Saccharomyces cerevisiae. Trends Microbiol. 8, 508-513. [DOI] [PubMed] [Google Scholar]
- Boldogh, I., Vojtov, N., Karmon, S., and Pon, L.A. (1998). Interaction between mitochondria and the actin cytoskeleton in budding yeast requires two integral mitochondrial outer membrane proteins, Mmm1p and Mdm10p. J. Cell Biol. 141, 1371-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh, I.R., Yang, H.C., Nowakowski, W.D., Karmon, S.L., Hays, L.G., Yates, J.R., 3rd, and Pon, L.A. (2001). Arp2/3 complex and actin dynamics are required for actin-based mitochondrial motility in yeast. Proc. Natl. Acad. Sci. USA 98, 3162-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, S.M., Delannoy, M., and Jensen, R.E. (1994). MMM1 encodes a mitochondrial outer membrane protein essential for establishing and maintaining the structure of yeast mitochondria. J. Cell Biol. 126, 1375-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, J.A. (1987). Effects of cytochalasin and phalloidin on actin. J. Cell Biol. 105, 1473-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan, G.I., Lewis, G.K., Ramsay, G., and Bishop, J.M. (1985). Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell Biol. 5, 3610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox, T.D., Folley, L.S., Mulero, J.J., McMullin, T.W., Thorsness, P.E., Hedin, L.O., and Costanzo, M.C. (1991). Analysis and manipulation of yeast mitochondrial genes. Methods Enzymol. 194, 149-165. [DOI] [PubMed] [Google Scholar]
- Gietz, R.D., and Schiestl, R.H. (1995). Transforming yeast with DNA. Methods Mol. Cell Biol. 5, 255-269. [Google Scholar]
- Goldberg, M.B. (2001). Actin-based motility of intracellular microbial pathogens. Microbiol. Mol. Biol. Rev. 65, 595-626, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, L.A., Phillips, J.D., and Trumpower, B.L. (1992). Deletion of subunit 9 of the Saccharomyces cerevisiae cytochrome bc1 complex specifically impairs electron transfer at the ubiquinol oxidase site (center P) in the bc1 complex. FEBS Lett. 313, 251-254. [DOI] [PubMed] [Google Scholar]
- Hanekamp, T., Thorsness, M.K., Rebbapragada, I., Fisher, E.M., Seebart, C., Darland, M.R., Coxbill, J.A., Updike, D.L., and Thorsness, P.E. (2002). Maintenance of mitochondrial morphology is linked to maintenance of the mitochondrial genome in Saccharomyces cerevisiae. Genetics 162, 1147-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, H., Fukuda, Y., Murata, K., and Kimura, A. (1983). Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153, 163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, T., Watabe, A., Toh, E.A., and Matsui, Y. (2002). Complex formation with Ypt11p, a rab-type small GTPase, is essential to facilitate the function of Myo2p, a class V myosin, in mitochondrial distribution in Saccharomyces cerevisiae. Mol. Cell Biol. 22, 7744-7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, B.A., Newman, S.M., Hallberg, R.L., Slaughter, C.A., Perlman, P.S., and Butow, R.A. (2000). In organello formaldehyde crosslinking of proteins to mtDNA: identification of bifunctional proteins. Proc. Natl. Acad. Sci. USA 97, 7772-7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher, O., Holder, J., Srinivasan, M., Leung, R.S., and Jensen, R.E. (1997). The Tim54p-Tim22p complex mediates insertion of proteins into the mitochondrial inner membrane. J. Cell Biol. 139, 1663-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarino, D.A., Boldogh, I., Smith, M.G., Rosand, J., and Pon, L.A. (1994). Yeast mitochondria contain ATP-sensitive, reversible actin-binding activity. Mol. Biol. Cell 5, 807-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M.S., McKenzie, A., Demarini, D.J., Shah, N.G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J.R. (1998). Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953-961. [DOI] [PubMed] [Google Scholar]
- MacAlpine, D.M., Perlman, P.S., and Butow, R.A. (2000). The numbers of individual mitochondrial DNA molecules and mitochondrial DNA nucleoids in yeast are co-regulated by the general amino acid control pathway. EMBO J 19, 767-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa, I., Sando, N., Kawano, S., Nakamura, S., and Kuroiwa, T. (1987). Isolation of morphologically intact mitochondrial nucleoids from the yeast, Saccharomyces cerevisiae. J. Cell Sci. 88, 431-439. [DOI] [PubMed] [Google Scholar]
- Nowakowski, D.W., Swayne, T.C., and Pon, L.A. (2001). Epitope tagging and visualization of nuclear-encoded mitochondrial proteins in yeast. Methods Cell Biol. 65, 257-276. [DOI] [PubMed] [Google Scholar]
- Nunnari, J., Marshall, W.F., Straight, A., Murray, A., Sedat, J.W., and Walter, P. (1997). Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Mol. Biol. Cell 8, 1233-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, K., Perlman, P.S., and Butow, R.A. (1998). The sorting of mitochondrial DNA and mitochondrial proteins in zygotes: preferential transmission of mitochondrial DNA to the medial bud. J. Cell Biol. 142, 613-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, K., Perlman, P.S., and Butow, R.A. (2001). Targeting of green fluorescent protein to mitochondria. Methods Cell Biol. 65, 277-283. [DOI] [PubMed] [Google Scholar]
- Pollard, T.D., and Borisy, G.G. (2003). Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453-465. [DOI] [PubMed] [Google Scholar]
- Pringle, J.R., Preston, R.A., Adams, A.E., Stearns, T., Drubin, D.G., Haarer, B.K., and Jones, E.W. (1989). Fluorescence microscopy methods for yeast. Methods Cell Biol. 31, 357-435. [DOI] [PubMed] [Google Scholar]
- Sherman, F. (1991). Getting started with yeast. Methods Enzymol. 194, 3-21. [DOI] [PubMed] [Google Scholar]
- Simon, V.R., Karmon, S.L., and Pon, L.A. (1997). Mitochondrial inheritance: cell cycle and actin cable dependence of polarized mitochondrial movements in Saccharomyces cerevisiae. Cell Motil. Cytoskeleton 37, 199-210. [DOI] [PubMed] [Google Scholar]
- Simon, V.R., Swayne, T.C., and Pon, L.A. (1995). Actin-dependent mitochondrial motility in mitotic yeast and cell-free systems: identification of a motor activity on the mitochondrial surface. J. Cell Biol. 130, 345-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M.G., Simon, V.R., O'Sullivan, H., and Pon, L.A. (1995). Organellecytoskeletal interactions: actin mutations inhibit meiosis-dependent mitochondrial rearrangement in the budding yeast Saccharomyces cerevisiae. Mol. Biol. Cell 6, 1381-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo, L.F., and Yaffe, M.P. (1994). Regulation of mitochondrial morphology and inheritance by Mdm10p, a protein of the mitochondrial outer membrane. J. Cell Biol. 126, 1361-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, B. (1981). Mitochondrial structure. In: The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance, ed. J. Strathern and J. R. Broach, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory, 471-504.
- Taunton, J., Rowning, B.A., Coughlin, M.L., Wu, M., Moon, R.T., Mitchison, T.J., and Larabell, C.A. (2000). Actin-dependent propulsion of endosomes and lysosomes by recruitment of N-WASP. J. Cell Biol. 148, 519-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, D.T., Andrews, P.D., Gourlay, C.W., and Ayscough, K.R. (2002). Sla1p couples the yeast endocytic machinery to proteins regulating actin dynamics. J. Cell Sci. 115, 1703-1715. [DOI] [PubMed] [Google Scholar]
- Yang, H.C., Palazzo, A., Swayne, T.C., and Pon, L.A. (1999). A retention mechanism for distribution of mitochondria during cell division in budding yeast. Curr. Biol. 9, 1111-1114. [DOI] [PubMed] [Google Scholar]