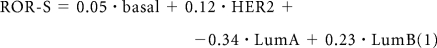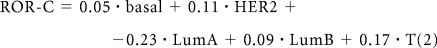Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes (original) (raw)
Abstract
Purpose
To improve on current standards for breast cancer prognosis and prediction of chemotherapy benefit by developing a risk model that incorporates the gene expression–based “intrinsic” subtypes luminal A, luminal B, HER2-enriched, and basal-like.
Methods
A 50-gene subtype predictor was developed using microarray and quantitative reverse transcriptase polymerase chain reaction data from 189 prototype samples. Test sets from 761 patients (no systemic therapy) were evaluated for prognosis, and 133 patients were evaluated for prediction of pathologic complete response (pCR) to a taxane and anthracycline regimen.
Results
The intrinsic subtypes as discrete entities showed prognostic significance (P = 2.26E-12) and remained significant in multivariable analyses that incorporated standard parameters (estrogen receptor status, histologic grade, tumor size, and node status). A prognostic model for node-negative breast cancer was built using intrinsic subtype and clinical information. The C-index estimate for the combined model (subtype and tumor size) was a significant improvement on either the clinicopathologic model or subtype model alone. The intrinsic subtype model predicted neoadjuvant chemotherapy efficacy with a negative predictive value for pCR of 97%.
Conclusion
Diagnosis by intrinsic subtype adds significant prognostic and predictive information to standard parameters for patients with breast cancer. The prognostic properties of the continuous risk score will be of value for the management of node-negative breast cancers. The subtypes and risk score can also be used to assess the likelihood of efficacy from neoadjuvant chemotherapy.
INTRODUCTION
Breast cancer is a heterogeneous disease with respect to molecular alterations, cellular composition, and clinical outcome. This diversity creates a challenge in developing tumor classifications that are clinically useful with respect to prognosis or prediction. Gene expression profiling by microarray has given us insight into the complexity of breast tumors and can be used to provide prognostic information beyond standard clinical assessment.1–7 For example, the 21-gene OncotypeDx assay (Genome Health Inc, Redwood City, CA) can be used to risk stratify early-stage estrogen receptor (ER) –positive breast cancer.4,5 Another strong predictor of outcome in ER-positive disease is proliferation or genomic grade.7–9 In addition, the 70-gene MammaPrint (Agendia, Huntington Beach, CA) microarray assay has shown prognostic significance in ER-positive and ER-negative early-stage node-negative breast cancer.2,3
The “intrinsic” subtypes luminal A (LumA), luminal B (LumB), HER2-enriched, basal-like, and normal-like have been extensively studied by microarray and hierarchical clustering analysis.1,6,10–12 Here, we study the utility of these subtypes alone and as part of a risk of relapse predictor in two cohorts: 1 patients receiving no adjuvant systemic therapy, and 2 patients undergoing paclitaxel, fluorouracil, doxorubicin, and cyclophosphamide (T/FAC) neoadjuvant chemotherapy. The risk of relapse models were compared with standard models using pathologic stage, grade, and routine biomarker status (ER and HER2).
METHODS
Samples and Clinical Data
Patient cohorts for training and test sets consisted of samples with data already in the public domain7,13–16 and fresh frozen and formalin-fixed paraffin-embedded (FFPE) tissues collected under institutional review board–approved protocols at the University of British Columbia (Vancouver, British Columbia, Canada), University of North Carolina (Chapel Hill, NC), Thomas Jefferson University (Philadelphia, PA), Washington University (St Louis, MO), and the University of Utah (Salt Lake City, UT). The training set for subtype prediction consisted of 189 breast tumor samples and 29 normal samples from heterogeneously treated patients given the standard of care dictated by their histology, stage, and clinical molecular marker status. The risk of relapse (ROR) models for prognosis in untreated patients were trained using the node-negative, untreated cohort of the Netherlands Cancer Institute (NKI) data set (n = 141).13 The subtype prediction and ROR models were independently tested for prognosis7,14,15 and chemotherapy response.16 The Hess et al data set16 used for prediction of chemotherapy sensitivity was not associated with long-term outcome data and was evaluated based on information for pathologic complete response (pCR). Clinical characteristics of the microarray training and test sets are presented in Table 1.17
Table 1.
Clinical Characteristics by Cohort
| Characteristic | Training Set | No Adjuvant Systemic Therapy* | Neoadjuvant Chemotherapy† |
|---|---|---|---|
| Samples | 189 | 761 | 133 |
| Median RFS, years | 4 | 9 | NA |
| Age, years | |||
| Mean | 58 | 53 | 52 |
| Standard deviation | 15 | 13 | 11 |
| ER | |||
| Positive | 114 | 544 | 82 |
| Negative | 77 | 195 | 51 |
| Node | |||
| Positive | 96 | 35 | 93 |
| Negative | 100 | 710 | 40 |
| HER2 | |||
| Positive | NA | 66 | 33 |
| Negative | NA | 192 | 99 |
| Tumor size, cm | |||
| ≤ 2 | 63 | 409 | 13 |
| > 2 | 136 | 339 | 120 |
| Grade | |||
| Low | 12 | 133 | 2 |
| Medium | 56 | 218 | 54 |
| High | 127 | 286 | 75 |
| Subtype | |||
| Luminal A | 23 | 269 | 37 |
| Luminal B | 12 | 168 | 27 |
| HER2-enriched | 31 | 120 | 29 |
| Basal-like | 56 | 128 | 27 |
| Normal-like | 12 | 76 | 13 |
Nucleic Acid Extraction
Total RNA was purified from fresh-frozen samples for microarray using the Qiagen RNeasy Midi Kit according to the manufacturer's protocol (Qiagen, Valencia, CA). The integrity of the RNA was determined using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). The High Pure RNA Paraffin Kit (Roche Applied Science, Indianapolis, IN) was used to extract RNA from FFPE tissues (2 × 10-μm sections or 1.5-mm punches) for quantitative reverse transcriptase polymerase chain reaction (qRT-PCR). Contaminating DNA was removed using Turbo DNase (Ambion, Austin, TX). The yield of total RNA was assessed using the NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies Inc, Rockland, DE).
Reverse Transcription and qPCR
First-strand cDNA was synthesized using Superscript III reverse transcriptase (first Strand Kit; Invitrogen, Carlsbad, CA) and a mixture of random hexamers and gene-specific primers. PCR amplification and fluorescent melting curve analysis was done on the LightCycler 480 using SYBR Green I Master Mix (Roche Applied Science). A detailed protocol of the PCR conditions can be found in the Appendix (online only).
Microarray
Total RNA isolation, labeling, and hybridizations on Agilent human 1Av2 microarrays or custom-designed Agilent human 22k arrays were performed using the protocol described in Hu et al.6 All microarray data have been deposited into the Gene Expression Omnibus18 under the accession number of GSE10886.
Identification of Prototypical Intrinsic Subtype Samples and Genes
To develop a clinical test that could make an intrinsic subtype diagnosis, we used a method to objectively select prototype samples for training and then predicted subtypes independent of clustering. To identify prototypic tumor samples, we started with an expanded “intrinsic” gene set comprised of genes found in four previous microarray studies.1,6,8,11 The normal-like class was represented using true “normals” from reduction mammoplasty or grossly uninvolved tissue, thus we have removed the normal-like class from all outcome analyses and consider this classification as a quality-control measure. A total of 189 breast tumors across 1,906 “intrinsic” genes were analyzed by hierarchical clustering (median centered by feature/gene, Pearson correlation, average linkage),19 and the sample dendrogram was analyzed using “SigClust”.20 A total of 122 breast cancers from 189 individuals profiled by qRT-PCR and microarray had significant clusters representing the “intrinsic” subtypes luminal A (LumA), luminal B (LumB), HER2-enriched, basal-like, and normal-like (Appendix Fig A1, online only). Four additional groups were identified in the training set as significantly reproducible clusters. All four of these groups have similar expression profiles as the luminal tumors and could represent intermediate states or tissue heterogeneity.
Gene Set Reduction Using Prototype Samples and qRT-PCR
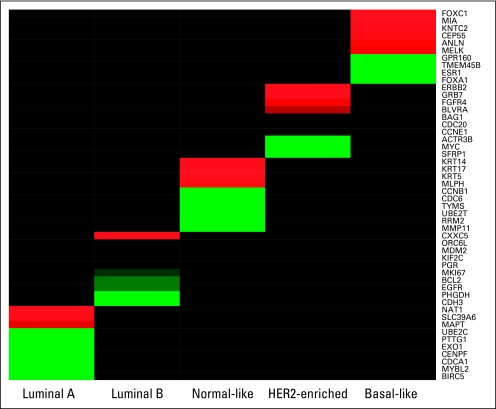
A minimized gene set was derived from the prototypic samples using the qRT-PCR data for 161 genes that passed FFPE performance criteria established in Mullins et al.21 Several minimization methods were used, including top “N” t test statistics for each group,22 top cluster index scores,23 and the remaining genes after “shrinkage” of modified t test statistics.24 Cross-validation (random 10% left out in each of 50 cycles) was used to assess the robustness of the minimized gene sets. The “N” t test method was chosen due to having the lowest cross-validation (random 10% left out of each iteration) error. The 50 genes selected and their contribution to distinguishing the different subtypes is provided in Appendix Figure A2 (online only).
Sample Subtype Prediction
The 50 gene set was compared for reproducibility of classification across three centroid-based prediction methods: Prediction Analysis of Microarray (PAM),24 a simple nearest centroid,6 and Classification of Nearest Centroid.25 In all cases, the subtype classification is assigned based on the nearest of the five centroids. Because of its reproducibility in subtype classification, the final algorithm consisted of centroids constructed as described for the PAM algorithm24 and distances calculated using Spearman's rank correlation. The centroids of the training set using the 50-gene classifier (henceforth called PAM50) are shown in Appendix Figure A3 (online only).
Prognostic and Predictive Models Using Clinical and Molecular Subtype Data
Univariate and multivariable analyses were used to determine the significance of the intrinsic subtypes (LumA, LumB, HER2-enriched, and basal-like) in untreated patients and in patients receiving neoadjuvant chemotherapy. For prognosis, subtypes were compared with standard clinical variables (tumor size [T], node status [N], ER status, and histologic grade), with time to relapse (ie, any event) as the end point. Subtypes were compared with grade and molecular markers (ER, progesterone receptor [PR], HER2) for prediction in the neoadjuvant setting because pathologic staging is not applicable. Likelihood ratio tests were done to compare models of available clinical data, subtype data, and combined clinical and molecular variables. Categoric survival analyses were performed using a log-rank test and visualized with Kaplan-Meier plots.
Developing Risk Models With Clinical and Molecular Data
The subtype risk model was trained with a multivariable Cox model using Ridge regression fit to the node-negative, untreated subset of the van de Vijver cohort.13 A ROR score was assigned to each test case using correlation to the subtype alone (1) (ROR-S) or using subtype correlation along with tumor size (2) (ROR-C):
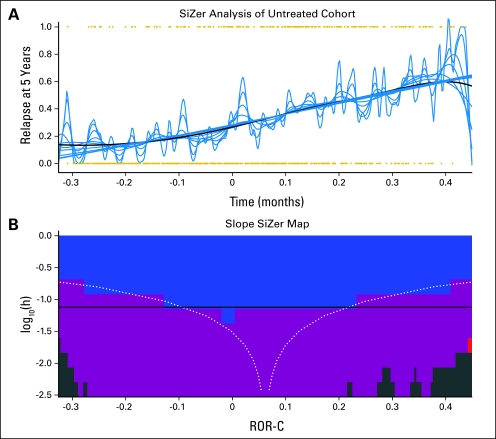
The sum of the coefficients from the Cox model is the ROR score for each patient. To classify samples into specific risk groups, we chose thresholds from the training set that required no LumA sample to be in the high-risk group and no basal-like sample to be in the low-risk group. Thresholds were determined from the training set and remained unchanged when evaluating test cases. SiZer analysis was performed to characterize the relationship between the ROR score and relapse-free survival26 (Appendix Fig A4, online only). The 95% CIs for the ROR score are local versions of binomial CIs, with the local sample size computed from a Gaussian kernel density estimator based on the Sheather-Jones choice of window width.27
Comparison of Relapse Prediction Models
Four models were compared for prediction of relapse: (1) a model of clinical variables alone (tumor size, grade, and ER status), (2) ROR-S, (3) ROR-C, and (4) a model combining subtype, tumor size, and grade. The C-index28 was chosen to compare the strength of the various models. For each model, the C-index was estimated from 100 randomizations of the untreated cohort into two thirds training set and one thirds test set. The C-index was calculated for each test set to form the estimate of each model, and C-index estimates were compared across models using the two sample t test.
RESULTS
Distribution of Intrinsic Subtypes in Comparison With Clinical Marker Status
Of the 626 ER-positive tumors analyzed in the microarray test set (Table 1), 73% were luminal (A or B), 11% were HER2-enriched, 5% were basal-like, and 12% were normal-like. Conversely, the ER-negative tumors comprised 11% luminal, 32% HER2-enriched, 50% basal-like, and 7% normal-like. The neoadjuvant study from Hess et al16 provided an opportunity to analyze the subtype distribution across clinical HER2 (HER2_clin_) status. Sixty-four percent (21 of 33) of HER2_clin_-positive were classified as HER2-enriched by gene expression. Only two (6%) of 33 HER2_clin_-positive tumors were classified as basal-like. Although the majority of the HER2_clin_-negative tumors were luminal (56%), 9% were classified as HER2-enriched and 24% were basal-like. Thus although the subtype diagnoses have markedly different distributions depending on ER or HER2 status, all subtypes were represented in ER-positive, ER-negative, HER2-positive, and HER2-negative categories. This finding demonstrates that ER and HER2 status alone are not accurate surrogates for true intrinsic subtype status. The intrinsic subtypes showed a significant impact on prognosis for relapse-free survival in untreated (no systemic therapy) patients and when stratified by ER status (Fig 1).
Fig 1.
PAM50 intrinsic subtype prognosis for relapse-free survival (RFS). (A) Outcome predictions according to the four tumor subtypes in a test set of 710 node-negative, no systemic adjuvant therapy patients. (B) Outcome by subtype in the subset of patients with estrogen receptor (ER) –positive disease from Figure 1A. (C) Outcome by subtype in patients with ER-negative disease. (D) Outcome by subtype in HER2_clin_-positive patients.
Risk of Relapse Models for Prognosis in Node-Negative Breast Cancer
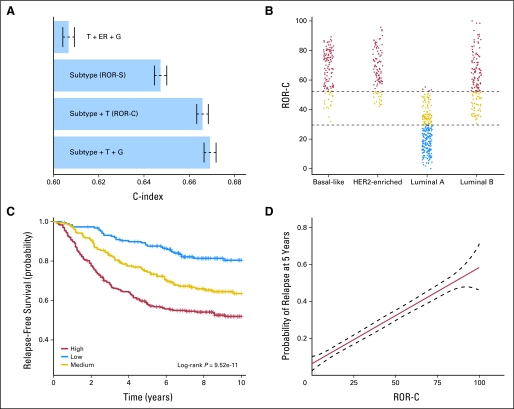
Cox models were tested using intrinsic subtype alone and together with clinical variables. Table 2 shows the multivariable analyses of these models in an independent cohort of untreated patients.7,13–15 In model A, subtypes, tumor size (T1 v greater), and histologic grade were found to be significant factors for ROR. The great majority of basal-like tumors (95.9%) were found to be medium or high grade, and therefore, in model B, which is an analysis without grade, basal-like becomes significant. Model C shows the significance of the subtypes in the node-negative population. All models that included subtype and clinical variables were significantly better than either clinical alone (P < .0001) or subtype alone (P < .0001). We trained a relapse classifier to predict outcomes within the context of the intrinsic subtypes and clinical variables. A node-negative, no systemic treatment cohort (n = 141) was selected from the van de Vijver microarray data set13 to train the ROR model and to select cut-offs (Appendix Fig A5, online only). Figure 2 provides a comparison of the different models using the C-index. There is a clear improvement in prediction with subtype (ROR-S) relative to the model of available clinical variables only (Fig 2A). A combination of clinical variables and subtype (ROR-C) is also a significant improvement over either individual predictor. However, information on grade did not significantly improve the C-index in the combined model, indicating that the prognostic value of grade had been superseded by information provided by the intrinsic subtype model. Figure 2 also presents the use of the ROR-C prognostic model for ROR in a test set of untreated node-negative patients. As was seen on the training data set, only the LumA group contained any low-risk patients (Fig 2B), and the three-class distinction of low, medium, and high risk was prognostic (Fig 2C). Lastly, Figure 2D shows that the ROR-C scores have a linear relationship with probability of relapse at 5 years.
Table 2.
Models of Relapse-Free Survival (untreated)
| Variable | Model A | Model B | Model C | |||
|---|---|---|---|---|---|---|
| Hazard Ratio | P | Hazard Ratio | P | Hazard Ratio | P | |
| Basal-like* | 1.33 | .330 | 1.79 | .030 | 1.58 | .066 |
| HER2-enriched* | 2.53 | .00012 | 3.25 | < .0001 | 2.90 | < .0001 |
| Luminal B* | 2.43 | < .0001 | 2.88 | < .0001 | 2.54 | < .0001 |
| ER status† | 0.83 | .38 | 0.83 | .34 | 0.83 | .32 |
| Tumor size‡ | 1.36 | .034 | 1.43 | .012 | 1.57 | .001 |
| Node status§ | 1.75 | .035 | 1.72 | .041 | — | — |
| Histologic grade‖ | 1.40 | .0042 | — | — | — | — |
| Full v subtype¶ | < .0001 | < .0001 | < .0001 | |||
| Full v clinical# | < .0001 | < .0001 | < .0001 |
Fig 2.
Risk of relapse (ROR) predictions using a test set of node-negative, no systemic therapy patients. (A) C-index analyses of four different Cox models using a test set of node-negative, untreated patients. (B) ROR-C (tumor size and subtype model) scores stratified by subtype. (C) Kaplan-Meier plots of the test set using cut points determined in training. (D) Analysis of the ROR-C model versus probability of survival shows a linear relationship (with the dashed lines showing the 95% CIs). ER, estrogen receptor; RFS, relapse-free survival.
Subtypes and Prediction of Response to Neoadjuvant T/FAC Treatment
The Hess et al16 study that performed microarray on tumors from patients treated with T/FAC allowed us to investigate the relationship between the subtypes and clinical markers and how each relates to pCR. Table 3 shows the multivariable analyses of the subtypes together with clinical molecular markers (ER, PR, HER2) and either with (model A) or without (model B) histologic grade. The only significant variables in the context of this study were the intrinsic subtypes. We found 94% sensitivity and 97% negative predictive value for identifying nonresponders to chemotherapy when using the ROR-S model to predict pCR (Fig 3A). The relationship between high-risk scores and a higher probability of pCR (Fig 3B) is consistent with the conclusion that indolent ER-positive tumors (LumA) are less responsive to chemotherapy. However, unlike ROR for prognosis, a plateau seems to be reached for the ROR versus probability of pCR, confirming the presence of significant chemotherapy resistance among the highest risk tumors.
Table 3.
Models of Neoadjuvant Response
| Variable | Model A | Model B | ||
|---|---|---|---|---|
| Odds Ratio | P | Odds Ratio | P | |
| Basal-like* | 2.96 | .021 | 3.06 | .016 |
| HER2-enriched* | 1.91 | .133 | 1.99 | .117 |
| Luminal B* | 2.42 | .036 | 2.43 | .035 |
| ER status† | −1.28 | .133 | −1.31 | .124 |
| HER2 status† | 0.80 | .236 | 0.85 | .208 |
| PR status† | −0.48 | .428 | −0.46 | .446 |
| Histologic grade‡ | 0.26 | .664 | — | — |
| Full v subtype§ | .175 | .105 | ||
| Full v clinical‖ | .016773 | .0066 |
Fig 3.
Relationship between risk of relapse (ROR) score and paclitaxel, fluorouracil, doxorubicin, and cyclophosphamide neoadjuvant response. (A) Receiver operating characteristic curve analysis of ROR-S (subtype only model) versus pathologic complete response (pCR) in the Hess et al16 test set. (B) ROR-S score versus probability of pCR. PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve.
Subtype Prediction and Outcome on FFPE Samples Using qRT-PCR
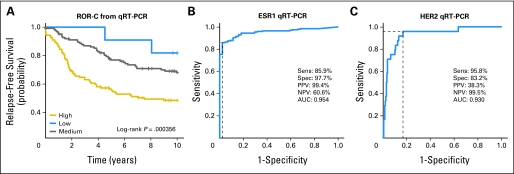
The subtype classifier and risk predictor were further validated using a heterogeneously treated cohort of 279 patients with FFPE samples archived between 1976 and 1995. The subtype classifications followed the same survival trends as seen in the microarray data, and the ROR score was significant for long-term relapse predictions (Appendix Fig A6A, online only). This old-age sample set was also scored for standard clinical markers (ER and HER2) by immunohistochemistry (IHC) and compared with the gene expression–based test. Analysis of ESR1 and ERBB2 by gene expression showed high sensitivity and specificity as compared with the IHC assay (Appendix Figs A6B and A6C). The advantages of using qRT-PCR versus IHC are that it is less subjective than visual interpretation and it is quantitative.
DISCUSSION
There have been numerous studies that have analyzed interactions between breast cancer intrinsic subtypes and prognosis,1,6,11 genetic alterations,29 and drug response.30 Because of the potential clinical value of subtype distinctions, we developed a standardized method of classification using a statistically derived gene and sample set that we have validated across multiple cohorts and platforms. The large and diverse test sets allowed us to evaluate the performance of the PAM50 assay at a population level and in relation to standard molecular markers. An important finding from these analyses is that all of the intrinsic subtypes are present and clinically significant in terms of outcome predictions in cohorts of patients diagnosed with either ER-positive or ER-negative tumors (Fig 1). Thus the molecular subtypes are not simply another method of classification that reflects ER status.
Stratification of the subtypes within HER2_clin_-positive samples did not show significance in outcome predictions; however, there were fewer numbers and less follow-up in this category. Nevertheless, there was clear separation of the curves for those HER2_clin_-positive patients classified as HER2-enriched (worse prognosis) compared with those with luminal subtypes (better prognosis). We found that 6% of HER2_clin_-positive tumors were classified as basal-like. It has been suggested that HER2_clin_-positive tumors expressing basal markers may have worse outcome when given a chemotherapeutic regimen of trastuzumab and vinorelbine.31
Approximately one third of the HER2-enriched expression subtype were not HER2_clin_-positive tumors, suggesting the presence of an ER-negative, nonbasal subtype that is not driven by HER2 gene amplification. The prototype samples selected to represent the HER2-enriched group had high expression of the 17q12-21 amplicon genes (HER2/ERBB2 and GRB7), FGFR4 (5q35), TMEM45B (11q24), and GPR160 (3q26). In addition, other growth factor receptors such as epidermal growth factor receptor are included within the PAM50 and could potentially also contribute to the HER2-enriched genomic classification.
We found that approximately 10% of breast cancers were classified as normal-like and can be either ER-positive or ER-negative and have an intermediate prognosis. Because the normal-like classification was developed by training on normal breast tissue, we suspect that the normal-like class is mainly an artifact of having a high percentage of normal “contamination” in the tumor specimen. Other explanations include a group of slow-growing basal-like tumors that lack expression of the proliferation genes or a potential new subtype that has been referred to as claudin-low tumors.32 Detailed histologic, immunohistochemical, and additional gene expression analyses of these cases are needed to resolve these issues. Because of the uncertainties, however, the normal-like samples were removed when modeling ROR.
The multivariable analysis for prognosis (ie, no systemic treatment) suggested that the best model was to use subtype with pathologic staging. Because pathologic staging is not available at diagnosis in the neoadjuvant setting, we used histologic grade and clinical biomarkers as the standard for prediction of chemotherapy response before resection. In this context, only the subtypes LumB and basal-like were predictive in the multivariable analysis that included histologic grade, ER, PR, and HER2 status (note that the Hess et al16 study did not incorporate trastuzumab into the regimen). The ROR score from the subtype-alone model was also the most predictive of neoadjuvant response. One of the major benefits of the ROR predictor is the identification of patients in the LumA group who are at a low ROR on the basis of pure prognosis and for whom the benefit from neoadjuvant therapy is unlikely. Thus the ROR predictor based on subtypes provides similar information as the OncotypeDx Recurrence Score for patients with ER-positive, node-negative disease.4,5 Furthermore, the PAM50 assay provides a ROR score for all patients, including those with ER-negative disease, and is highly predictive of neoadjuvant response when considering all patients.
In summary, the intrinsic subtype and risk predictors based on the PAM50 gene set added significant prognostic and predictive value to pathologic staging, histologic grade, and standard clinical molecular markers. The qRT-PCR assay can be performed using archived breast tissues, which will be useful for retrospective studies and prospective clinical trials.
Supplementary Material
[Data Supplement]
Appendix
Reverse Transcription and Real-Time Quantitative Polymerase Chain Reaction
First-strand cDNA was synthesized from 1.2 μg of total RNA using Superscript III reverse transcriptase (first Strand Kit; Invitrogen, Carlsbad, CA) and a mixture of random hexamers and gene-specific primers. The reaction was held at 55°C for 60 minutes and then 70°C for 15 minutes. The cDNA was washed on a QIAquick polymerase chain reaction (PCR) purification column (Qiagen Inc, Valencia, CA) and stored at −80°C in 25 mmol/L of Tris and 1 mmol/L of EDTA until further use. Each 5-μL PCR reaction included 1.25 ng (0.625 ngμL) of cDNA from samples of interest or 10 ng (5 ngμL) for reference, 2 pmol of both upstream and downstream primers, and LightCycler 480 SYBR Green I Master Mix (Roche Applied Science, Indianapolis, IN). Each run contained a single gene profiled in duplicate for test samples, reference sample, and negative control. The reference sample cDNA comprised an equal contribution of Human Reference Total RNA (Stratagene, La Jolla, CA) and the breast cell lines MCF7, ME16C, and SKBR3. PCR amplification was performed with the LightCycler 480 (Roche Applied Science, Indianapolis, IN) using an initial denaturation step (95°C, 8 minutes) followed by 45 cycles of denaturation (95°C, 4 seconds), annealing (56°C, 6 seconds with 2.5°C/s transition), and extension (72°C, 6 seconds with 2°C/sec transition). Fluorescence (530 nm) from the dsDNA dye SYBR Green I was acquired each cycle after the extension step. The specificity of the PCR was determined by postamplification melting curve analysis: samples were cooled to 65°C and slowly heated at 2°C/s to 99°C while continuously monitoring fluorescence (10 acquisitions/1°C).
Fig A1.
Hierarchical clustering and SigClust analysis of microarray data using 1,906 “intrinsic” genes and 189 samples. The SigClust algorithm statistically identifies significant/unique groups by testing the null hypothesis that a group of samples is from a single cluster, where a cluster is characterized as a multivariable normal distribution. SigClust was run at each node of the dendrogram beginning at the root and stopping when the test was no longer significant (P > .001). Statistical selection using SigClust identified nine significant groups, including the previously identified subtypes designated as luminal A (dark blue), luminal B (light blue), HER2-enriched (pink), normal-like (green), and basal-like (red).
Fig A2.
Focused heatmap of Classification by Nearest Centroids (ClaNC) selected genes for each subtype. The ClaNC algorithm was optimized to select 10 genes per class for a total of 50 genes. The 10 genes for each class are shown as red/green according to their expression in a class. Black indicates that gene was not selected for the given class.
Fig A3.
Heatmap of the centroid models of subtype. The centroids were constructed using the Classification by Nearest Centroids selected genes and calculated as described for the Prediction Analysis of Microarray algorithm. The expression values are shown as red/green according to their relative expression level.
Fig A4.
Developing a continuous risk score based on subtypes and clinical variables. (A) A family of smoothing functions illustrates the general linear relationship between the risk of relapse (ROR) score and relapse-free survival at 5 years. (B) Significance of the slopes in the smoothed lines is plotted for each bandwidth across the range of scores. Blue indicates a significant positive slope, purple indicates nonsignificant slope, and green indicates too few data for inference.
Fig A5.
Risk classification for training set of untreated patients using a combined model of intrinsic subtypes and clinical variables. (A) Risk of relapse based on the combined model with low-risk scores less than 29 (black), moderate-risk scores between 29 and 53 (green), and high-risk scores ≥ 53 (red). (B) Kaplan-Meier plot and significance of the risk score shown for 141 training set cases. LumA, luminal A; LumB, luminal B.
Fig A6.
Analysis of an old-aged formalin-fixed, paraffin-embedded patient cohort. (A) The combined risk of relapse (ROR)-C model predicting high, medium, and low risk of relapse in 279 patients from the University of British Columbia. (B) Receiver operating characteristic curve analysis of quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) data for ESR1 versus immunohistochemistry (IHC) data. (C) Receiver operating characteristic curve analysis for qRT-PCR data for HER2/ERBB2 versus HER2_clin_-positive (IHC and/or fluorescent in situ hybridization). PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve.
Footnotes
Supported by the Huntsman Cancer Institute/Foundation (P.S.B.), the ARUP Institute for Clinical and Experimental Pathology (P.S.B.), a National Cancer Institute (NCI) Strategic Partnering to Evaluate Cancer Signatures Grant No. U01 CA114722-01 (M.J.E.), an NCI Breast SPORE Grant No. P50-CA58223-09A1 (C.M.P.), a St Louis Affiliate of the Susan G. Komen Foundation CRAFT grant (M.J.E.), and the Breast Cancer Research Foundation (C.M.P. and M.J.E.). Additional support provided by the TRAC facility and Informatics at the Huntsman Cancer Center, supported in part by the NCI Cancer Center Support Grant No. P30 CA42014-19, and the tissue procurement facility at the Alvin J. Siteman Cancer Center at Washington University School of Medicine, which is funded in part by the NCI Cancer Center Support Grant No. P30 CA91842.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Philip S. Bernard, University Genomics Inc (U) Consultant or Advisory Role: Philip S. Bernard, University Genomics Inc (U) Stock Ownership: Matthew J. Ellis, University Genomics Inc; Charles M. Perou, University Genomics inc; Philip S. Bernard, University Genomics Inc Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Torsten O. Nielsen, Matthew J. Ellis, Charles M. Perou, Philip S. Bernard
Provision of study materials or patients: Juan Palazzo, Torsten O. Nielsen, Matthew J. Ellis, Charles M. Perou, Philip S. Bernard
Collection and assembly of data: Joel S. Parker, Michael Mullins, Maggie C.U. Cheang, Samuel Leung, David Voduc, Tammi Vickery, Sherri Davies, Christiane Fauron, Xiaping He, Zhiyuan Hu, John F. Quackenbush, Inge J. Stijleman, Juan Palazzo, J.S. Marron, Andrew B. Nobel, Elaine Mardis, Torsten O. Nielsen, Matthew J. Ellis, Charles M. Perou, Philip S. Bernard
Data analysis and interpretation: Joel S. Parker, Michael Mullins, Maggie C.U. Cheang, Samuel Leung, David Voduc, Sherri Davies, Christiane Fauron, Xiaping He, Zhiyuan Hu, J.S. Marron, Andrew B. Nobel, Torsten O. Nielsen, Matthew J. Ellis, Charles M. Perou, Philip S. Bernard
Manuscript writing: Joel S. Parker, David Voduc, Torsten O. Nielsen, Matthew J. Ellis, Charles M. Perou, Philip S. Bernard
Final approval of manuscript: Joel S. Parker, Michael Mullins, Maggie C.U. Cheang, Samuel Leung, David Voduc, Tammi Vickery, Sherri Davies, Christiane Fauron, Xiaping He, Zhiyuan Hu, John F. Quackenbush, Inge J. Stijleman, Juan Palazzo, J.S. Marron, Andrew B. Nobel, Elaine Mardis, Torsten O. Nielsen, Matthew J. Ellis, Charles M. Perou, Philip S. Bernard
REFERENCES
- 1.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van 't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 3.van't Veer LJ, Paik S, Hayes DF. Gene expression profiling of breast cancer: A new tumor marker. J Clin Oncol. 2005;23:1631–1635. doi: 10.1200/JCO.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 5.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 6.Hu Z, Fan C, Oh DS, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loi S, Haibe-Kains B, Desmedt C, et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J Clin Oncol. 2007;25:1239–1246. doi: 10.1200/JCO.2006.07.1522. [DOI] [PubMed] [Google Scholar]
- 8.Perreard L, Fan C, Quackenbush JF, et al. Classification and risk stratification of invasive breast carcinomas using a real-time quantitative RT-PCR assay. Breast Cancer Res. 2006;8:R23. doi: 10.1186/bcr1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sotiriou C, Wirapati P, Loi S, et al. Gene expression profiling in breast cancer: Understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98:262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 10.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 11.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan C, Oh DS, Wessels L, et al. Concordance among gene-expression-based predictors for breast cancer. N Engl J Med. 2006;355:560–569. doi: 10.1056/NEJMoa052933. [DOI] [PubMed] [Google Scholar]
- 13.van de Vijver MJ, He YD, van't Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Klijn JG, Zhang Y, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 15.Ivshina AV, George J, Senko O, et al. Genetic reclassification of histologic grade delineates new clinical subtypes of breast cancer. Cancer Res. 2006;66:10292–10301. doi: 10.1158/0008-5472.CAN-05-4414. [DOI] [PubMed] [Google Scholar]
- 16.Hess KR, Anderson K, Symmans WF, et al. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J Clin Oncol. 2006;24:4236–4244. doi: 10.1200/JCO.2006.05.6861. [DOI] [PubMed] [Google Scholar]
- 17.University of North Carolina Microarray Database. GEO Data Sets for Breast Cancer Research Published Papers (Clinical Data updated on 11-06-2007 for Data I, 4-7-2008 for Data II) https://genome.unc.edu/pubsup/breastGEO/
- 18.National Center for Biotechnology Information. Gene expression omnibus. http://www.ncbi.nlm.nih.gov/geo/
- 19.Eisen MB, Spellman PT, Brown PO, et al. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yufeng L, Hayes DL, Nobel A, et al. Statistical significance of clustering for high dimension low sample size data. J Am Stat Assoc. 2008;103:1281–1293. [Google Scholar]
- 21.Mullins M, Perreard L, Quackenbush JF, et al. Agreement in breast cancer classification between microarray and quantitative reverse transcription PCR from fresh-frozen and formalin-fixed, paraffin-embedded tissues. Clin Chem. 2007;53:1273–1279. doi: 10.1373/clinchem.2006.083725. [DOI] [PubMed] [Google Scholar]
- 22.Storey JD, Tibshirani R. Statistical methods for identifying differentially expressed genes in DNA microarrays. Methods Mol Biol. 2003;224:149–157. doi: 10.1385/1-59259-364-X:149. [DOI] [PubMed] [Google Scholar]
- 23.Dudoit S, Fridlyand J. A prediction-based resampling method for estimating the number of clusters in a dataset. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0036. RESEARCH0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tibshirani R, Hastie T, Narasimhan B, et al. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci U S A. 2002;99:6567–6572. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dabney AR. Classification of microarrays to nearest centroids. Bioinformatics. 2005;21:4148–4154. doi: 10.1093/bioinformatics/bti681. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhuri P, Marron JS. SiZer for exploration of structures in curves. J Am Stat Assoc. 1999;94:807–823. [Google Scholar]
- 27.Sheather SJ, Jones MC. A reliable data-based bandwidth selection method for kernel density esti-mation. J R Stat Soc. 1991;53:683–690. [Google Scholar]
- 28.The Design Library. C-index. http://lib.stat.cmu.edu/S/Harrell/Design.html.
- 29.Neve RM, Chin K, Fridlyand J, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rouzier R, Pusztai L, Delaloge S, et al. Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol. 2005;23:8331–8339. doi: 10.1200/JCO.2005.01.2898. [DOI] [PubMed] [Google Scholar]
- 31.Harris LN, You F, Schnitt SJ, et al. Predictors of resistance to preoperative trastuzumab and vinorelbine for HER2-positive early breast cancer. Clin Cancer Res. 2007;13:1198–1207. doi: 10.1158/1078-0432.CCR-06-1304. [DOI] [PubMed] [Google Scholar]
- 32.Herschkowitz JI, Simin K, Weigman VJ, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8:R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Data Supplement]