Actin and Endocytosis: Mechanisms and Phylogeny (original) (raw)
. Author manuscript; available in PMC: 2010 Feb 1.
Published in final edited form as: Curr Opin Cell Biol. 2009 Jan 29;21(1):20–27. doi: 10.1016/j.ceb.2009.01.006
Abstract
The regulated assembly of actin filament networks is a critical part of endocytosis, with critical temporal and spatial relationships between proteins of the endocytic and actin assembly machinery. Of particular importance has been a wealth of studies in budding and fission yeast. Cell biology approaches, combined with molecular genetics, have begun to uncover the complexity of the regulation of actin dynamics during the endocytic process. In a wide range of organisms, clathrin-mediated endocytosis appears to be linked to Arp2/3-mediated actin assembly. The conservation of the components, across a wide range eukaryotic species, suggests that the partnership between endocytosis and actin may be evolutionarily ancient.
Introduction
Actin assembly has been shown to be an essential element of endocytosis. Here, we review recent work on the molecular mechanisms involved, and we consider the breadth of species across which these mechanisms may hold. If the mechanisms are as widespread as they appear to be at this point, then endocytosis and membrane trafficking may be fundamental functions for actin in eukaryotes.
We focus first on actin and endocytosis mechanisms in Saccharomyces cerevisiae, because much recent progress comes from this model system. We then consider similarities in the process and the protein components among a diverse set of organisms. This high degree of conservation, among components and mechanisms, suggests that actin assembly and endocytosis have been functioning in concert for a long evolutionary time.
Evidence linking actin and endocytosis
The idea that the actin patch is the major site of endocytosis is now widely accepted, as described in recent reviews [1-4]. Genetics in yeast identified a large number of proteins involved in endocytosis, including many proteins known to control actin dynamics [5-7]. Immuno-electron microscopy revealed actin and actin-associated proteins on invaginations of the plasma membrane [8]. More recently, modern cell biological approaches have established a clear link between actin patch structures and sites of endocytosis at the plasma membrane. Two-color movies revealed that endocytic proteins and actin regulatory proteins colocalize in cortical actin patch structures [9]. Actin patch components have also been found to colocalize with internalizing membrane markers including the lipid dye FM4-64 and a fluorescent derivative of the alpha mating factor [10, 11].
Assembly and Movement: Localization Studies
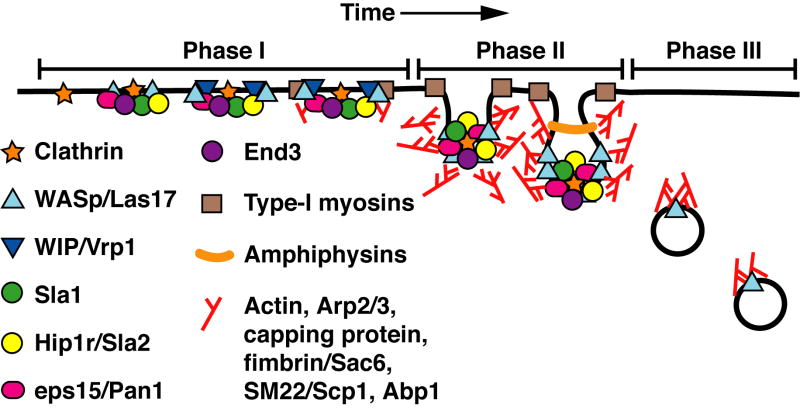
Live-cell imaging reveals endocytosis in budding yeast to be a dynamic process, with changes in the protein composition and the motile behavior of the endocytic site. The process can be considered in three broad phases, based on these changes in composition and movement (Figure 1).
Figure 1.
Model of actin patch assembly and movement during endocytosis in S. cerevisiae. The phases of patch movement we have defined and described in the text are overlaid on the model. This model is derived from the results of numerous works described and referenced herein.
During phase I, sites of endocytosis are initially marked by the recruitment of endocytic proteins. Later in phase I, proteins that regulate actin assembly appear. During this phase, the sites show very limited mobility at the plasma membrane. The first protein to be recruited is clathrin. To date, clathrin appears to mark all sites of endocytosis, based on colocalization studies with other endocytic proteins [12, 13]. After clathrin, proteins of the endocytic machinery, as well as the regulators of actin assembly WASp/Las17 and Eps15/Pan1, are recruited [9, 12, 14]. The localization of these first proteins to the patch does not depend on actin and many of these proteins are stabile at the membrane in the absence of actin polymerization [9, 12]. WASp-interacting protein (WIP/Vrp1) and then a type-I myosin, Myo5, follow shortly thereafter. Just prior to the end of this phase, which we define here by inward movement of the endocytic patch, actin polymerization begins, as revealed by the appearance of Arp2/3 complex, actin and most other actin-binding proteins [9, 15, 16]. The BAR-domain amphiphysin proteins, Rvs161 and Rvs167, then appear; they are the shortest-lived proteins of the endocytic patch, arriving after the onset of actin polymerization and just prior to the initiation of inward movement [12]. Amphiphysin proteins bind to curved membranes and can tubulate membranes in vitro [17], suggesting they may help drive invagination or vesicle scission in this setting.
Soon after actin polymerization is initiated, the actin patch makes a short movement into the cytoplasm, and this begins phase II in our operational definition. Nearly all patch proteins make this movement [9, 12, 16]. In some studies, C-terminal fusions of WASp/Las17 and type-I myosins with GFP appeared not to make this movement [9, 14, 16]. On the other hand, we found that, when overexpressed, an N-terminal fusion of WASp/Las17 with GFP was observed to move into the cytoplasm. However, we found that fusion of GFP to either the N- or C-terminus of WASp/Las17 resulted in a protein that was not fully functional, when actin patch motility was quantitatively examined [18].
Recently, Idrissi and colleagues used immuno-EM, with HA tags, to follow the location of endocytic and actin regulatory proteins with respect to endocytic membrane profiles [19]. The EM results support the idea that the short movement of Phase II corresponds vesicle invagination (Figure 1). In this study, WASp/Las17 and Myo5 both moved into the cytoplasm along with the endocytic invagination [19]. This apparent difference might be explained by the HA tag not interfering with function like the GFP tag does, but this remains to be tested. This detail is critical because movement of WASp/Las17 with the invaginating membrane would support a model where actin nucleation takes place on the endocytic vesicle membrane during invagination and as the vesicle moves away from the plasma membrane (Figure 2B).
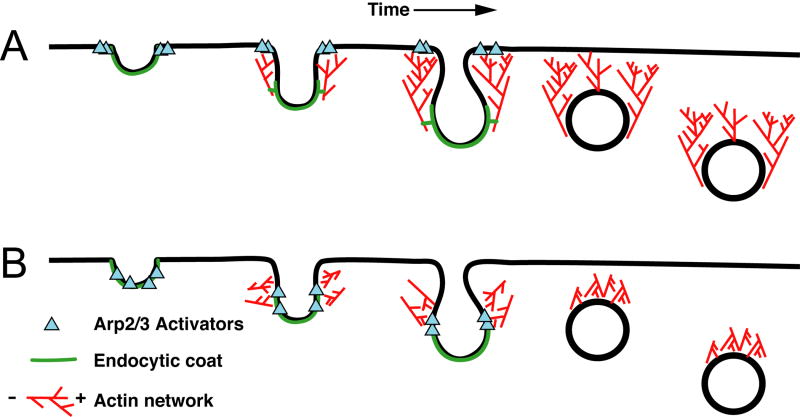
Figure 2.
Models of actin assembly during the invagination of the endocytic membrane. These models are derived from the results of numerous works described and referenced herein. The orientation of the actin filaments is indicated in the legend with a “+” for barbed ends and a “-” for pointed ends. Model A. The site of endocytosis is initially marked by recruitment of endocytic coat proteins and Arp2/3 activator proteins. The Arp2/3 activator proteins recruit Arp2/3 to nucleate an actin network from the plasma membrane. The network grows from these sites, with new actin monomers being added adjacent to the plasma membrane and the older parts of the network flowing into the cytoplasm. The endocytic coat proteins are anchored to this network, such that the flow of this network pulls the endocytic membrane into the cell. Model B. As in (A), sites of endocytosis are marked by recruitment of endocytic coat proteins and Arp2/3 activators. Immuno-EM studies suggest that the initial curvature of the membrane may occur prior to actin polymerization (see text). An actin network is nucleated from this invagination. The force of polymerization squeezes against this invagination, helping drive the endocytic coat into the cytoplasm, as well as providing lateral force for vesicle scission. Once scission occurs, the actin filaments are asymmetrically arranged around the endocytic vesicle and can propel its movement through the cytoplasm.
At some point during phase-II movement cofilin arrives [20], which may help promote dynamic turnover and/or disassembly of some of the actin filaments. After completing this short movement into the cytoplasm, essentially all endocytic proteins leave the vesicle [18, 21-23].
Membrane fission must then occur, to create an endocytic vesicle, which one assumes remains intimately linked with, perhaps identical to, an actin patch. Fission allows the endocytic vesicle / actin patch to move about the cytoplasm, which corresponds to the next phase of the process, phase III. During this time, actin patches make longer-range movements into and about the cytoplasm. The actin assembly machinery remains associated with the patch, which undergoes movements that are longer and faster than the phase-II movement [9, 15, 16, 20]. The fate of the endocytic vesicle remains poorly understood, in part because the earliest endosomal structures are not well defined. Actin patches appear to reach and fuse with structures that label with FM4-64 or fluorescent alpha-factor [10, 11], suggesting that endocytic vesicles can fuse with endosomes prior to or concomitant with actin disassembly.
Mechanisms for Actin Assembly: Mutational Analyses
The actin in patches is composed of a branched network of actin filaments [24] and their formation depends on Arp2/3 complex [25, 26]. Analysis of mutants in yeast has begun to provide insight into how the actin machinery might be harnessed to generate the forces and movements needed for endocytosis to occur, but much remains to be learned. Arp2/3-based nucleation requires and is promoted by actin filaments, so the assembly process is highly cooperative, with positive feedback, making it difficult to distinguish the initial molecular events, which start the process, from later ones that promote the ongoing process.
We know that recruitment of many of the early endocytic proteins is independent of actin [9, 12]. During this assembly process, two potential regulators of actin polymerization are recruited – WASp/Las17 and Eps15/Pan1. A simple model would feature these two proteins recruiting and activating Arp2/3 to nucleate actin filament formation. However, in mutants lacking the Arp2/3 binding regions of one or both of these proteins, actin filaments appear to form normally at endocytic sites [18, 27]. What nucleates such filaments remains to be identified. Type-I myosins in yeast can also bind Arp2/3 and thus may substitute in the absence of WASp/Las17 and Esp15/Pan1; however, type-I myosin localization to these sites depends at least in part on the presence of actin filaments [27]. Actin filaments themselves can bind Arp2/3, and yeast Arp2/3 has substantial nucleation activity on its own when assayed with yeast actin [28]. However, even if nucleation is a highly cooperative process, how the first actin filament is created or targeted remains unclear. Alternatives include nucleation by another molecule, such as a formin, and capture of an existing filament from the cytoplasm.
Once actin polymerization begins at the patch, a number of actin-binding proteins arrive. The role of these proteins during phase-I of endocytosis remains somewhat unclear. Mutation in almost any one regulator of actin dynamics increases the time at which endocytic sites remain at the membrane, prior to movement [12, 16, 18, 27]. Assembly of a proper actin network at this stage of endocytosis, prior to the initiation of inward movement, thus appears to be necessary, but how each component participates functionally remains to be defined.
The slow inward movement of the endocytic patch during phase II requires actin filaments as demonstrated by treatment of yeast cells with the actin-depolymerizing toxin Latrunculin A [9, 16]. Type-I myosin motors, Myo3 and Myo5, are necessary at this stage. In cells lacking Myo5, there is a decrease in both the frequency and the extent of this movement. In contrast, no defect is seen in cells lacking only Myo3, indicating that Myo5 and Myo3 functions do not simply overlap [18]. Myo5 and Myo3 appear to share some function, in that inward movement does not occur in cells lacking both proteins [18, 27]. The motor activity of the type-I myosins appears to be essential for normal inward movement [27]. In contrast, the ability of the myosin-I tails to bind to and activate Arp2/3, which was defined biochemically in concert with WIP/Vrp1 [27, 29, 30], appears to be dispensable [18, 27].
Patches do contain other proteins that can bind and regulate Arp2/3 activity in vitro, and more combinations of mutations will help to test the role of Arp2/3 activation. At this point, we know that phase-II movement takes place normally when the Arp2/3-binding acidic regions of WASp/Las17 and Pan1 are removed, alone or in combination [18, 27]. Furthermore, complete loss of Abp1 or Crn1, both potential regulators of Arp2/3 function, has no effect on this phase [12, 18]. However, WASp-interacting protein (WIP/Vrp1) is essential for this initial movement [27]. WIP/Vrp1 binds actin subunits as well as WASp/Las17 and the type-I myosins [31-33], suggesting the possibility that there may be cooperation among these proteins during this phase. In support of such a model, there appears to be redundancy between the type-I myosins and WASp/Las17 for this movement; cells lacking the Arp2/3 binding region of WASp/Las17 and Myo5 have a severe defect in both the frequency and extent of inward movement, and ones lacking the Arp2/3 binding region of WASp/Las17 and both type-I myosins have an even more severe defect [18, 27].
Other regulators of actin filament dynamics also seem to be important during phase II. Loss of actin capping protein impairs internalization of patches [16]. The actin filament crosslinkers fimbrin/Sac6 and SM22/Scp1 are also necessary for proper movement at this stage [12, 15]. The dendritic nucleation model proposed for Arp2/3 action includes a prominent role for capping protein but not filament crosslinking or bundling. However, one can readily imagine that filament side-to-side interactions might be important for remodeling the architecture of the filament network during endocytosis. Indeed, EM images of actin patches in situ show tufts of filaments protruding from the membrane [34], consistent with the existence of filament side interactions.
While it is clear that actin is critical for the slow inward movement of phase II, how the filaments are oriented relative to the invaginating endocytic structure and how these filaments are utilized to generate force is unclear. There is little evidence for how actin filaments are oriented in vivo. In sla2Δ cells long “comet tails” of actin, nucleated at the plasma membrane, form and flow away from the plasma membrane [9]. However, endocytosis is severely impaired in these cells and these actin structures may represent a structure not found normally, or a structure found only prior to the endocytic blockage in this mutant. This observation inspired a model where actin filaments are nucleated from a ring of WASp/Las17 and type-I myosin that surrounds the site of endocytic coat formation. Actin filaments, nucleated by Arp2/3 activated at these sites, push on the plasma membrane and cell wall, resulting in a flow of actin filaments away from the plasma membrane. These actin filaments are then attached to the endocytic coat and the flow of the filaments pulls the endocytic coat into the cytoplasm, generating an invagination [2] (Figure 2A). However, immuno-EM and light microscopy suggest that WASp/Las17 is not restricted to the plasma membrane at the base of the invagination [18, 19]. Furthermore, this model requires that actin filaments connect the endocytic coat proteins to the plasma membrane. However, actin binding proteins move in with the endocytic coat and fluorescent microscopy does not show evidence of actin binding proteins bridging the space between the actin patch and the plasma membrane, suggesting there may not be such a connection [9, 16].
In an alternative model, proposed by Merrifield for animal cells [35], actin filaments are nucleated around the endocytic coat. These filaments squeeze the endocytic invagination and push the endocytic membrane into the cytoplasm. This model does not require the actin network to connect the entire distance from the endocytic coat to the plasma membrane (Figure 2B). This type of model requires that the membrane have some curvature prior to the onset of actin polymerization; otherwise, the force of actin polymerization would counteract the initiation of invagination. Indeed, immuno-EM studies indicate that membrane curvature can be achieved prior to the accumulation of actin at the endocytic site [19]. In this case, actin filaments nucleated around the invagination generate pushing forces on the sides of an invagination, helping to extend the invagination into the cytoplasm. This model also provides a mechanism where actin polymerization could help promote membrane curvature for scission, and the model leaves the vesicle with an actin network to propel its movement after the vesicle is free from the plasma membrane.
Scission of the endocytic vesicle from the plasma membrane presumably takes place at the transition from the slow to fast movement of actin patches. Candidates for proteins involved in scission are the amphiphysins, Rvs161 and Rvs167. They arrive at the site of endocytosis just prior to inward movement and leave after moving only a relatively small amount into the cytoplasm [12]. This small distance moved might be the result of these proteins functioning at the neck of the invaginating vesicle. Indeed, EM images place Rvs167 at an intermediate point along long endocytic profiles [19]. In animal cells, amphiphysins tubulate membranes and link dynamin to clathrin coat proteins (reviewed in [36]). In yeast, mutants lacking one or both of the amphiphysins showed normal internalization, but endocytic proteins remained associated and frequently retracted, suggesting a defect in scission [12]. Whether actin functions during scission is an open question. Type-I myosins may play a role, based on the facts that Myo5-GFP intensity peaks just prior to fast movement, and a Myo5 tail mutation, in a myo3Δ null background, delays the onset of fast movement and causes the accumulation of membrane invaginations [14]. On the other hand, in another study, Myo5 was not observed at the patch at the time of the transition to fast movement [27].
Next, the actin patch, assumed to be an endocytic vesicle, makes a longer and more rapid movement into the cytoplasm. The major actin-binding proteins remain associated with the patch as it moves, and what powers this motion through the cytoplasm has been the subject of debate. On one hand, the motion cannot be due simply to inertia after the particle is pushed away from the plasma membrane, because of the small size of the particle and the high viscosity of the medium [37]. Some late actin patches have been observed to associate with actin cables and undergo long-range, retrograde movements along the mother-bud axis [11]. However, patches riding along cables does not appear to be necessary for late patch movement, because acute disruption of actin cables increased, not decreased, late patch movement [16]. An attractive model to reconcile these observations comes from Schizosaccharomyces pombe, where Arp2/3-mediated actin assembly is proposed to power the movement of patches, with cables serving as tracks to direct and constrain movement [38].
In some settings, late endocytic movements appear to be powered by actin polymerization from the surface of the vesicle, analogous to how Listeria moves in cytoplasm. In mammals, Arp2/3-powered movement has been seen with membrane bound vesicles in vivo and in vitro [39-44, 44, 45]. In yeast, several lines of evidence support this model. First, key components for dendritic nucleation are present on the particles, including actin, Arp2/3 complex, capping protein and cofilin, as well as other actin-binding proteins, including Abp1, fimbrin/Sac6 and SM22/Scp1 [9, 12, 15, 16, 20]. One critical question is whether an Arp2/3-interactor is present on the surface of the vesicle. Abp1, one such regulator, is present on the patch, but is dispensable for movement [9, 12, 18]. Mutations removing the Arp2/3 binding region of WASp/Las17 show defective late movement [18]. As noted above, GFP-WASp/Las17 can occasionally be seen moving into the cell with actin patches. In addition, mutants lacking the actin-binding proteins coronin/Crn1, capping protein, fimbrin/Sac6 and SM22/Scp1 have defects in the late movement of actin patches [15, 16, 18].
Evolution of actin and endocytosis
Proteins that compose and regulate the actin cytoskeleton, particularly ones involved in the formation of branched actin networks, are present and appear to be linked with endocytic machinery in a wide range of organisms, suggesting an evolutionarily ancient relationship. For example, orthologues of most of the components of actin patches in S. cerevisiae are also found at patches in S. pombe. Endocytosis occurs at actin patches in S. pombe, and actin polymerization is essential for endocytosis [46]. Furthermore, the roles for of actin regulatory proteins also appear to be conserved [47, 48]. The evolutionary distance between budding and fission yeast is very large, which alone suggests an ancient partnership between actin and endocytosis. Where it has been examined, much of the actin machinery also appears to have a conserved role in endocytosis in filamentous fungi and it localizes to dynamic structures similar to actin patches in yeasts [49-53].
Mammalian cells
The function of regulators of actin dynamics during endocytosis in mammalian cells has many similarities to what has been observed in fungi. In mammalian cells, N-WASp, Arp2/3, WIP and actin are recruited to clathrin coat structures (CCS) prior to their movement in to the cytoplasm [54-56]. Hip1r depletion results in actin tails on vesicles, which resembles how actin accumulates when its homolog, Sla2, is deleted in budding yeast [9, 55]. A type-I myosin, myosin 1E, also localizes to CCS [57]. Dynamic actin assembly is required for the formation, internalization and constriction of CCS cultured cells [58]. The accumulation of actin at CCS in mammalian cells is dependent on the Arp2/3 complex. While loss of N-WASp reduced the frequency at which actin accumulates at sites of clathrin mediated endocytosis, it did not reduce the peak amount of actin at these sights [56], suggesting another Arp2/3 activator may also be involved. The role of Abp1 is less clear. In cultured cells where mAbp1 was knocked down, transferrin uptake was severely reduced [59]. However, in neurons isolated from mAbp1 knockout mice, endocytosis was only moderately affected, but recycling of synaptic vesicles was severely impaired [60]. In Abp1 mutants in yeast, internalization is normal, but endocytic proteins are not uncoated from the vesicle [12, 18]. An interesting hypothesis is that the recycling defects seen in mAbp1 knockout neurons are a result of a failure to uncoat clathrin-coated vesicles. Interestingly, neurons from mice lacking synaptojanin1, whose yeast homolog also has a defect in uncoating endocytic vesicles, have a defect in recycling synaptic vesicles and in uncoating CCV [23, 61, 62].
Plants
Less is known about the potential link between actin and endocytosis in plants. Arabidopsis contains many actin-binding proteins, including capping protein, Arp2/3 complex and profilin, along with endocytic proteins such as clathrin, AP-2, AP180, dynamin, and Eps15. While WASp and Abp1 have not been found in Arabidopsis, WAVE/Scar proteins, which can activate Arp2/3, have been found in several plants and have been shown, in some cases, to have roles in cell morphogenesis [63-69]. Plants do have clathrin mediated endocytosis (reviewed in [70, 71]). Furthermore, multiple studies have shown that treatment of different plant tissues with toxins affecting actin dynamics inhibited the endocytic uptake of certain cargoes [72-75]. While the evidence suggests that endocytosis will function similarly in plants as in the organisms described above, studies showing a direct link between the clathrin mediated endocytic machinery and the actin cytoskeleton remain to be done.
Other Eukaryotes
If endocytosis is linked to actin assembly in fungi, mammals, and plants, then the connection must be evolutionarily ancient, extending back to the ancestor of all eukaryotes (See Figure 1.1 in [76]). To extend this analysis, we searched the genomes of other eukaryotes for genes encoding clathrin heavy chain and Arp2/3 complex components by BLAST. The pathogens Trypanosoma and Entamoeba contain Arp2/3 and clathrin, while other pathogens, including Cryptosporidia, Giardia, Plasmodia, Theileria, Toxoplasma, and Trichomonas, contain clathrin but not Arp2/3 complex 1. Among plants, red algae also have clathrin but not Arp2/3 complex2. Thus, among both unikonts and bikonts, some species have Arp2/3 genes while others do not, arguing for the presence of Arp2/3 in a common ancestor of all eukaryotes, with loss in certain species over time. Clathrin heavy chain genes were present in all of the genomes examined, suggesting it too was present in a common eukaryotic ancestor. Clathrin has membrane trafficking functions outside of endocytosis and may therefore be more necessary for eukaryotic life. If Arp2/3 complex is necessary for the link between actin and endocytosis, then this link may have been lost in some lineages. Functional studies of endocytosis in these other organisms will be required to address this issue properly. For example, endocytosis does occur in trypanosomes in association with clathrin [77], and whether it depends on actin assembly remains to be examined.
Acknowledgments
We acknowledge the support from the National Institutes of Health (GM38542 to JAC and GM077887 to BJG) for supporting our work described herein and preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ayscough KR. Coupling actin dynamics to the endocytic process in Saccharomyces cerevisiae. Protoplasma. 2005;22:681–88. doi: 10.1007/s00709-005-0107-5. [DOI] [PubMed] [Google Scholar]
- 2.Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- 3.Smythe E, Ayscough KR. Actin regulation in endocytosis. J Cell Sci. 2006;119:4589–4598. doi: 10.1242/jcs.03247. [DOI] [PubMed] [Google Scholar]
- 4.Moseley JB, Goode BL. The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiol Mol Biol Rev. 2006;70:605–645. doi: 10.1128/MMBR.00013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kubler E, Riezman H. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J. 1993;12:2855–2862. doi: 10.1002/j.1460-2075.1993.tb05947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munn AL, Stevenson BJ, Geli MI, Riezman H. end5, end6, and end7: mutations that cause actin delocalization and block the internalization step of endocytosis in Saccharomyces cerevisiae. Mol Biol Cell. 1995;6:1721–1742. doi: 10.1091/mbc.6.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raths S, Rohrer J, Crausaz F, Riezman H. end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. Journal of Cell Biology. 1993;120:55–65. doi: 10.1083/jcb.120.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mulholland J, Preuss D, Moon A, Wong A, Drubin D, Botstein D. Ultrastructure of the yeast actin cytoskeleton and its association with the plasma membrane. J Cell Biol. 1994;125:381–391. doi: 10.1083/jcb.125.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaksonen M, Sun Y, Drubin DG. A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell. 2003;115:475–487. doi: 10.1016/s0092-8674(03)00883-3. [DOI] [PubMed] [Google Scholar]
- 10.Toshima JY, Toshima J, Kaksonen M, Martin AC, King DS, Drubin DG. Spatial dynamics of receptor-mediated endocytic trafficking in budding yeast revealed by using fluorescent {alpha}-factor derivatives. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0601042103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huckaba TM, Gay AC, Pantalena LF, Yang HC, Pon LA. Live cell imaging of the assembly, disassembly, and actin cable-dependent movement of endosomes and actin patches in the budding yeast, Saccharomyces cerevisiae. J Cell Biol. 2004;167:519–530. doi: 10.1083/jcb.200404173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “12.Kaksonen M, Toret CP, Drubin DG. A modular design for the clathrin- and actin-mediated endocytosis machinery. Cell. 2005;123:305–320. doi: 10.1016/j.cell.2005.09.024.. Using TIRF microscopy the authors demonstrate that clathrin is the earliest protein recruited to all actin patches. The behavior of GFP-labeled actin patch components in a large number of mutant strains is presented along with a model placing actin patch components in to functional modules.
- “13.Newpher TM, Smith RP, Lemmon V, Lemmon SK. In vivo dynamics of clathrin and its adaptor-dependent recruitment to the actin-based endocytic machinery in yeast. Dev Cell. 2005;9:87–98. doi: 10.1016/j.devcel.2005.04.014.. The localization of clathrin is determined in live cells revealing its association with actin patches and sites of endocytosis.
- 14.Jonsdottir GA, Li R. Dynamics of yeast Myosin I: evidence for a possible role in scission of endocytic vesicles. Curr Biol. 2004;14:1604–1609. doi: 10.1016/j.cub.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 15.Gheorghe DM, Aghamohammadzadeh S, Smaczynska-de Rooij II, Allwood EG, Winder SJ, Ayscough KR. Interactions between the yeast SM22 homologue Scp1 and actin demonstrate the importance of actin bundling in endocytosis. J Biol Chem. 2008;283:15037–15046. doi: 10.1074/jbc.M710332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim K, Galletta BJ, Schmidt KO, Chang FS, Blumer KJ, Cooper JA. Actin-based motility during endocytosis in budding yeast. Mol Biol Cell. 2006;17:1354–1363. doi: 10.1091/mbc.E05-10-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- 18.Galletta BJ, Chuang DY, Cooper JA. Distinct Roles for Arp2/3 Regulators in Actin Assembly and Endocytosis. PLoS Biol. 2008;6:e1. doi: 10.1371/journal.pbio.0060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “”19.Idrissi FZ, Grotsch H, Fernandez-Golbano IM, Presciatto-Baschong C, Riezman H, Geli MI. Distinct acto/myosin-I structures associate with endocytic profiles at the plasma membrane. J Cell Biol. 2008;180:1219–1232. doi: 10.1083/jcb.200708060.. Immuno-EM is used to determine the localization of several critical endocytic and actin regulatory proteins along endocytic invaginations. This work provided strong support for the movement of proteins during phase II as seen by light microscopy, corresponding to invagination of the plasma membrane. This study provides spatial resolution of the localization of critical proteins that cannot be obtained by light microscopy.
- 20.Okreglak V, Drubin DG. Cofilin recruitment and function during actin-mediated endocytosis dictated by actin nucleotide state. J Cell Biol. 2007;178:1251–1264. doi: 10.1083/jcb.200703092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pishvaee B, Costaguta G, Yeung BG, Ryazantsev S, Greener T, Greene LE, Eisenberg E, McCaffery JM, Payne GS. A yeast DNA J protein required for uncoating of clathrin-coated vesicles in vivo. Nat Cell Biol. 2000;2:958–963. doi: 10.1038/35046619. [DOI] [PubMed] [Google Scholar]
- 22.Stefan CJ, Padilla SM, Audhya A, Emr SD. The phosphoinositide phosphatase Sjl2 is recruited to cortical actin patches in the control of vesicle formation and fission during endocytosis. Mol Cell Biol. 2005;25:2910–2923. doi: 10.1128/MCB.25.8.2910-2923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toret CP, Lee L, Sekiya-Kawasaki M, Drubin DG. Multiple pathways regulate endocytic coat disassembly in Saccharomyces cerevisiae for optimal downstream trafficking. Traffic. 2008;9:848–859. doi: 10.1111/j.1600-0854.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 24.Young ME, Cooper JA, Bridgman PC. Yeast actin patches are networks of branched actin filaments. J Cell Biol. 2004;166:629–635. doi: 10.1083/jcb.200404159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winter D, Podtelejnikov AV, Mann M, Li R. The complex containing actin-related proteins Arp2 and Arp3 is required for the motility and integrity of yeast actin patches. Curr Biol. 1997;7:519–529. doi: 10.1016/s0960-9822(06)00223-5. [DOI] [PubMed] [Google Scholar]
- 26.Winter DC, Choe EY, Li R. Genetic dissection of the budding yeast Arp2/3 complex: a comparison of the in vivo and structural roles of individual subunits. Proc Natl Acad Sci U S A. 1999;96:7288–7293. doi: 10.1073/pnas.96.13.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “27.Sun Y, Martin AC, Drubin DG. Endocytic internalization in budding yeast requires coordinated actin nucleation and myosin motor activity. Dev Cell. 2006;11:33–46. doi: 10.1016/j.devcel.2006.05.008.. Using GFP-fusions of actin patch proteins, the authors analyzed Arp2/3 regulator and type-I myosin function at sites of endocytosis.
- 28.Wen KK, Rubenstein PA. Acceleration of yeast actin polymerization by yeast ARP2/3 complex does not require an ARP2/3 activating protein. J Biol Chem. 2005;280:24168–24174. doi: 10.1074/jbc.M502024200. [DOI] [PubMed] [Google Scholar]
- 29.Geli MI, Lombardi R, Schmelzl B, Riezman H. An intact SH3 domain is required for myosin I-induced actin polymerization. Embo J. 2000;19:4281–4291. doi: 10.1093/emboj/19.16.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lechler T, Jonsdottir GA, Klee SK, Pellman D, Li R. A two-tiered mechanism by which Cdc42 controls the localization and activation of an Arp2/3-activating motor complex in yeast. Journal of Cell Biology. 2001;155:261–270. doi: 10.1083/jcb.200104094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lechler T, Shevchenko A, Li R. Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization. J Cell Biol. 2000;148:363–373. doi: 10.1083/jcb.148.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evangelista M, Klebl BM, Tong AH, Webb BA, Leeuw T, Leberer E, Whiteway M, Thomas DY, Boone C. A role for myosin-I in actin assembly through interactions with Vrp1p, Bee1p, and the Arp2/3 complex. J Cell Biol. 2000;148:353–362. doi: 10.1083/jcb.148.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaduva G, Martin NC, Hopper AK. Actin-binding verprolin is a polarity development protein required for the morphogenesis and function of the yeast actin cytoskeleton. J Cell Biol. 1997;139:1821–1833. doi: 10.1083/jcb.139.7.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodal AA, Kozubowski L, Goode BL, Drubin DG, Hartwig JH. Actin and septin ultrastructures at the budding yeast cell cortex. Mol Biol Cell. 2005;16:372–384. doi: 10.1091/mbc.E04-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merrifield CJ. Seeing is believing: imaging actin dynamics at single sites of endocytosis. Trends Cell Biol. 2004;14:352–358. doi: 10.1016/j.tcb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Ren G, Vajjhala P, Lee JS, Winsor B, Munn AL. The BAR domain proteins: molding membranes in fission, fusion, and phagy. Microbiol Mol Biol Rev. 2006;70:37–120. doi: 10.1128/MMBR.70.1.37-120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Life in moving fluids : the physical biology of flow. Princeton University Press; 1994. [Google Scholar]
- 38.Pelham RJ, Jr, Chang F. Role of actin polymerization and actin cables in actin-patch movement in Schizosaccharomyces pombe. Nat Cell Biol. 2001;3:235–244. doi: 10.1038/35060020. [DOI] [PubMed] [Google Scholar]
- 39.Rozelle AL, Machesky LM, Yamamoto M, Driessens MH, Insall RH, Roth MG, Luby-Phelps K, Marriott G, Hall A, Yin HL. Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol. 2000;10:311–320. doi: 10.1016/s0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- 40.Schafer DA, D’Souza-Schorey C, Cooper JA. Actin assembly at membranes controlled by ARF6. Traffic. 2000;1:892–903. doi: 10.1034/j.1600-0854.2000.011108.x. [DOI] [PubMed] [Google Scholar]
- 41.Taunton J, Rowning BA, Coughlin ML, Wu M, Moon RT, Mitchison TJ, Larabell CA. Actin-dependent Propulsion of Endosomes and Lysosomes by Recruitment of N-WASP. J Cell Biol. 2000;148:519–530. doi: 10.1083/jcb.148.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang F, Southwick FS, Purich DL. Actin-based phagosome motility. Cell Motil Cytoskeleton. 2002;53:81–88. doi: 10.1002/cm.10058. [DOI] [PubMed] [Google Scholar]
- 43.Southwick FS, Li W, Zhang F, Zeile WL, Purich DL. Actin-based endosome and phagosome rocketing in macrophages: activation by the secretagogue antagonists lanthanum and zinc. Cell Motil Cytoskeleton. 2003;54:41–55. doi: 10.1002/cm.10083. [DOI] [PubMed] [Google Scholar]
- 44.Merrifield CJ, Moss SE, Ballestrem C, Imhof BA, Giese G, Wunderlich I, Almers W. Endocytic vesicles move at the tips of actin tails in cultured mast cells. Nat Cell Biol. 1999;1:72–74. doi: 10.1038/9048. [DOI] [PubMed] [Google Scholar]
- 45.Benesch S, Lommel S, Steffen A, Stradal TE, Scaplehorn N, Way M, Wehland J, Rottner K. Phosphatidylinositol 4,5-biphosphate (PIP2)-induced vesicle movement depends on N-WASP and involves Nck, WIP, and Grb2. J Biol Chem. 2002;277:37771–37776. doi: 10.1074/jbc.M204145200. [DOI] [PubMed] [Google Scholar]
- 46.Gachet Y, Hyams JS. Endocytosis in fission yeast is spatially associated with the actin cytoskeleton during polarised cell growth and cytokinesis. J Cell Sci. 2005;118:4231–4242. doi: 10.1242/jcs.02530. [DOI] [PubMed] [Google Scholar]
- 47.Sirotkin V, Beltzner CC, Marchand JB, Pollard TD. Interactions of WASp, myosin-I, and verprolin with Arp2/3 complex during actin patch assembly in fission yeast. J Cell Biol. 2005;170:637–648. doi: 10.1083/jcb.200502053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee WL, Bezanilla M, Pollard TD. Fission Yeast Myosin-I, Myo1p, Stimulates Actin Assembly by Arp2/3 Complex and Shares Functions with WASp. Journal of Cell Biology. 2000;151:789–799. doi: 10.1083/jcb.151.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Araujo-Bazan L, Penalva MA, Espeso EA. Preferential localization of the endocytic internalization machinery to hyphal tips underlies polarization of the actin cytoskeleton in Aspergillus nidulans. Mol Microbiol. 2008;67:891–905. doi: 10.1111/j.1365-2958.2007.06102.x. [DOI] [PubMed] [Google Scholar]
- 50.Upadhyay S, Shaw BD. The role of actin, fimbrin and endocytosis in growth of hyphae in Aspergillus nidulans. Mol Microbiol. 2008;68:690–705. doi: 10.1111/j.1365-2958.2008.06178.x. [DOI] [PubMed] [Google Scholar]
- 51.McGoldrick CA, Gruver C, May GS. myoA of Aspergillus nidulans encodes an essential myosin I required for secretion and polarized growth. Journal of Cell Biology. 1995;128:577–587. doi: 10.1083/jcb.128.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuchs U, Steinberg G. Endocytosis in the plant-pathogenic fungus Ustilago maydis. Protoplasma. 2005;226:75–80. doi: 10.1007/s00709-005-0109-3. [DOI] [PubMed] [Google Scholar]
- 53.Read ND, Kalkman ER. Does endocytosis occur in fungal hyphae? Fungal Genet Biol. 2003;39:199–203. doi: 10.1016/s1087-1845(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 54.Merrifield CJ, Feldman ME, Wan L, Almers W. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol. 2002;4:691–698. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- 55.Merrifield CJ, Qualmann B, Kessels MM, Almers W. Neural Wiskott Aldrich Syndrome Protein (N-WASP) and the Arp2/3 complex are recruited to sites of clathrin-mediated endocytosis in cultured fibroblasts. Eur J Cell Biol. 2004;83:13–18. doi: 10.1078/0171-9335-00356. [DOI] [PubMed] [Google Scholar]
- 56.Benesch S, Polo S, Lai FP, Anderson KI, Stradal TE, Wehland J, Rottner K. N-WASP deficiency impairs EGF internalization and actin assembly at clathrin-coated pits. J Cell Sci. 2005;118:3103–3115. doi: 10.1242/jcs.02444. [DOI] [PubMed] [Google Scholar]
- 57.Krendel M, Osterweil EK, Mooseker MS. Myosin 1E interacts with synaptojanin-1 and dynamin and is involved in endocytosis. FEBS Lett. 2007;581:644–650. doi: 10.1016/j.febslet.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yarar D, Waterman-Storer CM, Schmid SL. A dynamic actin cytoskeleton functions at multiple stages of clathrin-mediated endocytosis. Mol Biol Cell. 2005;16:964–975. doi: 10.1091/mbc.E04-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mise-Omata S, Montagne B, Deckert M, Wienands J, Acuto O. Mammalian actin binding protein 1 is essential for endocytosis but not lamellipodia formation: functional analysis by RNA interference. Biochem Biophys Res Commun. 2003;301:704–710. doi: 10.1016/s0006-291x(02)02972-8. [DOI] [PubMed] [Google Scholar]
- 60.Connert S, Wienand S, Thiel C, Krikunova M, Glyvuk N, Tsytsyura Y, Hilfiker-Kleiner D, Bartsch JW, Klingauf J, Wienands J. SH3P7/mAbp1 deficiency leads to tissue and behavioral abnormalities and impaired vesicle transport. EMBO J. 2006;25:1611–1622. doi: 10.1038/sj.emboj.7601053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim WT, Chang S, Daniell L, Cremona O, Di Paolo G, De Camilli P. Delayed reentry of recycling vesicles into the fusion-competent synaptic vesicle pool in synaptojanin 1 knockout mice. Proc Natl Acad Sci U S A. 2002;99:17143–17148. doi: 10.1073/pnas.222657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cremona O, De Camilli P. Phosphoinositides in membrane traffic at the synapse. J Cell Sci. 2001;114:1041–1052. doi: 10.1242/jcs.114.6.1041. [DOI] [PubMed] [Google Scholar]
- 63.Zhang C, Mallery EL, Schlueter J, Huang S, Fan Y, Brankle S, Staiger CJ, Szymanski DB. Arabidopsis SCARs function interchangeably to meet actin-related protein 2/3 activation thresholds during morphogenesis. Plant Cell. 2008;20:995–1011. doi: 10.1105/tpc.107.055350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basu D, Le J, El-Essal S-D, Huang S, Zhang C, Mallery EL, Koliantz G, Staiger CJ, Szymanski DB. DISTORTED3/SCAR2 is a putative arabidopsis WAVE complex subunit that activates the Arp2/3 complex and is required for epidermal morphogenesis. Plant Cell. 2005;17:502–524. doi: 10.1105/tpc.104.027987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le J, Mallery EL, Zhang C, Brankle S, Szymanski DB. Arabidopsis BRICK1/HSPC300 is an essential WAVE-complex subunit that selectively stabilizes the Arp2/3 activator SCAR2. Curr Biol. 2006;16:895–901. doi: 10.1016/j.cub.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 66.Hussey PJ, Allwood EG, Smertenko AP. Actin-binding proteins in the Arabidopsis genome database: properties of functionally distinct plant actin-depolymerizing factors/cofilins. Philos Trans R Soc Lond B Biol Sci. 2002;357:791–798. doi: 10.1098/rstb.2002.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holstein SE, Oliviusson P. Sequence analysis of Arabidopsis thaliana E/ANTH-domain-containing proteins: membrane tethers of the clathrin-dependent vesicle budding machinery. Protoplasma. 2005;226:13–21. doi: 10.1007/s00709-005-0105-7. [DOI] [PubMed] [Google Scholar]
- 68.Holstein SE. Clathrin and plant endocytosis. Traffic. 2002;3:614–620. doi: 10.1034/j.1600-0854.2002.30903.x. [DOI] [PubMed] [Google Scholar]
- 69.Perroud PF, Quatrano RS. BRICK1 is required for apical cell growth in filaments of the moss Physcomitrella patens but not for gametophore morphology. Plant Cell. 2008;20:411–422. doi: 10.1105/tpc.107.053256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perez-Gomez J, Moore I. Plant endocytosis: it is clathrin after all. Curr Biol. 2007;17:R217–9. doi: 10.1016/j.cub.2007.01.045. [DOI] [PubMed] [Google Scholar]
- “71.Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, Friml J. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol. 2007;17:520–527. doi: 10.1016/j.cub.2007.01.052.. Using a combination of techniques the authors demonstrate that clatrin-mediated endocytosis is a major internalization pathway in plants.
- 72.Lisboa S, Scherer GE, Quader H. Localized endocytosis in tobacco pollen tubes: visualisation and dynamics of membrane retrieval by a fluorescent phospholipid. Plant Cell Rep. 2008;27:21–28. doi: 10.1007/s00299-007-0437-1. [DOI] [PubMed] [Google Scholar]
- 73.Baluska F, Hlavacka A, Samaj J, Palme K, Robinson DG, Matoh T, McCurdy DW, Menzel D, Volkmann D. F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol. 2002;130:422–431. doi: 10.1104/pp.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dhonukshe P, Grigoriev I, Fischer R, Tominaga M, Robinson DG, Hasek J, Paciorek T, Petrasek J, Seifertova D, Tejos R, et al. Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc Natl Acad Sci U S A. 2008;105:4489–4494. doi: 10.1073/pnas.0711414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baluska F, Wojtaszek P, Volkmann D, Barlow P. The architecture of polarized cell growth: the unique status of elongating plant cells. Bioessays. 2003;25:569–576. doi: 10.1002/bies.10282. [DOI] [PubMed] [Google Scholar]
- 76.Horner DS, Hirt RP. An Overview of Eukaryote Origins and Evolution: The Beauty of the Cell and the Fabulous Gene Phylogenies. In: Hirt RP, Horner DS, editors. Organelles, genomes, and eukaryote phylogeny : an evolutionary synthesis in the age of genomics. CRC Press; 2004. pp. 1–23. [Google Scholar]
- 77.Natesan SK, Peacock L, Matthews K, Gibson W, Field MC. Activation of endocytosis as an adaptation to the mammalian host by trypanosomes. Eukaryot Cell. 2007;6:2029–2037. doi: 10.1128/EC.00213-07. [DOI] [PMC free article] [PubMed] [Google Scholar]