Factors controlling cardiac myosin-isoform shift during hypertrophy and heart failure (original) (raw)
. Author manuscript; available in PMC: 2009 Jun 23.
Published in final edited form as: J Mol Cell Cardiol. 2007 Jul 21;43(4):388–403. doi: 10.1016/j.yjmcc.2007.07.045
Introduction
The capability of the heart to pump blood is intimately related to the ability of the myocardium to generate an active force and velocity of shortening. A great deal of evidence obtained from both clinical and experimental studies has indicated that, between these two determinants of contractility, loss of the ability of muscle fibers to generate an effective shortening velocity becomes a major defect in the failing heart [1]. Examination of different biophysical and biochemical parameters has revealed that a shift in the myosin-isoform distribution and a change in the cross bridge cycling characteristics of the sarcomere play a significant role in controlling the shortening velocity of cardiac muscle fibers [2–4]. With recent evidence confirming the presence of αMHC in human hearts and its disappearance in failing hearts, a new interest has arisen among investigators in finding ways of restoring and/or up-regulating the αMHC expression in the overloaded heart [5–8]. The basic premise is that reduction of cardiac hypertrophy combined with increased activity of αMHC could be beneficial in terms of increasing the myocardial contractility of a hemodynamically challenged heart [9]. This review focuses on the factors that control cardiac-specific expression of myosin heavy chain genes and the distribution of their protein isoforms in different pathophysiologic states of the heart.
Structural organization and developmental regulation of myosin isoforms
Myosin is a chemical-mechanical transducer of motion. It generates movements by transferring energy from hydrolysis of ATP into the sliding of myofilaments. In striated muscle, myosin is a principal molecule of the thick filaments. It overlaps with the thin (actin-containing) filaments in an orderly fashion, forming a repeated pattern of sarcomeres, which are contractile units. By immunofluorescence staining, myosin filaments are shown to be located in the ‘A’ band of the sarcomere [10]. Each myosin is basically a hexameric protein, consisting of two heavy chains (MHCs) and four lights chains (MLCs). The two MHCs (each 220 kDa) produce a long α-helical rod structure separated by two globular heads. By proteolytic cleavage, different regions of the myosin molecule can be separated. Two fragments, called meromysins, are released by digestion of the myosin molecule with the enzyme trypsin. The smaller fragment, light meromyosin, is derived from the α-helical rod portion of the molecule, whereas the larger, heavy meromyosin fragment includes the head, a small portion of the rod, and the light chains. Further digestion of the heavy meromyosin with papain, another proteolytic enzyme, removes the rest of the coiled coil rod portion (fragment S2) and leaves a globular protein, which includes the paired head of the MHCs and four light chains (fragment S1). This head region of the molecule contains ATPase activity and hence acts as a site of chemico-mechanical transduction [11].
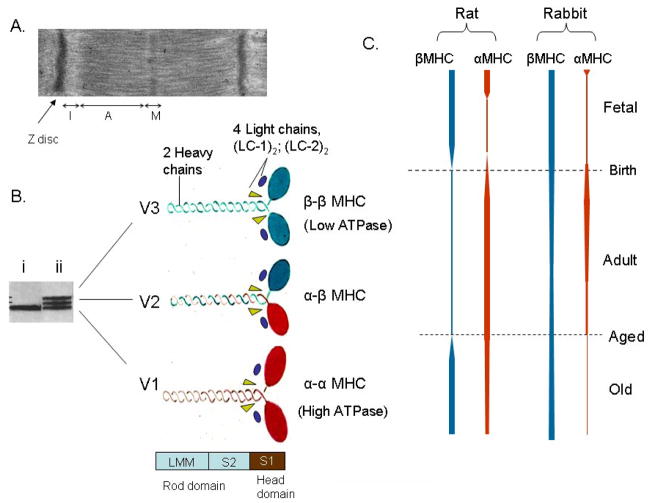
In cardiac myocytes, the two MHCs are encoded by two separate genes, designated as α and β genes [12]. The two MHCs, α and β, generate three myosin isoforms in the ventricles, named V1, V2, and V3, based upon their electrophoretic mobility in the native state. The three myosin isoforms differ in the composition of α and β MHCs, but have the same two pairs of MLCs, refereed as essential (LC-1) and regulatory (LC-2) light chains. The V1 myosin isoform is an αα homodimer and V3 is a ββ homodimer, whereas V2 is an α/β heterodimer (Fig. 1). The atria contain a separate myosin isoform (Va) that is made up of two αMHCs and a separate set of light chains (LC-1a and LC-2a) [11, 13].
Figure 1.
Structural organization and developmental regulation of MHC isoforms. (A) Transmission electron micrograph of rat heart muscle showing different regions of a sarcomere. MHC isoforms are located in the “A” band of the sarcomere. (B) A native gel showing separation of three ventricular myosin isoforms of an old aged (ii) and young adult (i) rat heart [17]. The composition of three (V1, V2 & V3) isofroms made up of 2 heavy chain subunits and 4 light chains is shown. Proteolytic fragment S1, corresponding to myosin head domain and two other proteolytic fragments, S2 and LMM (light meromyosins) corresponding to alpha-helical rod domain are shown. (C) Schematic representation of the developmental regulation of rat and rabbit heart myosin isoforms during fetal, adult and old age of the animal [15, 16, 154].
The expression of the two MHCs is developmentally regulated, and is controlled by numerous pathophysiologic stimuli. In rodents, both MHCs are detected in the developing cardiac myocytes as early as the 7.5th to 8th day of gestation, and in late fetal life αMHC is expressed predominantly in the atria and βMHC in the ventricles [14]. Immediately before birth, the expression of αMHC begins to increase in the ventricles and becomes the dominant (>90%) isoform in young adult animals (Fig. 1). As the animal ages, the αMHC level (~40% of total MHCs pool) becomes suppressed again, and βMHC becomes the major isoform [15, 16]. In domestic rabbits, the αMHC begins to rise just before birth, as in rats, and reaches levels almost equal to those of βMHC (50:50 α:β ratio) at the age of around two weeks of the animal. Thereafter, αMHC levels start to decline gradually with the age of the animal. In young adult rabbits, the ratio of α to β MHCs has been shown to be nearly 20:80, which gradually increases further in favor of βMHC as the animal ages [17, 18]. With the exception of rabbit, the developmental expression of MHC isoforms in other larger species has not been studied. Nonetheless, it is well established that the ventricular myosin composition is characteristic of a given mammalian species during adulthood [15, 17]. The fast-contracting hearts of rodents, such as the mouse, rat, and hamster express αMHC predominantly, whereas the slow-contracting hearts of guinea pigs, domestic rabbits, and pigs express mostly βMHC. There are, however, exceptions to this fact. The hearts of the hare and wild pig exhibit a large proportion of αMHC, suggesting that the living conditions of animals that are intermittently associated with high-velocity parameters of the myocardium lead to an isoform population in favor of the fast-myosin isoform, i.e. αMHC, whereas the opposite is true of animals with a sedentary life style [19]. In the adult human heart, αMHC has been detected at about 30% of the total MHC mRNA and 10–12% of the total myosin protein [7]. A recent study, however, suggests that αMHC may be as high as 70% of the total myosin pool in human neonates, and that this gradually decreases with age [20].
There are also gradations of MHC isoform expression in different regions of the ventricle of an adult heart. In an analysis of the entire left-ventricular free wall of a rabbit heart for myosin ATPase activity, a striking trans-mural gradation in the activity was observed [21]. The outermost epicardial region of the left ventricle contained αMHC predominantly, whereas the mid-wall and endocardial portions showed a checkerboard pattern, with cells expressing mostly βMHC [21]. A similar gradation of MHC expression has also been reported in rat hearts, particularly after induction of cardiac hypertrophy. The greatest amount of βMHC was seen to be expressed in the subendocardial region of a hypertrophied heart [22]. The physiologic consequences of this heterogeneous distribution of myosin isoforms are not known.
Functional consequences of myosin isoform shift
The mechanical properties of the heart are well correlated with the type of MHC isoform expressed [23]. Studies conducted on myofibrillar ATPase activity have documented that the speed with which muscle shortens (the velocity of shortening) is correlated with the ATP- hydrolyzing capacity of the myosin molecule [24]. The αMHC isoform of all of the species studied to date showed nearly three times higher ATPase activity than did the βMHC isoform. Many studies performed on single myocytes and isolated papillary muscle preparations, where the MHC ratio was altered by thyroid manipulation, have demonstrated that, indeed, a linear correlation exists between the maximal speed of shortening of unloaded cardiac muscle and the ratio of the α to βMHC isoform expression [25–27]. More tangible evidence for this finding came from in vitro motility assays where purified α and β MHC isoforms were mixed in different ratios and tested for their ability to slide fluorescence labeled actin [23, 28]. Warshaw’s group has shown in an in vitro motility assay, that the actin sliding velocity (V actin) of the myosin mixture was highest at 100% αMHC and gradually decreased as the proportion of the βMHC isoform was raised, reaching the lowest level at 100% βMHC isoform, thus confirming the linear correlation between V actin and α:βMHC isoforms ratio. Single molecule studies have also demonstrated that the higher actin sliding velocity of the αMHC isoform is related to a shorter attachment time of myosin after the power stroke and not to the displacement generated by the myosin power stroke [29, 30]. These studies have suggested that the kinetics (attachment time) rather than the mechanics (unitary displacement) of the myosin molecule accounts for the difference in the actin-sliding velocity of the α and βMHC isoforms [23, 28–30]. Based on these observations, it is generally believed that hearts expressing primarily αMHC isoform have a significantly higher velocity of muscle shortening; whereas hearts expressing mostly βMHC, which has low ATPase activity, have the ability to generate force with greater economy.
Differences in the velocity of shortening of the α and βMHC isofoms across the species are uniformly accepted; however, this is not the case for their force generating ability. The rat and mouse α and βMHC isoforms have been shown to generate the same amount of average force (isometric peak tension) [23, 28]. In contrast, the rabbit βMHC isoform has been shown to generate higher average force time integrals, compared to the rabbit αMHC isoform [23]. This difference is again explained on the basis of variations in kinetics (the fraction of time myosin is attached to actin during the entire cross-bridge cycle, also referred as duty ratio) of the power stroke generated by two MHC isoforms, and not by the unitary force, which is equal for both MHC isoforms of rabbits [23]. The structural basis of these differences in mechanical performance of the α and βMHC isoforms and among myosin isoforms of different species has been described by Alpert et al [23].
In terms of mechanical power, which is a product of the developed tension and the velocity of shortening, it appears that rodent hearts having mostly the αMHC isoform, which has a nearly three times higher speed of shortening, have a greater capacity of power generation, than do hearts in which the βMHC isoform predominates. This may not be the case for the rabbit heart, where the αMHC isofrom lags behind the force-generating ability of the βMHC isoform. Nevertheless, data obtained from mouse and rabbit hearts in which the MHC isoform ratio was experimentally altered, either by thyroid manipulation or by transgenesis, have indicated that hearts expressing high levels of αMHC are at an advantage under stress conditions over hearts expressing mostly the βMHC isofrom. Korte et al. have shown that the replacement of MHC isoforms from 100% α- to ~80% βMHC, by thyroid manipulation, reduced the power output of the left ventricle by nearly 65%, and the stroke volume and cardiac output by nearly half to the control values obtained from hearts with 100% αMHC [26]. Similar results have been reported by others studying the functional consequences of MHC isoform shift in transgenic mouse hearts, where 75% βMHC was forced expressed [31]. Krenz et al. noted that transgenic mice having a 25 to 75% α- to βMHC ratio were healthy and had no apparent defect in overall cardiac morphology, suggesting that the transition from α- to βMHC is benign in terms of the overall health of the animals [31]. However, when these hearts were stressed and analyzed for hemodynamic determinants, transgenic hearts with 75% βMHC had significantly reduced contractile ability, as assessed by diminished dp/dt max, compared to non-transgenic controls [32].
A similar conclusion has been drawn from studies with transgenic rabbit hearts, in which nearly 40% of endogenous βMHC was replaced by the αMHC isoform [6]. James et al have reported that the transgenic expression of the αMHC isoform in rabbit hearts has no apparent detrimental effects under basal conditions, and that these hearts are protected against tachycardia-induced cardiomyopathy [6]. Based on differences in the ATP requirement of two isoforms, it is generally considered that hearts expressing mostly βMHC are more economical, than are hearts expressing mainly αMHC. However, data obtained from studies on transgenic rabbit hearts have indicated that moderate expression of the αMHC isoform is advantageous for preserving the heart function under stress conditions, suggesting that the benefit to heart function presented by the αMHC isoform may outweigh the higher energy utilization by this isoform. The precise mechanism of the beneficial effect of the αMHC isoform for heart function remains unknown, however.
Regulation of MHC gene expression
The MHC is the only myofibrillar protein whose family members are clustered and not scattered on different chromosomes as are those encoding the MLCs and actins. The cardiac α- and βMHC genes are located on chromosome 14 in both man and mouse, and on chromosome 15 in the rat. They span a 51 kb DNA segment separated by ~4.5 kb intergenic sequences. In all species examined so far, which include human, mouse and rat, the two genes are linked in the 5′ to 3′ direction. The βMHC gene is located upstream from the 5′ end of the α gene. Each gene consists of 40 exons and 39 introns and transcribes ~7.0 kb of mature mRNA, with >90% nucleotide sequence identity [12, 33, 34]. However, the 3′UTR region of α- and βMHC mRNA transcripts is unique, and hence these sequences have been utilized extensively for analysis of the two gene transcripts. The expression of two MHC genes is controlled at both the transcriptional and post-transcriptional levels. In regard to the cardiac-specific expression of both genes, the immediate upstream promoter regions of the genes seem to be sufficient; but not efficient, as both of them require ~5.5 kb upstream sequences to be expressed strongly in cardiomyocytes. There is also evidence that the intergenic sequences between the α- and βMHC genes possess transcriptional activity on the opposite DNA strand. This intergenic transcription proceeds in the direction of the βMHC gene, thus yielding β-antisence RNA. Although the exact role of this anti-sense transcript is not yet understood, it has been found to be associated with MHC isoform switching in the heart in response to pathologic stimuli, such as hemodynamic overload and diabetes [35–37]. A similar anti-sense mediated regulation of the MHC genes has also been suggested for isoform switching in slow skeletal muscle fibers [38].
The transcriptional regulation of the MHC gene is complex, and is initially activated by the intricate genetic program of cardiac development. At least four different groups of transcription factors that are expressed early during the development of the heart are implicated in MHC gene expression. These include the following: (i) The GATA family of factors, which contain one or more Zn finger domains, they play a critical role in differentiation of both cardiac and hematopoietic cell lineages. (ii) A homeobox gene, Nkx2.5, which forms a complex with the GATA factors, has been implicated in the expression of cardiac-specific genes, but not of genes expressed in the skeletal-muscle-cell background. (iii) Members of the MADS family of proteins, which comprises serum response factor (SRF) binding to the C(Ar)G motif, and the MEF2 subfamily of factors that bind to AT-rich sequences; these factors have been described as being necessary for cell-specific expression of several cardiac and skeletal muscle genes. (iv) Factors that binds to M-CAT sequences, named TEF1 isoforms; they make a complex with SRF and MEF2 and are found to be necessary for muscle-specific expression of genes in both the cardiac and skeletal muscle-cell context. However, gene ablation of factors such as TEF1, GATA4, and Nkx2.5 has been found to have no effect on the expression of MHCs, while some proteins expressed in the cardiac muscle cell background, such as MLC2 and ANF, were found to be absent in Nkx2.5 null mice [39–46]. SRF knock-out was shown to attenuate the expression of both α- and βMHC transcripts [47]. In MEF2c null mice, MHC expression was down-regulated, but the expression of other cardiac genes such as MLC2 remained high [42]. These studies have indicated that a common as well as a unique gene-specific regulatory mechanism are likely to be involved in cardiac-specific muscle gene expression.
In addition to transcriptional activation, gene repression controlled by negative regulatory factors also seems to play a critical role in MHC gene expression. Our laboratory has identified a unique purine-rich negative regulatory (PNR) element in the first intronic region of the αMHC gene, which was found to be essential for the cardiac-specific expression of this gene [48]. The PNR element cooperated with the upstream SRF-binding sequences of the αMHC gene for cardiac-gene specificity [48, 49]. Deletion of this element allowed the expression of the αMHC gene in HeLa and other non-muscle cells, where it usually remains unexpressed. These studies were later confirmed by others who utilized αMHC gene regulatory sequences as a tool to generate cardiac-specific viral probes [50]. We subsequently cloned and characterized two single-strand DNA/RNA-binding proteins (PURα/β) which bind to the PNR element. These proteins were found to be capable of binding to the αMHC mRNA sequences as well, and they appeared to participate in both the transcriptional and translational regulation of αMHC gene expression [51]. Recently, a role of PURα/β has also been demonstrated in the regulation of βMHC gene expression in slow skeletal-muscle fibers. Whether this mechanism is also involved in the control of βMHC expression in cardiac myocytes is not known, however [52].
Effect of thyroid hormones
Among various extrinsic factors that are known to regulate MHC gene expression, thyroid hormone was found to be the most potent regulator of all members of the MHC gene family, but in a highly muscle-type specific manner [53]. The same gene which is upregulated by thyroid hormone in one tissue is unresponsive or down- regulated in other tissues [54]. The expression of the αMHC gene, whereas it is dependent upon thyroid hormone in the ventricles, remains unresponsive to the hormone in the atria of the same heart. A similar situation exists for the expression of the βMHC gene, which is repressed by thyroid hormone in the ventricular tissue, but remains almost un-responsive to the hormone in the slow skeletal-muscle fibers. The molecular mechanism of the thyroid-hormone action has been demonstrated to be mediated by binding to a nuclear protein, thyroid-hormone receptor (TR), a product of the c-erbA proto-oncogene [55, 56]. In the heart, mainly two TR isoforms, designated TRα1 and TRβ1, are present. A third isoform, TRα2, a splice variant of TRα1 that does bind to thyroid hormone, has also been identified [57]. In the transient transfection analysis, TRα1 was found to be capable of activating the αMHC promoter; whereas TRβ1 was found to be coupled with the repression of βMHC promoter activity [58]. TRα1 and TRβ1 are hormone-dependent transcription factors. They exercise their effect by binding to a hormone-responsive DNA element of the target gene. The thyroid-responsive element (TRE) has been characterized in the 5′ promoter region of the human, rat and mouse αMHC genes [56, 59, 60]. All of these genes contain a TRE with identical base-pair sequences and are able to confer thyroid hormone sensitivity of the gene in a heterologous system, suggesting that TRE of the αMHC gene by itself constitutes an enhancer and is sufficient for mediating the thyroid effect on the target gene.
The mechanism of thyroid hormone-dependent repression of the βMHC gene is, however, not yet understood. Sequences identical to those of the TRE that is present in the αMHC gene have not been found in the βMHC gene. Moreover, sequential deletion analysis of the human βMHC gene promoter region has revealed that the basal promoter region of the gene by itself is sufficient to elicit a thyroid effect. By foot-printing analysis, a sequence similar to the TRE half-site was identified adjacent to the TATA box of the βMHC gene [54]. This sequence is similar to the TRE half-site found in the basal promoter region of other negatively regulated genes, such as the α-subunit of the thyroid stimulating hormone and the lysozyme gene [61, 62]. Based on these reports, it is likely that transcriptional repression by thyroid hormone may be mediated by an element similar to that responsible for the positive regulation, but it is present as a single half-site to which the TR may bind as a monomer. An alternative mechanism could be that TR forms a heterodimer with other positively acting factors, thus nullifying their effects and ultimately leading to the repression of gene transcription. Such a mechanism has been described for down-regulation of genes by other nuclear receptors, such as glucocorticoids and retinoic-acid receptors [63].
Role of adrenergic stimulation
In addition to thyroid hormone, a wealth of evidence has come forward indicating the involvement of the adrenergic nervous system in the differential expression of the two cardiac MHC genes [64–75]. Experiments on adult rats expressing predominantly αMHC showed an increased expression of βMHC when they were treated with β-adrenergic antagonists [70]. A similar myosin isoform shift in favor of βMHC was found after denervation of the heart by either chemical or surgical sympathectomy [65, 69]. Furthermore, studies on a non-working heterotypically transplanted and hence denervated heart expressing mainly the βMHC isoform, when subjected to swimming exercise, exhibited an increased accumulation of αMHC, which could be prevented by pretreatment of the animal with β-adrenergic blockers [64].
Direct evidence for the involvement of this pathway in MHC gene regulation came first from experiments in which isolated hyperpermeable cardiac myocytes were used. In these cells, treatment with β-adrenergic agonists or with a membrane-permeable analogue of cAMP, which mediated an increase in the Ca2+-activated force, was found to be directly correlated with an increase in the myosin ATPase activity and expression of the αMHC isoform [72–75]. The same studies also indicated a role of the α-adrenergic pathway in the regulation of βMHC expression. Finally, by use of primary cultures of cardiac myocytes, it was demonstrated that adenylate cyclase activators and cAMP analogues activate the expression of the αMHC isoform (V1) and the αMHC mRNA transcript [66, 67]. The α1-adrenergic agonists or activators of the PK-c pathway, on the other hand, led to increased expression of the V3 myosin isoform and a relative abundance of βMHC mRNA [66, 67, 76]. However, in an earlier study using cultures of cardiac myocytes grown in a serum-free defined medium, no effect of either β- or α-adrenergic agonists on the expression MHC mRNA was found [77]. This discrepancy is likely to be due to differences in the culture conditions utilized by different investigators. In a later study, it was reported that, in serum-free cultures an effect of cAMP on αMHC mRNA expression could not be detected, but when the same cultures were supplemented with serum, the cAMP effect was found to be restored [67]. In addition, other studies documenting the depletion of myocardial catecholamines at an advanced age of the animal, when αMHC expression is down-regulated, also indirectly suggest a role of the adrenergic system in MHC gene regulation [1].
The effects of β-adrenergic agonists on the expression of MHC in larger animals and in man, where βMHC is expressed preferentially, have been studied as well. Administration of isoproterenol to the adult rabbit resulted in a shift of the isomyosin proportion in favor of αMHC synthesis [1, 68, 69]. Furthermore, in patients with congestive heart failure who received large doses of catecholamines for inotropic support, 40% more αMHC was found to be expressed as compared with matched controls of the same age, sex, and weight and duration of heart failure [69]. In a recent study, Bristow’s group differentiated between the role of two types of β-adrenergic receptors (ARs) in the activation of MHC gene expression. Using primary cultures of cardiac myocytes, they demonstrated that treatment of cells with high doses of isoproterenol leads to myocyte hypertrophy, that is associated with induction of βMHC and repression of αMHC gene expression [71]. This study further demonstrated that these changes are mediated by stimulation of β1AR and not by β2AR, as they are independent of the cAMP/PK-A pathway, but are prevented by inhibition of the Ca/CaMK pathway. Thus, this study, together with many earlier reports showing participation of the cAMP/PK-A pathway for αMHC induction, suggests that, whereas β1AR activation controls βMHC expression, β2AR stimulation contributes to induction of αMHC.
For determining the mechanism behind the adrenergic receptor stimulation-mediated change in MHC gene expression, the effect of cAMP on the αMHC gene promoter/reporter gene was analyzed. The results showed that cAMP transactivates the αMHC gene promoter in a cardiac and skeletal-muscle cell background, but not in non-muscle cells [67, 78]. Mutation analysis of the promoter uncovered an E-box/MCAT hybrid motif, which is responsible for the basal expression of the gene, and which mediates the cAMP-dependent activation of αMHC gene expression. In a separate study, Ojamaa et al. reported that the same motif also contributes to the contraction-mediated induction of αMHC gene expression in cardiac myocytes [79]. Further characterization of this motif revealed that two factors, Max and TEF1, bind to this motif; of these, TEF1 was found to be a substrate for cAMP-dependent phosphorylation [78, 80]. In a similar cell culture system, a conserved 20 bp sequence in the proximal promoter region of the βMHC gene was identified that could serve as a response element for the α1-adrenergic agonist and for PK-c dependent activation of the gene [81]. Interestingly, a core element located within this sequence is also an M-CAT binding site for the TEF1 protein, which was suggested also to be a substrate for phosphorylation by PK-C [81–83]. Thus, these reports implied that TEF1 binding to the M-CAT element is a functional end point for both α and β-adrenergic signaling pathways that mediate cardiac MHC gene expression. The precise mechanism by which phosphorylation of TEF1 by two different signaling pathways regulates the expression of the two MHC genes is not yet understood. However, there are other instances in which phosphorylation of a single transcription factor by different signaling mechanisms results in differences in gene regulation [84].
As noted above, factors involved in the initiation and maintenance of the cardiac myogenic program are likely to play a role in the regulation of the two cardiac MHC genes. Several studies have also implicated the role of the adrenergic system, particularly the β-adrenergic pathway, in cardiac myocyte development [85, 86]. The presence of clusters of the adrenergic cardiac cells capable of synthesizing and releasing catecholamines in both the ventricles and the atria of the heart has been documented [85]. These intra-cardiac adrenergic cells are found in the fetal heart at a developmental stage that precedes the sympathetic innervation. Furthermore, in studies in which targeted destruction of genes, encoding enzymes of catecholamine synthesis, and β-adrenergic receptor kinase which mediates phosphorylation and desensitization of the receptor, was carried out, mid-gastrulation death of embryos resulted, with severe phenotype anomalies of the heart [86]. Together, these studies provide strong support for the role of adrenergic pathways in the activation and/or maintenance of the cardiac muscle gene program.
Metabolic changes and diabetes
A great deal of evidence has also demonstrated that a change in the metabolic status of the heart influences myosin isoform expression. In general, decreased activity of the glycolytic pathway and a corresponding increase in free-fatty acid oxidation for energy production, as occurs in cases of diabetes, favors the synthesis of βMHC expression. Treatment of diabetic animals with insulin, feeding them with a high-fructose diet, or giving them a free-fatty acid mitochondrial transport inhibitor, methyl palmixirate, resulted in an increased rate of glycolytic flux and a shift to favor the synthesis of the αMHC isoform [87–89]. Increased expression of αMHC has also been reported when hypothyroid and hypophysectomized rats were fed a high fructose diet [90, 91]. In a separate study, Jordon et al. reported that the feeding of fructose protects rats against ischemia-reperfusion injury, independent of a change in insulin levels [92]. The precise mechanism of fructose-feeding mediated induction of αMHC expression and cardiac protection, however, remains unknown. There are other hormonal and nutritional alterations that are known to change the redistribution of MHC isoforms. Gonadectomy in euthyroid and spontaneously hypertensive rats was shown to result in loss of αMHC and increased synthesis of βMHC, which was completely reversible by testosterone replacement [91, 93]. This effect of testosterone on the MHC isoform shift was shown to be independent of any change in the hemodynamic load and of cardiac hypertrophy [93]. Other interventions such as intermittent starvation, prenatal under-nutrition, and hypoxia have also been shown to favor βMHC synthesis [94–96].
Cardiac hypertrophy
Cardiac hypertrophy develops in response to an increased workload on the heart, which can originate from either persistent physiologic activity or pathologic alterations in the cardiovascular system. Chronic exercise is known to induce physiologic hypertrophy, which is associated with increased expression of αMHC. Induction of αMHC is more evident with physiologic hypertrophy mediated by swimming than by running exercise. Swimming exercise for 8 to 10 wks has been shown to induce αMHC expression not only in controls, but also in spontaneously hypertensive rats and in animals with renal artery stenosis which expressed βMHC as a major myosin isoform [97–100]. As noted above, several independent studies have presented evidence for the role of an increased sympathetic drive in the induction of the αMHC isoform associated with physiologic hypertrophy, because it was found to be correlated with alterations in the activity of several key enzymes of catecholamine metabolism [98]. Also, swimming mediated induction of αMHC expression was found to be blocked by sympathectomy or pretreatment of animals with β-adrenergic receptor blockers [64, 65, 97, 100]. Recent studies carried out on human athletes and swimmers have also demonstrated increased cardiac sympathetic activity and formation of IGF1, associated with physiologic hypertrophy [64, 101, 102]. These studies together have strengthened the notion of enhanced adrenergic receptor activity for the induction of αMHC expression during physiologic hypertrophy. Chronic IGF-1 and GH infusion has also been shown to induce αMHC mRNA in normal control rats, without changing the levels of βMHC transcripts. However, theses effects of growth factors were not observed in hypophysectomized rats, indicating that IGF-1 and GH stimulate the synthesis of αMHC transcripts indirectly, depending upon the hormonal status of the animal [103]. In a recent study, Kinugawa et al. reported the induction of TRβ1 expression associated with running exercise-mediated physiologic hypertrophy [58]. As mentioned above, over expression of TRβ1 down regulates the βMHC expression. It is likely, therefore, that decreased βMHC levels may also contribute to the changed MHC ratio during physiologic hypertrophy [58].
A major shift in the myosin isoform distribution occurs during pathologic hypertrophy mediated by pressure and volume overload. This hypertrophy is associated with induction of βMHC at the expense of αMHC. This change from the α- to βMHC phenotype is taken as a hallmark of pathologic hypertrophy, which is more intense in pressure-overload than in volume-overload hypertrophy. In animal models of pressure overload, such as aortic constriction in rats, mice and rabbits, the relative proportion of αMHC has been shown to decrease during pathologic hypertrophy, but it was never found to be completely eliminated [104–107]. In human failing hearts the αMHC isoform, which is nearly 10% of the total MHC pool, decreased to nearly non-detectable levels, and this was suggested to be a critical determinant of transition of the heart from adaptive hypertrophy to failure [7, 9]. This idea is supported by experiments in which blocking of the MHC isoform shift by thyroid-hormone intervention prevented the functional decompensation of the heart in an aortic-banding model of heart failure [108]. However, because of the relatively low levels of αMHC in human hearts (~10% protein), others have disputed the concept of the shift in MHC isoforms as a major cause of muscle dysfunction of human failing hearts [109]. Alternatively, it has been suggested that the disappearance of αMHC in human failing hearts may be a compensatory mechanism aimed at preserving function by increasing the myocardial energy efficiency.
However, other studies carried out with isolated myocytes and transgenic animals have advocated a beneficial effect of αMHC isoform expression for preserving the heart function under stress conditions. In a study utilizing a single cell system, Herron et al demonstrated that cardiac myocytes expressing only 12% of the αMHC isoform produced a 52% greater power output than did those expressing only the βMHC isoform [25, 110]. As mentioned above, results obtained from transgenic rabbits, which have an MHC profile very similar to that of man, have indicated that hearts in which βMHC was replaced by 40% of αMHC were at an advantage under stress conditions in maintaining heart function [6]. This study also ruled out the possibility that decreased αMHC during human heart failure could be a compensatory mechanism for preserving the energy efficiency of the heart. Because, if this was the case then rabbit hearts with 40% αMHC would have fared worse under stress conditions than the non-transgenic control; obviously this did not happen [6].
Based on these findings, a new group of chemical agents has been designed which are capable of activating myosin ATPase activity (myosin activators). In experimental studies, myosin activators were found to increase the cardiac contractility of failing myocytes without changing intracellular levels of Ca2+, suggesting an inotropic potential of myosin activators for treatment of heart failure [5]. Some of these agents have also gone into the first phase of clinical trials and have shown encouraging results [111]. The functional significance of αMHC in human hearts was also supported by studies in which mutations in human αMHC, as with βMHC, was found to be associated with familial hypertrophic cardiomyopathy, an autosomal disorder associated with left ventricular hypertrophy and sudden death [112]. Although every experimental approach to MHC manipulation has its own set of limitations and none of them provide full proof of the concept whether one MHC isoform is better over the other, the majority of data available to date suggest that altering the MHC isoform ratio may eventually be beneficial for the treatment of a failing heart.
The reversibility of the MHC isoform shift after regression of cardiac hypertrophy has also been examined [113]. In rats, pressure overload hypertrophy was produced by aortic banding, and then MHC expression was analyzed in the overloaded hearts and following load removal (debanding). As expected, pressure overload resulted in induction of β- and repression of αMHC expression. Load removal led to regression of cardiac hypertrophy and reversal of αMHC levels to those of normal controls, whereas β-isoform levels recovered very slowly and remained elevated even 7 weeks after debanding [113]. These studies demonstrated that load-related signals change the expression of the α- and βMHC isoforms differently.
The mechanism of MHC gene expression during hypertrophy is complex, and both transcriptional and post-transcriptional regulatory mechanisms have been shown to play a role. Wiesner et al. reported a lack of coordination between the induction of βMHC protein and the mRNA transcripts in the aortic banding model of pressure-overload hypertrophy, suggesting that a post-transcriptional mechanism is involved in induction of βMHC during hypertrophy [114]. A similar conclusion was drawn from experiments in which the effect of contraction inhibitors on MHC expression was examined. In a model system of spontaneously beating cardiac myocytes, Qi et al. demonstrated that inhibition of myocyte contractility by treatment of cells with verapamil, KCl, or 2,3-butanedone monoxime (BDM) resulted in different effects on cellular Ca2+ levels, but all of these inhibitors down regulated the βMHC mRNA and up-regulated the αMHC mRNA expression. In contrast, in a transient transfection assay, all of these inhibitors had a similar negative regulatory effect on both α- and βMHC gene promoters, suggesting that increased αMHC mRNA levels resulted from a post-transcriptional mechanism [115]. By use of a transcription inhibitor, actinomycin-D, this study demonstrated that the half-life of the αMHC transcript was extended from 14 hours in contracting cells to 33 hours in non-contracting cells. These results indicated that intrinsic mechanical parameters, rather than Ca2+ alterations, influence the α- and βMHC expression involving a post-transcriptional mechanism. In a related experiment, Nikcevic et al. demonstrated that inhibition of contraction, but not of calcium, regulates the initiation of αMHC mRNA translation [116].
There is also ample evidence to suggest a role of gene transcription in the redistribution of the two myosin isoforms during hypertrophy and heart failure. A direct association of serum thyroid hormone levels with an α- to βMHC switch during cardiac hypertrophy has not been found; however, defects in the expression levels of thyroid receptors (TRα1, TRβ1 and TRα2) have been documented [58]. Treatment of animals with T4 or thyromimetic agent, DITPA, (diiodothyropropionic acid) reversed the myosin isoform switch as well as contractile dysfunctions associated with hypertrophy and heart failure, suggesting that a defect in thyroid-hormone signaling may, in part, contribute to the MHC isoform distribution accompanied with the failing heart [108, 117]. By promoter analysis of the αMHC gene, a number of negative regulatory DNA elements and their cognate binding factors have been identified. As mentioned above, we have identified binding sites of single-strand DNA/RNA binding proteins, PURα/β, in the 1st intronic region of the αMHC gene, as well as on its mature mRNA transcript. These proteins are strong repressors, because their over expression repressed the gene promoter activity as well as the translation ability of the αMHC mRNA. The expression level of the single-strand DNA binding proteins was found to be highly elevated in failing hearts of rabbits and man, where αMHC mRNA levels were suppressed, suggesting that PURα/β may, in part, contribute to down–regulation of αMHC expression during heart failure[51].
Recently, Leinwand’s group has shown increased expression of the YY1 factor in human failing hearts. In transient assays, YY1 repressed the activity of both the rat and human αMHC gene promoter when it was expressed ectopically in cardiac myocytes [118]. Over-expression of YY1 in cardiac myocytes by use of adenoviral vectors also resulted in suppressed expression of the endogenous αMHC gene. This group has identified another protein complex on the αMHC gene promoter whose activity is six-fold higher in human failing hearts. By DNA affinity-column purification followed by mass spectroscopy, they identified this complex as the heterodimer of Ku70/Ku80. Over- expression of Ku70 and Ku80 repressed the αMHC promoter activity and showed an additive effect when combined with YY1 [8]. YY1 has also been shown to interact physically and coordinate functionally with the PUR family of proteins for control of cell-specific gene regulation [119, 120]. It is, therefore, likely that these repressors, PURα/β, YY1 and Ku70/Ku80 either alone or in combination, contribute to the repression of αMHC promoter activity in some (if not all) aspects of heart failure.
The up-regulation of βMHC expression has been shown to be controlled by activation of the PK-C signaling pathway and elevation of intracellular Ca2+, both of which play a major role in the induction of cardiac hypertrophy [81, 121, 122]. Molkentin et al. have reported that elevated Ca2+ levels activate a calcium-dependent phospahatase, calcineurin, which dephosphorylates the transcription factor NFAT3 and thus enables it to translocate to the nucleus. Within the nucleus, NFAT3 binds to GATA4 and activates a discrete set of genes of the fetal gene program, which includes activation of β-MHC, skeletal α-actin and ANF gene expression [121]. Inhibition of calcineurin activity by immunosuppressant compounds, such as cyclosporine and FK506, prevents the development of pressure-overload cardiac hypertrophy and induction of βMHC expression. In a recent study, however, a direct role of GATA4 for the induction of βMHC expression during hypertrophy was not found [123]. Bisping et al. have shown that GATA4 knock-out mice subjected to aortic banding had overt heart failure and hypertrophy, which was associated with a massive increase in βMHC and ANF expression, thus disproving a direct role of GATA4 induction in the up-regulation of βMHC expression during pressure-overload hypertrophy [123]. In a separate study, participation of NFAT3 in the induction of βMHC gene expression during cardiac myocyte hypertrophy was also not found. By analyzing the rat βMHC promoter in cardiac myocytes, McLean et al. found that the four TEF1 binding M-CAT sites present in the proximal promoter region of the βMHC gene are required for its basal and phenylephrine-mediated activation [124]. These investigators did find a functional NFAT binding site in the βMHC promoter that can be activated by calcineurin, but this type of gene regulation was found to be restricted to expression of the βMHC gene in slow skeletal-muscle cells, and not in cardiac myocytes. Similar results were reported by others who analyzed the effect of different TEF1 isoforms for the α-1 adrenergic receptor-mediated activation of the rat βMHC gene [122]. These studies have demonstrated that the regulation of βMHC gene expression during the basal state and during hypertrophy is controlled by different mechanisms in cardiac myocytes and slow-skeletal muscle cells.
Chromatin modifying enzymes
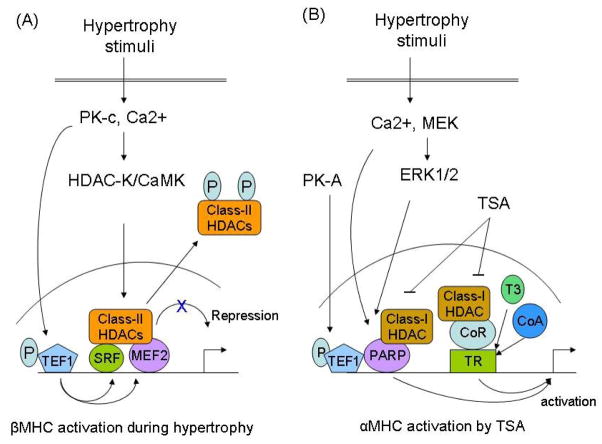
Recent advances in cell biology have uncovered the importance of chromatin remodeling, specifically histone acetylation and deacetylation, in the control of gene expression. The enzymes responsible for adding or removing acetyl groups are called histone acetyltransferase/acetylase (HAT) and histone deacetylase (HDAC), respectively [125]. Histone acetylation and deacetylation exert opposite effects on gene transcription, whereas former activates later suppresses the gene transcription. HDACs are divided into three main groups, class-I, II and III, depending upon their domain homology and the requirement of co-factors for the enzymatic activity [126]. There is compelling evidence that many factors that control cardiac MHC gene expression are associated with HAT and HDACs. MEF2 and SRF both physically bind to class-II HDACs, HDAC4 and HDAC5, and form a complex on the gene-regulatory sites, leading to repression of genes harboring MEF2 and SRF binding sites [127, 128]. Activation of Ca/CaMK signaling during hypertrophy has been shown to phosphorylate HDAC4/5, resulting in export of these enzymes from the nucleus to the cytoplasm; this leads to de-repression (reactivation) of target genes, such as βMHC, skeletal α-actin and ANF [128]. Both SRF and MEF2 have also been shown to collaborate with TEF1, a direct target of PK-C signaling, for gene regulation [41, 43]. It is thus likely that a combined effect of these factors, SRF, MEF2 and TEF1, controls βMHC expression during hypertrophy (Fig 2A).
Figure 2.
Models to explain HDAC-mediated regulation of MHC gene expression during hypertrophy. (A) Induction of βMHC gene expression by blocking the activity of class-II HDACs (HDAC 4/5). Class-II HDACs binds to SRF and MEF2 leading to repression of their gene transcription activity. Hypertrophy stimuli activate the activity of a kinase (Ca/calmodulin dependent kinase and/or HDAC kinase), which phosphorylates two serine residues conserved in class-II HDACs [128]. Upon phosphorylation, class-II HDACs are exported out from nucleus to cytoplasm, resulting in the de-repression of SRF and MEF2 transcription activity and induction of the βMHC expression from SRF and MEF2 binding sites [127, 128]. In a parallel pathway, activation of the PK-c signaling phosphorylates TEF1 leading to activation of its gene activation potential. TEF1 physically binds and functionally interacts with SRF and MEF2. It is therefore likely that a combined effect of these three regulators of gene expression controls the βMHC gene induction in response to hypertrophic stimuli. (B) Activation of αMHC gene expression by trichostatin A (TSA). The effect of TSA on αMHC induction is mediated by inhibition of the class-I HDACs and it occurs in euthyroid and not in hypothyroid animals (see text). In the absence of ligand (T3), thyroid receptor (TR) interacts with a multiprotein co-repressor (CoR) complex that contains class-I HDACs. This complex represses the gene transcription. In the presence of ligand (T3), the TR undergoes a conformational change, which results in replacement of the co-repressor complex with an activator complex (CoA) that contains HATs (p300/PCAF). Inhibition of HDACs by TSA enhances the activity of this activator complex with TR, resulting in the induction of the α-MHC gene expression. The activity of αMHC gene promoter is also controlled by change in activity of PARP1, which is activated by elevated levels of Ca2+ and increased activity of MEK/ERK1/2 signaling. PARP1 forms a repressor complex with class-I HDACs. Thus, TSA can also activate the αMHC gene expression by blocking the repressor activity of PARP1/class-I HDACs complex. In this context, another MHC gene regulator, TEF1, which physically binds to PARP1 can also modulate the PARP activity. TEF1 is phosphorylated by PK-A, and that stimulates its gene transcription activity [80]. Thus, we propose that the activation of PARP1 by TSA, and the activation of TEF1 by PK-A-signaling may have a co-operative effect for the activation of the αMHC gene expression (for details see text).
Inhibition of HDACs by a non-specific inhibitor, trichostatinA (TSA), an agent that inhibits both class-I and -II HDACs, has been shown to block cardiac hypertrophy and activate αMHC expression, without altering the levels of βMHC, in both in vitro and in vivo models of cardiac hypertrophy [129, 130]. This effect of TSA is likely to be contributed by its ability to inhibit class-I HDACs, as specific inhibitors of class-I HDACs were found to block cardiac hypertrophy, whereas inhibition of class-II HDACs was shown to sensitize the heart to hypertrophic stimuli [130, 131]. There is also evidence that the effect of TRs on MHC gene transcription is controlled by direct or indirect association of the receptor with HAT and HDACs. Danzi et al have shown that the TSA-mediated induction of αMHC expression occurs only in euthyroid, but not in hypothyroid animals, suggesting a role of thyroid-signaling in the induction of αMHC expression by TSA [132]. Based on studies with other T3-responsive genes, it has been proposed that class-I HDACs binds to a co-repressor complex which associates with the un-ligand-TR complex on the hormone responsive DNA binding site, leading to repression of gene transcription. In the presence of T3 and the HDAC-inhibitor, the ligand occupied TR complex goes through a conformational change that permits recruitment of a co-activator together with HAT, and this complex directs the activation of gene transcription from the TRE sites [133]. A similar mechanism is likely to participate for the TSA-dependent induction of αMHC expression in the presence of thyroid hormone (Fig 2B).
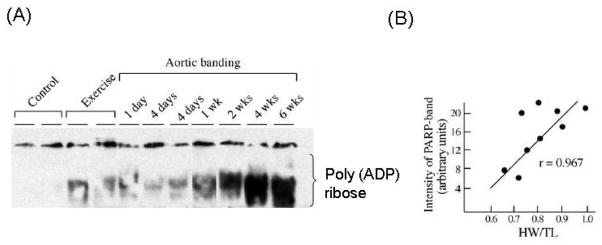
In addition to HAT and HDACs, another family of chromatin modifying enzymes, called poly(ADP-ribose) polymerase (PARP), has been found to play a role in the development of cardiac hypertrophy and MHC gene regulation [107, 134, 135]. PARP1, a prototype member of the large PARP family, has been shown to interact with TEF1 to form a complex on M-CAT binding sites of the cardiac troponinT and MHC genes, leading to their muscle-specific gene regulation [136, 137]. We as well as others have previously reported that PARP1 is activated during both physiologic and pathologic hypertrophy, and that its levels are further elevated in failing hearts [107, 135, 138]. A linear correlation was found between the intensity of PARP1 activation and the degree of cardiac hypertrophy, suggesting that the magnitude of PARP activation may have a role in the induction and progression of hypertrophy (Fig. 3) [107].
Figure 3.
Increased activity of PARP1 in physiologic and pathologic hypertrophy. (A) Heart nuclear extract of mice subjected to swimming or aortic banding was analyzed by western analysis using anti-poly (ADP) ribose antibody. (B) Linear-regression analysis of PARP activity with the degree of cardiac hypertrophy. These studies are previously published in ref [107].
PARP1 induction was also observed in cell-culture models of cardiac hypertrophy induced by treatment of cells with agonists such as angiotensin-II and phenylephrine [135, 139]. In regard to what is known about the intracellular stimuli of PARP1, at least two signaling pathways, an increased intracellular Ca2+ level and the activation of ERK1/2, have been found to activate PARP1 during hypertrophy [140, 141]. By analysis of PARP-inhibitors, it was found that PARP activates the TEF1-mediated gene transcription from the M-CAT sites [136]. In contrast, in a transient assay, over expression of PARP1 was found to repress the transcription of genes containing the M-CAT sites, again suggesting that PARP1 has a dual role in gene expression, activation followed by repression, depending upon the degree of PARP activation [107]. Recent studies have also shown that PARP1 is a target of acetylation and decatylation by p300/pCAF and class-I HDACs, respectively, but not by the class-II HDACs [142]. Acetylation of PARP1 has been shown to stabilize its interaction with other partner proteins, e.g., NFkB and TEF1 (unpublished observation), leading to activation of their target genes, whereas the opposite is true for deacetylation of PARP1 by class-I HDACs [142]. In the context of the effect of TSA on αMHC gene induction, it is therefore possible that some effects of the class-I HDAC inhibition by TSA could be mediated by acetylation of PARP1, resulting in de-repression of the transcriptional activity of the TEF1/PARP1 complex. In this situation, the phosphorylation of TEF1 by PK-A, which is known to activate TEF1, may have an additive role for further enhancing the transcription activity of this complex, leading to activation of the αMHC gene expression (Fig. 3B) [80]. Although this hypothesis needs to be formally tested, the induction of endogenous αMHC levels together with mild activation of PARP1 and activation of PK-A signaling during physiologic hypertrophy suggest that such a mechanism could operate to control the αMHC gene expression [72, 73, 107]. Another point worth noting about the effects of PARP1 is that mild PARP1 activation has the potential to relax the chromatin structure, leading to activation of gene transcription [143]. PARP1 has also been shown to control the activity of a member of the class-III HDACs (SIRT1), which has been shown to have an anti-hypertrophy potential, thus further indicating that PARP1 may have a role in the regulation of MHC isoform switch during hypertrophy [139, 144]. The role of PARP1 in cardiac disorders and that of its co-regulators in gene regulation have recently been reviewed [134, 145, 146].
MicroRNA and MHC gene expression
MicroRNAs (miRNA) are small, non protein encoding RNA transcripts, which base pair with specific RNAs. MiRNAs that match perfectly with target mRNA sequences cause degradation of the mRNA; whereas those that show imperfect match to the target mRNA generally results in translation inhibition [147]. Recently, Lagos-Quintana et al identified a microRNA (MiR-208) which is exclusively expressed in the heart [148]. This microRNA is encoded by intron 27 of the human and mouse αMHC gene, and it is extremely stable in cardiac myocytes, half life >12days [149]. Olson’s group has recently examined the functional significance of MiR208 in the heart [149]. They have shown that mice lacking MiR-208 transcripts were viable with no obvious abnormality in shape, size or structure of the heart [149]. When these mice were subjected to aortic-banding, they displayed a blunted hypertrophy response, and most notably they failed to up-regulate βMHC expression. While the expression of other stress-responsive genes, such as ANF and BNP was highly up-regulated in MiR-208 mutant mice, suggesting a critical role of MiR-208 transcripts in the induction of βMHC and cardiac remodeling during stress conditions. This study has also presented evidence that MiR208 has a profound role in thyroid hormone mediated antithetic regulation of α- and βMHC gene expression [149].
Future directions
The study of cardiac-specific muscle gene expression and the delineation of mechanisms that control the phenotype of terminally differentiated myocytes are essential for any future attempts to repair or replace the damaged cardiac tissue. The study of MHC gene expression has provided a basis for understanding both of these processes. Although many factors and stimuli capable of altering the MHC gene expression have been identified, the mechanism of MHC isoform shift, particularly the loss of αMHC during heart failure, is still not clear. Recent studies have suggested that the genomic order and tandem organization of the MHC genes on the same chromosome might be important for the coordinated regulation of these two genes. The intergenic sequences between the β- and αMHC genes have been shown to synthesize βMHC anti-sense RNA, which seems to correlate with the antithetic expression of these two transcripts in response to various stimuli [35]. Other studies have documented a role of microRNA-208 in antithetic expression of α- and βMHC isoforms in stress conditions. Further analysis is required for gaining insight into this type of mechanism of MHC gene regulation. Alterations in MHC expression by blocking of the activity of chromatin-remodeling enzymes are also of special interest, because the results have shown that a selective expression of αMHC can be achieved by trichostatin-A, an inhibitor of class-I and -II HDACs. This effect of trichostatin-A seems to be mediated by inhibition of class-I HDACs; however, a direct effect of a specific class-I HDAC inhibitor on the αMHC expression has not been studied yet. In addition, the effect of class-III HDACs (sirtuins, SIRT), which are also called longevity factors, needs to be examined for their potential to regulate MHC gene expression [150]. These proteins (SIRTs) have been shown to have anti-hypertrophic potential [144]. Also, many factors such as PARP1, Ku70, Nf-kB, and p300 that are known to participate in MHC gene regulation, and in the evolution of pathologic hypertrophy, have been shown to be targets of SIRT-dependent deacetylation [139, 151–153]. The study of the effect of SIRT1 on αMHC expression will also be of significance as both of them (SIRT1 and αMHC) are responsive to changes in the metabolic and oxidative states of myocytes. Above all, many results that have been collected by use of isolated cell systems, or with rodents as animal models, need to be re-examined in large animal models such as rabbits and pigs, whose myosin phenotype is similar to that of man.
Summary
The plasticity of cardiac myocytes is based largely on the multiplicity of muscle proteins, particularly a shift in the myosin heavy-chain (MHC) isoforms. In the mammalian heart, two MHCs, α and β, are expressed. They are encoded by two separate genes which are located on the same chromosome and are organized in tandem by ~4.5 kb intergenic sequences. The expression of the two MHC isoforms is developmentally regulated and can be modified by numerous pathophysiologic stimuli. Physiologic hypertrophy of the heart is generally associated with the induction of αMHC expression; however, during pathologic hypertrophy, βMHC is increased at the expense of αMHC. The relative distribution of α and βMHC isoforms has been shown to be directly related to the mechanical performance of the heart. Results obtained from transgenic animals (mouse and rabbit) have indicated that hearts expressing αMHC isoform are at an advantage under stress conditions, than the controls expressing mainly βMHC isoform. The mechanism of MHC gene expression during hypertrophy is complex, and is regulated at both the transcriptional and post-transcriptional levels. There is evidence that intergenic sequences between two MHC genes synthesize an anti-sense RNA, and an intron of the αMHC gene encodes a microRNA, that participate in antithetic regulation of two MHC genes in various pathologic states. Although a role of αMHC in heart function of larger animals and humans is not yet understood, a large body of evidence collected from small animal models suggests that altering the MHC isoform ratio may eventually be beneficial for the treatment of a failing heart.
Table 1.
A list of pathophysiologic stimuli controlling cardiac MHC isoforms expression.
| Stimuli | Mechanism | MHC isoform expression* | Ref. |
|---|---|---|---|
| Hormones: | |||
| Thyroid | |||
| Hyper. | TR | α-MHC ↑, β-MHC ↓ | [56] |
| Hypo | TR | β-MHC ↑, α-MHC ↓ | [59] |
| Diabetes | Reduced glycolytic flux | β-MHC ↑ | [87] |
| Gonadectomy | Reduced testosterone | β-MHC ↑, α-MHC ↓ | [93] |
| Adrenergic Drive: | |||
| Sympathectomy | Reduced catecholamines | α-MHC ↓ | [65] |
| Dobutamine | β2 AR/cAMP | α-MHC ↑ | [73] |
| Isoproterenol | β1 AR/Ca/CaMK | β-MHCa ↑ | [71] |
| Forskolin | cAMP | α-MHC ↑ | [66] |
| Norepinephrine | α1 AR/PK-c | β-MHC ↑ | [81] |
| Metabolism: | |||
| High fructose diet | ? | α-MHCb ↑ | [90] |
| Less FFA oxidation | ? | α-MHCb ↑ | [89] |
| Semi-starvation | ? | β-MHCb ↑ | [94] |
| Hypoxia | ? | β-MHCa ↑ | [96] |
| Function: | |||
| Exercise | Sympathetic drive/cAMP | α-MHC ↑ | [97] |
| Hemodynamic load | Multiple** | β-MHC ↑, α-MHC ↓ | [104] |
| Chromatin remodeling: | |||
| TrichostatinA | HDACs (Class I & II) inhibition | α-MHC ↑ | [129] |
Table 2.
Specific transcription factors implicated for regulation of α- and β-MHC gene promoter activity.
| Promoter | Transcription factor [reference] | Promoter activity |
|---|---|---|
| α-MHC | TRα1 [58], SRF[155], GATA4[156], MEF2 [157], TEF1[78], USF [79], Egr1[158]. | Activation |
| Ku70/Ku80 [8]*, YY1 [118]*, PURα/β [49], PARP1 [107]. | Repression | |
| β-MHC | TRβ1 [58]. | Repression |
| SRF [159]*, GATA4 [160], MEF2 [159]*, TEF1[82], PARP1 [137], AP1[161], NFAT3 [121, 124]. | Activation |
Acknowledgments
I thank Ms. Elisabeth Lanzl for critically reading the manuscript. These studies were supported by NIH RO1 grants HL-68083, HL77788 and HL83423 and AHA grant-in-aid N-150108.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spann J. Functional changes o in pathologic hypertrophy. In: Zak R, editor. Growth of the Heart in Health and Disease. New York: Raveen Press; 1984. pp. 421–466. [Google Scholar]
- 2.Hajjar RJ, Gwathmey JK. Cross-bridge dynamics in human ventricular myocardium. Regulation of contractility in the failing heart. Circulation. 1992;86:1819–26. doi: 10.1161/01.cir.86.6.1819. [DOI] [PubMed] [Google Scholar]
- 3.Mann DL, Urabe Y, Kent RL, Vinciguerra S, Cooper G. Cellular versus myocardial basis for the contractile dysfunction of hypertrophied myocardium. Circ Res. 1991;68:402–15. doi: 10.1161/01.res.68.2.402. [DOI] [PubMed] [Google Scholar]
- 4.Shroff SG, Motz W. Left ventricular systolic resistance in rats with hypertension and hypertrophy. Am J Physiol. 1989;257:H386–94. doi: 10.1152/ajpheart.1989.257.2.H386. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RL, Kawas RF, Pokrovskii MV, Godinez G, Lee JK, Mak J, et al. The cardaic myosin activator CK-1316719 increases myofibril ATPase activity and myocyte contractility in a rat model of heart failure. Circulation. 2006;114(suppl II):1440. [Google Scholar]
- 6.James J, Martin L, Krenz M, Quatman C, Jones F, Klevitsky R, et al. Forced expression of alpha-myosin heavy chain in the rabbit ventricle results in cardioprotection under cardiomyopathic conditions. Circulation. 2005;111:2339–46. doi: 10.1161/01.CIR.0000164233.09448.B1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res. 2000;86:386–90. doi: 10.1161/01.res.86.4.386. [DOI] [PubMed] [Google Scholar]
- 8.Sucharov CC, Helmke SM, Langer SJ, Perryman MB, Bristow M, Leinwand L. The Ku protein complex interacts with YY1, is up-regulated in human heart failure, and represses alpha myosin heavy-chain gene expression. Mol Cell Biol. 2004;24:8705–15. doi: 10.1128/MCB.24.19.8705-8715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braunwald E, Bristow MR. Congestive heart failure: fifty years of progress. Circulation. 2000;102:IV14–23. doi: 10.1161/01.cir.102.suppl_4.iv-14. [DOI] [PubMed] [Google Scholar]
- 10.Zak R. Contractile function as a determinant of muscle growth. Cell Muscle Motil. 1981;1:1–32. [Google Scholar]
- 11.Harrington WF, Rodgers ME. Myosin. Annu Rev Biochem. 1984;53:35–73. doi: 10.1146/annurev.bi.53.070184.000343. [DOI] [PubMed] [Google Scholar]
- 12.Mahdavi V, Chambers AP, Nadal-Ginard B. Cardiac alpha- and beta-myosin heavy chain genes are organized in tandem. Proc Natl Acad Sci U S A. 1984;81:2626–30. doi: 10.1073/pnas.81.9.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoh JF, McGrath PA, Hale PT. Electrophoretic analysis of multiple forms of rat cardiac myosin: effects of hypophysectomy and thyroxine replacement. J Mol Cell Cardiol. 1978;10:1053–76. doi: 10.1016/0022-2828(78)90401-7. [DOI] [PubMed] [Google Scholar]
- 14.Lyons GE, Schiaffino S, Sassoon D, Barton P, Buckingham M. Developmental regulation of myosin gene expression in mouse cardiac muscle. J Cell Biol. 1990;111:2427–36. doi: 10.1083/jcb.111.6.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lompre AM, Mercadier JJ, Wisnewsky C, Pantaloni P, d’Albis A, Schwartz K. Species and age-dependent changes in the relative amounts of cardiac myosin isoenzymes in mammals. Dev Biol. 1981;84:286. doi: 10.1016/0012-1606(81)90396-1. [DOI] [PubMed] [Google Scholar]
- 16.Lompre AM, Nadal-Ginard B, Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984;259:6437–46. [PubMed] [Google Scholar]
- 17.Clark WA, Jr, Chizzonite RA, Everett AW, Rabinowitz M, Zak R. Species correlations between cardiac isomyosins. A comparison of electrophoretic and immunological properties. J Biol Chem. 1982;257:5449–54. [PubMed] [Google Scholar]
- 18.Everett AW, Clark WA, Chizzonite RA, Zak R. Change in synthesis rates of alpha- and beta-myosin heavy chains in rabbit heart after treatment with thyroid hormone. J Biol Chem. 1983;258:2421–5. [PubMed] [Google Scholar]
- 19.Rupp H, Jacob R. Myocardial transition between fast and slow-type muscle as monitored by the population of myosin isoenzymes. In: Rupp H, editor. The Regulation of Heart Function. New York: Thieme Inc; 1986. pp. 271–291. [Google Scholar]
- 20.Herron TJ, Baghai M, Chaturvedi R, Kentish J. Age dependence of isometric crossbridge cycling and myosin heavy chain isoform expression in human ventricular myocytes (abstract) Biophys J. 2005;88:482a. [Google Scholar]
- 21.Eisenberg BR, Edwards JA, Zak R. Transmural distribution of isomyosin in rabbit ventricle during maturation examined by immunofluorescence and staining for calcium-activated adenosine triphosphatase. Circ Res. 1985;56:548–55. doi: 10.1161/01.res.56.4.548. [DOI] [PubMed] [Google Scholar]
- 22.Bugaisky LB, Anderson PG, Hall RS, Bishop SP. Differences in myosin isoform expression in the subepicardial and subendocardial myocardium during cardiac hypertrophy in the rat. Circ Res. 1990;66:1127–32. doi: 10.1161/01.res.66.4.1127. [DOI] [PubMed] [Google Scholar]
- 23.Alpert NR, Brosseau C, Federico A, Krenz M, Robbins J, Warshaw DM. Molecular mechanics of mouse cardiac myosin isoforms. Am J Physiol Heart Circ Physiol. 2002;283:H1446–54. doi: 10.1152/ajpheart.00274.2002. [DOI] [PubMed] [Google Scholar]
- 24.Barany M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967;50(Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herron TJ, McDonald KS. Small amounts of alpha-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res. 2002;90:1150–2. doi: 10.1161/01.res.0000022879.57270.11. [DOI] [PubMed] [Google Scholar]
- 26.Korte FS, Herron TJ, Rovetto MJ, McDonald KS. Power output is linearly related to MyHC content in rat skinned myocytes and isolated working hearts. Am J Physiol Heart Circ Physiol. 2005;289:H801–12. doi: 10.1152/ajpheart.01227.2004. [DOI] [PubMed] [Google Scholar]
- 27.Pagani ED, Julian FJ. Rabbit papillary muscle myosin isozymes and the velocity of muscle shortening. Circ Res. 1984;54:586–94. doi: 10.1161/01.res.54.5.586. [DOI] [PubMed] [Google Scholar]
- 28.VanBuren P, Harris DE, Alpert NR, Warshaw DM. Cardiac V1 and V3 myosins differ in their hydrolytic and mechanical activities in vitro. Circ Res. 1995;77:439–44. doi: 10.1161/01.res.77.2.439. [DOI] [PubMed] [Google Scholar]
- 29.Sugiura S, Kobayakawa N, Fujita H, Momomura S, Chaen S, Sugi H. Distinct kinetic properties of cardiac myosin isoforms revealed by in vitro studies. Adv Exp Med Biol. 1998;453:125–30. doi: 10.1007/978-1-4684-6039-1_15. [DOI] [PubMed] [Google Scholar]
- 30.Sugiura S, Kobayakawa N, Fujita H, Yamashita H, Momomura S, Chaen S, et al. Comparison of unitary displacements and forces between 2 cardiac myosin isoforms by the optical trap technique: molecular basis for cardiac adaptation. Circ Res. 1998;82:1029–34. doi: 10.1161/01.res.82.10.1029. [DOI] [PubMed] [Google Scholar]
- 31.Krenz M, Sanbe A, Bouyer-Dalloz F, Gulick J, Klevitsky R, Hewett TE, et al. Analysis of myosin heavy chain functionality in the heart. J Biol Chem. 2003;278:17466–74. doi: 10.1074/jbc.M210804200. [DOI] [PubMed] [Google Scholar]
- 32.Krenz M, Robbins J. Impact of beta-myosin heavy chain expression on cardiac function during stress. J Am Coll Cardiol. 2004;44:2390–7. doi: 10.1016/j.jacc.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 33.Kurabayashi M, Tsuchimochi H, Komuro I, Takaku F, Yazaki Y. Molecular cloning and characterization of human cardiac alpha- and beta-form myosin heavy chain complementary DNA clones. Regulation of expression during development and pressure overload in human atrium. J Clin Invest. 1988;82:524–31. doi: 10.1172/JCI113627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saez LJ, Gianola KM, McNally EM, Feghali R, Eddy R, Shows TB, et al. Human cardiac myosin heavy chain genes and their linkage in the genome. Nucleic Acids Res. 1987;15:5443–59. doi: 10.1093/nar/15.13.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haddad F, Bodell PW, Qin AX, Giger JM, Baldwin KM. Role of antisense RNA in coordinating cardiac myosin heavy chain gene switching. J Biol Chem. 2003;278:37132–8. doi: 10.1074/jbc.M305911200. [DOI] [PubMed] [Google Scholar]
- 36.Haddad F, Qin AX, Bodell PW, Zhang LY, Guo H, Giger JM, et al. Regulation of antisense RNA expression during cardiac MHC gene switching in response to pressure overload. Am J Physiol Heart Circ Physiol. 2006;290:H2351–61. doi: 10.1152/ajpheart.01111.2005. [DOI] [PubMed] [Google Scholar]
- 37.Luther HP, Morwinski R, Wallukat G, Haase H, Morano I. Expression of sense and naturally occurring antisense mRNA of myosin heavy chain in rat heart tissue and cultivated cardiomyocytes. J Mol Cell Cardiol. 1997;29:27–35. doi: 10.1006/jmcc.1996.0248. [DOI] [PubMed] [Google Scholar]
- 38.Pandorf CE, Haddad F, Roy RR, Qin AX, Edgerton VR, Baldwin KM. Dynamics of myosin heavy chain gene regulation in slow skeletal muscle: role of natural antisense RNA. J Biol Chem. 2006;281:38330–42. doi: 10.1074/jbc.M607249200. [DOI] [PubMed] [Google Scholar]
- 39.Chen CY, Schwartz RJ. Recruitment of the tinman homolog Nkx-2.5 by serum response factor activates cardiac alpha-actin gene transcription. Mol Cell Biol. 1996;16:6372–84. doi: 10.1128/mcb.16.11.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Z, Friedrich GA, Soriano P. Transcriptional enhancer factor 1 disruption by a retroviral gene trap leads to heart defects and embryonic lethality in mice. Genes Dev. 1994;8:2293–301. doi: 10.1101/gad.8.19.2293. [DOI] [PubMed] [Google Scholar]
- 41.Gupta M, Kogut P, Davis FJ, Belaguli NS, Schwartz RJ, Gupta MP. Physical interaction between the MADS box of serum response factor and the TEA/ATTS DNA-binding domain of transcription enhancer factor-1. J Biol Chem. 2001;276:10413–22. doi: 10.1074/jbc.M008625200. [DOI] [PubMed] [Google Scholar]
- 42.Lin Q, Schwarz J, Bucana C, Olson EN. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276:1404–7. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maeda T, Gupta MP, Stewart AF. TEF-1 and MEF2 transcription factors interact to regulate muscle-specific promoters. Biochem Biophys Res Commun. 2002;294:791–7. doi: 10.1016/S0006-291X(02)00556-9. [DOI] [PubMed] [Google Scholar]
- 44.Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–72. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 45.Sepulveda JL, Belaguli N, Nigam V, Chen CY, Nemer M, Schwartz RJ. GATA-4 and Nkx-2.5 coactivate Nkx-2 DNA binding targets: role for regulating early cardiac gene expression. Mol Cell Biol. 1998;18:3405–15. doi: 10.1128/mcb.18.6.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka M, Chen Z, Bartunkova S, Yamasaki N, Izumo S. The cardiac homeobox gene Csx/Nkx2.5 lies genetically upstream of multiple genes essential for heart development. Development. 1999;126:1269–80. doi: 10.1242/dev.126.6.1269. [DOI] [PubMed] [Google Scholar]
- 47.Balza RO, Jr, Misra RP. Role of the serum response factor in regulating contractile apparatus gene expression and sarcomeric integrity in cardiomyocytes. J Biol Chem. 2006;281:6498–510. doi: 10.1074/jbc.M509487200. [DOI] [PubMed] [Google Scholar]
- 48.Gupta M, Zak R, Libermann TA, Gupta MP. Tissue-restricted expression of the cardiac alpha-myosin heavy chain gene is controlled by a downstream repressor element containing a palindrome of two ets-binding sites. Mol Cell Biol. 1998;18:7243–58. doi: 10.1128/mcb.18.12.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta M, Sueblinvong V, Gupta MP. Single strand DNA/RNA-binding, Pur-B regulates serum rersponse fcator (SRF) medaited cardiac muscle gene expression. Can Physiool Pharmacol. 2006 doi: 10.1139/y07-009. in press. [DOI] [PubMed] [Google Scholar]
- 50.Aikawa R, Huggins GS, Snyder RO. Cardiomyocyte-specific gene expression following recombinant adeno-associated viral vector transduction. J Biol Chem. 2002;277:18979–85. doi: 10.1074/jbc.M201257200. [DOI] [PubMed] [Google Scholar]
- 51.Gupta M, Sueblinvong V, Raman J, Jeevanandam V, Gupta MP. Single-stranded DNA-binding proteins PURalpha and PURbeta bind to a purine-rich negative regulatory element of the alpha-myosin heavy chain gene and control transcriptional and translational regulation of the gene expression. Implications in the repression of alpha-myosin heavy chain during heart failure. J Biol Chem. 2003;278:44935–48. doi: 10.1074/jbc.M307696200. [DOI] [PubMed] [Google Scholar]
- 52.Ji J, Tsika GL, Rindt H, Schreiber KL, McCarthy JJ, Kelm RJ, Jr, et al. Pur{alpha} and Pur{beta} Collaborate with Sp3 To Negatively Regulate {beta}-Myosin Heavy Chain Gene Expression during Skeletal Muscle Inactivity. Mol Cell Biol. 2007;27:1531–43. doi: 10.1128/MCB.00629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Izumo S, Nadal-Ginard B, Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986;231:597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- 54.Morkin E. Regulation of myosin heavy chain genes in the heart. Circulation. 1993;87:1451–60. doi: 10.1161/01.cir.87.5.1451. [DOI] [PubMed] [Google Scholar]
- 55.Burnside J, Darling DS, Chin WW. A nuclear factor that enhances binding of thyroid hormone receptors to thyroid hormone response elements. J Biol Chem. 1990;265:2500–4. [PubMed] [Google Scholar]
- 56.Izumo S, Mahdavi V. Thyroid hormone receptor alpha isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature. 1988;334:539–42. doi: 10.1038/334539a0. [DOI] [PubMed] [Google Scholar]
- 57.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–9. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 58.Kinugawa K, Yonekura K, Ribeiro RC, Eto Y, Aoyagi T, Baxter JD, et al. Regulation of thyroid hormone receptor isoforms in physiological and pathological cardiac hypertrophy. Circ Res. 2001;89:591–8. doi: 10.1161/hh1901.096706. [DOI] [PubMed] [Google Scholar]
- 59.Flink IL, Morkin E. Interaction of thyroid hormone receptors with strong and weak cis-acting elements in the human alpha-myosin heavy chain gene promoter. J Biol Chem. 1990;265:11233–7. [PubMed] [Google Scholar]
- 60.Tsika RW, Bahl JJ, Leinwand LA, Morkin E. Thyroid hormone regulates expression of a transfected human alpha-myosin heavy-chain fusion gene in fetal rat heart cells. Proc Natl Acad Sci U S A. 1990;87:379–83. doi: 10.1073/pnas.87.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baniahmad A, Steiner C, Kohne AC, Renkawitz R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990;61:505–14. doi: 10.1016/0092-8674(90)90532-j. [DOI] [PubMed] [Google Scholar]
- 62.Chatterjee VK, Lee JK, Rentoumis A, Jameson JL. Negative regulation of the thyroid-stimulating hormone alpha gene by thyroid hormone: receptor interaction adjacent to the TATA box. Proc Natl Acad Sci U S A. 1989;86:9114–8. doi: 10.1073/pnas.86.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–95. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Advani SV, Malhotra A, Liang D, Geenen DL, Buttrick PM, Scheuer J. Swimming alternates the shift in myosin isozymes in the rat heterotopic cardiac isograft. Circulation. 1989;80(suppl II):1185. [Google Scholar]
- 65.Dowell RT. Myocardial contractile function and myofibrillar adenosine triphosphatase activity in chemically sympathectomized rats. Circ Res. 1976;39:683–9. doi: 10.1161/01.res.39.5.683. [DOI] [PubMed] [Google Scholar]
- 66.Gupta MP, Gupta M, Stewart A, Zak R. Activation of alpha-myosin heavy chain gene expression by cAMP in cultured fetal rat heart myocytes. Biochem Biophys Res Commun. 1991;174:1196–203. doi: 10.1016/0006-291x(91)91548-q. [DOI] [PubMed] [Google Scholar]
- 67.Gupta MP, Gupta M, Zak R. An E-box/M-CAT hybrid motif and cognate binding protein(s) regulate the basal muscle-specific and cAMP-inducible expression of the rat cardiac alpha-myosin heavy chain gene. J Biol Chem. 1994;269:29677–87. [PubMed] [Google Scholar]
- 68.Kawana M, Ischizuka S, Taria A, Kimata S, Hosoda S. Effect of cardiac sympathetic nerve activity on myosin isoenzymes of rabbit heart. Circulation. 1989;89(Suppl II):1893. [Google Scholar]
- 69.Kawana M, Kimata SI, Hosoda S. The change in expression of cardiac myosin isoenzymes by the stimulation of the sympathetic nerve and thyroxin. In: Hagano NtaNSD H., editor. The Adapted Heart. New York: Raven Press; 1994. pp. 393–401. [Google Scholar]
- 70.Pauletto P, Dalla Libera L, Vescovo G, Scannapieco G, Angelini A, Pessina AC, et al. Propranolol-induced changes in ventricular isomyosin composition in the rat. Am Heart J. 1985;109:1269–73. doi: 10.1016/0002-8703(85)90350-3. [DOI] [PubMed] [Google Scholar]
- 71.Sucharov CC, Mariner PD, Nunley KR, Long C, Leinwand L, Bristow MR. A beta1-adrenergic receptor CaM kinase II-dependent pathway mediates cardiac myocyte fetal gene induction. Am J Physiol Heart Circ Physiol. 2006;291:H1299–308. doi: 10.1152/ajpheart.00017.2006. [DOI] [PubMed] [Google Scholar]
- 72.Winegrad S, McClellan G, Tucker M, Lin LE. Cyclic AMP regulation of myosin isozymes in mammalian cardiac muscle. J Gen Physiol. 1983;81:749–65. doi: 10.1085/jgp.81.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winegrad S, McClellan G, Weisberg A, Lin LE, Weindling S, Horowits R. Beta-adrenergic regulation of cardiac myosin. Can J Physiol Pharmacol. 1987;65:606–9. doi: 10.1139/y87-102. [DOI] [PubMed] [Google Scholar]
- 74.Winegrad S, Weisberg A. Isozyme specific modification of myosin ATPase by cAMP in rat heart. Circ Res. 1987;60:384–92. doi: 10.1161/01.res.60.3.384. [DOI] [PubMed] [Google Scholar]
- 75.Winegrad S, Weisberg A, Lin LE, McClellan G. Adrenergic regulation of myosin adenosine triphosphatase activity. Circ Res. 1986;58:83–95. doi: 10.1161/01.res.58.1.83. [DOI] [PubMed] [Google Scholar]
- 76.Waspe LE, Ordahl CP, Simpson PC. The cardiac beta-myosin heavy chain isogene is induced selectively in alpha 1-adrenergic receptor-stimulated hypertrophy of cultured rat heart myocytes. J Clin Invest. 1990;85:1206–14. doi: 10.1172/JCI114554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gustafson TA, Bahl JJ, Markham BE, Roeske WR, Morkin E. Hormonal regulation of myosin heavy chain and alpha-actin gene expression in cultured fetal rat heart myocytes. J Biol Chem. 1987;262:13316–22. [PubMed] [Google Scholar]
- 78.Gupta MP, Amin CS, Gupta M, Hay N, Zak R. Transcription enhancer factor 1 interacts with a basic helix-loop-helix zipper protein, Max, for positive regulation of cardiac alpha-myosin heavy-chain gene expression. Mol Cell Biol. 1997;17:3924–36. doi: 10.1128/mcb.17.7.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ojamaa K, Samarel AM, Klein I. Identification of a contractile-responsive element in the cardiac alpha-myosin heavy chain gene. J Biol Chem. 1995;270:31276–81. doi: 10.1074/jbc.270.52.31276. [DOI] [PubMed] [Google Scholar]
- 80.Gupta MP, Kogut P, Gupta M. Protein kinase-A dependent phosphorylation of transcription enhancer factor-1 represses its DNA-binding activity but enhances its gene activation ability. Nucleic Acids Res. 2000;28:3168–77. doi: 10.1093/nar/28.16.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kariya K, Karns LR, Simpson PC. An enhancer core element mediates stimulation of the rat beta-myosin heavy chain promoter by an alpha 1-adrenergic agonist and activated beta-protein kinase C in hypertrophy of cardiac myocytes. J Biol Chem. 1994;269:3775–82. [PubMed] [Google Scholar]
- 82.Kariya K, Farrance IK, Simpson PC. Transcriptional enhancer factor-1 in cardiac myocytes interacts with an alpha 1-adrenergic- and beta-protein kinase C-inducible element in the rat beta-myosin heavy chain promoter. J Biol Chem. 1993;268:26658–62. [PubMed] [Google Scholar]
- 83.Stewart AF, Larkin SB, Farrance IK, Mar JH, Hall DE, Ordahl CP. Muscle-enriched TEF-1 isoforms bind M-CAT elements from muscle-specific promoters and differentially activate transcription. J Biol Chem. 1994;269:3147–50. [PubMed] [Google Scholar]
- 84.Karin M. Complexities of gene regulation by cAMP. Trends Genet. 1989;5:65–7. doi: 10.1016/0168-9525(89)90027-9. [DOI] [PubMed] [Google Scholar]
- 85.Huang MH, Friend DS, Sunday ME, Singh K, Haley K, Austen KF, et al. An intrinsic adrenergic system in mammalian heart. J Clin Invest. 1996;98:1298–1303. doi: 10.1172/JCI118916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, et al. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci U S A. 1996;93:12974–9. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dillmann WH. Influence of thyroid hormone administration on myosin ATPase activity and myosin isoenzyme distribution in the heart of diabetic rats. Metabolism. 1982;31:199–204. doi: 10.1016/0026-0495(82)90052-x. [DOI] [PubMed] [Google Scholar]
- 88.Dillmann WH. Fructose feeding increases Ca++-activated myosin ATPase activity and changes myosin isoenzyme distribution in the diabetic rat heart. Endocrinology. 1984;114:1678–85. doi: 10.1210/endo-114-5-1678. [DOI] [PubMed] [Google Scholar]
- 89.Dillmann WH. Methyl palmoxirate increases Ca2+-myosin ATPase activity and changes myosin isoenzyme distribution in the diabetic rat heart. Am J Physiol. 1985;248:E602–6. doi: 10.1152/ajpendo.1985.248.5.E602. [DOI] [PubMed] [Google Scholar]
- 90.Dillmann WH. Myosin isoenzyme distribution and Ca+2-activated myosin ATPase activity in the rat heart is influenced by fructose feeding and triiodothyronine. Endocrinology. 1985;116:2160–6. doi: 10.1210/endo-116-6-2160. [DOI] [PubMed] [Google Scholar]
- 91.Sheer D, Morkin E. Myosin isoenzyme expression in rat ventricle: effects of thyroid hormone analogs, catecholamines, glucocorticoids and high carbohydrate diet. J Pharmacol Exp Ther. 1984;229:872–9. [PubMed] [Google Scholar]
- 92.Jordan JE, Simandle SA, Tulbert CD, Busija DW, Miller AW. Fructose-fed rats are protected against ischemia/reperfusion injury. J Pharmacol Exp Ther. 2003;307:1007–11. doi: 10.1124/jpet.103.055970. [DOI] [PubMed] [Google Scholar]
- 93.Morano I, Gerstner J, Ruegg JC, Ganten U, Ganten D, Vosberg HP. Regulation of myosin heavy chain expression in the hearts of hypertensive rats by testosterone. Circ Res. 1990;66:1585–90. doi: 10.1161/01.res.66.6.1585. [DOI] [PubMed] [Google Scholar]
- 94.Dillmann WH, Berry S, Alexander NM. A physiological dose of triiodothyronine normalizes cardiac myosin adenosine triphosphatase activity and changes myosin isoenzyme distribution in semistarved rats. Endocrinology. 1983;112:2081–7. doi: 10.1210/endo-112-6-2081. [DOI] [PubMed] [Google Scholar]
- 95.Dowell RT, Martin AF. Perinatal nutritional modification of weanling rat heart contractile protein. Am J Physiol. 1984;247:H967–72. doi: 10.1152/ajpheart.1984.247.6.H967. [DOI] [PubMed] [Google Scholar]
- 96.Razeghi P, Essop MF, Huss JM, Abbasi S, Manga N, Taegtmeyer H. Hypoxia-induced switches of myosin heavy chain iso-gene expression in rat heart. Biochem Biophys Res Commun. 2003;303:1024–7. doi: 10.1016/s0006-291x(03)00478-9. [DOI] [PubMed] [Google Scholar]
- 97.Rupp H. Differential effect of physical exercise routines on ventricular myosin and peripheral catecholamine stores in normotensive and spontaneously hypertensive rats. Circ Res. 1989;65:370–7. doi: 10.1161/01.res.65.2.370. [DOI] [PubMed] [Google Scholar]
- 98.Rupp H, Felbier HR, Bukhari AR, Jacob R. Modulation of myosin isoenzyme populations and activities of monoamine oxidase and phenylethanolamine-N-methyltransferase in pressure loaded and normal rat heart by swimming exercise and stress arising from electrostimulation in pairs. Can J Physiol Pharmacol. 1984;62:1209–18. doi: 10.1139/y84-202. [DOI] [PubMed] [Google Scholar]
- 99.Rupp H, Jacob R. Response of blood pressure and cardiac myosin polymorphism to swimming training in the spontaneously hypertensive rat. Can J Physiol Pharmacol. 1982;60:1098–103. doi: 10.1139/y82-158. [DOI] [PubMed] [Google Scholar]
- 100.Scheuer J, Malhotra A, Hirsch C, Capasso J, Schaible TF. Physiologic cardiac hypertrophy corrects contractile protein abnormalities associated with pathologic hypertrophy in rats. J Clin Invest. 1982;70:1300–5. doi: 10.1172/JCI110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koziris LP, Hickson RC, Chatterton RT, Jr, Groseth RT, Christie JM, Goldflies DG, et al. Serum levels of total and free IGF-I and IGFBP-3 are increased and maintained in long-term training. J Appl Physiol. 1999;86:1436–42. doi: 10.1152/jappl.1999.86.4.1436. [DOI] [PubMed] [Google Scholar]
- 102.Neri Serneri GG, Boddi M, Modesti PA, Cecioni I, Coppo M, Padeletti L, et al. Increased cardiac sympathetic activity and insulin-like growth factor-I formation are associated with physiological hypertrophy in athletes. Circ Res. 2001;89:977–82. doi: 10.1161/hh2301.100982. [DOI] [PubMed] [Google Scholar]
- 103.Donath MY, Gosteli-Peter MA, Hauri C, Froesch ER, Zapf J. Insulin-like growth factor-I stimulates myofibrillar genes and modulates atrial natriuretic factor mRNA in rat heart. Eur J Endocrinol. 1997;137:309–15. doi: 10.1530/eje.0.1370309. [DOI] [PubMed] [Google Scholar]
- 104.Litten RZ, 3rd, Martin BJ, Low RB, Alpert NR. Altered myosin isozyme patterns from pressure-overloaded and thyrotoxic hypertrophied rabbit hearts. Circ Res. 1982;50:856–64. doi: 10.1161/01.res.50.6.856. [DOI] [PubMed] [Google Scholar]
- 105.Lompre AM, Schwartz K, d’Albis A, Lacombe G, Van Thiem N, Swynghedauw B. Myosin isoenzyme redistribution in chronic heart overload. Nature. 1979;282:105–7. doi: 10.1038/282105a0. [DOI] [PubMed] [Google Scholar]
- 106.Mercadier JJ, Lompre AM, Wisnewsky C, Samuel JL, Bercovici J, Swynghedauw B, et al. Myosin isoenzyme changes in several models of rat cardiac hypertrophy. Circ Res. 1981;49:525–32. doi: 10.1161/01.res.49.2.525. [DOI] [PubMed] [Google Scholar]
- 107.Pillai JB, Russell HM, Raman J, Jeevanandam V, Gupta MP. Increased expression of poly(ADP-ribose) polymerase-1 contributes to caspase-independent myocyte cell death during heart failure. Am J Physiol Heart Circ Physiol. 2005;288:H486–96. doi: 10.1152/ajpheart.00437.2004. [DOI] [PubMed] [Google Scholar]
- 108.Chang KC, Figueredo VM, Schreur JH, Kariya K, Weiner MW, Simpson PC, et al. Thyroid hormone improves function and Ca2+ handling in pressure overload hypertrophy. Association with increased sarcoplasmic reticulum Ca2+-ATPase and alpha-myosin heavy chain in rat hearts. J Clin Invest. 1997;100:1742–9. doi: 10.1172/JCI119699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Noguchi T, Camp P, Jr, Alix SL, Gorga JA, Begin KJ, Leavitt BJ, et al. Myosin from failing and non-failing human ventricles exhibit similar contractile properties. J Mol Cell Cardiol. 2003;35:91–7. doi: 10.1016/s0022-2828(02)00282-1. [DOI] [PubMed] [Google Scholar]
- 110.Herron TJ, Korte FS, McDonald KS. Loaded shortening and power output in cardiac myocytes are dependent on myosin heavy chain isoform expression. Am J Physiol Heart Circ Physiol. 2001;281:H1217–22. doi: 10.1152/ajpheart.2001.281.3.H1217. [DOI] [PubMed] [Google Scholar]
- 111.deGoma EM, Vagelos RH, Fowler MB, Ashley EA. Emerging therapies for the management of decompensated heart failure: from bench to bedside. J Am Coll Cardiol. 2006;48:2397–409. doi: 10.1016/j.jacc.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 112.Carniel E, Taylor MR, Sinagra G, Di Lenarda A, Ku L, Fain PR, et al. Alpha-myosin heavy chain: a sarcomeric gene associated with dilated and hypertrophic phenotypes of cardiomyopathy. Circulation. 2005;112:54–9. doi: 10.1161/CIRCULATIONAHA.104.507699. [DOI] [PubMed] [Google Scholar]
- 113.Gupta M, Zak R. Reversibility of load-induced changes in myosin heavy chain gene expression. Am J Physiol. 1992;262:R346–9. doi: 10.1152/ajpregu.1992.262.3.R346. [DOI] [PubMed] [Google Scholar]
- 114.Wiesner RJ, Ehmke H, Faulhaber J, Zak R, Ruegg JC. Dissociation of left ventricular hypertrophy, beta-myosin heavy chain gene expression, and myosin isoform switch in rats after ascending aortic stenosis. Circulation. 1997;95:1253–9. doi: 10.1161/01.cir.95.5.1253. [DOI] [PubMed] [Google Scholar]
- 115.Qi M, Puglisi JL, Byron KL, Ojamaa K, Klein I, Bers DM, et al. Myosin heavy chain gene expression in neonatal rat heart cells: effects of [Ca2+]i and contractile activity. Am J Physiol. 1997;273:C394–403. doi: 10.1152/ajpcell.1997.273.2.C394. [DOI] [PubMed] [Google Scholar]
- 116.Nikcevic G, Heidkamp MC, Perhonen M, Russell B. Mechanical activity in heart regulates translation of alpha-myosin heavy chain mRNA but not its localization. Am J Physiol. 1999;276:H2013–9. doi: 10.1152/ajpheart.1999.276.6.H2013. [DOI] [PubMed] [Google Scholar]
- 117.Morkin E, Pennock G, Spooner PH, Bahl JJ, Underhill Fox K, Goldman S. Pilot studies on the use of 3,5-diiodothyropropionic acid, a thyroid hormone analog, in the treatment of congestive heart failure. Cardiology. 2002;97:218–25. doi: 10.1159/000063110. [DOI] [PubMed] [Google Scholar]
- 118.Mariner PD, Luckey SW, Long CS, Sucharov CC, Leinwand LA. Yin Yang 1 represses alpha-myosin heavy chain gene expression in pathologic cardiac hypertrophy. Biochem Biophys Res Commun. 2005;326:79–86. doi: 10.1016/j.bbrc.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 119.Oei SL, Griesenbeck J, Schweiger M, Babich V, Kropotov A, Tomilin N. Interaction of the transcription factor YY1 with human poly(ADP-ribosyl) transferase. Biochem Biophys Res Commun. 1997;240:108–11. doi: 10.1006/bbrc.1997.7621. [DOI] [PubMed] [Google Scholar]
- 120.Zambrano N, De Renzis S, Minopoli G, Faraonio R, Donini V, Scaloni A, et al. DNA-binding protein Pur alpha and transcription factor YY1 function as transcription activators of the neuron-specific FE65 gene promoter. Biochem J. 1997;328(Pt 1):293–300. doi: 10.1042/bj3280293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–28. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stewart AF, Suzow J, Kubota T, Ueyama T, Chen HH. Transcription factor RTEF-1 mediates alpha1-adrenergic reactivation of the fetal gene program in cardiac myocytes. Circ Res. 1998;83:43–9. doi: 10.1161/01.res.83.1.43. [DOI] [PubMed] [Google Scholar]
- 123.Bisping E, Ikeda S, Kong SW, Tarnavski O, Bodyak N, McMullen JR, et al. Gata4 is required for maintenance of postnatal cardiac function and protection from pressure overload-induced heart failure. Proc Natl Acad Sci U S A. 2006;103:14471–6. doi: 10.1073/pnas.0602543103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McLean BG, Lee KS, Simpson PC, Farrance IK. Basal and alpha1-adrenergic-induced activity of minimal rat betaMHC promoters in cardiac myocytes requires multiple TEF-1 but not NFAT binding sites. J Mol Cell Cardiol. 2003;35:461–71. doi: 10.1016/s0022-2828(03)00049-x. [DOI] [PubMed] [Google Scholar]
- 125.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–49. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98:15–24. doi: 10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]
- 127.Davis FJ, Gupta M, Camoretti-Mercado B, Schwartz RJ, Gupta MP. Calcium/calmodulin-dependent protein kinase activates serum response factor transcription activity by its dissociation from histone deacetylase, HDAC4. Implications in cardiac muscle gene regulation during hypertrophy. J Biol Chem. 2003;278:20047–58. doi: 10.1074/jbc.M209998200. [DOI] [PubMed] [Google Scholar]
- 128.McKinsey TA, Zhang CL, Olson EN. Activation of the myocyte enhancer factor-2 transcription factor by calcium/calmodulin-dependent protein kinase-stimulated binding of 14-3-3 to histone deacetylase 5. Proc Natl Acad Sci U S A. 2000;97:14400–5. doi: 10.1073/pnas.260501497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Davis FJ, Pillai JB, Gupta M, Gupta MP. Concurrent opposite effects of trichostatin A, an inhibitor of histone deacetylases, on expression of alpha-MHC and cardiac tubulins: implication for gain in cardiac muscle contractility. Am J Physiol Heart Circ Physiol. 2005;288:H1477–90. doi: 10.1152/ajpheart.00789.2004. [DOI] [PubMed] [Google Scholar]
- 130.Kong Y, Tannous P, Lu G, Berenji K, Rothermel BA, Olson EN, et al. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation. 2006;113:2579–88. doi: 10.1161/CIRCULATIONAHA.106.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell. 2002;110:479–88. doi: 10.1016/s0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Danzi S, Dubon P, Klein I. Effect of serum triiodothyronine on regulation of cardiac gene expression: role of histone acetylation. Am J Physiol Heart Circ Physiol. 2005;289:H1506–11. doi: 10.1152/ajpheart.00182.2005. [DOI] [PubMed] [Google Scholar]
- 133.Wu Y, Koenig RJ. Gene regulation by thyroid hormone. Trends Endocrinol Metab. 2000;11:207–11. doi: 10.1016/s1043-2760(00)00263-0. [DOI] [PubMed] [Google Scholar]
- 134.Pacher P, Schulz R, Liaudet L, Szabo C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci. 2005;26:302–10. doi: 10.1016/j.tips.2005.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pillai JB, Gupta M, Rajamohan SB, Lang R, Raman J, Gupta MP. Poly(ADP-ribose) polymerase-1-deficient mice are protected from angiotensin II-induced cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2006;291:H1545–53. doi: 10.1152/ajpheart.01124.2005. [DOI] [PubMed] [Google Scholar]
- 136.Butler AJ, Ordahl CP. Poly(ADP-ribose) polymerase binds with transcription enhancer factor 1 to MCAT1 elements to regulate muscle-specific transcription. Mol Cell Biol. 1999;19:296–306. doi: 10.1128/mcb.19.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vyas DR, McCarthy JJ, Tsika GL, Tsika RW. Multiprotein complex formation at the beta myosin heavy chain distal muscle CAT element correlates with slow muscle expression but not mechanical overload responsiveness. J Biol Chem. 2001;276:1173–84. doi: 10.1074/jbc.M007750200. [DOI] [PubMed] [Google Scholar]
- 138.Xiao CY, Chen M, Zsengeller Z, Li H, Kiss L, Kollai M, et al. Poly(ADP-Ribose) polymerase promotes cardiac remodeling, contractile failure and translocation of apoptosis-inducing factor in a murine experimental model of aortic banding and heart failure. J Pharmacol Exp Ther. 2005;312:891–8. doi: 10.1124/jpet.104.077164. [DOI] [PubMed] [Google Scholar]
- 139.Pillai JB, Isbatan A, Imai S, Gupta MP. Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem. 2005;280:43121–30. doi: 10.1074/jbc.M506162200. [DOI] [PubMed] [Google Scholar]
- 140.Cohen-Armon M, Visochek L, Rozensal D, Kalal A, Geistrikh I, Klein R, et al. DNA-independent PARP-1 activation by phosphorylated ERK2 increases Elk1 activity: a link to histone acetylation. Mol Cell. 2007;25:297–308. doi: 10.1016/j.molcel.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 141.Homburg S, Visochek L, Moran N, Dantzer F, Priel E, Asculai E, et al. A fast signal-induced activation of Poly(ADP-ribose) polymerase: a novel downstream target of phospholipase c. J Cell Biol. 2000;150:293–307. doi: 10.1083/jcb.150.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hassa PO, Haenni SS, Buerki C, Meier NI, Lane WS, Owen H, et al. Acetylation of poly(ADP-ribose) polymerase-1 by p300/CREB-binding protein regulates coactivation of NF-kappaB-dependent transcription. J Biol Chem. 2005;280:40450–64. doi: 10.1074/jbc.M507553200. [DOI] [PubMed] [Google Scholar]
- 143.Poirier GG, de Murcia G, Jongstra-Bilen J, Niedergang C, Mandel P. Poly(ADP-ribosyl)ation of polynucleosomes causes relaxation of chromatin structure. Proc Natl Acad Sci U S A. 1982;79:3423–7. doi: 10.1073/pnas.79.11.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen IY, Lypowy J, Pain J, Sayed D, Grinberg S, Alcendor RR, et al. Histone H2A.z is essential for cardiac myocyte hypertrophy but opposed by silent information regulator 2alpha. J Biol Chem. 2006;281:19369–77. doi: 10.1074/jbc.M601443200. [DOI] [PubMed] [Google Scholar]
- 145.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–28. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 146.Szabo G, Liaudet L, Hagl S, Szabo C. Poly(ADP-ribose) polymerase activation in the reperfused myocardium. Cardiovasc Res. 2004;61:471–80. doi: 10.1016/j.cardiores.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 147.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–50. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 148.Lagos-Quintana M, Rauhut R, Meyer J, Borkhardt A, Tuschl T. New microRNAs from mouse and human. Rna. 2003;9:175–9. doi: 10.1261/rna.2146903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a MicroRNA. Science. 2007;316:575–9. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 150.Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–35. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- 151.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–2. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 152.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. Embo J. 2004;23:2369–80. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang J. Are poly(ADP-ribosyl)ation by PARP-1 and deacetylation by Sir2 linked? Bioessays. 2003;25:808–14. doi: 10.1002/bies.10317. [DOI] [PubMed] [Google Scholar]
- 154.Everett AW, Sinha AM, Umeda PK, Jakovcic S, Rabinowitz M, Zak R. Regulation of myosin synthesis by thyroid hormone: relative change in the alpha- and beta-myosin heavy chain mRNA levels in rabbit heart. Biochemistry. 1984;23:1596–9. doi: 10.1021/bi00303a002. [DOI] [PubMed] [Google Scholar]
- 155.Molkentin JD, Jobe SM, Markham BE. Alpha-myosin heavy chain gene regulation: delineation and characterization of the cardiac muscle-specific enhancer and muscle-specific promoter. J Mol Cell Cardiol. 1996;28:1211–25. doi: 10.1006/jmcc.1996.0112. [DOI] [PubMed] [Google Scholar]
- 156.Molkentin JD, Kalvakolanu DV, Markham BE. Transcription factor GATA-4 regulates cardiac muscle-specific expression of the alpha-myosin heavy-chain gene. Mol Cell Biol. 1994;14:4947–57. doi: 10.1128/mcb.14.7.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Molkentin JD, Markham BE. Myocyte-specific enhancer-binding factor (MEF-2) regulates alpha-cardiac myosin heavy chain gene expression in vitro and in vivo. J Biol Chem. 1993;268:19512–20. [PubMed] [Google Scholar]
- 158.Gupta MP, Gupta M, Zak R, Sukhatme VP. Egr-1, a serum-inducible zinc finger protein, regulates transcription of the rat cardiac alpha-myosin heavy chain gene. J Biol Chem. 1991;266:12813–6. [PubMed] [Google Scholar]
- 159.Huang WY, Chen JJ, Shih N, Liew CC. Multiple muscle-specific regulatory elements are associated with a DNase I hypersensitive site of the cardiac beta-myosin heavy-chain gene. Biochem J. 1997;327 (Pt 2):507–12. doi: 10.1042/bj3270507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hasegawa K, Lee SJ, Jobe SM, Markham BE, Kitsis RN. cis-Acting sequences that mediate induction of beta-myosin heavy chain gene expression during left ventricular hypertrophy due to aortic constriction. Circulation. 1997;96:3943–53. doi: 10.1161/01.cir.96.11.3943. [DOI] [PubMed] [Google Scholar]
- 161.von Harsdorf R, Edwards JG, Shen YT, Kudej RK, Dietz R, Leinwand LA, et al. Identification of a cis-acting regulatory element conferring inducibility of the atrial natriuretic factor gene in acute pressure overload. J Clin Invest. 1997;100:1294–304. doi: 10.1172/JCI119643. [DOI] [PMC free article] [PubMed] [Google Scholar]