DNA Replication Licensing and Progenitor Numbers Are Increased by Progesterone in Normal Human Breast (original) (raw)
Abstract
Proliferation in the nonpregnant human breast is highest in the luteal phase of the menstrual cycle when serum progesterone levels are high, and exposure to progesterone analogues in hormone replacement therapy is known to elevate breast cancer risk, yet the proliferative effects of progesterone in the human breast are poorly understood. In a model of normal human breast, we have shown that progesterone increased incorporation of 5-bromo-2′-deoxyuridine and increased cell numbers by activation of pathways involved in DNA replication licensing, including E2F transcription factors, chromatin licensing and DNA replication factor 1 (Cdt1), and the minichromosome maintenance proteins and by increased expression of proteins involved in kinetochore formation including Ras-related nuclear protein (Ran) and regulation of chromosome condensation 1 (RCC1). Progenitor cells competent to give rise to both myoepithelial and luminal epithelial cells were increased by progesterone, showing that progesterone influences epithelial cell lineage differentiation. Therefore, we have demonstrated that progesterone augments proliferation of normal human breast cells by both activating DNA replication licensing and kinetochore formation and increasing bipotent progenitor numbers.
Progesterone augments proliferation of normal human breast epithelial cells in matrix-embedded culture by activating DNA replication licensing and kinetochore formation, and by increasing bipotent progenitor numbers.
The human breast undergoes major developmental changes throughout adult life (1), orchestrated by the coordinated influence of endocrine and paracrine factors, which include the ovarian hormones, progesterone and estrogen. In the mouse, progesterone is both necessary and sufficient to stimulate the development of branching lobular alveolar structures of the mammary gland during pregnancy (2), supporting the idea that its influence on mouse mammary gland is proliferative. In the human, the role of progesterone in proliferation of the breast is controversial. In the uterus and breast cancer cell lines, progesterone is growth inhibitory (3), and it has been assumed that progesterone plays a similar role in the breast. However, proliferation in the nonpregnant human breast is highest in the luteal phase of the menstrual cycle when serum progesterone levels are high (4), but both estrogen and progesterone are present in the luteal phase circulation (2,4), leaving open the question of whether progesterone contributes to the higher proliferation observed during the luteal phase.
A role for progesterone in stimulating normal breast proliferation is likely to be critical in breast cancer. Women without ovaries have a reduced risk of breast cancer (5), implicating ovarian hormones in breast cancer development. Substantial data link estrogen with the development and progression of breast cancer (5), yet the role of progesterone has received less attention, despite large scale clinical studies showing that exposure to progesterone analogs in hormone replacement therapy results in elevated breast cancer risk (5). Ablation of the nuclear progesterone receptor (PR) in rodents dramatically reduces susceptibility to carcinogenesis (6), demonstrating an experimental link between PR signaling and mammary cancer. In addition, progesterone exposure increases genomic instability in p53 null mouse models of mammary carcinogenesis (7). On the basis of animal studies and the observed higher level of proliferation in the normal human breast during the luteal phase of the menstrual cycle, a proliferative role for progesterone has been suggested to underpin its association with increased breast cancer risk (4).
The epithelial compartment of the human breast is believed to develop by coordinated expansion of bipotent progenitor cells that give rise to both luminal and myoepithelial cell types. Although estrogen and progesterone influence human breast development, their role in expansion of epithelial progenitors is unknown. Delineating the effect of progesterone on the breast is critical in understanding its role in breast cancer because after early transforming events in the breast, which are postulated to occur in the reproductive years, initiated cells are exposed to a lifetime of cyclical progesterone. We developed and applied a physiologically relevant and hormone-responsive model to identify whether progesterone has the capacity to stimulate proliferation and influence progenitor populations in the normal human breast.
Materials and Methods
Matrix-embedded culture of normal primary breast epithelium
Normal breast tissue was obtained from reduction mammoplasty specimens (n = 5), with informed consent and approval from Human Research Ethics Committees of the Sydney West Area Health Service, the University of Sydney, and the San Hospital, New South Wales, Australia. Tissue was minced, collagenase digested (16 h, 37 C, 0.5 mg/ml in F-12 medium containing antibiotics and 10% fetal bovine serum), incubated for 2–3 h in collagenase (12.5 mg/ml), and filtered sequentially through 150- and 40-μm filters. The resultant organoids were frozen.
Organoids were resuspended in undiluted growth factor reduced Matrigel basement membrane (BD Biosciences, San Jose, CA), overlaid with MCDB170 medium supplemented with 2.5% pituitary extract (formula no. 95-0255DJ; Invitrogen, Carlsbad, CA) and incubated at 37 C in 5% CO2 for 12–14 d with medium renewal every 3–4 d. Primary breast cells in MCDB170 medium were also cultured in monolayer. Where indicated, cultures were treated with 100 nm progesterone (Sigma-Aldrich, Castle Hill, Australia) or vehicle. Where indicated, 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich) was incorporated by addition to culture medium at 10 μm final concentration for 6 h, starting 48 h before sample fixation, followed by replacement with fresh medium.
Primary suspension assay
Primary cultures were dissociated by digestion with dispase (50 U/ml; Becton Dickinson; 2 h, 37 C). Cells were pelleted, resuspended in 0.05% trypsin and 0.02% EDTA, incubated 2–5 min, followed by mechanical disruption by 4–5 passes through a blunt-ended needle until a single cell suspension was achieved. Cell suspensions were washed, resuspended in MEGM medium (Invitrogen) at 104 cells/ml, transferred into 10 cm2 Ultralow attachment plates (15 ml/plate; Corning, Corning, NY), and incubated at 37 C, 5% CO2, 7–13 d. Mammosphere numbers were counted over the entire culture, using a reference grid.
Aldefluor assay
Primary normal breast cultures were dissociated to single cells as above. Aldehyde dehydrogenase activity was measured using an Aldefluor assay kit (Stem Cell Technologies, Victoria, Australia) and positive cells measured by flow cytometry (FacsCalibur; BD Biosciences, North Ryde, New South Wales, Australia).
Sample processing
Thrombin clots were prepared from primary suspension cultures by centrifuging acini and resuspending into a thrombin clot (8). Matrigel cultures or thrombin clots were fixed, paraffin embedded, cut into 2-μm sections, and antigens retrieved (9).
Immunohistochemistry
Morphologic assessment was performed on matrix-embedded cultures and normal breast tissue stained with hematoxylin and eosin and periodic acid Schiff ± diastase. Immunoperoxidase staining was performed as previously published (8) using mouse monoclonal antibodies to PRA and PRB (clone 16 and San27, respectively; Novocastra, Newcastle, UK).
Periodic acid Schiff staining
Sections were dewaxed, treated with 1% periodic acid followed by De Tomasi Schiff’s reagent (Australian Biostain Pty. Ltd., Victoria, Australia) and counterstained with Mayer’s hematoxylin. Diastase pretreated sections were initially incubated with 2% α-amylase (Sigma-Aldrich). Control tissue was liver.
Immunofluorescence histochemistry
For multiple immunofluorescence staining, antigens were revealed sequentially by incubation of primary antibody followed by an appropriate biotinylated secondary antibody [goat antimouse or antirabbit (Dakocytomation, Glostrup, Denmark) or goat antirat (Abcam, Cambridge, UK)] and a streptavidin-conjugated fluorescent label (Alexa 594, 488, or 350, for the first, second, and third staining sequences, respectively; Invitrogen, Frederick, MD). For single immunofluorescence, antigens were revealed with Alexa 488. For same-host primary antibodies, we incubated overnight, between sequences, with goat antimouse Fab fragments (Cappell; ICN Biomedical, Aurora, CA). To ensure antibody specificity, adjacent sections were stained using PBS/0.5% Triton X-100 to replace the second (and/or third) sequence primary antibody.
Application of primary antibodies
Triple immunofluorescence antibodies included p63 (4A4, BD Biosciences, San Diego, CA), mucin-1 (Muc-1; Abcam), and CK5/6 (D5/16B4; Zymed, San Francisco, CA) or p63, CK18 (CY-90; Sigma, St. Louis, MO), and CK5 (Abcam).
Dual-immunofluorescence antibodies included PRA+PRB, or estrogen receptor (ER) (ID5; Dakocytomation) and p63 or Muc-1 (BC-2; Chemicon, Temecula, CA); PRB specific (Novocastra; clone San27) and PRA specific (Novocastra; clone 16); and regulator of chromosome condensation 1 (RCC1; ab54600; Abcam) and Ras-related nuclear protein 1 (Ran; Abcam).
Single immunofluorescence antibodies included RCC1; dimerization partner 1 (DP1; SPM178; Abcam); Ran; minichromosome maintenance (MCM)-2 (Abcam).
Fluorescent sections were mounted with Vectashield fluorescent mountant (Vector Laboratories, Burlingame, CA) and examined using a BX 60 fluorescence microscope (Olympus, Tokyo, Japan) with filters to detect Alexa 594 [band pass (BP) 545–580], Alexa 488 (BP 470–490), and Alexa 350 (BP 330–385) and a dual excitation filter to detect Alexa 594 and Alexa 488 simultaneously. Individual images were captured at each excitation using a SPOT charge-coupled device camera (SciTech, Victoria, Australia). Triple-stained images were derived by merging single excitation images and dual-stained images by using the dual-excitation filter.
Relative lineage marker abundance
Relative lineage marker abundance was determined by digital image analysis of multiple images (n = 10–30) of sections (n = 3 individual donors) dual stained for Muc-1 (luminal) or p63 (myoepithelial) using Image-Pro Plus software (version 5; Media Cybernetics Inc., Silver Spring, MD). Total cellular area of each section was quantitated using a gray-scale image taken under the dual filter and the area stained by each lineage marker quantitated by filtering the image through the appropriate red or green channel. Data were analyzed using SPSS software (version 12; SPSS Science, Chicago, IL). The Mann Whitney U test (significance level 5%) was used to compare luminal-myoepithelial cells in cultures vs. original samples.
Gene expression profiling
Progesterone effects on normal breast and breast cancer cells were measured by gene expression profiling using Illumina (San Diego, CA) human whole-genome 6v2 beadchips, containing probes to 42,648 genes, obtained from GeneWorks (Hindmarsh, South Australia, Australia). Gene expression in matrix-embedded and monolayer cultures of normal breast cells were compared with the original material by profiling expression on human 8000 cDNA microarrays (Australian Genome Research Facility, Parkville, Victoria, Australia) containing 7425 target cDNA sequences. Total RNA was isolated using the Stratagene Absolutely RNA microprep kit (Integrated Sciences, Sydney, Australia). For profiling on Illumina bead arrays, total RNA was amplified and labeled using Ambion Total Prep reagents (Applied Biosystems, Scoresby, Victoria, Australia) and Beadchips processed using Illumina reagents (GeneWorks). For profiling on cDNA microarrays, total RNA (100–500 ng) was amplified using Ambion MessageAmp II reagents (Applied Biosystems) and labeled by indirect incorporation of Cy3 and Cy5 into amplified RNA, as described (10). Arrays were scanned using a GenePix 4000B scanner and red-green fluorescent intensities were estimated using GenePixPro 6.0.1 software (Molecular Devices, Sunnyvale, CA).
Illumina microarray data were analyzed using Beadstudio software version 3 (Illumina, San Diego, CA). Average signal intensities were normalized using the cubic spline function in Beadstudio and the Illumina Custom function was used to assign a differential expression score and P value to each gene. Transcripts with detection and differential expression P < 0.01 in at least three of five normal breast samples were considered significantly different. When comparing numbers of progesterone-regulated genes between data sets a fold change cutoff of 1.5 was applied. Data from cDNA arrays were analyzed using the Bioconductor workspace environment (11) (www.bioconductor.org), background threshold filtered, and then normalized using the mArray package of Bioconductor (12), using the intensity-dependent within print-tip Lowess regression function. Differentially expressed genes were ranked according to their penalized t-statistic (13). Transcripts with an absolute penalized t statistic and fold change greater than 2 between two samples, were considered differentially expressed.
Enrichment of specific gene ontology categories within differentially expressed gene lists was determined using GO Miner (14) (discover. nci.nih.gov/gominer/) and the Database for Annotation, Visualization, and Integrated Discovery online tool, version 2.1 (15) (apps1.niaid. nih.gov/david/). Functional network analysis was performed using the Metacore online tool (GeneGO; www.genego.com). Significant enrichment of gene ontology categories in matrix-embedded and monolayer cultures, relative to the original material, was determined using GO Miner and visualized as a vector graphic.
Direct comparisons were only made between data generated using the same microarray platform and no cross-platform comparisons of gene numbers or ontologies were made.
Results
Bipotent epithelial progenitors are maintained in human breast culture
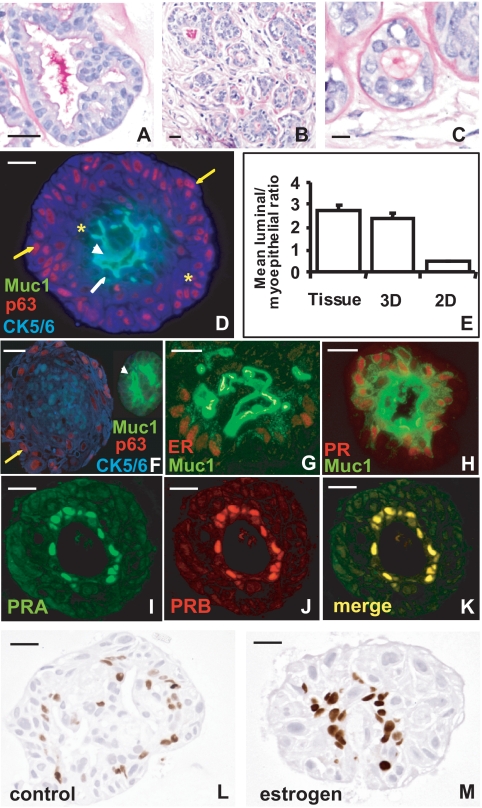
Primary human breast epithelial cells expanded to form numerous discrete multicellular structures that were predominantly round or lobulated in shape after 14 d. There was some morphologic heterogeneity in individual cultures, but they consistently showed evidence of mature glandular differentiation with multicellular acini formed by polarized epithelial cells with a distinct apical glycocalyx and intraluminal secretion (Fig. 1A). These features essentially recapitulated the lobular structure of the normal breast (Fig. 1, B and C). In addition, some cultures showed foci of squamous differentiation and/or cells with cytoplasmic clearing reminiscent of clear cell change that has been described in the normal breast (16). Acinar structures contained cells of different lineages at various stages of differentiation, including progenitor cells (CK5/6+; Muc-1−; p63−, Fig. 1D, asterisk); committed preluminal cells (CK5/6+; Muc-1+; p63−); premyoepithelial cells (CK5/6+; Muc-1−; p63+); and mature luminal cells (CK5/6−; Muc-1+; p63−) (Fig. 1D), demonstrating the preservation of bipotent epithelial progenitors. The proportion of cells expressing luminal or myoepithelial lineage markers in embedded culture was not different from the original samples (Fig. 1E; P = 0.7, Mann Whitney U test).
Figure 1.
Morphology, lineage markers, and ER and PR expression in matrix-embedded culture of normal breast acini. PAS staining with diastase pretreatment of normal breast cells in matrix-embedded culture (A) and normal breast tissue (B and C) showing distinct apical glycocalyx and intraluminal secretions. Triple-immunofluorescence staining of the progenitor marker CK5/6 (blue), myoepithelial marker p63 (red), and luminal epithelial marker Muc-1 (green) in sections prepared from primary cells grown in matrix-embedded cultures (D) and in primary cells grown in matrix-embedded culture after initial passage in monolayer (F; yellow or white arrows demonstrate committed premyoepithelial and preluminal cells, respectively; white arrowhead shows mature luminal cells; asterisks represent uncommitted progenitor cells). E, Ratio of luminal and myoepithelial markers in primary cells in matrix-embedded and monolayer culture. Dual-immunofluorescence staining of matrix-embedded cultures: (G and H) showing colocalization within the luminal compartment of ER (G) or PR (H; red) with the luminal epithelial marker Muc1 (green); (I–K) showing colocalization of PRA and PRB, PRA expression (I); PRB expression (J); merge (K). PR expression in normal breast cells in matrix-embedded culture; without (L) and with estrogen treatment (M). Scale bars, 20 μm.
The culture conditions largely preserved the functional characteristics of the primary cells because gene expression profiling revealed no significant difference in major gene ontology categories between primary cells before or after culture, other than proliferation and development (supplemental Table S1, published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org). Network mapping of ontology hierarchies enriched in cultured cells revealed significant nodes only in cell proliferation and tissue development ontology trees (Fig. 2A), reflecting the increase in cell number and morphogenesis after culture.
Figure 2.
Enriched functional categories in differentially expressed gene sets from normal breast cells in monolayer and matrix-embedded culture. Vector graphic representation of enrichment in specific functional categories organized into gene ontology hierarchies. Gene expression was compared on cDNA arrays of 7425 genes. Functional categories enriched in matrix-embedded cultures (A) and monolayer culture of primary normal breast cells (B) when compared with the original tissue. Significance of enrichment of individual categories (two sided Fisher exact test) is indicated by the black circles at each enriched node, with size inversely proportional to P value. Labels are included only for functional categories with significant P values for enrichment or which represent branching points in the gene ontology tree.
When primary cells were cultured in monolayer, there was preferential expansion of the myoepithelial component (Fig. 1E), and down-regulation of large numbers of gene categories particularly those involved in interaction with the extracellular environment and responses to metabolic stimuli (supplemental Table 1). Network mapping of ontology hierarchies enriched in monolayer culture revealed multiple highly significant nodes representing cellular response to external stimuli (Fig. 2B). Even if primary cells were returned to embedded Matrigel culture after a passage in monolayer, they did not resume the functional characteristics of in vivo tissue (not shown), and all acini derived from monolayer cultured cells contained either luminal epithelial cells, or myoepithelial cells, but never both (Fig. 1F), in contrast to what occurred when primary cells were placed directly into matrix-embedded culture (Fig. 1D). This demonstrated that bipotent epithelial progenitors, able to give rise to both luminal and myoepithelial cells, were lost on monolayer culture of primary cells. ER and PR were also lost in monolayer culture (not shown).
ER and PR are maintained and regulated in Matrigel-embedded primary human breast culture
ER and PR were expressed in primary cultures (Fig. 1), and there was frequent but not total colocation of ER and PR expression, as observed in normal human breast (17). ER and PR were colocalized with the luminal marker Muc-1 (Fig. 1, G and H), but not with the myoepithelial marker p63 (not shown). Dual staining for Muc-1 and p63 in sections adjacent to those stained for a single lineage marker and either PR or ER confirmed that both luminal and myoepithelial cells were present in each section (not shown), but receptors were seen only in luminal cells. PRA and PRB were coexpressed at similar levels (Fig. 1, I–K), as shown in normal human breast (18). There was marked PR positivity in the absence of estrogen (Fig. 1L), although PR levels were augmented by estrogen pretreatment (Fig. 1M; Mann Whitney test P = 0.01), demonstrating both constitutive and estrogen-dependent PR expression and confirming that ER was functional in this model. Progesterone also down-regulated its own receptor in this model, and numbers of PR+ acini (Fig. 3A) and PR+ cells per acinus (Fig. 3B) were reduced in progesterone-treated cultures (Mann Whitney test P < 0.0001 for each). PR was not significantly down-regulated in cultures treated with progesterone and estrogen (not shown), concordant with the lack of fluctuation in PR expression observed in the normal human breast during the menstrual cycle, when both hormones are present (4).
Figure 3.
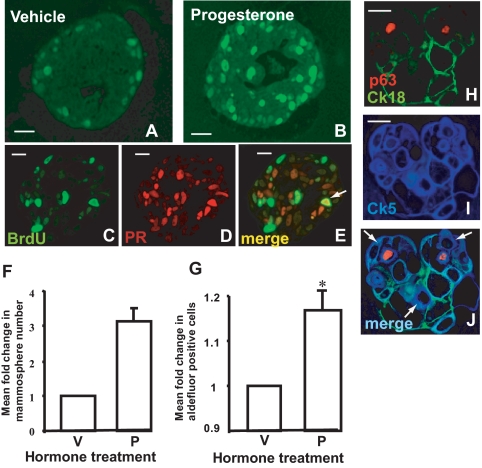
Progesterone responsiveness and proliferation of normal breast cells in matrix-embedded culture. PR expression and proliferation (BrdU incorporation) of normal breast cells in matrix-embedded culture determined by dual-immunofluorescence staining. Progesterone (P) down-regulated its own receptor as shown by fewer PR+ acini (shaded column) (A) and fewer cells per acinus expressing PR (B). C, Progesterone treatment increased the total number of cells per acinus incorporating BrdU. D, Progesterone treatment increased total cell numbers in cultures. E, Progesterone preferentially increased BrdU incorporation within PR+ acini. *, P < 0.05 Mann Whitney test; bars, sem. V, Vehicle.
Progesterone augmented normal breast proliferation and increased progenitor numbers
There was a significant increase in the mean number of proliferating cells per acinus after progesterone treatment (Fig. 4, A and B), as measured by incorporation of BrdU (Mann Whitney test, P = 0.0006, Fig. 3C). The proliferative effect of progesterone was also evidenced by an increase in the total number of cells after 14 d of culture (two tailed t test, P = 0.039, Fig. 3D). There was also a correlation between numbers of BrdU+ cells and PR+ cells (Spearman rank, P < 0.0001, not shown) and between proliferation and PR status of acini because proliferation in response to progesterone was higher in PR+ acini than PR− acini (Mann Whitney test, P = 0.001, Fig. 3E). Proliferation was restricted primarily to PR− cells, and only a small proportion (6–7%) of PR+ cells were also BrdU+ (Fig. 4, C–E).
Figure 4.
Progesterone increased proliferation and bipotent progenitor cell numbers. Progesterone increased proliferation (BrdU incorporation) of normal breast cells in matrix-embedded culture; BrdU incorporation without (A) and with progesterone treatment (B). Dual-immunofluorescence staining of matrix-embedded cultures: (C–E) showing colocalization of BrdU and PR; BrdU incorporation (C); PR expression (D); merge (E); arrow shows proliferating PR+ cell. Progesterone increased progenitor cells: progesterone treatment induced a mean 3.1-fold increase (F) in mammosphere numbers in primary suspension cultures (n = 2; normalized data, t test: paired two sample for means, one tail, P = 0.06), and a significant increase in aldefluor-positive progenitor cells by flow cytometry (G) (n = 4; t test: paired two sample for means, two tail, P = 0.005). *, P < 0.05; bars, sem. H–J, Triple immunofluorescence staining for uncommitted progenitor cells after primary suspension culture. H, Myoepithelial marker p63 (red) and luminal marker CK18 (green). I, Progenitor marker CK5 (blue). J, Merge, arrows show uncommitted progenitor cells. Scale bars, 20 μm.
Putative progenitors were measured after hormone treatment of matrix-embedded culture, using the mammosphere suspension assay, which preferentially selects for progenitor cells able to survive and form spheres in suspension culture (19) and by determining the proportion of cultured cells that were positive for the progenitor marker aldehyde dehydrogenase on flow cytometry (20). Progesterone increased the proportion of cells that formed mammospheres in suspension (Fig. 4F). Mammospheres, derived from single cells placed into suspension culture, grew into multicellular structures that contained both uncommitted progenitors and cells committed to either the luminal or the myoepithelial lineage (Fig. 4, H–J), demonstrating that the cells giving rise to these mammospheres had bipotent lineage capacity Progesterone also significantly increased the proportion of aldehyde dehydrogenase-positive cells (Fig. 4G), supporting the conclusion that progenitor cell proliferation was increased by progesterone.
Progesterone increases DNA replication licensing in normal breast
Gene expression profiles of five independent normal breast samples revealed 953 genes significantly regulated after progesterone treatment in Matrigel culture (supplemental Table 2). Gene ontology annotations showed that categories associated with proliferation, specifically cell cycle and DNA replication were among the most highly enriched by progesterone, and there was significant concordance between the independent human samples (Fig. 5A). RNA biogenesis and processing categories were also highly enriched.
Figure 5.
Distinct profiles of progesterone-regulated gene expression in normal and malignant breast cells. A, Primary normal breast epithelial cultures (Bre) from five individuals (25, 27, 52, 62, 64) were treated with 100 nm progesterone or vehicle for 6 h. Gene expression profiles were determined using Illumina whole-genome arrays. Enrichment analysis of differentially expressed genes demonstrated overrepresentation only of the functional categories shown. B, Primary normal breast epithelial cells or T-47D breast cancer cells were grown in matrix-embedded culture and treated (6 h) with 100 nm progesterone. RNA was isolated and gene expression profiling was performed on Illumina whole-genome expression arrays. Numbers of progesterone regulated genes in normal breast and T-47D breast cancer cell matrix-embedded cultures, and their overlap, are indicated. The number genes detected and not regulated by progesterone in either cell type are indicated in the rectangle.
Network analysis of progesterone-regulated genes revealed the E2F/DP1 complex as a critical regulatory node in a proliferation scheme linking replication licensing, passage through the G1/S transition and G2/M checkpoint control (Fig. 6A and supplemental Table 3). E2F controls cell division by directing the expression of key cell cycle and DNA replication licensing regulators (21,22,23,24). All three of the well-characterized activating E2Fs, E2F1, E2F2 and E2F3, were increased after 6 h progesterone exposure (2.3-, 1.8-, and 1.4-fold, respectively). These E2Fs are potent transcriptional activators, which when overexpressed, trigger movement into S phase (21). In addition to increased expression of E2F family members and binding partner DP1, Cdt1, and cdc7, which activate the prereplication complex (25,26), were increased (Fig. 6A), as were minichromosome maintenance proteins 2–5 (Fig. 6A), which make up the prereplication complex (27,28). CDC25A phosphatase, a key mediator of both the G1/S and G2/M transitions (29,30), also increased with progesterone exposure (Fig. 6A). In contrast, both CDC25B and CDC25C, which are primarily involved in G2/M regulation (29,30), were significantly decreased (Fig. 6A), suggesting a dynamic shift in control by this family of dual specificity phosphatases. Ran and RCC1, binding partners that are critical in coordinating spindle assembly and mitosis at the completion of S phase (31,32), were also increased after progesterone treatment (Fig. 6A), as were the nuclear lamina components lamin B1 and B2 (Fig. 6A). Lamin B proteins are important regulators of nuclear functions, including DNA replication (33,34), nuclear envelope formation (35), and mitotic spindle assembly (36). Incorporation of lamin B into mitotic spindles is Ran dependent (36), linking the functions of these two molecules.
Figure 6.
Progesterone activates a network of genes controlling DNA replication licensing and cell cycle progression. A, Functional analysis of genes regulated by progesterone in normal breast cultures revealed an enriched network of genes involved in regulation of DNA replication licensing, G1/S transition, G2/M progression, and kinetochore function. Filled arrows indicate increased and decreased expression. ORC, Origin recognition complexes. B, DNA replication licensing and cell proliferation genes increased by progesterone on expression profiling were confirmed. Histograms indicate relative mRNA transcript levels estimated by quantitative real-time RT-PCR. Open bars, vehicle; shaded bars, 6 h progesterone (100 nm). C, Images show immunodetection of the indicated protein products in normal breast cultures, after 48 h treatment with 100 nm progesterone (P) or vehicle (V).
Progesterone up-regulation of critical components of the DNA replication licensing machinery, including the origin of replication protein ORC1L, E2Fs, Cdt1, the MCMs and DP1 was confirmed by real-time PCR (Fig. 6B) and/or immunohistochemistry (Fig. 6C). Increased expression of cdc7, cdc25A, cyclinD1, cdk6, RCC1, and Ran was also confirmed, as was the coexpression of Ran and RCC1.
Progesterone increases Notch signaling in normal breast
Progesterone increased the expression of genes in the Notch pathway, which plays a critical role in regulating breast stem and progenitor cells (37). Notably, the human homologues of the Notch-delta ligand, delta-like 1 and 3 (DLL-1, DLL-3, supplemental Table 2) were increased by progesterone, as was the Notch receptor regulator presenilin 2 (supplemental Table 2). Delta- and presenilin-mediated cleavage of the receptor are required for release and nuclear translocation of the Notch intracellular signaling domain, which results in transcriptional effects on Notch target genes (38). The finding that critical mediators of Notch signal transduction are positively regulated by progesterone suggests that the progesterone-mediated increase in breast progenitor cells demonstrated by increased mammosphere formation and aldefluor positivity (Fig. 4) may be mediated by effects on the Notch pathway.
Progesterone regulated pathways differ in normal breast and breast cancer cells
The profile of progesterone-regulated transcripts in the normal breast did not overlap with PR-regulated transcripts previously observed in breast cancer cells (10). Furthermore, even when T-47D breast cancer cells, which express PR and are progesterone responsive, were placed into Matrigel culture under identical experimental conditions as used for primary normal breast, profiles of progesterone-regulated transcripts were predominantly nonoverlapping. There were only 100 transcripts in common (Fig. 5B, supplemental Table 4), demonstrating that the majority of progesterone-regulated genes in the normal breast are not progesterone targets in breast cancer cells. This difference in progesterone regulation may be due in part to having compared primary cells with an established cell line; however, progestin effects in T-47D cells and the MCF-10A minimally transformed human breast cell line, transduced with PR, were also largely distinct (not shown), underscoring the influence of transformation on hormone response. In further support of the difference in progesterone-regulated pathways in normal breast and breast cancer cells, we observed that the enrichment of functional categories seen in normal breast cells was not observed in T-47D cells (supplemental Table 5). Specific comparison of progesterone regulation of DNA replication-associated genes in normal breast and T-47D cells further confirmed this, showing that only two of these genes, cdk6 and cyclin G1, were PR targets in both normal breast and T-47D cells (supplemental Table 3).
Discussion
This study has demonstrated that progesterone increases proliferation and progenitor numbers, in a model of normal human breast that preserves key features of the in situ tissue. A major limitation in elucidating pathways of progesterone action in the human breast has been the limited number of suitable models that preserve the proliferative capacity, progenitor cell complement, and hormone responsiveness of normal breast in vivo. Hormone responsiveness is mediated by ER and PR in luminal epithelial cells, which associate in organized structures with receptor-negative myoepithelial cells (39,40). The epithelial lineages in the normal breast are derived from common progenitor cells, and maintenance of the association of luminal and myoepithelial cells, derived from appropriate progenitor populations (41), is crucial for maintenance of correct epithelial cell polarity and function (42).
Progesterone-responsive model of normal human breast
Primary normal human breast tissue in embedded culture recapitulates the morphology, cell lineages, functional gene expression characteristics, and ER and PR responsiveness of the breast in vivo. The ratio of luminal to myoepithelial cells after culture recapitulates that observed in the uncultured tissue, highlighting the fact that progenitor cells capable of giving rise to both epithelial cell lineages are retained in this model system. Primary cells placed into monolayer culture, even for a single passage, lose bipotent progenitors, and the myoepithelial lineage predominates, demonstrating the rapidity with which phenotypic changes and selection occur in normal breast cells, unless cultured under conditions that prevent this outcome.
Primary matrix-embedded culture of normal human breast cells retains progesterone responsiveness, as demonstrated by expression of PR, and progesterone effects on PR levels, proliferation and gene expression. Whereas it was recognized several decades ago that immortalized breast epithelial cells would recapitulate aspects of in vivo architecture and function when placed into three-dimensional culture (43), maintenance of hormone responsiveness in these systems has been an elusive goal because these immortalized cell lines lack ER and PR.
DNA replication licensing is under progesterone control in human breast
This study has demonstrated that progesterone is able to stimulate proliferation of normal human breast cells. Increased incorporation of BrdU and increased total cell numbers in progesterone-treated cultures demonstrated this phenomenon, and functional analysis of genes regulated by progesterone revealed that components of the DNA replication licensing machinery were progesterone targets. DNA replication licensing occurs in late M phase when replication origins are bound by origin recognition complexes with the ATPase Cdc6 (Fig. 6A). The replication licensing factor Cdt1 then recruits several MCM proteins, which form a DNA helicase complex, opening the DNA helix and allowing DNA copying by polymerase enzymes. It is crucial to ensure that DNA is replicated only once in each cell cycle, and this is normally achieved by tight regulation of the DNA replication licensing machinery (28). Once cells enter S phase, key components of the licensing machinery, notably Cdt1 in mammalian cells, are repressed to prevent rereplication (28,44). This study has now shown that critical components of the DNA replication licensing machinery are increased by progesterone in normal breast cells.
The E2F transcription factor heterodimerizes with DP1 to form an active E2F regulatory complex (21,22), and both E2F and DP1 were increased by progesterone. The E2F transcripts that were increased by progesterone were predominantly the activating E2Fs and the fact that several S-phase components were also increased by progesterone likely reflected this overall increase in the activating forms of this transcription factor. Progesterone also regulated proteins involved in spindle formation during mitosis because both Ran and its nuclear partner protein RCC1 were coexpressed and progesterone regulated (Fig. 6). Through its role in nuclear import, Ran is also an important regulator of S-phase progression by controlling the availability of key cell cycle factors in the nucleus. This study demonstrates that progesterone increases proliferation at a number of points within the replication cycle, most notably replication licensing.
In the uterus, progesterone inhibits estrogen-mediated proliferation, in contrast to its proliferative effect in normal breast demonstrated in this study, and in animal models, it has been shown that the inhibitory effect of progesterone is mediated by inhibition of replication licensing (45). Genes identified as up-regulated in association with the increased proliferation caused by progesterone in the normal breast in this study, are down-regulated by progesterone in the uterus (45), demonstrating that the same pathway is targeted by progesterone in breast and uterus but in different directions, consistent with the divergent physiological roles of progesterone in these tissues.
Progesterone augments putative progenitor numbers
The development of primary breast epithelial cells into ordered structures reminiscent of the normal breast tissue from which they were derived, with luminal to myoepithelial ratios that paralleled the ratio observed in normal breast, suggested that matrix-embedded cultures contained putative progenitors. The existence of putative progenitor cells in normal cultures was confirmed using two approaches. Cells that can be propagated in culture as floating mammospheres are considered to have stem/progenitor properties (19), and this was confirmed by the ability of cells in mammospheres to develop into both the luminal and myoepithelial lineages. Stem/progenitor cells also express the aldehyde dehydrogenase enzyme (20), and aldehyde dehydrogenase-positive cells were identified in normal breast cultures in this study. Treatment with progesterone caused an increase in these putative progenitor cells, demonstrating the capacity for progesterone to regulate the epithelial cell complement of the human breast.
In the T-47D PR+ breast cancer cell line, a small (1%) subpopulation of PR−, CD44+, CK5+ cells was shown to have tumor initiating properties in mice (46). Exposure to progestin increased PR positivity in this population, and, although peripheral to the findings of this study, the T-47D study demonstrates the capacity for progestins to influence cell populations with tumor initiating properties.
Progesterone likely acts in a paracrine manner on PR-negative progenitors because cells that were identified by BrdU incorporation as actively proliferating were mostly PR−. PR is known to affect proliferation via a number of paracrine effectors, including receptor activator of nuclear factor-κB ligand (47), Wnt-4 (48), and amphiregulin (49). Although no progesterone effect on Wnt, receptor activator of nuclear factor-κB ligand, or amphiregulin expression was detected in this study, the mechanisms underlying progesterone regulation of progenitor abundance may include effects on Notch signaling, a known downstream target of Wnt in the mouse and human (37,50). Notch ligands, and the presenilin enzyme, essential for receptor cleavage and release into the cytoplasm, were shown in this study to be increased by progesterone.
Progesterone regulation of gene expression is divergent in normal breast and breast cancer cells
To directly compare progesterone regulation of gene pathways in normal breast and breast cancer cells, PR+ T-47D breast cancer cells were placed into matrix-embedded culture and treated identically to normal breast cultures. The overlap in expressed genes was minimal, even for genes involved in proliferation. Because progesterone inhibits proliferation in T-47D cells (3), in contrast to the increased proliferation documented here in normal breast, we reasoned that the same proliferation genes may have been regulated in both systems, although in opposite directions, but this was not observed. This suggests that regulation of proliferation by progesterone in normal breast and T-47D breast cancer cells occurs by different mechanisms. It also shows that continuously cultured PR+ cancer cell lines, even in a microenvironment that reproduces some aspects of normal breast cell morphology, provide a view of progesterone action that is quite different from that revealed in primary normal breast and that manipulation of cell context and microenvironment cannot restore progesterone signaling pathways operative in primary tissue.
In summary, this study has shown that progesterone increased proliferation and the numbers of putative progenitor cells, demonstrating the capacity of this hormone to influence proliferation and epithelial cell lineage differentiation in the normal breast. The proliferative effect of progesterone was mediated by activation of pathway components involved in DNA replication licensing and by increased expression of proteins involved in kinetochore formation. Activation of DNA replication licensing and kinetochore formation and increased numbers of uncommitted progenitors in normal human breast as a result of exposure to progesterone demonstrate the mechanisms of augmented breast proliferation in response to progesterone. Aberrations in these pathways may underlie the contribution of progestins to increased breast cancer risk.
Supplementary Material
[Supplemental Data]
Acknowledgments
We thank Karen Byth for assistance with statistical analyses, Virginia James for performing PAS staining, and Jadranka Tomas for cutting paraffin sections.
Footnotes
This work was supported by the National Health and Medical Research Council of Australia. R.L.B. is a Cancer Institute New South Wales Fellow.
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 2, 2009
For editorial see page 2988
Abbreviations: BP, Band pass; BrdU, 5-bromo-2′-deoxyuridine; DP1, dimerization partner 1; ER, estrogen receptor; MCM, minichromosome maintenance; Muc-1, mucin-1; PR, progesterone receptor; Ran, Ras-related nuclear protein 1.
References
- Howard BA, Gusterson BA 2000 Human breast development. J Mammary Gland Biology Neoplasia 5:119–137 [DOI] [PubMed] [Google Scholar]
- Ismail PM, Amato P, Soyal SM, DeMayo FJ, Conneely OM, O'Malley BW, Lydon JP 2003 Progesterone involvement in breast development and tumorigenesis—as revealed by progesterone receptor “knockout” and “knockin” mouse models. Steroids 68:779–787 [DOI] [PubMed] [Google Scholar]
- Clarke CL, Sutherland RL 1990 Progestin regulation of cellular proliferation. Endocr Rev 11:266–301 [DOI] [PubMed] [Google Scholar]
- Pike MC, Spicer DV, Dahmoush L, Press MF 1993 Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev 15:17–35 [DOI] [PubMed] [Google Scholar]
- Santen RJ 2003 Risk of breast cancer with progestins: critical assessment of current data. Steroids 68:953–964 [DOI] [PubMed] [Google Scholar]
- Lydon JP, Ge G, Kittrell FS, Medina D, O'Malley BW 1999 Murine mammary gland carcinogenesis is critically dependent on progesterone receptor function. Cancer Res 59:4276–4284 [PubMed] [Google Scholar]
- Goepfert TM, McCarthy M, Kittrell FS, Stephens C, Ullrich RL, Brinkley BR, Medina D 2000 Progesterone facilitates chromosome instability (aneuploidy) in p53 null normal mammary epithelial cells. FASEB J 14:2221–2229 [DOI] [PubMed] [Google Scholar]
- Mote PA, Balleine RL, McGowan EM, Clarke CL 1999 Colocalization of progesterone receptors A and B by dual immunofluorescent histochemistry in human endometrium during the menstrual cycle. J Clin Endocrinol Metab 84:2963–2971 [DOI] [PubMed] [Google Scholar]
- Mote PA, Leary JA, Clarke CL 1998 Immunohistochemical detection of progesterone receptors in archival breast cancer. Biotech Histochem 73:117–127 [DOI] [PubMed] [Google Scholar]
- Graham JD, Yager ML, Hill HD, Byth K, O'Neill GM, Clarke CL 2005 Altered progesterone receptor isoform expression remodels progestin responsiveness of breast cancer cells. Mol Endocrinol 19:2713–2735 [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J 2004 Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5:R80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudoit S, Yang YH 2002 Bioconductor R packages for exploratory analysis and normalization of cDNA microarray data. In: Parmigiani G, Garrett ES, Irizarry RA, Zeger SL, eds. The analysis of gene expression data: methods and software. New York: Springer; 73–101 [Google Scholar]
- Smyth GK, Yang YH, Speed T 2003 Statistical issues in cDNA microarray data analysis. Methods Mol Biol 224:111–136 [DOI] [PubMed] [Google Scholar]
- Zeeberg BR, Feng W, Wang G, Wang MD, Fojo AT, Sunshine M, Narasimhan S, Kane DW, Reinhold WC, Lababidi S, Bussey KJ, Riss J, Barrett JC, Weinstein JN 2003 GoMiner: a resource for biological interpretation of genomic and proteomic data. Genome Biol 4:R28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis Jr G, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA 2003 DAVID: database for annotation, visualization, and integrated discovery. Genome Biol 4:P3 [PubMed] [Google Scholar]
- Rosen PP 1997 Breast pathology. New York: Lippincott-Raven [Google Scholar]
- Anderson E 2002 The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res 4:197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote PA, Bartow S, Tran N, Clarke CL 2002 Loss of coordinate expression of progesterone receptors A and B is an early event in breast carcinogenesis. Breast Cancer Res Treat 72:163–172 [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS 2003 In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 17:1253–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Ginestier C, Charafe-Jauffret E, Foco H, Kleer CG, Merajver SD, Dontu G, Wicha MS 2008 BRCA1 regulates human mammary stem/progenitor cell fate. Proc Natl Acad Sci USA 105:1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimarchi JM, Lees JA 2002 Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol 3:11–20 [DOI] [PubMed] [Google Scholar]
- DeGregori J, Johnson DG 2006 Distinct and overlapping roles for E2F family members in transcription, proliferation and apoptosis. Curr Mol Med 6:739–748 [DOI] [PubMed] [Google Scholar]
- Leone G, DeGregori J, Yan Z, Jakoi L, Ishida S, Williams RS, Nevins JR 1998 E2F3 activity is regulated during the cell cycle and is required for the induction of S phase. Genes Dev 12:2120–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani K, Iwanaga R, Nakamura M, Ikeda M, Yabuta N, Tsuruga H, Nojima H 1999 Cell growth-regulated expression of mammalian MCM5 and MCM6 genes mediated by the transcription factor E2F. Oncogene 18:2299–2309 [DOI] [PubMed] [Google Scholar]
- Sheu YJ, Stillman B 2006 Cdc7-Dbf4 phosphorylates MCM proteins via a docking site-mediated mechanism to promote S phase progression. Mol Cell 24:101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xouri G, Dimaki M, Bastiaens PI, Lygerou Z 2007 Cdt1 interactions in the licensing process: a model for dynamic spatiotemporal control of licensing. Cell Cycle 6:1549–1552 [DOI] [PubMed] [Google Scholar]
- Blow JJ, Dutta A 2005 Preventing re-replication of chromosomal DNA. Nat Rev Mol Cell Biol 6:476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani RA, Holzen TM 2007 Cell cycle regulation of DNA replication. Ann Rev Genet 41:237–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D, Kiyokawa H 2007 CDC25A levels determine the balance of proliferation and checkpoint response. Cell Cycle 6:3039–3042 [DOI] [PubMed] [Google Scholar]
- Boutros R, Lobjois V, Ducommun B 2007 CDC25 phosphatases in cancer cells: key players? Good targets? Nat Rev Cancer 7:495–507 [DOI] [PubMed] [Google Scholar]
- Moore JD 2001 The Ran-GTPase and cell-cycle control. Bioessays 23:77–85 [DOI] [PubMed] [Google Scholar]
- Dasso M 2006 Ran at kinetochores. Biochem Soc Trans 34:711–715 [DOI] [PubMed] [Google Scholar]
- Moir RD, Montag-Lowy M, Goldman RD 1994 Dynamic properties of nuclear lamins: lamin B is associated with sites of DNA replication. J Cell Biol 125:1201–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir RD, Spann TP, Herrmann H, Goldman RD 2000 Disruption of nuclear lamin organization blocks the elongation phase of DNA replication. J Cell Biol 149:1179–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Soler RI, Moir RD, Spann TP, Stick R, Goldman RD 2001 A role for nuclear lamins in nuclear envelope assembly. J Cell Biol 154:61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MY, Wang S, Heidinger JM, Shumaker DK, Adam SA, Goldman RD, Zheng Y 2006 A mitotic lamin B matrix induced by RanGTP required for spindle assembly. Science 311:1887–1893 [DOI] [PubMed] [Google Scholar]
- Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS 2004 Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res 6:R605–R615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini ME 2001 Notch and presenilin: a proteolytic mechanism emerges. Curr Opin Cell Biol 13:627–634 [DOI] [PubMed] [Google Scholar]
- Anderson E, Clarke RB, Howell A 1998 Estrogen responsiveness and control of normal human breast proliferation. J Mammary Gland Biol Neoplasia 3:23–35 [DOI] [PubMed] [Google Scholar]
- Lakhani SR, O'Hare MJ 2001 The mammary myoepithelial cell—Cinderella or ugly sister? Breast Cancer Res 3:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS 2003 Stem cells in normal breast development and breast cancer. Cell Prolif 36(Suppl 1):59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW 2002 Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci 115:39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Bissell MJ 2005 Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol 15:342–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook SS, Lin JJ, Dutta A 2007 Mechanisms to control rereplication and implications for cancer. Curr Opin Cell Biol 19:663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Deng Y, Pollard JW 2006 Progesterone blocks estrogen-induced DNA synthesis through the inhibition of replication licensing. Proc Natl Acad Sci USA 103:14021–14026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz KB, Dye WW, Harrell JC, Kabos P, Sartorius CA 2008 Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc Natl Acad Sci USA 105:5774–5779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM 2003 Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100:9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA 2000 Essential function of Wnt-4 in mammary gland development downstream of progesterone signalling. Genes Dev 14:650–654 [PMC free article] [PubMed] [Google Scholar]
- Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK 1995 Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol 9:691–705 [DOI] [PubMed] [Google Scholar]
- Ayyanan A, Civenni G, Ciarloni L, Morel C, Mueller N, Lefort K, Mandinova A, Raffoul W, Fiche M, Dotto GP, Brisken C 2006 Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci USA 103:3799–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Data]