Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement (original) (raw)
Abstract
David Moher and colleagues introduce PRISMA, an update of the QUOROM guidelines for reporting systematic reviews and meta-analyses
Systematic reviews and meta-analyses have become increasingly important in health care. Clinicians read them to keep up to date with their specialty,1 2 and they are often used as a starting point for developing clinical practice guidelines. Granting agencies may require a systematic review to ensure there is justification for further research,3 and some medical journals are moving in this direction.4 As with all research, the value of a systematic review depends on what was done, what was found, and the clarity of reporting. As with other publications, the reporting quality of systematic reviews varies, limiting readers’ ability to assess the strengths and weaknesses of those reviews.
Several early studies evaluated the quality of review reports. In 1987 Mulrow examined 50 review articles published in four leading medical journals in 1985 and 1986 and found that none met all eight explicit scientific criteria, such as a quality assessment of included studies.5 In 1987 Sacks and colleagues evaluated the adequacy of reporting of 83 meta-analyses on 23 characteristics in six domains.6 Reporting was generally poor; between one and 14 characteristics were adequately reported (mean 7.7, standard deviation 2.7). A 1996 update of this study found little improvement.7
In 1996, to address the suboptimal reporting of meta-analyses, an international group developed a guidance called the QUOROM statement (QUality Of Reporting Of Meta-analyses), which focused on the reporting of meta-analyses of randomised controlled trials.8 In this article, we summarise a revision of these guidelines, renamed PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses), which have been updated to address several conceptual and practical advances in the science of systematic reviews (see box).
Conceptual issues in the evolution from QUOROM to PRISMA
Completing a systematic review is an iterative process
The conduct of a systematic review depends heavily on the scope and quality of included studies: thus systematic reviewers may need to modify their original review protocol during its conduct. Any systematic review reporting guideline should recommend that such changes can be reported and explained without suggesting that they are inappropriate. The PRISMA statement (items 5, 11, 16, and 23) acknowledges this iterative process. Aside from Cochrane reviews, all of which should have a protocol, only about 10% of systematic reviewers report working from a protocol.9 Without a protocol that is publicly accessible, it is difficult to judge between appropriate and inappropriate modifications.
Conduct and reporting of research are distinct concepts
This distinction is, however, less straightforward for systematic reviews than for assessments of the reporting of an individual study, because the reporting and conduct of systematic reviews are, by nature, closely intertwined. For example, the failure of a systematic review to report the assessment of the risk of bias in included studies may be seen as a marker of poor conduct, given the importance of this activity in the systematic review process.10
Study-level versus outcome-level assessment of risk of bias
For studies included in a systematic review, a thorough assessment of the risk of bias requires both a study-level assessment (such as adequacy of allocation concealment) and, for some features, a newer approach called outcome-level assessment. An outcome-level assessment involves evaluating the reliability and validity of the data for each important outcome by determining the methods used to assess them in each individual study.11 The quality of evidence may differ across outcomes, even within a study, such as between a primary efficacy outcome, which is likely to be carefully and systematically measured, and the assessment of serious harms,12 which may rely on spontaneous reports by investigators. This information should be reported to allow an explicit assessment of the extent to which an estimate of effect is correct.11
Importance of reporting biases
Different types of reporting biases may hamper the conduct and interpretation of systematic reviews. Selective reporting of complete studies (such as publication bias),13 as well as the more recently empirically demonstrated “outcome reporting bias” within individual studies,14 15 should be considered by authors when conducting a systematic review and reporting its results. Although the implications of these biases on the conduct and reporting of systematic reviews themselves are unclear, some research has identified that selective outcome reporting may occur also in the context of systematic reviews.16
Terminology
The terminology used to describe a systematic review and meta-analysis has evolved over time. One reason for changing the name from QUOROM to PRISMA was the desire to encompass both systematic reviews and meta-analyses. We have adopted the definitions used by the Cochrane Collaboration.17 A systematic review is a review of a clearly formulated question that uses systematic and explicit methods to identify, select, and critically appraise relevant research, and to collect and analyse data from the studies that are included in the review. Statistical methods (meta-analysis) may or may not be used to analyse and summarise the results of the included studies. Meta-analysis refers to the use of statistical techniques in a systematic review to integrate the results of included studies.
Developing the PRISMA statement
A three-day meeting was held in Ottawa, Canada, in June 2005 with 29 participants, including review authors, methodologists, clinicians, medical editors, and a consumer. The objective of the Ottawa meeting was to revise and expand the QUOROM checklist and flow diagram as needed.
The executive committee completed the following tasks before the meeting: a systematic review of studies examining the quality of reporting of systematic reviews; a comprehensive literature search to identify methodological and other articles that might inform the meeting, especially in relation to modifying checklist items; and an international survey of review authors, consumers, and groups commissioning or using systematic reviews and meta-analyses (including the International Network of Agencies for Health Technology Assessment and the Guidelines International Network) to ascertain views of QUOROM, including the merits of the existing checklist items. The results of these activities were presented during the meeting and are summarised on the PRISMA website, www.prisma-statement.org/.
Only items deemed essential were retained or added to the checklist. Some additional items are nevertheless desirable, and review authors should include these, if relevant.18 For example, it is useful to indicate whether the systematic review is an update of a previous review19 and to describe any changes in procedures from those described in the original protocol.
Shortly after the meeting, a draft of the PRISMA checklist was circulated to the group, including those invited to the meeting but unable to attend. A disposition file was created containing comments and revisions from each respondent, and the checklist was subsequently revised 11 times. The group approved the checklist, flow diagram, and this summary paper.
Although no direct evidence was found to support retaining or adding some items, evidence from other domains was believed to be relevant. For example, item 5 asks authors to provide registration information about the systematic review, including a registration number if available. Although systematic review registration is not yet widely available,20 21 the participating journals of the International Committee of Medical Journal Editors22 now require all clinical trials to be registered in an effort to increase transparency and accountability.23 Those aspects are also likely to benefit systematic reviewers, possibly reducing the risk of an excessive number of reviews addressing the same question24 25 and providing greater transparency when updating systematic reviews.
The PRISMA statement
The PRISMA statement consists of a 27 item checklist (table 1) and a four phase flow diagram (figure) (also available as extra items on bmj.com for researchers to download and re-use). The aim of the PRISMA statement is to help authors improve the reporting of systematic reviews and meta-analyses. We have focused on randomised trials, but PRISMA can also be used as a basis for reporting systematic reviews of other types of research, particularly evaluations of interventions. PRISMA may also be useful for critical appraisal of published systematic reviews. However, the PRISMA checklist is not a quality assessment instrument to gauge the quality of a systematic review.
Table 1.
Checklist of items to include when reporting a systematic review or meta-analysis
| Section/topic | Item No | Checklist item | Reported on page No |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable, background, objectives, data sources, study eligibility criteria, participants, interventions, study appraisal and synthesis methods, results, limitations, conclusions and implications of key findings, systematic review registration number | |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (such as web address), and, if available, provide registration information including registration number | |
| Eligibility criteria | 6 | Specify study characteristics (such as PICOS, length of follow-up) and report characteristics (such as years considered, language, publication status) used as criteria for eligibility, giving rationale | |
| Information sources | 7 | Describe all information sources (such as databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | |
| Study selection | 9 | State the process for selecting studies (that is, screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | |
| Data collection process | 10 | Describe method of data extraction from reports (such as piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | |
| Data items | 11 | List and define all variables for which data were sought (such as PICOS, funding sources) and any assumptions and simplifications made | |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | |
| Summary measures | 13 | State the principal summary measures (such as risk ratio, difference in means). | |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (such as I2 statistic) for each meta-analysis | |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (such as publication bias, selective reporting within studies) | |
| Additional analyses | 16 | Describe methods of additional analyses (such as sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (such as study size, PICOS, follow-up period) and provide the citations | |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome-level assessment (see item 12). | |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present for each study (a) simple summary data for each intervention group and (b) effect estimates and confidence intervals, ideally with a forest plot | |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see item 15) | |
| Additional analysis | 23 | Give results of additional analyses, if done (such as sensitivity or subgroup analyses, meta-regression) (see item 16) | |
| Discussion | |||
| Summary of evidence | 24 | Summarise the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (such as health care providers, users, and policy makers) | |
| Limitations | 25 | Discuss limitations at study and outcome level (such as risk of bias), and at review level (such as incomplete retrieval of identified research, reporting bias) | |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (such as supply of data) and role of funders for the systematic review |
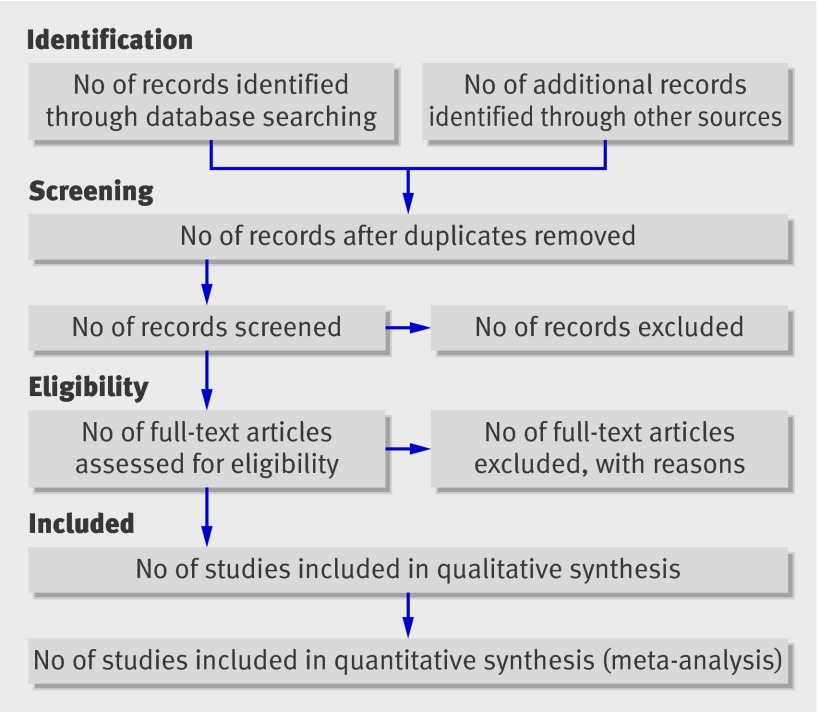
Flow of information through the different phases of a systematic review.
From QUOROM to PRISMA
The new PRISMA checklist differs in several respects from the QUOROM checklist, and table 2 lists the substantive specific changes. Generally, the PRISMA checklist “decouples” several items present in the QUOROM checklist and, where applicable, several checklist items are linked to improve consistency across the systematic review report.
Table 2.
Substantive specific changes between the QUOROM checklist and the PRISMA checklist (a tick indicates the presence of the topic in QUOROM or PRISMA)
| Section/topic and item | QUOROM | PRISMA | Comment |
|---|---|---|---|
| Abstract | √ | √ | QUOROM and PRISMA ask authors to report an abstract. However, PRISMA is not specific about format |
| Introduction: | |||
| Objective | √ | This new item (4) addresses the explicit question the review addresses using the PICO reporting system (which describes the participants, interventions, comparisons, and outcome(s) of the systematic review), together with the specification of the type of study design (PICOS); the item is linked to items 6, 11, and 18 of the checklist | |
| Methods: | |||
| Protocol | √ | This new item (5) asks authors to report whether the review has a protocol and if so how it can be accessed | |
| Search | √ | √ | Although reporting the search is present in both QUOROM and PRISMA checklists, PRISMA asks authors to provide a full description of at least one electronic search strategy (item 8). Without such information it is impossible to repeat the authors’ search |
| Assessment of risk of bias in included studies | √ | √ | Renamed from “quality assessment” in QUOROM. This item (12) is linked to reporting this information in the results (item 19). The new concept of “outcome level” assessment has been introduced |
| Assessment of risk of bias across studies | √ | This new item (15) asks authors to describe any assessments of risk of bias in the review, such as selective reporting within the included studies. This item is linked to reporting this information in the results (item 22) | |
| Discussion | √ | √ | Although both QUOROM and PRISMA checklists address the discussion section, PRISMA devotes three items (24-26) to the discussion. In PRISMA the main types of limitations are explicitly stated and their discussion required |
| Funding | √ | This new item (27) asks authors to provide information on any sources of funding for the systematic review. |
The flow diagram has also been modified. Before including studies and providing reasons for excluding others, the review team must first search the literature. This search results in records. Once these records have been screened and eligibility criteria applied, a smaller number of articles will remain. The number of included articles might be smaller (or larger) than the number of studies, because articles may report on multiple studies and results from a particular study may be published in several articles. To capture this information, the PRISMA flow diagram now requests information on these phases of the review process.
Endorsement
The PRISMA statement should replace the QUOROM statement for those journals that have endorsed QUOROM. We hope that other journals will support PRISMA; they can do so by registering on the PRISMA website. To emphasise to authors and others the importance of transparent reporting of systematic reviews, we encourage supporting journals to reference the PRISMA statement and include the PRISMA web address in their instructions to authors. We also invite editorial organisations to consider endorsing PRISMA and encourage authors to adhere to its principles.
The PRISMA explanation and elaboration paper
In addition to the PRISMA statement, a supporting explanation and elaboration document has been produced26 following the style used for other reporting guidelines.27 28 29 The process of completing this document included developing a large database of exemplars to highlight how best to report each checklist item, and identifying a comprehensive evidence base to support the inclusion of each checklist item. The explanation and elaboration document was completed after several face to face meetings and numerous iterations among several meeting participants, after which it was shared with the whole group for additional revisions and final approval. Finally, the group formed a dissemination subcommittee to help disseminate and implement PRISMA.
Discussion
The quality of reporting of systematic reviews is still not optimal.9 30 31 32 33 34 In a recent review of 300 systematic reviews, few authors reported assessing possible publication bias,9 even though there is overwhelming evidence for its existence13 and its impact on the results of systematic reviews.35 Even when the possibility of publication bias is assessed, there is no guarantee that systematic reviewers have assessed or interpreted it appropriately.36 Although the absence of reporting such an assessment does not necessarily indicate that it was not done, reporting an assessment of possible publication bias is likely to be a marker of the thoroughness of the conduct of the systematic review.
Several approaches have been developed to conduct systematic reviews on a broader array of questions. For example, systematic reviews are now conducted to investigate cost effectiveness,37 diagnostic38 or prognostic questions,39 genetic associations,40 and policy making.41 The general concepts and topics covered by PRISMA are relevant to any systematic review, not just those summarising the benefits and harms of a healthcare intervention. However, some modifications of the checklist items or flow diagram will be necessary in particular circumstances. For example, assessing the risk of bias is a key concept, but the items used to assess this in a diagnostic review are likely to focus on issues such as the spectrum of patients and the verification of disease status, which differ from reviews of interventions. The flow diagram will also need adjustments when reporting meta-analysis of individual patient data.42
We have developed an explanatory document to increase the usefulness of PRISMA.26 For each checklist item, this document contains an example of good reporting, a rationale for its inclusion, and supporting evidence, including references, whenever possible. We believe this document will also serve as a useful resource for those teaching systematic review methodology. We encourage journals to include reference to the explanatory document in their instructions to authors.
Like any evidence based endeavour, PRISMA is a living document. To this end we invite readers to comment on the revised version, particularly the new checklist and flow diagram, through the PRISMA website. We will use such information to inform PRISMA’s continued development.
The following people contributed to the PRISMA statement: Doug Altman, Centre for Statistics in Medicine (Oxford); Gerd Antes, University Hospital Freiburg (Freiburg, Germany); David Atkins, Health Services Research and Development Service, Veterans Health Administration (Washington DC, USA); Virginia Barbour, PLoS Medicine (Cambridge, UK); Nick Barrowman, Children’s Hospital of Eastern Ontario (Ottawa, Canada); Jesse A. Berlin, Johnson & Johnson Pharmaceutical Research and Development (Titusville NJ, USA); Jocalyn Clark, PLoS Medicine (at the time of writing, BMJ, London); Mike Clarke, UK Cochrane Centre (Oxford) and School of Nursing and Midwifery, Trinity College (Dublin, Ireland); Deborah Cook, Departments of Medicine, Clinical Epidemiology and Biostatistics, McMaster University (Hamilton, Canada); Roberto D’Amico, Università di Modena e Reggio Emilia (Modena, Italy) and Centro Cochrane Italiano, Istituto Ricerche Farmacologiche Mario Negri (Milan, Italy); Jonathan J Deeks, University of Birmingham (Birmingham); P J Devereaux, Departments of Medicine, Clinical Epidemiology and Biostatistics, McMaster University; Kay Dickersin, Johns Hopkins Bloomberg School of Public Health (Baltimore MD, USA); Matthias Egger, Department of Social and Preventive Medicine, University of Bern (Bern, Switzerland); Edzard Ernst, Peninsula Medical School (Exeter, UK); Peter C Gøtzsche, Nordic Cochrane Centre (Copenhagen, Denmark); Jeremy Grimshaw, Ottawa Hospital Research Institute (Ottawa, Canada); Gordon Guyatt, Departments of Medicine, Clinical Epidemiology and Biostatistics, McMaster University; Julian Higgins, MRC Biostatistics Unit (Cambridge, UK); John P A Ioannidis, University of Ioannina Campus (Ioannina, Greece); Jos Kleijnen, Kleijnen Systematic Reviews (York, UK) and School for Public Health and Primary Care (CAPHRI), University of Maastricht (Maastricht, Netherlands); Tom Lang, Tom Lang Communications and Training (Davis CA, USA); Alessandro Liberati, Università di Modena e Reggio Emilia, and Centro Cochrane Italiano, Istituto Ricerche Farmacologiche Mario Negri; Nicola Magrini, NHS Centre for the Evaluation of the Effectiveness of Health Care—CeVEAS (Modena, Italy); David McNamee, Lancet (London, UK); Lorenzo Moja, Centro Cochrane Italiano, Istituto Ricerche Farmacologiche Mario Negri; David Moher, Ottawa Methods Centre, Ottawa Hospital Research Institute (Ottawa, Canada); Cynthia Mulrow, Annals of Internal Medicine (Philadelphia PA, USA); Maryann Napoli, Center for Medical Consumers (New York, USA); Andy Oxman, Norwegian Health Services Research Centre (Oslo, Norway); Ba’ Pham, Toronto Health Economics and Technology Assessment Collaborative (Toronto, Canada) (at the time of first meeting of the group, GlaxoSmithKline Canada, Mississauga, Ontario); Drummond Rennie, University of California San Francisco (San Francisco CA, USA); Margaret Sampson, Children’s Hospital of Eastern Ontario (Ottawa, Canada); Kenneth F Schulz, Family Health International (Durham NC, USA); Paul G Shekelle, Southern California Evidence Based Practice Center (Santa Monica CA, USA); Jennifer Tetzlaff, Ottawa Methods Centre, Ottawa Hospital Research Institute; David Tovey, Cochrane Library, Cochrane Collaboration (Oxford, UK) (at the time of first meeting of the group, BMJ, London); Peter Tugwell, Institute of Population Health, University of Ottawa (Ottawa, Canada).
Author contributions: ICMJE criteria for authorship read and met—DM. Agree with the recommendations—DM, AL, JT, DGA. Wrote the first draft of the paper—DM, AL, DGA. Contributed to the writing of the paper—DM, AL, JT, DGA. Participated in regular conference calls, identified the participants, secured funds, planned the meeting, participated in the meeting, and drafted the manuscript—DM, AL, DGA. Participated in identifying the evidence base for PRISMA, refining the checklist, and drafting the manuscript—JT.
Funding: PRISMA was funded by the Canadian Institutes of Health Research; Università di Modena e Reggio Emilia, Italy; Cancer Research UK; Clinical Evidence BMJ Knowledge; the Cochrane Collaboration; and GlaxoSmithKline, Canada. AL is funded, in part, through grants of the Italian Ministry of University (COFIN-PRIN 2002 prot 2002061749 and COFIN-PRIN 2006 prot 2006062298). DGA is funded by Cancer Research UK. DM is funded by a University of Ottawa Research Chair. None of the sponsors had any involvement in the planning, execution, or writing of the PRISMA documents. No funder played a role in drafting this manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
In order to encourage dissemination of the PRISMA statement, this article is freely accessible on bmj.com and will also be published in PLoS Medicine, Annals of Internal Medicine, Journal of Clinical Epidemiology, and Open Medicine. The authors jointly hold the copyright of this article. For details on further use, see the PRISMA website (www.prisma-statement.org/).
Cite this as: BMJ 2009;338:b2535
References
- 1.Oxman AD, Cook DJ, Guyatt GH. Users’ guides to the medical literature. VI. How to use an overview. Evidence-Based Medicine Working Group. JAMA 1994;272:1367-71. [DOI] [PubMed] [Google Scholar]
- 2.Swingler GH, Volmink J, Ioannidis JP. Number of published systematic reviews and global burden of disease: database analysis. BMJ 2003;327:1083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canadian Institutes of Health Research. Randomized controlled trials registration/application checklist (12/2006). 2006. www.cihr-irsc.gc.ca/e/documents/rct_reg_e.pdf (accessed 19 May 2009).
- 4.Young C, Horton R. Putting clinical trials into context. Lancet 2005;366:107. [DOI] [PubMed] [Google Scholar]
- 5.Mulrow CD. The medical review article: state of the science. Ann Intern Med 1987;106:485-8. [DOI] [PubMed] [Google Scholar]
- 6.Sacks HS, Berrier J, Reitman D, Ancona-Berk VA, Chalmers TC. Meta-analysis of randomized controlled trials. N Engl J Med 1987;316:450-5. [DOI] [PubMed] [Google Scholar]
- 7.Sacks HS, Reitman D, Pagano D, Kupelnick B. Meta-analysis: an update. Mt Sinai J Med 1996;63:216-24. [PubMed] [Google Scholar]
- 8.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF, for the QUOROM group. Improving the quality of reporting of meta-analysis of randomized controlled trials: The QUOROM statement. Lancet 1999;354:1896-1900. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Tetzlaff J, Tricco AC, Sampson M, Altman DG. Epidemiology and reporting characteristics of systematic reviews. PLoS Med 2007;4:e78, 10.1371/journal.pmed.0040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moja LP, Telaro E, D’Amico R, Moschetti I, Coe L, Liberati A. Assessment of methodological quality of primary studies by systematic reviews: results of the metaquality cross sectional study. BMJ 2005;330:1053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al, for the GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schunemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, et al, for the ATS Documents Development and Implementation Committee. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med 2006;174:605-14. [DOI] [PubMed] [Google Scholar]
- 13.Dickersin K. Publication bias: recognizing the problem, understanding its origins and scope, and preventing harm. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication bias in meta-analysis—prevention, assessment and adjustments. Chichester: John Wiley, 2005:11-33.
- 14.Chan AW, Hrobjartsson A, Haahr MT, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291:2457-65. [DOI] [PubMed] [Google Scholar]
- 15.Chan AW, Krleza-Jeric K, Schmid I, Altman DG. Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research. CMAJ 2004;171:735-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silagy CA, Middleton P, Hopewell S. Publishing protocols of systematic reviews: comparing what was done to what was planned. JAMA 2002;287:2831-4. [DOI] [PubMed] [Google Scholar]
- 17.Green S, Higgins J, eds. Glossary. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. www.cochrane.org/resources/glossary.htm (accessed 19 May 2009).
- 18.Strech D, Tilburt J. Value judgments in the analysis and synthesis of evidence. J Clin Epidemiol 2008;61:521-4. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Tsertsvadze A. Systematic reviews: when is an update an update? Lancet 2006;367:881-3. [DOI] [PubMed] [Google Scholar]
- 20.University of York. Centre for Reviews and Dissemination, 2009. www.york.ac.uk/inst/crd/ (accessed 19 May 2009).
- 21.Joanna Briggs Institute. Protocols & work in progress, 2008. www.joannabriggs.edu.au/pubs/systematic_reviews_prot.php (accessed 19 May 2009).
- 22.De Angelis C, Drazan JM, Frizelle FA, Haug C, Hoey J, et al, for the International Committee Medical Journal Editors. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. CMAJ 2004;171:606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, et al. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet 2004;363:1341-5. [DOI] [PubMed] [Google Scholar]
- 24.Bagshaw SM, McAlister FA, Manns BJ, Ghali WA. Acetylcysteine in the prevention of contrast-induced nephropathy: a case study of the pitfalls in the evolution of evidence. Arch Intern Med 2006;166:161-6. [DOI] [PubMed] [Google Scholar]
- 25.Biondi-Zoccai GG, Lotrionte M, Abbate A, Testa L, Remigi E, et al. Compliance with QUOROM and quality of reporting of overlapping meta-analyses on the role of acetylcysteine in the prevention of contrast associated nephropathy: case study. BMJ 2006;332:202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al, for the PRISMA Group. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altman DG, Schulz KR, Moher D, Egger M, Davidoff F, et al, for the CONSORT group. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001;134:663-94. [DOI] [PubMed] [Google Scholar]
- 28.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, et al, for the STARD group. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD explanation and elaboration. Ann Intern Med 2003;138:W1-12. [DOI] [PubMed] [Google Scholar]
- 29.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, et al, for the STROBE initiative. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Ann Intern Med 2007;147:W163-94. [DOI] [PubMed] [Google Scholar]
- 30.Bhandari M, Morrow F, Kulkarni AV, Tornetta P. Meta-analyses in orthopaedic surgery: a systematic review of their methodologies. J Bone Joint Surg Am 2001;83-A:15-24. [PubMed]
- 31.Kelly KD, Travers A, Dorgan M, Slater L, Rowe BH. Evaluating the quality of systematic reviews in the emergency medicine literature. Ann Emerg Med 2001;38:518-26. [DOI] [PubMed] [Google Scholar]
- 32.Richards D. The quality of systematic reviews in dentistry. Evid Based Dent 2004;5:17. [DOI] [PubMed] [Google Scholar]
- 33.Choi PT, Halpern SH, Malik N, Jadad AR, Tramer MR, et al. Examining the evidence in anesthesia literature: a critical appraisal of systematic reviews. Anesth Analg 2001;92:700-9. [DOI] [PubMed] [Google Scholar]
- 34.Delaney A, Bagshaw SM, Ferland A, Manns B, Laupland KB. A systematic evaluation of the quality of meta-analyses in the critical care literature. Crit Care 2005;9:R575-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton AJ. Evidence concerning the consequences of publication and related biases. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication bias in meta-analysis—prevention, assessment and adjustments. Chichester: John Wiley, 2005:175-92.
- 36.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333:597-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ladabaum U, Chopra CL, Huang G, Scheiman JM, Chernew ME, et al. Aspirin as an adjunct to screening for prevention of sporadic colorectal cancer: a cost-effectiveness analysis. Ann Intern Med 2001;135:769-81. [DOI] [PubMed] [Google Scholar]
- 38.Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ 2001;323:157-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG. Replication validity of genetic association studies. Nat Genet 2001;29:306-9. [DOI] [PubMed] [Google Scholar]
- 41.Lavis J, Davies H, Oxman A, Denis J, Golden-Biddle K, et al. Towards systematic reviews that inform health care management and policy-making. J Health Serv Res Policy 2005;10:35-48. [DOI] [PubMed] [Google Scholar]
- 42.Stewart LA, Clarke MJ. Practical methodology of meta-analyses (overviews) using updated individual patient data. Cochrane Working Group. Stat Med 1995;14:2057-79. [DOI] [PubMed] [Google Scholar]