Sleep apnea: A proinflammatory disorder that coaggregates with obesity (original) (raw)
. Author manuscript; available in PMC: 2009 Aug 3.
Published in final edited form as: J Allergy Clin Immunol. 2008 May;121(5):1096–1102. doi: 10.1016/j.jaci.2008.04.002
Abstract
Both obesity and sleep apnea are prevalent health conditions that frequently coaggregate. Obesity-associated inflammation may influence asthma control; the relation of sleep apnea to asthma or allergic rhinitis may be bidirectional. Both obesity and sleep apnea are associated with augmented levels of inflammation and oxidative stress, and it is biologically plausible that the proinflammatory effects of one disorder influence the expression of the other disorder. This article elucidates mechanistic associations among obesity, sleep apnea, and systemic inflammation; highlights interrelationships between these factors with cardiopulmonary disease; and identifies specific areas for future research directions.
Keywords: Sleep apnea, obesity, inflammation, asthma
Sleep apnea, characterized by loud snoring and daytime sleepiness, is a prevalent disorder, affecting upwards of 2% to 4% of the population.1 It is caused by episodes of repetitive partial or complete upper airway collapse during sleep, which causes sleep disruption and fragmentation, as well as intermittent hypoxia, ventilatory overshoot hyperoxia, sympathetic nervous system surges, alterations in intrathoracic pressure, and local airway edema and inflammation. Sleep apnea also frequently coaggregates with obesity, and both conditions may independently or synergistically influence cardiopulmonary function and systemic inflammation. Moreover, sleep apnea and accompanying obesity may share common inflammatory pathways with asthma and allergic rhinitis. The relation of sleep apnea to asthma or allergic rhinitis may be bidirectional. To date, most research has been directed at understanding the links among sleep apnea, systemic inflammation, and cardiac responses. In this article, we present mechanistic associations between sleep apnea, obesity, and systemic inflammation. We highlight interrelationships between these factors and pulmonary as well as cardiac disease and identify specific areas for future research directions.
ACUTE PHYSIOLOGICAL RESPONSES TO SLEEP APNEA
Systemic responses
One mechanism by which sleep apnea may contribute to systemic inflammation is through increased sympathetic nervous system activation, which may be induced by upper airway closure, hypoxia, hypercarbia, and/or arousals associated with the respiratory disturbances. Sleep apnea–related enhanced sympathetic nervous system activity likely plays a pathophysiologic role in the development of the acute increases in blood pressure commonly observed at the termination of apneas. It is also implicated in the pathogenesis of sustained systemic hypertension. Augmented sympathetic nervous system activity also appears to be associated with cytokine expression and oxidative stress.2 In addition, elevated catecholamine levels may undergo auto-oxidation, and this has been considered to be a cause of catecholamine-induced cardiomyopathy, which may account for cardiovascular disease complications in individuals with sleep apnea.
Cyclical episodes of hypoxia-reoxygenation, analogous to cardiac ischemia/reoxygenation injury, occur repetitively in patients with sleep apnea, causing ATP depletion and xanthine oxidase activation, and increasing the generation of oxygen-derived free radicals. Responses to repetitive hypoxia-reoxygenation also include activation of endothelial cells and leukocytes, increased expression of adhesion molecules, activation of the proinflammatory transcription factor nuclear factor-κB,3 and activation of other stress genes and their products such as hypoxia inducible factor 1.4 Independent of gas exchange abnormalities, sleep apnea may contribute to systemic inflammation via effects on sleep continuity and duration, which may alter sympathetic nervous system activity and/or influence the hypothalamic pituitary adrenal axis and neurohumoral responses. Specific reductions in rapid eye movement sleep, in particular, have been reported to be associated with activation of lipid peroxidation and inhibition of antioxidants.5 These effects may explain the increased cardiovascular morbidity reported to be associated with short sleep duration in several epidemiologic surveys.6 However, it is also plausible that similar effects may contribute to respiratory morbidity.
Local upper airway inflammation
The intermittent obstruction of the upper airway that is the defining characteristic of sleep apnea leads to airflow turbulence, which may trigger local mucosal pharyngeal inflammation as well as systemic inflammatory responses and oxidative stress. Support for this includes reports of increases in IL-6,7 8-isoprostane concentrations, leukotriene B4, exhaled nitric oxide,8,9 and neutrophilia in the exhaled breath condensate or induced sputum and reduced pH in exhaled breath condensate of patients with sleep apnea compared with controls.10 However, in several of these studies, differences in inflammatory and oxidative markers were most significant when obese patients with sleep apnea were compared with nonobese controls, and were not significantly different when obese patients with sleep apnea were compared with obese subjects without sleep apnea,8 suggesting that underlying obesity may play a large role in mediating inflammatory responses, particularly in regard to elevations in exhaled IL-6 and F2-isoprostane levels (Table I11).
TABLE I.
Studies examining sleep apnea, obesity, and their relation to local upper airway inflammation
| Samplecharacteristics | Markers of interest | Findings | Effects independentof obesity? | |
|---|---|---|---|---|
| Carpagnano et al, 20027 | n = 28 | IL-6 in EBC | IL-6: higher in sleep apnea patients (8.7 ± 0.3 pg/mL) than healthy controls (1.6 ± 0.1 pg/mL; P < .0001). Obese controls had lower levels than sleep apnea patients (2.1 ± 0.2 pg/mL; P < .0001) | Exhaled IL-6: yes |
| Referral sample | 8-Isoprostane in EBC | 8-Isoprostane: levels were not higher in sleep apnea (7.4 ± 0.7 pg/mL) than obese subjects (5 ± 0.3 pg/mL; P = .4), but were higher than in healthy subjects (4.5 ± 0.5 pg/mL; P < .005). | Exhaled 8-isoprostane: no | |
| Depalo et al, 20088 | n = 43 | Exhaled NO | Exhaled NO: increased in patients with sleep apnea and obese patients (23.1 ± 2.1 and 17.9 ± 2.1 ppb) than in healthy subjects (7.2 ± 0.6 ppb; P < .001). | Exhaled NO: no |
| Referral sample | iNOS expression in induced sputum cells | iNOS: sleep apnea and obese patients showed increased expression in neutrophils and macrophages with respect to healthy subjects. | Sputum iNOS expression: no | |
| Petrosyan et al, 20079 | n = 45 | Exhaled NO | Exhaled NO: higher in patients with sleep apnea than in control subjects (P < .05). | Exhaled NO: no |
| Referral sample | Exhaled CO | Exhaled CO: higher in patients with sleep apnea than in control subjects (P < .05). | Exhaled CO: no | |
| 8-Isoprostane in EBC | 8-Isoprostane in EBC: elevated in patients with sleep apnea in comparison with obese subjects (P < .01). | Exhaled 8-isoprostane: yes | ||
| Leukotriene B4 in EBC | Leukotriene B4 in EBC: elevated in patients with sleep apnea in comparison with obese subjects (P < .01). | Leukotriene B4 in EBC: yes | ||
| Carpagnano et al, 200311 | n = 30 | |||
| Referral sample | 8-Isoprostane in EBC | 8-Isoprostane: higher in morning EBC (9.5 ± 1.9 pg/mL) and plasma (9.7 ± 1.5 pg/mL) of patients with sleep apnea compared with healthy obese subjects (6.7 ± 0.2 and 7.1 ± 0.3 pg/mL, respectively; P < .0001). | Exhaled 8-isoprostane: yes |
SYSTEMIC INFLAMMATION IN SLEEP APNEA IL-6
IL-6, a proinflammatory cytokine secreted by T cells and macrophages, increases in response to experimental intermittent hypercapnic hypoxia.12 Increased circulating levels of IL-6 levels have been demonstrated to be correlated with sleep apnea severity, 13 although one of the largest studies to examine this suggested that the increased levels of IL-6 could be largely attributable to increased obesity.14 The latter study, however, showed that even after considering the confounding influences of obesity, patients with sleep apnea have higher morning soluble IL-6 receptor levels, a marker with more expansive physiologic effects than IL-6 alone and also reported to be elevated in inflammatory pulmonary conditions such as asthma and sarcoidosis.15,16 The latter findings suggest that soluble IL-6 receptor signaling pathways may play a common role in the pathogenesis of both sleep apnea and asthma, potentially independent of obesity.
C-reactive protein
C-reactive protein (CRP) is an acute phase reactant produced by the liver and adipocytes and is influenced by IL-6 levels. It has emerged as a salient marker of cardiovascular risk. Although all reports of CRP and sleep apnea have reported CRP levels to be increased in sleep apnea, the data have been inconsistent as to whether this relationship is independent of obesity or explained by other confounders.17,18 However, several studies of relatively healthy samples, include a sample of men referred to a sleep laboratory screened to be free of underlying cardiovascular or metabolic disease, and 2 samples of children, demonstrated significant associations between CRP levels of sleep apnea severity.19,20 In addition, data from 2 small interventional trials have demonstrated reductions in CRP levels with continuous positive airway pressure (CPAP) treatment, consistent with sleep apnea as a reversible trigger for increased systemic inflammation.21,22
TNF-α
TNF-α is a cytokine involved in systemic inflammation. Data have demonstrated circulating levels TNF-α levels are significantly elevated in sleep apnea, independent of body mass index,23,24 and levels are associated with the degree of nocturnal sleep disturbance.24 There is evidence that TNF-α plays a role in mediating apoptotic death and cellular proliferation and contributes to atherosclerosis, and possibly to the pathogenesis of asthma. The latter effects may related to adverse effects of TNF-α on smooth muscle function.25
SLEEP APNEA AND OXIDATIVE STRESS
Oxidative stress is considered to be a fundamental cellular pathogenic process in metabolic and cardiovascular function and a key mediator of chronic inflammatory states such as asthma.26 Oxidative stress represents a state of imbalance between increased production of free radicals such as reactive oxygen species (ROS) and reduced antioxidant activity and may be assessed via direct measurements of ROS comprising superoxide, hydrogen peroxide, and the hydroxyl radical as well as enzymes such as nicotinamide adenine dinucleotide phosphate oxidases, superoxide dismutase, and glutathione peroxidase, which are involved with regulation of ROS production. Indirect measurements of oxidative stress may be ascertained by measurement of oxidized products of lipids, proteins, or DNA.
Measures of lipid peroxidation, malondialdehyde, thiobarbituric acid reactive substances, and oxidized low-density lipoprotein levels from urinary, plasma, exhaled condensate, or sputum samples have been the most studied measures of oxidative stress in sleep apnea. Measurement of F2-isoprostane is currently considered to be one of the most accurate methods to quantify lipid peroxidation. A pediatric study did not observe increased urinary F-2 isoprostanes in association with sleep apnea severity 27; however, the sample was small and included only a few children with severe levels of hypoxemia. Increased levels of overnight urinary 8-isoprostane levels have been reported in sleep apnea, with levels correlated with degree of hypoxia, and decreasing after CPAP treatment.28 Morning plasma malondialdehyde level has been reported to be significantly higher in subjects with sleep apnea compared with controls.29 Malondialdehyde levels also have been reported to be elevated in the intercostal muscles of individuals with sleep apnea, with elevations persisting even after long-term sleep apnea treatment.30 Inconsistent associations with sleep apnea have been reported when oxidative stress was quantified by using measures of thiobarbituric acid reactive substances,31–33 oxidized low-density lipoprotein,33,34 and circulating free nitrotyrosine.35 However, many of these measures are difficult to quantify accurately, and most studies have had small sample sizes. Neutrophil superoxide generation has been observed to be increased in sleep apnea and superoxide generation reversed with treatment.36 Oxidized DNA, measured by urinary 8-hydroxy-2’-deoxyguanosine excretion, was significantly higher in the subjects with severe sleep apnea than controls and correlated with degree of intermittent hypoxemia.26 Individuals with sleep apnea also have been reported to have lower total antioxidant capacity with normalization after treatment.37 Very little work to date has harnessed the power of microassay studies to understand sleep apnea stresses. However, 1 microarray study has shown that sleep apnea is associated with increased expression of genes involved in modulation of ROS, including heme oxygenase 1, superoxide dismutase 1 and 2, and catalase, and genes involved in cell growth, proliferation, and the cell cycle.38 It is plausible that the oxidative stresses associated with sleep apnea may contribute to an altered oxidant/antioxidant imbalance that may accompany asthma. Additional research is needed to clarify these potentially interacting mechanistic pathways, including the role of obesity in these relationships.
SLEEP APNEA AND THROMBOSIS, INSULIN RESISTANCE, AND IMMUNE FUNCTION
Coagulation factors
There is some evidence for a hypercoagulable state in sleep apnea, which may help explain the increased prevalence of vascular diseases in this population. Sleep apnea has been associated with increased levels of plasminogen activator inhibitor 1,39 fibrinogen,40 activated coagulation factors XIIa and VIIa, thrombin/antithrombin III complexes, and soluble P-selectin.41 Most studies have identified levels of hypoxemia to correlate with these levels. However, the studies have been inconsistent regarding the extent to which associations were independent of obesity and how levels changed with sleep apnea treatment. Coagulation and fibrinolysis also have been shown to play a role in the pathogenesis of asthma. For example, activated factor X may result in a dose-dependent increase in mucin production and collagen deposition, thereby mediating the asthmatic response.42 Sleep apnea-induced alterations in hemostasis may therefore contribute to airway hyperreactivity in susceptible patients.
Insulin resistance
Several observational studies have demonstrated associations between sleep apnea and insulin resistance that are independent of obesity.43 An interventional study demonstrated improved insulin sensitivity in patients with moderate to severe sleep apnea with treatment, with the best responses occurring in those with a body mass index <30 kg/m2.44 Because increased levels of insulin and diabetes have been associated with pulmonary dysfunction,45 it is possible that sleep apnea–related increases in insulin resistance may contribute to the morbidities associated with asthma and chronic obstructive lung disease.
Immunologic effects
All the mechanisms described earlier that may affect the sympathetic nervous system, cytokine and insulin levels, and oxidative stress likely will also influence other aspects of immunologic function. To date, most research has been directed at understanding the link between sleep apnea and cardiovascular disease, with an emphasis on actions of proinflammatory cytokines or the anti-inflammatory cytokine IL-10.46 However, sleep apnea and its attendant hypoxia have been associated with enhanced adhesion of neutrophils, an effect that may contribute to airway inflammation and bronchial hyperresponsiveness.46,47 Sleep apnea is also characterized by a “leptin resistant” state, and leptin has immune-modulating functions that may affect the asthma phenotype.48 Preliminary work has been reported on other immune-mediated mechanisms in sleep apnea, such as CD40-CD40 ligand interaction.49 Additional work is needed to assess sleep apnea–related immunologic perturbations more fully that may also influence airway and lung disease.
OBESITY AND SLEEP APNEA: INTERSECTING PATHWAYS PROMOTING INFLAMMATION
In middle-aged adults, obesity is the strongest but by no means the only risk factor for sleep apnea. Relative to stable weight, a 10% weight gain predicts an approximate 32% increase in sleep apnea severity and a 6-fold increased sleep apnea incidence; a 10% weight loss predicts a 26% decrease in severity.50 Obesity confers sleep apnea risk through alteration in upper airway structure (altered geometry and increased collapsibility), reduction in the functional residual capacity, and imbalance in the relationship between respiratory drive and load compensation. Central obesity, which is also associated with metabolic abnormalities, is particularly strongly associated with sleep apnea; that is, sleep apnea severity is associated with levels of waist circumference, upper body skin-fold thickness, and visceral adipose content.51 In addition, because sleep apnea often results in reduced sleep duration and sleep fragmentation, which may promote weight gain through effects on appetite-regulating hormones and metabolism,52 it is plausible that sleep apnea and obesity may be related through bidirectional pathways. Additional sleep apnea risk factors include male sex, increasing age, African American or Asian race, genetic factors, craniofacial structure, and/or neuromuscular disease. In younger and older populations, the strength of the association between sleep apnea and obesity is weaker, and conversely, the relative importance of other factors such as tonsillar size (in children) or frailty (in the elderly) increase.
Because of the frequent coaggregation of obesity and sleep apnea, it is often difficult to discern which health or pathophysiological effects are attributable to one or the other condition, and which effects may reflect additive or synergistic effects. As discussed, sleep apnea, through hypoxia/hyperoxia and sleep fragmentation, may cause or exacerbate proinflammatory states through effects on sympathetic hyperreactivity and/or oxidative stress. Many of the adverse metabolic and inflammatory effects of obesity are also considered to be mediated by similar prooxidative and sympathetic pathways, which include adipose tissue production of proinflammatory cytokines and chemokines such as TNF-α, IL-6, and leptin and reduced levels of adiponectin. Because in rodent models, background levels of obesity influence metabolic responses to intermittent hypoxemia,53 it is plausible that the proinflammatory responses to sleep apnea in human beings also are influenced by obesity, and conversely, that obesity-related effects on inflammation and cardiopulmonary disease are influenced by coexisting sleep apnea.
SLEEP APNEA, OBESITY, AND ASTHMA/ALLERGIC RHINITIS: AN INFLAMMATORY TRIAD
It is well recognized that asthma and sleep apnea are both prevalent health conditions that frequently coaggregate and share common risk factors such as obesity and prematurity and preferentially affect common population subgroups, specifically African Americans and individuals of low socioeconomic class.54 However, data to date do not provide definitive evidence of whether these associations are causal or of their directionality. Cross-sectional population-based data indicate that compared to subjects without asthma or nonwheezers, subjects with asthma or those who wheeze have higher rates of snoring and witnessed apneas,55,56 with associations stronger in younger compared with older individuals. In children, snoring also has been associated with exercise-induced asthma and atopy.57 Even after adjusting for obesity and race, persistent wheezing in childhood has been demonstrated to increase the likelihood of objectively measured sleep apnea more than 5-fold.58 A large 14-year longitudinal study showed that independent of obesity and other factors, a new diagnosis of asthma increased the incidence of snoring by almost 3-fold.59
Asthma and sleep apnea may both be characterized by generalized abnormalities of the nasopharynx and lower airway, which may coexist either because of common airway responses to inflammatory or atopic stimuli or because of genetic or other developmental factors that similarly affect the upper and lower airways. The potential importance of nasal pathology, including allergic rhinitis, in the link between asthma and sleep apnea is suggested by the results of a population-based study of adults that reported stronger associations of snoring with nasal congestion than with asthma.60 A large Swedish cohort study reported an approximate 2-fold higher odds of rhinitis in individuals with daytime sleepiness, snoring, and witnessed apneas (key symptoms of sleep apnea) even after adjustment for confounders.56 Also, optimal allergic rhinitis treatment decreases sleep apnea severity and prevents emergence of elongated facies in children, a known sleep apnea risk factor.61 It is also possible that stimulation of nasal mechanoreceptors because of sleep apnea–related snoring may trigger an inflammatory cascade resulting in rhinitis symptoms; therefore, a bidirectional relationship between sleep apnea and rhinitis may be in effect.
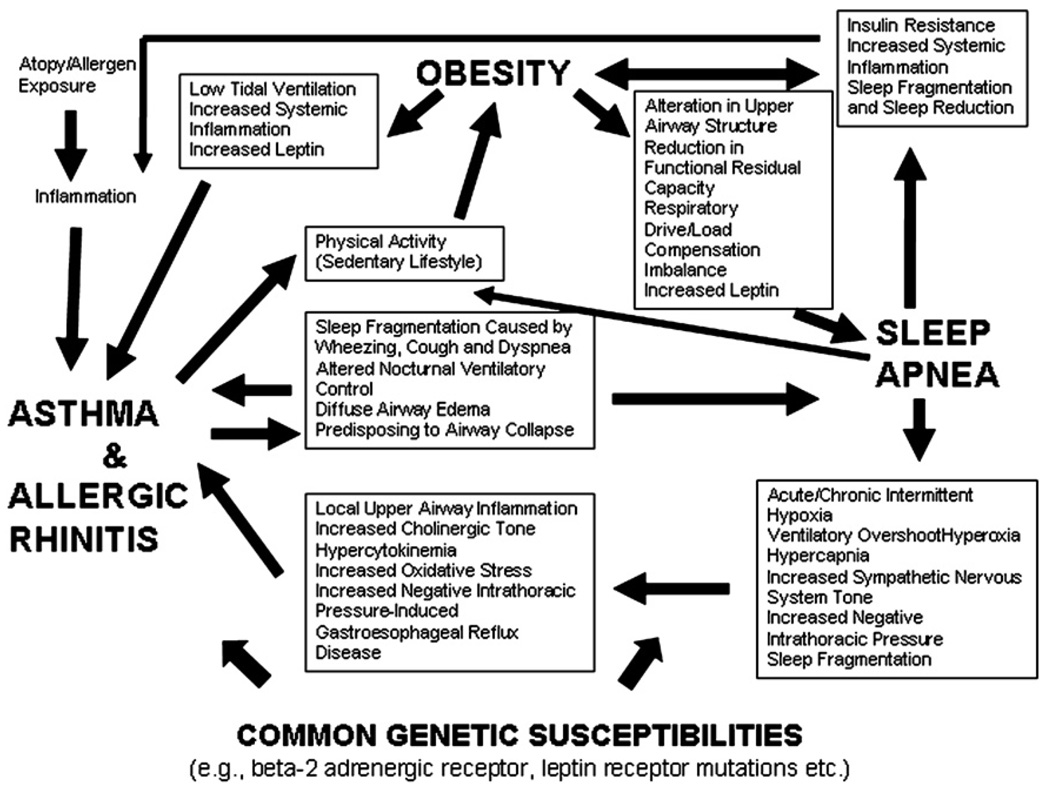
The pathogenetic links between obesity and asthma are reviewed in Dr Shore’s review in this issue of the Journal,62 and in this article, the links between obesity and sleep apnea are discussed. The overlapping inflammatory and physiologic mechanisms linking sleep apnea, obesity, and asthma/allergic rhinitis are depicted in Fig 1. All 3 conditions are characterized by low-grade inflammation and oxidative stress. Obesity and sleep apnea both may cause changes in thoracic mechanics that influence bronchial tone (ie, reduced lung capacity, increased negative intrathoracic pressures, and gastroesophageal reflux). Both sleep apnea and asthma may disrupt sleep and sleep-associated homeostatic control of airway function and ventilation. Atopy and allergic rhinitis, which may be strongly linked to asthma, also may increase the risk of sleep apnea by contributing to nasal obstruction through nasopharyngeal inflammation or adenotonsillary hypertrophy. Chronic nasal obstruction from allergic rhinitis occurring in early life also may lead to the development of long lower facies and relative mandibular retrognathia, which compromises upper airway size. Sleep apnea and asthma/allergic rhinitis also may share common developmental or genetic risk factors as proposed by the “unified airway” theory.63
Fig. 1.
A theoretical schema showing the multiple interrelated pathways among sleep-disordered breathing, obesity, and asthma. Sleep-disordered breathing, obesity, and asthma each are associated with numerous adverse physiological sequelae, many of which may exacerbate disease manifestations with the other 2 conditions.
Pathophysiologically, sleep apnea may be on the causal pathway between obesity and asthma. Plausible mechanisms by which sleep apnea may exacerbate asthma are through enhanced vagal cholinergic tone induced by apnea, upper airway repetitive obstruction–induced local airway inflammation, systemic inflammation, and apnea-related alterations in intrathoracic pressures, the latter resulting in gastroesophageal reflux and bronchoconstriction. It is also plausible that hypercytokinemia and oxidative stress generated during apnea-related hypoxemic episodes could contribute to airway inflammation and bronchial hyperreactivity. Conversely, asthma may exacerbate sleep apnea via nocturnal dyspnea, wheezing, or cough, resulting in sleep disruption, altered ventilatory control, and diffuse airway edema, increasing susceptibility to upper airway narrowing or collapse.
The extent to which sleep apnea operates as a mediator in the association between obesity and asthma in children was recently reported.64 In statistical models, both sleep apnea and obesity were independent predictors of wheeze. Consideration of sleep apnea in the statistical models significantly attenuated the association between obesity with wheeze but not between obesity and asthma, suggesting complex interactions between these disorders. Because it appeared that sleep apnea was a stronger confounder or intermediate risk factor for wheeze than asthma, the authors speculated that sleep apnea may be more strongly associated with “atypical” asthma or a special subset of asthma phenotypes.
Synergistic comorbidity because of the co-occurrence of hypoxemia and sleep disruption associated with each condition also has been suggested. In patients with both disorders, there is some evidence that treatment of the sleep apnea leads to reduced asthma symptoms, reduced bronchodilator use, and/or improved lung function and airway hyperreactivity.65–68 However, a recent intervention study in adults has shown increased levels of airway reactivity following 1 month of CPAP use for sleep apnea.69 The small size of these studies suggests the need for more research to understand optimal treatment approaches for these comorbid conditions.
OTHER PULMONARY–SLEEP APNEA INTERACTIONS
Overlap syndrome
The coexistence of chronic obstructive lung disease and sleep apnea, or “overlap syndrome,” is a well recognized entity. Patients with this disorder have more severe nocturnal hypoxemia than patients with only 1 disorder.70
Pulmonary hypertension
In sleep apnea, pulmonary hemodynamics may demonstrate transient perturbations in pulmonary artery pressure and pulmonary capillary wedge pressure coincident with respiratory events. There are some data indicating right ventricular dysfunction occurs in individuals with sleep apnea,71 but it is as yet unclear whether pulmonary hypertension occurs in sleep apnea without the presence of concomitant daytime hypoxia.
Obesity hypoventilation syndrome
Severe abnormalities in gas exchange occur in patients with obesity hypoventilation, which often includes variables degrees of obstructive sleep apnea. This condition is often associated with pulmonary restrictive patterns, pulmonary hypertension, and right ventricular dysfunction. Other than obesity, the specific risk factors for depressed ventilator responses in this condition are poorly understood.
FUTURE DIRECTIONS
Recent research provides compelling data linking sleep apnea, obesity, and inflammatory dysregulation. To date, the implications of these comorbidities and overlapping pathogenetic pathways predominantly have been related to cardiovascular disease. However, asthma is increasingly associated with obesity, and it is possible that the obese asthmatic phenotype is influenced by a unique set of risk factors that may include neutrophilic airway inflammation that may be modulated in concert with obesity-related inflammatory processes as well as sleep apnea–related stresses. In addition, despite the marked influence of pulmonologists in the area of sleep medicine, in general, there has been little research that has addressed the interactions between the upper and lower airways and how these anatomic and functional areas mutually influence each other as well as reflect common genetic and environmental influences. Research is also needed to address the clinical implications of comorbid asthma/allergic rhinitis and asthma, and how treatment of one condition may ameliorate the other. Preliminary work suggests, for example, that leukotriene blockers may improve apnea in snoring children.72
The role of immune dysregulation, the interaction between local (upper and lower airway) and systemic inflammatory and oxidative stress pathways, and the influence of sleep and circadian rhythm disruptions on both sleep apnea and asthma are important areas for research. From a clinical perspective, data are needed to guide approaches for screening for each disorder given the other, as well as understanding the role of interventions for one disorder and their effect on the other. Finally, because asthma and sleep apnea are both exacerbated by obesity, there remains an urgent need to prevent and treat obesity.
Acknowledgments
Supported by the Association of Subspecialty Professors, American College of Chest Physicians T. Franklin Williams Geriatric Development Research Award, National Heart, Lung, and Blood Institute grant K23 HL079114-01A2, an American Heart Association National Scientist Development Award (0530188N), and National Institutes of Health grant 1U54CA116867
Abbreviations used
CPAP
Continuous positive airway pressure
CRP
C-reactive protein
ROS
Reactive oxygen species
Footnotes
Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 4.Lavie L, Kraiczi H, Hefetz A, Ghandour H, Perelman A, Hedner J, et al. Plasma vascular endothelial growth factor in sleep apnea syndrome: effects of nasal continuous positive air pressure treatment. Am J Respir Crit Care Med. 2002;165:1624–1628. doi: 10.1164/rccm.20110-040OC. [DOI] [PubMed] [Google Scholar]
- 5.Kresiun VI, Rozhkovskiǐ IaV. [Disruption of the structural organization of brain mitochondrial membranes during deprivation of the paradoxal sleep phase and correction for it using lithonite] Ukr Biokhim Zh. 1991;63:59–65. [PubMed] [Google Scholar]
- 6.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 7.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest. 2002;122:1162–1167. doi: 10.1378/chest.122.4.1162. [DOI] [PubMed] [Google Scholar]
- 8.Depalo A, Carpagnano GE, Spanevello A, Sabato R, Cagnazzo MG, Gramiccioni C, et al. Exhaled NO and iNOS expression in sputum cells of healthy, obese and OSA subjects. J Intern Med. 2008;263:70–78. doi: 10.1111/j.1365-2796.2007.01875.x. [DOI] [PubMed] [Google Scholar]
- 9.Petrosyan M, Perraki E, Simoes D, Koutsourelakis I, Vagiakis E, Roussos C, et al. Exhaled breath markers in patients with obstructive sleep apnoea. Sleep Breath. 2007 Dec 11; doi: 10.1007/s11325-007-0160-8. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Ip MS, Lam B, Chan LY, Zheng L, Tsang KW, Fung PC, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–2171. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 11.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. 8-Isoprostane, a marker of oxidative stress, is increased in exhaled breath condensate of patients with obstructive sleep apnea after night and is reduced by continuous positive airway pressure therapy. Chest. 2003;124:1386–1392. doi: 10.1378/chest.124.4.1386. [DOI] [PubMed] [Google Scholar]
- 12.Tam CS, Wong M, Tam K, Aouad L, Waters KA. The effect of acute intermittent hypercapnic hypoxia treatment on IL-6, TNF-alpha, and CRP levels in piglets. Sleep. 2007;30:723–727. doi: 10.1093/sleep/30.6.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 14.Mehra R, Storfer-Isser A, Kirchner HL, Johnson N, Jenny N, Tracy RP, et al. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med. 2006;166:1725–1731. doi: 10.1001/archinte.166.16.1725. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama A, Kohno N, Hirasawa Y, Kondo K, Abe M, Inoue Y, et al. Evaluation of soluble IL-6 receptor concentration in serum and epithelial lining fluid from patients with interstitial lung diseases. Clin Exp Immunol. 1995;100:325–329. doi: 10.1111/j.1365-2249.1995.tb03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama A, Kohno N, Sakai K, Kondo K, Hirasawa Y, Hiwada K. Circulating levels of soluble interleukin-6 receptor in patients with bronchial asthma. Am J Respir Crit Care Med. 1997;156:1688–1691. doi: 10.1164/ajrccm.156.5.9610070. [DOI] [PubMed] [Google Scholar]
- 17.Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep. 2007;30:29–34. doi: 10.1093/sleep/30.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taheri S, Austin D, Lin L, Nieto FJ, Young T, Mignot E. Correlates of serum c-reactive protein (CRP): no association with sleep duration or sleep disordered breathing. Sleep. 2007;30:991–996. doi: 10.1093/sleep/30.8.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkin EK, Rosen CL, Kirchner HL, Storfer-Isser A, Emancipator JL, Johnson NL, et al. Variation of c-reactive protein levels in adolescents: association with sleep-disordered breathing and sleep duration. Circulation. 2005;111:1978–1984. doi: 10.1161/01.CIR.0000161819.76138.5E. [DOI] [PubMed] [Google Scholar]
- 20.Gozal D, Crabtree VM, Sans CapdevilaO, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007;176:188–193. doi: 10.1164/rccm.200610-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drager LF, Bortolotto LA, Figueiredo AC, Krieger EM, Lorenzi GF. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176:706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 22.Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, et al. Elevated levels of c-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107:1129–1134. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 23.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 25.Adner M, Rose AC, Zhang Y, Sward K, Benson M, Uddman R, et al. An assay to evaluate the long-term effects of inflammatory mediators on murine airway smooth muscle: evidence that TNFalpha up-regulates 5-HT(2A)-mediated contraction. Br J Pharmacol. 2002;137:971–982. doi: 10.1038/sj.bjp.0704928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamauchi M, Nakano H, Maekawa J, Okamoto Y, Ohnishi Y, Suzuki T, et al. Oxidative stress in obstructive sleep apnea. Chest. 2005;127:1674–1679. doi: 10.1378/chest.127.5.1674. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery-Downs HE, Krishna J, Roberts LJ, 2nd, Gozal D. Urinary f2-isoprostane metabolite levels in children with sleep-disordered breathing. Sleep Breath. 2006;10:211–215. doi: 10.1007/s11325-006-0079-5. [DOI] [PubMed] [Google Scholar]
- 28.Minoguchi K, Yokoe T, Tanaka A, Ohta S, Hirano T, Yoshino G, et al. Association between lipid peroxidation and inflammation in obstructive sleep apnoea. Eur Respir J. 2006;28:378–385. doi: 10.1183/09031936.06.00084905. [DOI] [PubMed] [Google Scholar]
- 29.Dikmenoglu N, Ciftci B, Ileri E, Guven SF, Seringec N, Aksoy Y, et al. Erythrocyte deformability, plasma viscosity and oxidative status in patients with severe obstructive sleep apnea syndrome. Sleep Med. 2006;7:255–261. doi: 10.1016/j.sleep.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Barreiro E, Nowinski A, Gea J, Sliwinski P. Oxidative stress in the external intercostal muscles of patients with obstructive sleep apnoea. Thorax. 2007;62:1095–1101. doi: 10.1136/thx.2006.069963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alzoghaibi MA, Bahammam AS. Lipid peroxides, superoxide dismutase and circulating IL-8 and GCP-2 in patients with severe obstructive sleep apnea: a pilot study. Sleep Breath. 2005;9:119–126. doi: 10.1007/s11325-005-0022-1. [DOI] [PubMed] [Google Scholar]
- 32.Lavie L, Vishnevsky A, Lavie P. Evidence for lipid peroxidation in obstructive sleep apnea. Sleep. 2004;27:123–128. [PubMed] [Google Scholar]
- 33.Svatikova A, Wolk R, Lerman LO, Juncos LA, Greene EL, McConnell JP, et al. Oxidative stress in obstructive sleep apnoea. Eur Heart J. 2005;26:2435–2439. doi: 10.1093/eurheartj/ehi440. [DOI] [PubMed] [Google Scholar]
- 34.Wali SO, Bahammam AS, Massaeli H, Pierce GN, Iliskovic N, Singal PK, et al. Susceptibility of LDL to oxidative stress in obstructive sleep apnea. Sleep. 1998;21:290–296. [PubMed] [Google Scholar]
- 35.Svatikova A, Wolk R, Wang HH, Otto ME, Bybee KA, Singh RJ, et al. Circulating free nitrotyrosine in obstructive sleep apnea. Am J Physiol Regul Integr Comp Physiol. 2004;287:R284–R287. doi: 10.1152/ajpregu.00241.2004. [DOI] [PubMed] [Google Scholar]
- 36.Schulz R, Mahmoudi S, Hattar K, Sibelius U, Olschewski H, Mayer K, et al. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162:566–570. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 37.Barcelo A, Barbe F, de la Pena M, Vila M, Perez G, Pierola J, et al. Antioxidant status in patients with sleep apnoea and impact of continuous positive airway pressure treatment. Eur Respir J. 2006;27:756–760. doi: 10.1183/09031936.06.00067605. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann MS, Singh P, Wolk R, Romero-Corral A, Raghavakaimal S, Somers VK. Microarray studies of genomic oxidative stress and cell cycle responses in obstructive sleep apnea. Antioxid Redox Signal. 2007;9:661–669. doi: 10.1089/ars.2007.1589. [DOI] [PubMed] [Google Scholar]
- 39.von Kanel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest. 2007;131:733–739. doi: 10.1378/chest.06-2006. [DOI] [PubMed] [Google Scholar]
- 40.Steiner S, Jax T, Evers S, Hennersdorf M, Schwalen A, Strauer BE. Altered blood rheology in obstructive sleep apnea as a mediator of cardiovascular risk. Cardiology. 2005;104:92–96. doi: 10.1159/000086729. [DOI] [PubMed] [Google Scholar]
- 41.Robinson GV, Pepperell JC, Segal HC, Davies RJ, Stradling JR. Circulating cardiovascular risk factors in obstructive sleep apnoea: Data from randomised controlled trials. Thorax. 2004;59:777–782. doi: 10.1136/thx.2003.018739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shinagawa K, Martin JA, Ploplis VA, Castellino FJ. Coagulation factor Xa modulates airway remodeling in a murine model of asthma. Am J Respir Crit Care Med. 2007;175:136–143. doi: 10.1164/rccm.200608-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Punjabi NM, Polotsky VY. Disorders of glucose metabolism in sleep apnea. J Appl Physiol. 2005;99:1998–2007. doi: 10.1152/japplphysiol.00695.2005. [DOI] [PubMed] [Google Scholar]
- 44.Harsch IA, Schahin SP, Radespiel-Troger M, Weintz O, Jahreiss H, Fuchs FS, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–162. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 45.Yeh HC, Punjabi NM, Wang NY, Pankow JS, Duncan BB, Cox CE, et al. Cross-sectional and prospective study of lung function in adults with type 2 diabetes mellitus: the Atherosclerosis Risk In Communities (ARIC) study. Diabetes Care. 2008;31:741–746. doi: 10.2337/dc07-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dyugovskaya L, Lavie P, Lavie L. Phenotypic and functional characterization of blood gammadelta T cells in sleep apnea. AmJ Respir Crit CareMed. 2003;168:242–249. doi: 10.1164/rccm.200210-1226OC. [DOI] [PubMed] [Google Scholar]
- 47.Kokura S, Wolf RE, Yoshikawa T, Granger DN, Aw TY. T-lymphocyte-derived tumor necrosis factor exacerbates anoxia-reoxygenation-induced neutrophil-endothelial cell adhesion. Circ Res. 2000;86:205–213. doi: 10.1161/01.res.86.2.205. [DOI] [PubMed] [Google Scholar]
- 48.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–586. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi K, Nishimura Y, Shimada T, Yoshimura S, Funada Y, Satouchi M, et al. Effect of continuous positive airway pressure on soluble cd40 ligand in patients with obstructive sleep apnea syndrome. Chest. 2006;129:632–637. doi: 10.1378/chest.129.3.632. [DOI] [PubMed] [Google Scholar]
- 50.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 51.Gami AS, Caples SM, Somers VK. Obesity and obstructive sleep apnea. Endocrinol Metab Clin North Am. 2003;32:869–894. doi: 10.1016/s0889-8529(03)00069-0. [DOI] [PubMed] [Google Scholar]
- 52.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Polotsky VY, Li J, Punjabi NM, Rubin AE, Smith PL, Schwartz AR, et al. Intermittent hypoxia increases insulin resistance in genetically obese mice. J Physiol. 2003;552:253–264. doi: 10.1113/jphysiol.2003.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics. 2002;110:315–322. doi: 10.1542/peds.110.2.315. [DOI] [PubMed] [Google Scholar]
- 55.Fitzpatrick MF, Martin K, Fossey E, Shapiro CM, Elton RA, Douglas NJ. Snoring, asthma and sleep disturbance in Britain: a community-based survey. Eur Respir J. 1993;6:531–535. [PubMed] [Google Scholar]
- 56.Larsson LG, Lindberg A, Franklin KA, Lundback B. Symptoms related to obstructive sleep apnoea are common in subjects with asthma, chronic bronchitis and rhinitis in a general population. Respir Med. 2001;95:423–429. doi: 10.1053/rmed.2001.1054. [DOI] [PubMed] [Google Scholar]
- 57.Teculescu DB, Caillier I, Perrin P, Rebstock E, Rauch A. Snoring in French preschool children. Pediatr Pulmonol. 1992;13:239–244. doi: 10.1002/ppul.1950130412. [DOI] [PubMed] [Google Scholar]
- 58.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children: associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–1532. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 59.Knuiman M, James A, Divitini M, Bartholomew H. Longitudinal study of risk factors for habitual snoring in a general adult population: the Busselton Health Study. Chest. 2006;130:1779–1783. doi: 10.1378/chest.130.6.1779. [DOI] [PubMed] [Google Scholar]
- 60.Young T, Finn L, Palta M. Chronic nasal congestion at night is a risk factor for snoring in a population-based cohort study. Arch Intern Med. 2001;161:1514–1519. doi: 10.1001/archinte.161.12.1514. [DOI] [PubMed] [Google Scholar]
- 61.Ng DK, Chan CH, Hwang GY, Chow PY, Kwok KL. A review of the roles of allergic rhinitis in childhood obstructive sleep apnea syndrome. Allergy Asthma Proc. 2006;27:240–242. doi: 10.2500/aap.2006.27.2855. [DOI] [PubMed] [Google Scholar]
- 62.Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–1093. doi: 10.1016/j.jaci.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Nayak AS. A common pathway: asthma and allergic rhinitis. Allergy Asthma Proc. 2002;23:359–365. [PubMed] [Google Scholar]
- 64.Sulit LG, Storfer-Isser A, Rosen CL, Kirchner HL, Redline S. Associations of obesity, sleep-disordered breathing, and wheezing in children. Am J Respir Crit Care Med. 2005;171:659–664. doi: 10.1164/rccm.200403-398OC. [DOI] [PubMed] [Google Scholar]
- 65.Chan CS, Woolcock AJ, Sullivan CE. Nocturnal asthma: role of snoring and obstructive sleep apnea. Am Rev Respir Dis. 1988;137:1502–1504. doi: 10.1164/ajrccm/137.6.1502. [DOI] [PubMed] [Google Scholar]
- 66.Guerrero M, Lepler L, Kristo D. The upper airway resistance syndrome masquerading as nocturnal asthma and successfully treated with an oral appliance. Sleep Breath. 2001;5:93–96. doi: 10.1007/s11325-001-0093-6. [DOI] [PubMed] [Google Scholar]
- 67.Guilleminault C, Quera-Salva MA, Powell N, Riley R, Romaker A, Partinen M, et al. Nocturnal asthma: snoring, small pharynx and nasal CPAP. Eur Respir J. 1988;1:902–907. [PubMed] [Google Scholar]
- 68.Thomas PS, Geddes DM, Barnes PJ. Pseudo-steroid resistant asthma. Thorax. 1999;54:352–356. doi: 10.1136/thx.54.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Devouassoux G, Levy P, Rossini E, Pin I, Fior-Gozlan M, Henry M, et al. Sleep apnea is associated with bronchial inflammation and continuous positive airway pressure-induced airway hyperresponsiveness. J Allergy Clin Immunol. 2007;119:597–603. doi: 10.1016/j.jaci.2006.11.638. [DOI] [PubMed] [Google Scholar]
- 70.Sanders MH, Newman AB, Haggerty CL, Redline S, Lebowitz M, Samet J, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167:7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]
- 71.Guidry UC, Mendes LA, Evans JC, Levy D, O’Connor GT, Larson MG, et al. Echocardiographic features of the right heart in sleep-disordered breathing: the Framingham Heart Study. Am J Respir Crit Care Med. 2001;164:933–938. doi: 10.1164/ajrccm.164.6.2001092. [DOI] [PubMed] [Google Scholar]
- 72.Goldbart AD, Goldman JL, Veling MC, Gozal D. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med. 2005;172:364–370. doi: 10.1164/rccm.200408-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]