Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti–CTLA-4 antibodies (original) (raw)
Abstract
Cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) is a critical negative regulator of immune responses. Uniquely among known inhibitory receptors, its genetic ablation results in a fulminating and fatal lymphoproliferative disorder. This central regulatory role led to the development of antibodies designed to block CTLA-4 activity in vivo, aiming to enhance immune responses against cancer. Despite their preclinical efficacy and promising clinical activity against late stage metastatic melanoma, the critical cellular targets for their activity remains unclear. In particular, debate has focused on whether the effector T cell (Teff) or regulatory T cell (T reg cell) compartment is the primary target of antibody-mediated blockade. We developed a mouse expressing human instead of mouse CTLA-4, allowing us to evaluate the independent contributions of CTLA-4 blockade of each T cell compartment during cancer immunotherapy in an in vivo model of mouse melanoma. The data show that although blockade on effector cells significantly improves tumor protection, unicompartmental blockade on regulatory cells completely fails to enhance antitumor responses. However, concomitant blockade of both compartments leads to a synergistic effect and maximal antitumor activity. We conclude that the combination of direct enhancement of Teff cell function and concomitant inhibition of T reg cell activity through blockade of CTLA-4 on both cell types is essential for mediating the full therapeutic effects of anti–CTLA-4 antibodies during cancer immunotherapy.
The T cell receptor CTL-associated antigen 4 (CTLA-4) is recognized as a critical inhibitory regulator of the early stages of T cell expansion, opposing the actions of CD28-mediated costimulation. Genetic disruption of CTLA-4 expression results in a polyclonal CD4-dominated lymphoproliferative syndrome, which is characterized by T cell infiltration of multiple organs and early lethality within 3–4 wk after birth (Waterhouse et al., 1995). This pathology is dependent on interactions of CD28 with its ligands B7-1 and B7-2, as indicated by the lack of disease in CTLA-4/B7-1/B7-2 triple KO (TKO) mice and the protection afforded by repeated administration of CTLA-4 Ig in CTLA-4 KO mice (Mandelbrot et al., 1999).
Based on the recognition of the importance of CTLA-4 in the regulation of immune responses, blocking antibodies against both mouse and human forms have been investigated for their potential to boost immunological responses against cancer. Data from preclinical models support a significant role for CTLA-4 blockade in the induction of durable antitumor immunity (Leach et al., 1996; Shrikant et al., 1999; van Elsas et al., 1999; Quezada et al., 2006). Furthermore, clinical trials with human anti–human CTLA-4 (aHuCTLA-4) mAbs show promising early results in the treatment of late-stage metastatic melanoma (Hodi et al., 2003; Phan et al., 2003; Ribas et al., 2005; Peggs et al., 2008). Despite growing experience of their use in the treatment of human cancers, the precise mechanisms underpinning CTLA-4–mediated control of the immune response and, more specifically, the antitumor activity of anti–CTLA-4 antibodies, remain elusive. Two independent, but not mutually exclusive, hypotheses invoke cell autonomous and non–cell autonomous mechanisms.
A cell autonomous mechanism of action for CTLA-4, with CTLA-4 acting when expressed in cis of CD28, is supported by several lines of data. Studies of both in vitro and in vivo systems show higher proliferative capacity of CTLA-4–deficient CD4+ and CD8+ T cells when compared with their WT counterparts (Chambers et al., 1998, 1999; Greenwald et al., 2001, 2002). Several mechanisms have been proposed to explain such cell autonomous inhibition, including CTLA-4 outcompeting CD28 for binding to its ligands B7-1 and B7-2 (for review see van der Merwe and Davis, 2003), as well as direct inhibitory signaling through the CTLA-4 cytoplasmic tail (Carreno et al., 2000). In contrast, it has remained more contentious whether CTLA-4 expressed in trans by CD4+CD25+Foxp3+ regulatory T cells (T reg cells) has a direct role in their suppressive function. Constitutive expression of CTLA-4 is a cardinal feature of T reg cells, although the majority of CTLA-4 is found intracellularly in both T reg and conventional T cells, even after activation. Two major findings have supported a non–cell autonomous inhibitory function of CTLA-4. The first is the dominant protection against lethal lymphoproliferation afforded by WT mixed chimerism in the bone marrow transplant model using CTLA-4−/− and WT donors (Bachmann et al., 1999). The second is the ability of CTLA-4 blockade to abrogate the protective effect mediated by CD4+CD25+ T cells in an adoptive transfer model of colitis (where CD4+CD45RBhigh T cell transfer into immunodeficient mice normally results in severe colitis; Read et al., 2000, 2006). However, interpretation of the impact of anti–CTLA-4 on T reg cells is confounded by the direct effects that CTLA-4 blockade has on non–T reg cells within the same systems, whereas isolation of Foxp3-expressing CD4+ T cells from CTLA-4−/− animals requires immunological manipulations that may have influenced T reg cell development (e.g., the use of TKO mice; Read et al., 2006). Nonetheless, the recent demonstration that T reg cell–specific CTLA-4 deficiency in conditional KO (CKO) mice is associated with a profound reduction in their suppressive capacity has definitively proven a role for CTLA-4 in T reg cell–mediated suppression (Wing et al., 2008). CKO mice developed a lethal autoimmune lymphoproliferative syndrome with a slightly slower tempo than CTLA-4−/− mice, illustrating the essential role that CTLA-4 plays in T reg cell function.
These recent findings have direct relevance for the clinical development of anti–CTLA-4 antibodies. They highlight the question of which are the relevant cellular targets for their antitumor activity. To date, effector T cells (Teff) have been thought to be the most relevant target for therapeutic activity, but the data from the CKO experiments suggest otherwise by highlighting the critical role that CTLA-4 plays in T reg cell function. The answer is important for the rational development of combinatorial immunotherapeutic strategies. The lack of functional CTLA-4–expressing T reg cell at tumor engraftment in the CKO studies proved sufficient to prevent sustained tumor growth in 60% of mice (Wing et al., 2008). Both we and others, however, have demonstrated that targeted depletion of T reg cells (either with anti-CD25 mAbs or in Foxp3-DTR transgenic mice) can be effective as a prophylaxis but not as a treatment once tumor engraftment is established, a setting which better reflects their clinical usage (Onizuka et al., 1999; Quezada et al., 2008). In addition, it is possible that quantitative or qualitative differences may exist between CTLA-4 ablation and antibody blockade. Thus, the relative importance of antibody-mediated blockade of CTLA-4 in trans on the regulatory compartment as compared with blockade in cis on the nonregulatory compartment in tumor models and clinical applications remains unclear.
In this study, we used mice expressing human instead of mouse CTLA-4 to allow assessment of the influence of uni- and bicompartmental blockade on T reg and non–T reg cell compartments during in vitro assays and in an in vivo tumor model of B16/BL6 melanoma. We show that unicompartmental antibody-mediated blockade of CTLA-4 on T reg cells modestly reduced suppressive capacity during in vitro suppressor assays. This effect was exaggerated by use of CTLA4−/− T reg cells derived from stable bone marrow chimeras, from which T reg cells could be isolated without significant contamination by activated effectors. Intriguingly, unicompartmental antibody-mediated blockade of CTLA-4 on T reg cells combined with a GM-CSF–secreting cellular vaccine (Gvax) had no impact on tumor growth kinetics when given after engraftment. Blockade of the non–T reg cell compartment in combination with Gvax resulted in delayed tumor growth and rejection in 12/30 (40%) mice, whereas blockade of both T reg and non–T reg cell populations resulted in rejection in 22/30 (73%; P = 0.0017). Our data are the first to demonstrate the critical importance of synergy between the independent contributions of CTLA-4 blockade in cis and in trans to antitumor activity, illustrating that both Teff and T reg cells are relevant targets for the therapeutic efficacy of anti–CTLA-4 antibodies.
RESULTS AND DISCUSSION
A transgenic mouse model for unicompartmental CTLA-4 blockade
Much of the difficulty complicating the interpretation of previous studies attempting to define the cellular target of CTLA-4 antibody in blocking experiments has been the presence of CTLA-4 on both activated effector and regulatory compartments. Thus, blockade has potentially affected both populations and assigning relative contributions to experimental endpoints has been impossible. To allow evaluation of unicompartmental CTLA-4 blockade, we have taken advantage of the fact that human CTLA-4 is capable of interacting with mouse B7-1 and B7-2 and generated mice expressing a chimeric CTLA-4 molecule composed of the human extracellular domain and the mouse transmembrane and cytoplasmic domains instead of full length mouse CTLA-4.
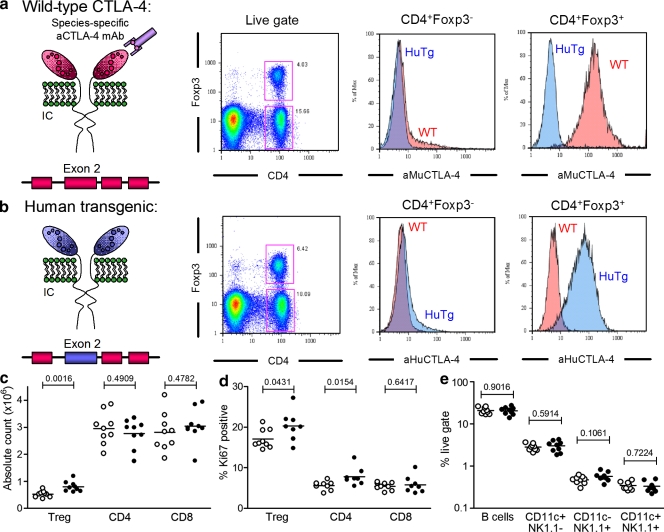
Both human and mouse CTLA-4 genes are composed of four exons, with overall sequence homology of corresponding proteins of 76% (67% in the extracellular [exon 2], 83% in the transmembrane [exon 3], and 100% in the intracytoplasmic [exon 4] domains). The gene product of exon 1 is a signal peptide not expressed in the mature protein. We created a chimeric 17-kb DNA construct containing putative upstream regulatory sequences required for CTLA-4 expression and in which the extracellular (exon 2) coding domain of mouse CTLA-4 was replaced with that of human CTLA-4. This allowed the generation of a human CTLA-4 transgenic (HuTg) mouse, which was then backcrossed into the CTLA-4−/− background (Fig. S1). This HuTg mouse permitted reconstitution of both in vitro and in vivo experimental systems in which the composition of T reg and non–T reg cell compartments (WT vs. HuTg) could be controlled. The species-specific binding of anti–CTLA-4 antibodies then allowed evaluation of unicompartmental CTLA-4 blockade. Intracellular staining for Foxp3 and mouse CTLA-4 confirmed the absence of expression of mouse CTLA-4 in HuTg T cells (Fig. 1 a). Anti–HuCTLA-4 revealed similar expression profiles of human CTLA-4 in HuTg CD4+Foxp3− and CD4+Foxp3+ populations (Fig. 1 b), as demonstrated for mouse CTLA-4 in WT mice (Fig. 1 a).
Figure 1.
Functional replacement of mouse CTLA-4 with the human CTLA-4 gene in vivo. (a and b) Flow cytometric analysis of intracellular Foxp3 and CTLA-4 in freshly isolated LN CD4+ T cells from WT and HuTg mice, labeled with aMUCTLA-4 (a) and aHuCTLA-4 (b). (c) Absolute CD4+Foxp3+, CD4+Foxp3−, and CD8+ T cell counts from age-matched WT (empty circles) and HuTg mice (filled circles; 8–10-mo-old mice; n = 8–9 per group). (d) Frequency of Ki67-expressing CD4+Foxp3+, CD4+Foxp3−, and CD8+ T cells in LN of WT (empty circles) and HuTg mice (filled circles). (e) Absolute cell counts for B cell, NK cell, and dendritic cell populations in LN of WT (empty circles) and HuTg mice (filled circles). Data represent three independent experiments. Horizontal bars in c–e represent mean values.
Homozygous HuTg mice were rescued from the lymphoproliferative phenotype of CTLA-4−/− mice, suggesting normal complementation of CTLA-4 expression in this model. These mice had a normal lifespan with no evidence of autoimmune disease over a period of followup of >12 mo. Similarly to a previously published human CTLA-4 knockin mouse (in which both mouse exon 2 and exon 3 were replaced with their human homologues [Lute et al., 2005]), there was no enlargement of lymphoid organs and no significant increase in absolute numbers of total T cells harvested from either LNs (Fig. 1 c) or spleens (not depicted). There was a small but significant increase in the number of CD4+Foxp3+ T cells in HuTg compared with WT mice, which could be explained by the increased levels of Ki67 in the HuTg CD4+ population (Fig. 1 d). This small increase in the number of CD4+Foxp3+ T cells had no impact on the overall phenotype of other T cells because the extent of activation in vivo in HuTg mice, as assessed by flow cytometry for surface CD44 and CD62L expression, was essentially indistinguishable from that in WT T cells (unpublished data). There were no differences between HuTg and WT mice in LNs (Fig. 1 e) or spleens (not depicted) in terms of numbers of B, dendritic, or NK cells. Furthermore, there was no evidence of increased levels of autoantibodies with age as assessed by antinuclear antibody (ANA) production. ELISAs showed that only 1/11 aged mice (11–12 mo old) had positive titers for ANA, compared with 0/8 in a control group of WT B6 mice (unpublished data). The incidence of ANA in B6 mice has been reported at ∼20% by 10 mo of age (Chen, et al., 2000), suggesting that the rates in HuTg mice are no higher. These data suggest a normal development of the myeloid and lymphoid compartments in these mice and indicate that human CTLA-4 can functionally interact with mouse B7 molecules to prevent the lymphoproliferative disease evident in CTLA-4−/− mice.
To further evaluate the regulation of CTLA-4 in HuTg mice, we performed in vitro analyses of CTLA-4 expression, comparing the temporal profiles of up-regulation in WT and HuTg mice after stimulation with anti-CD3 (Fig. S2). The expression patterns of both cell surface and intracellular CTLA-4 were very similar over 72 h when comparing WT and HuTg mice in either CD8+, CD4+FoxP3+, or CD4+FoxP3− compartments, suggesting comparable regulation in the WT and HuTg mice. Finally, we performed tumor challenge experiments in the HuTg mice with either the MC38 colonic carcinoma cell line or the B16 melanoma cell line (both syngeneic), treating with anti–CTLA-4 monotherapy (100 µg aHuCTLA-4, clone 147, on days 3, 6, and 9) or combined anti–CTLA-4 and an irradiated GM-CSF–secreting B16/BL6 cellular vaccine (Gvax; 106 cells on days 3, 6, and 9), respectively. Tumors were rejected with a similar kinetic to that previously demonstrated in WT mice treated with an anti–mouse CTLA-4 (aMuCTLA-4) mAb, suggesting that HuCTLA-4 plays a comparable role in vivo in these tumor model systems to that of MuCTLA-4 in WT mice (unpublished data).
Unicompartmental CTLA-4 blockade during in vitro suppressor assays results in a modest decrease in suppressive activity
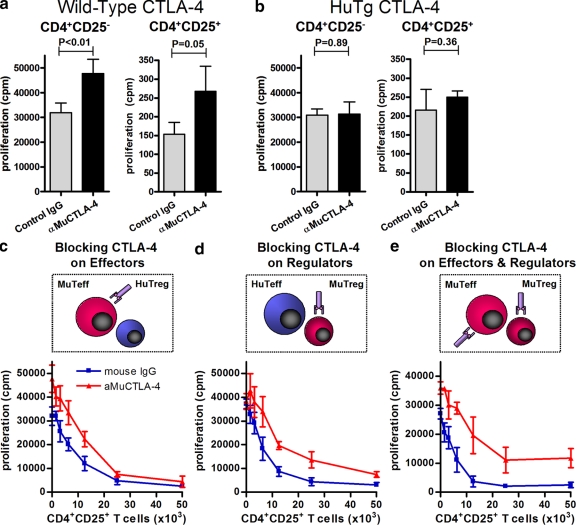
In accordance with the species-specific binding of anti–CTLA-4 mAb (Fig. 1, a and b), functional blockade with an aMuCTLA-4 (clone 9H10) was also species specific (Fig. 2, a and b). Purified populations of either CD4+CD25− or CD4+CD25+ WT T cells proliferated to a significantly greater degree in the presence of aMuCTLA-4 than control IgG in thymidine incorporation assays (Fig. 2 a), whereas neither population of purified cells from HuTg mice showed enhanced proliferation in the presence of aMuCTLA-4 (Fig. 2 b). The direct effect on purified effector and regulatory WT cells highlights the cell autonomous role of CTLA-4 in inhibiting T cell proliferation. The demonstration that aMuCTLA-4 produces functional blockade in a species-specific manner allowed us to examine the effects of unicompartmental blockade of either the T reg or non–T reg cell populations.
Figure 2.
Unicompartmental blockade with aMuCTLA-4 during in vitro suppressor assays suggests both cell autonomous and non–cell autonomous activities of CTLA-4. Suppressor assays were performed using mixtures of WT and HuTg cells. (a and b) Proliferation of 50,000 purified WT or HuTg CD4+CD25− and CD4+CD25+ T cells in response to T cell–depleted splenocytes and anti-CD3 and in the presence of control IgG or aMuCTLA-4, confirming the species specificity of the CTLA-4 blockade. (c) Unicompartmental blockade of 50,000 WT CD4+CD25− T cells with aMuCTLA-4, compared with control IgG during in vitro suppression by addition of increasing numbers of HuTg CD4+CD25+ T reg cells. (d) Unicompartmental blockade of WT CD4+CD25+ T reg cells with aMuCTLA-4, compared with control IgG during in vitro suppression of 50,000 HuTg CD4+CD25− T cells. (e) Bicompartmental blockade of both WT CD4+CD25− Teff and CD4+CD25+ T reg cells with aMuCTLA-4, compared with control IgG during in vitro suppressor assays. Data in a–e represent three or more independent experiments, and in each group replicates were performed as quintuplets. Error bars indicate SD.
Conventional suppressor assays were performed using mixtures of cells purified from WT and HuTg cells (Fig. 2, c–e). Blockade on WT CD4+CD25− T cells in the presence of HuTg T reg cells resulted in increased baseline proliferation, but titration of HuTg T reg cells resulted in equivalent suppression to that seen without aMuCTLA-4 at ratios of 1:1 (Fig. 2 c). By comparison, blockade on WT T reg cells did not increase baseline proliferation HuTg CD4+CD25− T cells (Fig. 2 d). Titration of increasing numbers of WT T reg cells largely suppressed proliferation but reproducibly failed to induce equivalent suppression of thymidine incorporation to that seen without aMuCTLA-4 (Fig. 2 d). These results could be explained by a slight reduction in the suppressive capacity of WT T reg cells after CTLA-4 blockade but do not exclude the possibility that the excess thymidine incorporation occurred within the WT T reg cell compartment in the presence of CTLA-4 blockade and that full suppression of CD4+CD25− T cells was achieved. Bicompartmental blockade in experiments where both CD4+CD25− and CD4+CD25+ T cells were WT showed a possible additive effect of blockade. The baseline proliferation of the non–T reg cell compartment was increased and titration of WT T reg cells resulted in a lesser degree of suppression, which failed to approach complete suppression at ratios of 1:1 (Fig. 2 e). These data suggest that antibody-mediated CTLA-4 blockade influences T cell proliferation by both cell autonomous and non–cell autonomous mechanisms, but with a more subtle effect on T reg cells.
Different antibodies may display subtle or more obvious differences in terms of mode of action or magnitude of effect. To confirm that these findings were specific neither to one antibody clone nor to blockade of WT CTLA-4, we performed parallel experiments using alternate anti–CTLA-4 antibodies. Results were comparable using the 9D9 aMu-CTLA-4 clone (not depicted) and the aHuCTLA-4 clone 147 (Fig. S3). Thus, the different antibodies seem largely equivalent in terms of in vitro suppressor assays.
Inhibitory effects of CTLA-4 during in vitro suppressor assays are exaggerated in CTLA-4 KO T cells
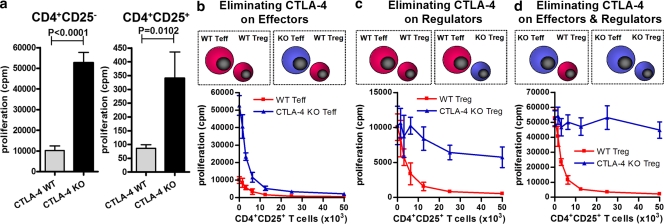
We reasoned that the apparently modest impact of blockade of CTLA-4 on the T reg cell compartment might reflect incomplete functional blockade, the presence and function of a ligand-independent form of CTLA-4 (Vijayakrishnan et al., 2004), or, alternatively, might indicate that suppression in this assay is dependent on alternate pathways. These explanations are not mutually exclusive. If the former were true genetic ablation of CTLA-4 might be associated with heightened effects, whereas this might not occur if alternate pathways were responsible for suppression. The selection of T reg cells based on CD25 expression from CTLA4−/− mice without significant contamination by activated Teff has proven difficult. Generation of CTLA-4/B7-1/B7-2 TKO mice allows isolation of T reg cells from a nonactivated background, but interpretation of subsequent experiments is potentially complicated both by the lack of B7 molecules on antigen-presenting cells during T cell development and on T cells themselves. The impact of the latter is particularly hard to predict. B7 molecules have been demonstrated to mediate reverse signaling into the cell on which they are expressed (Fallarino et al., 2003; Bodor et al., 2007). Furthermore, PD-L1 has recently been revealed to be a specific binding partner of B7-1 with an affinity intermediate between that for CTLA-4 and B7-1 and CD28 and B7-1, with evidence that PD-L1 and B7-1 can mediate bidirectional inhibitory signaling (Butte et al., 2007). We therefore sought to isolate CTLA-4–deficient T reg cells from an alternate source. Because mixed bone marrow chimeras generated from CD45.2+CTLA-4−/− and CD45.1+ WT donors have been shown to be protected from lymphoproliferation and capable of apparently normal responses to challenge with several pathogens (Bachmann et al., 1999, 2001), we purified CTLA-4−/− T reg cells from stable chimeras using the CD45.1 congenic marker to deplete WT (SJL) cells (Fig. S4). Mice were bled 10–12 wk after transplantation and only those displaying 40–60% SJL chimerism were used for subsequent experiments. The profile of CD25 staining on the CD45.1−Foxp3− T cells revealed no excess activation of the CTLA-4−/− non–T reg cells. Purified populations were <0.5% CD45.1+, 96–99% CD25+, and 86–92% Foxp3+.
CD4+CD25− and CD4+CD25+CTLA-4−/− T cells both showed enhanced proliferation in thymidine incorporation assays compared with WT controls (Fig. 3 a). The fold increase was greater than that seen with CTLA-4 blockade (Fig. 2 a), which is consistent with a greater impact of CTLA-4 ablation compared with antibody-mediated blockade. Despite higher baseline proliferation, WT T reg cells suppressed proliferation of CTLA-4−/− CD4+CD25− T cells to the same level as seen with WT CD4+CD25− T cells at ratios of 1:1 (Fig. 3 b). In contrast, CTLA-4−/− T reg cells were severely impaired in their ability to suppress WT CD4+CD25− T cells (Fig. 3 c). In this case, the difference was unlikely to be explained solely by an excess of proliferation in the CTLA-4−/− T reg cell compartment, as the differences in absolute counts (cpm) in the proliferation assays were well in excess of those demonstrated for the purified CTLA-4−/− CD4+CD25+ T cells (Fig. 3 a). Furthermore, the flow cytometry profiles of the CTLA-4−/− T cells (Fig. S4) and relatively modest increase in absolute levels of thymidine incorporation (Fig. 3 a) argue against a significant contamination of CTLA-4−/− T reg cells with CTLA-4−/− CD4+CD25− T cells. Suppression of CTLA-4−/− CD4+CD25− T cells by CTLA-4−/− T reg cells was virtually nonexistent in contrast to the full suppression mediated by WT T reg cells (Fig. 3 d). These data confirm that CTLA-4 has a role in both cell autonomous and non–cell autonomous (i.e., T reg cell–mediated) inhibitory regulation of non–T reg cells and also a cell autonomous role within the T reg cell compartment. These data are entirely consistent with those using CKO CTLA-4–deficient T reg cells (Wing et al., 2008).
Figure 3.
CTLA-4−/− T reg cell display diminished regulatory activity during in vitro suppressor assays. Suppressor assays were performed using mixtures of WT and CTLA-4−/− cells. (a) Proliferation of WT and CTLA-4−/− CD4+CD25− and CD4+CD25+ T cells in response to T cell–depleted splenocytes and anti-CD3, demonstrating cell autonomous inhibitory function of CTLA-4 in the proliferation of Teff and T reg cells. (b) Suppression of 50,000 WT or CTLA-4−/− CD4+CD25− Teff cells by increasing numbers of WT CD4+CD25+ T reg cells. (c) Suppression of 50,000 WT CD4+CD25− Teff cells by increasing numbers of WT or CTLA-4−/− CD4+CD25+ T reg cells. (d) Suppression of 50,000 CTLA-4−/− CD4+CD25− T cells by WT or CTLA-4−/− CD4+CD25+ T reg cells. Data in a–d represent three independent experiments, and in each group replicates were performed as quintuplets. Error bars indicate SD.
CTLA-4 blockade on both Teff and T reg cell compartments is required for maximal antitumor activity
Our data indicate that CTLA-4 expression is important for the suppressive capacity of T reg cells. However, the impact of antibody-mediated CTLA-4 blockade of the T reg cell compartment was relatively modest in the in vitro suppressor assays (Fig. 2 d). Furthermore, our previous work had shown that depletion of T reg cells after tumor engraftment effected very different outcomes from those seen with prophylactic depletion before tumor challenge (Quezada et al., 2008). The lack of impact of T reg cell depletion on tumor growth as an isolated therapeutic strategy combined with the modest impact in vitro led us to question which cellular targets were important for the therapeutic antitumor activity of blocking antibodies in vivo. To address this question, we reconstituted immunodeficient RAG2−/− mice with cells from WT and HuTg donors (Fig. S5). After pan–T cell selection of cells derived from LN and spleen, purified populations of CD4+CD25+ T cells were mixed with the combined CD4+CD25− and CD8+ fractions (henceforth referred to as Teff) in four combinations to allow blockade of both compartments, T reg cells only, Teff only, or neither compartment (i.e., WT:WT, HuTg:WT, WT:HuTg, and HuTg:HuTg). We aimed for the T reg cell compartment to be 3–5% of the total cells or up to 10% of the CD4+ population, hence mirroring physiological levels (Fig. S5 a). After adoptive transfer of 1.8–2.0 × 107 total T cells, mice were left for 10 wk to allow equilibration. At this point, the mice were bled to assess equivalence before tumor challenge (Fig. S5 b). In all cases, the relative proportion of CD4+CD25highFoxp3+ was higher than in the infused mixture, and with a significantly higher number in those reconstituted with HuTg T reg cells. This finding mirrored those presented in Fig. 1 (c and d). T cell populations were stable between 6 and 10 wk after infusion (unpublished data). CD4+CD25highFoxp3+ cells in the reconstituted mice could derive from several sources, including expansion of the transferred CD4+CD25+ T cells or from the CD4+CD25− T cells through either peripheral conversion of FoxP3− cells or up-regulation of CD25 on CD4+CD25−FoxP3+ T cells. To confirm that the majority derived from the CD4+CD25+ T cell inoculum, we used a congenic marker to trace the transferred populations (Fig. S5 c). At 6 and 10 wk after transfer, the majority of the FoxP3+ T reg cell compartment in blood, LNs, and spleen was derived from the CD25+ inoculum. A median of 88.4% (range 80.2–92.0%) of circulating T reg cells were derived from this population, and similar numbers were derived from the CD25+ inoculum in both LNs and spleens (median 88.6 and 92.3%, respectively).
After B16/BL6 tumor challenge, all mice received combination therapy with aMuCTLA-4 (clone 9H10, 100 µg every 3 d from day 3) and an irradiated GM-CSF–secreting B16/BL6 cellular vaccine (Gvax, 106 cells on days 3, 6, and 9; Fig. 4 a) as previously described (Quezada et al., 2006). Tumor growth in mice in which CTLA-4 blockade affected only the T reg cell compartment did not differ significantly from those in which it targeted neither population (Fig. 4 b). This is consistent with our previous experience of therapeutic T reg cell depletion with either anti-CD25 mAbs or in Foxp3-DTR transgenic mice (Quezada et al., 2008) and with the minimal impact that CTLA-4 blockade had on T reg cell function in the in vitro experiments (Fig. 2). Unicompartmental blockade of the Teff cells resulted in a significant delay in tumor growth and improved rejection rates and long-term tumor-free survival (Fig. 4, b and c). Combined survival from three experiments was 12/30 (40%; P = 0.001 compared with unicompartmental blockade of T reg cells). Blockade in this instance would also have included that of induced T reg cells, but the additional blockade of the entire T reg cell compartment markedly enhanced antitumor activity. Thus, bicompartmental blockade resulted in synergy of antitumor activity, with tumor rejection in the majority of mice and much delayed growth in the others (Fig. 4, b and c). 22/30 (73%) mice were long-term survivors (P = 0.0017 compared with unicompartmental blockade of Teff).
Figure 4.
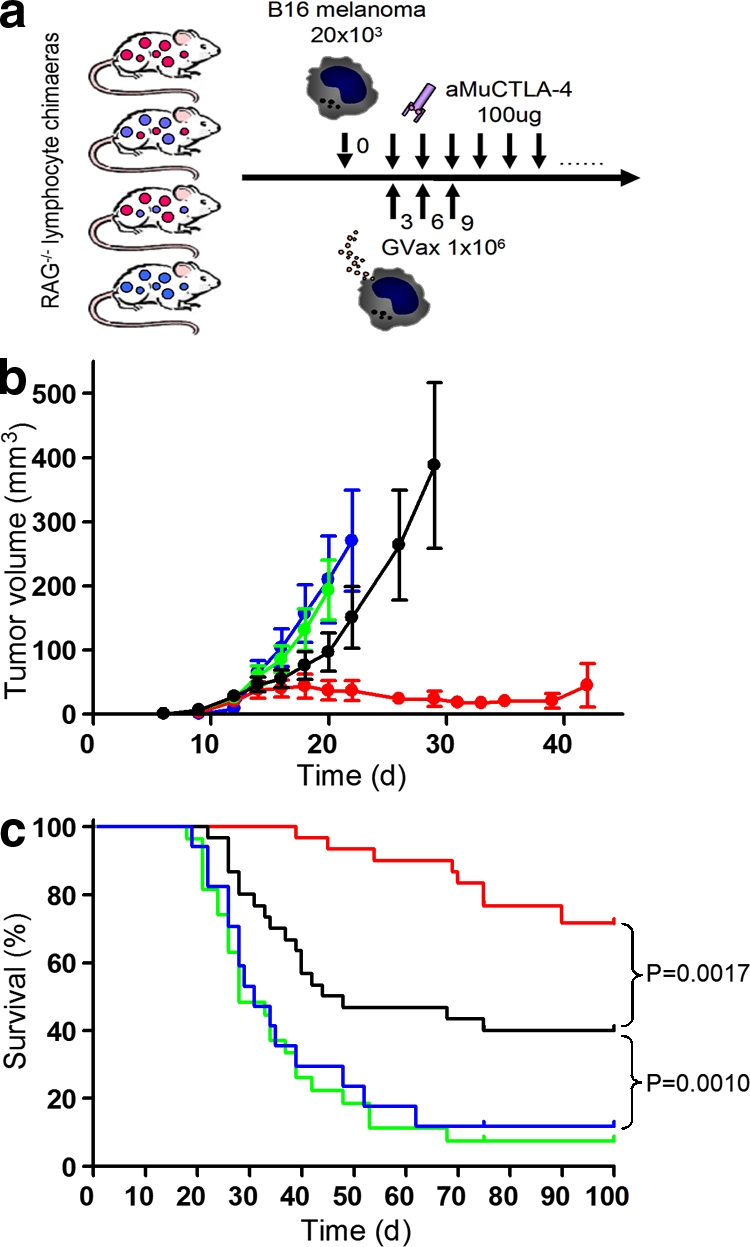
CTLA-4 blockade of effector and regulatory compartments synergizes to produce maximal antitumor activity. (a) Experimental design. RAG2-deficient mice were reconstituted with four combinations of WT and HuTg T reg and non–T reg (CD4+ and CD8+) cells. 10 wk later, they were challenged with 20 × 103 B16/BL6 melanoma cells intradermally. From day 3, they received combination immunotherapy with Gvax and aMuCTLA-4. (b) Tumor growth curves for mice reconstituted with WT Teff and T reg cells (red), HuTg Teff and WT T reg cells (green), WT Teff and HuTg T reg cells (black), and HuTg Teff and T reg cells (blue; n = 10 per group). Error bars indicate SEM. Data represent three independent experiments. (c) Survival curves with color coding as in b. Mice were euthanized when tumor volume exceeded 350 mm3. Pooled results from three independent experiments are shown.
These data are significant for several reasons, highlighting the differences between genetic ablation of a molecule and antibody-mediated blockade. They demonstrate the absolute requirement for blockade on the effector compartment for the initiation of antitumor activity by anti–CTLA-4 in a therapeutic setting and also confirm the importance of CTLA-4 for optimal T reg cell function. Furthermore, they demonstrate the apparent synergy of the effects of blockade on effector and regulatory cells, revealing that only concomitant blockade of both compartments culminates in maximal antitumor activity. This duality of immunostimulatory function is perhaps unique among currently available clinical therapeutics, although TNF receptor agonists may also influence the function of both compartments (Piconese et al., 2008). Finally, they demonstrate that although the expression of CTLA-4 may be critical for suppressive function, outcomes after antibody-mediated blockade may be more subtle. This has great significance for the development of combinatorial immunotherapies and helps to explain the enhancement of antitumor activity obtained by approaches combining CTLA-4 blockade and therapeutic T reg cell depletion (Sutmuller et al. 2001; Quezada et al., 2008). The maintenance of significant suppressor function, despite antibody-mediated blockade during the in vitro assays, is consistent with T reg cells being able to use multiple suppressive mechanisms. The relative importance of each is likely to vary according to the model under study, leading to apparent redundancy after antibody-mediated blockade in some settings, but not others, and helping to explain the seemingly contradictory results of earlier studies.
MATERIALS AND METHODS
Mice.
C57BL/6, RAG2−/−, and B6.SJL mice (6–8 wk old) were purchased from Taconic. CTLA-4−/− mice have been previously described (Chambers et al., 1997). HuTg mice were generated as documented in the text and in Fig. S1. In individual experiments, mice were age matched and used at 6–8 wk of age, except CTLA-4−/− mice which were 7–10 d of age. All animal experiments were approved by the Memorial Sloan-Kettering Cancer Center Institutional Animal Care and Use Committee.
Antibodies.
Anti–CTLA-4 (clone 9H10; BioExpress) was given i.p. for all in vivo studies. Anti-Ki67 FITC and aMuCTLA-4 PE (clone UC10-4F10-11; BD) were used according to manufacturer's instructions, whereas all other antibodies used for flow cytometry were purchased from eBioscience. For species-specific intracellular labeling, two anti–human (eBio20A and 14D3) and two anti–mouse (UC10-4F10-11 and 9H10) anti–CTLA-4 clones were used in independent experiments.
In vitro suppression assays.
CD4+CD25+ and CD4+CD25− T cells were purified from LNs with magnetic beads (Miltenyi Biotec) according to the manufacturer's instructions (>95% purity). To test in vitro suppressive activity, 50,000 CD4+CD25− T cells were plated in round-bottom 96-well plates in the presence of 150,000 T cell–depleted irradiated splenocytes, 10 µg/ml purified anti-CD3 and increasing amounts of CD4+CD25+ T reg cells. Anti–MuCTLA-4 (or mouse IgG control) was added at a final concentration of 50 µg/ml, which has been described to have maximum effect in in vitro cultures (Takahashi et al., 2000). Cells were incubated at 37°C for 72 h and pulsed with [3H]thymidine in the last 8 h of culture, at the end of which the plates were harvested and analyzed for [3H]thymidine incorporation. Groups were analyzed in quintuplicate, and experiments were repeated at least three times. For experiments in which aHuCTLA-4 was used, the mAb was also added at a final concentration of 50 µg/ml and human IgG was used as the control.
Cell lines.
The highly tumorigenic and poorly immunogenic melanoma cell line B16/BL6 was used for tumor challenge. B16/BL6-expressing GM-CSF, referred to in this paper as Gvax, was used for treatment of tumor-bearing mice. Both cell lines have been previously described (Quezada et al., 2006).
Bone marrow chimeras.
These were generated as previously described using bone marrow from the femurs of CD45.1+B6.SJL and CD45.2+CTLA4−/− mice Bachmann et al., 1999, 2001). Cells were mixed 50:50 and infused at a dose of 107 mononuclear cells per animal into RAG2−/− recipients 6 h after irradiation (300 cGy). 10–12 wk later, mice were bled to confirm mixed chimerism (40–60% CD45.1+). LNs were harvested, single cell suspensions prepared, and T cells selected using a pan–T cell purification kit (Miltenyi Biotech; negative selection). Anti-CD45.1 biotin was then added immediately before CD4+CD25+ T cell selection using a T reg cell selection kit (Miltenyi Biotech), allowing isolation of CD45.1−CTLA-4−/− Teff and T reg cells (Fig. S4).
Tumor challenge and treatments.
RAG2−/− were injected via the tail vein with mixtures of WT and HuTg Teff and T reg cells as outlined in the text and in Fig. S5. 10 wk later, mice were challenged in the flank intradermally at day 0 with 20,000 B16/BL6 melanoma cell, and then treated with Gvax and aMuCTLA-4 (as detailed in Fig. 4 a). 106 irradiated (15O Gy) Gvax cells were injected intradermally (100 µl) in the contralateral flank on days 3, 6, and 9, while at the same time points, 100 µg of aMuCTLA-4 was injected i.p. in 200 µl PBS. Mice were monitored every 2–3 d for tumor growth.
Statistical analyses.
Data were analyzed using Prism 4.0 (GraphPad Software, Inc.). Experiments were repeated two to three times as indicated. Statistical significance was determined by a Student's t test (between two groups or conditions) or analysis of variance with a post-hoc test (three or more groups or conditions). Data for tumor survival were analyzed according to the Kaplan-Meier method. The log-rank test was used to compare survival curves for different subgroups on univariate analyses. P-values <0.05 were considered statistically significant.
Online supplemental material.
Fig. S1 shows the scaled map of the 17-kb genomic DNA used for the generation of HuTg mice. Fig. S2 shows the regulation of cell surface and intracellular CTLA-4 expression in WT and HuTg mice in response to stimulation with anti-CD3. Fig. S3 shows in vitro suppressor assays using aHuCTLA-4 rather than aMuCTLA-4. Fig. S4 illustrates the approach used for isolation of CTLA-4−/− T reg cells from mixed bone marrow chimeras. Fig. S5 illustrates the reconstitution of RAG2−/− mice and subsequent assessment of peripheral blood, LN, and splenic phenotyping before tumor challenge. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20082492/DC1.
Acknowledgments
We would like to dedicate this work to the memory of Dr Cynthia Chambers.
We would like to acknowledge Mark Karlok, Jose Mendoza, and Chris Petromilli for their assistance in the generation of the HuTg CTLA-4 mice and Anne Trumble and Jocelyn Lu for technical assistance with some of the experiments.
K.S. Peggs is currently an investigator at the Department of Haematology, University College London Cancer Institute, University College London, UK, and receives funding from the Leukaemia Research Fund, UK. S.A. Quezada is a Research Fellow funded by the Irvington Institute Fellowship Program of the Cancer Research Institute USA and a junior member of the Millennium Nucleus on Immunology and Immunotherapy, Pontifícia Universidad Católica de Chile. J.P. Allison is an investigator of the Howard Hughes Medical Institute and holds the David H. Koch Chair in Immunological Studies at the Memorial Sloan-Kettering Cancer Center. This work was also supported by The Experimental Therapeutics Center of Memorial Sloan-Kettering Cancer Center funded by William H. Goodwin and Alice Goodwin.
Anti–CTLA-4 is currently in clinical development by Medarex and Bristol-Meyers Squibb. A.J. Korman is an employee of Medarex. J.P. Allison is a consultant for Medarex and Bristol-Meyers Squibb, and is an inventor of intellectual property that has been licensed to Medarex and Bristol-Myers Squibb by the University of California, Berkeley. The other authors have no other conflicting financial interests.
Footnotes
Abbreviations used: aHuCTLA-4, anti–human CTLA-4; aMuCTLA-4, anti–mouse CTLA-4; ANA, antinuclear antibody; CKO, conditional KO; CTLA-4, CTL-associated antigen 4; Gvax, GM-CSF–secreting cellular vaccine; HuTg, human CTLA-4 transgenic; Teff, effector T cell; TKO, triple KO; T reg cell, regulatory T cell.
References
- Bachmann M.F., Kohler G., Ecabert B., Mak T.W., Kopf M. 1999. Cutting edge: lymphoproliferative disease in the absence of CTLA-4 is not T cell autonomous.J. Immunol. 163:1128–1131 [PubMed] [Google Scholar]
- Bachmann M.F., Gallimore A., Jones E., Ecabert B., Acha-Orbea H., Kopf M. 2001. Normal pathogen-specific immune responses mounted by CTLA-4-deficient T cells: a paradigm reconsidered.Eur. J. Immunol. 31:450–458 [DOI] [PubMed] [Google Scholar]
- Bodor J., Fehervari Z., Diamond B., Sakaguchi S. 2007. ICER/CREM-mediated transcriptional attenuation of IL-2 and its role in suppression by regulatory T cells.Eur. J. Immunol. 37:884–895 [DOI] [PubMed] [Google Scholar]
- Butte M.J., Keir M.E., Phamduy T.B., Sharpe A.H., Freeman G.J. 2007. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses.Immunity. 27:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno B.M., Bennett F., Chau T.A., Ling V., Luxenberg D., Jussif J., Baroja M.L., Madrenas J. 2000. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression.J. Immunol. 165:1352–1356 [DOI] [PubMed] [Google Scholar]
- Chambers C.A., Cado D., Truong T., Allison J.P. 1997. Thymocyte development is normal in CTLA-4-deficient mice.Proc. Natl. Acad. Sci. USA. 94:9296–9301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C.A., Sullivan T.J., Truong T., Allison J.P. 1998. Secondary but not primary T cell responses are enhanced in CTLA-4-deficient CD8+ T cells.Eur. J. Immunol. 28:3137–3143 [DOI] [PubMed] [Google Scholar]
- Chambers C.A., Kuhns M.S., Allison J.P. 1999. Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates primary and secondary peptide-specific CD4(+) T cell responses.Proc. Natl. Acad. Sci. USA. 96:8603–8608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Koralov S.B., Kelsoe G. 2000. Complement C4 inhibits systemic autoimmunity through a mechanism independent of complement receptors CR1 and CR2.J. Exp. Med. 192:1339–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F., Grohmann U., Hwang K.W., Orabona C., Vacca C., Bianchi R., Belladonna M.L., Fioretti M.C., Alegre M.L., Puccetti P. 2003. Modulation of tryptophan catabolism by regulatory T cells.Nat. Immunol. 4:1206–1212 [DOI] [PubMed] [Google Scholar]
- Greenwald R.J., Boussiotis V.A., Lorsbach R.B., Abbas A.K., Sharpe A.H. 2001. CTLA-4 regulates induction of anergy in vivo.Immunity. 14:145–155 [DOI] [PubMed] [Google Scholar]
- Greenwald R.J., Oosterwegel M.A., van der Woude D., Kubal A., Mandelbrot D.A., Boussiotis V.A., Sharpe A.H. 2002. CTLA-4 regulates cell cycle progression during a primary immune response.Eur. J. Immunol. 32:366–373 [DOI] [PubMed] [Google Scholar]
- Hodi F.S., Mihm M.C., Soiffer R.J., Haluska F.G., Butler M., Seiden M.V., Davis T., Henry-Spires R., MacRae S., Willman A., et al. 2003. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients.Proc. Natl. Acad. Sci. USA. 100:4712–4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach D.R., Krummel M.F., Allison J.P. 1996. Enhancement of antitumor immunity by CTLA-4 blockade.Science. 271:1734–1736 [DOI] [PubMed] [Google Scholar]
- Lute K.D., May K.F., Jr., Lu P., Zhang H., Kocak E., Mosinger B., Wolford C., Phillips G., Caligiuri M.A., Zheng P., Liu Y. 2005. Human CTLA4 knock-in mice unravel the quantitative link between tumor immunity and autoimmunity induced by anti-CTLA-4 antibodies.Blood. 106:3127–3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelbrot D.A., McAdam A.J., Sharpe A.H. 1999. B7-1 or B7-2 is required to produce the lymphoproliferative phenotype in mice lacking cytotoxic T lymphocyte–associated antigen 4 (CTLA-4).J. Exp. Med. 189:435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onizuka S., Tawara I., Shimizu J., Sakaguchi S., Fujita T., Nakayama E. 1999. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody.Cancer Res. 59:3128–3133 [PubMed] [Google Scholar]
- Peggs K.S., Quezada S.A., Allison J.P. 2008. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy.Immunol. Rev. 224:141–165 [DOI] [PubMed] [Google Scholar]
- Phan G.Q., Yang J.C., Sherry R.M., Hwu P., Topalian S.L., Schwartzentruber D.J., Restifo N.P., Haworth L.R., Seipp C.A., Freezer L.J., et al. 2003. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma.Proc. Natl. Acad. Sci. USA. 100:8372–8377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piconese S., Valzasina B., Colombo M.P. 2008. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection.J. Exp. Med. 205:825–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada S.A., Peggs K.S., Curran M.A., Allison J.P. 2006. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells.J. Clin. Invest. 116:1935–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada S.A., Peggs K.S., Simpson T.R., Shen Y., Littman D.R., Allison J.P. 2008. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma.J. Exp. Med. 205:2125–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Malmstrom V., Powrie F. 2000. Cytotoxic T lymphocyte–associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation.J. Exp. Med. 192:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Greenwald R., Izcue A., Robinson N., Mandelbrot D., Francisco L., Sharpe A.H., Powrie F. 2006. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo.J. Immunol. 177:4376–4383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A., Camacho L.H., Lopez-Berestein G., Pavlov D., Bulanhagui C.A., Millham R., Comin-Anduix B., Reuben J.M., Seja E., Parker C.A., et al. 2005. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206.J. Clin. Oncol. 23:8968–8977 [DOI] [PubMed] [Google Scholar]
- Shrikant P., Khoruts A., Mescher M.F. 1999. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism.Immunity. 11:483–493 [DOI] [PubMed] [Google Scholar]
- Sutmuller R.P., van Duivenvoorde L.M., van Elsas A., Schumacher T.N., Wildenberg M.E., Allison J.P., Toes R.E., Offringa R., Melief C.J. 2001. Synergism of cytotoxic T lymphocyte–associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses.J. Exp. Med. 194:823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T.W., Sakaguchi S. 2000. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte–associated antigen 4.J. Exp. Med. 192:303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe P.A., Davis S.J. 2003. Molecular interactions mediating T cell antigen recognition.Annu. Rev. Immunol. 21:659–684 [DOI] [PubMed] [Google Scholar]
- van Elsas A., Hurwitz A.A., Allison J.P. 1999. Combination immunotherapy of B16 melanoma using anti–cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)–producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation.J. Exp. Med. 190:355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakrishnan L., Slavik J.M., Illes Z., Greenwald R.J., Rainbow D., Greve B., Peterson L.B., Hafler D.A., Freeman G.J., Sharpe A.H., et al. 2004. An autoimmune disease-associated CTLA-4 splice variant lacking the B7 binding domain signals negatively in T cells.Immunity. 20:563–575 [DOI] [PubMed] [Google Scholar]
- Waterhouse P., Penninger J.M., Timms E., Wakeham A., Shahinian A., Lee K.P., Thompson C.B., Griesser H., Mak T.W. 1995. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4.Science. 270:985–988 [DOI] [PubMed] [Google Scholar]
- Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., Sakaguchi S. 2008. CTLA-4 control over Foxp3+ regulatory T cell function.Science. 322:271–275 [DOI] [PubMed] [Google Scholar]