The Important Role of the Apoptotic Effects of Zinc in the Development of Cancers (original) (raw)
. Author manuscript; available in PMC: 2009 Aug 17.
Published in final edited form as: J Cell Biochem. 2009 Apr 1;106(5):750–757. doi: 10.1002/jcb.22049
Abstract
Zinc is a trace element that is essential for the normal function of cells. It is a cofactor for the structure and function of a wide range of cellular proteins including enzymes, transcription factors, and structural proteins. Recent studies have shown that zinc plays a role in the development of various cancers. Unfortunately no established common relationships of zinc with cancer development and progression have been identified. Zinc is known to have systemic effects such as regulation of the immune system as well as direct cellular effects resulting in regulation of gene expression, bioenergetics, metabolic pathways, signal transduction and cell invasion. Zinc is also reported to regulate cell proliferation and growth. In this review presentation we focus on the effects of zinc that are involved in the regulation of apoptosis in malignant cells. We selected the apoptotic effects of zinc because zinc is reported to both induce apoptosis in some cancers and to protect other cancer cells against apoptosis induced by other factors. The effects of zinc in the regulation of apoptosis appear to be cell type specific. More importantly the reported effects of zinc on cancer cells must be viewed from the perspective of the physiological regulation of zinc homeostasis. Thus one must be mindful of the experimental conditions under which zinc effects are investigated relative to the physiological and pathological conditions of cellular zinc distribution and concentrations that can exist in situ.
Keywords: zinc, cancer, apoptosis, prostate, breast, liver, pancreas, ovarian, zinc transporters
In recent years, evidence and information have evolved regarding the involvement and importance of zinc in the development of several cancers. Many potential effects and actions of zinc have been described in relation to different cancers. Unfortunately, relatively few established and consistent relationships have been identified concerning the role and mechanism of zinc effects in a specific cancer. For discussion purposes, the effects of zinc can be categorized as “systemic” effects or “malignant cell” effects. The former are those effects, such as immune system effects, that alter bodily function at the integrated systems level; which can then influence the development and progression of cancer. The latter are those direct cellular effects associated with the transformation of normal cells to malignant cells and with the manifestation of malignant capabilities of cancer cells. In regard to the direct cellular effects of zinc, one can identify four (perhaps more) important cellular actions that zinc can manifest in the development and progression of malignant cells.
- Alter gene expression via direct effects on target genes or via effects on transcription factors.
- Alter cellular metabolism that provides the bioenergetic and synthetic requirements.
- Alter cell migration and invasive activities.
- Alter cell proliferation and apoptosis.
Obviously, there exist levels of interdependency among these effects.
In this presentation we will focus on the apoptotic effects of zinc. We selected this subject for three reasons: 1) zinc effects on apoptosis are important in the regulation of normal and malignant cell growth/proliferation; 2) the reported apoptotic effects of zinc are complex, inconsistent and controversial; and hopefully this Prospects article will provide some degree of clarity to a confusing subject; and 3) the apoptotic effects provide a potential target for the development of anti-tumor agents.
Some Fundamental Considerations Regarding the Physiology of Zinc
Before addressing the issues of zinc and apoptosis, some important factors must be described and appreciated. In our view, for described cellular effects of zinc to be relevant, the experimental conditions should bear some reasonable representation of physiological and in situ conditions. Consequently, an understanding of the biochemistry and physiology of zinc in biological systems is essential (Beyersmann et al., 2001;Eide, 2006;Franklin et al., 2005;Truong-Tran et al., 2000a). For example, many reported studies involving the treatment of cells with zinc-supplemented medium have employed extremely high concentrations of zinc, which would never exist under physiological or even pathological conditions. Most mammalian cells in situ are exposed to the interstitial fluid, which is a filtrate of blood plasma. The zinc concentration of normal interstitial fluid is approximately 2–5 uM, while the free Zn++ ion concentration is extremely low (∼200 nM in plasma/interstitial fluid) (Magneson et al., 1987). However, many reports of zinc effects have been conducted with zinc concentrations ranging from 50 uM to 1000 uM. In most mammalian cells, the normal intracellular concentration of zinc is in the range of ∼100–500 uM. However ∼90% of this total zinc is tightly bound to proteins and is not a part of the mobile reactive pool of zinc. The mobile reactive pool of zinc is comprised of low molecular weight zinc ligands with relatively low binding affinities; such as ZnAspartate, ZnHistidine, ZnCysteine, ZnGlutamate, ZnCitrate, and ZnMetallothionein. The cellular free Zn++ ion concentration is in the pM-fM range; so that it is not a physiologically relevant pool of reactive zinc in most mammalian cells. One must recognize that the measurement of the total cellular zinc is not as important as the determination of the mobile reactive pool of zinc. In addition, zinc is not uniformly distributed within the cells; so that organelle sequestering of zinc also becomes a factor.
Zinc experimental studies generally involve manipulations of the cellular levels of zinc. This is most often achieved by zinc treatment of cells in the presence of an ionophore to facilitate its cellular uptake and increase the intracellular level of zinc. Conversely, cells are treated with a cell-permeable zinc chelator to bind/remove the intracellular zinc, which is often followed by restoration of zinc as previously described. The relationship of these manipulated cellular zinc levels to the range of “normal” cellular zinc levels must be considered in the translational interpretation of the results obtained. For example, a commonly employed experimental procedure in the studies of zinc protection against apoptosis is the treatment of cells with TPEN; which rapidly depletes the cellular level of zinc, followed by apoptosis (i.e. TPEN-induced apoptosis). However, cautious interpretation of zinc effects and its physiological relevance from such studies is required. TPEN exhibits a high zinc-binding affinity (logKf∼16), which could virtually remove the entire mobile reactive pool of zinc; as well as a significant fraction of immobile macromolecular-bound zinc. Such a cellular condition would not be achievable in normal or pathological conditions; and predictably would result in cell death. This is seemingly averted by the use of relatively low concentrations of TPEN (e.g. 10 uM) compared to ∼200 uM total cellular zinc and ∼20 uM mobile reactive zinc. However, this expectation is not exactly valid. The cell permeant property of TPEN insures its diffusion into and out of the cell. Also, as ZnTPEN is formed, it diffuses out of the cells. The amount of Zn extracted from the cell becomes dependent upon the relative volumes and concentrations (ie the total TPEN vs the total zinc of all the cells). Also, the use of “excess” TPEN is likely to decrease the cellular pool of available Mn and Fe, which are bound by TPEN (log Kf∼10 and 15, respectively). In the opposite situation, the use of a zinc ionophore such as pyrithione to incorporate zinc into cells can result in supraphysiological levels of cellular zinc.
While these experimental “tools” are useful and even often necessary, they require special attention in the design and control of zinc levels, and in the interpretation of experimental studies. This is especially relevant to the issue of zinc and apoptosis; more so since cellular zinc can have concentration-dependent biphasic (induction and inhibition) effects on apoptosis. This brief discussion does not define other important considerations, but is intended to exemplify the importance of and necessity to understand the biochemistry and physiology of zinc in biological systems.
Zinc Exhibits Cell-Specific Opposite Apoptotic Actions
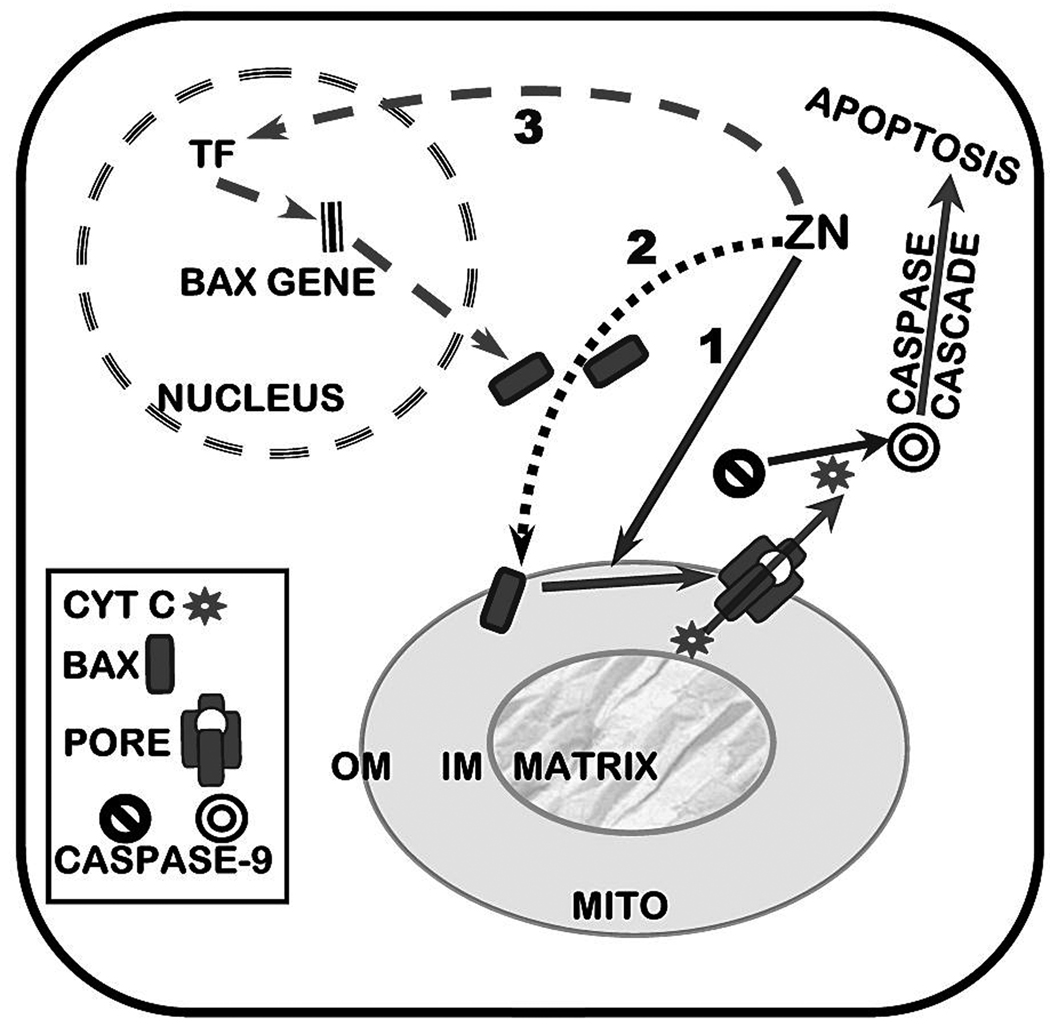
That zinc plays an important role in the determination and regulation of apoptosis in mammalian cells is well established (Bae et al., 2006;Chang et al., 2006;Chimienti et al., 2001;Chung et al., 2000). Presently, the mechanisms, factors, and pathways associated with this role of zinc are diverse, complex, cell-specific and poorly understood. A composite of many of the reported cellular effects of zinc relating to its apoptotic pathways can be summarized as represented in figure 1. Zinc can have direct effects on apoptosis through its direct action on the nucleus or its direct action on mitochondrial apoptogenesis as represented in A and B in figure 1. Zinc can also regulate or modulate cellular apoptotic signaling factors/pathways (mechanism C in figure 1) that then act directly on the nucleus (C1) or on mitochondrial apoptogenesis (C2 and D). Also, zinc can have a direct effect on mitochondria (B) which induces altered mitochondrial function, which produces conditions (E1) that activate the apoptotic signaling pathway (E2). While these are representative potential pathways, other complex pathways can exist. The pathway and the zinc effect (stimulation or inhibition) on apoptosis that is manifested are cell-specific.
Figure 1.
Representation of some zinc-induced apoptotic pathways. A. Direct effects on nucleus. B. Direct effects on mitochondria. C. Direct effect on apoptotic signaling pathways f1,f2,f3. C1/C2. Apoptotic signaling pathway effects on nucleus or mitochondria. D. Effects of mitochondrial factors on nucleus. E1/E2. Effects of mitochondrial factors mediated through the apoptotic signaling pathway.
A critical issue that must be addressed relates to the opposite actions of zinc that appear to be cell-type dependent. Zinc has been reported to induce apoptosis in many mammalian cell types, including prostate epithelial cells, neurones and glial cells, ovarian epithelial cells, esophageal epithelial cells, choriocarcinoma cells, hepatoma cells, and others. In contrast, in other cells (e.g. breast cells, lung epithelial cells, renal cells, macrophages, lymphocytes, thymocytes, pancreatic acinar cells, Hela cells, and others) zinc exhibits anti-apoptotic effects. Why and how these opposite actions are manifested remain critically important unanswered questions. Some reports have shown the absence of effects of zinc at near-physiological levels, but observe specific effects at unphysiological zinc levels. Moreover, in some cells exposure to low zinc levels induces apoptosis; whereas exposure to high zinc levels inhibits apoptosis (Provinciali et al., 1995). A difficulty in assessing such results is the absence of relevant information regarding the cellular level of zinc. The mechanisms and capabilities of cellular uptake and accumulation of zinc vary in different cell types. Often one cannot establish whether the observation results from a direct physiological effect of zinc on the apoptotic process or is an artifact effect due to manifestations of zinc on other conditions that will then influence the apoptotic process. One must assess the conditions employed when considering the possible translational relationship to the likely in situ conditions of the normal and malignant cells as we earlier described. With such issues in mind, we will proceed to describe the relationship of zinc as an inducer or inhibitor of apoptosis in some representative cancers.
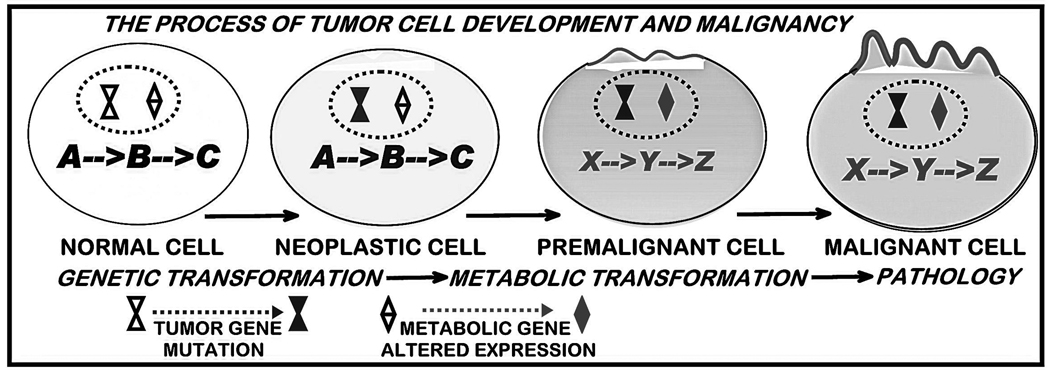
Zinc: A Genetic/Metabolic Transformation Stage in the Development of Cancer
To understand the role of zinc in the development and progression of cancer, we pose a common relationship that applies to virtually all cancers (figure 2). The initiation of malignancy requires the genetic transformation of a normal cell to a neoplastic cell that is endowed with malignant capability. To manifest its potential malignant activities, the neoplastic cell undergoes additional “genetic/metabolic” transformations that provide the cellular bioenergetic/synthetic, proliferation/growth and migration/invasive requirements of malignancy. These transformations define a “premalignant” stage. The establishment of the “metabolic” conditions then permits the development and progression of malignancy. In the absence of the “metabolic transformation”, the neoplastic cell will remain in an arrested condition or will be aborted. This process of malignancy can be applied to the zinc-apoptosis relationship.
Figure 2.
The concept of the genetic/metabolic transformation in the development of malignant cells.
Alterations in the cellular zinc status are associated with the malignant transformation and the malignant activities. Altered cellular zinc status can involve changes in the concentration of zinc, changes in the form of zinc, and changes in the sequestration of zinc. For this presentation, we will focus on the most identified zinc relationship in the malignant process; which is the change in the concentration of cellular zinc. In this discussion we will presume that such a change will be reflective of a change in the mobile reactive pool of zinc.
The accumulation of zinc in cells is dependent upon zinc transporters (for recent reviews see Eide, 2006;Taylor et al., 2007). The cellular level of zinc is firstly dependent upon zinc derived from extracellular sources, predominantly from interstitial fluid for most mammalian cells. To derive this zinc, a cell must possess a plasma membrane zinc uptake transporter; i.e. predominantly a ZIP-family transporter. Once within the cytosol, redistribution of zinc occurs by transporters (predominantly ZnT-family transporters) that sequester the zinc among organelles or export the zinc from the cell. Therefore zinc transporter expression and availability (especially ZIP transporters) constitute a critical factor associated with the genetic/metabolic transformation that is essential to the malignant process in most, if not all, cancers as represented in figure3. Presently, the best established examples of this are presented below in the description of prostate cancer and in pancreatic cancer, which show opposite relationships.
Figure 3.
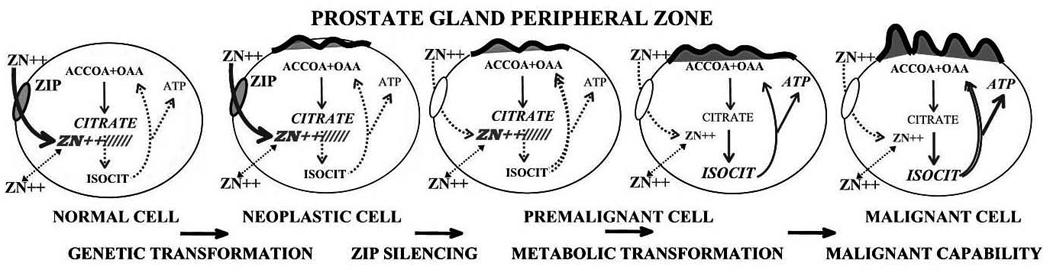
The role of Zip1 and zinc in the genetic/metabolic transformation of normal prostate cells to malignant cells.
Prostate Cancer
In prostate the normal epithelial cells are zinc accumulating cells and contain high levels of cellular zinc. In contrast, the malignant cells have lost the ability to accumulate zinc and contain low levels of zinc. The mechanism responsible for this “metabolic” transformation is the down regulation of hZIP1, which is a major transporter responsible for zinc uptake and accumulation in prostate cells (Costello et al., 2006) and (Franklin et al., 2007). In this way the malignant cells avoid the tumor suppressor effects of zinc, which includes the apoptogenic effect. For these reasons we refer to ZIP1 as a tumor suppressor gene and zinc as a tumor suppressor agent in prostate cancer.
Because most early studies described zinc as growth-promoting and an anti-apoptotic agent in mammalian cells , we were somewhat surprised to observed that the exposure of prostate cells to zinc inhibited cell growth (Franklin et al., 1995); and that this effect was due, in part, to its induction of apoptosis (Liang et al., 1999). Further studies revealed for the first time that the apoptotic effect is due to zinc-induced mitochondrial apoptogenesis (Feng et al., 2000). Most significantly, this action is due to a direct effect of cytoplasmic zinc on the mitochondria, which results in the release of cytochrome c, followed by activation of caspase-3 and the cascade that leads to downstream cellular apoptotic events (Feng et al., 2002). This zinc effect results from its ability to increase resident Bax-mitochondrial interaction that is associated with Bax-induced pore formation (Feng et al., 2008). This is a rapid apoptogenic effect that occurs within minutes following an increase in the cytosolic availability of zinc. Because the mitochondria of prostate cells such as PC-3 cells contain endogenous levels of resident Bax, the zinc-Bax associated pore formation does not require any additional cytosolic factors or interaction to induce the release of cytochrome c. Upstream or additional mitochondrial events such as ROS production, energy uncoupling activity, altered membrane potential, are not required for this zinc effect. Figure 4 provides a representation of this zinc-induced apoptogenic effect. This apoptogenic mechanism is an example of the direct zinc effect shown as pathway B and D in figure 1.
Figure 4.
Zinc induction of apoptogenesis in prostate cells. 1. Cytosolic zinc directly interacts with mitochondrial resident Bax to facilitate pore formation and release of cytochrome c for caspase activation leading to apoptosis. 2. Cytosolic zinc facilitates the insertion of cytosolic Bax into the mitochondrial membrane. Cytosolic zinc activates Bax gene-associated transcription factor, which increases expression and cellular level of Bax.
In concert with this action, zinc also increases the cellular level of Bax (Feng et al, 2008). No such increase occurs in either cellular or mitochondrial Bcl-2; so that the Bax/Bcl-2 ratio is increased, which is a pro-apoptotic condition. The increased production of Bax increases the level of Bax that is available for zinc-induced mitochondrial pore formation (figure 4). The increase in cellular Bax is attenuated by cycloheximide and is likely due to zinc induction of events that cause increased expression of the Bax gene (Feng et al, 2008). Since the promoter region of the Bax gene is devoid of consensus sequences for a metal-response element (MRE), a direct Bax gene interaction of zinc via the metal response element binding transcription factor-1 (MTF-1) is not likely. Other zinc-activated transcription factor response elements are present in the Bax promoter (for example Egr-1 and HIF1a) through which zinc might increase expression of the Bax gene. This effect of zinc is an example of apoptotic pathway C, C2, and D in figure 1. It is important to note that p53 is not involved in this pathway since it exists in p53 deficient PC-3 cells.
This direct zinc-induced mitochondrial apoptogenic effect is not observed in all cells. There are apparently specific cellular requirements for this action to be manifested in response to exposure of cells to zinc. First, there must be cellular uptake and cytosolic accumulation of zinc. The cytosolic zinc must contain a significant concentration of mobile reactive zinc that is required to manifest the mitochondrial Bax associated interaction. If one does not observe an effect of cell exposure to zinc, one must ascertain if the uptake and accumulation of cellular zinc is reflected by an increase in the level of mobile reactive zinc. Another factor is the cell type. While additional information is necessary, it appears that the mitochondria from different cells do not exhibit the same responsiveness or capability to this direct effect of zinc. This is a potentially critical factor in the differing responses and effects of various cells to zinc. It is noteworthy that Jiang et al (2001) also observed direct effects of zinc on mitochondria, which induced release of cytochrome c leading to apoptosis.
Another important issue is associated with this apoptogenic effect of zinc in prostate cells. Since the normal prostate epithelial cells evolved and exist as highly specialized zinc-accumulating cells, they must possess adapted mechanisms that prevent the potential adverse effects of high zinc levels. Also, there is very little cell turnover in the normal mature prostate gland. Consequently, zinc induction of apoptosis is suppressed in the normal cells in situ, most likely due to the presence of anti-apoptotic activities. For example, HIF-1a expression reportedly prevents the apoptotic effect of zinc in normal prostate glands (Park et al., 2007). Our identification of the role of Bax would suggest that the normal prostate glandular epithelial cells likely incorporate up-regulation of anti-apoptotic proteins such as Bcl-2 that prevent the mitochondrial apoptogenic effects of zinc. This is an important issue and relationship that needs to elucidated. An understanding of the absence of zinc induced apoptosis in normal prostate epithelium could provide insight for new approaches for the treatment and possible prevention of prostate cancer.
Breast Cancer
Several reports have established that zinc levels are markedly increased in breast cancer tissue as compared with non-cancerous tissue (Margalioth et al., 1983; Rizk et al., 1984; Santoliquido et al., 1976). Consistent with this association, Kagara et al (2007) reported that increased ZIP10 expression correlated with increasing aggressiveness and metastatic activity of the malignant cells. They further show that ZIP10 expression was required for optimal migration activity of malignant breast cells, and that zinc was essential for this capability. In contrast, ZIP6 (LIV1) expression was not associated with these effects. Unfortunately, no information was provided or was determined for the expression of ZIP10 in malignant breast tissue vs. non-malignant tissue.
Other studies have implicated the up-regulation of estrogen-stimulated ZIP6 (LIV1) expression in the progression of malignancy and metastasis (Manning et al., 1994; Manning et al., 1995; Taylor et al 2007). In addition, Kasper et al (2005) observed significant increased expression of ZIP6 in tumor tissue versus normal tissue in sections from the same subjects. However, the level of ZIP6 transporter protein did not correlate with the ZIP6 mRNA change, and did not show a significant increase in breast tumor tissue. Thus, some confusion exists concerning the significance of altered ZIP6 expression in breast cancer if it is not manifested by a corresponding change in the level of functional ZIP6 transporter. Taylor et al (2003) had shown that the ZIP6 transporter was plasma membrane associated in CHO (Chinese-hamster ovary) cells and functioned as a zinc-uptake transporter. However, Kasper et al (2005) identified the localization of ZIP6 to the endoplasmic reticulum of breast cancer cell lines ZR-75-1 and MCF-7, rather than to the plasma membrane as reported for CHO cells. They propose that this variance could be due to the natural localization of endogenous ZIP6 in the breast cells. They further suggest that ZIP6 functions to sequester cellular zinc which would re-distribute zinc for its effects. However, this would not account for the increase in total zinc associated with breast cancer tissue.
Once the cellular level of zinc is increased, the issue becomes the mechanism by which the malignant cells are protected from zinc associated apoptosis. One possible mechanism is provided by Ostrakhovitch and Cherian (2005) who found that zinc-induced apoptosis in MCF-7 breast cancer cells required the presence of a functional p53 expression. In these cells, zinc treatment initiated p53/ROS -mediated apoptotic events, which included translocation of p53 to mitochondria, dissipation of mitochondrial membrane potential, and direct mitochondrial translocation of Bax. Such events are involved in the induction of mitochondrial apoptogenesis that would be mediated via cytochrome c release and induction of the apoptotic caspase cascade. P53 negative cells did not elicit these effects of zinc.
These results indicate that zinc can act as an apoptogenic agent in breast epithelial cells via the pathway represented as C and C2 in figure 1. It then follows that malignant breast cells avoid this apoptogenic effect of zinc by the elimination of functional p53 or by the elimination of downstream factors that are required to mediate the p53-induced apoptosis. However, a conundrum exists as to why the malignant cells would exhibit an increase in zinc and the associated transporter changes in the first place. Although an increase in zinc in breast cancer is clearly established, the mechanism responsible for the change in zinc and the role of zinc in the malignant process are poorly understood and require much more investigation.
Pancreatic Cancer
Presently pancreatic cancer is one of the deadliest cancers, being virtually incurable and untreatable once the cancer has been identified. A better understanding of the important factors that contribute to the development and progression of pancreatic cancer is essential. A recent excellent study by Li et al (2007) has provided compelling evidence for an important role of zinc and the zinc uptake transporter ZIP4 in the development of pancreatic cancer. Analysis of human tissue sections revealed that malignant glands exhibited a marked increase in ZIP4 expression compared to surrounding normal tissue. The increased expression was accompanied by an increase in ZIP4 transporter protein. They additionally found that increased ZIP4 expression existed in pancreatic cancer cell lines; and that ZIP4 functions as a zinc uptake transporter. In in vitro studies, ZIP4 expression was associated with increased cellular accumulation of zinc and increased cell proliferation. Correspondingly, xenograft studies showed that ZIP4 expression significantly increased tumor cell zinc levels and tumor growth. This comprehensive study demonstrates a highly significant role of zinc in the development and progression of pancreatic cancer. Pursuant studies are now necessary to determine the mechanism and factors involved in the altered ZIP4 gene expression, and the mechanism for the proliferative action of zinc.
In a study involving the effect of zinc in pancreatic cancer cell lines, Donadelli et al (2008a) found that exposure of cells to TPEN to deplete cellular zinc induced apoptosis, which is reversed by the administration of zinc. Furthermore, they provide evidence that the anti-apoptotic effects of zinc involve mitochondrial changes including inhibition of caspase-3 activation and caspase-8 activity and also decreasing the ratio of apoptotic/antiapoptotic mitochondrial-related Bcl-family proteins. These growth promoting anti-apoptotic effects of zinc in pancreatic cancer cells are consistent with the study of Li et al.
However, in another study with a seemingly opposite action of zinc, Donadelli et al (2008b) report that zinc treatment of pancreatic cancer cells strongly inhibits cell growth due to a significant increase in zinc-induced apoptosis. They provide evidence that cellular zinc accumulation increased ROS production, which initiated the apoptotic effect. Neither caspase-3/caspase-8 activation nor mitochondrial proapoptotic proteins were involved in the apoptotic process. Instead, they suggest that zinc-dependent ROS production causes nuclear translocation of AIF that results in the apoptotic effect. There is no explanation presented for the seemingly conflicting effects of zinc presented in these two reports. As we discussed above in Section 2, the imposition of experimental conditions can and will provide differing cellular effects and results that might not reflect the physiological conditions of in situ cells and their environment. It seems probable that the different conditions employed to manipulate the cellular levels of zinc induce differing cellular conditions that manifest differing effects that are interpreted as zinc effects. Other possible factors and conditions might also contribute to the diversity of responses.
If, as the study of Li et al demonstrates, pancreatic cancer involves the metabolic transformation for increased uptake and accumulation of zinc in the malignant cells, the effect of zinc must be growth promoting; which likely involves an inhibitory effect on apoptosis. Despite the fact that zinc prevents apoptosis in many mammalian cells, there exists no established specific mechanism for this effect of zinc. Fukamachi et al (1998) showed that zinc treatment of human premonocytic U937 cells prevented apoptosis by decreasing Bax levels with no effect on Bcl-2; i,e. increased Bcl-2/Bax ratio. Several studies have reported inhibitory effects on various caspases in some cells as possible mechanisms for zinc prevention of apoptosis. Moreover, activation of caspase-3 seems to be a potentially important effect in airway epithelial cells (Truong-Tran et al., 2000b;Truong-Tran et al., 2001). Other anti-apoptotic direct effects of zinc have been described in various cells. At this time, the mechanism for a potential effect of accumulated zinc in pancreatic cancer is still a critical issue that needs to be resolved. Moreover, in addition to its effect on increased proliferation, one must wonder what effect the elevated increase in cellular zinc has on the intermediary metabolism of the malignant cells. Increased cellular zinc inhibits citrate oxidation, terminal oxidation, and respiration of mammalian cells (Costello et al., 1997;Costello et al., 2004). This relationship also needs to be investigated in the zinc-associated genetic/metabolic transformation in the development and progression of pancreatic malignancy.
Ovarian Cancer
In 1986 Lightman et al (1986) first reported that the zinc level of ovarian tumor tissue was significantly lower than the level in benign tissue samples. Since that early report, to the best of our knowledge, there has not been any follow-up studies reported to confirm this potentially important observation. There are several reports showing that the zinc concentration in serum is reduced in ovarian cancer patients. Consistent with a decrease in tumor zinc levels is the report of Bae et al. (2006), which showed that exposure of OVCAR-3 cells (ovarian cancer cell line) to zinc-supplemented medium results in decreased cell growth that involved increased apoptosis. They further observed that zinc treatment increased the cellular accumulation of zinc, which resulted in the inhibition of m-aconitase activity. As we had reported, accumulation of mitochondrial zinc causes inhibition of m-aconitase and citrate oxidation; and this is evidence of increased mobile reactive zinc as we showed for prostate and liver cells (Costello et al., 1981;Costello et al., 2000). Also, the induction of apoptosis involved an increase in Bax and Bax/Bcl-2 ratio and the activation of caspase-3. None of these effects were observed in NOSE cells (normal ovarian surface epithelial cells) since they did not accumulate zinc (Bae et al 2006). It is noteworthy that these effects of zinc accumulation in the OVCAR-3 cells are somewhat similar to the effects observed in prostate cancer cells. Another more recent study (Ding et al., 2008) showed that treatment of A2780 cells (human ovarian cancer cells) with zinc and zinc ionophores (clioquinol and PDTC) induces apoptosis and necrosis, which was associated with an inhibition of Akt and NF-kappaB signaling pathways. The composite of these clinical and experimental studies provide substantial evidence that zinc accumulation appears to play an important role in the development and progression of ovarian cancer. The studies suggest that zinc is an apoptogenic agent in ovarian epithelial cells, which the malignant cells avoid by a decrease in zinc accumulation. No information exists concerning the critical issue of the mechanism and/or transporters associated with the decrease in cellular zinc in the malignant cells.
Hepatocellular Cancer
There is corroborating clinical evidence that hepatocellular cancer tissue contains significantly lower zinc levels than normal parenchymal tissue. For example, Ebara et al (2000) reported for sixteen subjects a 55% decrease in the zinc level of hepatomas compared to the surrounding normal parenchyma. Similarly Liaw et al (1997) observed a 66% decrease; and Danielson and Steinnes (1970) observed a 62% decrease. Other reports confirm that zinc levels are decreased in heptocellular cancer (Gurusamy, 2007; Tashiro et al., 2003; Tashiro-Itoh et al., 1997). Despite this important clinical relationship, little information or reports exist regarding the mechanisms and factors responsible for the decrease in zinc and for the role of a decrease in zinc in the development of malignancy.
It is notable that cDNA microarray studies by Liu et al (2007) found that the ZIP14 gene was down regulated in hepatoma tissue. However such microarrays are of little significance if additional studies are not conducted to establish the functional implications of the ZIP14 transporter in the normal and malignant cells. In regard to apoptotic effects of zinc in hepatocellular cancer cells, Xu et al (1996) reported that zinc induced apoptosis in human hepatoma H-7 cells. The effect required c-myc and a proposed c-myc pathway to apoptosis which did not manifest an internucleosomal DNA fragmentation. Reaves et al (2000) reported that zinc depletion increases and zinc supplementation decreases p53 in HepG2 hepatoblastoma cells. Initially this response of p53 seems to be inconsistent with the decreased zinc levels in hepatocellular cancer. However, as pointed out by Reaves decreased cellular zinc causes p53 to take a mutant like form with decreased DNA binding capability. These studies and others establish that p53 expression and activity are responsive to the zinc status of the cell. Therefore it is reasonable to suggest that the decrease in zinc in hepatocellular cancer in situ attenuates the tumor-suppressor/apoptotic effects of p53 and/or c-myc. However, much more research is essential to elucidate the relationship of altered zinc in hepatocellular cancer.
10. Summary and Conclusions
We do not represent that the preceding series of cancers constitutes all of the cancers in which altered zinc relationships play an important role in the development of malignancy. It is evident from the preceding descriptions that altered zinc relationships differ in different cancers. However, it is also evident that the concept of the requirement for a genetic/metabolic transformation in the development of malignancy (figure 2) is appropriate. It is also important to recognize that our discussion did not attempt to identify or describe different forms of cancer within any of the cancer types. However, this is an important consideration. One of the most striking revelations is the distinct difference in the zinc relationship of pancreatic cancer from all of the other cancers that we described. The increased zinc accumulation and up regulation of ZIP 4 transporter in pancreatic cancer is particularly opposite to prostate cancer in which decreased zinc and down-regulation of ZIP1 are critical for malignant cell development and proliferation. It is also important to re-emphasize that the role of zinc in cancer includes metabolic/bioenergetic and migration/invasive effects as well as apoptotic/proliferation effects.
Perhaps the most profound conclusions of this review are: 1) Altered zinc relationships are critical in cancer development; 2) among the important effects of zinc is its role in apoptosis; 3) much more information and research are essential to elucidating the role, mechanisms and factors relating to zinc in various cancers; and 4) such information is critical to the understanding of the etiology and progression of cancer, and to the development of new approaches to the treatment and prevention of cancer.
Acknowledgement
The studies of RBF and LCC referred to in this paper were supported by NIH grants CA79903; CA71207.
References
- Bae SN, Lee YS, Kim MY, Kim JD, Park LO. Antiproliferative and apoptotic effects of zinc-citrate compound (CIZAR(R)) on human epithelial ovarian cancer cell line, OVCAR-3. Gynecol Oncol. 2006;103:127–136. doi: 10.1016/j.ygyno.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Beyersmann D, Haase H. Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals. 2001;14:331–341. doi: 10.1023/a:1012905406548. [DOI] [PubMed] [Google Scholar]
- Chang KL, Hung TC, Hsieh BS, Chen YH, Chen TF, Cheng HL. Zinc at pharmacologic concentrations affects cytokine expression and induces apoptosis of human peripheral blood mononuclear cells. Nutrition. 2006;22:465–474. doi: 10.1016/j.nut.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Chimienti F, Seve M, Richard S, Mathieu J, Favier A. Role of cellular zinc in programmed cell death: temporal relationship between zinc depletion, activation of caspases, and cleavage of Sp family transcription factors. Biochem Pharmacol. 2001;62:51–62. doi: 10.1016/s0006-2952(01)00624-4. [DOI] [PubMed] [Google Scholar]
- Chung KC, Park JH, Kim CH, Lee HW, Sato N, Uchiyama Y, Ahn YS. Novel biphasic effect of pyrrolidine dithiocarbamate on neuronal cell viability is mediated by the differential regulation of intracellular zinc and copper ion levels, NF-kappaB, and MAP kinases. J Neurosci.Res. 2000;59:117–125. [PubMed] [Google Scholar]
- Costello LC, Franklin RB. Aconitase activity, citrate oxidation, and zinc inhibition in rat ventral prostate. Enzyme. 1981;26:281–287. doi: 10.1159/000459195. [DOI] [PubMed] [Google Scholar]
- Costello LC, Franklin RB. The clinical relevance of the metabolism of prostate cancer; zinc and tumor suppression: connecting the dots. Mol Cancer. 2006;5:17. doi: 10.1186/1476-4598-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Franklin RB, Liu Y, Kennedy MC. Zinc causes a shift toward citrate at equilibrium of the m-aconitase reaction of prostate mitochondria. J Inorganic Biochem. 2000;78:161–165. doi: 10.1016/s0162-0134(99)00225-1. [DOI] [PubMed] [Google Scholar]
- Costello LC, Guan Z, Kukoyi B, Feng P, Franklin RB. Terminal oxidation and the effects of zinc in prostate and liver mitochondria. Mitochondrion. 2004;4:331–338. doi: 10.1016/j.mito.2004.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Liu Y, Franklin RB, Kennedy MC. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J Biol.Chem. 1997;272:28875–28881. doi: 10.1074/jbc.272.46.28875. [DOI] [PubMed] [Google Scholar]
- Danielsen A, Steinnes E. A study of some selected trace elements in normal and cancerous tissue by neutron activation analysis. J Nucl.Med. 1970;11:260–264. [PubMed] [Google Scholar]
- Ding WQ, Yu HJ, Lind SE. Zinc-binding compounds induce cancer cell death via distinct modes of action. Cancer Lett. 2008;271:251–259. doi: 10.1016/j.canlet.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Donadelli M, Dalla PE, Costanzo C, Scupoli MT, Scarpa A, Palmieri M. Zinc depletion efficiently inhibits pancreatic cancer cell growth by increasing the ratio of antiproliferative/proliferative genes. J Cell Biochem. 2008a;104:202–212. doi: 10.1002/jcb.21613. [DOI] [PubMed] [Google Scholar]
- Donadelli M, Dalla PE, Scupoli MT, Costanzo C, Scarpa A, Palmieri M. Intracellular zinc increase inhibits p53(-/-) pancreatic adenocarcinoma cell growth by ROS/AIF-mediated apoptosis. Biochim.Biophys.Acta. 2008b doi: 10.1016/j.bbamcr.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Ebara M, Fukuda H, Hatano R, Saisho H, Nagato Y, Suzuki K, Nakajima K, Yukawa M, Kondo F, Nakayama A, Sakurai H. Relationship between copper, zinc and metallothionein in hepatocellular carcinoma and its surrounding liver parenchyma. J Hepatol. 2000;33:415–422. doi: 10.1016/s0168-8278(00)80277-9. [DOI] [PubMed] [Google Scholar]
- Eide DJ. Zinc transporters and the cellular trafficking of zinc. Biochim.Biophys.Acta. 2006;1763:711–722. doi: 10.1016/j.bbamcr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Feng P, Li T, Guan Z, Franklin RB, Costello LC. The Involvement of Bax in Zinc-Induced Mitochondrial Apoptogenesis in Malignant Prostate Cells. Mol Cancer. 2008;7:25. doi: 10.1186/1476-4598-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate. 2002;52:311–318. doi: 10.1002/pros.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Liang JY, Li TL, Guan ZX, Zou J, Franklin RB, Costello LC. Zinc induces mitochondria apoptogenesis in prostate cells. Mol Urol. 2000;4:31–35. [PubMed] [Google Scholar]
- Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch.Biochem Biophys. 2007;463:211–217. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RB, Milon B, Feng P, Costello LC. Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front Biosci. 2005;10:2230–2239. doi: 10.2741/1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RB, Zou J, Costello LC. Effect of zinc on proliferation of human prostate cancer cells. Urol Res. 1995;23:266. [Google Scholar]
- Fukamachi Y, Karasaki Y, Sugiura T, Itoh H, Abe T, Yamamura K, Higashi K. Zinc suppresses apoptosis of U937 cells induced by hydrogen peroxide through an increase of the Bcl-2/Bax ratio. Biochem Biophys.Res.Commun. 1998;246:364–369. doi: 10.1006/bbrc.1998.8621. [DOI] [PubMed] [Google Scholar]
- Gurusamy K. Trace element concentration in primary liver cancers--a systematic review. Biol Trace Elem Res. 2007;118:191–206. doi: 10.1007/s12011-007-0008-x. [DOI] [PubMed] [Google Scholar]
- Jiang D, Sullivan PG, Sensi SL, Steward O, Weiss JH. Zn(2+) induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J Biol Chem. 2001;276:47524–47529. doi: 10.1074/jbc.M108834200. [DOI] [PubMed] [Google Scholar]
- Kagara N, Tanaka N, Noguchi S, Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 2007;98:692–697. doi: 10.1111/j.1349-7006.2007.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper G, Weiser AA, Rump A, Sparbier K, Dahl E, Hartmann A, Wild P, Schwidetzky U, Castanos-Velez E, Lehmann K. Expression levels of the putative zinc transporter LIV-1 are associated with a better outcome of breast cancer patients. Int J Cancer. 2005;117:961–973. doi: 10.1002/ijc.21235. [DOI] [PubMed] [Google Scholar]
- Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q. Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. ProcNatl Acad Sci.U.S.A. 2007;104:18636–18641. doi: 10.1073/pnas.0709307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang JY, Liu YY, Zou J, Franklin RB, Costello LC, Feng P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999;40:200–207. doi: 10.1002/(sici)1097-0045(19990801)40:3<200::aid-pros8>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw KY, Lee PH, Wu FC, Tsai JS, Lin-Shiau SY. Zinc, copper, and superoxide dismutase in hepatocellular carcinoma. Am.J Gastroenterol. 1997;92:2260–2263. [PubMed] [Google Scholar]
- Lightman A, Brandes JM, Binur N, Drugan A, Zinder O. Use of the Serum Copper/Zinc Ratio in the Differential Diagnosis of Ovarian Malignancy. Clin Chem. 1986;32:101–103. [PubMed] [Google Scholar]
- Liu Y, Zhu X, Zhu J, Liao S, Tang Q, Liu K, Guan X, Zhang J, Feng Z. Identification of differential expression of genes in hepatocellular carcinoma by suppression subtractive hybridization combined cDNA microarray. Oncol.Rep. 2007;18:943–951. [PubMed] [Google Scholar]
- Magneson GR, Puvathingal JM, Ray WJ. The concentrations of free Mg2+ and free Zn2+ in equine blood plasma. J Biol Chem. 1987;262:11140–11148. [PubMed] [Google Scholar]
- Manning DL, McClelland RA, Knowlden JM, Bryant S, Gee JM, Green CD, Robertson JF, Blamey RW, Sutherland RL, Ormandy CJ. Differential expression of oestrogen regulated genes in breast cancer. Acta Oncol. 1995;34:641–646. doi: 10.3109/02841869509094041. [DOI] [PubMed] [Google Scholar]
- Manning DL, Robertson JF, Ellis IO, Elston CW, McClelland RA, Gee JM, Jones RJ, Green CD, Cannon P, Blamey RW. Oestrogen-regulated genes in breast cancer: association of pLIV1 with lymph node involvement. Eur J Cancer. 1994;30A:675–678. doi: 10.1016/0959-8049(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Margalioth EJ, Schenker JG, Chevion M. Copper and zinc levels in normal and malignant tissues. Cancer. 1983;52:868–872. doi: 10.1002/1097-0142(19830901)52:5<868::aid-cncr2820520521>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Ostrakhovitch EA, Cherian MG. Role of p53 and reactive oxygen species in apoptotic response to copper and zinc in epithelial breast cancer cells. Apoptosis. 2005;10:111–121. doi: 10.1007/s10495-005-6066-7. [DOI] [PubMed] [Google Scholar]
- Park SE, Park JW, Cho YS, Ryu JH, Paick JS, Chun YS. HIF-1alpha promotes survival of prostate cells at a high zinc environment. Prostate. 2007;67:1514–1523. doi: 10.1002/pros.20641. [DOI] [PubMed] [Google Scholar]
- Provinciali M, DiStefano G, Fabris N. Dose-dependent opposite effect of zinc on apoptosis in mouse thymocytes. International Journal of Immunopharmacology. 1995;17:735–744. doi: 10.1016/0192-0561(95)00063-8. [DOI] [PubMed] [Google Scholar]
- Reaves SK, Fanzo JC, Arima K, Wu JY, Wang YR, Lei KY. Expression of the p53 tumor suppressor gene is up-regulated by depletion of intracellular zinc in HepG2 cells. J Nutr. 2000;130:1688–1694. doi: 10.1093/jn/130.7.1688. [DOI] [PubMed] [Google Scholar]
- Rizk SL, Sky-Peck HH. Comparison between concentrations of trace elements in normal and neoplastic human breast tissue. Cancer Res. 1984;44:5390–5394. [PubMed] [Google Scholar]
- Santoliquido PM, Southwick HW, Olwin JH. Trace metal levels in cancer of the breast. Surg.Gynecol.Obstet. 1976;142:65–70. [PubMed] [Google Scholar]
- Tashiro H, Kawamoto T, Okubo T, Koide O. Variation in the distribution of trace elements in hepatoma. Biol.Trace Elem.Res. 2003;95:49–63. doi: 10.1385/BTER:95:1:49. [DOI] [PubMed] [Google Scholar]
- Tashiro-Itoh T, Ichida T, Matsuda Y, Satoh T, Sugiyama M, Tanaka Y, Ishikawa T, Itoh S, Nomoto M, Asakura H. Metallothionein expression and concentrations of copper and zinc are associated with tumor differentiation in hepatocellular carcinoma. Liver. 1997;17:300–306. doi: 10.1111/j.1600-0676.1997.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Taylor KM, Morgan HE, Johnson A, Hadley LJ, Nicholson RI. Structure-function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters. Biochem J. 2003;375:51–59. doi: 10.1042/BJ20030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KM, Morgan HE, Smart K, Zahari NM, Pumford S, Ellis IO, Robertson JF, Nicholson RI. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol Med. 2007;13:396–406. doi: 10.2119/2007-00040.Taylor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD. The role of zinc in caspase activation and apoptotic cell death. Biometals. 2001;14:315–330. doi: 10.1023/a:1012993017026. [DOI] [PubMed] [Google Scholar]
- Truong-Tran AQ, Ho LH, Chai F, Zalewski PD. Cellular zinc fluxes and the regulation of apoptosis/gene-directed cell death. J Nutr. 2000a;130:1459S–1466S. doi: 10.1093/jn/130.5.1459S. [DOI] [PubMed] [Google Scholar]
- Truong-Tran AQ, Ruffin RE, Zalewski PD. Visualization of labile zinc and its role in apoptosis of primary airway epithelial cells and cell lines. Am.J Physiol.Lung Cell Mol Physiol. 2000b;279:L1172–L1183. doi: 10.1152/ajplung.2000.279.6.L1172. [DOI] [PubMed] [Google Scholar]
- Xu J, Xu Y, Nguyen Q, Novikoff PM, Czaja MJ. Induction of hepatoma cell apoptosis by c-myc requires zinc and occurs in the absence of DNA fragmentation. Am.J Physiol. 1996;270:G60–G70. doi: 10.1152/ajpgi.1996.270.1.G60. [DOI] [PubMed] [Google Scholar]