The Fusobacterium nucleatum Outer Membrane Protein RadD Is an Arginine-Inhibitable Adhesin Required for Inter-Species Adherence and the Structured Architecture of Multi-Species Biofilm (original) (raw)
. Author manuscript; available in PMC: 2009 Sep 10.
Summary
A defining characteristic of the suspected periodontal pathogen Fusobacterium nucleatum is its ability to adhere to a plethora of oral bacteria. This distinguishing feature is suggested to play an important role in oral biofilm formation and pathogenesis, with fusobacteria proposed to serve as central “bridging organisms” in the architecture of the oral biofilm bringing together species which would not interact otherwise. Previous studies indicate that these bacterial interactions are mediated by galactose- or arginine-inhibitable adhesins although genetic evidence for the role and nature of these proposed adhesins remains elusive. To characterize these adhesins at the molecular level, the genetically transformable F. nucleatum strain ATCC 23726 was screened for adherence properties, and arginine inhibitable adhesion was evident, while galactose-inhibitable adhesion was not detected. Six potential arginine binding proteins were isolated from the membrane fraction of F. nucleatum ATCC 23726 and identified via mass spectroscopy as members of the outer membrane family of proteins in F. nucleatum. Inactivation of the genes encoding these six candidates for arginine-inhibitable adhesion and two additional homologues revealed that only a mutant derivative carrying an insertion in Fn1526 (now designated as radD) demonstrated significantly decreased co-aggregation with representatives of the Gram-positive “early oral colonizers”. Lack of the 350 kDa outer membrane protein encoded by radD resulted in the failure to form the extensive structured biofilm observed with the parent strain when grown in the presence of Streptococcus sanguinis ATCC 10556. These findings indicate that radD is responsible for arginine-inhibitable adherence of F. nucleatum and provides definitive molecular evidence that F. nucleatum adhesins play a vital role in inter-species adherence and multispecies biofilm formation.
Keywords: Fusobacterium nucleatum, RadD. Arginine, Adhesin, Biofilm, Co-aggregation
Introduction
The oral cavity contains one of the most diverse communities in our body with an estimated 700 or more different bacterial species. Most of these bacteria are considered commensals, but a number of species are suspected to be opportunistic pathogens responsible for causing two of the most chronic and epidemic diseases that affect humans: dental caries and periodontal disease. Both commensals and suspected pathogens reside in oral biofilms, the highly organized mixed-species communities found in the mouth. They associate in a complex yet organized manner making the oral biofilm an excellent model system for the exploration of interspecies interactions and their role in biofilm architecture.
Oral biofilm bacteria have been grouped into early, intermediate and late colonizers (Palmer et al., 2003, Kolenbrander & London, 1993). The early colonizing bacteria are primarily represented by Gram-positive species which have the ability to adhere directly to the salivary pellicle and the tooth surface. Examples of early colonizers include streptococci and actinomyces, which possess surface components such as fimbriae and receptor polysaccharides that mediate inter- and intrageneric co-aggregation and allow for attachment to salivary proteins and the tooth surface (Palmer et al., 2003, Clark et al., 1989, Ruhl et al., 2004). Late colonizing bacteria are represented by a variety of Gram-negative bacteria including the periodontal pathogens Aggregatibacter actinomycetemcomitans, Treponema denticola and Porphyromonas gingivalis.
A key role as a “bridging” organism between early and late colonizers is attributed to the suspected periodontal pathogen Fusobacterium nucleatum because it is frequently found associated with both groups and coaggregates with many species present in the oral biofilm (Kolenbrander et al., 1989). Two distinct types of adherence have been found in fusobacteria, and these interactions are classified based on their inhibition by either D-galactose or L-arginine. The adherence to the predominantly Gram-negative late colonizing bacteria is associated with galactose-inhibitable interactions, while adherence to mainly Gram-positive early-colonizing species is associated with arginine-inhibitable interactions (Kolenbrander et al., 1989). By bridging the gap between two separate groups of oral bacteria the adhesin genes of F. nucleatum act outside the commonly observed narrow range of individual interspecies interactions, and join many bacteria together in one community (Kolenbrander & London, 1993). These adherence properties are not uniform among fusobacterial strains (Xie et al., 1991), leading to the possibility of strain-dependent differential influence on biofilm development. Thus, the ability of F. nucleatum to adhere to members of both the early and late-colonizing groups of bacteria results in this species acting as biofilm “mortar” and guiding biofilm architecture by bringing these two groups of bacteria together.
Interest in identifying the fusobacterial proteins involved in adherence (Han et al., 2005) has led to the biochemical isolation and characterization of adhesins from the membranes of F. nucleatum using affinity chromatography by three independent research groups (Takemoto et al., 1993, Murray et al., 1988, Edwards et al., 2007). Both the galactose- and arginine-inhibitable adhesins were found to be large proteins with estimated molecular mass of 300-330 kDa and ∼370 kDa respectively. The arginine-inhibitable adhesin was further confirmed by the recent finding that a spontaneous mutant strain of F. nucleatum ATCC 10953, deficient in its interaction with representative early colonizers, was lacking an outer membrane protein (OMP) of approximately 360 kDa (Edwards et al., 2007). Despite this progress, identification of these adhesins at a molecular level is lacking. Targeted approaches to studying these adhesins in co-aggregation and biofilm formation were hampered by the inability to create gene inactivation mutants in F. nucleatum, but recent advances in genetic systems for F. nucleatum have made gene inactivation and molecular analyses possible (Kaplan et al., 2005, Han et al., 2007). We used these approaches to create targeted gene inactivation mutants of candidate adhesin genes in the transformable F. nucleatum strain ATCC 23726. We report here that the arginine-inhibitable adherence in co-aggregation and biofilm formation is specifically mediated by radD, a gene encoding a 350 kDa putative autotransporter protein belonging to the F. nucleatum OMP family.
Results
Phenotypic characterization of F. nucleatum ATCC 23726 adherence
The current taxonomic designations of Fusobacterium nucleatum strains/subspecies do not correlate with their adherent properties (Xie et al., 1991). The adhesion properties of the genetically transformable F. nucleatum ssp. nucleatum strain ATCC 23726 were unknown and therefore examined in this study. Examination of the co-aggregation profile with a selection of oral bacterial species, previously characterized for interactions with F. nucleatum species (Kolenbrander et al., 1989), revealed strong interactions of ATCC 23726 with Gram-positive representatives of the early colonizers, while adhesion to the two Gram-negative oral species (T. denticola and V. atypica) was not apparent (Table 1, column “wt_”). In agreement with previously reported co-aggregation patterns, the observed interactions with Gram-positive early colonizers were sensitive to 50 mM arginine but not 50 mM galactose (Table 1, column “_wt in presence of inhibitor”). The visual assessment of co-aggregation was then confirmed via quantitative spectrophotometric measurement of co-aggregation. Strain ATCC 23726 did not display significant interactions with the Gram-negative species tested. Little difference was observed between co-aggregation of wild-type cells and those treated with 50 mM galactose (Fig. 1, 50 mM galactose) while 50 mM arginine inhibited co-aggregation reactions by 65-74% (Fig. 1, 50 mM arginine). These results indicate that ATCC 23726 contains an arginine-inhibitable adhesin for mediating interactions with Gram-positive early colonizers.
Table 1.
Effect of OMP mutations and inhibitors on F. nucleatum coaggregation with bacterial strainsa
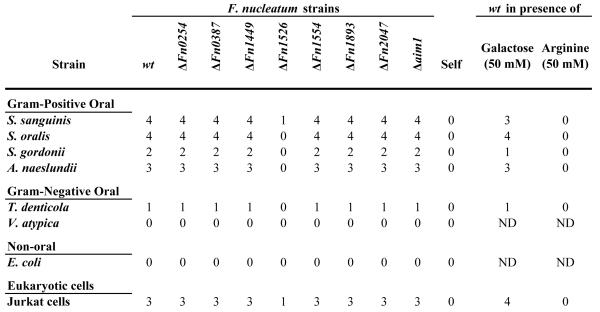
Fig. 1.
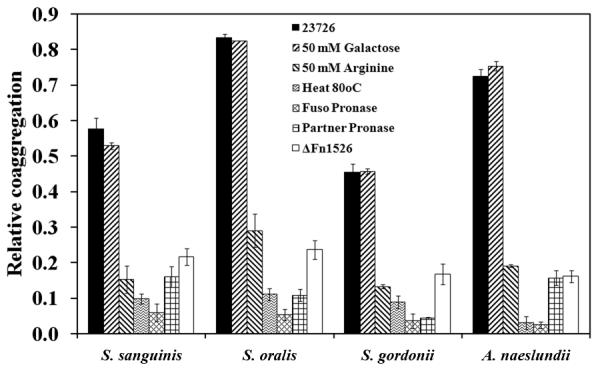
Spectrophotometric co-aggregation assay of F. nucleatum ATCC 23726 and Gram-positive partner strains. Wild-type F. nucleatum ATCC 23726 co-aggregated with each of the Gram-positive partner strains tested. Treatment with heat (80°C, 15 min.) and pronase (10 mg ml-1, 2 h) resulted in approximately 90% loss of co-aggregation in both partner strains. Addition of arginine inhibited co-aggregation with each strain, while galactose had no effect on co-aggregation. Disruption of the gene encoding Fn1526 resulted in a loss of co-aggregation comparable to the effect seen with the addition of arginine. Relative co-aggregation was determined by dividing the difference between the total turbidity of each partner strain and the co-aggregation supernatant turbidity by the total turbidity of each partner strain. The data presented are based on experiments conducted in triplicate. All co-aggregation values except for galactose inhibition (P = 0.01) were significantly different from wild-type (P >0.0001).
Heat and pronase treatments disrupt F. nucleatum co-aggregation with partner strains
Previous reports indicate that the arginine—inhibitable adhesin is mediated by a cell surface protein (Edwards et al., 2007, Takemoto et al., 1993). To confirm this observation for the F. nucleatum strain ATCC 23726 used in this study, cells were incubated at 80°C for 15 min or subjected to 10 mg ml-1 pronase treatment for 2 h at 37°C prior to the co-aggregation experiments. Heat-treated wild-type F. nucleatum exhibited an 80-95% decrease in co-aggregation with each of the Gram-positive partner strains tested (Fig. 1, Heat 80°C). Treatment of wild-type F. nucleatum with pronase resulted in a comparable decrease in co-aggregation, ranging from 88 to 96% (Fig. 1, Fuso Pronase). These findings indicated that the arginine adhesin was a heat-labile, pronase-sensitive cell surface protein or a non-proteinaceous structure associating with a cell surface protein. To determine the nature of the interacting component on the surface of the strains co-aggregating with F. nucleatum, the Gram-positive partner species were pronase treated and tested for adherence to untreated ATCC 23726. The observed decrease in co-aggregation (Fig. 1, Partner Pronase) was very similar to the reduction in interaction observed upon heat or Pronase treatment of F. nucleatum (Fig. 1, Heat 80°C or Fuso Pronase) indicating that both species in the co-aggregation reaction contain proteins on their surface that are involved in the interaction.
Isolation and identification of an arginine-inhibitable adhesin from F. nucleatum ATCC 23726 membranes
To identify this adhesin in F. nucleatum ATCC 23726 we isolated arginine-binding proteins from membranes using arginine agarose beads. Separation of the bound protein via SDS-PAGE revealed six high-molecular-weight bands with apparent molecular weights of approximately 210, 230, 243, 260, 302 and 350 kDa (Fig. 2, L-Arg purified, wt). Mass spectroscopy analysis of the individual protein bands revealed that all contained protein fragments corresponding to members of the “Fusobacterium OMP family” in F. nucleatum, specifically the ones encoded by Fn0254, Fn1526, Fn1554, Fn1893, Fn2047 and the previously characterized Fn2058 (aim1). Band A1 only contained peptides from a single protein, Fn1526 of the published genome of F. nucleatum ssp. nucleatum strain ATCC 25586 (Table 2). The other five bands (A2—A6) contained peptides from one or more proteins, but all contained peptides from the protein Fn1893 (Fig. 2). In addition to containing peptides from Fn1893: band A2 contained peptides from Fn1554; band A3 contained peptides from Fn2047 and Fn2058, and band A4 contained peptides from Fn0254. Bands A5 and A6 only contained peptides specific to Fn1893 (Fig. 2). Genome analysis showed that this group of proteins belongs to the type Va class of secretory proteins, or autotransporter proteins. Analysis of the gene sequences indicated that each protein has a C-terminal autotransporter domain that is characteristic of this class of proteins.
Fig. 2.
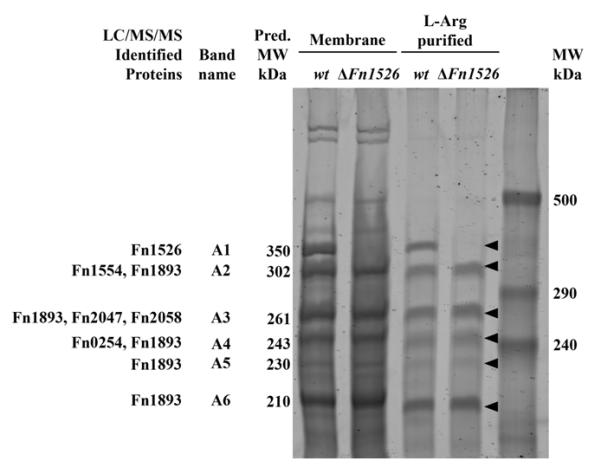
Purified membrane proteins and arginine binding proteins from F. nucleatum ATCC 23726 wild-type and Δ_Fn1526_ strains analyzed by 4% SDS-PAGE. Six arginine binding proteins were isolated from the wild-type strain and identified by LC/MS/MS. Gene inactivation of Fn1526 resulted in the absence of a 350 kDa protein in the membrane preparations from the Fn1526 mutant. The ‘Fn’ designation indicates the ORFs annotated in the F. nucleatum ATCC 25586 genome. Arrows indicate the six bands (A1-A6) that were analyzed by LC/MS/MS.
Table 2.
Sequence of tryptic peptide fragments from arginine binding protein band A1
| Tryptic peptide fragment sequence | Amino acid |
|---|---|
| KTEIKTRI | 49-56 |
| KEQENISQMLKE | 58-69 |
| KEADESMKDIELKI | 68-81 |
| KNPLEFVDKI | 152-161 |
| KAIGILQDVEGAGTAKT | 607-623 |
| KIALATEGSTINLKN | 696-709 |
| KSGEGTVNITNEKD | 1127-1140 |
| KISAENIALALQGTGANKI | 1794-1812 |
| KILNSGTLNLTKT | 1811-1823 |
| KTVNTDIQLGGEKT | 1911-1924 |
| KDFNISLGTNAKG | 2203-2215 |
| KLTFDLASGKL | 2277-2287 |
| KVGANGIALGTKG | 2317-2328 |
| KTLTVADSAYGIFSKG | 2609-2624 |
| RSISGVAEVLPNFSKG | 3020-3035 |
| RIPYNSLISGERY | 3087-3099 |
| RGDIYSNVQERM | 3142-3153 |
| KSYNELLSSYNKT | 3162-3174 |
| KSTGVLYLNDRE | 3202-3213 |
| KNEGLPLEVKG | 3342-3352 |
Creation and verification of gene inactivation mutants for F. nucleatum large membrane proteins
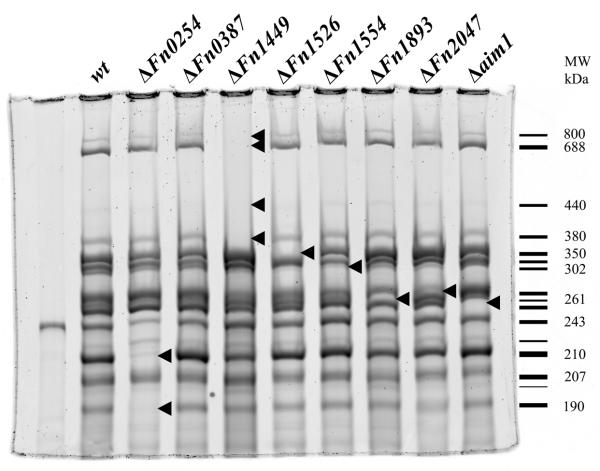
The results presented above indicated that F. nucleatum strain ATCC 23726 possessed at least six different candidate OMPs for the arginine-inhibitable adhesin. To determine the identity of the OMPs responsible for the arginine-inhibitable co-aggregation of fusobacteria with their Gram-positive early-colonizing partner strains, we individually inactivated the respective genes encoding ORFs of all candidate proteins. Because complete genome information was not available for the transformable strain ATCC 23726, we examined the genome of F. nucleatum ATCC 25586, a strain of the same subspecies nucleatum, for homologues to the arginine-binding proteins identified above. The F. nucleatum ATCC 25586 genome contains eight large OMPs in this family of proteins: Fn0254, Fn0387, Fn1449, Fn1526, Fn1554, Fn1893, Fn2047, and the previously characterized Fn2058 (aim1) (Table 1). All eight ORFs appear to be autotransporter proteins predicted to be secreted through type Va secretion systems. Based on this bioinformatic analysis and the results of the arginine binding protein isolation, we pursued a combined genetic and biochemical approach to identify the possible arginine-inhibitable adhesin(s). This approach involved (i) genetic insertional inactivation of each of the eight genes encoding autotransporter homologs, (ii) characterization of the membrane proteins of the mutants, and (iii) phenotypic screening for mutants defective in arginine-inhibitable co-aggregation with Gram-positive early colonizers and in agglutination with lymphocytes.
The ORF encoding Aim1 was previously inactivated and shown to play a role in the induction of apoptosis in lymphocytes (Kaplan et al., 2005). In addition to the Aim1 autotransporter, the ORFs for the remaining seven members of this family of OMPs were inactivated using previously established approaches (Kaplan et al., 2005, Haake et al., 2000). Plasmids for gene inactivation via single cross-over were created as described in Experimental procedures and the resulting mutant strains were confirmed by PCR analysis using primers flanking the insertion site and by Southern blotting (Fig. 3). The autotransporter domain of the autotransporter proteins is located at the C-terminal end of the protein, so a disruption in the N-terminal portion of these proteins should prevent their expression at the cell surface. Membrane fractions from each mutant strain were isolated and compared to the profile of the wild-type parent ATCC 23726 to determine the absence of any membrane proteins (Fig. 4). Gene inactivation of five ORFs (Fn1526, Fn1554, Fn1893, Fn2047, aim1) resulted in the absence of a single membrane protein each. The observed sizes of OMPs missing in the respective mutant strains correlated approximately with the predicted translation product of their encoding genes. Interestingly, inactivation of ORFs Fn0254 and Fn1449 resulted in two and four bands missing, respectively, indicating the modification and/or formation of multimers in the wild-type strain. Disruption of ORF Fn0387 did not result in the absence of a clearly discernable band compared to the wild-type profile, suggesting that the corresponding protein is not expressed at detectable levels under the conditions tested (Fig. 4). Fn1893 was identified in arginine-purified protein bands (Fig. 2) A6 (210 kDa), A5 (230 kDa), A4 (243 kDa), A3 (261 kDa) and A2 (302 kDa), while the Fn1893 mutant only lacks the 261 kDa band (Fig. 4). The processing required to purify the arginine-binding proteins may have resulted in cleavage of Fn1893 that contaminated several bands.
Fig. 3.
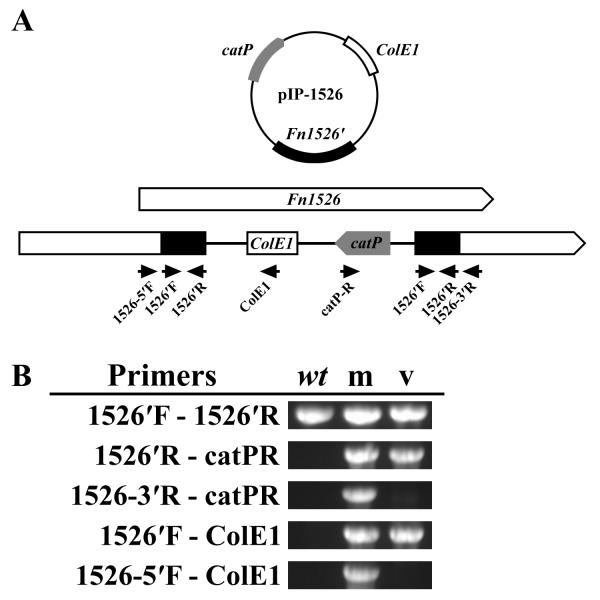
Analysis of the Δ_Fn1526_ gene inactivation mutant.
A. Organization of Fn1526 with insertion of the inactivation plasmid pIP-1526. Black arrows indicate the location of primers used for construction of the mutant and the analysis. Gene Fn1526 was shown as an example of the methodology for analyzing each insertion.
B. Confirmation of plasmid insertion into Fn1526 by PCR analysis of the Δ_Fn1526_ mutant (m) with wild-type (wt) and pIP-1526 vector (v) as controls. Fragments were amplified from the mutant strain, but not the controls, when using primer pairs where one was outside the insertion site (Fn1526-5′F/ColE1 and Fn1526-3′R/catPR).
Fig. 4.
Purified membrane proteins from eight OMP mutant strains analyzed by 4% SDS-PAGE. Arrows indicate protein bands missing in each gene inactivation mutant. The Fn1526 and four Fn1449 bands contained peptides for their respective proteins when analyzed by LC/MS/MS. The schematic to right represents each observed band in the wild-type strain with the relative molecular mass of the bands missing in the mutants indicated.
Co-aggregation of F. nucleatum mutant derivatives lacking individual OMPs
The initial assessment of the co-aggregation properties of the F. nucleatum OMP mutants with other oral bacteria was performed using a visual co-aggregation assay. Each wild-type interacting partner was evaluated for its ability to co-aggregate with each F. nucleatum OMP mutant. The F. nucleatum mutant strain lacking Fn1526 was clearly defective in co-aggregation with the Gram-positive early colonizers as compared to the parent strain (Table 1). Co-aggregation of the Fn1526 mutant was similar to co-aggregation inhibited by arginine. In contrast, none of the other OMP mutant derivatives displayed a detectable decrease in co-aggregation with any of the co-aggregation partners when compared to the wild-type strain.
The Fn1526 mutant was examined in a spectrophotometric assay for a quantitative assessment of its co-aggregation-deficient phenotype. The values obtained confirmed the results of the visual co-aggregation assay indicating low levels of co-aggregation with Gram-positive early colonizers for the Fn1526 mutant compared to the wild-type strain (Fig. 1, Fn1526). The extent of co-aggregation defect with the representatives of the Gram-positive early colonizing species in this mutant derivative was similar to the effect of arginine in blocking co-aggregation of the wild-type parent F. nucleatum ATCC 23726 (p = 0.01). To rule out the possibility that Fn1526 possess arginine protease activity cleavage experiments were performed and showed no reaction when F. nucleatum was incubated with BApNA under conditions when co-aggregation occurs (data not shown). Finally, we purified the arginine binding proteins from the Fn1526 mutant and demonstrated that the band containing Fn1526 (Fig. 2, band A1) was not purified from this mutant. Taken together, these data indicate that the Fn1526 gene product is the arginine-inhibitable adhesin present in F. nucleatum ATCC 23726.
Agglutination of F. nucleatum with lymphocytes
Previous studies demonstrated that the agglutination of fusobacteria with human lymphocytes is also sensitive to the addition of arginine (Tuttle et al., 1992). To examine if this interaction is mediated by the same protein required for interaction with bacterial, co-aggregation partners, we first determined if F. nucleatum ATCC 23726 was able to induce agglutination of Jurkat cells. The wild-type strain induced agglutination with Jurkat cells and the addition of 50 mM arginine, but not 50 mM galactose, inhibited this reaction (Table 1, Jurkat cells). Next, all the autotransporter mutant strains were examined for agglutination with Jurkat cells. The Fn1526 mutant failed to agglutinate when mixed with Jurkat cells, while the remaining mutants exhibited agglutination similar to the wild-type strain (Table 1, Jurkat cells). These results demonstrate that agglutination with Jurkat cells is mediated in an arginine-inhibitable manner by the same OMP encoded by Fn1526 that plays a role in F. nucleatum co-aggregation.
Lack of the Fn1526 gene product impairs F. nucleatum biofilm formation with S. sanguinis
The adherence of the Gram-positive early colonizer S. sanguinis to fusobacteria is hypothesized to be relevant for oral biofilm formation (Kolenbrander et al., 1989, Kolenbrander & London, 1993). Previous studies demonstrate that these two organisms when mixed together form the characteristic in vivo biofilm features called “corncobs” (Lancy et al., 1983). However, confirmation of the relevance of the arginine-inhibitable co-aggregation between these two species in biofilm formation is still lacking. The identification and inactivation of Fn1526 as the gene encoding the OMP required in F. nucleatum for the arginine-inhibitable co-aggregation with S. sanguinis now put us into the unique position to evaluate this interaction in biofilm formation.
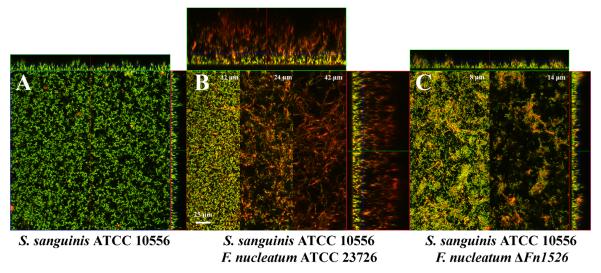
We examined the Fn1526 mutant in comparison to the parental wild-type strain for the ability to form biofilms with the Gram-positive early colonizer S. sanguinis ATCC 10556. In monospecies biofilm experiments S. sanguinis formed a homogeneous approximately 20 μm thick biofilm (Fig. 5 A). In contrast, the biofilm generated during co-culture of F. nucleatum with S. sanguinis was 80 μm thick and could be separated into three different layers based on the decrease in number of streptococci and increase in number of fusiform cells (Fig. 5 B). The lower layer included the first 20 μm and was comprised of a mat of intermingled streptococci and fusiform bacteria to which the streptococci were attached. Visual morphology of the S. sanguinis in this layer was similar to that grown in the monoculture biofilm (Fig. 5B, 12 μm). The intermediate layer (located at about 20-40 μm from the bottom of the well) consisted mainly of F. nucleatum that formed a loose interwoven network of very elongated (10-50 μm in length) fusiform cells (Fig. 5B, 24 μm). This striking cell morphology is in stark contrast to planktonic cells of this strain which are fusiform and only about 5 μm in length. S. sanguinis cells were found attached to F. nucleatum cells in this layer. The top layer of the biofilm (starting at about 40 μm from the bottom of the well) contained mostly elongated fusiform bacteria with few attached streptococci and extended 80 μm above the base of the biofilm (Fig. 5B, 42 μm).
Fig. 5.
Typical biofilm growth after 48 h visualized by CSLM. Biofilms were stained with HEX-labeled F. nucleatum specific probes (red) and counterstained with STYO9 (green).
A. Monoculture of S. sanguinis ATCC 10556.
B. Co-culture of F. nucleatum ATCC 23726 and S. sanguinis ATCC 10556 produced an 80-μm-thick biofilm. Sections from 12, 24 and 42 μm above the growth surface demonstrate the variation in the biofilm morphology as the height of the biofilm increases.
C. Co-culture of F. nucleatum Δ_Fn1526_ with S. sanguinis ATCC 10556. Sections from 8, and 14 μm above the growth surface demonstrate the variation in the biofilm morphology as the height of the biofilm increases.
The biofilm formed by co-culture of S. sanguinis with the Fn1526 mutant derivative of F. nucleatum greatly differed in appearance from the one observed for the combination with the wild-type strain. The biofilm grew only 30 μm tall and contained significantly fewer F. nucleatum cells when visually compared the biofilm formed with wild-type F. nucleatum. The morphology of the predominantly S. sanguinis containing lower layer of the biofilm was similar to the one developed in monoculture or in the presence of wild-type F. nucleatum cells (Fig. 5 C, 8 μm). In contrast to the loose network of fusiform cells built by wild-type F. nucleatum grown in co-culture with S. sanguinis, the Fn1526 mutant cells only formed small aggregates on top of the S. sanguinis biofilm in repeated experiments (Fig. 5 C, 14 μm). These results suggest that the arginine-inhibitable adhesin, encoded by Fn1526, is necessary for F. nucleatum to form elaborate biofilms with S. sanguinis in vitro.
The gene Fn1526 is part of a four gene operon
The Fn1526 homolog present in the completed genome of the F. nucleatum strain ATCC 25586, the same ssp. nucleatum as the transformable strain ATCC 23726, is annotated as the last gene of a putative four gene operon that includes upstream genes Fn1527, Fn1528 and Fn1529. To determine if these genes are similarly organized and if they are part of the same transcriptional unit, genomic DNA (gDNA) and RNA were isolated from wild-type cells of ATCC 23726, and the RNA was reverse transcribed to generate cDNA. A detailed PCR analysis was performed using primer pairs that would amplify a section from the 5′ end of Fn1526 to the 3′ end of Fn1529 (Fig. 6). Amplification of the expected 1.2 kb band from gDNA confirmed that ORFs are similarly organized in ATCC 23726, and from cDNA confirmed that Fn1527, Fn1528 and Fn1529 are transcribed together from the same promoter upstream of Fn1529. No corresponding band was evident in the control RNA template that was not subjected to reverse transcription, demonstrating the lack of genomic DNA contamination. Amplification from the 3′ end of Fn1526 to the 5′ end of Fn1525 produced a product from genomic DNA, but not from cDNA confirming that Fn1526 is the last gene transcribed in the operon (Fig. 6). The relationship between the proteins encoded by this operon is unknown, but the presence of a homologous operon in the genomes of F. nucleatum vincentii and F. nucleatum polymorphum, as revealed by genomic analyses, indicates that it is conserved among F. nucleatum subspecies. We have designated FN1526 as _rad_D based on the role of the Fn1526 gene as an arginine (R)-inhibitable adhesin, and it location at the end of a four gene operon (ORF “D”).
Fig. 6.
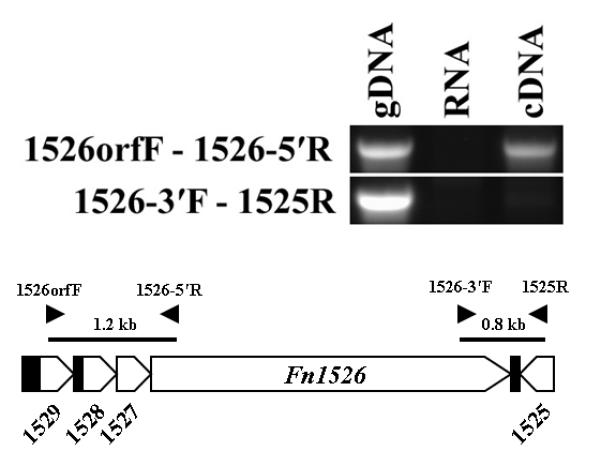
Analysis of the Fn1526 operon in F. nucleatum ATCC 23726. The operon in F. nucleatum ATCC 25586 is organized with three genes upstream of Fn1526. Amplification of fragments from ATCC 23726 genomic DNA and cDNA using primers in Fn1529 (Fn1526orfF) and Fn1526 (Fn1526-5′R) indicate that the organization of the genes in ATCC 23726 is the same at that of ATCC 25586, and that Fn1526 to Fn1529 are transcribed as a single unit. No fragments were amplified using primers spanning gene Fn1525 (1525R) and Fn1526 (Fn1526-3′F). RNA was used as a negative control for genomic DNA contamination. Black arrowheads indicate the position of primers used in this analysis.
Discussion
Several groups have isolated adhesin proteins from F. nucleatum strains (Takemoto et al., 1993, Edwards et al., 2007, Murray et al., 1988, Han et al., 2005) and demonstrated their involvement in adherence to oral bacteria and salivary proteins. In a recent paper Edwards et al. revealed that a F. nucleatum strain of the subspecies polymorphum lacking a large OMP due to a random mutation was deficient in arginine inhibitable co-aggregation to streptococcal strains and secretory IgA. In this study, we present the genetic basis for the arginine-inhibitable adhesin, RadD, encoded in the genome of F. nucleatum ssp. nucleatum ATCC 23726. Delineation of the radD gene and its insertional inactivation, described here, enabled studies providing direct evidence for the role of this protein in interspecies interactions and biofilm architecture.
Similar to previous reports of adhesin protein purification in F. nucleatum ssp. polymorphum, RadD was identified by taking advantage of the ability of adhesins to bind the small molecules that are associated with inhibiting their adherence to other bacteria and cells (Takemoto et al., 1993, Edwards et al., 2007). We used F. nucleatum strain ATCC 23726 because it is amenable to the generation of specific gene inactivation mutants (Kinder Haake et al., 2006, Haake et al., 2000, Kaplan et al., 2005). Since we did not identify any galactose-inhibitable interactions in strain ATCC 23726 we focused on the arginine-inhibitable adherence mediating co-aggregation with Gram-positive bacterial species (Fig. 1, Table 1). In contrast to previous reports isolating a single adhesin protein from the F. nucleatum ssp. polymorphum based on its ability to bind to arginine agarose beads (Takemoto et al., 1995, Edwards et al., 2007), six surface proteins were recovered in the eluate from F. nucleatum ATCC 23726. Interestingly, all six proteins could be assigned to the same family of type Va class of secretory proteins or autotransporters.
Gene inactivation of the six candidate adhesin OMPs and two additional OMPs from the same family of proteins was undertaken, and subsequent analyses demonstrated that only a mutation in radD resulted in any decrease in co-aggregation (Fig. 1, Table 2). RadD, with an estimated molecular weight of 350 kDa, was the largest protein isolated in the eluate and has a similar mass to arginine-binding proteins previously isolated from F. nucleatum ssp. polymorphum strain (Edwards et al., 2007, Takemoto et al., 1993). Consistent with the identification of RadD as the arginine-inhibitable adhesin of F. nucleatum, the radD mutant exhibited decreased co-aggregation with streptococci and A. naeslundii (Fig. 1, Table 1). This absence of co-aggregation by the radD mutant suggests that RadD is the only adhesin required for F. nucleatum ATCC 23726 interactions with Gram-positive strains.
The nature of the RadD cognate receptor on the surface of the Gram-positive interacting partner species was investigated by pronase treatment of the Gram-positive interacting strains, and the observed inhibition of co-aggregation revealed that the RadD receptor on the Gram-positive cell is also likely a protein (Fig. 1). Furthermore, the Gram-positive strains apparently do not possess adhesins interacting with a molecule present on F. nucleatum other than RadD since no co-aggregation was observed with the radD mutant (Table 1, Fig. 1). The specificity of these interactions indicates that F. nucleatum interacts through discrete adhesins and the adherence properties conferred are likely to constitute an important strategy for survival in the oral cavity and the complex biofilm environment. Other bacterial adhesins are known to play a significant role in oral biofilm formation. The Gram-positive early colonizer A. naeslundii possesses type 2 fimbriae that attach to receptor polysaccharides on the surface of S. sanguinis (Yoshida et al., 2006). Disruption of either the fimbriae or the polysaccharide receptor results in disruption of biofilm formation (Yoshida et al., 2006). Oral streptococci and A. naeslundii possess fimbriae that serve to attach to proline-rich proteins and salivary proteins that coat the tooth surface. Strains that lacked these genes failed to bind to the specified proteins (Ruhl et al., 2004). The insertional inactivation of genes in F. nucleatum has only recently been achieved (Haake et al., 2000; Kaplan et al., 2005), which now enables addressing significant questions concerning the role of F. nucleatum adhesins in biofilm formation to be addressed.
Co-aggregation studies identified bacteria that F. nucleatum strains have the potential to associate with in the oral cavity, but it is critical to test the relevance of these interactions to biofilm formation. We used a F. nucleatum-S. sanguinis multispecies biofilm model in which both species were inoculated simultaneously and the resulting complex biofilm was the result of spatial self-organization by the two species. We were able to grow a structured biofilm 80 μm thick (Fig. 5 B) where the long filamentous F. nucleatum cells formed an interwoven aggregate of cells on top of the cocci that covered the bottom of the chamber and adhered to F. nucleatum. Biofilm growth with the radD mutant and S. sanguinis resulted in limited F. nucleatum incorporation and diminished biofilm development, indicating a central role for RadD. This finding substantiates previous research on co-aggregation patterns of F. nucleatum with other bacteria (Kolenbrander et al., 1989), demonstrating the relevance of the arginine-inhibitable adherence in biofilm formation as predicted by the earlier studies.
F. nucleatum ATCC 23726 has eight autotransporter OMPs with molecular weights greater than 200 kDa. The RadD autotransporter characterized in this study was demonstrated to have a central role in fusobacterial adherence, Fn2058, or _aim_1 was previously identified as an apoptosis inducing protein (Kaplan et al., 2005), whereas the function of the six remaining OMPs is currently unknown. Phylogenetic analysis of the predicted protein sequences of these proteins indicated that they are similar and have conserved N-terminal domains that may serve as a signal sequences and C-terminal autotransporter domains. The N-terminal and C-terminal domains also appear to be conserved in OMPs present in F. nucleatum ATCC 10953 and ATCC 49256 suggesting that the secretion mechanism may be conserved in the sequenced F. nucleatum strains (data not shown). A large region in the N-terminal portion of these OMPs displays a great amount of variability similar to findings in other species (Henderson et al., 2004), yet all are predicted to contain a high proportion of β-strands (data not shown). This indicates that the F. nucleatum OMP passenger domains are likely β-helical in structure. β-helices are large filamentous structures that possess loops connecting β-strands that make up the central core of the molecules. The β-helix structure of autotransporter passenger domains is a common motif that is hypothesized to play a role in their ATP independent secretion to the cell surface (Henderson et al., 2004, Junker et al., 2006). The autotransporter passenger domains in other bacteria have multiple activities that function in adherence and biofilm formation (Sven Laarmann, 2002, Charbonneau & Mourez, 2007, Korotkova et al., 2006). The wide array of adherence properties found in F. nucleatum strains, represented by galactose-inhibitable, arginine-inhibitable, lysine-inhibitable adherence as well as adherence to fibronectin, laminin, and collagen binding may be mediated by different motifs found in the passenger domains or other through protein and non-protein molecules associated with the OMPs.
It is known that F. nucleatum strains vary in the adherence properties that they possess. This variability suggests that individual strains may colonize distinct ecological niches and may exert significant influence on the subsequent biofilm development within a specific ecological niche. In particular, since galactose-inhibitable adhesins are associated with adherence to Gram-negative oral bacteria that include key periodontal pathogens, the presence of this adhesin on a F. nucleatum strains may correlate to the potential for periodontal disease in patients. Further analysis of the significance of RadD and the other adhesins in multispecies biofilm formation is necessary. The results presented here strongly support a role for the RadD adhesin as an architectural protein in the formation of the F. nucleatum — S. sanguinis biofilm, and provide an important first step in the process of understanding oral biofilm formation on a molecular level.
Experimental procedures
Bacteria and culture conditions
Fusobacterium nucleatum ATCC 23726 and mutant derivatives were cultivated on Columbia agar or in Columbia broth (Difco, Detroit, MI) under anaerobic conditions (5% H2, 5% CO2, 90% N2) at 37°C. Thiamphenicol (MP Biomedicals, Irvine, CA) at 5 μg ml-1 was used for selection and maintenance of strains possessing the _cat_P determinant. Bacterial strains used in co-aggregation assays were grown anaerobically in BHI (Difco, Detroit, MI) at 37°C: Streptococcus sanguinis ATCC 10556, Streptococcus oralis ATCC 10557, Streptococcus gordonii ATCC 10558, Actinomyces naeslundii ATCC 12104, and Veillonella atypica PK1910 (in BHI supplemented with 1% lactic acid) (Hughes et al., 1988). Treponema denticola ATCC 35405 was grown in TYGVS anaerobically at 37°C as previously described (Ohta et al., 1986). Escherichia coli JM101 was grown aerobically at 37°C in Luria-Bertani broth. Jurkat cell line ATCC TIB-152 clone E6-1 was maintained in RPMI 1640 supplemented with 1 mM sodium pyruvate, 0.1 mM non-essential amino acids, 10 U ml-1 penicillin, 10 mg ml-1 streptomycin (Life Technologies, Grand Island, NY), and 10% fetal calf serum (Irvine Scientific, Santa Ana, CA) (Table 3).
Table 3.
Bacterial strains, Plasmids and Primers used in this study
| Bacterial strains | Relevant characteristics/sequence | Source |
|---|---|---|
| F. nucleatum | ||
| ATCC 23726 | ssp. nucleatum | ATCC |
| Δ_Fn0254_ | ATCC 23726 Fn0254 ::pIP_0254_ | This study |
| Δ_Fn0387_ | ATCC 23726 Fn0387 ::pIP_0387_ | This study |
| Δ_Fn1449_ | ATCC 23726 Fn1449::pIP_1449_ | This study |
| Δ_Fn1526_ | ATCC 23726 Fn1526 ::pIP1526 | This study |
| Δ_Fn1554_ | ATCC 23726 Fn1554 ::pIP_1554_ | This study |
| Δ_Fn1893_ | ATCC 23726 Fn1893 ::pIP_1983_ | This study |
| Δ_Fn2047_ | ATCC 23726 Fn2047 ::pIP_2047_ | This study |
| aim1 | ATCC 23726 aim1 ::pIP_aim1_ | This study |
| E. coli | ||
| DH5a | Hanahan, D. (1983) | |
| JM109 | New England Biolabs | |
| Other | ||
| Streptococcus sanguinis ATCC 10556 | ATCC | |
| Streptococcus oralis ATCC 10557 | ATCC | |
| Streptococcus gordonii ATCC 10558 | ATCC | |
| Actinomyces naeslundii ATCC 12104 | ATCC | |
| Actinomyces viscosus ATCC 15987 | ATCC | |
| Veillonella atypica PK1910 | Hughes, C. V. (1988) | |
| Treponema denticola ATCC 35405 | ATCC |
| Plasmid | Description | Purpose | Source |
|---|---|---|---|
| pHS31 | F. nucleatum suicide vector; CmR | Suicide plasmid | Kaplan, C. W., (2005) |
| pIP0254 | pHS31 with Fn0254′ inserted into SnaBI site | Gene inactivation plasmid | This study |
| pIP0387 | pHS31 with Fn0387′ inserted into SnaBI site | Gene inactivation plasmid | This study |
| pIP1449 | pHS31 with Fn1449′ inserted into SnaBI site | Gene inactivation plasmid | This study |
| pIP1526 | pHS31 with Fn1526′ inserted into SnaBI site | Gene inactivation plasmid | This study |
| pIP1554 | pHS31 with Fn1554′ inserted into SnaBI site | Gene inactivation plasmid | This study |
| pIP1893 | pHS31 with Fn1893′ inserted into SnaBI site | Gene inactivation plasmid | This study |
| pIP2047 | pHS31 with Fn2047′ inserted into SnaBI site | Gene inactivation plasmid | This study |
| pIPaim1 | pHS31 with aim1′ inserted into SnaBI site | Gene inactivation plasmid | This study |
| Primer | Sequence | Purpose | Source |
|---|---|---|---|
| 0254′F | 5′-TGGAGCAGAAGCAGGAGAA-3′ | Gene inactivation | This study |
| 0254′R | 5′-TTGTTCCTTTTCCATCAGCA-3′ | Gene inactivation | This study |
| 0387′F | 5′-GGAAGAGCCACAGGATTTGA-3′ | Gene inactivation | This study |
| 0387′R | 5′-CCACTTGCTCCTTCACCATT-3′ | Gene inactivation | This study |
| 1554′F | 5′-GAATGGCAGGATTTGCTTCA-3′ | Gene inactivation | This study |
| 1554′R | 5′-TTGGTTAGTTCCCTTTGCGTA-3′ | Gene inactivation | This study |
| 1893′F | 5′-TCAGCAACAGCAACAAGTCC-3′ | Gene inactivation | This study |
| 1893′R | 5′-CCTATTTGCCCAACATTTCC-3′ | Gene inactivation | This study |
| 2047′F | 5′-AAAAATCGGCTGGGTTAAAAT-3′ | Gene inactivation | This study |
| 2047′R | 5′-TTCCTCCATCTCCTGCTGTT-3′ | Gene inactivation | This study |
| 2058′F | 5′-CTGTTGGGAAAGAAGGAGTTG-3′ | Gene inactivation | This study |
| 2058′R | 5′-TTGAATAAAGGGCTGCTGTG-3′ | Gene inactivation | This study |
| 1449′F | 5′-TCAAAAAGCATCAGCAGGAA-3′ | Gene inactivation | This study |
| 1449′R | 5′-CCTACCACACCATCTGTAGCAA-3′ | Gene inactivation | This study |
| 1526′F | 5′-AAAAGCTGGTGCTGGAAAAA-3′ | Gene inactivation | This study |
| 1526′R | 5′-CTGCTGCTATTCCTGTTGCT-3′ | Gene inactivation | This study |
| catPF | 5′-TTAGGACGGCAATCAATCAA-3′ | Insertion analysis | This study |
| catPR | 5′-AAACGGCAAATGTGAAATCC-3′ | Insertion analysis | This study |
| 1526-5′F | 5′-GCATCAGGAGGCTCTCTATCA-3′ | Insertion analysis | This study |
| 1526-3′R | 5′-TTTGCTCCTGTTGCATTTTC-3′ | Insertion analysis | This study |
| ColE1 | 5′-GCAGAGCGAGGTATGTAGGC-3′ | Insertion analysis | This study |
| 1526orfF | 5′-CAGCTCCAGAAGAAAGAAAAACA-3′ | Operon analysis | This study |
| 1526-5′R | 5′-TCTGCATAAGAAAAACCACCATTA-3′ | Operon analysis | This study |
| 1526-3′F | 5′-AGAAAGAAGAAGAGCAAGAGTAGCA-3′ | Operon analysis | This study |
| 1525R | 5′-TGAGTGGTGGACAAAAACAAAG-3′ | Operon analysis | This study |
| FUSO | 5′-CTAATGGGACGCAAAGCTCTC-3′ | FISH probe | Sunde, P.T. (2003) |
Co-aggregation Assay
Co-aggregation assays were performed in co-aggregation buffer (CAB) 150 mM NaCl, 1 mM Tris, 0.1 mM CaCl2, 0.1 mM MgCl2 as previously described with minor modifications (Cisar et al., 1979). Cells were collected, washed and resuspended in phosphate buffered saline (PBS) containing 0.02% NaN3 and then stored at 4°C until use. Equal numbers of bacterial cells were diluted in CAB or 25% saliva to a final concentration of 2 × 109 cells ml-1 in a reaction tube. The tubes were vortexed for 5 seconds and graded on a 0-4 scale after 10 min based on degree of co-aggregation. A score of 0 was assigned for no visible co-aggregation and a score of 4 for complete sedimentation with a clear supernatant. No clumping of individual partner strains was observed in our experimental controls.
Spectrophotometric co-aggregation assay
Co-aggregation experiments were performed essentially identical to the visual assay except that after 10 min of incubation, the co-aggregation reactions were centrifuged at low speed (100 g) for 1 minute to pellet co-aggregating cells while leaving the non-aggregated bacteria in suspension. The supernatant was then removed without disturbing the pellet, and the optical density of the recovered supernatant was measured at 600 nm. For inhibition assays, 50 mM of either D-galactose or L-arginine was added to the reaction tube containing the F. nucleatum strain and vortexed before addition of the co-aggregation partner strain. Relative co-aggregation was determined by dividing the difference between the total turbidity of each partner strain and the co-aggregation supernatant turbidity by the total turbidity of each partner strain.
Bacterial membrane isolation
Bacterial cells (500 ml) were pelleted by centrifugation and resuspended in buffer (20 mM sodium phosphate buffer with 150 mM NaCl, pH 7.2) containing protease inhibitors. Cells were lysed via three passes through a French press at 12000 PSI. Cell debris was removed by centrifugation (10 000 g at 4°C for 10 min). Membranes were pelleted by ultracentrifugation (150 000 g at 4°C for 60 min) and resuspended in buffer. Membrane protein concentrations were determined with the BCA protein assay kit (Pierce, Rockford, IL). Proteins (30 μg) were resolved by electrophoresis on a 4% acrylamide gel.
Arginine adhesin purification
Bacterial membranes (1 mg) were diluted in 4 ml CAB containing n-octyl-β-D-glucopyranoside (100 mM) and incubated at 4°C overnight with end-over-end mixing according to previously described protocols (Edwards et al., 2007, Takemoto et al., 1993). Debris was removed from the tube by centrifugation at 4750 g for 10 min. Supernatant was removed and incubated at 4°C overnight with end-over-end mixing with L-arginine agarose (500 μl) which was washed three times in CAB. After incubation the L-arginine agarose was washed in 10 vols (40 ml) CAB. Proteins adhering to the agarose beads were eluted by mixing the beads gently in 4 ml CAB containing 150 mM L-arginine. Supernatant was removed and dialyzed against 8 L of ddH2O at 4°C overnight. Isolated proteins were concentrated by freeze drying and resuspended in 100 μl buffer (20 mM sodium phosphate buffer with 150 mM NaCl, pH 7.2). Proteins (30 μg) were resolved by electrophoresis on a 4% acrylamide gel.
Protein identification by liquid chromatography-mass spectrometry
Proteins (25 μg) were separated on (4%) SDS-PAGE gels which were then fixed for 1 h in fixing buffer (10% methanol, 7% acetic acid), stained overnight in SYPRO Ruby and destained in wash buffer (10% methanol, 7% acetic acid). Bands of interest were excised from the gel and in-gel tryptic digested (Shevchenko et al., 1996). The dried peptides were resuspended in 0.1% formic acid (FA) and identified on an Applied Biosystems (Foster City, CA USA) QSTAR® XL (QqTOF) mass spectrometer. The resulting peptide MS/MS spectra was searched against the F. nucleatum ATCC 25586 genome translated in all six reading frames using the Mascot search engine (Matrix Science, London, UK).
Construction of Fusobacterium nucleatum gene inactivation mutants
Internal gene fragments were amplified for each OMP using Taq DNA polymerase and gene inactivation primers (Table 3) phosphorylated with polynucleotide kinase (New England Biolabs, Ipswich, MA) according to the manufacturer’s protocol. Fragments were cloned into the F. nucleatum suicide vector pHS31 (Kaplan et al., 2005) which was digested with SnaBI (New England Biolabs, Ipswich, MA), dephosphorylated with Calf Intestinal Phosphatase (New England Biolabs, Ipswich, MA) and gel purified. The individual OMP fragments were ligated to the digested vector using T4 DNA ligase (New England Biolabs, Ipswich, MA) to generate the respective integration plasmids (Table 3). E. coli DH5α (Hanahan, 1983) was transformed with the ligation reactions and the resulting integration plasmids were confirmed by restriction analysis and PCR (data not shown). Plasmids were purified by cesium-chloride ethidium bromide density gradient (Sambrook, 1989) and transformed into F. nucleatum as previously described (Haake et al., 2000, Kaplan et al., 2005). F. nucleatum gene inactivation mutants were verified by southern blot and PCR analysis.
Nucleic acid isolation
Genomic DNA was extracted from stationary phase cells following standard protocols. Total RNA was extracted from mid-log phase cells using a hot-phenol protocol (Merritt et al., 2005). Three microGrams of total RNA was used for cDNA synthesis using Stratascript RT (Stratagene, La Jolla, CA) according to the manufacturer’s protocol.
Biofilm Growth
Eight-well chambered coverglasses (Nunc, Rochester, NY) were pre-conditioned with 100 μl of 50% saliva diluted in ddH2O that was centrifuged for 1 minute at 10 000 g to remove debris. The chambers were sterilized under UV light for 1 h before inoculation. Overnight cultures of F. nucleatum (10 μl containing ∼1 × 107 cells) and S. sanguinis (10 μl of a 1/10th dilution containing ∼1 × 105 cells) were inoculated into growth chamber wells containing 400 μl of filter-sterilized BHI Saliva Broth (BHISB, 25% BHI and 25% Saliva). Samples were incubated overnight under anaerobic conditions (5% H2, 5% CO2, 90% N2) at 37°C. After 24 h 300 μl of the medium was removed and replaced with fresh medium. After 48 h the medium was removed and biofilms were fixed with in 400 μl of 3.7% formaldehyde in PBS for 1 h before labeling with FISH probes and general nucleic acid dye.
Fluorescent in Situ Hybridization and Biofilm Staining
F. nucleatum present in biofilms were specifically labeled using FISH. Biofilms were fixed in 3.7% formaldehyde diluted in PBS for 1 hr. Biofilms were washed 3 times with 150 mM NaCl 0.25% BSA prior to hybridization. The Hex conjugated F. nucleatum “FUSO” probe (Sunde et al., 2003) was diluted to a final concentration of 5 μM with preheated 20% stringency buffer (0.9 M NaCl, 20 mM Tris-HCl, 20% formamide, 0.01% SDS). Diluted probe (100 μl) was added to each biofilm and hybridized in the dark at 46°C for 3 hrs. Biofilms were washed three times with 20% stringency buffer (900 mM NaCl, 20 mM Tris-HCl, 5 mM EDTA, 0.01% SDS), counterstained with the nucleaic acid staining dye SYTO9 to reveal S. sanguinis (Invitrogen, Carlsbad, CA) and visualized using confocal laser scanning microscopy (CSLM).
Confocal laser scanning microscopy
Specimens were examined using a PASCAL 5 confocal laser scanning microscope (Zeiss, Jena, Germany). The scanning module of the system was mounted onto an inverted microscope (Axiovert 200M) and samples were viewed through a 40× oil-immersion objective (Achroplan/N.A. 1.3). Excitation of 488 nm with an argon laser in combination with a 505-530 nm bandpass emission filter was used for SYTO9 fluorescence imaging. The Hex fluorophore was visualized using 543 nm excitation with a helium-neon laser and a 560 nm longpass emission filter.
Statistical analysis
Student t-test was performed using Excel 2007 (Microsoft, Seattle, WA).
Acknowledgements
We thank Dr. Howard K. Kuramitsu, State University of New York, Buffalo, for his suggestions on this manuscript. We thank Dr. Melissa Sondej for her technical assistance. The protein identification by liquid chromatography-mass spectrometry was performed by the UCLA W. M. Keck Proteomic Center. This study was supported by NIH T32 training grant in oral and craniofacial biology, a research grant from C3 Jian Inc. (W.S.), and NIH/NIDCR PHS Grant No. DE015348 (S.K.H.).
Footnotes
This manuscript is free of any conflict of interest or financial disclosure issues.
REFERENCES
- Charbonneau M-E, Mourez M. Functional organization of the autotransporter adhesin involved in diffuse adherence. J. Bacteriol. 2007;189:9020–9029. doi: 10.1128/JB.01238-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisar JO, Kolenbrander PE, McIntire FC. Specificity of co-aggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979;24:742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WB, Beem JE, Nesbitt WE, Cisar JO, Tseng CC, Levine MJ. Pellicle receptors for Actinomyces viscosus type 1 fimbriae in vitro. Infect. Immun. 1989;57:3003–3008. doi: 10.1128/iai.57.10.3003-3008.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AM, Grossman TJ, Rudney JD. Association of a high-molecular weight arginine-binding protein of Fusobacterium nucleatum ATCC 10953 with adhesion to secretory immunoglobulin A and co-aggregation with Streptococcus cristatus. Oral Microbiology and Immunology. 2007;22:217–224. doi: 10.1111/j.1399-302X.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- Haake SK, Yoder SC, Attarian G, Podkaminer K. Native plasmids of Fusobacterium nucleatum: characterization and use in development of genetic systems. J Bacteriol. 2000;182:1176–1180. doi: 10.1128/jb.182.4.1176-1180.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Ikegami A, Chung P, Zhang L, Deng CX. Sonoporation is an efficient tool for intracellular fluorescent dextran delivery and one-step double-crossover mutant construction in Fusobacterium nucleatum. Appl. Environ. Microbiol. 2007;73:3677–3683. doi: 10.1128/AEM.00428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YW, Ikegami A, Rajanna C, Kawsar HI, Zhou Y, Li M, Sojar HT, Genco RJ, Kuramitsu HK, Deng CX. Identification and characterization of a novel adhesin unique to oral fusobacteria. J. Bacteriol. 2005;187:5330–5340. doi: 10.1128/JB.187.15.5330-5340.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala’Aldeen D. Type V protein secretion pathway: the autotransporter story. Microbiol. Mol. Biol. Rev. 2004;68:692–744. doi: 10.1128/MMBR.68.4.692-744.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes CV, Kolenbrander PE, Andersen RN, Moore LV. Co-aggregation properties of human oral Veillonella spp.: relationship to colonization site and oral ecology. Appl. Environ. Microbiol. 1988;54:1957–1963. doi: 10.1128/aem.54.8.1957-1963.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker M, Schuster CC, McDonnell AV, Sorg KA, Finn MC, Berger B, Clark PL. Pertactin beta-helix folding mechanism suggests common themes for the secretion and folding of autotransporter proteins. PNAS. 2006;103:4918–4923. doi: 10.1073/pnas.0507923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan CW, Lux R, Huynh T, Jewett A, Shi W, Haake SK. Fusobacterium nucleatum Apoptosis-inducing Outer Membrane Protein. J Dent Res. 2005;84:700–704. doi: 10.1177/154405910508400803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haake S. Kinder, Yoder S, Gerardo S. Hunt. Efficient gene transfer and targeted mutagenesis in Fusobacterium nucleatum. Plasmid. 2006;55:27–38. doi: 10.1016/j.plasmid.2005.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander PE, Andersen RN, Moore LV. Co-aggregation of Fusobacterium nucleatum, Selenomonas flueggei, Selenomonas infelix, Selenomonas noxia, and Selenomonas sputigena with strains from 11 genera of oral bacteria. Infect Immun. 1989;57:3194–3203. doi: 10.1128/iai.57.10.3194-3203.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander PE, London J. Adhere today, here tomorrow: oral bacterial adherence. J Bacteriol. 1993;175:3247–3252. doi: 10.1128/jb.175.11.3247-3252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova N, Cota E, Lebedin Y, Monpouet S, Guignot J, Servin AL, Matthews S, Moseley SL. A subfamily of Dr adhesins of Escherichia coli bind independently to decay-accelerating factor and the N-domain of carcinoembryonic antigen. J. Biol. Chem. 2006;281:29120–29130. doi: 10.1074/jbc.M605681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancy P, Jr, Dirienzo JM, Appelbaum B, Rosan B, Holt SC. Corncob formation between Fusobacterium nucleatum and Streptococcus sanguis. Infect. Immun. 1983;40:303–309. doi: 10.1128/iai.40.1.303-309.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt J, Qi F, Shi W. A unique nine-gene comY operon in Streptococcus mutans. Microbiology. 2005;151:157–166. doi: 10.1099/mic.0.27554-0. [DOI] [PubMed] [Google Scholar]
- Murray PA, Kern DG, Winkler JR. Identification of a galactose-binding lectin on Fusobacterium nucleatum FN-2. Infect Immun. 1988;56:1314–1319. doi: 10.1128/iai.56.5.1314-1319.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta K, Makinen KK, Loesche WJ. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect. Immun. 1986;53:213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RJ, Jr., Gordon SM, Cisar JO, Kolenbrander PE. Co-aggregation-mediated interactions of streptococci and actinomyces detected in initial human dental plaque. J. Bacteriol. 2003;185:3400–3409. doi: 10.1128/JB.185.11.3400-3409.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl S, Sandberg AL, Cisar JO. Salivary receptors for the proline-rich protein-binding and lectin-like adhesins of oral actinomyces and streptococci. J Dent Res. 2004;83:505–510. doi: 10.1177/154405910408300614. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 1989. [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Sunde PT, Olsen I, Gobel UB, Theegarten D, Winter S, Debelian GJ, Tronstad L, Moter A. Fluorescence in situ hybridization (FISH) for direct visualization of bacteria in periapical lesions of asymptomatic root-filled teeth. Microbiology %R 10.1099/mic.0.26077-0. 2003;149:1095–1102. doi: 10.1099/mic.0.26077-0. [DOI] [PubMed] [Google Scholar]
- Laarmann D. C. T. J. S. J. B. J. W. S. G. Sven. The Haemophilus influenzae Hia autotransporter harbours two adhesive pockets that reside in the passenger domain and recognize the same host cell receptor. Molecular Microbiology. 2002;46:731–743. doi: 10.1046/j.1365-2958.2002.03189.x. [DOI] [PubMed] [Google Scholar]
- Takemoto T, Hino T, Yoshida M, Nakanishi K, Shirakawa M, Okamoto H. Characteristics of multimodal co-aggregation between Fusobacterium nucleatum and streptococci. Journal of Periodontal Research. 1995;30:252–257. doi: 10.1111/j.1600-0765.1995.tb02130.x. [DOI] [PubMed] [Google Scholar]
- Takemoto T, Ozaki M, Shirakawa M, Hino T, Okamoto H. Purification of arginine-sensitive hemagglutinin from Fusobacterium nucleatum and its role in co-aggregation. J Periodontal Res. 1993;28:21–26. doi: 10.1111/j.1600-0765.1993.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Tuttle RS, Strubel NA, Mourad J, Mangan DF. A non-lectin-like mechanism by which Fusobacterium nucleatum 10953 adheres to and activates human lymphocytes. Oral Microbiol Immunol. 1992;7:78–83. doi: 10.1111/j.1399-302x.1992.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Xie H, Gibbons RJ, Hay DI. Adhesive properties of strains of Fusobacterium nucleatum of the subspecies nucleatum, vincentii and polymorphum. Oral Microbiol Immunol. 1991;6:257–263. doi: 10.1111/j.1399-302x.1991.tb00488.x. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Palmer RJ, Yang J, Kolenbrander PE, Cisar JO. Streptococcal receptor polysaccharides: recognition molecules for oral biofilm formation. BMC Oral Health. 2006;6:S12. doi: 10.1186/1472-6831-6-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]