Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma (original) (raw)
. Author manuscript; available in PMC: 2010 Aug 15.
Abstract
Acquired platinum resistance is a serious problem in the treatment of ovarian carcinomas. However, the mechanism of the drug resistance has not been elucidated. Here, we show functional significance of restoration of BRCA2 protein by secondary BRCA2 mutations in acquired drug resistance of _BRCA2_-mutated ovarian carcinoma. Three ovarian cancer cell lines (PEO1, PEO4 and PEO6) were derived from a BRCA2 mutation (5193C>G (Y1655X)) carrier with ovarian carcinoma with acquired cisplatin resistance and a secondary BRCA2 mutation (5193C>T (Y1655Y)) that canceled the inherited mutation. PEO1 was BRCA2-deficient and sensitive to cisplatin and a poly(ADP-ribose) polymerase inhibitor, AG14361, while PEO4 was resistant. PEO4 and PEO6, derived from ascites at the time of relapse with cisplatin resistance, had the secondary mutation and were BRCA2-proficient. In vitro cisplatin/AG14361 selection of PEO1 led to restoration of BRCA2 due to another secondary BRCA2 mutation. BRCA2 depletion sensitized BRCA2-restored PEO1 clones and PEO4 to cisplatin/AG14361. Thus, restoration of BRCA2 due to secondary BRCA2 mutation is involved in acquired drug resistance of _BRCA2_-mutated ovarian carcinoma.
Keywords: BRCA2, cisplatin, drug resistance, ovarian cancer, PARP inhibitor
Introduction
Chemotherapy with platinum compounds, such as cisplatin and carboplatin, is initially effective for most patients with ovarian carcinomas. However, the majority eventually become refractory to platinum treatment (1). BRCA2 is a tumor suppressor gene responsible for familial breast/ovarian cancer. BRCA2 controls homologous recombination by regulating RAD51 (2, 3). BRCA1- and BRCA2- (BRCA1/2) deficient cells are hypersensitive to cisplatin and poly(ADP-ribose) polymerase (PARP) inhibitors (4-6). Tumors from heterozygous BRCA1/2 mutation carriers usually show loss of heterozygosity at the BRCA1/2 loci and are presumed to be BRCA1/2 deficient (7-9). Consistently, women with _BRCA1/2_-mutated ovarian carcinoma have a better prognosis than those without BRCA1/2 mutation if they receive platinum-based therapy (10), and PARP inhibitors are becoming a therapeutic option in _BRCA1/2_-mutated cancers (6). However, even patients with _BRCA1/2_-mutated ovarian carcinoma frequently experience recurrence with platinum resistance.
Acquired resistance to cisplatin in vitro in a _BRCA2_-mutated pancreatic cancer cell line, Capan-1, is mediated by secondary mutations in BRCA2 that restore the wild-type BRCA2 reading frame (11, 12). Three cases of platinum-resistant recurrent ovarian cancer with secondary BRCA2 mutation have been reported (11, 12), suggesting involvement of secondary BRCA2 mutation in platinum-resistance of ovarian cancer. However, because of lack of a human BRCA2-deficient ovarian cancer cell line model, the functional significance of secondary BRCA2 mutations in ovarian cancer has not been demonstrated. Here, we report the first human BRCA2-deficient ovarian cancer cell line model and the functional significance of secondary BRCA2 mutations in acquired drug sensitivity of ovarian cancer cells.
Materials and Methods
Cell lines
Ovarian cancer cell lines (PEO1, 2008, 2008+FANCF, C13*, OAW42, TOV-21G, TOV-112D, OV-90, PA-1, ES-2, Caov-3, SK-OV-3, SW626, NIH:OVCAR-3, DOV13, RMG-1, A2780, IGROV-1, OVCAR-5, OVCAR-8, A1847, OAW28, COLO720E, 59M) (13) and PEO6 (14) were described previously. PEO1 and PEO4 (15) were gifts from Dr. S. Williams (Fox Chase Cancer Center). Cell lines were grown in DMEM with 10% fetal calf serum in a humidified 5% CO2-containing atmosphere at 37°C. Gamma irradiation was delivered using a JL Shepherd Mark I Cesium Irradiator (JL Shepherd & associates, San Fernando, CA). PEO1 cells were selected in cisplatin (4μM) (Sigma, St. Louis, MO) or a PARP inhibitor (AG14361 (4μM), a gift of Pfizer) -containing medium for 4 weeks. Drug-resistant colonies were picked and expanded in normal medium. Initially, 14 cisplatin-selected clones were picked, but three of them (C4-3, C4-8, and C4-9) were omitted, because C4-8 and C4-9 did not grow and C4-3 turned out to be a mixed population. Therefore, 11 cisplatin-selected clones were analyzed. Initially, 12 AG14361-selected clones were picked, but nine of them were omitted, because they did not grow. Therefore, three AG14361-selected clones were analyzed.
Western blot analysis and immunofluorescence microscopy
BRCA2 western blotting using anti-BRCA2 Ab-1 (OP95, EMD Biosciences) and Ab-2 (PC146, EMD Biosciences) and immunostaining for RAD51 were done as described (11).
BRCA2 sequencing
Extraction of genomic DNA and RNA, reverse transcription, PCR and sequencing of BRCA2 were done as described (11). All nucleotide numbers refer to the cDNA human sequence of BRCA2 (accession no. U43746; version U43746.1 GI: 1161383) (GenBank).
Drug sensitivity assays
Cisplatin/AG14361 sensitivity of cells was determined by crystal violet assay as described (11).
siRNA transfection
Expression of BRCA2 was knocked down by transient transfection of siRNA directed against BRCA2 (#1 (5’-AACAACAATTACGAACCAAAC-3’), #2 (5'-CAGGACACAATTACAACTAAA-3')) and negative control (5’-AATTCTCCGAACGTGTCACGT-3’) as described (11). Final concentration of siRNA was 50nM. Two or three days after transfection, cells were used for drug sensitivity assays, western blotting and immunofluorescence experiments.
In vitro homologous recombination assay
V-C8-DR-GFP cells were transfected with either pcDNA3.1 vector, pcBAsce1 vector containing the I-SceI restriction endonuclease gene, or pcBAsce1 plus various FLAG tagged BRCA2-pcDNA3.1 constructs. After 72 h, cells were harvested and the number of green fluorescent protein (GFP)–expressing cells was assessed by flow cytometry. In parallel, we determined transfection/expression efficiency for BRCA2 by fluorescently labeling cells from these transfection experiments with an anti-FLAG antibody and counting the number of FLAG-BRCA2–expressing cells per 1,000 cells using a fluorescence microscope. The ratio of GFP-expressing cells induced by wild-type or mutant BRCA2 compared with vector control in the homologous recombination assay was then plotted after adjustment for transfection efficiency (11).
Clinical specimens
Tissue samples from the _BRCA2_-mutated ovarian cancer patient from whom the ovarian cancer cell lines, PEO1, PEO4 and PEO6, were derived were obtained from the University of Edinburgh. The study was approved by Institutional Review Boards of Fred Hutchinson Cancer Research Center.
Results and Discussion
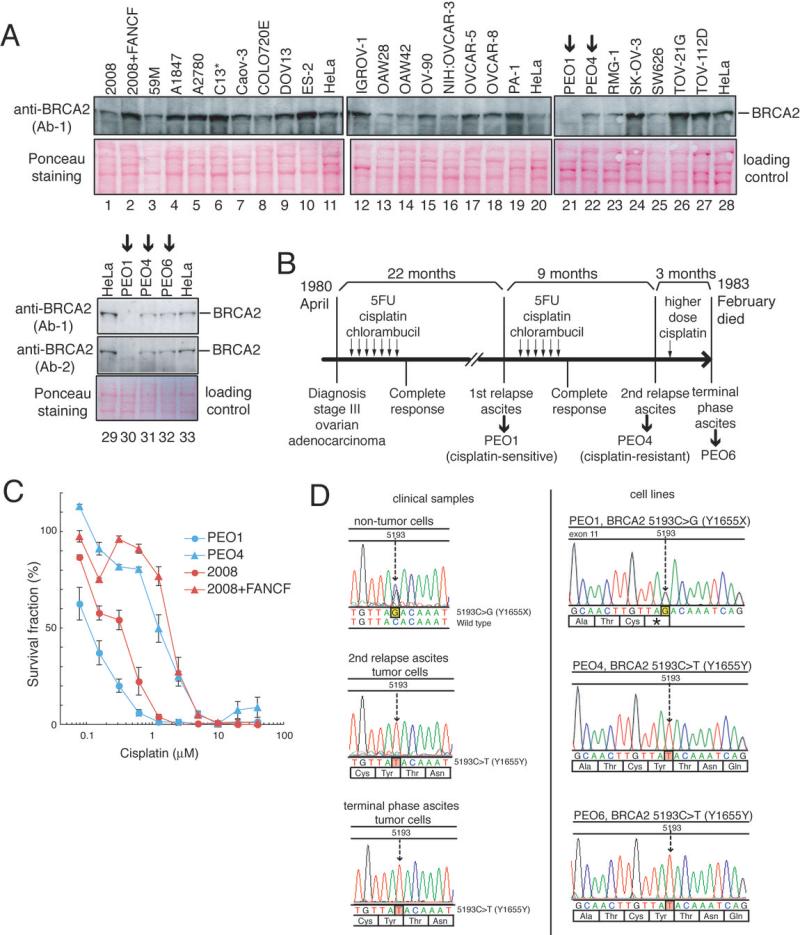
We tested 25 ovarian cancer cell lines for expression of BRCA2 protein and found that BRCA2 protein was undetectable in PEO1 (Fig 1A). PEO1 was derived from the ascites of a patient with poorly differentiated ovarian serous adenocarcinoma that was still clinically responsive to cisplatin at the first relapse (14, 15). The clinical course of this patient is summarized in Fig 1B. Remarkably, in two ovarian cancer cell lines (PEO4 and PEO6) derived from the same patient obtained when her carcinoma had acquired clinical cisplatin resistance, BRCA2 protein was detectable (Fig 1A). PEO1 was sensitive to cisplatin and a PARP inhibitor (AG14361), while PEO4 was resistant (Figs 1C and 2D), consistent with the BRCA2 protein expression status.
Figure 1. PEO1 is a _BRCA2_-deficient ovarian cancer cell line.
(Full-length blots are presented in Supplemental Fig. S5A-B)
A. BRCA2 western blotting of 25 ovarian cancer cell lines.
B. Clinical course of the patient with an ovarian cancer from which PEO1, PEO4 and PEO6 were derived (reconstituted using published information (14, 15)). Timings of collection of samples used to generate the cell lines were also shown.
C. Cisplatin sensitivity assessed by crystal violet assay. 2008 and 2008+FANCF are a cisplatin-sensitive and a cisplatin-resistant control, respectively (13).
D. DNA sequences of BRCA2. In PEO1, a nonsense mutation (5193C>G, Y1655X) was observed. In PEO4 and PEO6, a silent mutation on the same base (5193C>T, Y1655Y) was observed. In the non-tumor cells of the patient, a heterozygous mutation (5193C>G) was detected. In tumor cells from the ascites both at the second relapse and at the terminal phase, a secondary BRCA2 mutation (5193C>T) was detected.
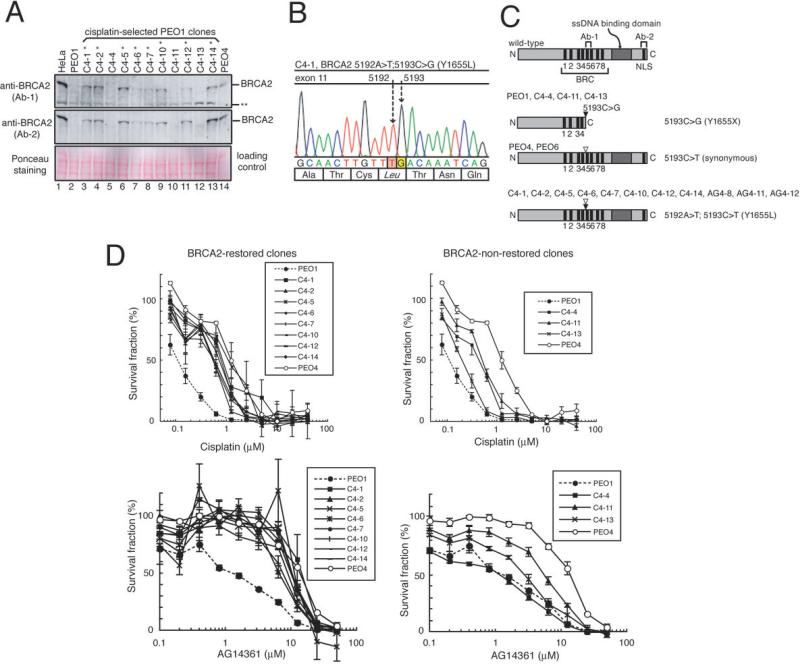
Figure 2. Restoration of BRCA2 protein by secondary BRCA2 mutation in cisplatin-selected PEO1 clones.
(Full-length blots are presented in Supplemental Fig. S5C)
A. BRCA2 western blotting of the 11 clones of PEO1 generated by selecting the cells in the presence of cisplatin. Eight PEO1 clones indicated with a single asterisk restored BRCA2 protein expression. A double asterisk indicates a band presumed to be non-specific.
B. DNA sequence of BRCA2 in C4-1. In addition to the original mutation (5193C>G), a secondary mutation (5192A>T) was observed. The same mutation was observed in C4-2, C4-5, C4-6, C4-7, C4-10, C4-12 and C4-14 (data not shown). The mutation was confirmed in cDNAs (data not shown).
C. Schematic presentation of BRCA2 proteins encoded by transcripts in PEO1 clones, PEO4 and PEO6. The secondary genetic changes (shown as white arrow heads) cancel the nonsense mutation caused by the original mutation (5193C>G, black arrow heads), and the encoded BRCA2 proteins have intact C-terminal regions containing a single strand DNA (ssDNA) binding domain and nuclear localization signals (NLS). The regions that the BRCA2 antibodies (Ab-1 and Ab-2) recognize are depicted.
D. Cisplatin/AG14361 sensitivity of the PEO1 clones. Mean values of at least three independent experiments ± SEM are shown. All of the cisplatin-selected PEO1 clones and PEO4 are cisplatin resistant compared to parental PEO1 (p<0.05, LD50 data were compared by unpaired t test). All of the cisplatin-selected PEO1 clones (except for C4-4) and PEO4 were AG14361 resistant compared to parental PEO1 (p<0.05, LD50 data were compared by unpaired t test).
The patient turned out to be a heterozygous carrier of BRCA2.5193C>G (Y1655X) nonsense mutation (Fig 1D). Consistently, PEO1 had the hemizygous nonsense mutation (5193C>G). PEO1 is the first and only human BRCA2-deficient ovarian cancer cell line described, to the best of our knowledge.
Interestingly, PEO4 and PEO6 had a silent mutation (5193C>T, Y1655Y) instead (Fig 1D). In neoplastic cells in the ascites from which PEO4 and PEO6 were derived, at the relapse with clinical cisplatin resistance, 5193C>T was also detected. Thus, the secondary mutation (5193C>T) which canceled the inherited nonsense mutation (5193C>G) occurred in vivo.
Next, we selected PEO1 cells in cisplatin-containing medium (4μM) for four weeks in vitro and obtained 11 clones out of two million cells. Eight of them restored BRCA2 protein (Fig 2A) and had a secondary BRCA2 mutation (5192A>T) (Figs 2B and 2C). This mutation is a single base pair substitution that changed the stop codon into an amino acid coding triplet leading to the restoration of the open reading frame (5192A>T; 5193C>G, Y1655L).
The eight BRCA2-restored PEO1 clones and PEO4 were cisplatin- and AG14361-resistant compared to parental PEO1 (Fig 2D), consistent with functionality of the restored BRCA2 proteins. Two of the three clones without BRCA2 restoration (C4-4, and C4-11) were cisplatin resistant, while the other clone (C4-13) was only slightly resistant. One of the cisplatin-resistant clones without restored BRCA2 (C4-4) was still sensitive to AG14361, consistent with the lack of BRCA2. Two cisplatin-resistant clones without restored BRCA2 (C4-11 and C4-13) showed intermediate sensitivity to AG14361, suggesting the existence of alternative mechanisms for the drug resistance.
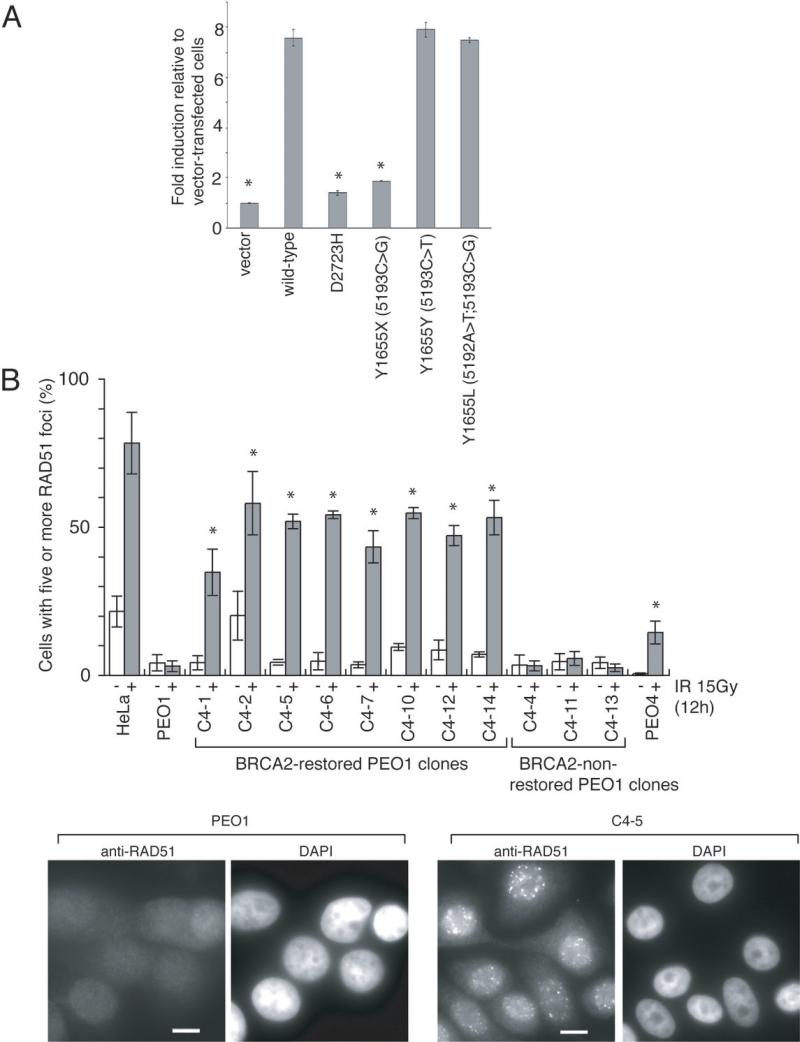
Next, we analyzed the homologous recombination-based DNA double-strand-break repair function of the novel BRCA2 proteins using an I-SceI-dependent DR-GFP reporter assay in BRCA2-deficient V-C8 cells (16, 17) (Fig 3A and Supplemental Fig S1). In this assay, GFP expression correlates with the occurrence of homologous recombination. Transfection of a wild-type BRCA2 construct or constructs with the secondary BRCA2 mutations ((5192A>T; 5193C>G, Y1655L) or (5193C>T, Y1655Y)) resulted in seven to eight-fold more GFP-positive cells compared to control, whereas transfection of the 5193C>T (Y1655X) mutant construct resulted in impaired induction of GFP-positive cells. These results indicate that the secondary-mutated BRCA2 proteins efficiently promote homologous recombination.
Figure 3. The restored BRCA2 proteins with secondary BRCA2 mutations are functional.
A. Quantitation of homologous recombination induced by I-SceI in VC8-DR-GFP cells transiently transfected with wild-type and mutant forms of FLAG-tagged human BRCA2 cDNA. The proportion of GFP-positive cells for each construct relative to vector control is shown (mean ± SEM). Asterisks (*) indicate significant difference with wild-type BRCA2 cDNA-transfected cells (p<0.05, unpaired t test).
B. Ionizing radiation (IR)-induced RAD51 foci formation is restored in the BRCA2-restored PEO1 clones. Indicated cells were irradiated (15 Gy) and fixed 12 hours after IR. Cells were immunostained with RAD51 antibody. Representative pictures of immunostained cells after IR are shown, together with quantification of the cells with at least five RAD51 foci before (−, white bars) and 12 hours after IR (+, grey bars). Mean values of at least three independent experiments ± SEM are shown. Asterisks (*) indicate significant difference with irradiated parental PEO1 cells (p<0.05, unpaired t test). Scale bar = 20 μm. Counterstains for the DNA-specific dye 4’, 6-diamidino-2-phenylindole dihydrochloride (DAPI) are also shown.
Functional BRCA2 is required for ionizing radiation-induced RAD51 foci formation (4). Consistently, in parental PEO1 cells and the three BRCA2-non-restored PEO1 clones, ionizing radiation-induced RAD51 foci formation was severely impaired (Fig 3B). In contrast, it was restored in the eight BRCA2-restored PEO1 clones and in PEO4, again suggesting that the secondary-mutated BRCA2 proteins are functional.
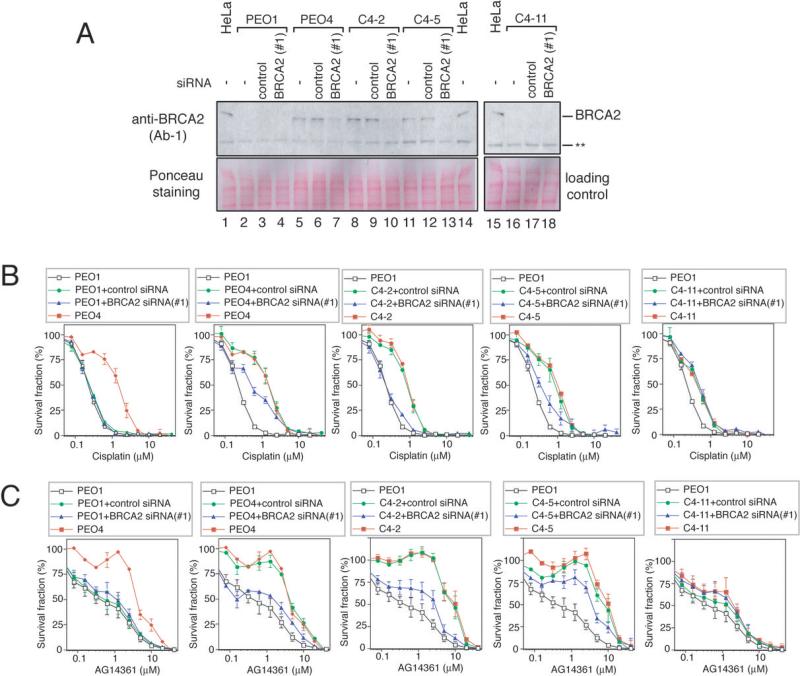
Next, we depleted BRCA2 in two BRCA2-restored clones (C4-2 and C4-5) and PEO4 by siRNA transfection (Fig 4). The BRCA2-depleted cells became sensitive to cisplatin/AG14361, indicating that the restored BRCA2 proteins are critical for the acquired cisplatin/AG14361 resistance. We confirmed this result using another BRCA2 siRNA (Supplemental Fig S2). In contrast, BRCA2 siRNA had no effect on drug sensitivity of a BRCA2-non-restored clone C4-11 and parental PEO1 (Fig 4).
Figure 4. BRCA2 restoration is critical for acquired drug resistance.
(Full-length blots are presented in Supplemental Fig. S5D)
A. BRCA2 western blotting of PEO1, PEO4, C4-2, C4-5, and C4-11 cells treated with indicated siRNA. BRCA2 siRNA #1 was used in this experiment.
B. Cisplatin sensitivity assessed by crystal violet assay. PEO4, C4-2 and C4-5 were resistant to cisplatin. BRCA2 siRNA (#1)- treated PEO4, C4-2 and C4-5 cells are cisplatin-sensitive compared to control siRNA-treated cells. (p<0.05, LD50 data were compared by unpaired t test). BRCA2 siRNA had no effect on cisplatin sensitivity of PEO1 and C4-11 cells. (mean ± SEM, n=3)
C. AG14361 sensitivity assessed by crystal violet assay. PEO4, C4-2 and C4-5 were resistant to AG14361. BRCA2 siRNA (#1)-treated PEO4, and C4-2 cells were AG14361-sensitive compared to control siRNA-treated cells (p<0.05, LD50 data were compared by unpaired t test). BRCA2 siRNA (#1)-treated C4-5 cells tended to be AG14361-sensitive compared to control siRNA-treated cells, but the difference is not statistically significant (p=0.067). BRCA2 siRNA (#1) had no effect on AG14361 sensitivity of PEO1 and C4-11 cells. (mean ± SEM, n=3)
We also selected PEO1 cells in AG14361-containing medium (4μM) for four weeks and obtained three clones out of one million cells. These clones restored BRCA2 protein, harbored the same secondary mutation (5192A>T), showed restored RAD51 foci formation, and were resistant to both cisplatin and AG14361 (Supplemental Fig S3A-D). Depletion of BRCA2 sensitized these clones to cisplatin and AG14361 (Supplemental Fig S3E-F, S4), indicating that restored BRCA2 was critical for the drug resistance.
These findings provide compelling evidence that restoration of functional BRCA2 protein by secondary BRCA2 mutation has a critical role in acquired platinum/PARP inhibitor resistance of _BRCA2_-mutated ovarian carcinomas. This concept has two important clinical implications. First, it suggests the importance of testing BRCA2 mutation status in recurrent ovarian carcinomas in BRCA2 mutation carriers to predict their response to platinum and PARP inhibitors. Second, it provides a theoretical basis for a strategy to overcome platinum/PARP inhibitor resistance. If the mechanism of resistance is restoration of BRCA2, inhibiting BRCA2 function is a logical way to re-sensitize the tumor to the drugs.
We may be able to apply the concept to other genes regulating DNA repair, such as BRCA1 and ATM. Indeed, secondary mutations of BRCA1 occur in _BRCA1_-mutated ovarian cancer with platinum resistance (18). Whether we can apply the concept to sporadic ovarian carcinomas with reduced BRCA1 or BRCA2 expression (19) is also an important issue to be addressed in the future.
Supplementary Material
Suppl data
Acknowledgments
We thank Dr. Williams for reagents, Pfizer for AG14361, Dr. M Rowley for FLAG-BRCA2 western blotting, and Drs. MC King, BY Karlan and N Urban for critical reading of the manuscript.
Financial Support: This work was supported by the National Institutes of Health/National Cancer Institute [R01CA125636 to T.T., K08CA96610-01 to E.M.S., R01CA116167 and R01CA116201 to F.J.C.]; Searle Scholars Program (to T.T.); V Foundation (to T.T.); Hartwell Innovation Fund (to T.T.); start-up funds from the Fred Hutchinson Cancer Research Center (to T.T.); and the Yvonne Betson Trust (to E.M.S.).
References
- 1.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–16. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 2.Chen PL, Chen CF, Chen Y, Xiao J, Sharp ZD, Lee WH. The BRC repeats in BRCA2 are critical for RAD51 binding and resistance to methyl methanesulfonate treatment. Proc Natl Acad Sci U S A. 1998;95:5287–92. doi: 10.1073/pnas.95.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–72. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 4.Yuan SS, Lee SY, Chen G, Song M, Tomlinson GE, Lee EY. BRCA2 is required for ionizing radiation-induced assembly of Rad51 complex in vivo. Cancer Res. 1999;59:3547–51. [PubMed] [Google Scholar]
- 5.Bhattacharyya A, Ear US, Koller BH, Weichselbaum RR, Bishop DK. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899–903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 6.Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26:3785–90. doi: 10.1200/JCO.2008.16.0812. [DOI] [PubMed] [Google Scholar]
- 7.Neuhausen SL, Marshall CJ. Loss of heterozygosity in familial tumors from three BRCA1-linked kindreds. Cancer Res. 1994;54:6069–72. [PubMed] [Google Scholar]
- 8.Collins N, McManus R, Wooster R, et al. Consistent loss of the wild type allele in breast cancers from a family linked to the BRCA2 gene on chromosome 13q12-13. Oncogene. 1995;10:1673–5. [PubMed] [Google Scholar]
- 9.Gudmundsson J, Johannesdottir G, Bergthorsson JT, et al. Different tumor types from BRCA2 carriers show wild-type chromosome deletions on 13q12-q13. Cancer Res. 1995;55:4830–2. [PubMed] [Google Scholar]
- 10.Chetrit A, Hirsh-Yechezkel G, Ben-David Y, Lubin F, Friedman E, Sadetzki S. Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: the national Israeli study of ovarian cancer. J Clin Oncol. 2008;26:20–5. doi: 10.1200/JCO.2007.11.6905. [DOI] [PubMed] [Google Scholar]
- 11.Sakai W, Swisher EM, Karlan BY, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–20. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards SL, Brough R, Lord CJ, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–5. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi T, Tischkowitz M, Ameziane N, et al. Disruption of the Fanconi anemia-BRCA pathway in cisplatin-sensitive ovarian tumors. Nat Med. 2003;9:568–74. doi: 10.1038/nm852. [DOI] [PubMed] [Google Scholar]
- 14.Langdon SP, Lawrie SS, Hay FG, et al. Characterization and properties of nine human ovarian adenocarcinoma cell lines. Cancer Res. 1988;48:6166–72. [PubMed] [Google Scholar]
- 15.Wolf CR, Hayward IP, Lawrie SS, et al. Cellular heterogeneity and drug resistance in two ovarian adenocarcinoma cell lines derived from a single patient. Int J Cancer. 1987;39:695–702. doi: 10.1002/ijc.2910390607. [DOI] [PubMed] [Google Scholar]
- 16.Wu K, Hinson SR, Ohashi A, et al. Functional evaluation and cancer risk assessment of BRCA2 unclassified variants. Cancer Res. 2005;65:417–26. [PubMed] [Google Scholar]
- 17.Farrugia DJ, Agarwal MK, Pankratz VS, et al. Functional assays for classification of BRCA2 variants of uncertain significance. Cancer Res. 2008;68:3523–31. doi: 10.1158/0008-5472.CAN-07-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swisher EM, Sakai W, Karlan BY, Wurz K, Urban N, Taniguchi T. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68:2581–6. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilton JL, Geisler JP, Rathe JA, Hattermann-Zogg MA, DeYoung B, Buller RE. Inactivation of BRCA1 and BRCA2 in ovarian cancer. J Natl Cancer Inst. 2002;94:1396–406. doi: 10.1093/jnci/94.18.1396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl data