An alternative pathway mediates the mouse and human cone visual cycle (original) (raw)
. Author manuscript; available in PMC: 2010 Oct 13.
Published in final edited form as: Curr Biol. 2009 Sep 24;19(19):1665–1669. doi: 10.1016/j.cub.2009.07.054
Summary
One of the fundamental mysteries of the human visual system is the continuous function of cone photoreceptors in bright daylight. As visual pigment is destroyed, or bleached, by light [1], cones require its rapid regeneration, which in turn involves rapid recycling of the pigment’s chromophore. The canonical visual cycle for rod and cone pigments involves recycling of their chromophore from all-trans retinol to 11-cis retinal in the pigment epithelium, adjacent to photoreceptors [2]. However, shortcomings of this pathway indicate the function of a second, cone-specific, mechanism for chromophore recycling [3]. Indeed, biochemical [3–7] and physiological [8] studies on lower species have described a cone-specific visual cycle additional to the long-known pigment epithelium pathway. Two important questions remain, however: what is the role of this pathway in the function of mammalian cones and is it present in higher mammals, including humans. Here we show that mouse, primate, and human neural retinas promote pigment regeneration and dark adaptation selectively in cones, but not in rods. This pathway supports rapid dark adaptation of mammalian cones and extends their dynamic range in background light independently of the pigment epithelium. This pigment-regeneration mechanism is essential for our daytime vision and appears to be evolutionarily conserved.
Results
Cone Dark Adaptation in the Isolated Mouse Retina
We measured flash sensitivity of single photoreceptors before and after exposure to bright light to determine whether the mouse retina, isolated from the pigment epithelium, is able to promote cone pigment regeneration and dark adaptation. We used a suction electrode to record membrane current from individual mouse rods and cones in retina removed from the pigment epithelium. Rod recordings were done from wild type (WT, C57BL/6) mice and cone recordings were done from rod transducin α-subunit knockout (Trα−/−) mice, as described previously [9].
We compared the dark current and sensitivity of photoreceptors before (dark adapted) and after 40-s exposure to bright light followed by recovery in darkness (bleached, DA). With subsequent 4-h dark incubation, exposure of rods in isolated retina to bleaching light produced significant decrease in dark current, from 15 pA to 5 pA, and 155-fold decrease in their flash sensitivity, from 3.1 ± 0.5 ×10−1 (n = 6) pA photon−1 μm2 for dark adapted cells to 2.0 ± 0.2 × 10−3 (n = 6) pA photon−1 μm2 after bleach (Figure 1A). In contrast, identical bleach followed by 2-h dark incubation of cones in Trα−/− retinas had no effect on the dark current and the sensitivity recovered to 50% of its dark adapted value, from 1.57 ± 0.35 × 10−4 (n = 5) pA photon−1 μm2 to 0.78 ± 0.25 × 10−4 (n = 5) pA photon−1 μm2 (Figure 1B). Cone recovery following a bleach took place in the absence of pigment epithelium indicating that substantial cone pigment regeneration occurred within the mouse retina. Rod pigment regeneration under the same conditions was insignificant even following longer dark incubation. These results are consistent with our recent findings from wild type mice using whole-retina electroretinogram (ERG) recordings (Wang et al., see also Fig 1C, D). Notably, cone ERG recovery could be observed after subsequent second and third exposures to the bleaching light (Figure 1D). Considering the low level of free 11-cis retinal in the mouse retina [10], this result indicates that cone pigment regeneration in the isolated retina was driven by recycling chromophore released from bleached visual pigment rather than by a store of retinoid. A simple explanation for the incomplete cone pigment regeneration would be that the retina was damaged during dissection, or that chromophore released from bleached cones was lost to the perfusion solution, thereby reducing the chromophore available for regeneration.
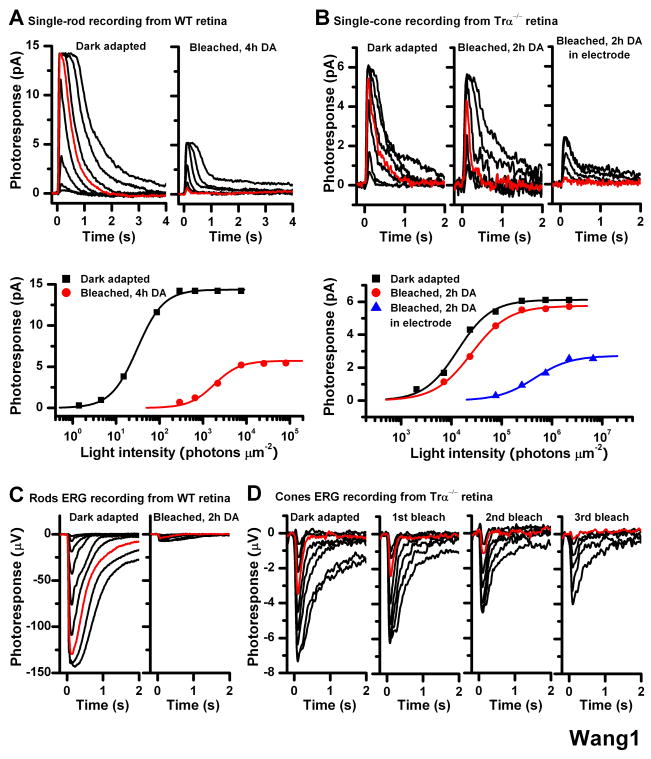
Figure 1.
Effect of bleach on rod and cone single-cell responses in isolated mouse retina Single-cell suction recordings of flash intensity-response families from rods of wild type mice (A) and cones of Trα−/− mice (B). Top panels show test flash responses from individual cells recorded in dark-adapted state (left) or following a 40-s 500 nm light bleach and 4-h (rod) or 2-h (cone) dark recovery period (right). Right-most panel in (B) shows responses from a cone that was bleached and held in the recording electrode during the dark recovery period. Here and in all subsequent figures, photoresponses were generated by 20-ms test flashes delivered at time 0 and of intensity increasing in 0.5 log unit steps. Red traces represent photoresponses to 291 photons μm−2, 500 nm for rods (A) and to 75,814 photons μm−2, 500 nm for cones (B). Bottom panels show the corresponding intensity-response relations for these cells, fit with Michaelis-Menten function R/Rmax = I/(I+IO), where R/Rmax is the normalized response amplitude, I is the flash intensity, and IO is the intensity required to produce half-saturating response. The desensitization produced by the bleach was persistent in rods but largely reversed in cones from isolated retina in the absence of pigment epithelium
(C) Rod ERG responses from isolated wild type mouse retina in darkness (left) and following a bleach and 2-h dark recovery period (right). Red traces represent photoresponses to 1,977 photons μm−2 500 nm
(D) Cone ERG responses from Trα−/− retina in dark-adapted state (left) and after each of three subsequent bleaches, followed by dark recovery period (right three panels). The substantial desensitization induced by the bleach was persistent in rods but was largely reversed in cones
Methods: Mice were dark adapted overnight, euthanized by CO2 asphyxiation, and the eyes enucleated under dim red light. Under infrared light, the eyeballs were hemisected, and the retinas were removed and placed in L-15 medium saturated with pure oxygen. To block Müller cells function, prior to isolating the retina the eyecup was incubated with 10 mM L-α-AAA dissolved in L-15 medium, pH 7.4, for 2.5 h in oxygen-saturated chamber at room temperature. Single-cell suction recordings for rods and cones were done as described previously [9, 19]. Rod and cone electroretinogram (ERG) photoresponses from isolated mouse retina were done as described previously [8, 20, 21]. By pharmacologically blocking synaptic transmission (see Supplemental Experimental Procedures), we recorded the photoreceptor component (a-wave) of isolated retina ERG responses. Test flashes at 500 nm were delivered from an optical bench using a set of calibrated neutral density filters. The signals were amplified, low-pass filtered at 30 Hz (8-pole Bessel) and digitized at 100 Hz for further analysis. Flash sensitivity was calculated from the linear region of the intensity-response curve as the ratio of response amplitude and flash intensity.
Role of Müller Glia in the Retina Visual Cycle
Biochemical studies in cone-dominant retinas [3, 5] as well as recordings from salamander retina [8] suggest that retinal Müller glia might be part of the retina visual cycle. To investigate the role of Müller cells in mammalian cone pigment regeneration, we first perturbed the physical contact between a cone and the rest of retina, including Müller cells. We achieved that by drawing the inner segment of a cone from a piece of retina into a suction electrode. We found that cone dark adaptation was blocked and could not be observed in this configuration (Figure 1B, right).
Next, we selectively disrupted the function of Müller cells by pre-incubating the retina for 2.5 h with the glial cell metabolic inhibitor α-aminoadipic acid (L-α-AAA), a well known selective gliotoxin [11], prior to recordings. Such treatment provides an effective block of the retina visual cycle in salamander retina [8]. L-α-AAA pre-incubation resulted in weaker immunostaining of glial processes for Müller cell-specific glutamine synthetase (GS) and loss of Müller cell nuclei (Figure 2A). The gliotoxin did not affect the function of dark-adapted cones (Figure 2B, left) but dramatically inhibited the recovery of cone response amplitude in the isolated retina following a bleach (Figure 2B, center). Cone sensitivity also declined, from 1.75 ± 0.38 × 10−4 (n = 4) pA photon −1 μm2 in dark-adapted cells to 5.12 ± 1.41 × 10−6 (n = 4) pA photon −1 μm2 following the bleach. Thus, blocking Müller cell function, either physically or pharmacologically, inhibited the mouse retina visual cycle and blocked pigment regeneration and dark adaptation in cones.
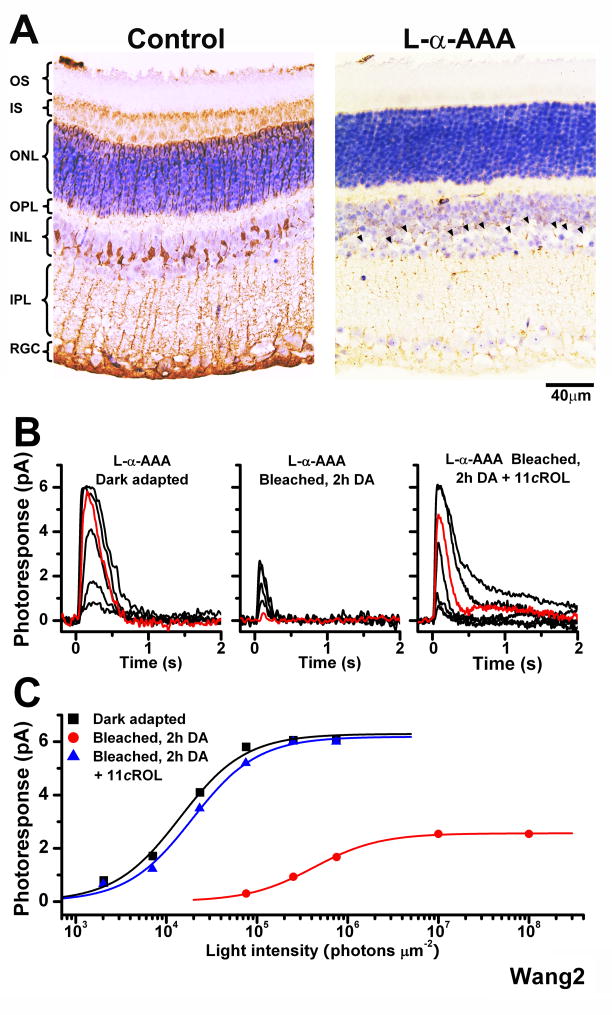
Figure 2.
Effect of the Müller cell inhibitor L-α-AAA on the recovery of mouse cone sensitivity following a bleach
(A) Comparison of the morphology of control retina (left) and retina incubated in 10 mM L-α-AAA gliotoxin for 2.5 h (right). Missing Müller cell nuclei in right panel are indicated by arrowheads
(B) Cone suction recordings from isolated Trα−/− retina pretreated with 10 mM L-α-AAA in darkness for 2.5 h and then transferred to control solution prior to recordings. Cone test-flash responses from retina in dark-adapted state (left), bleached and incubated in darkness for 2 h (middle), and bleached and incubated in darkness for 2 h in the presence of 11-cis retinol (11_c_ROL, right). Red traces represent photoresponses to 75,814 photons μm−2, 500 nm
(C) Intensity-response relations of the cells from (B) fitted with Michaelis-Menten function. The recovery of sensitivity and amplitude of cones from isolated retina were blocked by the gliotoxin, but brought back by the addition of 11-cis retinol.
Application of exogenous 11-cis retinol reversed the cone desensitization in the bleached gliotoxin-treated mouse retina and promoted recovery of cone sensitivity to dark-adapted levels, 1.52 ± 0.04 × 10−4 (n = 5) pA photon−1 μm2 (Figure 2B, right; see also Figure 2C). This result indicates that the gliotoxin did not affect the ability of cones to oxidize 11-cis retinol or to regenerate their pigment but rather blocked the supply of recycled chromophore, most likely 11-cis retinol, from Müller cells to cones. In contrast, 11-cis retinol was not able to promote rod pigment regeneration and had no effect on the sensitivity of bleached rods (data not shown).
Role of the Retina Visual Cycle in Cone Dark and Background Adaptation
To study the recovery of cone sensitivity in real time and determine the kinetics of chromophore recycling by the cone-specific retina visual cycle, we recorded rod and cone ERG photoresponses from isolated mouse retina. The Trα−/− retina restored cone sensitivity to half of its dark-adapted level within 5 min following a bleach (Figure 3A). Consistent with our single-cell results, this recovery was inhibited when the function of Müller cells was blocked by L-α-AAA (Figure 3A). Thus, the Müller cell-mediated retina visual cycle was rapid and could promote the dark adaptation of mouse cones at a rate identical to the one measured in vivo [12].
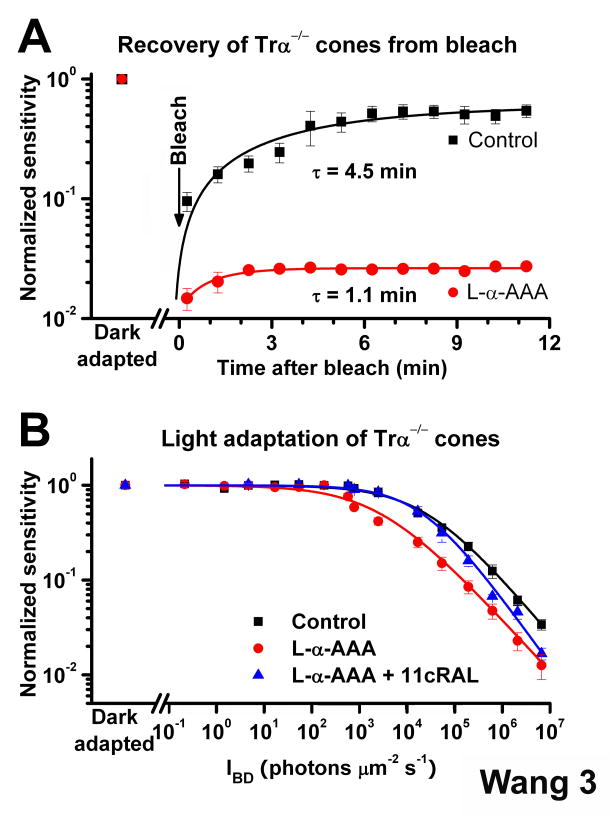
Figure 3.
Effect of the retina visual cycle on mouse cone dark- and light adaptation (A) Recovery of cone sensitivity following a bleach at time 0 from ERG recordings of isolated Trα−/− retina in control solution (black, n = 5) and Trα−/− retina pre-treated for 2.5 h with 10 mM L-α-AAA (red, n = 5). The isolated Trα−/− retina promoted rapid cone dark adaptation at body temperature that could be inhibited by the gliotoxin L-α-AAA. Data are fitted by single exponential functions
(B) Cone desensitization in background light from ERG recordings of isolated Tr-α−/− retina. Data are fitted with the Weber-Fechner relation _S/SDA = (1 + IB/IO)_− 1, where S is the light-adapted sensitivity, SDA is the dark-adapted sensitivity, IB is the intensity of the background, and IO is the background that reduced sensitivity to half of SDA. IO was 25,165 photons μm−2 s−1 for cones in control retinas (n = 7) and was reduced to 2,747 photons μm−2 s−1 in L-α-AAA treated retinas (n = 8). Exogenous 11-cis retinal reversed the effect of gliotoxin, with IO = 20,749 photons μm −2 s−1 (n = 7). All sensitivity measurements were normalized to the corresponding dark-adapted value. Error bars give SEM.
To establish whether the rapid cone pigment regeneration by the retina visual cycle affects the dynamic range of cones in background light, we compared light adaptation of cones in isolated retina with and without functional retina visual cycle. Blocking the retina visual cycle using L-α-AAA resulted in a substantial shift to the left in the cone background adaptation curve (Figure 3B). The background light required to reduce sensitivity to half of its dark adapted level decreased 9.2-fold, from 25,165 photons μm−2 s−1 (n = 7) in control retina to 2,747 photons μm−2 s−1 (n = 8) in L-α-AAA-treated retina. Applying exogenous 11-cis retinal reversed the effect of L-α-AAA on background adaptation in low intensity backgrounds (Figure 3B). The efficiency of 11-cis retinal in rescuing background adaptation declined with increasing light intensity, most likely because the limited supply of exogenous chromophore could not keep up with the high rate of pigment bleaching in brighter backgrounds. These results indicate that in background light conditions the retina visual cycle provides chromophore for cones to broaden their functional range.
Functional Retina Visual Cycle in the Primate and Human Retina
To investigate whether a visual cycle is present in the primate and human retinas, we studied their cones using whole-retina ERG recordings. As primate eye enucleation was performed in bright light, both rods and cones were initially bleached. Following 3-h dark incubation of primate retina attached to the pigment epithelium in eyecup, we observed prominent rod (slow, high sensitivity) and cone (fast, low sensitivity) components in the ERG response (Figure 4A, left) consistent with a dark adaptation of both rods and cones. However, after this retina was isolated from the eyecup, bleached, and then incubated in darkness for 3 h, the rod component was abolished whereas the cone component recovered essentially completely (Figure 4A, right). Similarly, a light-adapted primate retina removed from the eyecup immediately after enucleation and incubated for 1 h in darkness exhibited a robust cone response, but no rod response (Figure 4B). Following a subsequent bleach and 1-h dark incubation, the cone response amplitude recovered essentially completely and cone sensitivity recovered to 30% (n = 8) of the pre-bleach level (Figure 4B).
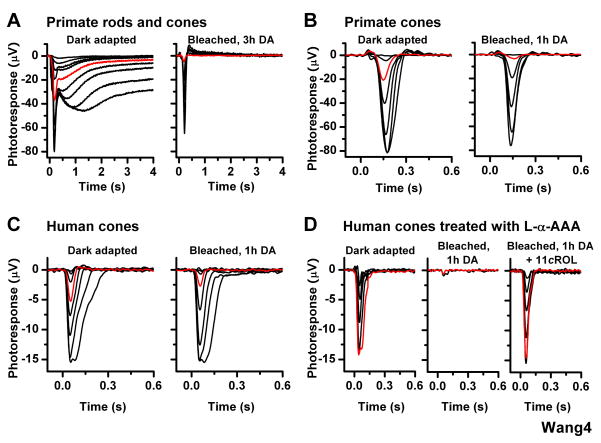
Figure 4.
Effect of bleach on cone ERG responses in isolated primate and human retinas (A) Primate rod (slow, high sensitivity) and cone (fast, low sensitivity) ERG responses from retina dark adapted in eyecup after enucleation in bright light (left). Following a subsequent bleach of the isolated retina, the rod component was abolished whereas the cone component recovered substantially (right)
(B) Cone ERG responses from primate retina, dark adapted in the absence of pigment epithelium after enucleation in bright light (left) and following subsequent bleach and 1-h dark incubation (right). Primate cone response amplitude and sensitivity recovered substantially following the bleach
(C) Cone ERG responses from human retina, dark adapted in the absence of pigment epithelium after enucleation in bright light (left) and following subsequent bleach and 1-h dark incubation (right). Human cone response amplitude and sensitivity recovered substantially following the bleach
(D) Cone ERG recordings from human retina pretreated with 10 mM L-α-AAA in darkness for 2.5 h and then transferred to control solution prior to recordings. Cone test-flash responses from retina in dark-adapted state (left) and bleached and dark-adapted for 1 h in control solution (middle) or in the presence of 11-cis retinol (11_c_ROL, right). The gliotoxin blocked recovery of sensitivity and amplitude of human cones from isolated retina, but exogenous 11-cis retinol reversed that effect. Red traces represent photoresponses to 18,560 photons μm−2 560 nm (A–C) and to 663,500 photons μm−2, 560 nm (D).
Finally, we performed recordings from freshly harvested human retina. As enucleation and retina removal were performed in bright light, both rods and cones were initially bleached. Following incubation in darkness for 3 h, we observed robust cone responses but no rod responses (Figure 4C, left). The ability of the isolated human retina to promote cone dark adaptation was further demonstrated by the recovery of cone sensitivity following a 40-s bleach and 1-h dark incubation (Figure 4C, right). Similarly to the case of mouse, inhibiting the function of Müller cells with gliotoxin in isolated human retina blocked the recovery of cone sensitivity (Figure 4D). Subsequent treatment with exogenous 11-cis retinol reversed the effect of the bleach and promoted cone-specific dark adaptation (Figure 4D). In contrast, exogenous 11-cis retinal induced dark adaptation and response recovery in both rods and cones of gliotoxin-treated bleached human retina (data not shown). Together, these results demonstrate that, similarly to salamander and mouse, the primate and human retinas promote cone-selective pigment regeneration and dark adaptation independently of the pigment epithelium.
Discussion
Our results establish the function of a mechanism by which the mammalian neural retina recycles chromophore and enables pigment regeneration independently of the pigment epithelium. This visual cycle promotes the rapid pigment regeneration and dark adaptation selectively in cones, but not in rods. The recycling of chromophore released from bleached cone pigment involves the retinal Müller glia, where all-trans retinol is converted most likely to 11-cis retinol. We demonstrate the robust function of this pathway in the retinas of mice, primates, and humans. Together with our previous salamander results [8] and recent biochemical studies from zebrafish [13], chicken [6], and ground squirrel [3], our findings indicate that the retina visual cycle is evolutionally conserved. The presence of a retina visual cycle in a wide range of species, from amphibian to human, also points to its critical role in cone photoreceptor function.
Functional Role of the Mammalian Retina Visual Cycle
The neural retina visual cycle plays an important role for mammalian daytime vision. We find that, following an essentially complete bleach, the mammalian retina promotes cone dark adaptation at rates comparable to the rates of recovery of cone sensitivity _in vivo_[12, 14]. Thus, the neural retina visual cycle helps explain the substantially faster pigment regeneration and dark adaptation of mammalian cones compared to rods. In addition, we show that this rapid pigment regeneration enables mammalian cones to maintain sufficient pigment levels to function in steady bright light, that bleaches their pigment at a high rate, and in that way extends the functional range of cones. As the canonical pathway for recycling chromophore through the pigment epithelium is too slow to keep up with the high rate of pigment bleaching in steady bright light [3], the neural retina visual cycle should be critical for maintaining adequate levels of pigment and the continuous function of cones during the day. Together, our results demonstrate that the mammalian retina visual cycle is essential for maintaining our daytime vision, mediated by the cones, in bright and rapidly changing light conditions during the day.
Cone Specificity of the Retina Visual Cycle
We find that the presumptive product of the retina visual cycle, 11-cis retinol, promotes pigment regeneration and dark adaptation only in mammalian cones but not in rods. The exclusive ability of mammalian cones to utilize 11-cis retinol for pigment regeneration grants the cone-selectivity of the retina visual cycle. The enzyme responsible for converting 11-cis retinol to 11-cis retinal in cones remains unknown. Previous biochemical studies from mouse retina have failed to detect enzymatic activities consistent with a retina visual cycle [3] most likely due to the small number of cones (3%) in the mouse retina. In addition, two studies of all-cone Nrl−/− mice lacking RPE65, a key component of the pigment epithelium visual cycle, also could not find evidence for chromophore recycling in the mouse retina, further raising doubts about the function of a cone-specific visual cycle in mammals with rod-dominant retinas [15, 16]. However, our results clearly demonstrate the presence of a visual cycle in mouse, primate, and human retinas indicating the function of an 11-cis retinol dehydrogenase in their cones.
Human Retina Visual Cycle and Cone Disorders
The characterization of a cone-specific visual cycle in the human retina calls attention to its possible clinical implication for cone visual disorders. Deficits in the canonical visual cycle have been implicated in multiple visual disorders and the mechanisms by which chromophore deficiency affects rod function have been well characterized [17]. However, understanding of the mechanisms by which such disorders affect cones is lagging behind. Our characterization of a human cone-specific visual cycle indicates that cones might be affected differently from rods in disorders of the pigment epithelium visual cycle. One example of such disorder is Leber congenital amaurosis (LCA) associated with chromophore deficiency due to mutations in RPE65. Notably, in LCA patients cone sensitivity is less severely affected than rod sensitivity [18] indicating a possible role for the retina visual cycle in maintaining higher chromophore level in cones compared to rods. In addition, possible deficiencies in the retina visual cycle are likely to selectively disturb the function of cones. Finally, it would be important to consider how the function of the retina visual cycle is affected with age and whether gradual deterioration of that pathway is linked to age-related cone disorders, most notably age-related macular degeneration.
Supplementary Material
01
Acknowledgments
We thank Janis Lem for the Trα−/− mice, Rosalie Crouch for the gift of 11-cis retinal and 11-cis retinol, Milam Brantley for the gift of α-GS antibody, Dan Moran, Dana Abendschein, Chad Faulkner, and Mary-Kay Harmon for the donation of primate eyes, and William Harbour and Lori Worley for the donation of human retina. We also thank King-Wai Yau, Carter Cornwall, Peter Lukasiewicz, and Rosalie Crouch for comments on the manuscript. Supported by Career Development Award from Research to Prevent Blindness, NIH grant EY 019312, and unrestricted grant from Research to Prevent Blindness and EY 02687 (Department of Ophthalmology & Visual Sciences at Washington University).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- 2.Saari JC. Biochemistry of visual pigment regeneration: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2000;41:337–348. [PubMed] [Google Scholar]
- 3.Mata NL, Radu RA, Clemmons RC, Travis GH. Isomerization and oxidation of vitamin A in cone-dominant retinas: a novel pathway for visual-pigment regeneration in daylight. Neuron. 2002;36:69–80. doi: 10.1016/s0896-6273(02)00912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bustamante JJ, Ziari S, Ramirez RD, Tsin AT. Retinyl ester hydrolase and the visual cycle in the chicken eye. Am J Physiol. 1995;269:R1346–1350. doi: 10.1152/ajpregu.1995.269.6.R1346. [DOI] [PubMed] [Google Scholar]
- 5.Das SR, Bhardwaj N, Kjeldbye H, Gouras P. Muller cells of chicken retina synthesize 11-cis-retinol. Biochem J. 1992;285(Pt 3):907–913. doi: 10.1042/bj2850907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trevino SG, Villazana-Espinoza ET, Muniz A, Tsin AT. Retinoid cycles in the cone-dominated chicken retina. J Exp Biol. 2005;208:4151–4157. doi: 10.1242/jeb.01881. [DOI] [PubMed] [Google Scholar]
- 7.Villazana-Espinoza ET, Hatch AL, Tsin AT. Effect of light exposure on the accumulation and depletion of retinyl ester in the chicken retina. Exp Eye Res. 2006;83:871–876. doi: 10.1016/j.exer.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Wang JS, Estevez ME, Cornwall MC, Kefalov VJ. Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nat Neurosci. 2009;12:295–302. doi: 10.1038/nn.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikonov SS, Kholodenko R, Lem J, Pugh EN., Jr Physiological features of the S- and M-cone photoreceptors of wild-type mice from single-cell recordings. J Gen Physiol. 2006;127:359–374. doi: 10.1085/jgp.200609490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyubarsky AL, Pugh EN., Jr Over 98% of 11-cis retinal in the dark-adapted mouse eye is bound to rod and cone opsins. Invest Ophthalmol Vis Sci. 2007;48:3246–3246. [Google Scholar]
- 11.Jablonski MM, Iannaccone A. Targeted disruption of Muller cell metabolism induces photoreceptor dysmorphogenesis. Glia. 2000;32:192–204. doi: 10.1002/1098-1136(200011)32:2<192::aid-glia80>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 12.DeMarco PJ, Jr, Katagiri Y, Enzmann V, Kaplan HJ, McCall MA. An adaptive ERG technique to measure normal and altered dark adaptation in the mouse. Doc Ophthalmol. 2007;115:155–163. doi: 10.1007/s10633-007-9078-5. [DOI] [PubMed] [Google Scholar]
- 13.Fleisch VC, Schonthaler HB, von Lintig J, Neuhauss SC. Subfunctionalization of a retinoid-binding protein provides evidence for two parallel visual cycles in the cone-dominant zebrafish retina. J Neurosci. 2008;28:8208–8216. doi: 10.1523/JNEUROSCI.2367-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rushton WA. Visual adaptation. Proc R Soc Lond B Biol Sci. 1965;162:20–46. doi: 10.1098/rspb.1965.0024. [DOI] [PubMed] [Google Scholar]
- 15.Feathers KL, Lyubarsky AL, Khan NW, Teofilo K, Swaroop A, Williams DS, Pugh EN, Jr, Thompson DA. Nrl-knockout mice deficient in Rpe65 fail to synthesize 11-cis retinal and cone outer segments. Invest Ophthalmol Vis Sci. 2008;49:1126–1135. doi: 10.1167/iovs.07-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenzel A, von Lintig J, Oberhauser V, Tanimoto N, Grimm C, Seeliger MW. RPE65 is essential for the function of cone photoreceptors in NRL-deficient mice. Invest Ophthalmol Vis Sci. 2007;48:534–542. doi: 10.1167/iovs.06-0652. [DOI] [PubMed] [Google Scholar]
- 17.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, Pang JJ, Sumaroka A, Windsor EA, Wilson JM, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi G, Yau KW, Chen J, Kefalov VJ. Signaling properties of a short-wave cone visual pigment and its role in phototransduction. J Neurosci. 2007;27:10084–10093. doi: 10.1523/JNEUROSCI.2211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heikkinen H, Nymark S, Koskelainen A. Mouse cone photoresponses obtained with electroretinogram from the isolated retina. Vision Res. 2008;48:264–272. doi: 10.1016/j.visres.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Nymark S, Haldin C, Tenhu H, Koskelainen A. A new method for measuring free drug concentration: retinal tissue as a biosensor. Invest Ophthalmol Vis Sci. 2006;47:2583–2588. doi: 10.1167/iovs.05-1116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01