Deficiency of type I IFN receptor in lupus-prone NZM 2328 mice decreases dendritic cell numbers and activation and protects from disease (original) (raw)
. Author manuscript; available in PMC: 2010 Nov 1.
Published in final edited form as: J Immunol. 2009 Oct 7;183(9):6021–6029. doi: 10.4049/jimmunol.0803872
Abstract
Type I interferons are potent regulators of innate and adaptive immunity and are implicated in the pathogenesis of systemic lupus erythematosus. Here we report that clinical and pathological lupus nephritis and serum anti-nuclear antibody levels are greatly attenuated in NZM 2328 mice deficient in type I IFN receptors (IFNAR). To determine if the inflammatory environment in NZM 2328 mice leads to IFNAR-regulated changes in dendritic cells (DC), the number, activation, and function of DC subsets were compared in 2 and 5 month-old (clinically healthy) female NZM and NZM-IFNAR-/- mice. Numbers of activated CD40hi plasmacytoid DC (pDC) were significantly increased in renal lymph nodes of 2 month-old NZM but not NZM-IFNAR-/- mice, suggesting an early IFNAR-dependent expansion and activation of pDC at disease sites. Relative to NZM spleens, NZM-IFNAR-/- spleens in 5 month-old mice were significantly decreased in size and contained reduced numbers of conventional DC (cDC) subsets, but not pDC. Splenic and renal lymph node NZM-IFNAR-/- DC analyzed directly ex vivo expressed significantly less CD40, CD86 and PDL1 than NZM DC. Upon activation with synthetic TLR9 ligands in vitro, splenic NZM-IFNAR-/- DC produced less IL-12p40/70 and TNFα than NZM DC. The limited IFNAR-/- DC response to endogenous activating stimuli correlated with reduced numbers of splenic activated memory CD4+ T cells and CD19+ B cells in older mice. Thus, IFNAR signaling significantly increases DC numbers, acquisition of antigen presentation competence, and pro-inflammatory function prior to onset of clinically apparent lupus disease.
Keywords: Systemic lupus erythematosus, dendritic cells, autoimmunity, cell activation, cell differentiation
Introduction
Type I interferons (IFNs), including multiple IFNα species, IFNβ and certain other IFNs, are potent regulators of both innate and adaptive immunity (1). Acting via the two-chain IFNα/β receptor (IFNAR), type I IFNs modulate the differentiation, proliferation, and survival of B cells, T cells, NK cells, macrophages, and dendritic cells (DC) as well as their respective cytokine production and signaling responses (1).
DC are critical for both innate and adaptive immunity, and can be divided into plasmacytoid DC (pDC) and conventional DC (cDC) populations. Following initial activation of pDC or cDC through either TLR-dependent or TLR-independent pathways, the IFN produced can act in an autocrine-paracrine manner via IFNAR to amplify the ongoing proinflammatory response (2, 3). Thus, IFNAR expression in both pDC and cDC is likely essential for the strength and duration of inflammatory responses under both physiologic and pathophysiologic conditions. Indeed, Type I IFNs and IFNAR increase the functional competence of DC, thus promoting adaptive T cell and B cell immunity (4-9).
It is well established that DC production of, and responses to, type I IFNs are central to effective antiviral immune responses (10). However, dysregulation of type I IFN-mediated pathways involving DC can also contribute to the development of systemic autoimmunity, including systemic lupus erythematosus (SLE), in susceptible hosts (10). SLE patients harbor elevated serum levels of IFNα, and their blood leukocytes show increased expression of type I IFN-regulated genes (11-14).
Consistent with the role of type I IFN in DC survival and activation, abnormalities in DC numbers, phenotype, and function have been identified in human SLE (15-19). SLE patients harbor activated myeloid DC and monocytes in peripheral blood, likely due to abundant IFN produced by pDC consequent to chronic TLR signaling triggered by endogenous TLR7 and/or TLR9 ligands (20-22). Increased DC activation and expression of type I IFN-regulated molecules likely contribute to the breach of lymphocyte self tolerance (13, 14, 21, 23). Moreover, elevated splenic and bone marrow (BM) DC numbers and activation state are features of SLE-prone mice, including the [NZB×NZW]F1 and NZM 2328 strains, suggesting a dysregulation of the type I IFN pathways in DC in these mice (24-31).
Prior studies of IFNAR deficiency in strains of mice with incomplete SLE phenotypes (B6.Nba2, [B6.Nba2×NZW]F1, 129×C57BL/6/lpr, or NZB), or in chemically-induced SLE in mice bearing a 129Sv background, pointed to a contribution for IFNAR signaling in autoantibody production and development of renal disease (32-35). In stark contrast, it is the absence of IFNAR signaling in MRL/lpr mice that heightens autoantibody production and end-organ disease (36). These conflicting results highlight the need to more fully assess the role for IFNAR in development and progression of SLE, and to determine how IFNAR deficiency alters numbers or function of specific immune cells during SLE.
The (NZB×NZW)F1-derived recombinant inbred NZM 2328 line is a spontaneous SLE model resembling the human disease more closely than other models (37). In the present work, we have generated IFNAR-deficient NZM 2328 mice to assess the role for the IFNAR in SLE disease manifestations and immune dysfunction. We herein report that development of SLE nephritis and pathogenic autoantibodies are greatly attenuated in female NZM 2328 mice lacking IFNAR. Concurrently, DC numbers and their antigen-presentation competence and proinflammatory function are considerably reduced prior to the onset of clinical disease, implicating IFNAR engagement on DC as a vital process in the development of SLE.
Materials and Methods
Mice
Mice were maintained at the University of Southern California (Los Angeles, CA), and the experiments were approved by the Institutional Animal Care and Use Committee. Mice lacking the α-chain of the IFNα/β receptor (IFNAR1, encoded by the Ifnar1 gene located on the distal segment of chromosome 16) on the 129/SvEv background were kindly provided by Dr. Moskophidis from the Medical College of Georgia (38). These IFNα/β receptor (IFNAR) deficient mice were backcrossed onto the SLE-prone NZM 2328 mice using a marker assisted selection protocol as described (39). The studies presented herein used homozygous IFNAR knock-out (KO) mice of at least the N6 generation of backcross, at which time the mice showed the NZM genotype at all markers tested. Genomic DNA extracted from tail clippings was PCR-amplified with the primers: 5’-AAGATGTGCTGTTCCCTTCCTCTGCTCTGA-3’ and 5’-ATTATTAAAAGAAAAGACGAGGCGAAGTGG-3’ for 30 cycles at 94°C for 1 min, 57°C for 1 min, and 72°C for 2 min. The band size for the intact IFNAR gene fragment is 150bp; whereas the band size for the disrupted IFNAR gene is 1.3kb.
IFNα treatment
Aliquots of recombinant adenovirus containing the INFα subtype 5 under the control of the CMV promoter/enhancer was used in vivo as described (40) with minor modifications. Female mice 8-9 weeks of age were injected once in the tail vein with 1-2×109 IFNα adenovirus particles. Negative control mice received AdCMVpLpA vector lacking a cDNA insert at 109 virus particles per i.v. injection.
Serum autoantibody concentrations
Sera were assayed for levels of IgG anti-chromatin, anti-histone, and anti-dsDNA autoantibodies by ELISA as described with minor changes (41). Positive control sera from 5 female, 36 to 38 week old, (NZB×NZW)F1 mice were combined together and assayed at 1:200 dilution on each plate, and the average OD of these sera for each autoantigen was arbitrarily set at a dilution value of 100 (U). Values for the test sera were calculated as (ODserum/ODcontrol) X 100. In contrast to IgG autoantibodies, IgM autoantibody levels do not correlate with lupus development in this model system (39, 41).
Assessment of nephritis
The development of proteinuria was assessed twice weekly using Albustix assay strips (Bayer, Elkhart, IN) using a scale of 0 to +4. Severe proteinuria was defined as >300mg/dl (3+ or more) on two consecutive examinations. For the assessment of renal histology, paraffin sections (3μm) were stained with hematoxylin and eosin (H&E), and periodic acid-Schiff (PAS) and were scored in a blinded fashion. Lupus nephritis was classified histopathologically according to the scheme developed by the World Health Organization (WHO) and widely used in human SLE.
Analyses of cells by flow cytometry
For analyses of DC, spleens and lymph nodes were digested to a single cell suspension with collagenase type D (Roche) (1 mg/ml) and DNAse I (Roche) (0.1 mg/ml) in Hank's Balanced Salt Solution at 37°C for 30 min, after which red cells (in spleen) were lysed. BM cells were released from the spine and red cells lysed. After washing, cells were immediately processed for flow cytometry as described (42). To detect DC subsets and activation status, cells directly isolated from the spleen, lymph nodes or BM were preincubated with anti-CD16/32, and labeled with optimally titered mAbs in FACS buffer (PBS, 5% newborn calf serum, 0.1% sodium azide). Fluorochrome- or biotin labeled mAbs specific for CD11c, CD11b, B220, Ly6C, CD8α, F4/80, CD19, CD115 and activated caspase-3 (rabbit IgG) were obtained from BD Biosciences. PE-labeled mAbs specific for CD40, CD86, PDL1, MHCII (M5/114.15.2), and PDCA-1 were obtained from eBioscience or Miltenyi. PE- or FITC-conjugated streptavidin was used to detect the biotinylated mAb. Samples were run on a FACSCalibur or LSRII instrument and data analyzed with FlowJo software.
For lymphocyte subset analyses, mouse spleen mononuclear cells, obtained after mechanical disruption and red cell lysis, were stained with combinations of fluorochrome-conjugated mAb specific for murine CD3, CD4, CD5, CD8, CD11b, CD19, CD21, CD23, CD44, CD62L, CD69, NK1.1, IgM, or IgD (BD Pharmingen and eBioscience) and analyzed by flow cytometry.
Analyses of DC production of cytokines after TLR9 stimulation
To detect production of IL-12 p40/p70 in DC subsets, splenocytes or DC from BM cultures were activated for 16-18 hr directly ex vivo in the presence of brefeldin A (for the final 10-12 hr) with 5 μg/ml CpG-B oligodeoxynucleotides (ODN) (Integrated DNA Technologies, Coralville, IA) prior to using a combination of mAbs for cell surface markers and intracellular cytokine staining with anti-IL-12p40/p70 mAb (C15.6) (BD Biosciences). To detect production of TNFα, splenocytes were activated for 6 hr in the presence of brefeldin A prior to intracellular staining with anti-TNFα mAb (MIP6-XT22) (BD Biosciences).
Cytokine-driven cultures models of DC differentiation
BM cells were cultured in RPMI 1640 with 10% FCS (Omega Scientific, Tarzana, CA), 2 mM glutamine, 100U penicillin/0.1 mg streptomycin/ml, 10 mM HEPES buffer, 50 μM 2-ME, and 1 mM sodium pyruvate. In the GM-CSF-driven model of DC differentiation, cultures were set up using total BM cells (5×105 cell/ml), including red cells, as described (42). Cells were harvested on day 7 and analyzed by flow cytometry for DC surface markers. GM-CSF induced DC were activated for 16 hr from day 6-7 with 5 μg/ml CpG-B ODN. In the Flt3 Ligand (FL)-driven culture model, DC were generated from total BM cells (after red cell lysis) at 1-3×106/ml in cultures supplemented with FL-IgGFc (400 ng/ml) as described (43). Cells were harvested on day 7–9, counted and analyzed by flow cytometry. FL-induced DC were activated by incubation for 16 hr with 10 μg/ml CpG-A ODN (Coley Pharmaceutical Group) (44).
Statistical analyses
Analyses were performed using SigmaStat software (SPSS, Chicago, IL) or SAS (version 9.1; SAS Institute, Inc., Cary, NC). When necessary (Fig. 6A-F, Fig. S2, C-D), raw results were log-transformed to achieve normality and/or to satisfy the equal variance test. Mean fluorescence intensity (MFI) was evaluated as relative MFI obtained by dividing an individual MFI value by the WT (Control) mean of a specific experiment. Relative MFI is a normalizing method producing an MFI rate. Data were summarized using means and standard errors and displayed through box plots and bar charts. Parametric testing between two groups was performed by the t test, and parametric testing among three groups was performed by one-way ANOVA. When combining experiments for analysis, a two-way ANOVA was performed. These data were summarized using least square means due to the unbalanced data. Survival data were analyzed by the log rank test with multiple comparisons by the Holm-Sidak method. Results were considered statistically significant when p < 0.05.
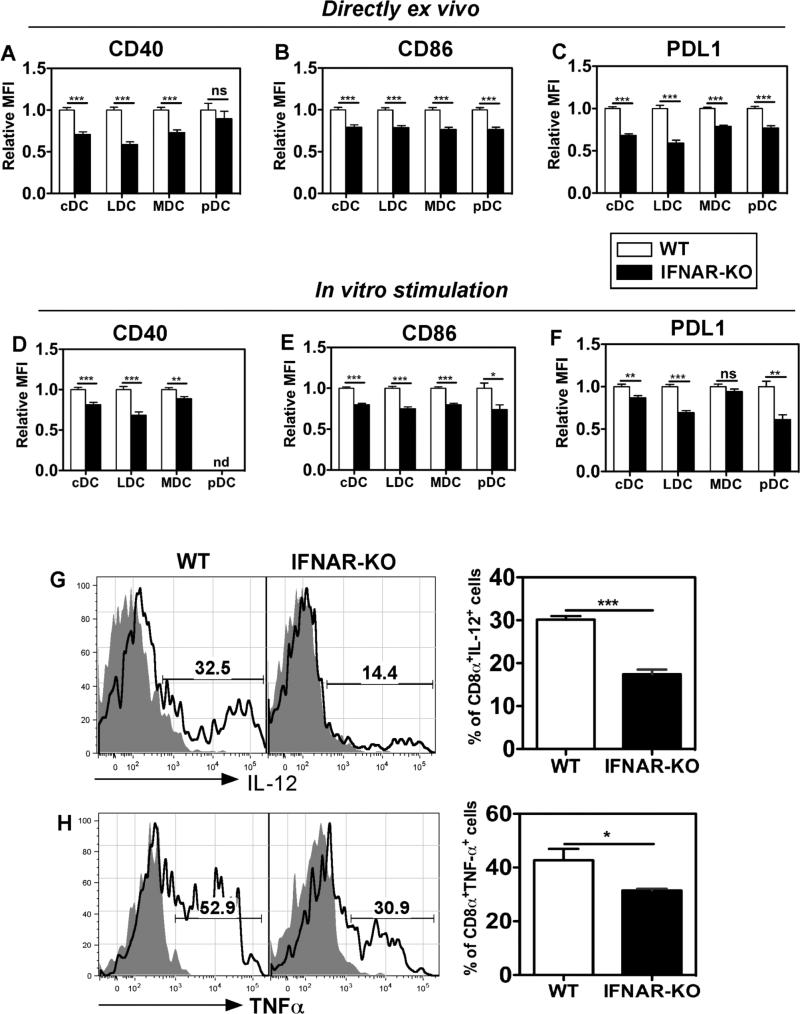
Fig. 6. IFNAR-/- DC are less activated in vivo and show a reduced capacity for activation upon TLR9 stimulation in vitro.
(A-C) Splenic DC from IFNAR-KO mice were less activated in vivo. Splenic DC subsets, as gated in Fig. S1, were analyzed directly ex vivo for surface expression of (A) CD40 (B) CD86 and (C) PDL1. (D-F) Splenic DC from IFNAR-KO mice showed less upregulation of costimulatory molecules after in vitro activation by TLR9 ligands. Splenic DC were stimulated for 16 hr in vitro with CpG-B ODN (5 μg/ml) and DC subsets analyzed for expression of (D) CD40, (E) CD86 and (F) PDL1. For panels A-F, shown are mean + SEM of relative MFI values of each costimulatory molecule on the indicated splenic DC subsets in WT (open bar) or IFNAR-KO (closed bar) mice. The data were compiled from 3-4 independent experiments, each with groups of 4-5 mice (20 weeks of age), using a statistical normalization method. Raw data were log-transformed to achieve normality and/or to satisfy the equal variance test. *p<0.05; **p<0.01; ***p<0.001; nd, not determined. (G) IFNAR-KO LDC produced less IL-12p40/70 than WT LDC. Splenocytes were activated in vitro for 18 hr with CpG-B ODN (5 μg/ml), with brefeldin A added for the last 10 hr, prior to detection of IL-12+ LDC by surface and intracellular staining. Shown are representative histograms of IL-12p40/70 expression (black line) as compared to unstimulated cells (shaded histogram), and the mean + SEM values for WT and IFNAR-KO DC, n=4-5; ***p<0.0001. Data are representative of 3 independent experiments. (H) IFNAR-KO LDC produced less TNFα than WT LDC. Splenocytes were activated in vitro for 6 hr with CpG-B ODN (5 μg/ml), in the presence of brefeldin A, prior to detection of TNFα+ LDC. Shown are representative histograms of TNFα expression (black line) as compared to unstimulated cells (shaded histogram), and the mean + SEM values for WT and IFNAR-KO DC, n=4; *p=0.039. Data are representative of 2 independent experiments.
Results
Generation of NZM 2328 mice unresponsive to IFNα
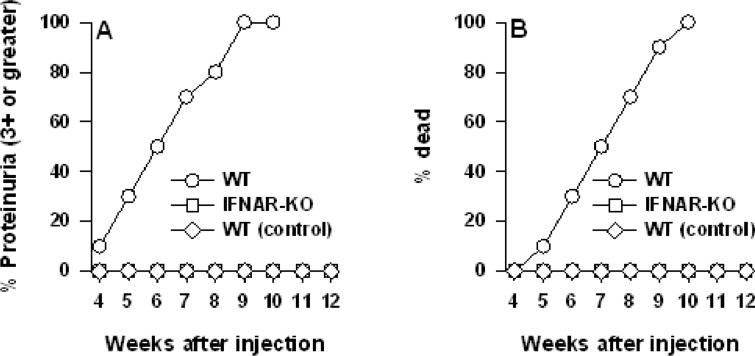
We have transferred the IFNAR1 null mutation into the lupus susceptible (NZB×NZW)F1-derived recombinant inbred line NZM 2328. To demonstrate that IFNAR-/- NZM 2328 mice are functionally incapable of responding to IFNα in vivo, a replication-deficient mouse rIFNα adenovirus was administered to wild-type (WT) or IFNAR-/- (IFNAR-KO) female mice at 8-9 weeks of age, before any autoimmune phenotype was evident. As negative controls, mice were injected with PBS or with the adenovirus vector lacking IFNα cDNA. As shown previously in (NZB×NZW)F1 mice (40), WT mice developed severe proteinuria within 3-6 weeks after injection of the IFN-expressing adenovirus and died within 6-10 weeks (Fig. 1). In contrast, IFNAR-/- mice remained completely healthy during a follow up period of 12 weeks after injection. These results conclusively confirm the inability of these IFNAR-/- NZM 2328 mice to respond to IFNα in vivo.
Fig. 1. In vivo adenovirus-mediated delivery of mIFNα fails to induce proteinuria and death in NZM 2328 mice deficient in IFNAR.
8-9 week old WT NZM 2328 female mice (n=16) and IFNAR deficient (IFNAR-KO) NZM 2328 mice (n=13) were injected with IFNα-producing adenovirus as described in M&M. A third group of WT female mice (n=7) labeled “control” were injected with an adenovirus lacking the IFNα construct. Data are presented as the percent cumulative prevalence of severe (+3, >300mg/dl) proteinuria and as percent cumulative mortality at the ages shown.
IFNAR deficiency attenuates disease development in NZM 2328 mice
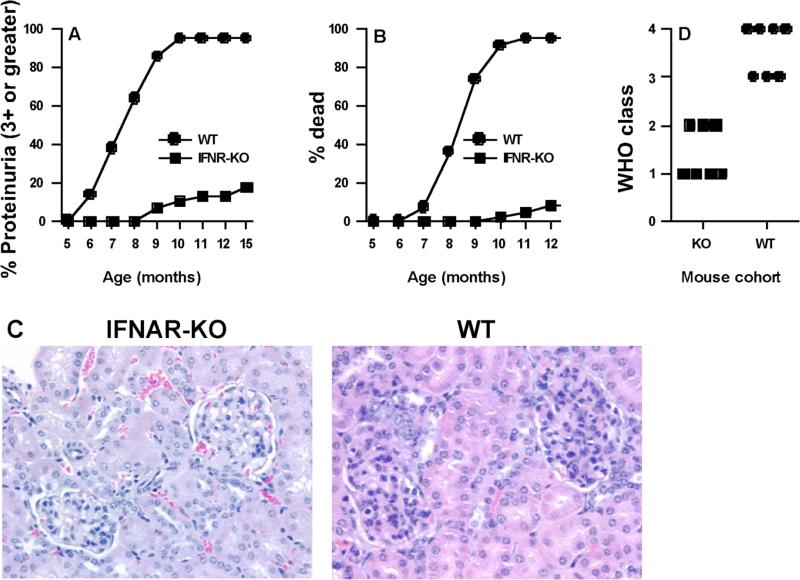
NZM 2328 females began developing severe proteinuria at 6 months of age and began dying 1-2 months later, with ~95% mortality by 10-11 months (p<0.001, Fig. 2A-B). In contrast, >90% of IFNAR-/- females were alive at 12 months of age, and >80% were disease-free at 15 months of age. Histological examination of kidney sections showed milder GN in IFNAR-/- females than in age matched WT female mice (Fig. 2C-D).
Fig. 2. IFNAR deficiency protects NZM 2328 mice from proteinuria, kidney pathology, and mortality.
Shown are cumulative (A) proteinuria and (B) mortality of NZM 2328 WT and IFNAR-deficient (KO) female mice with age. A total of 21-24 mice for each group were monitored up to 12 mo of age. Data are presented as the percent cumulative prevalence of severe (+3, >300mg/dl) proteinuria and as percent cumulative mortality at the ages shown. (C) A representative H&E stained kidney section (×400) from an 11 mo old IFNAR-deficient female mouse (Left) showing mild glomerular infiltration, is compared to an 8.5 mo old WT NZM 2328 female mouse (Right) showing marked glomerular hypercellularity. (D) Quantification of GN was done using the WHO scoring system (class 1 to class 4). IFNAR-KO mice at 10-12 months of age were compared to WT mice at 7-9 months of age. Please note that WT of older age were not available because these mice die by this age.
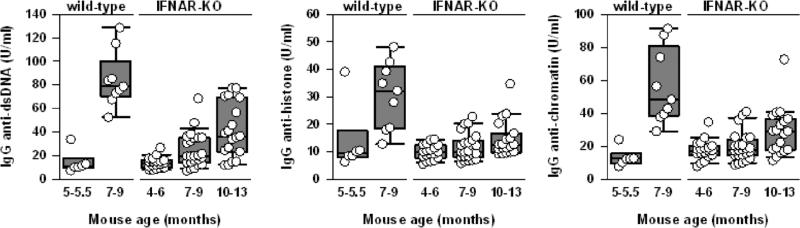
By 7-9 months of age, IFNAR-/- mice showed significant reductions in circulating levels of IgG anti-dsDNA, anti-histone, and anti-chromatin autoantibodies in comparison to corresponding levels in WT (p<0.001 for each comparison, Fig. 3). Indeed, even at 10-13 months of age, circulating autoantibody levels were much lower in IFNAR-/- mice than in WT mice (p<0.001 for each comparison).
Fig. 3. Delayed production of IgG autoantibodies in IFNAR-deficient mice.
Comparison of serum IgG anti-dsDNA, anti-histone, and anti-chromatin Ab levels in WT NZM 2328 mice and IFNAR-deficient female mice at the indicated ages. The composite results are plotted as box plots, with median (line inside box), 25th and 75th percentiles (box boundaries) and 10th and 90th percentiles (bars extending from boxes) indicated. All three types of autoantibodies are significantly higher in 7-9 month old WT mice compared to IFNAR-/- mice at 7-9 or 10-13 months of age with p<0.001.
Young NZM 2328 mice show an IFNAR-dependent increase in activated pDC only in renal lymph nodes
Altered numbers of pDC and cDC have been found in blood and target organs of SLE patients (15-19, 22, 45). In addition, most lines of lupus-prone mice harbor elevated numbers of activated DC as disease progresses with age, although this has not been linked to IFNAR function (24-31). One important question is whether DC in lupus-prone mice are activated in young mice prior to disease onset, which would suggest that intrinsic defects in DC contribute to lupus susceptibility. Indeed, in previous studies of 2 month old lupus-prone B6.Sle3 or NZM 2410 mice, splenic DC displayed elevated levels of costimulatory molecules, although their numbers were not increased (24, 25).
To determine if DC were activated or increased in number in young healthy NZM 2328 mice in an IFNAR-dependent manner, we used flow cytometry to analyze myeloid cells in the spleen, renal lymph nodes (rLN) and BM of 2 month old WT NZM and NZM IFNAR-/- mice. In these mice, numbers of total cells, cDC, pDC, monocytes and macrophages in the spleen and BM were not different, and DC and macrophage populations in the spleen did not show evidence of activation, as assessed by costimulatory molecule expression (HA, EC and SK, data not shown).
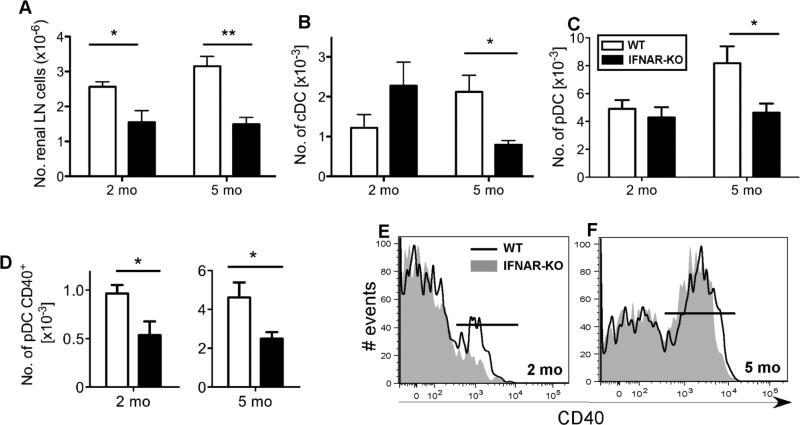
In contrast, the total number of rLN cells was significantly reduced in 2 month old IFNAR-/- mice relative to WT mice (Fig. 4A). While total numbers of cDC (CD11chi B220−) and pDC (CD11cint B220+ PDCA-1+) in rLN were not different (Fig. 4B-C; Fig. S1), rLN in IFNAR-/- mice did harbor a reduced percentage and number of activated CD40hi pDC (Fig. 4D-E). These data show that prior to activation of DC in other lymphoid organs, pDC in rLN are preferentially activated, via an IFNAR-dependent pathway. This local pDC activation, as well as the general increase in rLN cell numbers, likely reflects early IFNAR-dependent renal specific disease activity that occurs prior to onset of more systemic disease in NZM mice.
Fig. 4. Two and five month old NZM 2328 mice show an IFNAR-dependent increase in activated pDC in renal lymph nodes.
(A) IFNAR deficiency decreases the total number of rLN cells. rLN were digested with collagenase and total viable cell numbers determined by hemocytometer counts. (B) Numbers of rLN cDC are reduced in 5 month old IFNAR-KO mice. (C) Numbers of rLN pDC (gated as shown in Fig. S1) are reduced in 5 month old IFNAR-KO mice. (D) Numbers of activated CD40hi pDC are reduced in 2 and 5 month old IFNAR-KO mice. For panels A-D, shown are the mean + SEM values for WT (open bar) and IFNAR-KO (closed bar) cells, n=5; *p<0.05, **p<0.01. (E) The percentage of rLN pDC expressing high levels of CD40 is shown for representative 2 month old WT (18%, black line) and IFNAR-KO (10%, shaded gray) mice. (F) The percentage of rLN pDC expressing high levels of CD40 is shown for representative 5 month old WT (57%, black line) and IFNAR-KO (56%, shaded gray) mice.
By 5 months of age, numbers of total cells, cDC and pDC in rLN were significantly increased, relative to numbers at 2 months of age, in WT but not IFNAR-/- mice (Fig. 4A-C). An increase in numbers of activated CD40hi pDC was observed in rLN of both WT and IFNAR-/- mice, although the numbers continued to be significantly higher in WT mice (Fig. 4D). A similar expansion of activated pDC was not found in cutaneous LN (SB and SK, data not shown), suggesting that pDC preferentially accumulate proximal to active disease sites.
Numbers of splenic conventional DC, but not plasmacytoid DC, are reduced in 5 month old IFNAR-deficient NZM 2328 mice
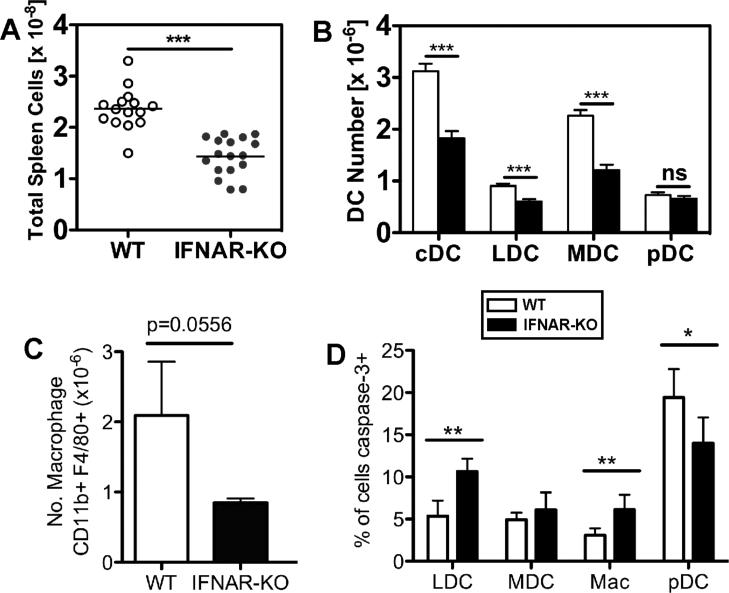
We also quantified splenic DC subsets in ~5 month old mice, prior to their development of severe proteinuria or elevated serum autoantibodies. Relative to WT NZM 2328 spleens, which are notably larger than age-matched healthy C57BL/6 spleens (HA and SK, data not shown), NZM IFNAR-/- spleens contained significantly fewer cells, (p<0.0001, Fig. 5A). These data suggest that 5 month old NZM 2328 IFNAR-/- mice do not have the splenomegaly often observed in lupus-prone mice.
Fig. 5. Reduction of numbers of total splenocytes, splenic macrophages, and splenic cDC, but not pDC, in 5 month old IFNAR-/- mice.
(A) IFNAR deficiency reduces the splenomegaly characteristic of NZM 2328 mice. Spleens were digested with collagenase, red cells lysed and total viable cell numbers determined by hemocytometer counts. Shown are the values for individual 5 month old mice with the mean value indicated by the bar. The mean + SEM for WT spleens was 2.36 + 0.104 ×108, n=15 and for IFNAR-KO spleens was 1.43 + 0.089 ×108, n=17, ***p<0.0001. (B) Numbers of splenic cDC but not pDC were reduced in IFNARKO mice. Splenic DC subsets were identified using multi-parameter flow cytometry according to the gating scheme shown in Fig. S1. Shown are the mean + SEM numbers of each DC subset, n=15-17; ***p<0.0001; ns, not significant. (C) Numbers of splenic macrophages tend to be reduced in IFNAR-KO mice. Shown are the mean + SEM, n=5; p=0.0556. (D) IFNAR deficiency alters the percentages of myeloid cells that express activated caspase-3. Shown are the mean + SEM of each cell subset, n=5; *p<0.05, **p<0.01.
Conventional CD11chi B220− MHCII+ DC (cDC) were subdivided into CD8α+ lymphoid (LDC) or CD8α− myeloid (MDC), while pDC were identified as CD11cint B220+ PDCA-1+ (Fig. S1). Relative to WT spleens from 5 month old mice, IFNAR−/− spleens contained reduced numbers of cDC (MDC and LDC) (Fig. 5B), although the frequency of cDC was identical in WT and IFNAR-/- mice, 1.3% vs. 1.2% of total live splenocytes in this example (Fig. S1). The ratio of MDC to LDC was not altered. The ~40% reduction in cDC numbers was comparable to the reduction in total splenocyte numbers. In contrast, the frequency of pDC was elevated in IFNAR-/- mice (in this example, 0.84% vs. 0.4% of total live splenocytes), with the result that total pDC numbers did not differ significantly in the two lines of mice (Fig. 5B, Fig. S1). Numbers of splenic CD11b+ F4/80+ CD11clo macrophages tended to decrease in the IFNAR-/- mice, although this difference did not quite reach significance (p=0.0556) (Fig. 5C). These data show that IFNAR-mediated events lead to increased numbers of splenic cDC, but not pDC, prior to onset of overt clinical disease or accumulation of autoantibodies. This contrasts with previous observations in mice bearing a non-autoimmune 129Sv background, in which no differences in organ size or the frequency of DC in various organs in IFNAR-/- and WT mice were reported (6, 46, 47). Taken together, these data suggest that in healthy 129Sv mice, maintenance of homeostatic DC numbers does not require IFNAR, while in lupus-prone NZM 2328 mice, IFNAR promotes increased splenic and rLN DC numbers.
Analyses of cells in the BM showed a trend to decreased numbers of monocytes (CD115+ F4/80+) and cDC (CD11c+ CD11b+ CD19−), but not pDC (CD11c+ B220+ Ly6C+) or B cells (CD19+) in IFNAR-/- relative to WT mice (EC and SK, data not shown). These data suggest that IFNAR deficiency does not significantly alter numbers of mature cells in the BM.
NZM-IFNAR-/- DC are less activated in vivo
DC in SLE patients and murine models of lupus often show elevated costimulatory molecule expression (e.g. CD86, CD40), reflecting their increased activation state and hyperstimulatory capacity (17, 24, 25, 28, 31, 45). To determine the effects of IFNAR deficiency on the activation status of splenic DC in 5 month old mice, the DC subsets identified in Fig. 5 were assessed directly ex vivo (without culture or additional stimulation) for surface expression of the costimulatory molecules CD40, CD86, and PDL1 (Fig. 6A-C). Relative to WT DC, IFNAR-/- cDC subsets, especially LDC, showed decreased CD40 expression (~30-40% reduction in mean fluorescence intensity (MFI)), whereas pDC expression of CD40 was variable and not significantly different in the two lines of mice (Fig. 6A). IFNAR-/- cDC subsets (especially LDC) and pDC also showed decreased expression of CD86 and PDL1 (~20-40% reduction in MFI, Fig. 6B,C). Surface MHC class II levels on splenic DC, and CD86 levels on splenic macrophages were not different in the two lines of mice (HA and SK, data not shown). These data show that in 5 month old NZM 2328 mice, IFNAR promotes an activated splenic DC phenotype prior to development of overt clinical lupus. In contrast, pDC and cDC in 129Sv and 129Sv-IFNAR-/- mice did not differ in their low expression of CD40 or CD86 costimulatory molecules when analyzed directly ex vivo (46). Thus the difference in costimulatory molecule expression between NZM and NZM-IFNAR-/- DC is due to IFNAR regulated activation of DC in vivo by endogenous stimulators that arise even before clinical lupus develops in NZM mice.
IFNAR-deficiency alters DC apoptosis
The increase in splenic cDC numbers in 5 month old WT mice could be due to increased survival mediated by IFNAR signaling. Indeed, DC in B6.Sle3 mice were less apoptotic than WT B6 DC after a period of in vitro culture (25). Apoptosis is characterized by the accumulation of the active form of caspase-3 (48). In addition, activated caspase-3 levels decrease after DC stimulation by TLR ligands, thus facilitating endosomal trafficking that leads to new peptide-MHCII complex formation (49). Therefore, we hypothesized that DC in WT NZM mice might contain less activated caspase-3 if they are chronically activated and longer-lived than DC in IFNAR-/- mice. We used intracellular flow cytometry directly ex vivo to measure the percentage of DC and macrophages bearing activated caspase-3 (48). Relative to WT cells, a greater percentage (~2-fold) of IFNAR-/- LDC and macrophages contained active caspase-3 (Fig. 5D). These data are consistent with the reduced numbers of activated cDC in IFNAR-/- mice. In contrast, a greater percentage of WT pDC contained active caspase-3 (Fig. 5D). This may account for the absence of elevated pDC numbers in WT spleens, despite the increase in spleen size.
NZM-IFNAR-/- DC show a reduced capacity for activation upon TLR9 stimulation in vitro
Signaling through TLR7 and TLR9 by engagement of endogenous nucleic acid ligands may importantly contribute to the inflammatory state in SLE (21). Indeed, SLE patients have elevated serum levels of the pro-inflammatory cytokine IL-12 (50). Thus, we determined whether IFNAR expression in NZM DC influences TLR9-induced signaling pathways that lead to increased cell surface costimulatory molecules and production of inflammatory cytokines.
Splenocytes were incubated 16 hr in culture with the TLR9 ligand CpG-B ODN. Relative to WT DC, IFNAR-/- MDC, LDC, and pDC showed less increase in surface CD40, CD86, and PDL1 expression after activation with TLR9 ligands, with the most marked difference noted in LDC (Fig. 6D-F). Intracellular cytokine staining showed that a reduced percentage of IFNAR-/- LDC were capable of producing IL-12p40/p70 and TNFα (Fig. 6G,H); similar results were obtained with MDC (HA and SK, data not shown). IL-6 production by WT and IFNAR-/- cDC did not differ (HA and SK, data not shown). These data are consistent with earlier reports using 129Sv mice that IFNAR signaling promotes cDC and pDC activation in response to synthetic TLR9 ligands in vitro or in vivo (46, 47). Thus, in NZM 2328 mice, IFNAR affects the magnitude of DC activation and pro-inflammatory cytokine production in response to stimulation via TLR9, possibly contributing to the ability of DC to promote breaches in T cell tolerance.
NZM WT bone marrow cells have an IFNAR-dependent reduction in DC precursors responsive to FL and GM-CSF in vitro
Recent reports showed that IFNα acting via IFNAR stimulates the proliferation of normally quiescent BM hematopoietic stem cells (HSC) in vivo (51, 52). Chronic IFNα stimulation of HSC led to their depletion and compromised their function, including the ability to repopulate the BM of irradiated mice. Therefore, we hypothesized that elevated type I IFN and IFNAR signaling in NZM WT mice would lead to decreased numbers of functional HSC, which might reduce the frequency of DC precursors in BM.
We used two cytokine-driven (GM-CSF and FL) DC development models to study the differentiation potential of BM DC precursors in WT and IFNAR-/- mice. Since GM-CSF and FL regulate DC differentiation in vivo, these cytokine-driven culture models have typically been used to determine factors that modulate DC differentiation (53). Indeed, published reports assessing DC differentiation in these culture models showed that IFNα or IFNAR inhibited MDC differentiation in non-inflammatory conditions (4, 32, 54, 55).
The FL driven model yields pDC (CD11c+B220+PDCA-1+) and cDC (CD11c+B220-CD11b+ MDC and CD11c+B220−CD11blo/− LDC) (43). After differentiation of WT or IFNAR-/- BM cells for 8 days in FL-containing medium, numbers of total live cells in IFNAR-/- cultures were increased relative to WT cultures (Fig. S2A). The numbers of each DC subset also were significantly increased in IFNAR-/- cultures. DC in these cultures were not activated, as determined by their low level of CD40 and CD86 expression (Fig. S2C,D and data not shown). These data suggest that NZM WT mice have an IFNAR-dependent impairment in DC precursor activity, perhaps caused by elevated amounts of IFNα already present in 5 month old mice.
In contrast to effects on differentiation, IFNα can promote the survival of developed pDC in culture (4). Upon activation of DC in FL-driven cultures with the TLR9 ligand CpG-A ODN, the total numbers of live cells in IFNAR-/- BM cultures were reduced relative to WT cultures (Fig. S2B). Numbers of IFNAR-/- pDC and LDC were significantly reduced, while numbers of MDC were not significantly different (Fig. S2B). These data show that in the presence of activating stimuli, IFNAR signaling promotes increased numbers of DC, perhaps due to enhancement of cell survival.
We also studied how IFNAR deficiency impacted GM-CSF mediated DC differentiation from monocytes and myeloid precursors, a pathway important for generation of tissue DC during homeostasis and inflammation in vivo (53). The GM-CSF driven model yields myeloid (CD11c+CD11b+) DC, comprising ~50-80% of the cells in culture on day 7 (42). In the absence of activating stimuli, the total number of cells in culture on day 7 was higher in IFNAR-/- (mean + SE was 2.6 + 0.19 × 106, n=7) than WT (mean + SE was 1.7 + 0.22 × 106, n=5) cultures (p=0.014) (SB and SK, data not shown). Both the frequency and absolute numbers of MDC were increased in IFNAR-/- cultures (Fig. S2E), as we observed in FL driven cultures. These data are consistent with an IFNAR-dependent decrease in numbers of GM-CSF-responsive myeloid progenitors in NZM BM and published reports (4, 32, 54, 55).
IFNAR-/- DC in cytokine-driven BM cultures are less activated by TLR9 ligands
To compare the ability of DC derived from WT or IFNAR-/- BM cells in FL-driven cultures to become activated by TLR9 ligands, we assessed surface levels of CD40 and CD86 after incubation with CpG-A ODN. Relative to WT DC, IFNAR-/- MDC, LDC and pDC showed significantly reduced levels of CD40 or CD86 after incubation with TLR9 ligands, as we observed with splenic DC (Fig. S2C,D).
WT and IFNAR-/- DC from GM-CSF-driven cultures also were activated for 16 hr with the TLR9 ligand CpG-B ODN, followed by assessment of IL-12p40/70 production by intracellular flow cytometry. A greater percentage of WT DC produced IL-12p40/p70, consistent with a role for IFNAR signaling in IL-12 production (Fig. S2F). While baseline levels of surface MHCII, CD40, and CD86 did not significantly differ between the two DC populations, WT DC showed slightly higher expression of these molecules than IFNAR-/- DC (SB and SK_, data not shown_). These experiments with BM-derived MDC are consistent with our results with freshly isolated splenic DC in showing that IFNAR deficiency on the NZM 2328 background leads to reduced DC activation in response to synthetic TLR9 ligands, as well as the endogenous TLR9 ligands present in vivo.
IFNAR deficient NZM 2328 mice harbor reduced lymphocyte numbers, with a marked reduction in activated memory CD4+ T cells
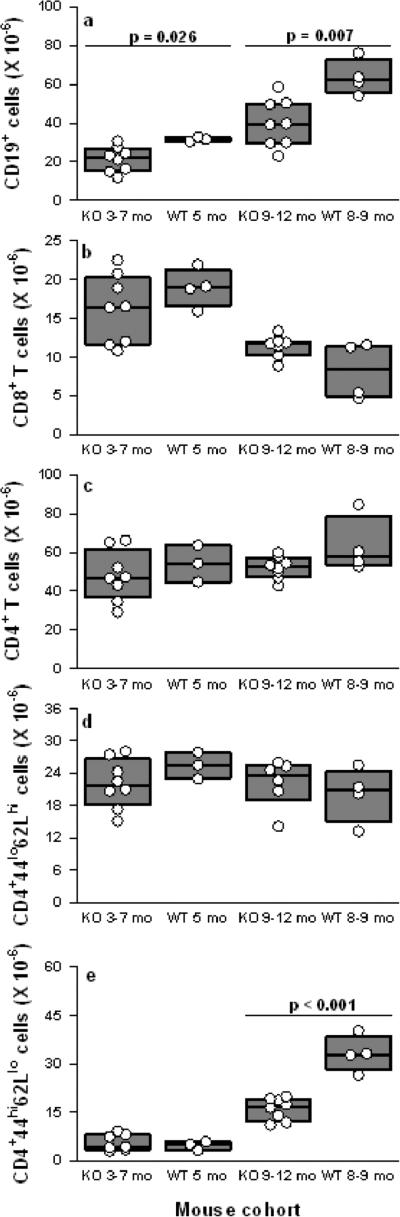
Increased numbers of activated DC promote autoreactive B and T cell responses in murine autoimmune models (25, 56). Autocrine IFNα and IFNβ acting via IFNAR enhance DC survival and activation, thereby increasing the ability of DC to stimulate adaptive T cell immunity and enhance humoral immunity by increasing B cell proliferation and Ig class switching (4-9). IFNAR signaling also may have direct effects in lymphocytes (57). To determine if IFNAR signaling and/or increased numbers of activated DC led to altered numbers or phenotypes of lymphocytes in NZM 2328 mice, we compared numbers of B and T cell subsets in WT and IFNAR-/- mice at 5 months of age prior to development of overt clinical disease, and at 8-12 months of age, when clinical disease is apparent in WT mice. At 5 months of age, WT mice had greater numbers of CD19+ B cells, (p=0.026, Fig. 7A), which included an increase in CD21int CD23+ follicular B cells (Fig. S3). However, at 5 months of age, no differences in numbers of CD4+ or CD8+ T cells were found between WT and IFNAR-/- mice (Fig. 7B,C). By 8-9 months, the number of CD19+ B cells was significantly increased in WT but not IFNAR-/- mice, (p=0.007, Fig. 7A), which included increased numbers of CD5+ B cells (Fig. S3). These increased B cell numbers in WT mice are consistent with the increased production of anti-nuclear autoantibodies that we also observed in WT mice at this age (Fig. 3).
Fig. 7. IFNAR deficiency prevents expansion of splenic CD19+ B cells and activated memory CD4+ T cells.
For B and T lymphocyte subsets, numbers shown are millions of cells per spleen of WT or IFNAR-KO mice at the indicated ages. Older (10-12 month) WT mice were not available because the majority had died by 9-10 months of age. The composite results are plotted as box plots. The lines inside the boxes indicate the medians; the outer borders of the boxes indicate 25th and 75th percentiles; the bars extending from the boxes indicate the 10th and 90th percentiles. Flow cytometry was used to identify (A) CD19+ B lymphocytes; (B) CD8+ T cells; (C) CD4+ T cells; (D) CD4+CD44loCD62Lhigh naive T cells and (E) CD4+CD44highCD62Llow/neg activated memory T cells. Significant differences between IFNAR-deficient and WT mice are indicated in the plots.
Notably, 8-9 month old WT mice had a significantly expanded population of activated memory CD4+ CD44hi CD62Llo T cells, which was not present in the IFNAR-/- mice (Fig. 7E). Numbers of naive CD4+ CD44lo CD62Lhi T cells were not different in WT and IFNAR-/- mice at either age 5 or 8-9 months (Fig. 7D). These data suggest that the increased numbers of activated DC observed at 5 months of age (prior to onset of clinical disease) in WT mice lead to the priming and subsequent maintenance of activated memory CD4+ T cells that ultimately fuel onset and maintenance of overt clinical disease.
Discussion
We have shown that IFNAR deficiency on the NZM 2328 background significantly reduces the incidence of lupus nephritis, autoantibody production, and mortality. Our analyses of DC prior to the onset of clinical disease in 2 and 5 month old NZM 2328 mice show that IFNAR signaling promotes increased numbers of activated pDC in the renal LN and cDC in the spleen. As disease progresses, these activated DC correlate with elevated numbers of activated effector CD4+ T cells and production of pathogenic autoantibodies. These data implicate IFNAR engagement in DC as a critical factor at early pre-clinical stages of lupus disease.
Increased numbers of DC bearing high levels of costimulatory molecules accumulate with age in most murine models of lupus, including the MRL/lpr, NZB, [NZB×NZW]F1, BXSB, [NZB×BXSB]F1, NZM 2328, NZM 2410, B6.Sle1 and B6.Sle3 strains (24-31). The increased DC numbers could be due to new DC differentiation or to extended DC survival mediated by endogenous TLR ligands and IFNAR signaling. New DC arise during inflammation after differentiation from inflammatory monocytes recruited to lymphoid organs and tissues (53). In addition, TLR9 activation induces lymphoid progenitors to switch their developmental program and differentiate to myeloid cells, including DC and macrophages (58). Interestingly, elevated numbers of activated DC, which accumulate due to increased new development or extended survival during chronic inflammation, have been shown to promote loss of self tolerance. An extended lifespan of activated DC can induce SLE-like autoimmunity in C57BL/6 mice, as shown by experiments that inhibited DC apoptosis after stimulation by TLR ligands in vivo (56, 59). Similarly, lupus disease in mice with multiple extra copies of the TLR7 gene was associated with elevated numbers of DC, consistent with chronic TLR7 signaling inducing new DC development or extended DC survival (60). Our data show that in the context of the inflammation and chronic TLR signaling in NZM 2328 mice, IFNAR likely acts via one of these mechanisms to promote the increased numbers of activated cDC and pDC. Interestingly, the effect of IFNAR on activated DC numbers is specific to lupus-prone mice, since this effect of IFNAR deficiency was not observed in 129Sv mice (47).
In young (2 month old) mice, we identified an IFNAR-dependent population of CD40hi pDC that preferentially accumulated in rLN; a general increase in rLN size was also IFNAR-dependent. This was notable since we did not observe populations of activated DC or macrophages in the spleen or BM at this age. These data suggest that an early event during pre-clinical stages of lupus nephritis is the recruitment of pDC to the kidney or rLN, leading to the accumulation of activated pDC at these sites. We provide evidence that IFNAR has an important role in this tissue-specific accumulation, consistent with reports that IFNAR signaling increases pDC activation, survival, and migration (4, 46). In human SLE patients, elevated numbers of pDC are observed in kidney or skin but not in blood, which also suggests that pDC are preferentially recruited to active disease sites (15, 50, 61, 62).
In 5 month old WT NZM mice, pDC numbers in rLN increased, yet splenic pDC numbers remained comparable in NZM WT and IFNAR-/- mice. The most likely explanation is that IFNAR signaling does promote pDC survival and activation in NZM 2328 mice, but the activated pDC are preferentially recruited to target tissues such as kidney and their draining LN as we have noted. It is also possible that activated splenic pDC are converted to cDC, as occurs during viral infection or upon in vivo exposure to type I IFN (63, 64).
In NZM 2328 mice, IFNAR promotes activated cDC and pDC phenotypes in vivo prior to development of severe lupus symptoms. In contrast, pDC and cDC in unstimulated 129Sv and 129Sv-IFNAR-/- mice did not differ in low expression of CD40 or CD86 costimulatory molecules (46). This difference in costimulatory molecule expression between NZM and NZM-IFNAR-/- DC is likely due to activation of DC in vivo by endogenous stimulators that occur as lupus begins to develop in NZM mice. Similar comparisons of NZM2410 to Balb/c mice (24) or B6.Sle1 and B6.Sle3 to B6 mice (25, 28) showed that DC in lupus-prone mice exhibit a hyperstimulatory phenotype prior to disease onset.
We found that IFNAR deficiency had a profound effect on activation of pDC and CD8α+ LDC. During homeostasis, LDC and pDC help to maintain T cell self tolerance (53, 65). Upon bacterial or viral infection, LDC are critical for priming of naive T cells. Activated pDC also may have a significant role in antigen presentation to T cells at sites of inflammation and in local LN (66). Thus, the IFNAR-regulated LDC and pDC activation that occurs prior to overt clinical disease is likely to contribute to the loss of T cell tolerance necessary to initiate the adaptive immune phase of lupus disease. Indeed, our analyses of 8-9 month old mice showed an expansion of activated memory T cells and marginal zone B cells in WT but not IFNAR-/- mice.
Our data with NZM 2328 mice are consistent with prior reports of the effect of IFNAR deficiency in spontaneous models with partial lupus phenotypes or in experimental lupus-like models. Deficiency of IFNAR in 129Sv mice inhibited autoantibody production, severe renal disease and expression of type I IFN-stimulated genes in a chemically induced (2,6,10,14-tetramethylpentadecane) lupus model (35). NZB mice lacking the IFNAR α chain had significantly reduced anti-erythrocyte autoantibodies, hemolytic anemia and anti-DNA-Abs (32). IFNAR-deficient B6.Nba2, [B6.Nba2×NZW]F1, and 129×C57BL/6/lpr mice showed decreased autoantibody production and renal disease and did not develop splenomegaly or lymphadenopathy (33, 34). We now show that disease protection in these IFNAR-deficient lupus models may occur because sustained DC activation does not occur, which prevents chronic lymphocyte responses. Paradoxically, IFNAR deficiency in MRL/lpr mice worsened lymphoproliferation, autoantibody production, and end-organ disease, suggesting that type I IFNs may suppress autoimmunity induced by Fas deficiency on the MRL genetic background (36). These distinct effects of IFNAR deficiency on lupus disease in different models could relate to the various mechanisms by which DC induce tolerance. While “immature” DC lacking costimulatory molecules or inflammatory cytokines promote tolerance by failing to activate lymphocytes, in some settings activated “mature” DC are required to induce T regulatory cells (67). Thus, it is possible that disease-suppressing T regulatory cells in MRL/lpr mice are dependent upon IFNAR signaling in DC, and this mechanism is less critical in NZM 2328 mice.
NZM 2328 mice exhibit a disease course and sex bias that most closely resembles human SLE. Thus, our finding that IFNAR deficiency decreases numbers of activated DC and reduces lupus severity and mortality provides additional rationale for human SLE therapy involving IFNAR blockade or anti-IFNα mAbs.
Supplementary Material
1
Supplementary Figure Legends
Fig. S1. Definition of DC subsets in the spleen and rLN using flow cytometry. (A-C) These panels show the strategy to identify pDC in the rLN. (A) Identification of B220+ cells. (B) B220+ cells contain a population of PDCA-1+ cells. (C) The majority of B220+ PDCA-1+ cells are CD11c+. (D-G) These panels show the gating strategy to identify splenic DC subsets. (D) After gating out autofluorescent cells, cDC were identified as CD11chi. In this representative example, the percentage of cDC in total live splenocytes was the same for WT (1.3%, gray solid line) and IFNAR-KO (1.2%, black dotted line). (E) CD11chi cells gated in panel C were divided into CD8α+ LDC and CD8α− MDC. (F,G) pDC within the fraction of CD11cint cells indicated in panel C were identified by coexpression of B220 and PDCA-1. In this representative example, the percentage of pDC in total live splenocytes in IFNAR-KO spleens (0.84%) (G) was ~2-fold higher than in WT spleens (0.4%) (F). Flow cytometry data were analyzed using FlowJo software and are shown in biexponential plots.
Fig. S2. IFNAR deficiency alters the differentiation and function of DC in cytokine-driven culture models. (A) Numbers of live cells and differentiated DC subsets were increased from IFNAR-KO BM, relative to WT BM, in non-inflammatory conditions. BM cells from WT or IFNAR-KO were cultured in FL for 8 days. The frequency of LDC, MDC and pDC were assessed using flow cytometry and absolute numbers of DC were calculated based on hemocytometer counts of harvested cells. Shown are data pooled from 2 experiments, n=4-5, *p<0.05. WT, open bars; IFNAR-KO, closed bars. (B) Upon activation by TLR9 ligands, numbers of live cells and DC subsets were decreased in cultures generated from IFNAR-KO BM. Cells on day 7 of culture were activated for 16 hr with CpG-A ODN, followed by assessment of the frequency and number of DC subsets. Shown are data pooled from 2 experiments, n=6-7, **p<0.01; ***p<0.001. (C,D) IFNAR-/- DC show less expression of surface CD40 and CD86 after activation by TLR9 ligands. Cells were activated for 16 hr with CpG-ODN, followed by assessment of (C) CD40 and (D) CD86 surface expression on DC subsets. Shown are representative flow cytometry data histograms (WT, gray solid line; IFNAR-KO, black dotted line; shaded histogram, unstimulated WT cells) and a compilation of normalized MFI values (WT, open bar; IFNAR-KO, closed bar). Raw data were log-transformed to achieve normality and/or to satisfy the equal variance test. CD40 data were pooled from 2 experiments, n=4-6, and CD86 data were pooled from 3 experiments, n=7-10, ***p<0.001. (E) IFNAR decreases the differentiation of myeloid DC in non-inflammatory conditions. BM cells from WT or IFNAR-KO mice were cultured in GM-CSF for 7 days. The frequency of CD11c+ CD11b+ DC was assessed using flow cytometry, and absolute numbers of DC were calculated based on hemocytometer counts of harvested cells. Data are representative of 4 independent experiments. Shown are the mean + SEM of values pooled from 2 experiments, n=5-7; ***p<0.0001; *p=0.02. (F) IFNAR promotes IL-12p40/70 production by DC. DC were stimulated for 16 hr with CpG-B ODN (5 μg/ml), with brefeldin A added for the last 12 hr. The presence of IL-12 in DC was assessed using surface marker and intracellular cytokine staining. Shown are the percentage of DC expressing IL-12p40/70, mean + SEM of values pooled from 2 experiments, n=8-9, ***p<0.0001.
Fig. S3. Effect of IFNAR deficiency on splenic B cell subsets. For B lymphocyte subsets, (A) CD21hi CD23lo B cells, (B) CD21int CD23+ follicular B cells, (C) CD5+ B cells, numbers shown are millions of cells per spleen of WT or IFNAR-KO mice at the indicated ages. The composite results are plotted as box plots. The lines inside the boxes indicate the medians; the outer borders of the boxes indicate 25th and 75th percentiles; the bars extending from the boxes indicate the 10th and 90th percentiles.
Abbreviations
BM
bone marrow
cDC
conventional DC
DC
dendritic cell
FL
Flt3 Ligand
GN
glomerulonephritis
IFNAR
type I IFN receptor
IFNAR-KO
IFNAR knockout mice
LDC
lymphoid DC
MDC
myeloid DC
MFI
mean fluorescence intensity
ODN
oligodeoxynucleotide
pDC
plasmacytoid DC
rLN
renal lymph node
SLE
systemic lupus erythematosus
Footnotes
1
This work was supported by the Arthritis National Research Foundation (to SK and COJ), the Arthritis Foundation OK Chapter (to SK) and NIH grants R01 AR050193 (to WS) and R01 AI057473 (to COJ).
References
- 1.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 2.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 3.Honda K, Takaoka A, Taniguchi T. Type I interferon gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192:219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585–589. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 6.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 7.Luft T, Pang KC, Thomas E, Hertzog P, Hart DN, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- 8.Le Bon A, Schiavoni G, D'Agostino G, Gresser I, Belardelli F, Tough DF. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 9.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 11.Hooks JJ, Jordan GW, Cupps T, Moutsopoulos HM, Fauci AS, Notkins AL. Multiple interferons in the circulation of patients with systemic lupus erythematosus and vasculitis. Arthritis Rheum. 1982;25:396–400. doi: 10.1002/art.1780250406. [DOI] [PubMed] [Google Scholar]
- 12.Hooks JJ, Moutsopoulos HM, Geis SA, Stahl NI, Decker JL, Notkins AL. Immune interferon in the circulation of patients with autoimmune disease. N Engl J Med. 1979;301:5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 13.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cederblad B, Blomberg S, Vallin H, Perers A, Alm GV, Ronnblom L. Patients with systemic lupus erythematosus have reduced numbers of circulating natural interferon-alpha- producing cells. J Autoimmun. 1998;11:465–470. doi: 10.1006/jaut.1998.0215. [DOI] [PubMed] [Google Scholar]
- 16.Decker P, Kotter I, Klein R, Berner B, Rammensee HG. Monocyte-derived dendritic cells over-express CD86 in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2006 doi: 10.1093/rheumatology/kel061. [DOI] [PubMed] [Google Scholar]
- 17.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 18.Zhuang H, Narain S, Sobel E, Lee PY, Nacionales DC, Kelly KM, Richards HB, Segal M, Stewart C, Satoh M, Reeves WH. Association of anti-nucleoprotein autoantibodies with upregulation of Type I interferon-inducible gene transcripts and dendritic cell maturation in systemic lupus erythematosus. Clin Immunol. 2005;117:238–250. doi: 10.1016/j.clim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Ding D, Mehta H, McCune WJ, Kaplan MJ. Aberrant phenotype and function of myeloid dendritic cells in systemic lupus erythematosus. J Immunol. 2006;177:5878–5889. doi: 10.4049/jimmunol.177.9.5878. [DOI] [PubMed] [Google Scholar]
- 20.Ronnblom L, Alm GV. A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J Exp Med. 2001;194:F59–63. doi: 10.1084/jem.194.12.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 22.Palucka AK, Banchereau J, Blanco P, Pascual V. The interplay of dendritic cell subsets in systemic lupus erythematosus. Immunol Cell Biol. 2002;80:484–488. doi: 10.1046/j.1440-1711.2002.01112.x. [DOI] [PubMed] [Google Scholar]
- 23.Kyogoku C, Tsuchiya N. A compass that points to lupus: genetic studies on type I interferon pathway. Genes Immun. 2007 doi: 10.1038/sj.gene.6364409. [DOI] [PubMed] [Google Scholar]
- 24.Colonna L, Dinnall JA, Shivers DK, Frisoni L, Caricchio R, Gallucci S. Abnormal costimulatory phenotype and function of dendritic cells before and after the onset of severe murine lupus. Arthritis Res Ther. 2006;8:R49. doi: 10.1186/ar1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu J, Liu X, Xie C, Yan M, Yu Y, Sobel ES, Wakeland EK, Mohan C. T cell hyperactivity in lupus as a consequence of hyperstimulatory antigen-presenting cells. J Clin Invest. 2005;115:1869–1878. doi: 10.1172/JCI23049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lian ZX, Kikuchi K, Yang GX, Ansari AA, Ikehara S, Gershwin ME. Expansion of bone marrow IFN-alpha-producing dendritic cells in New Zealand Black (NZB) mice: high level expression of TLR9 and secretion of IFN-alpha in NZB bone marrow. J Immunol. 2004;173:5283–5289. doi: 10.4049/jimmunol.173.8.5283. [DOI] [PubMed] [Google Scholar]
- 27.Adachi Y, Taketani S, Toki J, Ikebukuro K, Sugiura K, Oyaizu H, Yasumizu R, Tomita M, Kaneda H, Amoh Y, Ito T, Okigaki M, Inaba M, Ikehara S. Marked increase in number of dendritic cells in autoimmune-prone (NZW x BXSB)F1 mice with age. Stem Cells. 2002;20:61–72. doi: 10.1634/stemcells.20-1-61. [DOI] [PubMed] [Google Scholar]
- 28.Wan S, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol. 2007;178:271–279. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi M, Hashimoto M, Ichiyama K, Yoshida R, Hanada T, Muta T, Komune S, Kobayashi T, Yoshimura A. Ifi202, an IFN-inducible candidate gene for lupus susceptibility in NZB/W F1 mice, is a positive regulator for NF-kappaB activation in dendritic cells. Int Immunol. 2007;19:935–942. doi: 10.1093/intimm/dxm054. [DOI] [PubMed] [Google Scholar]
- 30.Ishikawa S, Nagai S, Sato T, Akadegawa K, Yoneyama H, Zhang YY, Onai N, Matsushima K. Increased circulating CD11b+CD11c+ dendritic cells (DC) in aged BWF1 mice which can be matured by TNF-alpha into BLC/CXCL13-producing DC. Eur J Immunol. 2002;32:1881–1887. doi: 10.1002/1521-4141(200207)32:7<1881::AID-IMMU1881>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 31.Bagavant H, Deshmukh US, Wang H, Ly T, Fu SM. Role for nephritogenic T cells in lupus glomerulonephritis: progression to renal failure is accompanied by T cell activation and expansion in regional lymph nodes. J Immunol. 2006;177:8258–8265. doi: 10.4049/jimmunol.177.11.8258. [DOI] [PubMed] [Google Scholar]
- 32.Santiago-Raber ML, Baccala R, Haraldsson KM, Choubey D, Stewart TA, Kono DH, Theofilopoulos AN. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J Exp Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braun D, Geraldes P, Demengeot J. Type I Interferon controls the onset and severity of autoimmune manifestations in lpr mice. J Autoimmun. 2003;20:15–25. doi: 10.1016/s0896-8411(02)00109-9. [DOI] [PubMed] [Google Scholar]
- 34.Jorgensen TN, Roper E, Thurman JM, Marrack P, Kotzin BL. Type I interferon signaling is involved in the spontaneous development of lupus-like disease in B6.Nba2 and (B6.Nba2 x NZW)F(1) mice. Genes Immun. 2007 doi: 10.1038/sj.gene.6364430. [DOI] [PubMed] [Google Scholar]
- 35.Nacionales DC, Kelly-Scumpia KM, Lee PY, Weinstein JS, Lyons R, Sobel E, Satoh M, Reeves WH. Deficiency of the type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56:3770–3783. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hron JD, Peng SL. Type I IFN protects against murine lupus. J Immunol. 2004;173:2134–2142. doi: 10.4049/jimmunol.173.3.2134. [DOI] [PubMed] [Google Scholar]
- 37.Waters ST, Fu SM, Gaskin F, Deshmukh US, Sung SS, Kannapell CC, Tung KS, McEwen SB, McDuffie M. NZM2328: a new mouse model of systemic lupus erythematosus with unique genetic susceptibility loci. Clin Immunol. 2001;100:372–383. doi: 10.1006/clim.2001.5079. [DOI] [PubMed] [Google Scholar]
- 38.Price GE, Gaszewska-Mastarlarz A, Moskophidis D. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. J Virol. 2000;74:3996–4003. doi: 10.1128/jvi.74.9.3996-4003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacob CO, Zang S, Li L, Ciobanu V, Quismorio F, Mizutani A, Satoh M, Koss M. Pivotal role of stat4 and stat6 in the pathogenesis of the lupus-like disease in the New Zealand mixed 2328 mice. J Immunol. 2003;171:1564–1571. doi: 10.4049/jimmunol.171.3.1564. [DOI] [PubMed] [Google Scholar]
- 40.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black x New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174:2499–2506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 41.Jacob CO, Pricop L, Putterman C, Koss MN, Liu Y, Kollaros M, Bixler SA, Ambrose CM, Scott ML, Stohl W. Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand mixed 2328 mice deficient in BAFF. J Immunol. 2006;177:2671–2680. doi: 10.4049/jimmunol.177.4.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J Immunol. 2004;172:1426–1436. doi: 10.4049/jimmunol.172.3.1426. [DOI] [PubMed] [Google Scholar]
- 43.Carreras E, Turner S, Paharkova-Vatchkova V, Mao A, Dascher C, Kovats S. Estradiol Acts Directly on Bone Marrow Myeloid Progenitors to Differentially Regulate GM-CSF or Flt3 Ligand-Mediated Dendritic Cell Differentiation. J Immunol. 2008;180:727–738. doi: 10.4049/jimmunol.180.2.727. [DOI] [PubMed] [Google Scholar]
- 44.Vollmer J, Weeratna R, Payette P, Jurk M, Schetter C, Laucht M, Wader T, Tluk S, Liu M, Davis HL, Krieg AM. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol. 2004;34:251–262. doi: 10.1002/eji.200324032. [DOI] [PubMed] [Google Scholar]
- 45.Monrad S, Kaplan MJ. Dendritic cells and the immunopathogenesis of systemic lupus erythematosus. Immunol Res. 2007;37:135–145. doi: 10.1007/BF02685895. [DOI] [PubMed] [Google Scholar]
- 46.Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O'Garra A, Vicari A, Trinchieri G. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201:1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, Trinchieri G, Caux C, Garrone P. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fox R, Aubert M. Flow cytometric detection of activated caspase-3. Methods Mol Biol. 2008;414:47–56. doi: 10.1007/978-1-59745-339-4_5. [DOI] [PubMed] [Google Scholar]
- 49.Santambrogio L, Potolicchio I, Fessler SP, Wong SH, Raposo G, Strominger JL. Involvement of caspase-cleaved and intact adaptor protein 1 complex in endosomal remodeling in maturing dendritic cells. Nat Immunol. 2005;6:1020–1028. doi: 10.1038/ni1250. [DOI] [PubMed] [Google Scholar]
- 50.Tucci M, Quatraro C, Lombardi L, Pellegrino C, Dammacco F, Silvestris F. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 2008;58:251–262. doi: 10.1002/art.23186. [DOI] [PubMed] [Google Scholar]
- 51.Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 52.Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med. 2009;15:696–700. doi: 10.1038/nm.1973. [DOI] [PubMed] [Google Scholar]
- 53.Naik SH. Demystifying the development of dendritic cell subtypes, a little. Immunol Cell Biol. 2008;86:439–452. doi: 10.1038/icb.2008.28. [DOI] [PubMed] [Google Scholar]
- 54.Honda K, Mizutani T, Taniguchi T. Negative regulation of IFN-alpha/beta signaling by IFN regulatory factor 2 for homeostatic development of dendritic cells. Proc Natl Acad Sci U S A. 2004;101:2416–2421. doi: 10.1073/pnas.0307336101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ichikawa E, Hida S, Omatsu Y, Shimoyama S, Takahara K, Miyagawa S, Inaba K, Taki S. Defective development of splenic and epidermal CD4+ dendritic cells in mice deficient for IFN regulatory factor-2. Proc Natl Acad Sci U S A. 2004;101:3909–3914. doi: 10.1073/pnas.0400610101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen M, Wang YH, Wang Y, Huang L, Sandoval H, Liu YJ, Wang J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–1164. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- 57.Le Bon A, Durand V, Kamphuis E, Thompson C, Bulfone-Paus S, Rossmann C, Kalinke U, Tough DF. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J Immunol. 2006;176:4682–4689. doi: 10.4049/jimmunol.176.8.4682. [DOI] [PubMed] [Google Scholar]
- 58.Welner RS, Pelayo R, Nagai Y, Garrett KP, Wuest TR, Carr DJ, Borghesi LA, Farrar MA, Kincade PW. Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection. Blood. 2008 doi: 10.1182/blood-2008-04-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, Fink PJ, Chervonsky AV. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fiore N, Castellano G, Blasi A, Capobianco C, Loverre A, Montinaro V, Netti S, Torres D, Manno C, Grandaliano G, Ranieri E, Schena FP, Gesualdo L. Immature myeloid and plasmacytoid dendritic cells infiltrate renal tubulointerstitium in patients with lupus nephritis. Mol Immunol. 2008;45:259–265. doi: 10.1016/j.molimm.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 62.Werth VP. Cutaneous lupus: insights into pathogenesis and disease classification. Bull NYU Hosp Jt Dis. 2007;65:200–204. [PubMed] [Google Scholar]
- 63.Zuniga EI, McGavern DB, Pruneda-Paz JL, Teng C, Oldstone MB. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol. 2004;5:1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toma-Hirano M, Namiki S, Miyatake S, Arai K, Kamogawa-Schifter Y. Type I interferon regulates pDC maturation and Ly49Q expression. Eur J Immunol. 2007;37:2707–2714. doi: 10.1002/eji.200737173. [DOI] [PubMed] [Google Scholar]
- 65.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 67.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
Supplementary Figure Legends
Fig. S1. Definition of DC subsets in the spleen and rLN using flow cytometry. (A-C) These panels show the strategy to identify pDC in the rLN. (A) Identification of B220+ cells. (B) B220+ cells contain a population of PDCA-1+ cells. (C) The majority of B220+ PDCA-1+ cells are CD11c+. (D-G) These panels show the gating strategy to identify splenic DC subsets. (D) After gating out autofluorescent cells, cDC were identified as CD11chi. In this representative example, the percentage of cDC in total live splenocytes was the same for WT (1.3%, gray solid line) and IFNAR-KO (1.2%, black dotted line). (E) CD11chi cells gated in panel C were divided into CD8α+ LDC and CD8α− MDC. (F,G) pDC within the fraction of CD11cint cells indicated in panel C were identified by coexpression of B220 and PDCA-1. In this representative example, the percentage of pDC in total live splenocytes in IFNAR-KO spleens (0.84%) (G) was ~2-fold higher than in WT spleens (0.4%) (F). Flow cytometry data were analyzed using FlowJo software and are shown in biexponential plots.
Fig. S2. IFNAR deficiency alters the differentiation and function of DC in cytokine-driven culture models. (A) Numbers of live cells and differentiated DC subsets were increased from IFNAR-KO BM, relative to WT BM, in non-inflammatory conditions. BM cells from WT or IFNAR-KO were cultured in FL for 8 days. The frequency of LDC, MDC and pDC were assessed using flow cytometry and absolute numbers of DC were calculated based on hemocytometer counts of harvested cells. Shown are data pooled from 2 experiments, n=4-5, *p<0.05. WT, open bars; IFNAR-KO, closed bars. (B) Upon activation by TLR9 ligands, numbers of live cells and DC subsets were decreased in cultures generated from IFNAR-KO BM. Cells on day 7 of culture were activated for 16 hr with CpG-A ODN, followed by assessment of the frequency and number of DC subsets. Shown are data pooled from 2 experiments, n=6-7, **p<0.01; ***p<0.001. (C,D) IFNAR-/- DC show less expression of surface CD40 and CD86 after activation by TLR9 ligands. Cells were activated for 16 hr with CpG-ODN, followed by assessment of (C) CD40 and (D) CD86 surface expression on DC subsets. Shown are representative flow cytometry data histograms (WT, gray solid line; IFNAR-KO, black dotted line; shaded histogram, unstimulated WT cells) and a compilation of normalized MFI values (WT, open bar; IFNAR-KO, closed bar). Raw data were log-transformed to achieve normality and/or to satisfy the equal variance test. CD40 data were pooled from 2 experiments, n=4-6, and CD86 data were pooled from 3 experiments, n=7-10, ***p<0.001. (E) IFNAR decreases the differentiation of myeloid DC in non-inflammatory conditions. BM cells from WT or IFNAR-KO mice were cultured in GM-CSF for 7 days. The frequency of CD11c+ CD11b+ DC was assessed using flow cytometry, and absolute numbers of DC were calculated based on hemocytometer counts of harvested cells. Data are representative of 4 independent experiments. Shown are the mean + SEM of values pooled from 2 experiments, n=5-7; ***p<0.0001; *p=0.02. (F) IFNAR promotes IL-12p40/70 production by DC. DC were stimulated for 16 hr with CpG-B ODN (5 μg/ml), with brefeldin A added for the last 12 hr. The presence of IL-12 in DC was assessed using surface marker and intracellular cytokine staining. Shown are the percentage of DC expressing IL-12p40/70, mean + SEM of values pooled from 2 experiments, n=8-9, ***p<0.0001.
Fig. S3. Effect of IFNAR deficiency on splenic B cell subsets. For B lymphocyte subsets, (A) CD21hi CD23lo B cells, (B) CD21int CD23+ follicular B cells, (C) CD5+ B cells, numbers shown are millions of cells per spleen of WT or IFNAR-KO mice at the indicated ages. The composite results are plotted as box plots. The lines inside the boxes indicate the medians; the outer borders of the boxes indicate 25th and 75th percentiles; the bars extending from the boxes indicate the 10th and 90th percentiles.