Charge neutralization and collapse of the C-terminal tail of alpha-synuclein at low pH (original) (raw)
Abstract
Alpha-synuclein (αS) is the primary component of Lewy bodies, the pathological hallmark of Parkinson's Disease. Aggregation of αS is thought to proceed from a primarily disordered state with nascent secondary structure through intermediate conformations to oligomeric forms and finally to mature amyloid fibrils. Low pH conditions lead to conformational changes associated with increased αS fibril formation. Here we characterize these structural and dynamic changes using solution state NMR measurements of secondary chemical shifts, relaxation parameters, residual dipolar couplings, and paramagnetic relaxation enhancement. We find that the neutralization of negatively charged side-chains eliminates electrostatic repulsion in the C-terminal tail of αS and leads to a collapse of this region at low pH. Hydrophobic contacts between the compact C-terminal tail and the NAC (non-amyloid-β component) region are maintained and may lead to the formation of a globular domain. Transient long-range contacts between the C-terminus of the protein and regions N-terminal to the NAC region are also preserved. Thus, the release of long-range contacts does not play a role in the increased aggregation of αS at low pH, which we instead attribute to the increased hydrophobicity of the protein.
Keywords: α-synuclein, intrinsically disordered proteins, Parkinson's, amyloid, protein aggregation
Introduction
Parkinson's disease (PD), the most common movement disorder and second most common neurodegenerative disease, afflicts between one and two percent of the population over the age of 651 and the affected population increases markedly with age.2 Symptoms of PD (resting tremor, bradykinesia/akinesia, rigor and postural instability) are caused by the degeneration of dopaminergic neurons in the substantia nigra, but the precise mechanism by which neuronal damage occurs is unknown.3 The main pathological hallmark of PD is the presence of cytosolic inclusion bodies, Lewy bodies (LBs), which are also found in related neurological disorders including Multiple System Atrophy and Dementia with Lewy Bodies.4 LBs are largely comprised of tangles of amyloid fibrils, of which the primary component is α-Synuclein (αS),5 a 14.5 kD protein of undetermined function, which in vitro has been shown to form amyloid fibrils identical to those found in LBs.6 Although ∼90% of PD is idiopathic, there are early onset heritable, autosomal dominant forms of the disease, which have been traced to mutations in the αS gene. At present there are three known mutants of αS associated with Parkinsonism, A53T,7 A30P,8 and E46K.9 Furthermore, duplication10,11 or triplication12 of the αS gene leading to overexpression of the protein can also cause PD.
The primary structure of αS can be divided into two domains, a lipid-binding domain consisting of residues 1–102 and the C-terminal tail of the protein, consisting of the remaining C-terminal residues (see Fig. 1). The N-terminal lipid binding domain contains a series of imperfect 11-residue repeats, each of which contains a more highly conserved KTKEGV consensus sequence, resulting in a net positive charge for this domain, while the C-terminal tail contains a large number of acidic residues, resulting in a net negative charge. The N-terminal lipid binding domain also contains the relatively hydrophobic NAC (non-amyloid-β component of amyloid plaques) region, consisting of residues 61–95.14 The aggregated, fibrillar form of αS displays the typical, highly ordered, cross-beta structure of amyloid fibrils, with the ordered fibril core containing residues ∼30–100.15–18 Diverse oligomeric forms of the protein have also been observed, including short, ring-like protofibrils, short chain protofibrils,19 and other more globular forms with different secondary structure contents.20 Because some of the PD linked αS mutations accelerate fibril formation (A53T, E46K)21–25 while others inhibit it (A30P),19 but all increase oligomer formation, oligomers have been proposed to be the toxic forms of the protein.19,21,26,27
Figure 1.
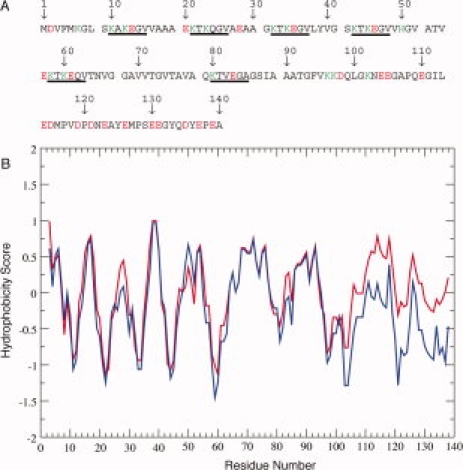
(A) Primary Sequence of αS. The 11-mer imperfect repeats are separated by spaces and the consensus KTKEGV sequence within each repeat is underlined. Charged residues (at neutral pH) are shown in red (−) and green (+). (B) Hydrophobicity scores for αS pH 7.4 (blue) and pH 3.4 (red) calculated with ProtScale [http://ca.expasy.org/tools/protscale.html] using the method of Cowan et al.13
The formation of amyloid fibrils by αS occurs via a nucleation dependent pathway,28 with access to the pathway controlled by conformational intermediates. An understanding of the conformational transitions associated with such intermediates will improve our understanding of the αS fibril formation process, and a large number of structural studies of αS have been undertaken with this goal in mind. αS exists in a number of conformations. When isolated in dilute solution at neutral pH, the protein shows a primarily disordered conformation without a well defined secondary or tertiary structure,29 although both nascent secondary structure preferences30 and transient tertiary interactions31,32 are detectable. At Low pH, however, αS has been reported to adopt a more compact conformation with altered secondary structure content,33 and the formation of this low pH intermediate conformation is maximal at pH 3.0.33,34 At the same time, low pH accelerates αS fibril formation, indicating that the induced conformational changes favor fibril formation. Here we present a detailed study of the conformation of αS at pH 3.0, using NMR secondary chemical shifts, paramagnetic relaxation enhancement, and residual dipolar couplings. We find that the normally highly acidic and extended C-terminal tail of αS, which becomes fully protonated and therefore uncharged, collapses to a compact conformational ensemble, consistent with a marked increase in its hydrophobicity. Long-range transient contacts are largely unaffected, although a slight increase in hydrophobic interactions between the C-terminal tail and the NAC region is indicated, suggesting that at low pH the NAC and C-terminal tail regions may together form a globular hydrophobic domain capable of driving αS aggregation.
Results
Secondary chemical shifts
To examine the effects of low pH on secondary structure propensity, we calculated the deviation of the pH 3.0 Cα chemical shifts from tabulated “random-coil” values and compared them with values previously reported at pH 7.4 (see Fig. 2). These deviations, or secondary shifts, are sensitive indicators of secondary structural propensity, with positive deviations indicating a tendency towards helical conformations and negative deviations a tendency towards more extended conformations.36–38 Throughout the N-terminal lipid binding domain generally positive values are observed, indicating a slight helical propensity at both pH values, with good agreement between the two conditions. In the C-terminal tail, however, the data under the two different conditions diverge dramatically. At pH 7.4, the Cα secondary shift data in the C-terminal tail region exhibit two distinct negative value lobes separated by a sharp positive peak. In contrast, at pH 3.0, the region corresponding to the first negative lobe exhibits all positive values and only three positions in the second negative lobe maintain negative values, with the remainder exhibiting positive values. The data indicate a transition of the C-terminal tail from more extended conformations at neutral pH to more compact or possibly helical conformations at low pH. This observation is consistent with CD data indicating a slightly increased helicity for αS at low pH.39
Figure 2.
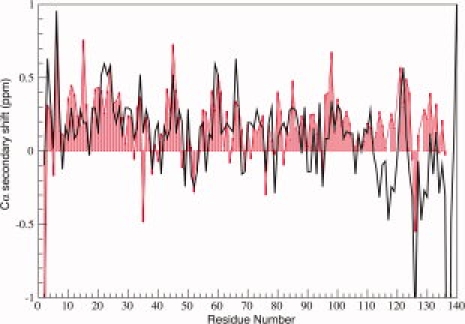
NMR Cα secondary shifts for αS at pH 3.0 (red bars) and pH 7.4 (black line). Positive values indicate propensity for helical structure while negative values indicate a preference for more extended conformations. Data at neutral pH are as previously reported.35
Paramagnetic relaxation enhancement
The ability of spin-labels such as MTSL to broaden NMR resonances of residues in the spatial proximity of the label (paramagnetic relaxation enhancement, PRE) has been widely used as tool to investigate protein conformation in denatured states.40–42 PRE measurements can readily detect interactions between residues up to ∼25 Å from the spin label. We measured PRE in αS at pH 3.0 using a number of single cysteine mutants (S9C, G31C, E61C, A85C, E110C, and E130C) conjugated to MTSL (see Fig. 3). Where possible (S9C, A85C, and E110C) these data were directly compared with data previously obtained at neutral pH.43,44
Figure 3.
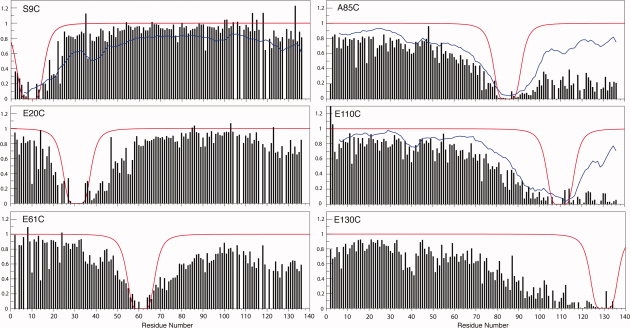
PRE measurements for MTSL-conjugated αS single cysteine mutants at pH 3.0. Missing values correspond to overlapped or unassigned resonances. The location of each cysteine mutation is indicated for each panel. Where available, smoothed data for corresponding mutants at neutral pH43,44 are shown (blue lines). The broadening expected based on a statistical random coil model is also shown (red lines).
Labels in the C-terminal tail
For spin labels at either position 110 or 130 extensive broadening was observed throughout the C-terminal tail of αS at pH 3.0. In contrast, for a label at position 110 at neutral pH, the broadening in the C-terminal tail decreased monotonically for resonances C-terminal to the labeling site. These results indicate that the C-terminal tail is more compact at low pH, consistent with the secondary shift data above. Broadening for residues directly preceding the C-terminal tail, with labels at positions 110 and 130, exhibited a slow monotonic decrease, which extended well into the NAC region of the protein, indicating a favorable interaction between this region and the C-terminal tail at low pH. A similar pattern, but with slightly less broadening, was observed at neutral pH (for labels at position 110), indicating that contacts between the C-terminal tail and the NAC region are not perturbed, and may instead be slightly enhanced at low pH. Broadening was also observed in more N-terminal regions, particularly for residues 1–10 and in the region around residue 40, indicating that transient contacts between the C-terminal tail and the N-terminus of the protein previously observed at neutral pH are also preserved at low pH.
Labels in the NAC region
With a spin labels at position 85, extensive broadening was observed in the C-terminal tail region of αS at pH 3.0, with resonance intensities dramatically lower than those observed at neutral pH. This confirms that NAC contacts with the C-terminal tail persist at low pH. Furthermore, because of the observed compactness of the C-terminal tail at low pH, any contacts with the NAC domain would be expected to bring the entire region into the proximity of the spin label at position 85, accounting for the increased degree of resonance broadening when compared with neutral pH.
Labels N-terminal to the NAC region
Spin labels attached at positions 31 and 61 lead to slightly asymmetric broadening patterns, with the effect extending further in the C-terminal direction than in the N-terminal direction. In both cases, the extent of broadening was significantly greater than that predicted using an ideal random coil model, indicating that the polypeptide chain around both labeling sites is more compact than a statistical random coil. Labels at both sites also lead to some degree of broadening in the C-terminal tail region of the protein, suggesting that long-range contacts between the N- and C-terminal domain previously observed at neutral pH are maintained to some extent at low pH.
The most N-terminal spin label, at position 9, produced a broadening pattern that approaches that predicted by an ideal random coil model, suggesting that at low pH, this region of αS is not more compact than a statistical random coil. This results differs significantly from what is seen at neutral pH, where the broadening extends much further, suggesting a significantly more compact conformation. At low pH, like at neutral pH, a weak broadening effect is also observed in the C-terminal tail region, again indicating that transient long-range contacts between the N- and C-terminal regions are maintained.
Backbone dynamics
NMR relaxation rate constants provide information on protein motions.45,46 We measured backbone 15N _R_1 and _R_2 relaxation rates as well as steady state 1H-15N heteronuclear NOE (hNOE) values for αS at pH 3.0 and compared them to previous measurements at neutral pH (see Fig. 4). All three parameters report on fast (ps-ns) motions of the NH bond vector, while _R_2 is also sensitive to μs-ms time scale conformational or chemical exchange. Lower values for either _R_2 or hNOE data indicate increased mobility.
Figure 4.
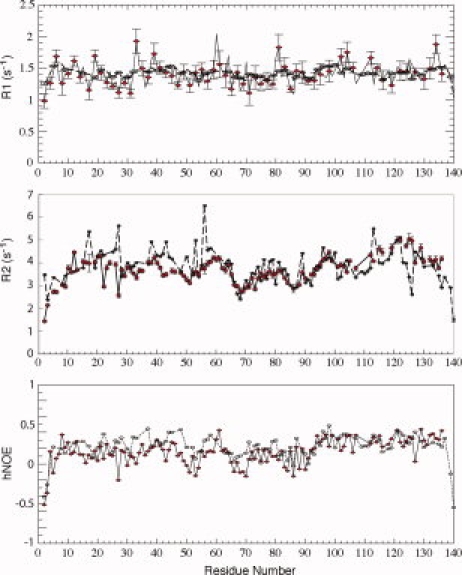
NMR relaxation parameters for αS at pH 3.0 (red diamonds) and pH 7.4 (open circles, dashed lines), including R1, R2 and the steady state 1H-15N heteronuclear NOE (hNOE). Data at neutral pH are as previously reported.43
All three relaxation parameters are consistent with a highly disordered polypeptide at both pH values, with average hNOE values of around 0.2, average _R_2 values near 3 s−1 (typical values for well structured proteins of similar size would be on the order of 10 s−1 for _R_2 and 0.8 for hNOE) and an _R_2/_R_1 ratio near one, indicating mobility approaching the extreme narrowing limit. Interestingly, a slight decrease in the hNOE values in the NAC region of the protein, with an alternating rise and fall pattern, is present at both pH values. This feature has been noted before35 but remains without a clear explanation.
The _R_1 data at pH 3.0 are closely similar to those observed at neutral pH throughout the protein. For both the hNOE and _R_2 data, a slight decrease is observed for residues N-terminal to the NAC region (∼20–60) at pH 3.0 (average R2 of 3.7 +/− 0.4 s−1 vs. 4.3 +/− 0.6 s−1 and average hNOE of 0.12 +/− 0.11 vs. 0.27 +/− 0.09), suggesting a slight increase in mobility for this region of the protein relative to neutral pH. Perhaps surprisingly, the C-terminal tail also exhibits a close agreement between data at both pH values. The one exception, however, is the stretch of residues from 123 to 126, which show an increase of _R_2 at low pH, suggesting a decrease in mobility. This region corresponds closely to the region between the lobes of negative secondary chemical shifts (see above) and high amplitude RDCs (see below) at neutral pH.
Residual dipolar couplings
Residual dipolar couplings (RDCs) contain information about the orientation of bond vectors and can act as a source of additional structural information.47 The interpretation of RDCs in disordered proteins, however, remains an active area of research.42 For statistical random coils, RDCs are predicted to have a characteristic profile, uniform throughout the polypeptide except at the termini, where they approach zero due to increasingly isotropic flexibility.48 Deviations from such a profile may be an indication of secondary structure preferences with a decrease of RDC amplitude correlated with local compactness or flexibility.49 For αS, it has also been suggested that large amplitude RDCs in the C-terminal tail of the protein may report on long-range transient contacts between the C-terminus and regions in the N-terminal lipid-binding domain.32
We measured RDCs for αS at pH 3.0 aligned in C8E5 bicelles and compared them to previous measurements at pH 7.443 (see Fig. 5). Throughout the N-terminal lipid-binding domain, RDCs are closely similar at low and high pH. In the C-terminal tail of the protein, a bi-lobed feature is seen in the neutral pH RDC data, which suggests two extended segments joined by a flexible linker, consistent with secondary chemical shift and dynamics measurements. At pH 3.0, however, this bi-lobed feature is essentially absent, with most RDCs in the C-terminal tail showing small amplitude or positive values indicative of more compact and/or helical structure. This change is consistent with the changes in secondary chemical shifts observed in this region (see Fig. 2).
Figure 5.
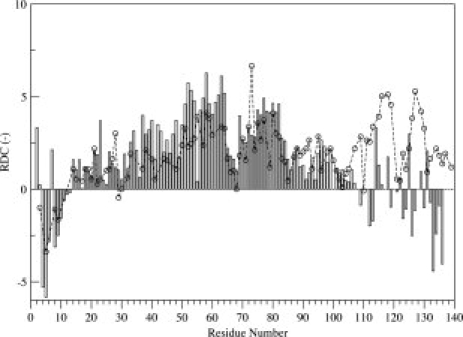
1H-15N RDCs measured for αS aligned in C8E5 bicelles at pH 3.0 (bars) and 7.4 (open circles, dashed line). By convention, RDCs are shown inverted.
Discussion
Studies of the structural characteristics of aggregation-prone and aggregation-inhibited forms of αS have been widely undertaken in order to clarify those features that control the entry of the protein into oligomerization and aggregation pathways. Observation of C-to-N terminal contacts in WT αS,31,32 and data suggesting such contacts are abrogated in aggregation prone mutants50 supported a proposed model in which long-range contacts prevented the formation of fibrilization pathway intermediates by occluding the hydrophobic NAC region of the protein. This model, however, was not supported by more recent studies of other synuclein family members, where the predicted correlation between long-range contacts and aggregation propensity was not observed.43 Furthermore, the conclusion that PD-linked mutations perturb such contacts at all was called into question.42,43 An alternate model suggests that changes in basic physicochemical properties of the protein sequence, such as net charge, secondary structure propensity and hydrophobicity are primarily responsible for the different aggregation propensities of synuclein variants.35,51,52 Here, we have investigated structural changes induced in αS at low pH, where aggregation is strongly enhanced,33 in order to further characterize structural changes associated with increased αS oligomerization and fibril formation.
The N-terminal lipid-binding domain of αS is largely unchanged at low pH
Our results, based on NMR chemical shift, PRE, RDC and relaxation measurements, indicate that secondary structure propensity, backbone dynamics, and local compactness within the N-terminal lipid-binding domain of αS are highly similar at low and neutral pH. The primary exception is found near the N-terminus of the protein, where PRE data suggest the polypeptide chain samples a more expanded ensemble of conformations at low pH. This may result from the presence of the N-terminal amino group plus three nearby lysine residues (K6, K10, K12) within the span of 12 residues, which through electrostatic repulsion might lead to a preference for more extended chain conformations at low pH. At neutral pH, two acidic residues (D2 and E13) would mitigate this effect. Somewhat surprisingly, the decrease in local compactness is not accompanied by any clear change in either backbone dynamics (on the ps-ns time scale) or secondary structure preferences.
The C-terminal tail of αS collapses at low pH
In contrast to the N-terminal lipid-binding domain, the C-terminal ∼40 residues of αS experience dramatic changes in going from neutral to low pH. At the level of secondary structure, a preference for extended structure, especially in two distinct lobes, is replaced by a preference for more compact or helical conformations, as seen in both chemical shift and RDC data and, accordingly, local compactness as measured by PRE is greatly increased. The collapse of the C-terminus is not accompanied by large changes in fast time-scale motions, suggesting that the more highly compact ensemble retains a high degree of internal motion. An exception to this occurs for residues 123–126, which lie between the two lobes of more highly extended conformations (evident in the chemical shift and RDC data) observed at neutral pH. These residues exhibit greater flexibility at pH 7.4, and become less mobile at pH 3.0. A possible explanation is that these residues behave as a flexible hinge between two more rigid and extended chain segments at neutral pH, but experience more restricted motions due to greater excluded volume effects in the context of a locally compact ensemble at low pH.
Long-range C- to N-terminal contacts are largely unperturbed at low pH
Transient long-range contacts between the C- and N-terminal domains of αS were originally detected using PRE measurements,31,32 which revealed a broadening effect on resonances near the N-terminus of the protein and in the NAC region for spin-label sites in the C-terminal tail. The PRE data presented here indicate that such long-range contacts are largely preserved in αS at pH 3.0. Specifically, broadening is observed in the C-terminal tail for spin labels attached in the NAC region and vice versa. The implied contacts between the NAC region and the C-terminal tail may in fact be slightly enhanced at low pH, as judged by a slightly increased broadening effect in the NAC region for spin labels located in the C-terminal tail (Fig. 3, E110C), consistent with the hydrophobic nature of the NAC region and the increased hydrophobicity of the C-terminal tail at pH 3.0 (see Fig. 1). However, the increased extent of the broadening evident in the C-terminal tail with a label placed in the NAC region likely originates from the increased compactness of the tail, which, for any given contact event, brings a greater fraction of tail residues into the effective broadening range of the spin label.
Broadening is also observed in the C-terminal tail for a spin label attached at several sites N-terminal to the NAC region and vice versa. These contacts are thought to be mediated by electrostatic interactions and might therefore be expected to be eliminated by the neutralization of the C-terminal tail at low pH. However, the pKa of the C-terminal carboxyl group in peptides is ∼2.0, indicating that its negative charge would be retained at pH 3.0, while the positive charge of the N-terminal region increases at low pH. Thus, electrostatic interactions could still mediate such transient long-range contact. Furthermore, because of the compact nature of the C-terminal tail, even interactions involving only the very C-terminus of the polypeptide could bring much of the tail into the proximity of any contact site, leading to more extensive broadening effects, as observed, than might otherwise be anticipated.
Role of low pH in αS aggregation
Our results reveal that the primary effects of low pH on the structural properties of αS are the collapse of the C-terminal tail, while transient long-range contacts are not substantially reduced or altered. These changes are a consequence of the protonation of a large number of acidic residues in the C-terminal tail of the protein. At neutral pH, the C-terminal tail carries a net charge of −15, spread over 38 residues. The electrostatic repulsion that results from this high charge density (−0.39/residue) is likely the cause of the observed preference of this region for extended structures. At low pH, this net charge is reduced to −1 (the C-terminal carboxyl group), greatly increasing the hydrophobicity of the tail region and leading it to adopt a more highly collapsed ensemble of conformations. In contrast, the N-terminal lipid-binding domain of αS carries a net charge of +6 at neutral pH. At pH 3.0, this charge increases to +17, but is spread over 102 residues. This results in a lower charge density (+0.17/residue), which does not appear to create a sufficient degree of electrostatic repulsion to induce a preference for extended conformations, except to some degree near the very N-terminus of the protein.
Because long-range contacts are not decreased, and are perhaps slightly increased, at low pH, the release of such contacts cannot explain the increased propensity of the protein to aggregate at low pH. This is consistent with recent findings that the absence of long-range contacts does not lead to enhanced aggregation for synuclein family members β- and γ-synuclein, as well as with observations that the E46K PD-linked mutation, which enhances αS aggregation, actually exhibits increased long-range C- to N-terminal contacts.53 Other factors that have been proposed to determine αS aggregation include the physicochemical properties of the protein sequence, such as secondary structure propensity, net charge, and hydrophobicity. The net charge of αS at neutral pH, −9, decreases as the pH is lowered, reaching zero at its pI value (predicted to be at pH 4.7) consistent with an increase in the aggregation rate of the protein.33 As the pH is lowered further, however, the net charge increases again, reaching a value of +16 at pH 3.0. This would be expected to decrease rather than increase the aggregation rate of the protein, in contrast to what is observed. In addition to the increase in net charge, however, at pH values below the pI, the titration of acidic side chains becomes essentially complete, resulting in a greatly increased hydrophobicity of the C-terminal tail of the protein. Thus, while the net charge of αS increases at pH 3.0, the hydrophobicity of the protein increases.
The increased aggregation rate of αS at pH values below its pI suggests that the increase in the overall hydrophobicity of the protein overcomes the increase in its net charge. Two arguments may help to explain this result. First, although the net charge of the protein is higher at pH 3.0 than at neutral pH, the charge density is lower, leading to reduced intra-molecular electrostatic repulsion and suggesting a correspondingly decreased potential for inter-molecular repulsion. Second, upon collapse of the hydrophobic C-terminal tail at pH 3.0, contacts between the tail and the NAC region bring more residues from each region into the proximity of the other, suggesting that the two subdomains together may form a single collapsed hydrophobic domain, possibly with molten globule like characteristics. Such states are known to mediate the aggregation of numerous proteins by promoting inter-molecular hydrophobic interactions, and would be consistent with the amyloidogenic αS intermediate originally proposed to exist at low pH.33 Such a scenario would also be consistent with small angle x-ray scattering data indicating a decreased radius of gyration and the presence of globular structure for αS at pH 3.0,33 and could account for the increased binding of the hydrophobic dye ANS to αS at low pH. Interestingly, this argument suggests that rather than inhibiting αS aggregation, long-range contacts can enhance the aggregation of the protein by forming collapsed hydrophobic domains that promote inter-molecular interactions.
Methods
Protein preparation
Recombinant αS was expressed, grown, and purified as described previously.30 Single-cysteine mutants were produced by site directed mutagenesis of the WT plasmid construct (kindly provided by Dr. Peter Lansbury, Harvard Medical School). Truncated protein was produced by addition of a stop codon at position 103. Recombinant mutants were labeled by growing cells in M9 minimal media made using either 15N ammonium chloride for single labeled or 13C glucose and 15N ammonium chloride for double labeled proteins. Cells were incubated at 37°C until OD600 ∼0.6 was reached. Protein purity was verified by the appearance of a single band in SDS-PAGE gels, by comparison of NMR spectra where appropriate and by mass spectrometry in the case of mutant proteins.
NMR spectroscopy
NMR samples were prepared by dissolving lyophilized protein in buffer solution (100 m_M_ NaCl, 10 m_M_ Na2HPO4, 10%/90% D2O/H2O pH 3.0) and filtering the resulting solutions over Millipore 100,000 MWCO centrifugation filters. Typical protein concentrations were 70 μ_M_ by weight before filtration. Sample pH was monitored in the NMR tube by a MI-412 pH probe (Microelectrodes, Bedford, NH) and adjusted by addition of HCl and/or NaOH. Samples were prepared immediately before measurement. pH titration was conducted by measurement of HSQCs for a single sample of 15N labeled C-terminally truncated αS (residues 1–102) at 10°C, titrating pH between HSQC spectra by addition of μL amounts of HCl and NaOH. HSQC peak assignments were transferred sequentially from pH 7.4 to pH 2.5. Overall sample dilution was kept below 10% over the entire titration. All measurements were made on either a 600 MHz Varian INOVA spectrometer with a cryoprobe attachment (Weill Medical College of Cornell University) or 700 MHz, 800 MHz, or 900 MHz Bruker AVANCE spectrometers (New York Structural Biology Center). All NMR data were processed with NMRPipe54 and analyzed with NMRViewJ.55 Spectra were referenced indirectly to DSS and ammonia using the known chemical shift of water. Random coil values obtained from linear hexapeptides in 1M urea at pH 5.0 and 25°C56 were used to calculate Cα secondary shifts, using the values for protonated glutamate and aspartate.57
Resonance assignments
To assign the NMR backbone resonances of αS at pH 3.0 we first used 2D 1H-15N HSQC spectra to monitor a titration of C-terminally truncated αS (residues 1–102) from pH 7.4 down to pH 2.1 using 11 intermediate pH values, and transferred the known pH 7.4 1H-15N resonance assignments30 to all other pH values. Reversability was verified by overlaying initial and final spectra at the same pH. Only minor line broadening was observed throughout the titration, with peak shifts observed only for the titratable residues and their near neighbors at pH values near their respective pKas (data not shown). To verify the transferred peak assignments at pH 3.0, we measured 3D NMR spectra (HNCO, HNCACO, HNCACB, and CBCACONH) for truncated αS at this pH and then repeated the same suite of NMR measurements on the full length (1–140) protein under the same conditions to assign the remainder of the peaks.
Dynamics
R1 measurements employed delay times of 75, 150, 200, 300, 400, 500, 600, 800, 1000, and 1200 ms, with repeat collection at 150, 400, and 800 ms. _R_2 measurements were made using a CPMG pulse sequence with delay times of 6, 18, 26, 34, 50, 66, 98, 130, 178 and 246 ms, with repeat collection at 6, 66, and 246 ms. Steady-state 1H-15N heteronulcear NOE data were recorded as interleaved NOE and NONOE spectra. _R_1 and _R_2 measurements employed 1024 × 512 points while hNOE data were collected using 2048 × 640 points in the proton and nitrogen dimensions respectively.
Residual dipolar couplings
RDC samples were made by addition of 15N-labeled αS stock solutions (prepared as above) to bicelle solutions made with 7.4% (w/v) pentaethylene glycol monooctyl ether (C8E5)/octanol (from Sigma). The measurements were made using 2D HSQC based IPAP experiments,58 with deuterium quadrupolar splitting used as a measure of sample alignment. Measurements were performed on an 800 MHz Bruker AVANCE instrument with 6400 × 512 points in the proton and nitrogen dimensions respectively.
Paramagnetic relaxation enhancement
Protein samples were prepared by dissolving lyophilized 15N-labeled αS single cysteine mutants S9C, G31C, E61C, A85C, E110C, and E130C in buffer (100 m_M_ NaCl, 10 m_M_ Na2HPO4, 10%/90% D2O/H2O pH 3.0). The spin label agent 1-oxy-2,2,5,5-tetramethyl-d-pyroline-3methyl-methanethiosulfate (MTSL, Toronto Research Chemicals) was added in 10-fold excess to the protein solutions, and allowed to equilibrate for 1 h at room temperature. Excess MTSL was removed by passing the solution three times over spin columns packed with Sephadex G-25 matrix. Each sample was divided in two, and TCEP (tris(2-carboxyethyl)phosphine) was added to the control sample to reduce the MTSL from the protein. Sample pH was measured in the NMR tube by a Microelectrodes MI-412 pH probe and adjusted by addition of HCl. HSQC spectra were collected for each pair (MTSL labeled vs. MTSL+TCEP) at 10°C with 6400 × 512 points on a Varian 600 MHz cryoprobe-equipped spectrometer. “Random coil” fit functions were calculated as detailed previously.43
Conclusions
At highly acidic pH, the neutralization of charged residues in the C-terminal tail of αS leads to an increased hydrophobic character and a collapse of the tail region to a more compact ensemble of conformations. Hydrophobic contacts between the collapsed C-terminal tail and the NAC region are maintained, and may lead to the formation of a single compact domain with molten-globule like properties, including ANS binding. At the same time, transient electrostatic contacts between the C-terminus and regions N-terminal to the NAC domain are also maintained. Thus, disruption of long-range contacts does not play a role in enhancing αS aggregation at low pH. While the overall net charge of the protein increases, the individual charges are spread out, causing only weak intra-molecular electrostatic repulsion and any inter-molecular repulsion appears to be compensated by the increased hydrophobicity of the protein, which is likely the primary driving force for increased αS aggregation below the pI of the protein. In general, changes in the basic physicochemical properties of the protein, including hydrophobicity, net charge, and secondary structure propensity, appear to best explain the effects of conditions, mutations, and sequence variations on the aggregation rates of the synucleins.
Acknowledgments
This work was supported by a gift from Herbert and Ann Siegel. The authors thank Dr. Peter Lansbury (Harvard Medical School) for the kind gift of expression vectors, Drs. Clay Bracken (Weill Cornell Medical College) and Mike Goger, Kaushik Dutta, and Shibanyi Bhattacharya (New York Structural Biology Center) for assistance in NMR data collection and processing, and Trudy Ramlall for protein production. D.E. is a member of the New York Structural Biology Center, which is a STAR center supported by the New York State Office of Science, Technology and Academic Research, and received funds from NIH, USA, the Keck Foundation, New York and the NYC Economic Development Corporation for the purchase of 900 MHz spectrometers.
References
- 1.Lang AE, Lozano AM. Parkinson's disease. Second of two parts. N Engl J Med. 1998;339:1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- 2.Schrag A, Ben-Shlomo Y, Quinn NP. Cross sectional prevalence survey of idiopathic Parkinson's disease and Parkinsonism in London. BMJ. 2000;321:21–22. doi: 10.1136/bmj.321.7252.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 4.Goedert M. α-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 5.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 6.Conway KA, Harper JD, Lansbury PT., Jr Fibrils formed in vitro from α-synuclein and two mutant forms linked to Parkinson's disease are typical amyloid. Biochemistry. 2000;39:2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 7.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 8.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson's disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 9.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 10.Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Agid Y, Durr A, Brice A. Causal relation between α-synuclein gene duplication and familial Parkinson's disease. Lancet. 2004;364:1169–1171. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- 11.Ahn TB, Kim SY, Kim JY, Park SS, Lee DS, Min HJ, Kim YK, Kim SE, Kim JM, Kim HJ, Cho J, Jeon BS. α-Synuclein gene duplication is present in sporadic Parkinson disease. Neurology. 2008;70:43–49. doi: 10.1212/01.wnl.0000271080.53272.c7. [DOI] [PubMed] [Google Scholar]
- 12.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. α-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 13.Cowan R, Whittaker RG. Hydrophobicity indices for amino acid residues as determined by high-performance liquid chromatography. Pept Res. 1990;3:75–80. [PubMed] [Google Scholar]
- 14.Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Der-Sarkissian A, Jao CC, Chen J, Langen R. Structural organization of α-synuclein fibrils studied by site-directed spin labeling. J Biol Chem. 2003;278:37530–37535. doi: 10.1074/jbc.M305266200. [DOI] [PubMed] [Google Scholar]
- 16.Del Mar C, Greenbaum EA, Mayne L, Englander SW, Woods VL., Jr Structure and properties of α-synuclein and other amyloids determined at the amino acid level. Proc Natl Acad Sci USA. 2005;102:15477–15482. doi: 10.1073/pnas.0507405102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heise H, Hoyer W, Becker S, Andronesi OC, Riedel D, Baldus M. Molecular-level secondary structure, polymorphism, and dynamics of full-length α-synuclein fibrils studied by solid-state NMR. Proc Natl Acad Sci USA. 2005;102:15871–15876. doi: 10.1073/pnas.0506109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilar M, Chou HT, Luhrs T, Maji SK, Riek-Loher D, Verel R, Manning G, Stahlberg H, Riek R. The fold of α-synuclein fibrils. Proc Natl Acad Sci USA. 2008;105:8637–8642. doi: 10.1073/pnas.0712179105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci USA. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaylor J, Bodner N, Edridge S, Yamin G, Hong DP, Fink AL. Characterization of oligomeric intermediates in α-synuclein fibrillation: FRET studies of Y125W/Y133F/Y136F α-synuclein. J Mol Biol. 2005;353:357–372. doi: 10.1016/j.jmb.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 21.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 22.Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC, Wypych J, Biere AL, Citron M. Both familial Parkinson's disease mutations accelerate α-synuclein aggregation. J Biol Chem. 1999;274:9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 23.Giasson BI, Uryu K, Trojanowski JQ, Lee VM. Mutant and wild type human α-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274:7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- 24.Choi W, Zibaee S, Jakes R, Serpell LC, Davletov B, Crowther RA, Goedert M. Mutation E46K increases phospholipid binding and assembly into filaments of human α-synuclein. FEBS Lett. 2004;576:363–368. doi: 10.1016/j.febslet.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 25.Greenbaum EA, Graves CL, Mishizen-Eberz AJ, Lupoli MA, Lynch DR, Englander SW, Axelsen PH, Giasson BI. The E46K mutation in α-synuclein increases amyloid fibril formation. J Biol Chem. 2005;280:7800–7807. doi: 10.1074/jbc.M411638200. [DOI] [PubMed] [Google Scholar]
- 26.Conway KA, Lee SJ, Rochet JC, Ding TT, Harper JD, Williamson RE, Lansbury PT., Jr Accelerated oligomerization by Parkinson's disease linked α-synuclein mutants. Ann N Y Acad Sci. 2000;920:42–45. doi: 10.1111/j.1749-6632.2000.tb06903.x. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Uversky VN, Fink AL. Effect of familial Parkinson's disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human α-synuclein. Biochemistry. 2001;40:11604–11613. doi: 10.1021/bi010616g. [DOI] [PubMed] [Google Scholar]
- 28.Wood SJ, Wypych J, Steavenson S, Louis JC, Citron M, Biere AL. α-Synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson's disease. J Biol Chem. 1999;274:19509–11952. doi: 10.1074/jbc.274.28.19509. [DOI] [PubMed] [Google Scholar]
- 29.Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT., Jr NACP, a protein implicated in Alzheimer's disease and learning, is natively unfolded. Biochemistry. 1996;35:13709–13715. doi: 10.1021/bi961799n. [DOI] [PubMed] [Google Scholar]
- 30.Eliezer D, Kutluay E, Bussell R, Jr, Browne G. Conformational properties of α-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 31.Dedmon MM, Lindorff-Larsen K, Christodoulou J, Vendruscolo M, Dobson CM. Mapping long-range interactions in α-synuclein using spin-label NMR and ensemble molecular dynamics simulations. J Am Chem Soc. 2005;127:476–477. doi: 10.1021/ja044834j. [DOI] [PubMed] [Google Scholar]
- 32.Bertoncini CW, Jung YS, Fernandez CO, Hoyer W, Griesinger C, Jovin TM, Zweckstetter M. Release of long-range tertiary interactions potentiates aggregation of natively unstructured α-synuclein. Proc Natl Acad Sci USA. 2005;102:1430–1435. doi: 10.1073/pnas.0407146102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uversky VN, Li J, Fink AL. Evidence for a partially folded intermediate in α-synuclein fibril formation. J Biol Chem. 2001;276:10737–10744. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 34.Morris AM, Finke RG. α-Synuclein aggregation variable temperature and variable pH kinetic data: a re-analysis using the Finke-Watzky 2-step model of nucleation and autocatalytic growth. Biophys Chem. 2009;140:9–15. doi: 10.1016/j.bpc.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Bussell R, Jr, Eliezer D. Residual structure and dynamics in Parkinson's disease-associated mutants of α-synuclein. J Biol Chem. 2001;276:45996–46003. doi: 10.1074/jbc.M106777200. [DOI] [PubMed] [Google Scholar]
- 36.Wishart DS, Sykes BD, Richards FM. The chemical shift index: a fast and simple method for the assignment of protein secondary structure through NMR spectroscopy. Biochemistry. 1992;31:1647–1651. doi: 10.1021/bi00121a010. [DOI] [PubMed] [Google Scholar]
- 37.Wishart DS, Sykes BD. The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J Biomol NMR. 1994;4:171–180. doi: 10.1007/BF00175245. [DOI] [PubMed] [Google Scholar]
- 38.Eliezer D. Characterizing residual structure in disordered protein States using nuclear magnetic resonance. Methods Mol Biol. 2007;350:49–67. doi: 10.1385/1-59745-189-4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uversky VN, Li J, Fink AL. Evidence for a partially folded intermediate in α-synuclein fibril formation. J Biol Chem. 2001;276:10737–10744. doi: 10.1074/jbc.M010907200. [DOI] [PubMed] [Google Scholar]
- 40.Gillespie JR, Shortle D. Characterization of long-range structure in the denatured state of staphylococcal nuclease. I. Paramagnetic relaxation enhancement by nitroxide spin labels. J Mol Biol. 1997;268:158–169. doi: 10.1006/jmbi.1997.0954. [DOI] [PubMed] [Google Scholar]
- 41.Lietzow MA, Jamin M, Dyson HJ, Wright PE. Mapping long-range contacts in a highly unfolded protein. J Mol Biol. 2002;322:655–662. doi: 10.1016/s0022-2836(02)00847-1. [DOI] [PubMed] [Google Scholar]
- 42.Eliezer D. Biophysical characterization of intrinsically disordered proteins. Curr Opin Struct Biol. 2009;19:23–30. doi: 10.1016/j.sbi.2008.12.004. Doi: 10.1016/j.sbi.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sung YH, Eliezer D. Residual structure, backbone dynamics, and interactions within the synuclein family. J Mol Biol. 2007;372:689–707. doi: 10.1016/j.jmb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sung YH. 2008. Structure and interactions of the synucleins; p. 212. Ph.D. thesis Cornell University Ithaca, New York. [Google Scholar]
- 45.Palmer AG., III Probing molecular motion by NMR. Curr Opin Struct Biol. 1997;7:732–737. doi: 10.1016/s0959-440x(97)80085-1. [DOI] [PubMed] [Google Scholar]
- 46.Kay LE. Protein dynamics from NMR. Nat Struct Biol. 1998;5:513–517. doi: 10.1038/755. [DOI] [PubMed] [Google Scholar]
- 47.Tjandra N, Bax A. Direct measurement of distances and angles in biomolecules by NMR in a dilute liquid crystalline medium. Science. 1997;278:1111–1114. doi: 10.1126/science.278.5340.1111. [DOI] [PubMed] [Google Scholar]
- 48.Louhivuori M, Paakkonen K, Fredriksson K, Permi P, Lounila J, Annila A. On the origin of residual dipolar couplings from denatured proteins. J Am Chem Soc. 2003;125:15647–15650. doi: 10.1021/ja035427v. [DOI] [PubMed] [Google Scholar]
- 49.Mohana-Borges R, Goto NK, Kroon GJ, Dyson HJ, Wright PE. Structural characterization of unfolded states of apomyoglobin using residual dipolar couplings. J Mol Biol. 2004;340:1131–1142. doi: 10.1016/j.jmb.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Bertoncini CW, Fernandez CO, Griesinger C, Jovin TM, Zweckstetter M. Familial mutants of α-synuclein with increased neurotoxicity have a destabilized conformation. J Biol Chem. 2005;280:30649–30652. doi: 10.1074/jbc.C500288200. [DOI] [PubMed] [Google Scholar]
- 51.Chiti F, Stefani M, Taddei N, Ramponi G, Dobson CM. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature. 2003;424:805–808. doi: 10.1038/nature01891. [DOI] [PubMed] [Google Scholar]
- 52.Rivers RC, Kumita JR, Tartaglia GG, Dedmon MM, Pawar A, Vendruscolo M, Dobson CM, Christodoulou J. Molecular determinants of the aggregation behavior of α- and β-synuclein. Protein Sci. 2008;17:887–898. doi: 10.1110/ps.073181508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rospigliosi CC, McClendon S, Schmid AW, Ramlall TF, Barre P, Lashuel HA, Eliezer D. The E46K Parkinson's-linked mutation enhances C-to N-terminal contacts in α-synuclein. J Mol Biol. 2009;388:1022–1032. doi: 10.1016/j.jmb.2009.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson BA, Blevins RA. NMR View: a computer program for the visualization and analysis of NMR data. J Biomol NMR. 1994;4:603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- 55.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 56.Wishart DS, Bigam CG, Holm A, Hodges RS, Sykes BD. 1H, 13C and 15N random coil NMR chemical shifts of the common amino acids. I. Investigations of nearest-neighbor effects. J Biomol NMR. 1995;5:67–81. doi: 10.1007/BF00227471. [DOI] [PubMed] [Google Scholar]
- 57.Howarth OW, Lilley DM. Carbon-13-NMR of Peptides and Proteins. Prog NMR Spectrosc. 1978;12:1–40. [Google Scholar]
- 58.Ottiger M, Delaglio F, Bax A. Measurement of J and dipolar couplings from simplified two-dimensional NMR spectra. J Magn Reson. 1998;131:373–378. doi: 10.1006/jmre.1998.1361. [DOI] [PubMed] [Google Scholar]