FTY720 (fingolimod) in Multiple Sclerosis: therapeutic effects in the immune and the central nervous system (original) (raw)
Abstract
FTY720 (fingolimod) is a first-in-class sphingosine 1-phosphate (S1P) receptor modulator that was highly effective in Phase II clinical trials for Multiple Sclerosis (MS). FTY720 is phosphorylated in vivo by sphingosine kinase-2 to form the active moiety FTY720-phosphate that binds to four of the five G protein-coupled S1P receptor subtypes. Studies using conditional S1P1 receptor-deficient and sphingosine kinase-deficient mice showed that the egress of lymphocytes from lymph nodes requires signalling of lymphocytic S1P1 receptors by the endogenous ligand S1P. The S1P mimetic FTY720-phosphate causes internalization and degradation of cell membrane-expressed S1P1, thereby antagonizing S1P action at the receptor. In models of human MS and demyelinating polyneuropathies, functional antagonism of lymphocytic S1P1 slows S1P-driven egress of lymphocytes from lymph nodes, thereby reducing the numbers of autoaggressive TH17 cells that recirculate via lymph and blood to the central nervous system and the sciatic/ischiatic nerves. Based on its lipophilic nature, FTY720 crosses the blood–brain barrier, and ongoing experiments suggest that the drug also down-modulates S1P1 in neural cells/astrocytes to reduce astrogliosis, a phenomenon associated with neurodegeneration in MS. This may help restore gap-junctional communication of astrocytes with neurons and cells of the blood–brain barrier. Additional effects may result from (down-) modulation of S1P3 in astrocytes and of S1P1 and S1P5 in oligodendrocytes. In conclusion, FTY720 may act through immune-based and central mechanisms to reduce inflammation and support structural restoration of the central nervous system parenchyma. Beyond the autoimmune indications, very recent studies suggest that short-term, low-dose administration of FTY720 could help treat chronic (viral) infections. Differential effects of the drug on the trafficking of naïve, central memory and effector memory T cell subsets are discussed.
Keywords: S1P (sphingosine 1-phosphate), FTY720 (fingolimod), Multiple Sclerosis, autoimmunity, central nervous system, immunotherapy, infection
Introduction
Since 2002, a large number of studies reported efficacy of FTY720 in models of autoimmune diseases, particularly experimental autoimmune encephalomyelitis (EAE), a model of human Multiple Sclerosis (MS) (Brinkmann et al., 2002; Fujino et al., 2003; Webb et al., 2004; Kataoka et al., 2005; Balatoni et al., 2007; Brinkmann, 2007; Foster et al., 2009). More recent data showed that the drug was also highly effective in rat experimental autoimmune neuritis (EAN), a model of human demyelinating polyneuropathies (Zhang et al., 2009). Meanwhile, clinical Phase II studies suggest that the drug may provide an effective treatment for human relapsing remitting MS (Kappos et al., 2006; O'Connor et al., 2009).
In vivo, FTY720 is phosphorylated by sphingosine kinase (SphK)-2 (Brinkmann et al., 2002; Zemann et al., 2006) to yield the biologically active (S)-configured FTY720-phosphate (FTY720-P) (Albert et al., 2005). FTY720-P represents a close structural analogue of sphingosine 1-phosphate (S1P), a sphingolipid mediator that regulates multiple biological processes through binding to five G protein-coupled S1P receptors (Chun et al., 2002; Hla, 2004; Brinkmann, 2007). The biological activity of FTY720 and its phosphates was first assessed in S1P receptor assays, which detect agonist-induced GTP[γ-35S] binding as functional readout of receptor activation. It was found that the (S)-configured FTY720-P [but not the (R)-FTY720-P or parent FTY720] acts as a full agonist at S1P1 (0.3 nM), S1P4 (0.6 nM) and S1P5 (0.3 nM) and with approximately 10-fold lower potency at S1P3 (3.1 nM), but has no activity at S1P2 (>10 000 nM) (Brinkmann et al., 2002; Mandala et al., 2002; Albert et al., 2005). Comparable low-nM affinities to the receptors were reported using competitive receptor binding assays (Mandala et al., 2002). The differing receptor affinities and potencies between FTY720-P and S1P suggest the possibility of inducing distinct responses in target cells in vivo, either via agonistic signalling or via functional antagonism/internalization of S1P receptors.
As described below, conditional deletion of S1P1 receptors from lymphocytes (Matloubian et al., 2004) or neural cells/astrocytes (Choi et al., 2008) mimicked the effects of FTY720 in vivo. This suggests that the drug acts as a functional antagonist of S1P1 to interrupt the pro-inflammatory S1P-S1P1 axis, modulating MS pathology on immune and central levels.
Sphingosine 1-phosphate
S1P is generated from intracellular sphingosine, a break-down product of the cell membrane constituent sphingomyelin, and all cells are thought to be able to generate S1P in the process of normal sphingolipid turnover (Hla, 2004). During this process, sphingomyelin is degraded via ceramide to sphingosine, which is then phosphorylated by SphK1 and SphK2 to yield S1P. Platelets had long been considered to be the major source of plasma S1P; however, recent studies revealed the importance of erythrocytes as a major supply (Pappu et al., 2007). Although both erythrocytes and platelets can produce S1P, only platelets synthesize and release FTY720-P (Kihara and Igarashi, 2008). The release of S1P from cells may involve the ATP-binding cassette family of transporters that catalyse the transport of lipids from the inner to the outer leaflet of the plasma membrane (Van Meer and Lisman, 2002), and FTY720/FTY720-P may also use this transport system (Honig et al., 2003).
During embryogenesis, S1P is critically involved in the development of the cardiovascular (Allende et al., 2003) and the central nervous system (CNS) (Mizugishi et al., 2005). In the adult, plasma S1P is tightly associated with albumin and lipoproteins, particularly high-density lipoprotein (Okajima, 2002), and a significant concentration gradient of S1P exists between plasma and interstitial fluids. Tissue levels of S1P are generally low, whereas plasma S1P levels are high and many fold above the dissociation constant _K_d for S1P receptors (Toman and Spiegel, 2002; Schwab and Cyster, 2007; Kim et al., 2009). Excessive production of S1P can occur at inflammatory sites as a result of cell activation by pro-inflammatory stimuli, including interleukin-1, tumour necrosis factor and vascular endothelial growth factor (Alvarez et al., 2007; Brinkmann, 2007), leading to activation of S1P receptors. The different S1P levels in tissues/body fluids at steady state, as well as its local production at inflammatory sites, appears to be crucial to the maintenance of directed immune cell trafficking (Matloubian et al., 2004), the maintenance of the vascular tone and endothelial barriers (McVerry and Garcia, 2004) and the gap-junctional communication of neural cells in the CNS (Rouach et al., 2006; Brinkmann, 2007).
S1P receptors
S1P binds to five related G protein-coupled receptors (GPCRs), termed S1P1–5 (formerly Edg-1, -5, -3, -6 and -8 respectively) (Chun et al., 2002; Hla, 2004). S1P1, S1P2 and S1P3 receptors are widely expressed in the immune, cardiovascular and central nervous systems, with S1P1 being the dominant receptor also on lymphocytes/leukocytes (Chae et al., 2004). S1P4 is specifically expressed in lymphoid tissue (Graeler and Goetzl, 2002), and S1P5 is present in spleen and white matter tracts of the CNS [primarily on oligodendrocytes (OGC)] (Jaillard et al., 2005). To date, S1P receptor expression in tissues has primarily been studied at the mRNA level, due to a lack of specific monoclonal antibodies. The reported mRNA levels may not always match receptor protein expression, and they do not unravel the distribution in intracellular compartments versus the cell membrane, the latter being critical for S1P-dependent cell migration (Matloubian et al., 2004; Pappu et al., 2007). Furthermore, S1P receptor expression patterns significantly change with the activation status of cells (Matloubian et al., 2004; Miron et al., 2008), and receptor expression in cell membranes is modulated by S1P levels present in body fluids/tissues (Shiow et al., 2006). Finally, S1P receptors cross-talk to various growth factor receptors that are also regulated by cell activation processes and modulated by their specific ligands (Igarashi et al., 2003; Pyne et al., 2003; Alvarez et al., 2007). It is therefore difficult to model S1P/S1P receptor homeostasis and modulation that occurs in vivo by using in vitro cultures of serum/S1P-starved cells. Thus, the mechanistic data discussed below refer primarily to in vivo analysis of S1P receptor-deficient mice or reverse pharmacology approaches using in vivo treatment with S1P or functional antagonists of S1P receptors.
Regulation of egress from lymph nodes by lymphocytic S1P1
Lymphocyte egress from lymphoid organs must be controlled during a normal immune response. Within the first hours after an antigenic stimulus (i.e. an infection), exit from the draining lymph nodes (LNs) is blocked to increase the number of antigen-specific T cells in the node, a phenomenon known since 50 years as ‘shutdown’ (Schwab and Cyster, 2007). Only recently it was found that ‘shutdown’ is associated with a striking down-modulation of S1P1 mRNA expression in LN T cells (Matloubian et al., 2004). A key role of S1P and S1P1 receptors in LN egress was finally demonstrated by using foetal liver chimeric mice with specific deletion of the receptor from haematopoietic cells (Matloubian et al., 2004). In such mice, S1P1-deficient thymocytes and T cells were unable to egress from thymus and LNs respectively. Further, elimination of the receptor ligand S1P (via genetic deletion of SphKs) also blunted lymphocyte egress, suggesting that signalling of S1P at lymphocytic S1P1 receptors was promoting egress. Accordingly, restoration of S1P to plasma of SphK-deficient mice rescued egress of wild type but not S1P1-deficient lymphocytes (Pappu et al., 2007). Visualization of the branched organization of LN cortical sinuses showed that S1P1-deficient T cells probed the (LYVE-1+) lymphatic vascular endothelial surface of cortical sinuses but failed to enter. In contrast, wild-type T cells probed and entered the cortical sinuses, were then captured in a region of flow, passaged to medullary sinuses and were flushed into the subcapsular space and the efferent lymph (Grigorova et al., 2009) (Figure 1).
Figure 1.
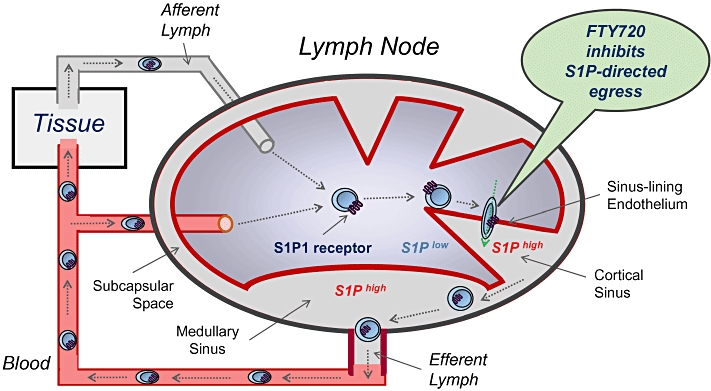
FTY720 inhibits the egress of lymphoytes from lymph nodes. T cells recirculate between blood and lymph nodes (LNs) in search for foreign antigen, entering LNs either from blood via high endothelial venules or from tissues via afferent lymphatics. Once in the LNs, the cells up-regulate sphingosine 1-phosphate (S1P)1 on cell membranes as a consequence of the S1Plow environment. To egress from LNs, T cells transmigrate through the sinus-lining endothelium into the S1Phigh cortical sinus. Imaging studies showed that this process depends on lymphocytic S1P1, as only wild type but not S1P1-deficient T cells transmigrate through the endothelium. In the cortical sinuses, T cells are captured in a region of flow, passaged to medullary sinuses and flushed into the subcapsular space and the efferent lymph. Treatment with FTY720 causes internalization of lymphocytic S1P1 on T cells, thereby inhibiting the egress from LNs.
Treatment of wild-type mice with FTY720 mimicked the effects of S1P1 deletion from haematopoietic cells, strongly suggesting that the drug acts as a functional antagonist of S1P1 receptors to prevent egress (Matloubian et al., 2004). Accordingly, FTY720 treatment caused an internalization of membrane-expressed S1P1 in LN T cells in vivo (Pham et al., 2008), and the drug could promote ubiquitinylation and proteasomal degradation of the receptor in endothelial cells in vitro (Oo et al., 2007). Western blot analysis further revealed that treatment of mice with FTY720 dose-dependently reduced S1P1 receptor protein in cytosolic and membrane fractions of organ tissues (unpublished), suggesting that receptor degradation also occurs in vivo. It is likely that the 10-fold higher concentrations of FTY720 and its phosphate in LNs compared with blood (Sensken et al., 2008) provide some tissue specificity of S1P1 modulation.
All together, the data propose an obligatory role of lymphocytic S1P1 receptors in the egress from lymphoid organs, and suggest that FTY720 down-modulates lymphocytic S1P1 to slow S1P-S1P1-dependent egress into cortical sinuses of LNs. As a consequence, autoaggressive T cells remain in the antigen-draining LNs, and this reduces their recirculation via blood and lymph to the CNS and abrogates EAE/MS relapses and central inflammation (Fujino et al., 2003). Importantly, FTY720 neither affected the functionality of T cells (Pinschewer et al., 2000; Brinkmann et al., 2001; Kursar et al., 2008) nor the motility of CNS-resident T cells (Bartholomäus et al., 2008), and this could be important for the maintenance of central immunesurveillance under FTY720 therapy.
Experiments using green-fluorescent protein-transgenic bone marrow chimeric mice showed that the development of single positive thymocytes in the thymus was normal. Furthermore, mature T cells developed and recirculated to blood and lymphoid organs. Similar maximal levels of T cell chimerizm were achieved in FTY720-treated and untreated mice, but the egress of T cells from thymus and the repopulation of lymphoid organs was delayed by FTY720 (Metzler et al., 2008). These data suggest that in FTY720-treated animals, the trafficking of T cell precursors from bone marrow to the thymus is grossly normal, and that a repopulation of lymphoid and peripheral organs with T cells occurs, however, with delayed kinetics.
Does endothelial S1P1 contribute to inhibition of T cell egress?
Initial reports suggested that S1P receptor drugs like FTY720 may act as pure agonists (rather than functional antagonists) and activate endothelial S1P1 to increase barrier function in LNs and prevent egress (Mandala et al., 2002; Sanchez et al., 2003). This concept was further supported by: (i) short-term in vitro studies with serum/S1P-deprived cells that show activation if re-exposed to S1P receptor ligands (Mullershausen et al., 2009); (ii) T cell motility studies in explanted LNs (Wei et al., 2005); and (iii) the finding that novel direct competitive S1P1 antagonists did not block lymphocyte egress in vivo (Sanna et al., 2006). However, in contrast to this concept, administration of the natural S1P receptor agonist S1P promoted, rather than inhibited, egress in SphK-deficient mice (Pappu et al., 2007). Furthermore, studies with explanted LNs may not appreciate S1P gradients and physiological flow that exist between LNs and efferent lymph in vivo, and the ineffective S1P1 antagonists may not be present at sufficiently high concentrations in LNs to mask the endogenous S1P gradient. Accordingly, mice with as little as 3–5% of plasma S1P concentrations of wild-type mice showed normal egress and, thus, synthetic antagonists would have to be exceedingly efficient (Pappu et al., 2007). Indeed, in contrast to the ineffective S1P1 antagonists, S1P1-blocking antibodies with high effective avidity for the receptor were able to inhibit egress, suppressed T cell chemotaxis to S1P in vivo and reduced the severity of colitis (Liao et al., 2009). These considerations further support the concept that natural S1P acts as an agonist at lymphocytic S1P1 receptors to promote egress, and that FTY720 slows egress by down-modulating/internalizing lymphocytic S1P1 (Brinkmann et al., 2004; Matloubian et al., 2004).
Internalization of GPCRs occurs via endocytosis after ligand binding and provides a general mechanism to terminate receptor signalling and regulate receptor degradation and recycling (Marchese et al., 2008). Specific sorting signals, including intracytosolic domains of GPCRs and endocytic adaptor proteins, regulate trafficking of the internalized receptor-ligand complexes through the intracellular endosomal-lysosomal system. The internalized receptor-ligand complexes may continue to signal from endosomes for several hours (Mullershausen et al., 2009), until the ligand is finally shed and the receptor either recycled to the cell membrane or degraded (Marchese et al., 2008). In case of S1P binding to S1P1, internalized receptors are rapidly recycled, whereas S1P1-FTY720-P complexes are sorted from endosomes to lysosomes and fed into the proteasomal degradation pathway (Oo et al., 2007).
Differential trapping of naive and memory T cell subsets
FTY720 differentially affects the recirculation of naive and memory T cells subsets between LNs and the blood (Figure 2). In MS patients, FTY720 primarily reduced the numbers of CCR7+ CD45RA+ naive T cells and of CCR7+ CD45RA− central memory T cells in blood, presumably because these cells express the homing receptor CCR7, recirculate through LNs on a regular basis and, thus, can be trapped by FTY720 (Mehling et al., 2008a). In contrast, CCR7− CD45RA− and CCR7− CD45RA+ effector memory T cell subsets remained in blood. Upon restimulation in vitro, these blood T cells displayed a reduced potential to secrete IL-2 and to proliferate but rapidly produced IFNγ. FTY720 did not directly affect proliferation and cytokine expression (Brinkmann et al., 2001; Mehling et al., 2008a), suggesting that the altered IL-2/IFNγ ratios in the remaining blood T cells reflected a functional effector memory phenotype. Earlier reports had already shown that antigen-specific, long-lived memory T cells preferentially home to non-lymphoid tissue where they can proliferate in response to IL-7 and IL-15 (Sallusto et al., 1999; Masopust et al., 2001; Tan et al., 2002), and that CD8 cells isolated from such tissues exhibit direct cytolytic activity ex vivo (Masopust et al., 2001; Sallusto et al., 2004). Thus, effector memory T cells constitute a reservoir of tissue-resident cells whose function is likely not to be affected by FTY720. In immune-experienced humans, FTY720 preferentially traps CD4 T cells in LNs since they contain predominantly CCR7+ naive and central memory T cells, whereas the blood CD8 T cell pool consists primarily of CCR7− effector memory T cells that are not trapped in LNs (Figure 2).
Figure 2.
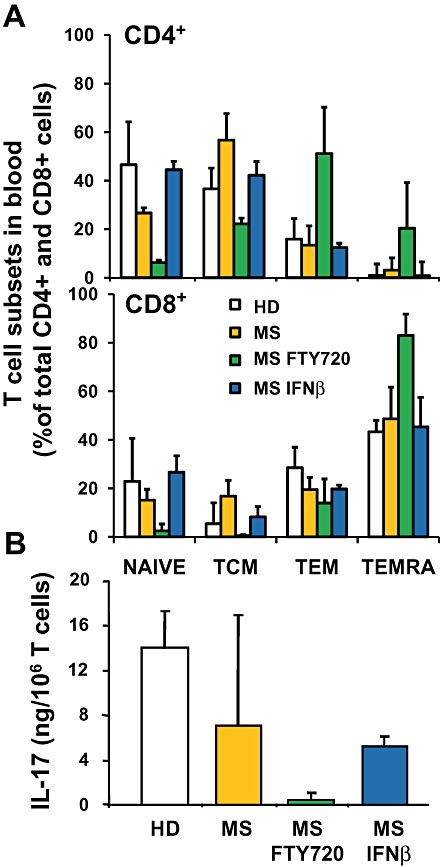
Redistribution of naive, memory and TH17 T cell subsets in FTY720-treated Multiple Sclerosis (MS) patients. (A) The percentage of naive and memory T cell subsets within blood CD4+ and CD8+populations was determined by flow cytometry. The characterized subsets are CCR7+ CD45RA+naive T cells (naive), CCR7+ CD45RA− central memory T cells (TCM), CCR7− CD45RA− effector memory T cells (TEM) and CCR7− CD45RA+ effector memory T cells (TEMRA). The subsets were determined in healthy controls (HD, _n_= 7), drug-untreated MS patients (MS, _n_= 3), IFNβ-treated MS patients (MS-IFNβ, _n_= 3) and FTY720-treated MS patients (MS-FTY720, _n_= 5). (B) The production of IL-17 by equal numbers of anti-CD3/anti-CD28-stimulated CD3+ T cells isolated from indicated donor populations was determined by ELISA (HD, _n_= 5; MS, _n_= 4; MS FTY720, _n_= 6; MS-IFNβ, _n_= 6). Data represent means ± SD.
Trapping of TH17 cells
There is now evidence that the T cells trapped in LNs by FTY720 contain the pro-inflammatory CD4+ TH17 subset (Mehling et al., 2008b). Th17 cells produce IL-17 and IL-22, thereby inducing a massive tissue reaction owing to the broad distribution of the IL-17 and IL-22 receptors. TH17 cells transmigrate efficiently across the blood–brain barrier (BBB) and disrupt tight junctions, highly express granzyme B and kill human neurons, and promote CNS inflammation through additional CD4 T cell recruitment (Kebir et al., 2007). Accordingly, large numbers of TH17 cells are found in brain tissues from MS patients, particularly at the borders of acute and chronic active lesions (Tzartos et al., 2008). In FTY720-treated MS patients, the numbers of circulating CD4+ TH17-like cells (which produced large amounts of IL-17 upon restimulation) were reduced by >95%, and this related to the preferential trapping of CD4+ (compared with CD8+) T cells in LNs. In line with the human data, FTY720 attenuated lesional TH17 cell accumulation in rat EAN, a model of human demyelinating polyneuropathies (Zhang et al., 2009). In sciatic nerves of EAN rats, TH17 cells were found around blood vessels and correlated with severity of neurological signs. FTY720 strikingly reduced TH17 cells in sciatic nerves and this correlated to trapping of these cells in LNs. The data are consistent with the possibility that TH17 cells contribute to the pathogenesis of MS and polyneuropathies, and that FTY720 prevents trafficking of such cells to the CNS and the sciatic nerves (Figure 3).
Figure 3.
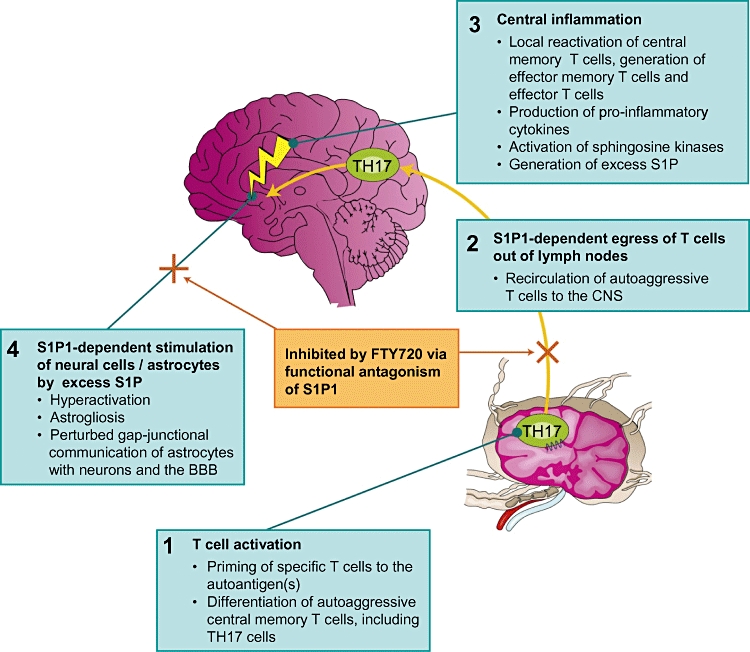
Proposed mode of action of FTY720 in Multiple Sclerosis. The model is based on experiments using conditional sphingosine 1-phosphate (S1P)1 receptor-deficient and sphingosine kinase-deficient mice, and in vivo analysis of FTY720 and S1P. The data suggest that FTY720 functionally antagonizes S1P-S1P1-dependent immune and central processes. This reduces: (i) the egress of TH17 cells from lymphoid organs and their recirculation to the central nervous system (CNS); and (ii) the astrogliosis associated with Multiple Sclerosis, to restore gap-junctional communication between CNS cells. It remains to be determined whether (down-) modulation of S1P3 in astrocytes and of S1P1 and/or S1P5 in oligodendrocytes further contributes to the therapeutic effects.
S1P receptors and dendritic cells
Dendritic cells (DCs) represent the major antigen-presenting cell population that is crucial to the induction of primary immune responses. Depending on their maturation stage and tissue origin, DCs can express mRNA for all S1P receptors (Lan et al., 2005; Idzko et al., 2006), and skin DCs showed activation of S1P1 and S1P3 genes after stimulation by antigen and migration into the LNs (Czeloth et al., 2005). Treatment of mice with FTY720 or the S1P1-selective agent SEW2871 slightly increased the numbers of CD11c+ DCs in blood, while decreasing blood lymphocyte counts (Lan et al., 2005). Later studies proposed that the anti-inflammatory effects of FTY720 in models of asthma (Idzko et al., 2006), skin transplantation (Lan et al., 2008) and allergic contact dermatitis (Reines et al., 2009) may relate, at least in part, to a reduction of DC numbers in the antigen-draining LNs. However, the FTY720-induced redistribution of lymphocytes from blood to LNs may have caused relative rather than absolute changes in DC counts in the analysed tissues, and the anti-inflammatory effect of the drug could relate solely to T cell trapping in LNs. Indeed, general effects of FTY720 on DC function in vivo are unlikely, given the unimpaired antigen-specific T and B cell activation and proliferation observed in models of vaccination and viral/bacterial infections (Pinschewer et al., 2000; Xie et al., 2003; Kursar et al., 2008; Marsolais et al., 2009). Conditional deletion of S1P receptors in macrophage/DC lineages may be needed to determine in more detail the immunological consequences of S1P receptor modulation in DCs.
S1P1 receptor modulation and infection
Effects of FTY720 on anti-viral and anti-bacterial immune responses were studied in models of lymphocytic choriomeningitis virus (LCMV), vesicular stomatitis virus (Pinschewer et al., 2000) and Listeria monocytogenes (Lm) infection (Kursar et al., 2008). Administration of FTY720 at EAE-therapeutic doses of 0.3 mg·kg·day−1 did not impair the generation of specific cytotoxic CD8 T cells in either model, and did not affect the induction of anti-viral humoral immunity that requires functional DCs, CD4+ T cells and B cells (Pinschewer et al., 2000). However, the drug reduced the numbers of antigen-specific naive T cells being recruited from other sites/LNs to the antigen-draining LNs (Xie et al., 2003), and slowed the egress kinetics of antigen-activated T cells from the LNs (Pinschewer et al., 2000; Xie et al., 2003; Kursar et al., 2008). Together these effects may be relevant particularly to primary infections where immune responses develop in the absence of preformed memory T cells present in non-lymphoid tissues and bone marrow.
Interestingly, FTY720 treatment did not affect Lm-specific memory responses in wild-type mice and in lymphotoxin-β receptor-deficient mice that lack functional LNs, and the drug only marginally changed the frequencies and numbers of Lm-specific T cells in LNs, spleen and liver, allowing clearance of the bacteria (Kursar et al., 2008). However, a recall response was profoundly inhibited if intravenously infected mice were splenectomized subsequently to recovery from a primary infection, suggesting a key role of the spleen in generating the observed recall response. It has been suggested that FTY720 may not trap T cells in the spleen because, unlike in LNs, lymphocytes do not egress from the spleen via lymphatics (Mandala et al., 2002; Hofmann et al., 2006). The spleen is specialized to present blood-borne antigens, whereas LNs filter lymph draining from skin or mucosal surfaces, and Peyer's patches obtain antigen by transepithelial transport from the intestinal lumen (Schwab and Cyster, 2007). Taken together, potential effects of S1P receptor drugs on anti-infective immunity may depend on the invasion route of the infectious agent and the numbers of preformed memory T cells in the infected non-lymphoid tissues and the bone marrow.
Notably, FTY720 completely cleared a severe chronic LCMV infection if administered only transiently for 3 days and at very low dosing of 0.004 mg·kg−1 (Premenko-Lanier et al., 2008). Under these conditions, FTY720 treatment augmented LCMV-specific CD4 and CD8 T cell responses, particularly in LNs that are also a significant site of LCMV virus production and pathology. It has been shown earlier that optimal activation of LN T cells by antigen (in the absence of S1P receptor drugs) required T cell receptor-induced down-modulation of S1P1 to delay T cell egress (Matloubian et al., 2004). In situations of chronic infection, suboptimal down-modulation of S1P1 may occur (as a result of low-antigen exposure), and this could be compensated by FTY720. After withdrawal of the drug, the fully activated T cells could now recirculate and fight the virus in the periphery. The demonstration of anti-viral activity of FTY720 is so far limited to the single example of the murine LCMV model, and it remains to be determined whether the effect could translate to the successful treatment of other chronic infections, including those that afflict humans.
A more recent study evaluated effects of a transient, low-dose FTY720 regimen in simian human immunodeficiency virus (SHIVSF162P3)-infected rhesus macaques (Kersh et al., 2009). Under the applied conditions, FTY720 did not induce significant deviations from the natural pattern of viral control and did not change T cell activity throughout the drug course. It is possible that in this model, CD8+ T cells were already controlling the infection to a maximal extent, without FTY720 administration, which could not be improved by the drug. More work is needed to reveal whether FTY720 could be beneficial in more pathogenic SHIV, simian immunodeficiency virus or HIV.
Down-modulation of S1P1 receptors on neural cells
Besides their role in the immune system, S1P receptors may also be critical to the regulation of neural cell migration/function, particularly during central inflammation in MS. In the CNS, S1P receptors are expressed in astrocytes (Sorensen et al., 2003; Wu et al., 2008), OGCs (Jaillard et al., 2005), neurons (Kimura et al., 2006) and microglia/macrophages (Kimura et al., 2006). Furthermore, increased production of S1P has been detected locally in the spinal cord after contusion injury (Kimura et al., 2006), and injection of natural S1P directly into the striata of mice produced astrogliosis (Sorensen et al., 2003), a phenotype also seen in human MS. A recent study demonstrated a functional role of S1P synthesis and S1P receptor expression, particularly S1P3, in astrocyte proliferation leading to astrogliosis during the terminal stages of neurodegeneration in Sandhoff disease, a prototypical neuronopathic lysosomal storage disorder (Wu et al., 2008). Because astrocyte responses are involved in many types of neurodegeneration, the SphK/S1P receptor signalling axis may be generally important during the pathogenesis of neurodegenerative diseases. Interestingly, an abstract reporting preliminary results using a conditional deletion of S1P1 supports a role for astrocytic S1P1 during the progression of EAE (Choi et al., 2008). In this model, astrogliosis was a histopathological feature of EAE, but was attenuated by either conditional S1P1 deficiency in astrocytes or by FTY720 treatment. Taken together, the above data may indicate a role for both S1P1 and S1P3 in promoting astrogliosis, perhaps with differential involvement in early acute and later chronic disease.
FTY720 readily crosses the BBB (Meno-Tetang et al., 2006), resulting in high pM concentrations of free FTY720-P in the cerebrospinal fluid (Foster et al., 2007). At 100 pM concentration, the bioactive _S_-configured FTY720-P internalizes S1P1 and S1P3 receptors in transfected cell lines by 50–80% (unpublished). Thus, the drug may also down-modulate and functionally antagonize these receptors in astrocytes in vivo to modulate their functional properties, without changing astrocyte numbers, and this could reduce the reported negative effects of increased S1P levels and astrocytes on gap junctions between neural cells (Rouach et al., 2006; Choi et al., 2008). This may help restore effective communication of astrocytes with neurons and with endothelial cells in the BBB, as gap junction channels connect the cytoplasm of contacting cells and coordinate electric and metabolic activity (Rouach et al., 2006) (Figure 3).
It remains to be determined whether FTY720 affects other cells of the CNS. In OGCs, FTY720 could produce process extension or retraction in vitro, depending on culture conditions, and induced reciprocal and cyclic modulation of S1P1 and S1P5 mRNA levels (Antel and Miron, 2008; Miron et al., 2008). OGCs expressed particularly high levels of S1P5 mRNA; however, genetic deletion of S1P5 in vivo did not affect the myelination process (Jaillard et al., 2005). In vitro, activation of S1P receptors and down-stream signalling pathways by FTY720 is often observed if cell cultures are performed with serum-starved cells in the absence of S1P (Chen et al., 2001; Osinde et al., 2007). In contrast, the drug down-modulates and functionally antagonizes the very same receptors in vivo to suppress for example S1P-S1P1-dependent egress of T cells from LNs (Matloubian et al., 2004; Grigorova et al., 2009). The data imply that conditional S1P receptor knock-out approaches in specific CNS cell lineages in vivo are needed to unravel the role of individual S1P receptor subtypes in the pathophysiology of EAE/MS.
In neurons, transactivation of distinct S1P receptors by neurite growth factor in vitro modulated neuronal development in a reciprocal manner, whereby S1P1 acted in opposition to S1P2 and S1P5 to coordinate neurite extension (Milstien et al., 2007). Neurons express S1P1 (Chae et al., 2004); however, preliminary data suggest that conditional deletion of S1P1 in neuronal cell lineages may not alter EAE scores and FTY720 efficacy (Choi et al., 2008). In contrast, the sub-cellular distribution of S1P and SphKs in neurons may be critical to their survival (Maceyka et al., 2005), as S1P induced neuronal apoptosis when accumulating above a certain threshold in the endoplasmatic reticulum (Hagen et al., 2009). It remains to be determined whether FTY720 could directly affect neurodegeneration by modulating the intracellular SphK/S1P metabolism to alter S1P-sphingosine-ceramide rheostats.
Experimental autoimmune encephalomyelitis
FTY720 is highly effective as prophylactic and therapeutic treatment in EAE, the rodent model of human MS (Brinkmann et al., 2002; Fujino et al., 2003; Webb et al., 2004; Chiba, 2005; Kataoka et al., 2005; Brinkmann, 2007). As discussed above, the therapeutic effects of FTY720 in all autoimmune models may relate primarily to a reduced recruitment of autoaggressive T cells to the disease-relevant tissues and, in MS, to a direct reduction of astrogliosis.
Recent studies analysed in more detail the effects of FTY720 on inflammation, BBB leakiness, demyelination and nerve conductance in a rat model of EAE (Balatoni et al., 2007; Foster et al., 2009). Therapeutic treatment reversed central inflammation by blocking T cell infiltration, and this was associated with a down-regulation of inflammatory genes and vascular adhesion molecules. Drug treatment further decreased expression of matrix metalloproteinase gene MMP-9 and increased its counter-regulator, the tissue inhibitor of metalloproteinases TIMP-1, resulting in a proteolytic balance that favours preservation of BBB integrity. Accordingly, there was no evidence for immunoglobulin precipitation in the CNS of FTY720-treated animals. Most importantly, late-stage rescue therapy started up to 1 month after EAE onset also reversed inflammatory infiltrates and demyelination, and normalized disturbances to visual and somatosensory evoked action potentials. None of the FTY720-treated animals showed active demyelinating lesions, but they showed Schwann cell remyelinated areas in the spinal cord, whereas vehicle controls exhibited actively demyelinating and inactive demyelinated lesions. In conclusion, the data indicate a rapid blockade of ongoing disease by FTY720, and structural restoration of the CNS parenchyma, which is likely due to inhibition of autoimmune T cell infiltration and direct modulation of neural cells/astrocytes.
Clinical trials
FTY720 was highly effective in Phase II clinical trials involving 255 patients with relapsing remitting MS (Kappos et al., 2006). The median total number of gadolinium-enhanced lesions on magnetic resonance imaging was lower with 1.25 mg of FTY720 (1 lesion, P < 0.001) and 5.0 mg (3 lesions, _P_= 0.006) than with placebo (5 lesions). The annualized relapse rate was 0.77 in the placebo group, as compared with 0.35 in the group given 1.25 mg of FTY720 (_P_= 0.009) and 0.36 in the group given 5.0 mg (_P_= 0.01). During the 2 year extension phase, patients who switched from placebo to FTY720 also showed clear reductions in annualized relapse rates and lesion counts compared with the placebo phase (O'Connor et al., 2009).
FTY720 was generally well tolerated and the safety profile was in line with previous experience (Kappos et al., 2006; O'Connor et al., 2009). The transient reduction of heart rate observed in all FTY720 clinical trials may relate to a short, S1P1-dependent activation of the G protein-gated potassium channel IKAch in atrial myocytes, prior to internalization and/or desensitization of the S1P1 receptors by the drug (Mazurais et al., 2002; Koyrakh et al., 2005; Brinkmann, 2007). A reduction of heart rate comparable to FTY720 was also reported in a Phase I clinical trial exploring the novel S1P1-selective agonist ACT-128800 (http://www1.actelion.com/en/investors/events/actelion-day-2009.page). Earlier studies in mice suggested a preferential role for S1P3 in heart rate regulation (Sanna et al., 2004); however, species differences may exist. Indeed, S1P1 mRNA and protein are strongly expressed in human ventricular, septal and atrial cardiomyocytes, whereas S1P3 is only weakly expressed in cardiomyocytes from both atria and ventricles (Mazurais et al., 2002). The mild increase in blood pressure observed in the FTY720 clinical trials may relate to a down-modulation of S1P1 in vascular endothelium (Oo et al., 2007), which would reduce activation of the vasodilatory endothelial nitic oxide synthase pathway by endogenous S1P (Igarashi et al., 2003; Brinkmann, 2007).
An abstract summarizing results from the 1 year Phase III TRANSFORMS study of FTY720 in relapsing remitting MS has recently been published, and the data suggest superior efficacy of oral FTY720 at 0.5 and 1.25 mg doses compared with a standard of care, the injectable interferon-β 1a (IFNβ) (Avonex®) (Cohen et al., 2009 and Trial watch: Phase III promise for oral MS therapy. _Nat Rev Drug Discov_2009; 8: 98–99). Two other ongoing studies – FREEDOMS and FREEDOMS II – are 2 year placebo-controlled Phase III studies to assess the impact of FTY720 in reducing the frequency of relapses and slowing the progression of disability. In depth analyses of all Phase III studies will soon provide a comprehensive assessment of FTY720's benefit–risk profile.
Conclusions
Current data suggest that FTY720 acts as a functional antagonist of S1P1 receptors. Down-modulation of S1P1 on lymphocytes slows egress kinetics of pro-inflammatory T(H17) cells from LNs, preventing their recirculation to the CNS to reduce central inflammation. Functional antagonism of S1P1 in astrocytes may directly reduce MS-associated astrogliosis to improve gap-junctional communication between cells and allow structural restoration of the CNS parenchyma. Recent clinical trials indicate that FTY720 could provide an effective treatment for human relapsing remitting MS. Further studies are needed to confirm whether short-term, low-dose FTY720 treatment regimens could translate into effective treatments for chronic infections, including those that afflict humans.
Acknowledgments
I gratefully acknowledge the helpful discussions with P. Burtin and S. Aradhye, Novartis Clinical Development. Furthermore, I thank J. Chun and J.W. Choi, The Scripps Research Institute, La Jolla, CA, USA, and L. Kappos and M. Mehling, University Hospital Basel, Switzerland, for fruitful collaborations, and C. Vedrine and K. Kristofic for excellent technical assistance.
Glossary
Abbreviations:
BBB
blood–brain barrier
CNS
central nervous system
DC
dendritic cell
EAE
experimental autoimmune encephalomyelitis
EAN
experimental autoimmune neuritis
FTY720-P
FTY720-phosphate
LCMV
lymphocytic choriomeningitis virus
Lm
Listeria monocytogenes
LN
lymph node
MS
Multiple Sclerosis
OGC
oligodendrocyte
S1P
sphingosine 1-phosphate
SphK
sphingosine kinase
Statement of conflict of interest
V.B. is an employee of the Novartis Institutes of BioMedical Research.
References
- Albert R, Hinterding K, Brinkmann V, Guerini D, Mueller-Hartwieg C, Knecht H, et al. Novel immunomodulator FTY720 is phosphorylated in rats and humans to form a single stereoisomer. identification, chemical proof, and biological characterization of the biologically active species and its inactive enantiomer. J Med Chem. 2005;48:5373–77. doi: 10.1021/jm050242f. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. ). Guide to Receptors and Channels (GRAC) Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allende ML, Yamashita T, Proia RL. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3667. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Antel J, Miron V. Central nervous system effects of current and emerging multiple sclerosis-directed immuno-therapies. Clin Neurol Neurosurg. 2008;110:951–957. doi: 10.1016/j.clineuro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Balatoni B, Storch MK, Swoboda EM, Schönborn V, Koziel A, Lambrou GN, et al. FTY720 sustains and restores neuronal function in the DA rat model of MOG-induced experimental autoimmune encephalomyelitis. Brain Res Bull. 2007;74:307–316. doi: 10.1016/j.brainresbull.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Bartholomäus I, Schläger C, Brinkmann V, Wekerle H, Flügel A. Intravital 2-photon imaging of encephalitogenic effector cells during fingolimod (FTY720) treatment of experimental autoimmune encephalomyelitis. World Congress on Treatment and Research in Multiple Sclerosis (ECTRIMS), Montreal, Canada. Poster P7.
- Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Chen S, Feng L, Pinschewer D, Nikolova Z, Hof R. FTY720 alters lymphocyte homing and protects allografts without inducing general immunosuppression. Transplant Proc. 2001;33:530–531. doi: 10.1016/s0041-1345(00)02126-6. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;4:1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Chae SS, Proia RL, Hla T. Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat. 2004;73:141–150. doi: 10.1016/j.prostaglandins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Chen S, Bacon KB, Garcia G, Liao R, Pan ZK, Sullivan SK, et al. FTY720, a novel transplantation drug, modulates lymphocyte migratory responses to chemokines. Transplant Proc. 2001;33:3057–3063. doi: 10.1016/s0041-1345(01)02306-5. [DOI] [PubMed] [Google Scholar]
- Chiba K. FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther. 2005;108:308–319. doi: 10.1016/j.pharmthera.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Choi JW, Herr D, Lee C-W, Teo S, Kennedy G, Chun J. S1P1 receptor signalling on cells of astrocytic lineages in experimental autoimmune encephalomyelitis: a role in disease progression and the efficacy of fingolimod (FTY720) World Congress on Treatment and Research in Multiple Sclerosis (ECTRIMS) 2008, Montreal, Canada. Poster P29.
- Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, et al. International union of pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- Cohen J, Pelletier J, Kappos L, Montalban X, Hartung HP, Comi G, et al. Oral fingolimod (FTY720) versus Interferon beta-1a in relapsing remitting Multiple Sclerosis: Results from a Phase III study (TRANSFORMS) 61st Annual Meeting of the American Academy of Neurology, Seattle, Washigton, U.S.A. [S21.004.
- Czeloth N, Bernhardt G, Hofmann F, Genth H, Foerster R. Sphingosine-1-phosphate mediates migration of mature dendritic cells. J Immunol. 2005;175:2960–2967. doi: 10.4049/jimmunol.175.5.2960. [DOI] [PubMed] [Google Scholar]
- Foster CA, Howard LM, Schweitzer A, Persohn E, Hiestand PC, Balatoni B, et al. Brain penetration of the oral immunomodulatory drug FTY720 and its phosphorylation in the central nervous system during experimental autoimmune encephalomyelitis: consequences for mode of action in multiple sclerosis. J Pharmacol Exp Ther. 2007;323:469–475. doi: 10.1124/jpet.107.127183. [DOI] [PubMed] [Google Scholar]
- Foster CA, Mechtcheriakova D, Storch MK, Balatoni B, Howard LM, Bornancin F, et al. FTY720 rescue therapy in the Dark Agouti rat model of experimental autoimmune encephalomyelitis: expression of central nervous system genes and reversal of blood-brain-barrier damage. Brain Pathol. 2009;9:254–266. doi: 10.1111/j.1750-3639.2008.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino M, Funeshima N, Kitazawa Y, Kimura H, Amemiya H, Suzuki S, et al. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats by FTY720 treatment. J Pharmacol Exp Ther. 2003;305:70–77. doi: 10.1124/jpet.102.045658. [DOI] [PubMed] [Google Scholar]
- Graeler M, Goetzl EJ. Activation-regulated expression and chemotactic function of sphingosine 1-phosphate receptors in mouse splenic T cells. FASEB J. 2002;16:1874–1878. doi: 10.1096/fj.02-0548com. [DOI] [PubMed] [Google Scholar]
- Grigorova IL, Schwab SR, Phan TG, Pham TH, Okada T, Cyster JG. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat Immunol. 2009;10:58–65. doi: 10.1038/ni.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen N, Van Veldhoven PP, Proia RL, Park H, Merrill AH, Jr, Van Echten-Deckert G. Subcellular origin of sphingosine-1-phosphate is essental for its toxic effect in lyase deficient neurons. J Biol Chem. 2009;284:11346–11353. doi: 10.1074/jbc.M807336200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T. Physiological and pathological actions of sphingosine 1-phosphate. Semin Cell Dev Biol. 2004;15:513–520. doi: 10.1016/j.semcdb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Brinkmann V, Zerwes HG. FTY720 preferentially depletes naive T cells from peripheral and lymphoid organs. Int Immunopharmacol. 2006;6:1902–1910. doi: 10.1016/j.intimp.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Honig SM, Fu S, Mao X, Yopp A, Gunn MD, Randolph GJ, et al. FTY720 stimulates multidrug transporter- and cysteinyl leukotriene-dependent T cell chemotaxis to lymph nodes. J Clin Invest. 2003;111:627–637. doi: 10.1172/JCI16200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idzko M, Hammad H, van Nimwegen M, Kool M, Müller T, Soullié T, et al. Local application of FTY720 to the lung abrogates experimental asthma by altering dendritic cell function. J Clin Invest. 2006;116:2935–2944. doi: 10.1172/JCI28295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factors receptors. Proc Natl Acad Sci USA. 2003;100:10664–10669. doi: 10.1073/pnas.1934494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillard C, Harrison S, Stankoff B, Aigrot MS, Calver AR, Duddy G, et al. Edg8/S1P5: an oligodendroglial receptor with dual function on process retraction and cell survival. J Neurosci. 2005;25:1459–1469. doi: 10.1523/JNEUROSCI.4645-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Sugahara K, Shimano K, Teshima K, Koyama M, Fukunari A, et al. FTY720, sphingosine 1-phosphate receptor modulator, ameliorates experimental autoimmune encephalomyelitis by inhibition of T cell infiltration. Cell Mol Immunol. 2005;2:439–448. [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersh EN, Luo W, Adams DR, Mitchell J, Garcia-Lerma JG, Butera S. Evaluation of the lymphocyte trafficking drug FTY720 in SHIVSF162P3-infected rhesus macaques. J Antimicrob Chemother. 2009;63:758–762. doi: 10.1093/jac/dkp008. [DOI] [PubMed] [Google Scholar]
- Kihara A, Igarashi Y. Production and release of sphingosine 1-phosphate and the phosphorylated form of the immunomodulator FTY720. Biochim Biophys Acta. 2008;1781:496–502. doi: 10.1016/j.bbalip.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Kim RH, Takabe K, Milstien S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta. 2009;1791:692–696. doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A, Ohmori T, Ohkawa R, Madoiwa S, Mimuro J, Murakami T, et al. Essential roles of sphingosine 1-phosphate/S1P1 receptor axis in the migration of neural stem cells toward a site of spinal cord injury. Stem Cells. 2006;25:115–124. doi: 10.1634/stemcells.2006-0223. [DOI] [PubMed] [Google Scholar]
- Koyrakh L, Roman MI, Brinkmann V, Wickman K. The heart rate decrease caused by the sphingosine 1-phosphate receptor agonist FTY720 results from activation of the G protein-gated potassium channel IKACh. Am J Transplant. 2005;5:529–536. doi: 10.1111/j.1600-6143.2005.00754.x. [DOI] [PubMed] [Google Scholar]
- Kursar M, Jänner N, Pfeffer K, Brinkmann V, Kaufmann SH, Mittrücker HW. Requirement of secondary lymphoid tissues for the induction of primary and secondary T cell responses against Listeria monocytogenes. Eur J Immunol. 2008;38:127–138. doi: 10.1002/eji.200737142. [DOI] [PubMed] [Google Scholar]
- Lan YY, De Creus A, Colvin BL, Abe M, Brinkmann V, Coates PT, et al. The sphingosine-1-phosphate receptor agonist FTY720 modulates dendritic cell trafficking in vivo. Am J Transplant. 2005;5:2649–2659. doi: 10.1111/j.1600-6143.2005.01085.x. [DOI] [PubMed] [Google Scholar]
- Lan YY, Tokita D, Wang Z, Wang HC, Zhan J, Brinkmann V, et al. Sphingosine 1-phosphate receptor agonism impairs skin dendritic cell migration and homing to secondary lymphoid tissue: association with prolonged allograft survival. Transpl Immunol. 2008;20:88–94. doi: 10.1016/j.trim.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Liao JJ, Huang MC, Fast K, Gundling K, Yadav M, Van Brocklyn JR, et al. Immunosuppressive human anti-lymphocyte autoantibodies specific for the type 1 sphingosine 1-phosphate receptor. FASEB J. 2009;23:1786–1796. doi: 10.1096/fj.08-124891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Sankala H, Hait NC, Le Stunff H, Liu H, Toman R, et al. SphK1 and SphK2, sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolism. J Biol Chem. 2005;280:37118–37129. doi: 10.1074/jbc.M502207200. [DOI] [PubMed] [Google Scholar]
- McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal. 2004;17:131–139. doi: 10.1016/j.cellsig.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, et al. Alteration of lymphocyte trafficking by sphingosine 1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolais D, Hahm B, Walsh KB, Edelmann KH, McGavern D, Hatta Y, et al. A critical role for the sphingosine analog AAL-R in dampening the cytokine response during influenza virus infection. Proc Natl Acad Sci USA. 2009;106:1560–1565. doi: 10.1073/pnas.0812689106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Mazurais D, Robert P, Gout B, Berrebi-Bertrand I, Laville MP, Calmels T. Cell type-specific localization of human cardiac S1P receptors. J Histochem Cytochem. 2002;50:661–670. doi: 10.1177/002215540205000507. [DOI] [PubMed] [Google Scholar]
- Mehling M, Brinkmann V, Antel J, Bar-Or A, Goebels N, Vedrine C, et al. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology. 2008a;71:1261–1267. doi: 10.1212/01.wnl.0000327609.57688.ea. [DOI] [PubMed] [Google Scholar]
- Mehling M, Lindberg RL, Kuhle J, Vedrine C, Kappos L, Brinkmann V. Oral fingolimod (FTY720) treatment reduces peripheral IL-17-producing TH17 cells in patients with multiple sclerosis. ECTRIMS 2008, Montreal, Canada. Poster P697.
- Meno-Tetang GM, Li H, Mis S, Pyszczynski N, Heining P, Lowe P, et al. Physiologically based pharmacokinetic modeling of FTY720 (2-amino-2[2-(-4-octylphenyl)ethyl]propane-1,3-diol hydrochloride) in rats after oral and intravenous doses. Drug Metab Dispos. 2006;34:1480–1487. doi: 10.1124/dmd.105.009001. [DOI] [PubMed] [Google Scholar]
- Metzler B, Gfeller P, Wieczorek G, Li J, Nuesslein-Hildesheim B, Brinkmann V. Modulation of T cell homeostasis and alloreactivity under continuous FTY720 exposure. Int Immunol. 2008;20:633–644. doi: 10.1093/intimm/dxn023. [DOI] [PubMed] [Google Scholar]
- Milstien S, Gude D, Spiegel S. Sphingosine 1-phosphate in neural signalling and function. Acta Paediatr Suppl. 2007;96:40–43. doi: 10.1111/j.1651-2227.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- Miron VE, Jung CG, Kim HJ, Kennedy TE, Soliven B, Antel JP. FTY720 modulates human oligodendrocyte progenitor process extension and survival. Ann Neurol. 2008;63:61–71. doi: 10.1002/ana.21227. [DOI] [PubMed] [Google Scholar]
- Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL. Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol. 2005;25:11113–11121. doi: 10.1128/MCB.25.24.11113-11121.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullershausen F, Zecri F, Cetin C, Billich A, Guerini D, Seuwen K. Persistent signaling induced by FTY720-phosphate is mediated by internalized S1P1 receptors. Nat Chem Biol. 2009;6:377–378. doi: 10.1038/nchembio.173. [DOI] [PubMed] [Google Scholar]
- O'Connor P, Comi G, Montalban X, Antel J, Radue EW, de Vera A, et al. Oral fingolimod (FTY720) in multiple sclerosis: two-year results of a phase II extension study. Neurology. 2009;72:73–79. doi: 10.1212/01.wnl.0000338569.32367.3d. [DOI] [PubMed] [Google Scholar]
- Okajima S. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta. 2002;1582:132–137. doi: 10.1016/s1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- Osinde M, Mullershausen F, Dev KK. Phosphorylated FTY720 stimulates ERK phosphorylation in astrocytes via S1P receptors. Neuropharmacology. 2007;52:1210–1218. doi: 10.1016/j.neuropharm.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Oo ML, Thangada S, Wu MT, Liu CH, Macdonald TL, Lynch KR, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- Pham TH, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha i-coupled receptors to promote T cell egress. Immunity. 2008;28:122–133. doi: 10.1016/j.immuni.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne NJ, Waters C, Moughal NA, Sambi BS, Pyne S. Receptor tyrosine kinase-GPCR signal complexes. Biochem Soc Trans. 2003;31:1220–1225. doi: 10.1042/bst0311220. [DOI] [PubMed] [Google Scholar]
- Pinschewer DD, Ochsenbein AF, Odermatt B, Brinkmann V, Hengartner H, Zinkernagel RM. FTY720 immunosuppression impairs effector T-cell peripheral homing without affecting induction, expansion, and memory. J Immunol. 2000;164:5761–5770. doi: 10.4049/jimmunol.164.11.5761. [DOI] [PubMed] [Google Scholar]
- Premenko-Lanier M, Moseley NB, Pruett ST, Romagnoli PA, Altman JD. FTY720 treatment promotes immune-mediated clearance of a chronic viral infection. Nature. 2008;454:894–898. doi: 10.1038/nature07199. [DOI] [PubMed] [Google Scholar]
- Reines I, Kietzmann M, Mischke R, Tschernig T, Lüth A, Kleuser B, et al. Topical application of sphingosine-1-phosphate and FTY720 attenuate allergic contact dermatitis reaction through inhibition of dendritic cell migration. J Invest Dermatol. 2009;129:852–853. doi: 10.1038/jid.2008.454. [DOI] [PubMed] [Google Scholar]
- Rouach N, Pébay A, Même W, Cordier J, Ezan P, Etienne E, et al. S1P inhibits gap junctions in astrocytes: involvement of G and Rho GTPase/ROCK. Eur J Neurosci. 2006;23:1453–1464. doi: 10.1111/j.1460-9568.2006.04671.x. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, et al. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J Biol Chem. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2:396–398. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- Sensken SC, Bode C, Graler MH. Accumulation of FTY720 in lymphoid tissues contributes to prolonged efficacy. J Pharmacol Exp Ther. 2008;328:963–969. doi: 10.1124/jpet.108.148163. [DOI] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdicková N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Sorensen SD, Nicole O, Peavy RD, Montoya LM, Lee CJ, Murphy TJ, et al. Common signaling pathways link activation of murine PAR-1, LPA, and S1P receptors to proliferation of astrocytes. Mol Pharmacol. 2003;64:1199–1209. doi: 10.1124/mol.64.5.1199. [DOI] [PubMed] [Google Scholar]
- Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:49–52. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toman RE, Spiegel S. Lysophospholipid receptors in the nervous system. Neurochem Res. 2002;27:619–627. doi: 10.1023/a:1020219915922. [DOI] [PubMed] [Google Scholar]
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meer G, Lisman Q. Sphingolipid transport: rafts and translocators. J Biol Chem. 2002;277:25855–25858. doi: 10.1074/jbc.R200010200. [DOI] [PubMed] [Google Scholar]
- Webb M, Tham CS, Lin FF, Lariosa-Willingham K, Yu N, Hale J, et al. Sphingosine 1-phosphate receptor agonists attenuate relapsing–remitting experimental autoimmune encephalitis in SJL mice. J Neuroimmunology. 2004;153:108–121. doi: 10.1016/j.jneuroim.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Wei SH, Rosen H, Matheu MP, Sanna MG, Wang SK, Jo E, et al. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol. 2005;6:1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- Wu YP, Mizugishi K, Bektas M, Sandhoff R, Proia RL. Sphingosine kinase 1/S1P receptor signaling axis controls glial proliferation in mice with Sandhoff disease. Hum Mol Genet. 2008;17:2257–2264. doi: 10.1093/hmg/ddn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JH, Nomura N, Koprak SL, Quackenbush EJ, Forrest MJ, Rosen H. Sphingosine-1-phosphate receptor agonism impairs the efficiency of the local immune response by altering trafficking of naive and antigen-activated CD4+ T cells. J Immunol. 2003;170:3662–3670. doi: 10.4049/jimmunol.170.7.3662. [DOI] [PubMed] [Google Scholar]
- Zemann B, Kinzel B, Müller M, Reuschel R, Mechtcheriakova D, Urtz N, et al. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107:1454–1458. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Zhang Z, Schluesener HJ. FTY720 attenuates lesional interleukin-17 cell accumulation in rat experimental autoimmune neuritis. Neuropathol Appl Neurobiol. 2009 doi: 10.1111/j.1365-2990.2009.01016.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]