Continued Impact of Pneumococcal Conjugate Vaccine on Carriage in Young Children (original) (raw)
. Author manuscript; available in PMC: 2010 Jul 1.
Published in final edited form as: Pediatrics. 2009 Jul;124(1):e1–11. doi: 10.1542/peds.2008-3099
Abstract
OBJECTIVES
The goals were to assess serial changes in Streptococcus pneumoniae serotypes and antibiotic resistance in young children and to evaluate whether risk factors for carriage have been altered by heptavalent pneumococcal conjugate vaccine (PCV7).
METHODS
Nasopharyngeal specimens and questionnaire/medical record data were obtained from children 3 months to <7 years of age in primary care practices in 16 Massachusetts communities during the winter seasons of 2000–2001 and 2003–2004 and in 8 communities in 2006–2007. Antimicrobial susceptibility testing and serotyping were performed with S pneumoniae isolates.
RESULTS
We collected 678, 988, and 972 specimens during the sampling periods in 2000–2001, 2003–2004, and 2006–2007, respectively. Carriage of non-PCV7 serotypes increased from 15% to 19% and 29% (P < .001), with vaccine serotypes decreasing to 3% of carried serotypes in 2006–2007. The relative contribution of several non-PCV7 serotypes, including 19A, 35B, and 23A, increased across sampling periods. By 2007, commonly carried serotypes included 19A (16%), 6A (12%), 15B/C (11%), 35B (9%), and 11A (8%), and high-prevalence serotypes seemed to have greater proportions of penicillin nonsusceptibility. In multivariate models, common predictors of pneumococcal carriage, such as child care attendance, upper respiratory tract infection, and the presence of young siblings, persisted.
CONCLUSIONS
The virtual disappearance of vaccine serotypes in S pneumoniae carriage has occurred in young children, with rapid replacement with penicillin-nonsusceptible nonvaccine serotypes, particularly 19A and 35B. Except for the age group at highest risk, previous predictors of carriage, such as child care attendance and the presence of young siblings, have not been changed by the vaccine.
Keywords: Streptococcus pneumoniae, pneumococcal conjugate vaccine, antibiotic resistance, serotype, colonization
WHAT's KNOWN ON THIS SUBJECT
IPD rates decreased after the release of PCV7 but now are increasing because of non-PCV7 isolates. We showed previously that serial collection of colonizing strains can forecast increases in non-PCV7 isolates that may be sources of later disease.
WHAT THIS STUDY ADDS
This study shows rapid, nearly complete replacement of colonizing PCV7 strains by non-PCV7 strains in young children. Some previously common risk factors for carriage have changed, which suggests that serotype changes may be challenging our previous knowledge of pneumococcal transmission.
The heptavalent pneumococcal conjugate vaccine (PCV7) has reduced substantially the rates of invasive pneumococcal disease (IPD) in children.1–5 It also has produced modest decreases in rates of common childhood noninvasive diseases such as otitis media.6 Through herd immunity, it has reduced rates of IPD in adults and immunocompromised hosts,1,2,7 including disease among HIV-positive individuals.8
The ability of this vaccine to maintain reductions in IPD rates has been questioned, however, because nonvaccine serotypes have increased in the absence of vaccine serotypes.9–11 Evidence is mounting that nonvaccine serotypes are responsible for increasing proportions of IPD,1,12–16 which raises the question of whether reductions in IPD rates may be attenuated without use of a vaccine targeting broader serotypes. Numerous reports have highlighted the increase in nonvaccine serotype 19A involvement in both local disease and IPD14–17 and provided evidence for recombination events between vaccine serotypes and nonvaccine serotypes that can confer an antibiotic-resistant profile to a previously susceptible serotype.18–21
Changes in the serotype distribution of IPD are inextricably connected to the serotype distribution of Streptococcus pneumoniae colonization among children.9–11,22 Because colonization rates are highest in young children, children serve as a natural reservoir for invasive strains and represent the environment in which continued evolution of S pneumoniae (including resistance and invasiveness factors) occurs. Therefore, it is both critical and informative to understand the changing population biology of this pathogen under the combined selective pressures of universal immunization and frequent antibiotic use in this age group.23–25 We sampled the nasopharynx of young children in 16 Massachusetts communities in 200126 and 2004,10 during pediatric office visits, and we observed substantial serotype replacement by nonvaccine serotypes and increases in penicillin-nonsusceptible S pneumoniae (PNSP) strains among nonvaccine serotypes in 2004, compared with 2001.10,22 We showed that the increase in PNSP was driven by both clonal expansion of resistant strains in 15A and 35B serotypes and increasing strain diversity in 19A serotypes.22
We now report continuing analysis of S pneumoniae carriage in 8 of the Massachusetts communities, to evaluate ongoing changes in serotype replacement and penicillin resistance. We include an assessment of the impact of recent changes in cutoff points for penicillin nonsusceptibility established by the Clinical and Laboratory Standards Institute (CLSI) and adopted by the Food and Drug Administration in March 2008.27,28 These elevated breakpoints are intended to better reflect clinical relevance but may obscure incremental increases in resistance.
In addition, because colonization often is a precursor to IPD, we were interested in assessing whether known predictors of colonization have changed in association with the introduction of PCV7. In particular, we wanted to assess whether the strength of association with previously known predictors, such as group child care and young siblings, was reduced after PCV7 immunization.
METHODS
Data Collection
Nasopharyngeal specimens were collected from children 3 months to <7 years of age in pediatric practices in 8 Massachusetts communities during the winter respiratory virus season, from October 2006 to April 2007. These 8 communities were a subset of 16 geographically distinct areas chosen on the basis of evidence that few children crossed community boundaries for pediatric care.29 Similarly designed and conducted specimen collections were performed in these 16 communities during the winter respiratory virus seasons of 2001 (March to May)26 and 2003–2004 (November to April),10 as part of a cluster-randomized trial of a community-level intervention to promote judicious antibiotic-prescribing for children.29 Eight practices participated in 2007. These 8 practices were among the largest of the 23 participating practices in 2004 and the 31 practices in 2001.
Parents of participating children completed a brief survey (which was used previously) on possible predictors of pneumococcal carriage, with questions on group child care, young siblings, recent antibiotic use, and concurrent illness.26 Parents of participating children also consented to subsequent medical record review for information on vaccines received, recent antibiotic use, and symptoms and diagnoses at the time of the visit. Parental consent was obtained by study staff members, and nasopharyngeal swabs were obtained by trained nurses during well-child or sick visits. All study procedures were approved by the Harvard Pilgrim Health Care institutional review board.
Microbiologic Processing
Nasopharyngeal samples were processed within 24 hours. All pneumococcal isolates underwent antibiotic susceptibility testing and serotyping (using the Quellung reaction) as described previously.10,26 CLSI susceptibility breakpoints were used to classify organisms as susceptible, intermediate, or resistant to the following antibiotics: penicillin, amoxicillin, ceftriaxone, erythromycin, clindamycin, trimethoprim-sulfamethoxazole, levofloxacin, and vancomycin.27,28
Data Analysis
To assess whether analyses should be restricted to the 8 communities that participated in all 3 sampling periods or whether data from all 16 communities could be used in the evaluation of trends, we performed 2 analyses. First, we assessed whether the populations of serotypes were significantly distinct among communities within the same sampling period. We did this by using Monte Carlo hypothesis testing,30 repeatedly reassigning serotypes randomly to communities within a sampling period, and assessing where the observed serotype distribution lay among the many random assessments. Specific permutations of serotypes were compared by using a classification index described elsewhere.31 Second, we used a second Monte Carlo test to assess whether communities retained a unique mixture of serotypes over time. The intracommunity distribution of serotypes between sampling periods was compared by using the classification index. This was subsequently compared with observed distributions when random reassignments of communities as pairs were made. The first assessment showed no evidence that communities differed in profiles from the grouped communities. The second assessment detected no evidence that single communities were stable over time. Together, these tests support the use of all communities available in each of the 3 sampling periods (see Appendix). In addition, previous work with prescribing data from several large health insurers29 showed identical antibiotic usage rates (1.5 antibiotics per person-year) among children 2 to 4 years of age when communities included in 2007 were compared with those not included.
We calculated the proportions of children carrying any S pneumoniae isolate and non-PCV7 serotypes during the 3 sampling periods. In contrast to the 2007 sample, previous sampling periods included infants <3 months of age. Therefore, we excluded all infants <3 months of age, for comparability across periods. Age groups assessed were 3 to <6 months, 6 to <24 months, 24 to <36 months, and 36 to <84 months.10,26 We also calculated the proportions of children carrying S pneumoniae isolates that were not susceptible to antibiotics. Previous susceptibility breakpoints for penicillin (minimal inhibitory concentration [MIC] of ≤0.06 mg/L)28 were compared with new 2008 breakpoints (MIC of ≤2.0 mg/L) established by the CLSI.27 Two definitions of multidrug resistance were used, that is, resistance to the _β_-lactam class plus 1 other antibiotic class (multidrug-resistant group 1) and resistance to ≥3 antibiotic classes (multidrug-resistant group 2).10 Penicillins, cephalosporins, and carbapenems were considered a single class.
We further evaluated whether the prevalence of serotype-specific carriage in 2007 was associated with the proportion of serotype-specific penicillin nonsusceptibility. To do this, we created a scatterplot and fit a logistic regression curve, reporting the odds ratio (OR) for the association between antibiotic resistance and serotype prevalence.
Changes in specific serotypes were calculated on the basis of their respective proportions in previously reported sampling periods.10 Fisher's exact tests were used to assess differences among the sampling periods. All analyses were performed with SAS 9.1 (SAS Institute, Cary, NC).
We calculated the proportions of children with characteristics (determined on the basis of survey or chart review) associated with pneumococcal carriage in previous sampling periods,26 including age, group child care, young siblings, recent antibiotic use, and acute respiratory tract infection (RTI). Acute RTI was defined as confirmation of symptoms on questionnaires or any medical record diagnosis of any upper or lower RTI, including otitis media, sinusitis, bronchitis, pharyngitis, cough illness, and pneumonia. These characteristics were entered into generalized linear mixed models predicting carriage of S pneumoniae, PNSP, and non-PCV7 serotypes. From models restricted to individual sampling periods, we calculated not only the ORs of various covariates for the specified outcomes but also the probabilities of carriage associated with certain combinations of covariates. In a second set of models predicting the same outcomes, we included data from all sampling periods and evaluated sampling year as a covariate. In these models, based upon effect modification sampling year was assessed for all significant variables. All models accounted for clustering of data within communities by using general linear mixed models.32
RESULTS
Nasopharyngeal Colonization With S pneumoniae
Nasopharyngeal specimens were obtained from 2638 children. According to sampling period, there were 678, 988, and 972 child participants in 2001, 2004, and 2007, respectively. Community and individual characteristics are provided in Tables 1 and 2. PCV7 vaccine penetration (receipt of ≥1 dose) increased across the sampling periods but particularly between 2001 and 2004. Among children ≥12 months of age, vaccine penetration increased from 38% in 2001 to 79% in 2004 and 98% in 2007. Among children <12 months of age, vaccine penetration increased from 83% in 2001 to 96% in 2004 and 98% in 2007.
TABLE 1.
Characteristics of Participating Massachusetts Communities, Including Pneumococcal Carriage
| Community | Population | Family Income, Median, $ | Nonwhite, % | 2001 | 2004 | 2007 | |||
|---|---|---|---|---|---|---|---|---|---|
| N | Carriage, n (%) | N | Carriage, n (%) | N | Carriage, n (%) | ||||
| 1 | 28 000 | 67 000 | 3 | 34 | 11 (32) | 43 | 6 (14) | ||
| 2 | 30 000 | 50 000 | 7 | 86 | 23 (27) | 63 | 9 (14) | ||
| 3 | 30 000 | 59 000 | 3 | 41 | 20 (49) | 73 | 10 (14) | 104 | 25 (24) |
| 4 | 34 000 | 56 000 | 2 | 31 | 5 (16) | 78 | 29 (37) | 123 | 34 (28) |
| 5 | 37 000 | 93 000 | 10 | 25 | 6 (24) | 42 | 12 (29) | ||
| 6 | 38 000 | 48 000 | 3 | 33 | 6 (18) | 79 | 21 (27) | 82 | 25 (30) |
| 7 | 40 000 | 45 000 | 5 | 54 | 12 (22) | 30 | 8 (27) | ||
| 8 | 46 000 | 36 000 | 7 | 32 | 8 (25) | 53 | 13 (25) | 118 | 42 (36) |
| 9 | 52 000 | 56 000 | 5 | 30 | 8 (27) | 39 | 6 (15) | ||
| 10 | 52 000 | 76 000 | 5 | 36 | 11 (31) | 80 | 22 (28) | 155 | 55 (35) |
| 11 | 67 000 | 52 000 | 7 | 39 | 13 (33) | 42 | 2 (5) | ||
| 12 | 80 000 | 41 000 | 15 | 45 | 8 (18) | 58 | 15 (26) | 142 | 37 (26) |
| 13 | 94 000 | 40 000 | 39 | 71 | 20 (28) | 95 | 14 (15) | 124 | 46 (37) |
| 14 | 102 000 | 32 000 | 8 | 45 | 19 (42) | 73 | 25 (34) | ||
| 15 | 1 15 000 | 33 000 | 19 | 44 | 8 (18) | 72 | 23 (32) | ||
| 16 | 139 000 | 47 000 | 25 | 32 | 8 (25) | 68 | 16 (24) | 124 | 30 (24) |
TABLE 2.
Characteristics of Massachusetts Children Who Provided Nasopharyngeal Specimens
| n (%) | |||
|---|---|---|---|
| 2001 | 2004 | 2007 | |
| Total | 678 | 988 | 972 |
| Age | |||
| 3 to <6 mo | 59 (9) | 49 (5) | 76 (8) |
| 6 to <24 mo | 256 (38) | 349 (35) | 428 (44) |
| 24 to <36 mo | 104 (15) | 143 (14) | 130 (13) |
| 36 to <84 mo | 259 (38) | 447 (45) | 338 (35) |
| Recent antibiotic use (within 2 mo) | 287 (42) | 288 (39) | 390 (40) |
| Child care attendance | 301 (47) | 480 (51) | 458 (50) |
| RTI at time of specimen collection | 192 (29) | 263 (27) | 448 (46) |
| No. of young siblings | |||
| 0 | 339 (53) | 722 (75) | 500 (54) |
| 1 | 239 (37) | 205 (21) | 345 (37) |
| >1 | 62 (10) | 35 (4) | 85 (9) |
Pneumococcal carriage varied significantly across the years, with 27%, 23%, and 30% of children carrying S pneumoniae in 2001, 2004, and 2007, respectively (P = .003). The unadjusted proportions of children colonized with S pneumoniae within age groups differed among the sampling periods. Among children 3 to <6 months of age, carriage rates increased over time (2001: 10%; 2004: 22%; 2007: 30%; P = .02); among children ≥36 months of age, there was variability but not a monotonic trend (2001: 30%; 2004: 19%; 2007: 26%; P = .006). However, much of this unadjusted variation based upon year within age groups was explained by variations in RTI rates (Table 3). Age, RTI, and other potential confounders were adjusted for in the final model (described below).
TABLE 3.
Proportions of Children With Pneumococcal Colonization, Stratified According to Age, Sampling Year, and Presence of Upper RTI
| Age Group | % (n) | ||
|---|---|---|---|
| 2001 | 2004 | 2007 | |
| RTI | |||
| <6 mo | 22.2 (2) | 36.4 (4) | 40.9 (9) |
| 6 to <24 mo | 42.4 (36) | 33.0 (34) | 39.8 (76) |
| 24 to <36 mo | 60.6 (20) | 61.9 (26) | 63.8 (44) |
| ≥36 mo | 41.5 (27) | 19.6 (21) | 27.1 (45) |
| No RTI | |||
| <6 mo | 8.3 (4) | 18.4 (7) | 25.9 (14) |
| 6 to <24 mo | 23.6 (39) | 22.7 (55) | 26.6 (63) |
| 24 to <36 mo | 17.1 (12) | 26.5 (26) | 32.8 (20) |
| ≥36 mo | 26.2 (50) | 19.8 (65) | 24.4 (42) |
Emergence of Nonvaccine Serotypes
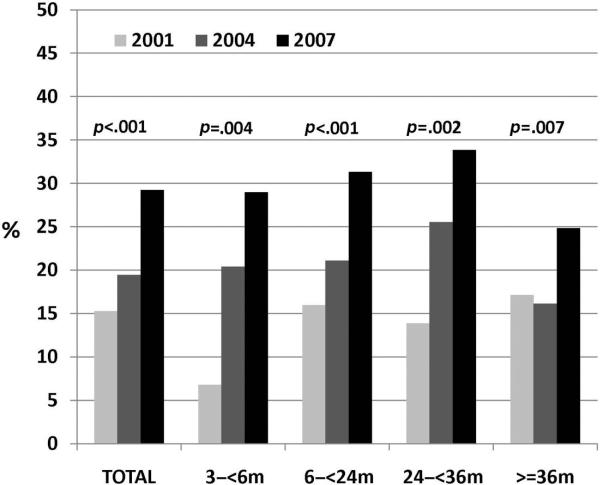
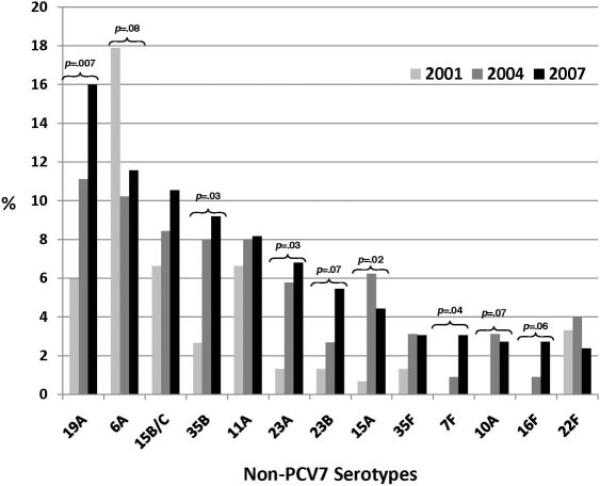
The proportion of children colonized with nonvaccine serotypes increased markedly across the 3 sampling periods. Non-PCV7 carriage rates were 15% (97 of 643 children) in 2001, 19% (191 of 982 children) in 2004, and 29% (284 of 972 children) in 2007 (P < .001). Increases were noted for all age groups (Fig 1). By 2007, the most commonly carried serotypes were 19A (16%), 6A (12%), 15B/C (11%), 35B (9%), and 11A (8%). The most notable absolute increases were in serotype 19A (from 6% to 11% to 16%; P = .007), 35B (from 3% to 8% to 9%; P = .03), and 23A (from 1% to 6% to 7%; P = .03). Overall, 35 nonvaccine serotypes were identified across all samples. Individually, with the exception of 6A and 22F, increases were seen among the 13 most common nonvaccine serotypes when 2001 and 2007 were compared (Fig 2). The remaining nonvaccine serotypes (not depicted in Fig 2) constituted 52% (2001), 28% (2004), and 14% (2007) of total serotypes. Among S pneumoniae carriers, carriage of vaccine serotypes decreased from 36% (n = 54) in 2001 to 15% (n = 34) in 2004 and 3% (n = 10) in 2007 (P < .001). The further segregation of serotype 6A into recently discovered 6A and 6C components through specialized means is reported elsewhere.33 The proportion of 6C among combined 6A/6C serotypes increased from 12% in 2001 to 22% in 2004 and 81% in 2007.33
FIGURE 1.
Proportions of children carrying non-PCV7 pneumococcal serotypes within each sampling period, according to age. P values of ≤.05 based on 2-tailed Fisher's exact tests are indicated.
FIGURE 2.
Distribution of non-PCV7 pneumococcal serotypes, as proportions of total serotypes, according to sampling period. P values of ≤.1 based on 2-tailed Fisher's exact tests evaluating differences in serotype-specific proportional carriage are indicated.
Changes in Antimicrobial Susceptibility Patterns
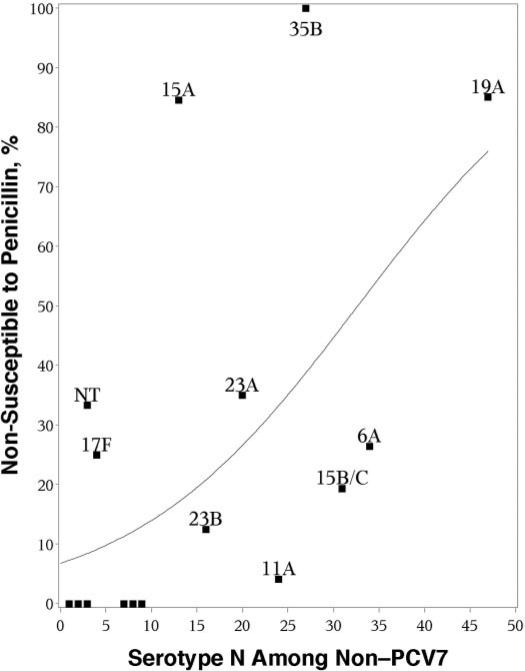
Changes in antibiotic susceptibility among nonvaccine serotypes are presented in Table 4. Proportional antibiotic resistance differed significantly across the 3 sampling periods for clindamycin, ceftriaxone, and strains with intermediate resistance to penicillin. The most common nonvaccine serotypes by 2007 were those with the highest proportional penicillin nonsusceptibility (using MIC of >0.06 mg/L). A logistic regression fit to the scatterplot of serotype-specific penicillin nonsusceptibility and the number of isolates of a given serotype in 2007 revealed an OR of 1.08 (95% confidence interval [CI]: 1.06–1.1) (Fig 3). This suggests an association between serotype-specific prevalence and increases in the proportional penicillin nonsusceptibility of that serotype.
TABLE 4.
Antibiotic Resistance Among Nonvaccine Serotypes According to Surveillance Period
| Year | N | % (n) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Penicillin, Intermediate-Level Resistancea | Penicillin, High-Level Resistance | PNSPb | Erythromycin, NS | Clindamycin, NS | Trimethoprim-Sulfamethoxazole, NS | Ceftriaxone, NS | MDR1c | MDR2d | ||
| 2001 | 97 | 13 (13) | 15 (15) | 29 (28) | 15 (14) | 0 | 30 (28) | 11 (10) | 21 (20) | 6 (6) |
| 2004 | 191 | 26 (50) | 9 (18) | 36 (68) | 21 (40) | 9 (17) | 18 (35) | 2 (3) | 21 (41) | 14 (27) |
| 2007 | 284 | 25 (70) | 12 (35) | 37 (105) | 19 (55) | 7 (20) | 19 (55) | 5 (13) | 20 (58) | 13 (38) |
| Pe | .03 | .32 | .35 | .52 | .004 | .07 | .003 | .96 | .11 |
FIGURE 3.
Scatterplot examining the association between the prevalence of pneumococcal isolates among non-PCV7 isolates and their proportional penicillin nonsusceptibility (MIC of >0.06 mg/L) in 2007.
Although the proportion of non-PCV7 isolates with intermediate nonsusceptibility (MIC of 0.12–1.0 mg/L) to penicillin seemed to increase with the use of previous breakpoints, new breakpoints showed that high-level penicillin resistance decreased significantly among nonvaccine serotypes, from 7.2% in 2001 to 1.1% in 2004 and 3.5% in 2007 (P = .02). All nonsusceptible isolates were classified as having intermediate susceptibility with the use of revised breakpoints.
Changes in Predictors of Carriage
Models assessing predictors of S pneumoniae carriage showed that group child care was a strong predictor of S pneumoniae, PNSP, and non-PCV7 carriage in 2007, as in previous studies. However, age and RTI had different effects across the sampling periods, as shown by the significance of interaction terms between year and these covariates (Table 5). When these effects were assessed in stratified models for each sampling period, older children seemed relatively less likely to carry S pneumoniae, compared with younger children, after the introduction of PCV7 (Table 5). This effect was attributable to the increase in carriage among the youngest age group (<6 months). RTI remained predictive of S pneumoniae carriage in all sampling periods, but this association was somewhat decreased in later time periods because of a lack of effect on PNSP carriage.
TABLE 5.
Predictors of Pneumococcal Carriage, PNSP Carriage, and Non-PCV7 Carriage According to Sampling Year
| Predictors of Carriage | OR (95% CI) | P for Interaction with Yeara | ||
|---|---|---|---|---|
| 2001 | 2004 | 2007 | ||
| Streptococcus pneumoniae | ||||
| Age | .02 | |||
| 3 to <6 mo | 1 | 1 | 1 | |
| 6 to <24 mo | 4.0 (1.5–11.0)b | 1.2 (0.6–2.6) | 1.2 (0.7–2.2) | |
| 24 to <36 mo | 2.0 (0.7–6.0) | 1.3 (0.6–2.9) | 1.1 (0.6–2.3) | |
| ≥36 mo | 2.6 (1.0–7.3) | 0.6 (0.3–1.2) | 0.5 (0.3–1.0)b | |
| RTI | 2.4 (1.7–3.6)b | 1.3 (1.0–1.9) | 1.4 (1.0–1.9)b | .03 |
| Group child care | 2.4 (1.6–3.7)b | 2.0 (1.4–2.9)b | 2.2 (1.6–3.2)b | .90 |
| Young siblings | .79 | |||
| 0 | 1 | 1 | 1 | |
| 1 | 1.5 (1.0–2.2)b | 1.3 (0.9–1.9) | 1.3 (0.9–1.8) | |
| >1 | 2.8 (1.5–5.2)b | 2.0 (0.9–4.3) | 1.7 (1.0–2.8)b | |
| Recent antibiotic use | 0.6 (0.4–0.9)b | 0.7 (0.5–1.0)b | 0.7 (0.5–0.9)b | .85 |
| PNSPc | ||||
| Age | .11 | |||
| 3 to <6 mo | 1 | 1 | 1 | |
| 6 to <24 mo | 5.2 (0.6–42.1) | 0.5 (0.2–1.3) | 1.0 (0.4–2.2) | |
| 24 to <36 mo | 1.6 (0.2–15.1) | 0.7 (0.3–1.9) | 0.5 (0.2–1.5) | |
| ≥36 mo | 1.8 (0.2–14.6) | 0.3 (0.1–0.7)b | 0.4 (0.1–0.9)b | |
| RTI | 4.2 (2.3–7.9)b | 0.9 (0.6–1.5) | 0.9 (0.6–1.4) | <.0001 |
| Group child care | 3.6 (1.8–7.3)b | 2.2 (1.3–3.7)b | 2.3 (1.4–3.9)b | .50 |
| Young siblings | .54 | |||
| 0 | 1 | 1 | 1 | |
| 1 | 1.1 (0.6–2.1) | 1.0 (0.5–1.8) | 0.9 (0.6–1.5) | |
| >1 | 1.6 (0.6–4.7) | 3.0 (1.1–7.6)b | (0.4–2.2) | |
| Recent antibiotic use | 1.4 (0.7–2.5) | 1.7 (1.0–2.6)b | 1.2 (0.8–1.9) | .66 |
| Non-PCV7 | ||||
| Age | .29 | |||
| 3 to <6 mo | 1 | 1 | 1 | |
| 6 to <24 mo | 2.4 (0.8–7.3) | 1.1 (0.5–2.4) | 1.2 (0.7–2.3) | |
| 24 to <36 mo | 1.3 (0.4–4.5) | 1.2 (0.5–2.7) | 1.2 (0.6–2.4) | |
| ≥36 mo | 1.6 (0.5–5.0) | 0.5 (0.2–1.2) | 0.5 (0.3–1.1) | |
| RTI | 1.9 (1.2–3.1)b | 1.3 (0.9–1.8)b | 1.3 (1.0–1.8) | .29 |
| Group child care | 2.7 (1.6–4.7)b | 2.2 (1.5–3.2)b | 2.2 (1.5–3.2)b | .78 |
| Young siblings | .96 | |||
| 0 | 1 | 1 | 1 | |
| 1 | 1.2 (0.7–1.9) | 1.2 (0.8–1.8) | 1.2 (0.9–1.7) | |
| >1 | 2.0 (1.0–4.2) | 1.3 (0.5–3.1) | 1.7 (1.0–2.8)b | |
| Recent antibiotic use | 0.5 (0.3–0.8)b | 0.7 (0.5–0.9)b | 0.7 (0.5–0.9)b | .68 |
In addition to the relative increase in risk (ORs) indicated in Table 5, we provided calculated estimates of carriage prevalence for children with specific risk factors, on the basis of model-based predicted probabilities of non-PCV7 carriage (Table 6). For example, in 2007, a child 6 to <24 months of age without additional risk factors had a 24% risk of non-PCV7 carriage. This 24% risk increased to 41% if the child was in group child care, and risk was as high as 61% if the child also had young siblings and a concurrent RTI. Recent antibiotic use only mildly mitigated the risk of non-PCV7 carriage (from 61% to 52%). These risks were quite similar for children 3 to <6 months and 24 to <36 months of age, but risks were 25% to 50% lower for children ≥36 months of age. Overall, non-PCV7 carriage ranged from 5% to 61%, depending on age and risk factors.
TABLE 6.
Probability of Non-PCV7 Carriage According to the Presence of Select Individual Predictors
| Age, mo | Child Care | >1 Young Sibling | RTI | Recent Antibiotic Use | Probability of Non-PCV7 Carriage | |
|---|---|---|---|---|---|---|
| 2001 | 2004 | 2007 | ||||
| 3 to <6 | .05 | .17 | .20 | |||
| X | .13 | .31 | .36 | |||
| X | X | X | .37 | .42 | .56 | |
| X | X | X | X | .23 | .32 | .46 |
| 6 to <24 | .12 | .18 | .24 | |||
| X | .27 | .33 | .41 | |||
| X | X | X | .58 | .44 | .61 | |
| X | X | X | X | .42 | .34 | .52 |
| 24 to <36 | .07 | .19 | .23 | |||
| X | .17 | .34 | .40 | |||
| X | X | X | .44 | .46 | .60 | |
| X | X | X | X | .29 | .36 | .51 |
| ≥36 | .08 | .10 | .12 | |||
| X | .19 | .19 | .23 | |||
| X | X | X | .48 | .27 | .41 | |
| X | X | X | X | .33 | .20 | .32 |
DISCUSSION
Some replacement in pneumococcal carriage with nonvaccine serotypes has been reported since the release of PCV7.9–11,22 This was associated with initial sharp decreases in rates of IPD attributable to vaccine serotypes,1–5,7,8 followed by increases in rates of IPD attributable to nonvaccine strains.1,12–17 Some of these effects on colonization and IPD rates were demonstrated in Massachusetts.10,22,34,35 Through the continued sampling of young children in Massachusetts communities, we now show that nonvaccine serotypes have virtually completely replaced colonizing vaccine serotypes within the 7 years since the release of PCV7. Replacement was correlated temporally with vaccine penetration and was rapid and relatively complete, compared with expectations based on incomplete effects on colonization during vaccine trials.36–38 These results suggest that the risk of pneumococcal disease, although clearly lower than in the prevaccine era, may continue to change in the absence of immunization against noncovered serotypes.
As vaccine serotypes disappear, nonvaccine serotypes have been increasing rapidly in all age groups. Common nonvaccine serotypes found in 2004 have become even more prevalent, with the proportions of infrequent serotypes decreasing. We report significant increases in 19A, 35B, 23A, and 7F prevalence, as well as trends toward increased prevalence of 23B and 16F. Although 19A has already been shown to be responsible for an increasing proportion of IPD, it remains to be seen whether these other serotypes will have similar invasive potential.
We suggest that the selective advantage of penicillin nonsusceptibility may explain in part the increase in specific serotypes. High rates of antibiotic use for common conditions such as acute otitis media may provide pressure in favor of resistant strains among colonized children.39–41 Previous genetic typing work suggested that clonal expansion of serotypes 19A, 35B, and 15A has favored strains that are resistant to penicillin, such as sequence type 199 among 19A serotypes, sequence type 558 among serogroup 35B isolates, and sequence type 63 among 15A serotypes.22,42
The revised national breakpoints for penicillin susceptibility are intended to reflect more closely the likelihood of clinical failure in response to antibiotic therapy. However, attention to the expansion of clones associated with resistance genes and incremental increases in MICs for common antibiotics can warn of impending loss of therapeutic efficacy before detection with these higher laboratory thresholds. Continued evaluation of the changing epidemiological features of pneumococcal serotypes, strain types, and phenotypic resistance is important during a time when the population dynamics of S pneumoniae are changing rapidly and invasive potential remains to be seen.
Despite the nearly complete replacement of vaccine serotypes by nonvaccine serotypes, we found that predictors of carriage remained quite similar between 2001 and 2007, with the exception of age and RTI. This change in age as a risk factor came as a surprise and seemed to be associated with higher colonization rates in young infants. Because we had no previous hypothesis in this direction, additional work will be required to determine whether this apparent trend is real. In later sampling periods, we found that RTI was less strongly associated with S pneumoniae carriage, a finding that seemed to be mediated by a loss of effect on PNSP carriage. Whether this is related to the changing serotype representation of PNSP isolates is not known. Child care persisted as the strongest predictor of pneumococcal carriage, with young siblings and RTI continuing to confer added independent risk. Together, these risk factors conferred a 2.5- to 3-fold increased risk of carriage in all age groups examined.
The persistence of pneumococcal carriage in 30% of young children through the post-PCV7 era and the continued influence of these common characteristics on carriage suggest that reductions in IPD rates will be influenced by the capacity of specific serotypes to produce IPD when carried, rather than by a vaccine-induced reduction in overall carriage rates. Knowledge of the dynamic changes in carried serotypes will be invaluable for understanding invasive potential. For example, we observed minimal evidence of serotype 3 colonization (<1%) in this large multi-community study of young children. Recent reports of increased rates of serotype 3 IPD in some areas of the United States might be better understood in the context of whether increases in serotype 3 carriage prevalence occurred previously.43,44
There are several limitations to this study. We sampled serially during winter respiratory virus seasons to assess changes in the S pneumoniae reservoir during peak times of annual carriage and transmission risk. We cannot know whether changes in carriage or the distributions of S pneumoniae serotypes are similar throughout the year or whether we adjusted completely for differences in respiratory illness rates between sampling periods. In addition, sampling in physician offices may bias our data toward serotypes that are cultured more easily during respiratory illnesses. The frequency of well-child visits and our adjustment for respiratory illness should have mitigated these effects, and the inclusion of children with respiratory illnesses allows assessment of pneumococcal carriage related to a common illness state in this age group. Finally, we did not examine the possibility that children carried multiple strains simultaneously. If this was common, this might have altered perceptions of antibiotic resistance and the relative proportions of nonvaccine serotypes.
CONCLUSIONS
We report the virtual disappearance of vaccine serotypes among young children in multiple Massachusetts communities. Under the abrupt and powerful selective pressure of immunization against select pneumococcal serotypes, it was plausible that overall pneumococcal colonization would decrease substantially. Instead, replacement with nonvaccine serotypes has enabled S pneumoniae prevalence to remain at ~30%, with persisting risk factors for pneumococcal carriage, such as child care attendance. Furthermore, increases were seen among serotypes with elevated penicillin MICs, such as 19A and 35B. This work and previous work continue to suggest that serotype-specific increases may be favored by penicillin resistance.22 Given antibiotic selection pressures and the demonstrated ability of S pneumoniae to acquire genetic factors that promote both resistance and invasion, we should be prepared for continuing changes in the incidence of local disease and IPD, as well as the responsible serotypes. Understanding the population biology underlying the “moving target” of pneumococcal colonization should help us understand the long-term impact of pneumococcal vaccines, and potentially other vaccines, that target only a subset of strains within a species.
ACKNOWLEDGMENTS
This study was funded by the National Institutes of Health (grant R01 AI066304). Dr Hanage is a Royal Society University Research Fellow.
We thank Kelly Welch, Lizzie Ford, Shannon Opel, and Verna Moran, RN, for their dedication to this project, and we extend our appreciation to the following participating practices: Alan Bulotsky, MD, and Associates (Brockton, MA), Berkshire Pediatric Associates (Pittsfield, MA), Cape Ann Pediatricians (Gloucester, MA), Children's Health Care (Newburyport, MA), Harvard Vanguard Medical Associates (Chelmsford, MA), Medical Associates Pediatrics (Leominster, MA), Middleboro Pediatrics (Lakeville, MA), and Needham Pediatrics (Needham, MA). We also extend our gratitude to the many parents and children, without whose volunteerism this study would not have been possible.
ABBREVIATIONS
PCV7
heptavalent pneumococcal conjugate vaccine
IPD
invasive pneumococcal disease
PNSP
penicillin-nonsusceptible Streptococcus pneumoniae
CLSI
Clinical and Laboratory Standards Institute
MIC
minimal inhibitory concentration
OR
odds ratio
CI
confidence interval
RTI
respiratory tract infection
APPENDIX: CUSTOM MONTE CARLO TESTS TO ASSESS 8 VERSUS 16 COMMUNITIES
The question we addressed with these tests was whether we should use all of the communities we had at each time point or whether we should use the 8 communities observed at all 3 time points (or use something more complicated). We operationalized this by assessing whether the assortment of communities from which we sampled could be treated as a population or whether they were sufficiently distinct that each represented a unique subpopulation. We divided the question into 2 tests, namely, whether there were significant differences between communities at each time point and whether there was consistency within communities across time points. Stable communities, all distinct, might result in apparently mixed populations at each time point.
The tests both depend on a classification index.31 This is constructed as
where ρ_ij_ is the frequency of serotype i in population j and ρ‒ is the average frequency of ρ across the 2 populations. When the 2 populations have identical proportions of each serotype, the index is 0; as the populations become more dissimilar, the index increases toward 1.
In the first test, we calculate the pairwise classification index between each of the pairs of communities within each season. Then we take their average; this is the observed statistic. We perform a Monte Carlo process, in which we permute the cases by reassigning them randomly to communities. This is a random reordering of the community membership. Effectively, each individual can be assigned to any community with a probability equal to the proportion of individuals actually observed in that community. We then calculate the average classification index on the basis of these Monte Carlo-assigned communities. This Monte Carlo process is repeated many times, resulting in many Monte Carlo statistics. If the true data show a great deal of difference in the distribution of serotypes between communities, then the observed statistic should be markedly different from the mass of Monte Carlo statistics. Technically, the null hypothesis that the communities are not distinct is assessed with a Monte Carlo P value. This is simply the rank of the observed statistic among the Monte Carlo statistics divided by the number of statistics in total. For example, if 999 Monte Carlo statistics were calculated and the observed statistic was greater than 995 of them, then the P value would be .005. Under the alternative hypothesis, the observed distribution would show greater difference between communities than would be expected according to chance, as expressed by the Monte Carlo statistics. The observed P values were all large (2001: P = .693; 2004: P = .396; 2007: P = .488), which provided no evidence against the null hypothesis and was consistent with the possibility that the communities reflected a broader population.
In the second test, we perform a similar Monte Carlo test but, in this case, we calculate the classification index between consecutive time points for each community. The average of these is the observed statistic in this case. The Monte Carlo process for this step involves not reassigning individuals randomly but pairing communities randomly across time. In other words, community A at time 1 may be paired with any of the communities at time 2. The average classification index is calculated. This process is repeated. The Monte Carlo P value (constructed as described above) assesses the null hypothesis that there is no continuity within a community, consistent with mixing across a broader population. The P values were .442 for the 16 communities between 2000–2001 and 2003–2004 and .698 for the 8 communities between 2003–2004 and 2007–2008. There was no evidence against the null hypothesis of no community stability.
Footnotes
FINANCIAL DISCLOSURE: Dr Pelton receives research support from Wyeth Lederle. Dr Hanage has served as a consultant for GlaxoSmithKline.
REFERENCES
- 1.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to nonpneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998 –2004. J Infect Dis. 2007;196(9):1346–1354. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 2.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 3.Black S, Shinefield H, Baxter R, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004;23(6):485–489. doi: 10.1097/01.inf.0000129685.04847.94. [DOI] [PubMed] [Google Scholar]
- 4.Poehling KA, Talbot TR, Griffin MR, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006;295(14):1668–1674. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease: United States, 1998 –2003. MMWR Morb Mortal Wkly Rep. 2005;54(36):893–897. [PubMed] [Google Scholar]
- 6.Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344(6):403–409. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 7.Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294(16):2043–2051. doi: 10.1001/jama.294.16.2043. [DOI] [PubMed] [Google Scholar]
- 8.Flannery B, Heffernan RT, Harrison LH, et al. Changes in invasive pneumococcal disease among HIV-infected adults living in the era of childhood pneumococcal immunization. Ann Intern Med. 2006;144(1):1–9. doi: 10.7326/0003-4819-144-1-200601030-00004. [DOI] [PubMed] [Google Scholar]
- 9.Ghaffar F, Barton T, Lozano J. Effect of the 7-valent pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae in the first 2 years of life. Clin Infect Dis. 2004;39(7):930–938. doi: 10.1086/423379. [DOI] [PubMed] [Google Scholar]
- 10.Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics. 2005;116(3) doi: 10.1542/peds.2004-2338. Available at: www.pediatrics.org/cgi/content/full/116/3/e408. [DOI] [PubMed]
- 11.Pelton SI, Loughlin AM, Marchant CD. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr Infect Dis J. 2004;23(11):1015–1022. doi: 10.1097/01.inf.0000143645.58215.f0. [DOI] [PubMed] [Google Scholar]
- 12.Singleton RJ, Hennessy TW, Bulkow LR. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297(16):1784–1792. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 13.Byington CL, Samore MH, Stoddard GJ, et al. Temporal trends of invasive disease due to Streptococcus pneumoniae among children in the Intermountain West: emergence of nonvaccine sero-groups. Clin Infect Dis. 2005;41(1):21–29. doi: 10.1086/430604. [DOI] [PubMed] [Google Scholar]
- 14.Park SY, Moore MR, Bruden DL, et al. Impact of conjugate vaccine on transmission of antimicrobial-resistant Streptococcus pneumoniae among Alaskan children. Pediatr Infect Dis J. 2008;27(4):335–340. doi: 10.1097/INF.0b013e318161434d. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs MR, Good CE, Beall B, Bajaksouzian S, Windau AR, Whitney CG. Changes in serotypes and antimicrobial susceptibility of invasive Streptococcus pneumoniae strains in Cleveland: a quarter century of experience. J Clin Microbiol. 2008;46(3):982–990. doi: 10.1128/JCM.02321-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention Emergence of antimicrobial-resistant serotype 19A Streptococcus pneumoniae: Massachusetts, 2001–6. MMWR Morb Mortal Wkly Rep. 2007;56(41):1077–1080. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction: eight states, 1998 –2005. MMWR Morb Mortal Wkly Rep. 2008;57(6):144–148. [PubMed] [Google Scholar]
- 18.Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298(15):1772–1778. doi: 10.1001/jama.298.15.1772. [DOI] [PubMed] [Google Scholar]
- 19.Pichichero ME, Casey JR. Evolving microbiology and molecular epidemiology of acute otitis media in the pneumococcal conjugate vaccine era. Pediatr Infect Dis J. 2007;26(10 suppl):S12–S16. doi: 10.1097/INF.0b013e318154b25d. [DOI] [PubMed] [Google Scholar]
- 20.Muñoz-Almagro C, Jordan I, Gene A, Latorre C, Garcia-Garcia JJ, Pallares R. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin Infect Dis. 2008;46(2):174–182. doi: 10.1086/524660. [DOI] [PubMed] [Google Scholar]
- 21.Brueggemann AB, Pai R, Crook DW, Beall B. Vaccine escape recombinants emerge after pneumococcal vaccination in the United States. PLoS Pathog. 2007;3(11):e168. doi: 10.1371/journal.ppat.0030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanage WP, Huang SS, Lipsitch M, et al. Diversity and antibiotic resistance among nonvaccine serotypes of Streptococcus pneumoniae carriage isolates in the post PCV7 era. J Infect Dis. 2007;195(3):347–352. doi: 10.1086/510249. [DOI] [PubMed] [Google Scholar]
- 23.Hare ME, Gaur AH, Somes GW, Arnold SR, Shorr RI. Does it really take longer not to prescribe antibiotics for viral respiratory tract infections in children? Ambul Pediatr. 2006;6(3):152–156. doi: 10.1016/j.ambp.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Nash DR, Harman J, Wald ER, Kelleher KJ. Antibiotic prescribing by primary care physicians for children with upper respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(11):1114–1119. doi: 10.1001/archpedi.156.11.1114. [DOI] [PubMed] [Google Scholar]
- 25.McCaig LF, Besser RE, Hughes JM. Trends in antimicrobial prescribing rates for children and adolescents. JAMA. 2002;287(23):3096–3102. doi: 10.1001/jama.287.23.3096. [DOI] [PubMed] [Google Scholar]
- 26.Finkelstein JA, Huang SS, Daniel J, et al. Antibiotic-resistant Streptococcus pneumoniae in the heptavalent pneumococcal conjugate vaccine era: predictors of carriage in a multicommunity sample. Pediatrics. 2003;112(4):862–869. doi: 10.1542/peds.112.4.862. [DOI] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational Supplement. Clinical and Laboratory Standards Institute; Wayne, PA: 2007. Publication M100-S17. [Google Scholar]
- 28.Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; Sixteenth Informational Supplement. Clinical and Laboratory Standards Institute; Wayne, PA: 2004. Publication M100-S16. [Google Scholar]
- 29.Finkelstein JA, Huang SS, Kleinman K, et al. Impact of a 16-community trial to promote judicious antibiotic use in Massachusetts. Pediatrics. 2008;121(1) doi: 10.1542/peds.2007-0819. Available at: www.pediatrics.org/cgi/content/full/121/1/e15. [DOI] [PubMed]
- 30.Dwass M. Modified randomization tests for nonparametric hypotheses. Ann Math Stat. 1957;28(1):181–187. [Google Scholar]
- 31.Jolley KA, Wilson DJ, Kriz P, McVean G, Maiden MC. The influence of mutation, recombination, population history, and selection on patterns of genetic diversity in Neisseria meningitidis. Mol Biol Evol. 2005;22(3):562–569. doi: 10.1093/molbev/msi041. [DOI] [PubMed] [Google Scholar]
- 32.Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. J Am Stat Assoc. 1993;88(421):9–25. [Google Scholar]
- 33.Nahm MH, Lin J, Finkelstein JA, Pelton SI. Increase in the prevalence of the newly discovered pneumococcal serotype 6C in the nasopharynx after introduction of pneumococcal conjugate vaccine. J Infect Dis. 2009;199(3):320–325. doi: 10.1086/596064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu K, Pelton SI, Karumuri S, Heisy-Grove D, Klein JO. Population based surveillance for childhood invasive pneumococcal disease in the era of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2005;24(1):17–23. doi: 10.1097/01.inf.0000148891.32134.36. [DOI] [PubMed] [Google Scholar]
- 35.Pelton SI, Huot H, Finkelstein JA, et al. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26(6):468–472. doi: 10.1097/INF.0b013e31803df9ca. [DOI] [PubMed] [Google Scholar]
- 36.Dagan R, Melamed R, Muallem M, et al. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis. 1996;174(6):1271–1278. doi: 10.1093/infdis/174.6.1271. [DOI] [PubMed] [Google Scholar]
- 37.Mbelle N, Huebner RE, Wasas AD, Kimura A, Chang I, Klugman KP. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis. 1999;180(4):1171–1176. doi: 10.1086/315009. [DOI] [PubMed] [Google Scholar]
- 38.Dagan R, Givon-Lavi N, Zamir O, Fraser D. Effect of a nonavalent conjugate vaccine on carriage of antibiotic-resistant Streptococcus pneumoniae in day-care centers. Pediatr Infect Dis J. 2003;22(6):532–540. doi: 10.1097/01.inf.0000069761.11093.c3. [DOI] [PubMed] [Google Scholar]
- 39.Chung A, Perera R, Brueggemann AB, et al. Effect of antibiotic prescribing on antibiotic resistance in individual children in primary care: prospective cohort study. BMJ. 2007;335(7617):429. doi: 10.1136/bmj.39274.647465.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dagan R, Barkai G, Leibovitz E, Dreifuss E, Greenberg D. Will reduction of antibiotic use reduce antibiotic resistance? The pneumococcus paradigm. Pediatr Infect Dis J. 2006;25(10):981–986. doi: 10.1097/01.inf.0000239266.20642.26. [DOI] [PubMed] [Google Scholar]
- 41.Guillemot D, Varon E, Bernede C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin G-nonsusceptible Streptococcus pneumoniae. Clin Infect Dis. 2005;41(7):930–938. doi: 10.1086/432721. [DOI] [PubMed] [Google Scholar]
- 42.Pai R, Moore MR, Pilishvili T, et al. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J Infect Dis. 2005;192(11):1988–1995. doi: 10.1086/498043. [DOI] [PubMed] [Google Scholar]
- 43.Bender JM, Ampofo K, Korgenski K, et al. Pneumococcal necrotizing pneumonia in Utah: does serotype matter? Clin Infect Dis. 2008;46(9):1346–1352. doi: 10.1086/586747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pelton SI. Replacement pneumococcal disease in perspective. Clin Infect Dis. 2008;46(9):1353–1355. doi: 10.1086/586748. [DOI] [PubMed] [Google Scholar]