History of Foot Ulcer Increases Mortality Among Individuals With Diabetes: Ten-year follow-up of the Nord-Trøndelag Health Study, Norway (original) (raw)
Abstract
OBJECTIVE
To compare mortality rates for individuals with diabetes with and without a history of foot ulcer (HFU) and with that for the nondiabetic population.
RESEARCH DESIGN AND METHODS
This population-based study included 155 diabetic individuals with an HFU, 1,339 diabetic individuals without an HFU, and 63,632 nondiabetic individuals who were all followed for 10 years with mortality as the end point.
RESULTS
During the follow-up period, a total of 49.0% of diabetic individuals with an HFU died, compared with 35.2% of diabetic individuals without an HFU and 10.5% of those without diabetes. In Cox regression analyses adjusted for age, sex, education, current smoking, and waist circumference, having an HFU was associated with more than a twofold (2.29 [95% CI 1.82–2.88]) hazard risk for mortality compared with that of the nondiabetic group. In corresponding analyses comparing diabetic individuals with and without an HFU, an HFU was associated with 47% increased mortality (1.47 [1.14–1.89]). Significant covariates were older age, male sex, and current smoking. After inclusion of A1C, insulin use, microalbuminuria, cardiovascular disease, and depression scores in the model, each was significantly related to life expectancy.
CONCLUSIONS
AN HFU increased mortality risk among community-dwelling adults and elderly individuals with diabetes. The excess risk persisted after adjustment for comorbidity and depression scores, indicating that close clinical monitoring might be warranted among individuals with an HFU, who may be particularly vulnerable to adverse outcomes.
Hospital-based studies have shown that mortality rates in individuals with diabetic foot ulcers are about twice those observed in individuals with diabetes without foot ulcers (1,2). A diabetic foot ulcer reflects the presence of underlying pathological conditions, and the risk of recurrent ulcers is high (3,4). It has been suggested that the elevated mortality rate among individuals with diabetic foot ulcers is related to comorbid disease such as cardiovascular disease and nephropathy (5) or to psychological factors including depression (6). Although the mortality rate in individuals with diabetes is high, no large population-based studies have examined the impact on mortality of a history of foot ulcers (HFU) among individuals with diabetes.
The purpose of this study was to compare mortality rates for individuals with diabetes reporting an HFU with those for individuals without an HFU and the nondiabetic population. These issues were investigated in the Nord-Trøndelag Health Study (HUNT 2), which includes a very large population-based sample of men and women from a well-defined geographic area. Participants with self-reported diabetes were well characterized with regard to their diabetes, and information on demographics, lifestyle, and prevalent disease including depression was available.
RESEARCH DESIGN AND METHODS
The HUNT 2 study was conducted during 1995–1997 and was approved by the Norwegian Data Inspectorate and the Regional Committee for Medical Research Ethics. Participation was voluntary, and each participant signed a consent form.
The HUNT 2 study was described previously (7,8). In brief, all inhabitants of Nord-Trøndelag County aged ≥20 years were invited to participate (n = 92,434). A questionnaire was mailed to each person along with an invitation to attend a clinical examination. Of those invited, 65,604 individuals (71%) attended. Participants who responded positively to the question, “Do you have or have you had diabetes?” were classified as having diabetes (n = 1,972) and were invited to take part in the diabetes substudy. Those who in an additional questionnaire answered positively to the question, “Have you had a foot ulcer that required more than three weeks to heal?” were classified as having an HFU (n = 155), and those who responded negatively were classified as having diabetes without an HFU (n = 1,339). Those classified as having diabetes but who did not take part in the diabetes substudy or did not answer the foot ulcer question were excluded from the analyses (n = 478) (7). Some 63,632 participants reported not having diabetes. Thus, the current study includes a total of 65,126 participants.
In HUNT 2, a nonfasting venous serum sample was analyzed for glucose; for those who reported diabetes, an EDTA whole-blood sample was also analyzed for A1C. Those who reported diabetes were given a follow-up appointment (74.8% participated) at which a fasting blood sample was drawn and analyzed for glucose, C-peptide, and GAD antibodies. Participants who reported diabetes received tubes for collecting three consecutive first morning urine samples. Among the 1,494 participants with or without an HFU, 94.1% returned the samples, which were analyzed for albumin and creatinine (8). An albumin-to-creatinine ratio >2.5 mg/mmol in at least two of the three urine samples was used to define microalbuminuria, as recommended by Hallan et al. (9).
Other variables included age, sex, BMI (weight in kilograms divided by the square of height in meters), and waist circumference. Education was categorized as <10 years or ≥10 years. Smoking was classified as current smoking or not. The baseline questionnaire included information about angina pectoris, myocardial infarction, and stroke; those who responded positively to one or more of these items were defined as having cardiovascular disease. Hypertension was defined as blood pressure of ≥140/90 mmHg or as current use of antihypertensive drugs. Exercise was dichotomized as <1 h of physical activity per week or ≥1 h. Other diabetes-related questions from the diabetes substudy included treatment, diabetes duration, eye problems due to diabetes, and amputation. Those reporting amputation of a toe, calf/knee, or femur were categorized as having any lower-limb amputation.
Depression was assessed by the Hospital Anxiety and Depression Scale (HADS) (10,11). This instrument includes seven items measuring depression (HADS-D subscale). Each item is scored from 0 to 3; thus, the maximum score is 21 on each of the subscales. Higher scores indicate higher levels of symptom load. Missing substitution was performed for individuals who responded to five or six of the seven HADS-D questions. This was done by multiplying the score obtained by 7/5 if five of the seven questions were answered and by 7/6 if six questions were answered. Such missing substitution was needed for 5.8% of the HADS-D scale; 4.6% of the respondents answered fewer than five questions on the HADS-D and were excluded. Caseness was defined by a score of ≥8 on the HADS-D. This cutoff level has been shown to optimally balance sensitivity and specificity on receiver-operating characteristic curves (11) and was also applied in our study. Factor analysis of HADS in HUNT was reported to result in a two-factor solution consistent with the two subscales, anxiety and depression. Cronbach's α values for internal consistency for the anxiety and depression subscales in HUNT were reported as 0.80 and 0.76, respectively (12).
Follow-up
Participants were followed for up to 10 years with mortality as the end point. Information on mortality was obtained from the Norwegian Causes of Death Registry using the Norwegian 11-digit personal identity number unique for each resident. Information on individuals who emigrated from Nord-Trøndelag County during the follow-up period was estimated to be negligible (<0.5%, http://www.ssb.no/english/subjects/02/02/20/innvutv_en/tab-2009–05-07–02-en.html).
Mortality diagnoses were coded according to the ICD-10. The main mortality diagnoses were categorized into diseases as follows: diabetes (E10–14), ischemic heart disease (I20–25), cerebrovascular disease (I60–69), other circulatory diseases (I00–15, I26–28, I30–52, I70–79, I80–99), renal disease (N00–39), cancer (C), and other diseases (A, B, D, E00–07, E15–90, F–H, J–M, N40–99, O–Y).
Statistical analyses
Power calculations were performed before the study and showed a statistical power of 78% to detect an increased risk of 33% among the foot ulcer group compared with the population with diabetes without an HFU, assuming a mortality of 30% during the follow-up in the latter group. We used t tests and χ2 tests to compare characteristics of the three subgroups at baseline.
Cox proportional hazards regression analyses were used to estimate mortality rate ratios (hazard ratios [HRs]) and 95% CI from the date of inclusion in the study (1995–1997) to 31 December 2005. We created dummy variables for the diabetic patients without an HFU and the diabetic patients with an HFU such that the HR for each category represents the comparison of that category with the HR for the nondiabetic population. Preliminary, simple Cox regression analyses were performed for all baseline covariates and all-cause mortality. For covariates with >2% missing data in the foot ulcer group, separate “unknown” categories were used. This involved education (n = 16), waist circumference (n = 5), microalbuminuria (n = 10), and depression (n = 11).
Multiple Cox proportional hazards regression analyses were then performed with adjustment for other known risk factors for mortality. Covariates were organized thematically in blocks, and increasingly complex models were developed by adding one set of variables at a time using forced entry. We chose this model because diabetes increases the risk of cardiovascular disease and therefore the development of cardiovascular disease is in the causal pathway, leading from diabetes to a higher risk of death (13).
Variable selection in multivariable modeling was made a priori based on previous knowledge, and assessment of the variable in relation to time, cause, and effect. For example, a history of amputation was not taken into the model because this most probably occurred after a diabetic foot ulcer. Severity of illness (judged by insulin use and A1C), microalbuminuria, a history of cardiovascular disease, and depression (HADS-D score ≥8) were entered into the model.
The two diabetic groups were first compared with the nondiabetic population after adjustment for demographic factors, lifestyle variables, cardiovascular disease, and depression. Covariates in model 1 included age (continuous), male sex (no or yes), level of education (high, low, or unknown), current smoking (no or yes), and high waist circumference of ≥102 cm in men or ≥88 cm in women (no, yes, or unknown). Covariates in model 2 included cardiovascular disease status (no or yes) and depression (HADS-D score ≥8) (no, yes, or unknown).
Analyses involving only the diabetic groups were adjusted similarly for age, male sex, level of education, current smoking, and waist circumference (model 3). The following additional factors were also included: cardiovascular status (no or yes) and depression (HADS-D score ≥8) (no, yes, or unknown) (model 4), microalbuminuria (no, yes, or unknown), A1C (continuous), and insulin use (no or yes) (model 5).
Cox regression analyses were also performed to test for possible interactions between the main exposure (nondiabetic subjects and diabetic subjects with and without an HFU) and the other covariates in the model among individuals with diabetes. Kaplan-Meier survival curves were estimated to describe all-cause mortality in the subgroups. Statistical significance was assigned as P < 0.05. Statistical analyses were conducted using SPSS (version 16.0).
RESULTS
Baseline characteristics
Compared with the nondiabetic sample, those with an HFU were older and had higher BMI, waist circumference, and depression scores; a higher proportion were male and physically inactive with low education, angina pectoris, myocardial infarction, stroke, and hypertension; and a lower proportion were smokers. In a comparison of the two diabetic groups, those with an HFU had higher mean waist circumference and A1C, and a larger proportion were physically inactive, used insulin, had a long diabetes duration, had microalbuminuria, and had a history of stroke, peripheral vascular surgery, eye problems due to diabetes, and lower-limb amputations (Table 1).
Table 1.
Description of the study population; the HUNT 2 study
| Nondiabetic subjects* | Diabetic subjects without an HFU* | Diabetic subjects with an HFU* | P † | P ‡ | |
|---|---|---|---|---|---|
| n | 63,632 | 1,339 | 155 | ||
| Demographic characteristics | |||||
| Age (years) | 49.7 ± 17.3 | 65.6 ± 13.6 | 67.2 ± 14.0 | <0.001 | 0.157 |
| Male sex (%) | 46.7 | 49.7 | 56.8 | 0.012 | 0.097 |
| Single (%) | 40.1 | 38.1 | 45.8 | 0.150 | 0.064 |
| Education (≥10 years) (%) | 64.0 | 37.7 | 33.8 | <0.001 | 0.367 |
| Lifestyle characteristics | |||||
| BMI (kg/m2) | 26.3 ± 4.1 | 28.9 ± 4.8 | 29.3 ± 5.3 | <0.001 | 0.396 |
| Waist circumference (cm) | 86.2 ± 11.6 | 95.0 ± 12.0 | 98.2 ± 12.3 | <0.001 | 0.002 |
| Physical activity <1 h/week (%) | 19.8 | 27.5 | 37.2 | <0.001 | 0.026 |
| Current smokers (%) | 29.0 | 16.8 | 11.1 | <0.001 | 0.070 |
| Cardiovascular disease status | |||||
| Self-reported stroke (%) | 1.8 | 5.0 | 12.2 | <0.001 | <0.001 |
| Self-reported myocardial infarction (%) | 3.0 | 12.6 | 15.3 | <0.001 | 0.345 |
| Self-reported angina pectoris (%) | 4.6 | 18.5 | 22.0 | <0.001 | 0.307 |
| Hypertension | 23.9 | 56.4 | 57.4 | <0.001 | 0.81 |
| Subgroups of diabetes | |||||
| Type 1 (%) | — | 16.9 | 26.0 | ||
| Type 2 (%) | — | 83.1 | 74.0 | ||
| Diabetes-specific variables | |||||
| A1C (% units) | — | 8.1 ± 1.7 | 8.4 ± 2.0 | — | 0.015 |
| Insulin use (%) | — | 31.8 | 43.5 | — | 0.004 |
| Microalbuminuria§ | — | 27.3 | 40.0 | — | 0.001 |
| Duration of diabetes (years) (median) | — | 6.0 | 10.0 | — | 0.001 |
| Peripheral vascular surgery (%) | — | 2.7 | 10.7 | — | <0.001 |
| Eye problems due to diabetes (%) | — | 11.9 | 24.8 | — | <0.001 |
| Any lower limb amputations (%) | — | 0.7 | 5.2 | — | <0.001 |
| Psychological assessment | |||||
| HADS-D score (0–21) | 3.5 ± 3.1 | 4.3 (SD 3.4) | 4.7 (SD 3.6) | <0.001 | 0.180 |
| HADS-D (score ≥8) (%) | 10.8 | 17.1 | 18.8 | 0.002 | 0.614 |
| HADS-D (score ≥11) (%) | 3.2 | 6.0 | 7.6 | 0.002 | 0.439 |
Mortality
During the follow-up period, 49% of the 155 diabetic individuals with an HFU died compared with 35.2% of the 1,339 diabetic individuals without an HFU and 10.5% of the 63,632 nondiabetic individuals. Among individuals with an HFU, the main causes of death were cardiovascular events (48.7%), diabetes (23.7%), and cancer (14.5%). Corresponding figures among those with diabetes without an HFU were 50.1, 11.7, and 18.6% and among the nondiabetic group were 44.9, 0.5, and 27.5%, respectively. The mortality rates from cardiovascular causes were not statistically different between the diabetic groups, although patients with an HFU had more prevalent cardiovascular disease and more cardiovascular disease risk factors at baseline than those without an HFU. After adjustment for age, sex, education, smoking, and waist circumference diabetic individuals with and without an HFU had a significantly higher mortality rate than that for the nondiabetic group (HR 2.29 [95% CI 1.82–2.88] and 1.70 [1.54–1.86], respectively) (Table 2, model 1). Covariates significantly associated with increased mortality risk were older age, male sex, low education, smoking, and larger waist circumference. The risk of mortality associated with having an HFU did not change markedly when cardiovascular disease and depression (HADS-D score ≥8) also were included in the model (Table 2, model 2).
Table 2.
Results of unadjusted and adjusted Cox proportional hazards models for all-cause mortality in diabetic participants without an HFU compared with nondiabetic participants and diabetic participants with a history of HFU compared with the nondiabetic population (models 1 and 2) and in diabetic participants with an HFU compared with those without an HFU (models 3–5)
| Unadjusted | Model 1 | Model 2 | Unadjusted | Model 3 | Model 4 | Model 5 | |
|---|---|---|---|---|---|---|---|
| n | 64,109* | 64,109 | 64,109 | 1,435† | 1,435 | 1,435 | 1,435 |
| Nondiabetic subjects | Ref.‡ | Ref.‡ | Ref.‡ | — | — | — | — |
| Diabetes without an HFU | 4.21 (3.83–4.62) | 1.70 (1.54–1.86) | 1.62 (1.48–1.78) | Ref.‡ | Ref.‡ | Ref.‡ | Ref.‡ |
| Diabetes with an HFU | 6.80 (5.40–8.55) | 2.29 (1.82–2.88) | 2.20 (1.75–2.77) | 1.68 (1.31–2.16) | 1.47 (1.14–1.89) | 1.46 (1.14–1.89) | 1.41 (1.09–1.82) |
| Age (years) | 1.12 (1.11–1.12) | 1.12 (1.12–1.12) | 1.11 (1.11–1.12) | 1.10 (1.09–1.11) | 1.11 (1.09–1.12) | 1.10 (1.09–1.11) | 1.10 (1.09–1.11) |
| Male sex | 1.41 (1.35–1.48) | 1.74 (1.66–1.83) | 1.67 (1.58–1.75) | 1.05 (0.88–1.25) | 1.49 (1.23–1.79) | 1.46 (1.20–1.76) | 1.44 (1.18–1.74) |
| Education (<10 years)§ | 4.08 (3.86–4.32) | 1.10 (1.04–1.17) | 1.08 (1.02–1.14) | 1.93 (1.55–2.39) | 0.98 (0.78–1.22) | 0.94 (0.75–1.19) | 0.98 (0.78–1.24) |
| Current smoking | 0.85 (0.80–090) | 1.64 (1.54–1.73) | 1.64 (1.55–1.74) | 1.09 (0.87–1.37) | 1.80 (1.42–2.27) | 1.76 (1.39–2.23) | 1.75 (1.38–2.22) |
| Waist circumference >102 or 88 cm§ | 1.97 (1.87–2.01) | 1.14 (1.08–1.20) | 1.12 (1.06–1.18) | 1.17 (0.98–1.39) | 1.16 (0.96–1.39) | 1.11 (0.92–1.33) | 1.13 (0.94–1.35) |
| Cardiovascular disease‖ | 6.38 (6.06–6.73) | 1.56 (1.48–1.65) | 2.56 (2.15–3.04) | 1.53 (1.28–1.83) | 1.50 (1.25–1.80) | ||
| Depression (score ≥8)§ | 2.32 (2.18–2.47) | 1.35 (1.27–1.44) | 1.49 (1.20–1.86) | 1.37 (1.10–1.72) | 1.35 (1.08–1.69) | ||
| Microalbuminuria§¶ | — | — | 2.34 (1.96–2.81) | 1.55 (1.25–1.82) | |||
| A1C | — | — | 1.11 (1.06–1.16) | 1.07 (1.02–1.13) | |||
| Insulin use | — | — | 1.02 (0.85–1.23) | 1.37 (1.13–1.66) |
Among individuals with diabetes, after adjustment for age, sex, education, smoking, and waist circumference, an HFU was associated with a 47% increased risk of mortality. Covariates significantly associated with mortality were older age, male sex, and smoking (Table 2, model 3). The association between an HFU and mortality did not change markedly when cardiovascular disease and depression (HADS-D score ≥8) were included in the model. When A1C, insulin use, and microalbuminuria entered the model, the HR for an HFU was slightly reduced to 1.41 [95% CI 1.09–1.82] (Table 2, model 5). Significant predictors for reduced life expectancy in the final model were older age, male sex, smoking, the presence of cardiovascular disease and depression (HADS-D score ≥8), microalbuminuria, A1C, and insulin use. To study the effect of A1C among diabetic individuals with an HFU only, we repeated the analyses restricted to this subgroup and found an effect of A1C that was slightly stronger than that among all individuals with diabetes, although not significant (HR 1.11 [0.97–1.28]).
We included those with missing information for education, waist circumference, microalbuminuria, and depression as separate subgroups in the Cox regression analyses. In general, the categories for missing values tended to have higher HR estimates (not shown in the table), although these were not significant, which probably reflects the small numbers. We also performed additional analyses that excluded individuals with diabetes who reported a history of amputation, but this did not alter the results markedly. Diabetes classification and diabetes duration were also included in the Cox regression analyses. The estimated effects of an HFU changed only marginally, and these covariates were not significantly associated to mortality.
A total of 478 individuals with diabetes did not participate in the substudy on diabetes or did not answer the question on foot ulcers. To assess the validity of the findings among those with diabetes, we compared those who completed the foot ulcer question with those who did not, with regard to demographics, prevalent disease, and health behaviors and found that those who did not complete this question had more advanced disease.
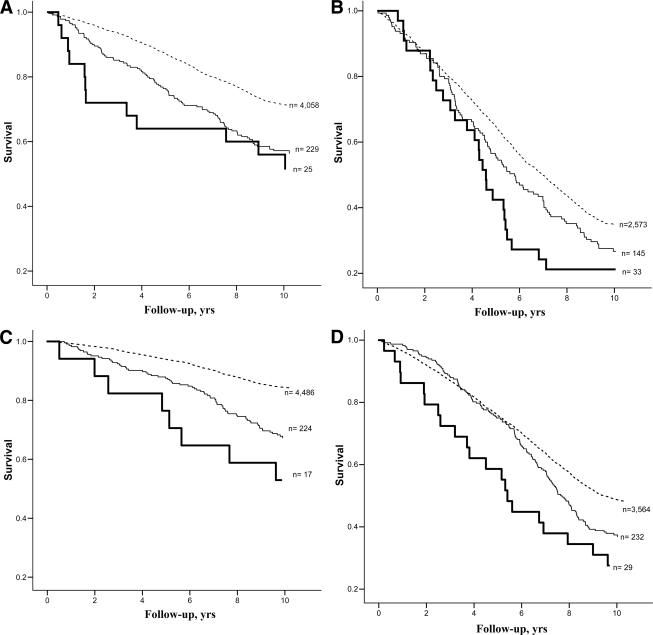
To illustrate the excess mortality attributable to diabetes with and without an HFU, Kaplan-Meier curves were drawn for data stratified into age-groups 65–74 and ≥75 years. As seen in Fig. 1, participants with diabetes and an HFU consistently had the highest mortality rates.
Figure. 1.
Kaplan-Meier survival curves (all-cause mortality) comparing nondiabetes, diabetes, and diabetes with an HFU subgroups by sex and age. Dotted line, nondiabetes; thin line, diabetes without an HFU; thick line, diabetes with an HFU. A: Estimates for men aged 65–74 years. B: Estimates for men aged ≥75 years. C: Estimates for women aged 65–74 years. D: Estimates for women aged ≥75 years.
Tests for interactions revealed interaction in model 5. This finding showed that the effect of age was less important for those with an HFU (P = 0.040).
CONCLUSIONS
In this 10-year follow-up study, an HFU was associated with more than a twofold elevated risk of mortality compared with the nondiabetic group and an ∼40% higher mortality compared with participants with diabetes but without an HFU. Compared with diabetes without an HFU, the excess risk was explained only partly by older age, male sex, higher A1C, current smoking, insulin use, microalbuminuria, cardiovascular disease, and depression.
This large community-based study showed that foot ulceration increases mortality risk among individuals with diabetes. As far as we are aware, this is the first such study to identify a higher mortality rate in individuals with diabetes and an HFU among community-dwelling adults and elderly individuals. Previous studies have to our knowledge included samples from hospitals, foot clinics, or outpatient settings (2,14,15). A substantial proportion of patients with foot ulcers are treated in primary care settings, and with the increasing prevalence of diabetes worldwide (16), the number of patients with diabetes and an HFU will increase over the next decade. Most of these patients are expected to have limited or infrequent access to multidisciplinary treatment teams (17). The present study underlines the importance of organizing future health care services with follow-up routines that allow for close clinical monitoring of individuals with an HFU in primary care.
In a 5-year observational study in Sweden, patients with diabetic foot ulcers attending a foot clinic had a twofold increase in mortality rates compared with nondiabetic individuals, after adjustment for age and sex (1). We found a similar increased risk after adjustment for additional potential confounders and after a longer follow-up period. In a study of ambulatory male patients with diabetes (2), the relative risk of death during 4 years of follow-up was 2.39 in those who developed a new foot ulcer compared with those who did not. The excess mortality rate was substantially higher than that in our study. This difference might reflect the more advanced illness in hospital-based patients; however, the study by Boyko et al. (2) was conducted between 1990 and 1994, and diabetes treatment has improved in recent years (18). Although the survival rate among individuals with an HFU might have improved in recent years, our data indicate continued excess mortality for those with an HFU. In addition, those with an HFU had a larger extent of severe diabetes complications compared with those with diabetes without an HFU. Further, among those with an HFU, a higher proportion of deaths was caused by diabetes and its complications, whereas the effect of age was less important among those with an HFU.
To our knowledge, our results relating poor glycemic control to higher mortality in individuals with diabetes and an HFU are novel and in contrast to the results presented by Winkley et al. (4), who reported that better glycemic control was significantly associated with higher mortality in individuals with a diabetic foot ulcer after 18 months of follow-up. Our findings underline the importance of early identification of foot ulcers and intensified treatment at an early stage.
Depression has previously been associated with increased mortality in individuals with diabetes (19,20). Ismail et al. (6) found that one-third of individuals with their first foot ulcer suffered from depression and that this condition was associated with increased mortality. Results from the present study support an increased risk of mortality among those depressed, over and beyond the increased risk associated with an HFU. Systematic monitoring and treatment of depression among those with an HFU should be considered (4).
Previous longitudinal studies of individuals with diabetes and an HFU have included mainly hospital or foot clinic patients (1,2,14). The present long-term study of >60,000 men and women including 1,494 individuals with validated diabetes (21) support these previous findings.
As with all large-scale epidemiologic studies, ours also has inherent shortcomings. During the 10-year follow-up period, new cases of diabetes probably developed, but the only information we have among nondiabetic subjects is that 0.5% of deaths were diabetes-related. The inclusion of an unknown number of subjects with diabetes in the nondiabetic group at baseline may influence the findings. Among those without known diabetes, a total of 62,757 delivered a nonfasting blood glucose (venous serum). The 217 individuals with a nonfasting glucose >11 mmol/l were contacted and advised to contact their general practitioner. In the analysis of the present study these ∼0.003% (217 of 62,757) were not defined as having diabetes owing to uncertainty. Because this is a very low number, it is unlikely that any of the risk estimates have been influenced by these individuals. It is likely that these procedures underestimated the number of subjects with diabetes. Further, among individuals who reported an HFU at baseline, we have no information about the development of HFU after baseline. A closer follow-up of these individuals would have enabled more detailed analyses to determine the real causes of the increased mortality in this group. We found that the diabetic individuals who did not respond to the questionnaire on foot ulcers reported otherwise more advanced disease (7), corresponding to results from other studies of nonresponders (22). The mortality risk associated with an HFU in the present study might therefore have been underestimated.
In previous studies the threshold of microalbuminuria varied from 2.5 to 3.5 mg/mmol for men and women (9). In the present study we used a cutoff of 2.5 mg/mmol for both sexes. Thus, the results of the present study might overestimate the proportion of women with microalbuminuria. Finally, compared with other studies (1,23), a relatively low proportion of participants reported a history of amputation, which may be explained by recruitment procedures that made it difficult for house-bound or institutionalized individuals to participate. Conversely, these two studies are from specialized foot care clinics and probably included individuals with more advanced disease and complications.
In summary, an HFU in those with diabetes among community-dwelling adults and elderly individuals was significantly related to increased mortality. This excess risk persisted after adjustment for relevant covariates of comorbidity and depression scores, thus indicating that close clinical monitoring is warranted among individuals with an HFU, who may be particularly vulnerable for adverse outcomes.
Acknowledgments
The Nord-Trøndelag Health Study (the HUNT 2 study) is a collaboration between the HUNT Research Centre, Faculty of Medicine, Norwegian University of Science and Technology, Verdal, Norway; the Norwegian Institute of Public Health, Oslo, Norway; and the Nord-Trøndelag County Council. This study was supported by Bergen University College. The diabetes part of HUNT 2 has received support from the Norwegian Diabetes Association and GlaxoSmithKline Norway.
No potential conflicts of interest relevant to this article were reported.
The abstract “A Prospective Study on Diabetic Foot Ulcers and Mortality: the Nord-Trondelag Health Study, Norway” is part of the Oral Presentation session entitled “Foot Care on the IDF,” 19 October 2009.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Apelqvist J, Larsson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Intern Med 1993; 233: 485– 491 [DOI] [PubMed] [Google Scholar]
- 2.Boyko EJ, Ahroni JH, Smith DG, Davignon D. Increased mortality associated with diabetic foot ulcer. Diabet Med 1996; 13: 967– 972 [DOI] [PubMed] [Google Scholar]
- 3.Boyko EJ, Ahroni JH, Cohen V, Nelson KM, Heagerty PJ. Prediction of diabetic foot ulcer occurrence using commonly available clinical information: the Seattle Diabetic Foot Study. Diabetes Care 2006; 29: 1202– 1207 [DOI] [PubMed] [Google Scholar]
- 4.Winkley K, Stahl D, Chalder T, Edmonds ME, Ismail K. Risk factors associated with adverse outcomes in a population-based prospective cohort study of people with their first diabetic foot ulcer. J Diabetes Complications 2007; 21: 341– 349 [DOI] [PubMed] [Google Scholar]
- 5.Apelqvist J, Larsson J. What is the most effective way to reduce incidence of amputation in the diabetic foot? Diabetes Metab Res Rev 2000; 16( Suppl. 1): S75– S83 [DOI] [PubMed] [Google Scholar]
- 6.Ismail K, Winkley K, Stahl D, Chalder T, Edmonds M. A cohort study of people with diabetes and their first foot ulcer: the role of depression on mortality. Diabetes Care 2007; 30: 1473– 1479 [DOI] [PubMed] [Google Scholar]
- 7.Iversen MM, Midthjell K, Østbye T, Tell GS, Clipp E, Sloane R, Nortvedt MW, Uhlving S, Hanestad BR. History of and factors associated with diabetic foot ulcers in Norway: the Nord-Trøndelag Health Study. Scand J Public Health 2008; 36: 62– 68 [DOI] [PubMed] [Google Scholar]
- 8.Holmen J, Midthjell K, Krüger Ø, Langhammer A, Holmen TL, Bratberg GH, Vatten L, Lund-Larsen PG. The Nord-Trøndelag Health Study 1995–97 (HUNT 2): objectives, contents, methods and participation. Nor J Epidemiology 2003; 13: 19– 32 [Google Scholar]
- 9.Hallan H, Romundstad S, Kvenild K, Holmen J. Microalbuminuria in diabetic and hypertensive patients and the general population: consequences of various diagnostic criteria: the Nord-Trondelag Health Study (HUNT). Scand J Urol Nephrol 2003; 37: 151– 158 [DOI] [PubMed] [Google Scholar]
- 10.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361– 370 [DOI] [PubMed] [Google Scholar]
- 11.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review J Psychosom Res 2002; 52: 69– 77 [DOI] [PubMed] [Google Scholar]
- 12.Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry 2001; 179: 540– 544 [DOI] [PubMed] [Google Scholar]
- 13.Boyko EJ. Progress in the estimation of mortality due to diabetes. Diabetes Care 2005; 28: 2320– 2321 [DOI] [PubMed] [Google Scholar]
- 14.Young MJ, McCardle JE, Randall LE, Barclay JI. Improved survival of diabetic foot ulcer patients 1995–2008: possible impact of aggressive cardiovascular risk management. Diabetes Care 2008; 31: 2143– 2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care 2003; 26: 491– 494 [DOI] [PubMed] [Google Scholar]
- 16.Wild SH, Forouhi NG. What is the scale of the future diabetes epidemic, and how certain are we about it? Diabetologia 2007; 50: 903– 905 [DOI] [PubMed] [Google Scholar]
- 17.Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D, Tennvall GR, Reike H, Spraul M, Uccioli L, Urbancic V, Van Acker K, Van Baal J, Van Merode F, Schaper N. Delivery of care to diabetic patients with foot ulcers in daily practice: results of the Eurodiale Study, a prospective cohort study. Diabet Med 2008; 25: 700– 707 [DOI] [PubMed] [Google Scholar]
- 18.Dale AC, Vatten LJ, Nilsen TI, Midthjell K, Wiseth R. Secular decline in mortality from coronary heart disease in adults with diabetes mellitus: cohort study. BMJ 2008; 337: 99– 102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Norris SL, Gregg EW, Cheng YJ, Beckles G, Kahn HS. Depressive symptoms and mortality among persons with and without diabetes. Am J Epidemiol 2005; 161: 652– 660 [DOI] [PubMed] [Google Scholar]
- 20.Katon WJ, Rutter C, Simon G, Lin EHB, Ludman E, Ciechanowski P, Kinder L, Young B, Von Korff M. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care 2005; 28: 2668– 2672 [DOI] [PubMed] [Google Scholar]
- 21.Midthjell K, Holmen J, Bjørndal A, Lund-Larsen G. Is questionnaire information valid in the study of a chronic disease such as diabetes? The Nord-Trøndelag diabetes study. J Epidemiol Community Health 1992; 46: 537– 542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drivsholm T, Eplov LF, Davidsen M, Jørgensen T, Ibsen H, Hollnagel H, Borch-Johnsen K. Representativeness in population-based studies: a detailed description of non-response in a Danish cohort study. Scand J Public Health 2006; 34: 623– 631 [DOI] [PubMed] [Google Scholar]
- 23.Ramsey SD, Newton K, Blough D, McCulloch DK, Sandhu N, Reiber GE, Wagner EH. Incidence, outcomes, and cost of foot ulcers in patients with diabetes. Diabetes Care 1999; 22: 382– 387 [DOI] [PubMed] [Google Scholar]