Receptors of anthrax toxin and cell entry (original) (raw)
. Author manuscript; available in PMC: 2010 Dec 1.
Published in final edited form as: Mol Aspects Med. 2009 Sep 2;30(6):406–412. doi: 10.1016/j.mam.2009.08.007
Abstract
Anthrax toxin-receptor interactions are critical for toxin delivery to the host cell cytoplasm. This review summarizes what is known about the molecular details of the protective antigen (PA) toxin subunit interaction with either the ANTXR1 and ANTXR2 cellular receptors, and how receptor-type can dictate the low pH threshold of PA pore formation. The roles played by cellular factors in regulating the endocytosis of toxin-receptor complexes is also discussed.
Keywords: Anthrax Toxin, Receptors, Endocytosis, LRP6, ARAP3
1. Introduction
Bacillus anthracis produces a tripartite A-B toxin that is an essential virulence factor for the development of anthrax disease [1]. The toxin is comprised of two alternative A-subunits, lethal factor (LF) and edema factor (EF), and a single receptor-binding B-subunit, designated as protective antigen (PA). LF is a zinc-dependent metalloproteinase that inactivates cellular signaling pathways through proteolysis of MAP kinase kinase proteins [2, 3]. EF is a calcium- and calmodulin-dependent adenylate cyclase [4]. PA combines with LF to form lethal toxin (LeTx), and with EF to form edema toxin (EdTx). Toxin entry into cells involves a) PA-receptor binding and proteolytic activation to generate PA63; b) formation of the PA63 heptameric prepore that binds EF and/or LF; c) internalization of the toxin-receptor complexes by cellular endocytosis; and d) low pH-induced PA pore formation and translocation of EF and LF subunits across endosomal membranes.
2. PA-Receptor interactions
The first step of intoxication involves binding of the 83kD PA subunit (PA83) to specific cellular receptors. This step is followed by cleavage, by a cell surface furin-like activity, to generate the heptameric PA63 prepore complex (PA63)7 that can bind up to 3 molecules of either EF or LF [5]. Alternatively, PA83 can be processed by cellular proteases contained in body fluids to generate the PA63 heptamer that is then capable of binding the toxin A subunits and also the cellular receptors [6].
Two types of cell surface receptor have been identified that bind either PA83 or (PA63)7: ANTXR1 (also known as tumor endothelial marker-8 (TEM8), and ANTXR2 (also known as capillary morphogenesis protein 2 (CMG2) [7, 8]. These receptors are type 1 transmembrane proteins with a single membrane-spanning domain. ANTXR1 and ANTXR2 each contain an extracellular domain that is highly related to von Willebrand factor type A (VWA) domains and integrin inserted (I) domains. The VWA domains of these receptors are the sites of PA interaction. The PA63 heptamer can bind up to seven molecules of the receptor I domain, leading to substantial receptor clustering at the cell surface [9].
The VWA domains of ANTXR1 and ANTXR2 contain a metal ion-dependent adhesion site (MIDAS) that is characteristic of other similar domains including integrin inserted (I) domains. The MIDAS consists of a (DxSxS..T..D) motif, where x is a variable amino acid and the dots represent variable spacing of amino acids, and it binds a divalent cation at the PA-receptor binding interface [9, 10]. Several features of the PA-receptor interaction are similar to those seen with the open, or high affinity-binding, conformation that is associated with integrin-ligand interactions [9-13]. Consistently, the affinity of the ANTXR1 VWA domain for PA in the presence of different divalent cations (Kd in the μM range) is reminiscent of that seen with integrin-ligand interactions [14]. By contrast, the affinity of PA for the VWA domain of ANTXR2 is approximately 1000-fold higher [15].
The reason for the striking difference in binding affinities seen with both receptors is not yet well understood. However, X-ray crystal structures of PA-ANTXR2 VWA complexes have shown that there is approximately 2000 Å of buried protein surface at the PA-binding interface. The ANTXR2 VWA domain contacts the base of PA domain 4, and residues located in the β4-α4 loop region of this receptor domain make intimate contacts with the base of PA domain 2 [9, 10]. Particularly important residues in this regard are G153, which permits a tight turn in this loop region of the receptor and L154 which makes hydrophobic contacts with the side chains of PA residues L340 and T349 (Fig. 1).
Figure 1.
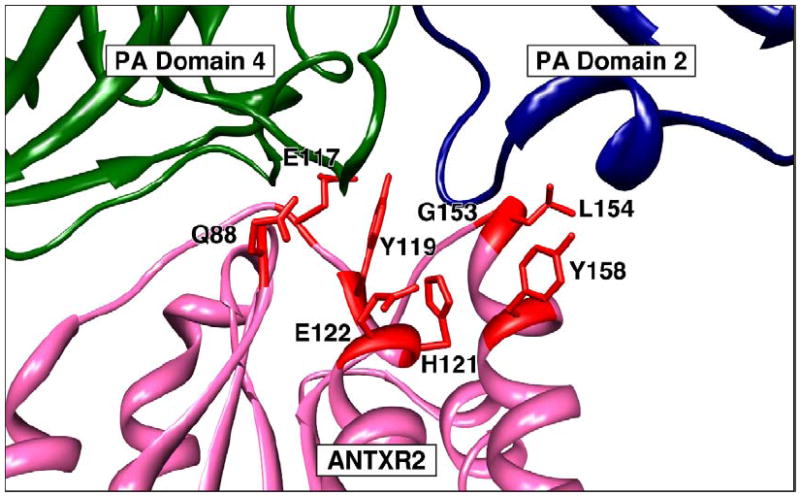
ANTXR2 determinants that dictate the low pH threshold of PA-heptamer pore formation.
A detailed structure of the PA-ANTXR1 complex is not yet available but it is anticipated that PA domain 4 will make similar contacts to this receptor. However, since the amino acid residues that correspond to G153 and L154 of ANTXR2 are strikingly different in ANTXR1 (glutamic acid and aspartic acid, respectively), it may be that the latter receptor makes little, if any, contacts with PA domain 2. If so, this could help explain the differences in PA binding affinities between the two receptors since it would reduce the size of the PA-ANTXR1 interface to one that more closely resembles integrin-ligand interactions.
The membrane spanning domain (MSD) of ANTXR1 contains a leucine zipper motif and has been shown to have self-association properties leading to homo-oligomer formation prior to PA-binding [17]. Indeed, receptor self-association seems to be important for toxin entry because mutations that disrupted ANTXR1 dimer formation reduced the rate of (PA63)7 pore formation in cells [17]. The MSD of ANTXR2 has similar features and can also promote self-association of that receptor [17]. However, in that case it is not yet known if receptor multimerization impacts the efficiency of toxin entry. It also remains to be determined whether ANTXR1 and ANTXR2 can form mixed oligomers on cells that express both receptor types.
While initial experiments demonstrated that the cytoplasmic tail of ANTXR1 is not strictly essential for toxin entry [18], the cytoplasmic regions of both anthrax toxin receptors appear to have multiple roles. It has been shown for ANTXR1, that the cytoplasmic tail modulates PA-binding affinity presumably through its interaction with cytoplasmic factors [19]. Thus, it has been proposed that ANTXR1, like integrins, can exist in either a low or high affinity ligand-binding state and that the switch could occur through an inside-out signaling mechanism [19]. The molecular details of how the cytoplasmic region regulates the binding affinity of the ANTXR1 VWA domain for PA remains to be elucidated. Also, it is not known if ANTXR2 can exist in both high and low affinity binding states since it exhibits features that are consistent only with a high affinity binding state [9, 13]. In addition, the cytoplasmic tail is important in regulating the half-life of the protein. It has in particular been shown that palmitoylation of cytoplasmic cysteine residues increase the half-life of the proteins at the plasma membrane by preventing its premature clearance for the cell surface [20]. Moreover the cytoplasmic tail is involved, as for most if not all transmembrane proteins, in guiding and promoting the endocytosis process in particular when triggered by the toxin ([20], see below). In the absence of most of the cytoplasmic tail or when the MSD and the cytoplasmic tail is replaced by a GPI-anchor, endocytosis still occurs [18], albeit presumably with different kinetics/efficiencies and via different routes.
ANTXR1 and ANTXR2 are ubiquitously expressed, and their normal functions are associated with binding to extracellular matrix components and are consistent with events associated with angiogenesis [21-27]. Intriguingly, EdTx can stimulate the upregulation of expression of both receptor types in macrophages by a mechanism that requires its associated adenylate cyclase activity [28]. Thus EdTx can serve to amplify the effects of anthrax toxin through upregulating receptor levels on the surfaces of susceptible cell types. It is currently unclear if Bacillus anthracis has evolved to use these two types of receptor only because they serve as efficient portals of entry into the cell or whether there are additional aspects of these molecules (e.g. their associated cell-signaling pathways) that are also important for various aspects of anthrax pathogenesis.
Knowledge about the molecular details of PA-receptor interactions has been used to develop new approaches for perturbing this first step of intoxication as candidate therapies for anthrax disease. These methods employ either monoclonal antibodies (reviewed in [29]), soluble receptor decoys [14, 30, 31] or polyvalent peptide inhibitors [32]. This information has also been used to generate altered forms of anthrax toxin that interact specifically with either ANTXR1 or ANTXR2. Amino acid substitutions of residue D683, which contributes to coordinating the metal ion bound to the receptor MIDAS, yields altered forms of PA that interact specifically with ANTXR2. These mutant forms of PA have been used to demonstrate the importance of that receptor for lethal toxin killing in rodents [33]. The N657Q mutation also generates a form of PA that binds preferentially to ANTXR2 [35]. By contrast, the R659S and M662R double amino acid substitutions enhanced the selectivity of PA binding to ANTXR1 over ANTXR2 [35]. Such ANTXR1-specific amino acid substitutions, when combined with matrix metalloproteinase-activated forms of PA, may find utility as candidate cancer therapies that employ lethal toxin. These altered forms of PA should allow for very specific toxin targeting to tumor vasculature cells that selectively overexpress ANTXR1 [35, 36].
3. Molecular requirements for anthrax toxin endocytosis
From the point of view of the toxin, considering that (PA63)7 is the receptor of EF and LF, endocytosis of PA83 and monomeric PA63 should not occur to any significant extent to avoid unproductive endocytosis of the toxin –EF and LF being left outside the cell. The anthrax toxin has thus evolved to bind to a receptor that does not undergo constitutive endocytosed but regulated, or ligand-induced, endocytosis. This exclusion of the toxin receptor from constitutive endocytosis appears to be due to S-palmitoylation of at least two cysteine residues in the cytoplasmic tail of both receptors [20]. S-palmitoylation is a reversible modification that consists in the addition of C16 carbon saturated fatty acyl chain to cytoplasmic cysteine residues via a thioester linkage. Upon toxin binding, the toxin receptor complex is redistributed at the cell surface from the glycerophopholipidic region of the plasma membrane to specialized domains rich in cholesterol, so-called lipid rafts [38]. Association with lipid rafts was shown to be a prerequisite for efficient endocytosis. This relocalization allows the encounter between toxin engaged receptors and the E3 ubiquitin ligase Cbl that triggers receptor ubiquitination on a juxtamembranous lysine (K352 in ANTXR1 and K350 in ANTXR2) [20]. The toxin receptor complex is supposedly then packaged in to clathrin-coated vesicles as suggested by the observation that endocytosis depends of the small GTPase dynamin, involved in fission of vesicles from the plasma membrane, and the clathrin accessory protein Eps15 [38]. A direct role of clathrin has however not yet been shown.
4. Role of LRP6
While searching for genes that are essential for endocytosis of anthrax toxin –using an expressed sequence tag (EST) screen to silence chromosomal genes–, Cohen and coworkers identified LRP6 (lipoprotein-receptor-related protein 6) [39]. LRP6 is a type I membrane protein with a large extracellular domain, and a 220 amino acid long cytoplasmic tail and is an essential component of the canonical Wnt signaling pathway [40-42]. Using immunoprecipitation, they found that LRP6 could interact with ANTXR1, findings that were confirmed by Abrami et al. [43] and extended to ANTXR2. Interestingly, it was observed that the toxin receptors can regulate the level of LRP6 in cells, overexpression and silencing of the receptors leading to the post-translational down regulation of LRP6 [43]. Upon toxin binding, LRP6 was found to undergo tyrosine phosphorylation, redistribution to detergent resistant membranes, endocytosis and degradation, showing that LRP6 is part of the toxin-receptor complex [43]. Whether LRP6 affects endocytosis of the toxin however remains controversial. Decreasing LRP6 levels in cells has been reported either to slow down –but not block– the kinetics of anthrax toxin endocytosis [39, 43] or have no effect [44]. The consensus however is that there is no absolute requirement for LRP6 in anthrax endocytosis, not surprisingly since in endocytosis absolute requirements, except for the receptors, are never, or almost never, observed and observed effects are generally kinetic. In agreement with this consensus, mouse embryonic fibroblasts (MEFs) from _lrp_6 knock out mice were found to be sensitive to the toxin [45].
5. Role of ARAP3
In their EST based screen, Cohen and co-workers found a second gene involved in anthrax toxin endocytosis: the multidomain protein ARAP3 (Arf GAP and Rho GAP with ankyrin repeat and PH domains) [46]. ARAP3 was initially isolated based on its ability to bind phosphoinositides and in particular phosphatidyl inositol phosphorylated at positions 3, 4 and 5 of the inositol ring (PIP3) [47]. ARAP3 has GAP (GTPase activating protein) domains for the two small GTPases ARF and Rho [48]. ARFs, or ADP-ribosylation factors, control membrane trafficking and cytoskeletal organization [49]. Rho-family GTPases, which include Rac1, Cdc42 and RhoA, play central roles in controlling actin cytoskeletal dynamics [50]. ARAP3 has been shown to be involved in cell attachment [51] and lamelipodia formation after growth factor stimulation [52]. It was also found to interact with CIN85, a multidomain adaptor protein [53] which interacts with the E3 ubiquitin ligase Cbl [54] which in turn mediates down-regulation of epidermal growth factor (EGF) receptors and as mentioned is also involved in anthrax toxin endocytosis [20]. At present, it is unclear how ARAP3 contributes to toxin endocytosis. Two possibilities are that ARAP3 promotes the recruitment of Cbl to the receptor or affects actin dynamics in a way that promotes toxin uptake. The involvement of actin in the intoxication process has not been investigated. An interaction between ANTXR1 and actin has however been observed and proposed to play a role in determining the affinity state of the receptor [19]. Also ANTXR1 has been shown to promote cell spreading, an actin dependent process [55].
6. Trafficking of the anthrax toxin along the endocytic pathway
Once the large heterooligomeric complex –composed of PA7mer, EF/LF and the receptors is taken up by the cell, it is delivered to early endosomes were it is incorporated into intraluminal vesicles [56]. It has been well established that sorting into multivesicular endosomes is triggered by the presence of mono or K63 linked ubiquitin on the cytoplasmic tail of transmembrane proteins, which interacts with the ESCRT (Endosomal Sorting Complex Required for Transport) machinery [57]. Although the involvement of ESCRT proteins has not been address for the anthrax uptake, it seems likely that this would be the case.
The acidic pH of the early endosome subsequently triggers the membrane insertion of PA7mer as well as the partial unfolding of EF and LF, which can then be translocated through the lumen of the PA channel to the other side of the membrane. When PA7mer inserts into the membrane of intraluminal vesicles, translocation of EF/LF leads to their release into the lumen of the intraluminal vesicles, i.e still away from their cytoplasmic target or cofactors –MAP kinase kinases for LF and calmodulin for EF.
At the level of early endosomes, formation of intraluminal vesicles can occur, but this is believed to be a one way trafficking route. In contrast, in late endosomes, the subsequent stage of the endocytic pathway, while intraluminal vesicles can form they can also undergo back fusion with the limiting membrane [58]. Thus to be released into the cytoplasm, the anthrax toxin enzymatic subunits must first be transported to late endosomes in a microtubule dependent manner [56, 59, (Fig. 2)]. It is important to note that, by being packaged in the lumen of the intraluminal vesicles, which is topologically equivalent to the cytoplasm, EF and LF are not in contact with lysosomal proteases. Therefore, anthrax toxin may have evolved to exploit this cellular entry pathway because it protects the enzymatic components from degradation. Little is known about the mechanisms that govern back fusion of intraluminal vesicles, nor are the kinetics and frequency of the process known. It is however tempting to speculate that this sophisticated delivery system would firstly allow the enzymatic subunits be delivery in close proximity of their targets, without having to rely on long-range diffusion, and secondly provide a slow delivery system of the toxin. Although the half-life of EF or LF in the cytoplasm has not been determined, it has been shown that LF is submitted to the N-end rule and that the identity of the N-terminal amino acid determines the potency of the toxin both in cells and mice [60]. These observations suggest that LF is fairly rapidly targeted to the proteasome and thus sequestering it from the cytoplasm and delivering the toxin gradually could well prolong its effect and increase its potency. This however remains to be shown.
Figure 2. Schematic representation of anthrax toxin endocytosis and cytoplasmic delivery.
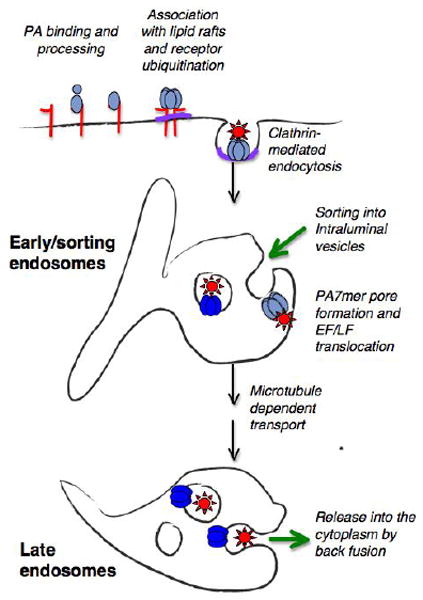
PA83 binds to one of the anthrax toxin receptors and is processed to PA63 by members of the furin family. PA63 subsequently heptamerizes in to PA7mer and concomitantly binds EF and LF. Processing and heptamerization of PA trigger a series of posttranslational modifications in the receptors tails that in turn trigger clathrin mediated endocytosis. In particular ubiquitination of the receptors is though to target the toxin receptor complex to intraluminal vesicles in early endosomes. At the acidic endosomal pH, PA7mer inserts into the membrane of intraluminal vesicles lead to the translocation of EF and LF into the luminal of these vesicles. Carrier intermediates are subsequently form, transported to late endosomes, where back fusion of intraluminal vesicles with the limiting membrane finally lead to release of the enzymatic subunits into the cytoplasm. There LF can process its targets such as MAP kinase kinases and EF can encounter calmodulin and become an active adenylate cyclase.
7. Receptor type dictates the pH threshold of pore formation
By bridging the base regions of PA domains 2 and 4, the ANTXR2 VWA domain has been shown to act as an effective molecular clamp that prevents PA63 prepore to pore conversion at neutral pH. Specifically, the bound receptor VWA domain acts as a structural impediment to the major conformational rearrangements in the 2- 3 loop region and in the flanking beta strands of PA domain 2 which leads to the formation of the transmembrane 14-strand β-barrel pore [9, 10]. In order for these structural changes to occur there either has to be a significant weakening or release of all receptor contacts. Biochemical and biophysical data are consistent with a model in which pore formation is accompanied by a weakening of PA-receptor contacts [61, 62]. In particular, NMR analysis has demonstrated (PA63)7 dissociation from the ANTXR2 VWA domain following low pH treatment [62].
Despite the similarities between the two types of anthrax toxin receptor, they display a striking difference in their associated pH thresholds of (PA63)7 pore formation. Indeed, the pH threshold for pore formation when PA is bound to ANTXR1 (pH 6.2) is a full 1.0 pH unit higher than that seen with ANTXR2 (pH 5.2) [61, 63]. Therefore, PA heptamer pore formation might occur preferentially in early endosomes (~ pH 6.5) when the toxin is bound to ANTXR1 but in late endosomes (pH 5-6), when bound to ANTXR2. The pH thresholds that are associated with pore formation when the PA heptamer is bound with different ratios of ANTXR1 versus ANTXR2 remain to be established.
The distinct pH thresholds of pore formation seen with the two receptors is primarily due to differences in their interaction with PA domain 2. Consistent with this idea, residues G153 and L154 of ANTXR2 which make contact with the base of PA domain 2 (Fig. 1) are the major determinants of the lower pH threshold of pore formation that is associated with that receptor. Substitution of these residues with those found at the corresponding positions of ANTXR1 gives rise to a recombinant receptor with the same pH threshold of pore formation as ANTXR1 [16]. ANTXR2 residue Y119, which is conserved also in ANTXR1 and that interacts with amino acid residues located at the interface between PA domains 2 and 4, is also an important determinant of the pH threshold of pore formation [16, 64]. Other ANTXR2 residues that contribute to the low pH threshold of PA pore formation include residue Q88 and E117 involved in PA domain 4 contact, and residues H121, E122, and Y158 that are involved in PA domain 2 interaction [16, 64].
8. Summary and Future perspectives
In summary much has been learned about anthrax toxin-receptor interactions since the discovery of the first of these receptors almost 8 years ago. High resolution structural information is available on the interaction between PA and one of these receptors, and important aspects of toxin-receptor interactions leading to cellular entry have been elucidated. In our opinion we believe that some of the most interesting outstanding questions that remain about these interactions are: a) how does the toxin signal its endocytosis and what is the repertoire of cellular factors involved in toxin endocytosis and trafficking? In particular what is the mechanism of accelerated entry associated with LRP6? b) What are the dynamic conformational changes in the toxin-receptor complex that give rise to pore formation at acidic pH? c) How does back fusion of intraluminal vesicles occur in late endosomes and does the toxin promote this event in some way? d) and finally what does the toxin tell us about the natural ligands of ANTXR1/2? How do the receptors respond to ligand binding? What is the physiological role of these receptors that would make them different/non redundant with integrins. Times ahead promise to be exciting and informative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moayeri M, Leppla SH. The roles of anthrax toxin in pathogenesis. Curr Opin Microbiol. 2004;7(1):19–24. doi: 10.1016/j.mib.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Duesbery NS, Webb CP, Leppla SH, Gordon VM, Klimpel KR, Copeland TD, Ahn NG, Oskarsson MK, Fukasawa K, Paull KD, Vande Woude GF. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280(5364):734–7. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 3.Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecucco C. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem Biophys Res Commun. 1998;248(3):706–11. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- 4.Leppla SH. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982;79(10):3162–6. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mogridge J, Cunningham K, Collier RJ. Stoichiometry of anthrax toxin complexes. Biochemistry. 2002;41(3):1079–82. doi: 10.1021/bi015860m. [DOI] [PubMed] [Google Scholar]
- 6.Panchal RG, Halverson KM, Ribot W, Lane D, Kenny T, Abshire TG, Ezzell JW, Hoover TA, Powell B, Little S, Kasianowicz JJ, Bavari S. Purified Bacillus anthracis lethal toxin complex formed in vitro and during infection exhibits functional and biological activity. J Biol Chem. 2005;280(11):10834–9. doi: 10.1074/jbc.M412210200. [DOI] [PubMed] [Google Scholar]
- 7.Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414(6860):225–9. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- 8.Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2003;100(9):5170–4. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacy DB, Wigelsworth DJ, Melnyk RA, Harrison SC, Collier RJ. Structure of heptameric protective antigen bound to an anthrax toxin receptor: a role for receptor in pH-dependent pore formation. Proc Natl Acad Sci U S A. 2004;101(36):13147–51. doi: 10.1073/pnas.0405405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santelli E, Bankston LA, Leppla SH, Liddington RC. Crystal structure of a complex between anthrax toxin and its host cell receptor. Nature. 2004;430(7002):905–8. doi: 10.1038/nature02763. [DOI] [PubMed] [Google Scholar]
- 11.Bradley KA, Mogridge J, Jonah G, Rainey A, Batty S, Young JA. Binding of anthrax toxin to its receptor is similar to alpha integrin-ligand interactions. J Biol Chem. 2003;278(49):49342–7. doi: 10.1074/jbc.M307900200. [DOI] [PubMed] [Google Scholar]
- 12.Bradley KA, Young JA. Anthrax toxin receptor proteins. Biochem Pharmacol. 2003;65(3):309–14. doi: 10.1016/s0006-2952(02)01455-7. [DOI] [PubMed] [Google Scholar]
- 13.Lacy DB, Wigelsworth DJ, Scobie HM, Young JA, Collier RJ. Crystal structure of the von Willebrand factor A domain of human capillary morphogenesis protein 2: an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2004;101(17):6367–72. doi: 10.1073/pnas.0401506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scobie HM, Thomas D, Marlett JM, Destito G, Wigelsworth DJ, Collier RJ, Young JA, Manchester M. A soluble receptor decoy protects rats against anthrax lethal toxin challenge. J Infect Dis. 2005;192(6):1047–51. doi: 10.1086/432731. [DOI] [PubMed] [Google Scholar]
- 15.Wigelsworth DJ, Krantz BA, Christensen KA, Lacy DB, Juris SJ, Collier RJ. Binding stoichiometry and kinetics of the interaction of a human anthrax toxin receptor, CMG2, with protective antigen. J Biol Chem. 2004;279(22):23349–56. doi: 10.1074/jbc.M401292200. [DOI] [PubMed] [Google Scholar]
- 16.Scobie HM, Marlett JM, Rainey GJ, Lacy DB, Collier RJ, Young JA. Anthrax toxin receptor 2 determinants that dictate the pH threshold of toxin pore formation. PLoS ONE. 2007;2(3):e329. doi: 10.1371/journal.pone.0000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Go MY, Kim S, Partridge AW, Melnyk RA, Rath A, Deber CM, Mogridge J. Self-association of the transmembrane domain of an anthrax toxin receptor. J Mol Biol. 2006;360(1):145–56. doi: 10.1016/j.jmb.2006.04.072. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Leppla SH. Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J Biol Chem. 2003;278(7):5227–34. doi: 10.1074/jbc.M210321200. [DOI] [PubMed] [Google Scholar]
- 19.Go MY, Chow EM, Mogridge J. The cytoplasmic domain of anthrax toxin receptor 1 affects binding of the protective antigen. Infect Immun. 2009;77(1):52–9. doi: 10.1128/IAI.01073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abrami L, Leppla SH, van der Goot FG. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J Cell Biol. 2006;172(2):309–20. doi: 10.1083/jcb.200507067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell SE, Mavila A, Salazar R, Bayless KJ, Kanagala S, Maxwell SA, Davis GE. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J Cell Sci. 2001;114(Pt 15):2755–73. doi: 10.1242/jcs.114.15.2755. [DOI] [PubMed] [Google Scholar]
- 22.Bonuccelli G, Sotgia F, Frank PG, Williams TM, de Almeida CJ, Tanowitz HB, Scherer PE, Hotchkiss KA, Terman BI, Rollman B, Alileche A, Brojatsch J, Lisanti MP. ATR/TEM8 is highly expressed in epithelial cells lining Bacillus anthracis’ three sites of entry: implications for the pathogenesis of anthrax infection. Am J Physiol Cell Physiol. 2005;288(6):C1402–10. doi: 10.1152/ajpcell.00582.2004. [DOI] [PubMed] [Google Scholar]
- 23.Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61(18):6649–55. [PubMed] [Google Scholar]
- 24.Hotchkiss KA, Basile CM, Spring SC, Bonuccelli G, Lisanti MP, Terman BI. TEM8 expression stimulates endothelial cell adhesion and migration by regulating cell-matrix interactions on collagen. Exp Cell Res. 2005;305(1):133–44. doi: 10.1016/j.yexcr.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Nanda A, Carson-Walter EB, Seaman S, Barber TD, Stampfl J, Singh S, Vogelstein B, Kinzler KW, St Croix B. TEM8 interacts with the cleaved C5 domain of collagen alpha 3(VI) Cancer Res. 2004;64(3):817–20. doi: 10.1158/0008-5472.can-03-2408. [DOI] [PubMed] [Google Scholar]
- 26.Rmali KA, Al-Rawi MA, Parr C, Puntis MC, Jiang WG. Upregulation of tumour endothelial marker-8 by interleukin-1beta and its impact in IL-1beta induced angiogenesis. Int J Mol Med. 2004;14(1):75–80. [PubMed] [Google Scholar]
- 27.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289(5482):1197–202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 28.Maldonado-Arocho FJ, Fulcher JA, Lee B, Bradley KA. Anthrax oedema toxin induces anthrax toxin receptor expression in monocyte-derived cells. Mol Microbiol. 2006;61(2):324–37. doi: 10.1111/j.1365-2958.2006.05232.x. [DOI] [PubMed] [Google Scholar]
- 29.Schneemann A, Manchester M. Monoclonal antibody therapies for anthrax. Future Microbiology. 2009;4:35–43. doi: 10.2217/17460913.4.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma S, Thomas D, Marlett J, Manchester M, Young JA. Efficient neutralization of antibody-resistant forms of anthrax toxin by a soluble receptor decoy inhibitor. Antimicrob Agents Chemother. 2009;53(3):1210–2. doi: 10.1128/AAC.01294-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vuyisich M, Gnanakaran S, Lovchik JA, Lyons CR, Gupta G. A dual-purpose protein ligand for effective therapy and sensitive diagnosis of anthrax. Protein J. 2008;27(5):292–302. doi: 10.1007/s10930-008-9137-0. [DOI] [PubMed] [Google Scholar]
- 32.Basha S, Rai P, Poon V, Saraph A, Gujraty K, Go MY, Sadacharan S, Frost M, Mogridge J, Kane RS. Polyvalent inhibitors of anthrax toxin that target host receptors. Proc Natl Acad Sci U S A. 2006;103(36):13509–13. doi: 10.1073/pnas.0509870103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scobie HM, Wigelsworth DJ, Marlett JM, Thomas D, Rainey GJ, Lacy DB, Manchester M, Collier RJ, Young JA. Anthrax toxin receptor 2-dependent lethal toxin killing in vivo. PLoS Pathog. 2006;2(10):e111. doi: 10.1371/journal.ppat.0020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scobie HM, Young JA. Divalent metal ion coordination by residue T118 of anthrax toxin receptor 2 is not essential for protective antigen binding. PLoS ONE. 2006;1:e99. doi: 10.1371/journal.pone.0000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen KH, Liu S, Bankston LA, Liddington RC, Leppla SH. Selection of anthrax toxin protective antigen variants that discriminate between the cellular receptors TEM8 and CMG2 and achieve targeting of tumor cells. J Biol Chem. 2007;282(13):9834–45. doi: 10.1074/jbc.M611142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alfano RW, Leppla SH, Liu S, Bugge TH, Duesbery NS, Frankel AE. Potent inhibition of tumor angiogenesis by the matrix metalloproteinase-activated anthrax lethal toxin: implications for broad anti-tumor efficacy. Cell Cycle. 2008;7(6):745–9. doi: 10.4161/cc.7.6.5627. [DOI] [PubMed] [Google Scholar]
- 37.Alfano RW, Leppla SH, Liu S, Bugge TH, Herlyn M, Smalley KS, Bromberg-White JL, Duesbery NS, Frankel AE. Cytotoxicity of the matrix metalloproteinase-activated anthrax lethal toxin is dependent on gelatinase expression and B-RAF status in human melanoma cells. Mol Cancer Ther. 2008;7(5):1218–26. doi: 10.1158/1535-7163.MCT-08-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrami L, Liu S, Cosson P, Leppla SH, van der Goot FG. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J Cell Biol. 2003;160(3):321–8. doi: 10.1083/jcb.200211018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei W, Lu Q, Chaudry GJ, Leppla SH, Cohen SN. The LDL receptor-related protein LRP6 mediates internalization and lethality of anthrax toxin. Cell. 2006;124(6):1141–54. doi: 10.1016/j.cell.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 40.Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407(6803):535–8. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- 41.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407(6803):530–5. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 42.Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13(1):149–56. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 43.Abrami L, Kunz B, Deuquet J, Bafico A, Davidson G, van der Goot FG. Functional interactions between anthrax toxin receptors and the WNT signaling protein LRP6. Cell Microbiol. 2008;10:2509–2519. doi: 10.1111/j.1462-5822.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- 44.Ryan PL, Young JA. Evidence against a human cell-specific role for LRP6 in anthrax toxin entry. PLoS ONE. 2008;3(3):e1817. doi: 10.1371/journal.pone.0001817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young JJ, Bromberg-White JL, Zylstra C, Church JT, Boguslawski E, Resau JH, Williams BO, Duesbery NS. LRP5 and LRP6 are not required for protective antigen-mediated internalization or lethality of anthrax lethal toxin. PLoS Pathog. 2007;3(3):e27. doi: 10.1371/journal.ppat.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu Q, Wei W, Kowalski PE, Chang AC, Cohen SN. EST-based genome-wide gene inactivation identifies ARAP3 as a host protein affecting cellular susceptibility to anthrax toxin. Proc Natl Acad Sci U S A. 2004 doi: 10.1073/pnas.0407794101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krugmann S, Anderson KE, Ridley SH, Risso N, McGregor A, Coadwell J, Davidson K, Eguinoa A, Ellson CD, Lipp P, Manifava M, Ktistakis N, Painter G, Thuring JW, Cooper MA, Lim ZY, Holmes AB, Dove SK, Michell RH, Grewal A, Nazarian A, Erdjument-Bromage H, Tempst P, Stephens LR, Hawkins PT. Identification of ARAP3, a novel PI3K effector regulating both Arf and Rho GTPases, by selective capture on phosphoinositide affinity matrices. Mol Cell. 2002;9(1):95–108. doi: 10.1016/s1097-2765(02)00434-3. [DOI] [PubMed] [Google Scholar]
- 48.Krugmann S, Williams R, Stephens L, Hawkins PT. ARAP3 is a PI3K- and rap-regulated GAP for RhoA. Curr Biol. 2004;14(15):1380–4. doi: 10.1016/j.cub.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 49.Inoue H, Randazzo PA. Arf GAPs and their interacting proteins. Traffic. 2007;8(11):1465–75. doi: 10.1111/j.1600-0854.2007.00624.x. [DOI] [PubMed] [Google Scholar]
- 50.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16(10):522–9. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Stacey TT, Nie Z, Stewart A, Najdovska M, Hall NE, He H, Randazzo PA, Lock P. ARAP3 is transiently tyrosine phosphorylated in cells attaching to fibronectin and inhibits cell spreading in a RhoGAP-dependent manner. J Cell Sci. 2004;117(Pt 25):6071–84. doi: 10.1242/jcs.01526. [DOI] [PubMed] [Google Scholar]
- 52.Krugmann S, Andrews S, Stephens L, Hawkins PT. ARAP3 is essential for formation of lamellipodia after growth factor stimulation. J Cell Sci. 2006;119(Pt 3):425–32. doi: 10.1242/jcs.02755. [DOI] [PubMed] [Google Scholar]
- 53.Kowanetz K, Husnjak K, Holler D, Kowanetz M, Soubeyran P, Hirsch D, Schmidt MH, Pavelic K, De Camilli P, Randazzo PA, Dikic I. CIN85 associates with multiple effectors controlling intracellular trafficking of epidermal growth factor receptors. Mol Biol Cell. 2004;15(7):3155–66. doi: 10.1091/mbc.E03-09-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haglund K, Shimokawa N, Szymkiewicz I, Dikic I. Cbl-directed monoubiquitination of CIN85 is involved in regulation of ligand-induced degradation of EGF receptors. Proc Natl Acad Sci U S A. 2002;99(19):12191–6. doi: 10.1073/pnas.192462299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Werner E, Kowalczyk AP, Faundez V. Anthrax toxin receptor 1/tumor endothelium marker 8 mediates cell spreading by coupling extracellular ligands to the actin cytoskeleton. J Biol Chem. 2006;281(32):23227–36. doi: 10.1074/jbc.M603676200. [DOI] [PubMed] [Google Scholar]
- 56.Abrami L, Lindsay M, Parton RG, Leppla SH, van der Goot FG. Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J Cell Biol. 2004;166:645–651. doi: 10.1083/jcb.200312072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458(7237):445–52. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 58.Falguieres T, Luyet PP, Gruenberg J. Molecular assemblies and membrane domains in multivesicular endosome dynamics. Exp Cell Res. 2009;315(9):1567–73. doi: 10.1016/j.yexcr.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 59.Dal Molin F, Tonello F, Ladant D, Zornetta I, Zamparo I, Di Benedetto G, Zaccolo M, Montecucco C. Cell entry and cAMP imaging of anthrax edema toxin. EMBO J. 2006;25(22):5405–13. doi: 10.1038/sj.emboj.7601408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta PK, Moayeri M, Crown D, Fattah RJ, Leppla SH. Role of N-terminal amino acids in the potency of anthrax lethal factor. PLoS ONE. 2008;3(9):e3130. doi: 10.1371/journal.pone.0003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rainey GJ, Wigelsworth DJ, Ryan PL, Scobie HM, Collier RJ, Young JA. Receptor-specific requirements for anthrax toxin delivery into cells. Proc Natl Acad Sci U S A. 2005;102(37):13278–83. doi: 10.1073/pnas.0505865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajapaksha M, Eichler JF, Hajduch J, Anderson DE, Kirk KL, Bann JG. Monitoring anthrax toxin receptor dissociation from the protective antigen by NMR. Protein Sci. 2009;18(1):17–23. doi: 10.1002/pro.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolfe JT, Krantz BA, Rainey GJ, Young JA, Collier RJ. Whole-cell voltage clamp measurements of anthrax toxin pore current. J Biol Chem. 2005;280(47):39417–22. doi: 10.1074/jbc.M509049200. [DOI] [PubMed] [Google Scholar]
- 64.Liu S, Leung HJ, Leppla SH. Characterization of the interaction between anthrax toxin and its cellular receptors. Cell Microbiol. 2007;9(4):977–87. doi: 10.1111/j.1462-5822.2006.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]