Functional extracellular eosinophil granules: novel implications in eosinophil immunobiology (original) (raw)
. Author manuscript; available in PMC: 2010 Dec 1.
Published in final edited form as: Curr Opin Immunol. 2009 Aug 24;21(6):694–699. doi: 10.1016/j.coi.2009.07.011
Abstract
Human eosinophils contain within their cytoplasmic granules multiple preformed proteins, including over three dozen cytokines with nominal Th1, Th2 and immunoregulatory capabilities and four distinctive cationic proteins. The secretion of these granule-derived proteins within eosinophils occurs principally by a mechanism whereby selected proteins are mobilized into vesicles for transport to and release at the cell surface. In contrast, the enigmatic presence of membrane-bound cell free granules extruded from eosinophils has been long recognized in tissues associated with eosinophilia, including allergic diseases and responses to helminths. Functional capabilities for extracellular granules have recently been demonstrated. Eosinophil granules express cytokine receptors on their membranes and function, upon extrusion from eosinophils, as independent secretory organelles releasing granule constituents in response to activating cytokines and chemokines. We provide an update on the processes that mediate selective protein secretion from within eosinophil granules both as intracellular organelles and, as novelty demonstrated, as cell-free extracellular structures.
Background
Eosinophils are granulocytic leukocytes notably associated with allergic conditions, anthelminthic host defense and immunoregulatory responses. Eosinophils contain notable cationic proteins stored within their cytoplasmic granules, including eosinophil peroxidase (EPO), major basic protein (MBP), eosinophil cationic protein (ECP) and eosinophil derived neurotoxin (EDN), which are capable of inducing tissue damage and dysfunction [1]. Furthermore, eosinophils store within their granules substantial quantities of preformed chemokines, growth factors and diverse cytokines with nominal Th1, Th2 and regulatory capacities [2,3]. Eosinophils rapidly and selectively secrete these granule preformed proteins in response to differing stimuli [3-7]; and this capacity for differential secretion of granule-derived cytokines and other proteins is central to the range of activities of eosinophils in varied inflammatory and immunoregulatory responses.
Eosinophil “degranulation”
By electron microscopy (EM), mature eosinophils have a single population of “specific” granules, membrane-bound organelles that contain a crystalloid core surrounded by a matrix. Unlike mast cells or basophils that undergo acute exocytotic degranulation upon cross-linking of their FcεRs, a physiologic mechanism to elicit comparable acute degranulation of eosinophils has not been identified. Exocytosis of granules, with fusion of granules to plasma membranes to release all granule contents in toto, is rarely seen in vivo, except when eosinophils are on the surface of large multicellular helminthic parasites. Cross-linking FcRs for IgG and IgA elicits release of eosinophil cationic granule proteins; but this FcR-mediated “degranulation” has subsequently been shown to be cytolytic for eosinophils and notably releases substantial numbers of free membrane-bound granules from most eosinophils.
In contrast to acute “exocytotic degranulation” the ultrastructure of “activated” eosinophils in vivo reveals that eosinophil granule contents are released by alternative mechanisms of granule secretion. By EM, “activated” eosinophils, e.g., those in asthmatic airways, demonstrate granule alterations, including losses of the crystalloid core and/or the granule matrix, indicative of mechanisms for the intracellular mobilization of granule contents. These alterations within intracellular granules provide evidence for the selective release of ultrastructurally imaged components of eosinophil granules, a process termed “piecemeal degranulation” (PMD) [2,8-11]. During PMD, eosinophil specific granules undergo progressive emptying of some, but not all of, their protein contents. Granule contents are selectively mobilized into spherical and tubular vesicles that need to disengage from granules, cross the cytoplasm and fuse with the plasma membrane to release their specific granule-derived protein cargo. Insights into the functioning of granules during PMD have come from previous work of our group. Melo and colleagues, using transmission electron microscopy (TEM) and electron tomography (ET) techniques, demonstrated that eosinophil secretory granules contain elaborate internal membranous vesiculotubular structures able to sequester and relocate granule products during eosinophil PMD [12]. Stimulated granules release both small spherical vesicles and elongated curved tubular vesicles, both of which have been shown to mediate transport of MBP and IL-4 [6,7]. Subcellular fractionation studies documented that for at least seven cytokines, including IL-4, IL-6, IFN-γ, TNF-α and IL-10, granules are the predominant intracellular sites of storage of these preformed cytokines within eosinophils [3]. Interestingly, granules are also rich sites of localization of chemokine (e.g., CCR3) and cytokine (e.g., IL-4Rα) receptors [5]. Moreover, during secretion of IL-4, IL-4 is transported and carried in secretory vesicles bound to its cognate receptor, IL-4Rα [5]. Subsequently, IL-15 has been likewise documented during its secretion to be obligately chaperoned by complexing with its IL-15 receptor α in other cell types, including transfected cell lines[13]. These findings elucidate mechanisms whereby vesicles derived from granules within eosinophils can transport specific granule-derived proteins, including cytokines chaperoned by their likely non-signaling receptors, and contribute to the selective secretion processes of eosinophils.
Diverse studies of PMD secretion by stimulated eosinophils have demonstrated that different agonists can elicit the differential secretion of certain preformed, granule-derived cytokines without releasing other cytokines [3,5,6,14-17]. Stimulus-induced release of proteins from human eosinophils is a tightly regulated, highly selective process that occurs within minutes of agonist stimulation; and additional insights will be needed to fully delineate the mechanisms that mediate the selective mobilization of specific granule-derived proteins from within intracellular granules of stimulated eosinophils.
Free extracellular eosinophil granules and disease
Besides “piecemeal degranulation,” cytolysis, which involves release and deposition of clusters of free extracellular granules upon lysis of the cell, has been suggested as a potential eosinophil “degranulation” process. EM studies that can recognize the distinct core-containing ultrastructure of membrane bound, extracellular eosinophil granules have suggested that cytolysis is a common mechanism of for the release and deposition of membrane-bound granules extracellularly in tissue biopsies of patients with atopic dermatitis [18], nasal allergy [19,20], nasal polyps [21] and in other upper airway respiratory mucosal disorders [22]. The functioning of cell-free granules and their capacities to mobilize their contents of preformed proteins had not been never been investigated.
The presence of free extracellular eosinophil granules has been recognized in association with diverse eosinophilic disorders. The first recognition of free eosinophil granules in sputum of asthmatics was reported in the early 1880's [23]. Over the years, the presence and importance of free eosinophil granules in tissue sites have been underestimated and at times attributed to “crush artifacts”. However, to date, eosinophil lysis and generation of membrane-bound free eosinophil granules have repeatedly been depicted in the literature by different groups [18,24-32]. With their unique ultrastructure, free extracellular eosinophil granules have been recognized in the airways or tissues in association with asthma and rhinitis [23,25,32]. Clusters of intact extracellular eosinophil granules were found in asthmatic sputum samples [23] and in association with sinus tissue of patients with chronic rhinosinusitis [25,32]. Analysis of the nasal mucus of patients with nasal allergy [20] and skin of patients with chronic idiophatic urticaria [27] revealed the existence of cytolytic eosinophils and extracellular deposition of eosinophil granules. EM analyses of skin biopsies of patients presenting with atopic dermatitis showed the presence of membrane-bound eosinophil granules outside the cell between collagen bundles and in dermis without recognizable adjacent eosinophils [18]. The reaction of the mammalian host to the parasites is associated with eosinophils and their released granule proteins, mainly after anthelminthic chemotherapy. For instance, it was shown that granules from necrotic eosinophils were regularly found on the surface of damaged microfilariae of Onchocerca volvulus after treatment with amocarzine [28]. In patients presenting advanced gastric carcinoma, TEM images revealed extracellular deposition of clusters of membrane-bound extracellular granules admixed with lipid bodies adjacent to late apoptotic eosinophils [29]. In patients with eosinophilic esophagitis, blind histologic evaluation of esophageal biopsy specimens revealed significantly increased numbers of degranulated eosinophils (defined as free eosinophil granules) in the esophageal epithelium [31]. In a different study, after analyzing biopsies of patients with eosinophilic esophagitis, eosinophil granules were also extensively found extracellularly [30]. Free eosinophil granules have also been found in histopathologic analyses of subcutaneous fat necrosis lesions in newborns [33].
As noted the functional importance of extracellular eosinophil granules has been unclear, although one report noted that the presence of free extracellular granules correlated with the severity of urticaria [27]. Moreover, Uller and colleagues found that anti-Fas mAb-induced cytolysis of murine airways eosinophils with release of cell-free granules aggravated rather than resolved experimental airways inflammation [34]. The release of free extracellular granules might potentially explain the observed exacerbation of the inflammatory response, but an underlying mechanism by which extracellular eosinophil granules might augment inflammation was unknown.
Eosinophil granules function as secretory-competent organelles
We have recently shown that isolated human eosinophil granules function extracellularly as secretion competent organelles [35,36]. Granules express on their outer membranes the G protein-coupled receptor (GPCR) for eotaxin, CCR3, and the IFN-γ receptor α chain. On isolated granules, both receptors express ligand-binding domains on their membranes. Receptor activation elicited intragranule signal transduction that lead to the differential secretion of granule-stored cytokines and other proteins. After stimulation with IFN-γ and eotaxin, granules secreted ECP and hydrolytic enzymes such as β-hexosaminidase. IFN-γ also induced differential secretion of cytokines eliciting the release of lL-4 and IL-6, but not IL-13, from isolated granules. Inhibitors of tyrosine kinases, protein kinase C (PKC), and p38 mitogen-activated protein kinase (MAPK) inhibited IFN-γ-dependent release of effector proteins from the granules, whereas the p38 MAPK inhibitor and pertussis toxin, an inhibitor of Gi-coupled GPCRs, blocked eotaxin-dependent granule content release. Western blotting showed the increased phosphorylation of tyrosine residues and p38 MAPK in granules in response to either eotaxin or IFN-γ stimulus compared with unstimulated granules. Moreover, granule secretion was suppressed when the intragranular membranotubular network was collapsed, as assessed after granule treatment with brefeldin A, suggesting a role for this intragranular membranous system on granule secretion, in the same fashion brefeldin A was shown previously to act within granules in intact eosinophils [12].
These findings are remarkable because they provide additional understanding about the capacity of eosinophils to contribute to modulating host and inflammatory responses after eosinophil cytolysis. Cytolytic release of intact eosinophil granules yields extracellular organelles fully capable of ligand-elicited active secretory responses able to act as functional “cluster bombs” amplifying the differential secretory properties of eosinophils and contributing to the persistence and exacerbation of the inflammatory response. More recently, we have identified functional receptors for cysteinyl leukotrienes on cell-free extracellular granule membranes, sensitive to inhibition by montelukast and a P2Y12 receptor antagonist, identifying novel mechanisms whereby cysteinyl leukotrienes may modulate granule secretion (unpublished data).
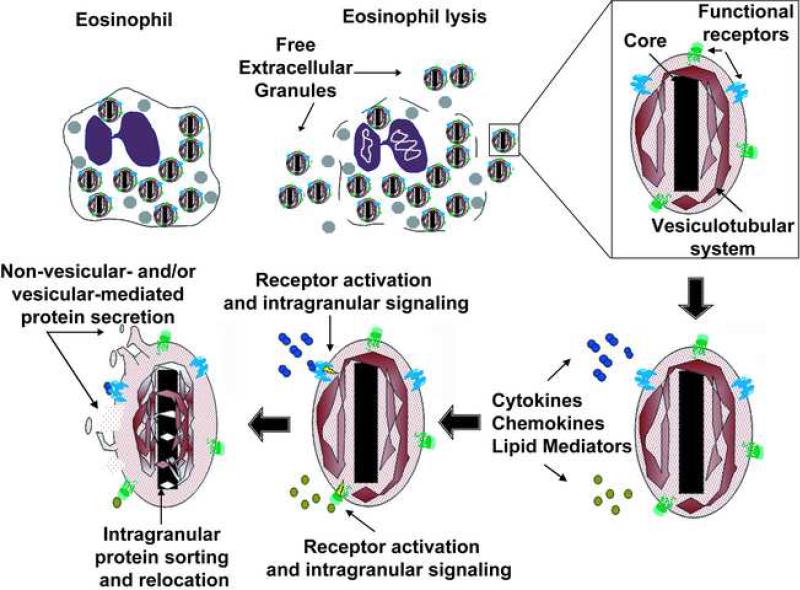
To date, one of the major challenges regarding extracellular granules as secretory organelles, remains in understanding the mechanism by which proteins are released. For cytoplasmic granules, during the process of eosinophil “piecemeal degranulation” and granule emptying, ET studies showed mobilization of granule content from granular subcompartments to the granule surface. Moreover, ET analysis followed by three dimension reconstruction and modeling revealed that there is an area of continuity between the intragranular membranous network and the limiting granule membrane [12]. For extracellular granules, our findings indicate a role for the intragranular vesiculotubular network during extracellular granule secretion, as assessed after granule brefeldin A treatment. However, whether intragranular vesicular structures fuse with the plasma membrane, leading to release of a granular subcompartment content or if there are vesicles coming out from the granule membrane carrying granule cargo, still needs to be elucidated (Figure 1).
Figure 1.
Schematic representation of the eosinophil post-cytolytic mechanisms that leads extracellular granule deposition and activation.
Likewise, these findings are intriguing for eosinophil cell biology. We have previously shown that certain cytokine and chemokine receptors are present on eosinophil granules and have an importance as a mechanism used by eosinophils to selectively and rapid secrete cytokines [5]. Interestingly, the granule surface membrane topology of receptors (CCR3, IFN-γ receptor α chain), [35], with ligand-binding domains displayed on the outer granule membranes, not only allows granules to function extracellularly, but also suggests that they granule expressed receptors may potentially serve as intracrine mediators of eosinophil granule-derived secretion. Excepting the GPCR receptor for cell-permeant estrogens, GPCRs have classically been considered to transduce signals at the plasma membrane [37]. More recently, in addition to its conventional plasma membrane expression, GPCRs for lipid mediators such as prostaglandins and cysteinyl leukotrienes have been immunolocalized at nuclear membranes [38-40]. For GPCRs such as the leukotrienes and prostaglandin receptors, activated by hydrophobic ligands that can be synthesized at the nuclear membrane or lipid bodies [41,42], it is possible to predict roles for these receptors in intracellular compartments, since they could have easy access to their ligands. For cytokine receptors, it is also feasible that the ligands themselves may be released into intracellular compartments during their biosynthesis and maturation or have specific cell uptake mechanisms.
Free granules from other leukocytes have rarely been found or recognized. The presence of free neutrophil granules in tissues of patients with chronic rhinosinusitis was reported in one study [32]. Moreover, by EM, the presence of CCR3 and IL-10 receptors has been demonstrated on the membranes of intracellular mast cell [43] and neutrophil [44] granules, respectively. However, whether granules from other leukocytes express receptors or are capable to work extracellularly as independent organelles is still unknown.
Concluding remarks and questions for the future
There remain important questions regarding the secretory capacities of eosinophil granules. Current knowledge is only beginning to understand the functional biology and responses of eosinophil granules. Eosinophils contain morphologically unique cytoplasmic granules; and evolving understanding has recognized that that eosinophil granules contain secretory machinery that enables granules to both selectively mobilize and secrete specific protein contents intracellularly and to function as secretory “organelles” extracellularly when eosinophil granules are extruded or otherwise released following eosinophil cytolysis in vivo. Important questions remain about the functioning of granule secretory components and their properties on cytoplasmic and extracellular free granules. For instance, how do cytokine and chemokine receptors, so richly localized intracellularly to eosinophil granules, traffic to and from granule membranes? Are granule membrane-expressed cytokine and chemokine receptors phosphorylated, desensitized and internalized in a manner analogous to cell surface receptors? Are granules sites for de novo protein synthesis of granule-stored proteins? Also remaining to be defined is the energy source for granule secretion. ATP or proton motive forces are potential candidates. However, whether granules express nucleoside transporters, known to mediate the majority of influx and efflux of nucleosides across membranes, or proton pump ATPases on their membranes, remain to be investigated. Previous work reported the expression of a vacuolar H+-ATPase (V-ATPase) in eosinophil granules whose function was related to the control of intragranular pH and exocytosis [45,46] . However, whether V-ATPases are involved in extracellular granule protein secretion remains to be elucidated.
Acknowledgments
This work was supported by the National Institutes of Health Grants AI020241, AI022571 and AI051645.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1••.Blanchard C, Rothenberg ME. Biology of the eosinophil. Adv Immunol. 2009;101:81–121. doi: 10.1016/S0065-2776(08)01003-1. [The authors review the eosinophil biology and the eosinophil contributions to inflammatory and adaptative immune responses.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2••.Melo RC, Spencer LA, Dvorak AM, Weller PF. Mechanisms of eosinophil secretion: large vesiculotubular carriers mediate transport and release of granule-derived cytokines and other proteins. J Leukoc Biol. 2008;83:229–236. doi: 10.1189/jlb.0707503. [This study provides important background on the organization of the human eosinophil secretory pathway highlighting the importance of large vesiculotubular carriers in eosinophil intracellular trafficking and secretion of proteins.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Spencer LA, Szela CT, Perez SA, Kirchhoffer CL, Neves JS, Radke AL, Weller PF. Human eosinophils constitutively express multiple Th1, Th2, and immunoregulatory cytokines that are secreted rapidly and differentially. J Leukoc Biol. 2009;85:117–123. doi: 10.1189/jlb.0108058. [Interesting study showing that human blood eosinophil granules are the predominant intracellular sites of storage of preformed IL-4, IL-6, IFN-γ, TNF-α and IL-10 within eosinophils. Findings in this study also demonstrate the ability of human eosinophils to rapidly and differentially secrete these cytokines in response to specific stimuli.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moqbel R, Coughlin JJ. Differential secretion of cytokines. Sci STKE. 2006;338:26. doi: 10.1126/stke.3382006pe26. [DOI] [PubMed] [Google Scholar]
- 5.Spencer LA, Melo RC, Perez SA, Bafford SP, Dvorak AM, Weller PF. Cytokine receptor-mediated trafficking of preformed IL-4 in eosinophils identifies an innate immune mechanism of cytokine secretion. Proc Natl Acad Sci U S A. 2006;103:3333–3338. doi: 10.1073/pnas.0508946103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melo RC, Spencer LA, Perez SA, Ghiran I, Dvorak AM, Weller PF. Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic. 2005;6:1047–1057. doi: 10.1111/j.1600-0854.2005.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melo RC, Spencer LA, Perez SA, Neves JS, Bafford SP, Morgan ES, Dvorak AM, Weller PF. Vesicle-mediated secretion of human eosinophil granule-derived major basic protein. Lab Invest. 2009;89:769–781. doi: 10.1038/labinvest.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karawajczyk M, Seveus L, Garcia R, Bjornsson E, Peterson CG, Roomans GM, Venge P. Piecemeal degranulation of peripheral blood eosinophils: a study of allergic subjects during and out of the pollen season. Am J Respir Cell Mol Biol. 2000;23:521–529. doi: 10.1165/ajrcmb.23.4.4025. [DOI] [PubMed] [Google Scholar]
- 9.Erjefalt JS, Greiff L, Andersson M, Adelroth E, Jeffery PK, Persson CG. Degranulation patterns of eosinophil granulocytes as determinants of eosinophil driven disease. Thorax. 2001;56:341–344. doi: 10.1136/thorax.56.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dvorak AM, Ackerman SJ, Furitsu T, Estrella P, Letourneau L, Ishizaka T. Mature eosinophils stimulated to develop in human-cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. II. Vesicular transport of specific granule matrix peroxidase, a mechanism for effecting piecemeal degranulation. Am J Pathol. 1992;140:795–807. [PMC free article] [PubMed] [Google Scholar]
- 11.Dvorak AM, Furitsu T, Letourneau L, Ishizaka T, Ackerman SJ. Mature eosinophils stimulated to develop in human cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. Part I. Piecemeal degranulation of specific granules and distribution of Charcot-Leyden crystal protein. Am J Pathol. 1991;138:69–82. [PMC free article] [PubMed] [Google Scholar]
- 12.Melo RC, Perez SA, Spencer LA, Dvorak AM, Weller PF. Intragranular vesiculotubular compartments are involved in piecemeal degranulation by activated human eosinophils. Traffic. 2005;6:866–879. doi: 10.1111/j.1600-0854.2005.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.Duitman EH, Orinska Z, Bulanova E, Paus R, Bulfone-Paus S. How a cytokine is chaperoned through the secretory pathway by complexing with its own receptor: lessons from interleukin-15 (IL-15)/IL-15 receptor alpha. Mol Cell Biol. 2008;28:4851–4861. doi: 10.1128/MCB.02178-07. [The study implicates a critical role for the receptor of IL-15 in mediating secretion of IL-15 in different transfected cell lines and potentially in primary monocytes and macrophages. Findings in this study support the novel implication that in eosinophils the receptor for IL-4 mediates the transport of IL-4 within eosinophil secretory granules.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacy P, Mahmudi-Azer S, Bablitz B, Hagen SC, Velazquez JR, Man SF, Moqbel R. Rapid mobilization of intracellularly stored RANTES in response to interferon-gamma in human eosinophils. Blood. 1999;94:23–32. [PubMed] [Google Scholar]
- 15.Bandeira-Melo C, Sugiyama K, Woods LJ, Weller PF. Cutting edge: eotaxin elicits rapid vesicular transport-mediated release of preformed IL-4 from human eosinophils. J Immunol. 2001;166:4813–4817. doi: 10.4049/jimmunol.166.8.4813. [DOI] [PubMed] [Google Scholar]
- 16.Tedla N, Bandeira-Melo C, Tassinari P, Sloane DE, Samplaski M, Cosman D, Borges L, Weller PF, Arm JP. Activation of human eosinophils through leukocyte immunoglobulin-like receptor 7. Proc Natl Acad Sci U S A. 2003;100:1174–1179. doi: 10.1073/pnas.0337567100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bandeira-Melo C, Sugiyama K, Woods LJ, Phoofolo M, Center DM, Cruikshank WW, Weller PF. IL-16 promotes leukotriene C(4) and IL-4 release from human eosinophils via CD4- and autocrine CCR3-chemokine-mediated signaling. J Immunol. 2002;168:4756–4763. doi: 10.4049/jimmunol.168.9.4756. [DOI] [PubMed] [Google Scholar]
- 18.Cheng JF, Ott NL, Peterson EA, George TJ, Hukee MJ, Gleich GJ, Leiferman KM. Dermal eosinophils in atopic dermatitis undergo cytolytic degeneration. J Allergy Clin Immunol. 1997;99:683–692. doi: 10.1016/s0091-6749(97)70031-9. [DOI] [PubMed] [Google Scholar]
- 19.Erjefalt JS, Greiff L, Andersson M, Matsson E, Petersen H, Linden M, Ansari T, Jeffery PK, Persson CG. Allergen-induced eosinophil cytolysis is a primary mechanism for granule protein release in human upper airways. Am J Respir Crit Care Med. 1999;160:304–312. doi: 10.1164/ajrccm.160.1.9809048. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe K, Misu T, Inoue S, Edamatsu H. Cytolysis of eosinophils in nasal secretions. Ann Otol Rhinol Laryngol. 2003;112:169–173. doi: 10.1177/000348940311200211. [DOI] [PubMed] [Google Scholar]
- 21.Erjefalt JS, Andersson M, Greiff L, Korsgren M, Gizycki M, Jeffery PK, Persson GA. Cytolysis and piecemeal degranulation as distinct modes of activation of airway mucosal eosinophils. J Allergy Clin Immunol. 1998;102:286–294. doi: 10.1016/s0091-6749(98)70098-3. [DOI] [PubMed] [Google Scholar]
- 22.Erjefalt JS, Persson CG. New aspects of degranulation and fates of airway mucosal eosinophils. Am J Respir Crit Care Med. 2000;161:2074–2085. doi: 10.1164/ajrccm.161.6.9906085. [DOI] [PubMed] [Google Scholar]
- 23.Persson CG, Erjefalt JS. “Ultimate activation” of eosinophils in vivo: lysis and release of clusters of free eosinophil granules (Cfegs). Thorax. 1997;52:569–574. doi: 10.1136/thx.52.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Persson CG, Erjefalt JS. Eosinophil lysis and free granules: an in vivo paradigm for cell activation and drug development. Trends Pharmacol Sci. 1997;18:117–123. doi: 10.1016/s0165-6147(97)01042-0. [DOI] [PubMed] [Google Scholar]
- 25.Greiff L, Erjefalt JS, Andersson M, Svensson C, Persson CG. Generation of clusters of free eosinophil granules (Cfegs) in seasonal allergic rhinitis. Allergy. 1998;53:200–203. doi: 10.1111/j.1398-9995.1998.tb03871.x. [DOI] [PubMed] [Google Scholar]
- 26.Farinelli P, Gattoni M, Delrosso G, Boggio P, Raselli B, Merlo E, Valente G, Colombo E. Eosinophilic granules in subcutaneous fat necrosis of the newborn: what do they mean? J Cutan Pathol. 2008;35:1073–1074. doi: 10.1111/j.1600-0560.2007.00975.x. [DOI] [PubMed] [Google Scholar]
- 27.Toyoda M, Maruyama T, Morohashi M, Bhawan J. Free eosinophil granules in urticaria: a correlation with the duration of wheals. Am J Dermatopathol. 1996;18:49–57. doi: 10.1097/00000372-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Gutierrez-Pena EJ, Knab J, Buttner DW. Immunoelectron microscopic evidence for release of eosinophil granule matrix protein onto microfilariae of Onchocerca volvulus in the skin after exposure to amocarzine. Parasitol Res. 1998;84:607–615. doi: 10.1007/s004360050459. [DOI] [PubMed] [Google Scholar]
- 29.Caruso RA, Ieni A, Fedele F, Zuccala V, Riccardo M, Parisi E, Parisi A. Degranulation patterns of eosinophils in advanced gastric carcinoma: an electron microscopic study. Ultrastruct Pathol. 2005;29:29–36. doi: 10.1080/019131290882303. [DOI] [PubMed] [Google Scholar]
- 30.Chehade M, Sampson HA, Morotti RA, Magid MS. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:319–328. doi: 10.1097/MPG.0b013e31806ab384. [DOI] [PubMed] [Google Scholar]
- 31.Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2007;119:206–212. doi: 10.1016/j.jaci.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Ponikau JU, Sherris DA, Kephart GM, Kern EB, Congdon DJ, Adolphson CR, Springett MJ, Gleich GJ, Kita H. Striking deposition of toxic eosinophil major basic protein in mucus: implications for chronic rhinosinusitis. J Allergy Clin Immunol. 2005;116:362–369. doi: 10.1016/j.jaci.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 33.Tajirian A, Ross R, Zeikus P, Robinson-Bostom L. Subcutaneous fat necrosis of the newborn with eosinophilic granules. J Cutan Pathol. 2007;34:588–590. doi: 10.1111/j.1600-0560.2006.00665.x. [DOI] [PubMed] [Google Scholar]
- 34.Uller L, Rydell-Tormanen K, Persson CG, Erjefalt JS. Anti-Fas mAb-induced apoptosis and cytolysis of airway tissue eosinophils aggravates rather than resolves established inflammation. Respir Res. 2005;6:90. doi: 10.1186/1465-9921-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Neves JS, Perez SA, Spencer LA, Melo RC, Reynolds L, Ghiran I, Mahmudi-Azer S, Odemuyiwa SO, Dvorak AM, Moqbel R, et al. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc Natl Acad Sci U S A. 2008;105:18478–18483. doi: 10.1073/pnas.0804547105. [This is the first investigation reporting that extracellular eosinophil granules can work as independent organelles after eosinophil lysis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Neves JS, Perez SA, Spencer LA, Melo RC, Weller PF. Subcellular fractionation of human eosinophils: isolation of functional specific granules on isoosmotic density gradients. J Immunol Methods. 2009;344:64–72. doi: 10.1016/j.jim.2009.03.006. [This paper describes in detail the isolation of functional membrane-bound humam eosinophil specific granules by subcellular fractionation on an isoosmotic iodinated density gradient.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koelle MR. Heterotrimeric G protein signaling: Getting inside the cell. Cell. 2006;126:25–27. doi: 10.1016/j.cell.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 38•.Boivin B, Vaniotis G, Allen BG, Hebert TE. G protein-coupled receptors in and on the cell nucleus: a new signaling paradigm? J Recept Signal Transduct Res. 2008;28:15–28. doi: 10.1080/10799890801941889. [The authors revise and discuss the presence and functionality of GPCR signaling complexes at intracellular compartments besides the plasma membrane.] [DOI] [PubMed] [Google Scholar]
- 39.Nielsen CK, Campbell JI, Ohd JF, Morgelin M, Riesbeck K, Landberg G, Sjolander A. A novel localization of the G-protein-coupled CysLT1 receptor in the nucleus of colorectal adenocarcinoma cells. Cancer Res. 2005;65:732–742. [PubMed] [Google Scholar]
- 40.Jiang Y, Borrelli LA, Kanaoka Y, Bacskai BJ, Boyce JA. CysLT2 receptors interact with CysLT1 receptors and down-modulate cysteinyl leukotriene dependent mitogenic responses of mast cells. Blood. 2007;110:3263–3270. doi: 10.1182/blood-2007-07-100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bozza PT, Magalhaes KG, Weller PF. Leukocyte lipid bodies - Biogenesis and functions in inflammation. Biochim Biophys Acta. 2009;1791:540–551. doi: 10.1016/j.bbalip.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brock TG, Anderson JA, Fries FP, Peters-Golden M, Sporn PH. Decreased leukotriene C4 synthesis accompanies adherence-dependent nuclear import of 5-lipoxygenase in human blood eosinophils. J Immunol. 1999;162:1669–1676. [PubMed] [Google Scholar]
- 43.Price KS, Friend DS, Mellor EA, De Jesus N, Watts GF, Boyce JA. CC chemokine receptor 3 mobilizes to the surface of human mast cells and potentiates immunoglobulin E-dependent generation of interleukin 13. Am J Respir Cell Mol Biol. 2003;28:420–427. doi: 10.1165/rcmb.2002-0155OC. [DOI] [PubMed] [Google Scholar]
- 44.Elbim C, Reglier H, Fay M, Delarche C, Andrieu V, El Benna J, Gougerot-Pocidalo MA. Intracellular pool of IL-10 receptors in specific granules of human neutrophils: differential mobilization by proinflammatory mediators. J Immunol. 2001;166:5201–5207. doi: 10.4049/jimmunol.166.8.5201. [DOI] [PubMed] [Google Scholar]
- 45.Bankers-Fulbright JL, Kephart GM, Bartemes KR, Kita H, O'Grady SM. Platelet-activating factor stimulates cytoplasmic alkalinization and granule acidification in human eosinophils. J Cell Sci. 2004;117:5749–5757. doi: 10.1242/jcs.01498. [DOI] [PubMed] [Google Scholar]
- 46.Kurashima K, Numata M, Yachie A, Sai Y, Ishizaka N, Fujimura M, Matsuda T, Ohkuma S. The role of vacuolar H(+)-ATPase in the control of intragranular pH and exocytosis in eosinophils. Lab Invest. 1996;75:689–698. [PubMed] [Google Scholar]