Functional enhancement and protection of dopaminergic terminals by RAB3B overexpression (original) (raw)
Abstract
In Parkinson's disease (PD), dopaminergic (DA) neurons in the substantia nigra (SN, A9) are particularly vulnerable, compared to adjacent DA neurons within the ventral tegmental area (VTA, A10). Here, we show that in rat and human, one RAB3 isoform, RAB3B, has higher expression levels in A10 compared to A9 neurons. RAB3 is a monomeric GTPase protein that is highly enriched in synaptic vesicles and is involved in synaptic vesicle trafficking and synaptic transmission, disturbances of which have been implicated in several neurodegenerative diseases, including PD. These findings prompted us to further investigate the biology and neuroprotective capacity of RAB3B both in vitro and in vivo. RAB3B overexpression in human dopaminergic BE (2)-M17 cells increased neurotransmitter content, [3H] dopamine uptake, and levels of presynaptic proteins. AAV-mediated RAB3B overexpression in A9 DA neurons of the rat SN increased striatal dopamine content, number and size of synaptic vesicles, and levels of the presynaptic proteins, confirming in vitro findings. Measurement of extracellular DOPAC, a dopamine metabolite, following l-DOPA injection supported a role for RAB3B in enhancing the dopamine storage capacity of synaptic terminals. RAB3B overexpression in BE (2)-M17 cells was protective against toxins that simulate aspects of PD in vitro, including an oxidative stressor 6-hydroxydopamine (6-OHDA) and a proteasome inhibitor MG-132. Furthermore, RAB3B overexpression in rat SN both protected A9 DA neurons and resulted in behavioral improvement in a 6-OHDA retrograde lesion model of PD. These results suggest that RAB3B improves dopamine handling and storage capacity at presynaptic terminals, and confers protection to vulnerable DA neurons.
Keywords: differential vulnerability, Parkinson's disease, RAB, synaptic vesicles
In Parkinson's disease (PD), dopaminergic (DA) neurons of the ventral tegmental area (VTA, A10) are largely spared, even though they are immediately adjacent to DA neurons in the substantia nigra (SN) pars compacta (A9) that are degenerating in PD (1). Recently, we and others have demonstrated that rodent A9 and A10 DA neurons have distinct gene expression profiles despite their many similarities (2–4), creating biochemical identities that underlie the different thresholds of vulnerability to pathophysiological processes (5). Indeed, altering expression of several differentially expressed genes both in vitro and in vivo affect the vulnerability to neurotoxins, providing a proof-of-principle for this concept (3, 6).
Microarray analysis of two subgroups of midbrain DA neurons in rat indicated that RAB3B mRNA levels were much more elevated in DA neurons of the VTA (A10) than DA neurons of the SN (A9) (2). RAB proteins are monomeric GTPase proteins and form the largest family in the Ras superfamily of GTPases. They are localized to the cytoplasmic face of vesicles and organelles and recognized for their key roles in vesicle budding, transport, docking, and fusion. Among these, RAB3 proteins (RAB3A-D) are enriched in synaptic vesicles in neurons and modulate vesicle trafficking at synaptic terminals. They regulate Ca2+-triggered neurotransmitter exocytosis (7) and interact with a complex of SNARE proteins including SNAP-25, and effector proteins including RIM, rabphilin 3, synpasin, and calmodulin (8, 9). In addition, RAB3 may have an important role in vesicle transport to synaptic terminals by interacting with an RAB3 effector protein, RAB3-GEP (GDP-GTP exchange protein) and specific transport motor proteins that are known to transport synaptic vesicles, such as KIF1A and KIF1Bβ (10). In support of this role, RAB3-GEP knockout mice showed reduced number and size of synaptic vesicles in hippocampal cultures (11). Furthermore, neuropathologic studies and disease models of neurodegenerative diseases have suggested compromise of synaptic transmission and vesicle trafficking, processes in which RAB3 proteins serve important functions (12, 13). In PD models, for example, α-synuclein overexpression disrupted vesicle trafficking between the endoplasmic reticulum (ER) and Golgi apparatus and overexpressing RAB1 protein, a facilitator of ER to Golgi trafficking, attenuated α-synuclein-mediated toxicity in models of PD (14). Furthermore, RAB homeostasis was generally disturbed by α-synuclein in yeast and overexpressing RAB3A and RAB8A was protective in neuronal models of α-synucleinopathy (15).
The biology of the RAB3B protein, together with literature implicating processes in which it is intimately involved in neurodegenerative diseases including PD, prompted us to investigate the biological consequences of increased neuronal RAB3B levels and the ability of RAB3B to confer neuroprotection in PD-relevant models both in vitro and in vivo.
Results
RAB3B Is Enriched in VTA (A10) DA Terminals Compared to SN (A9) Terminals.
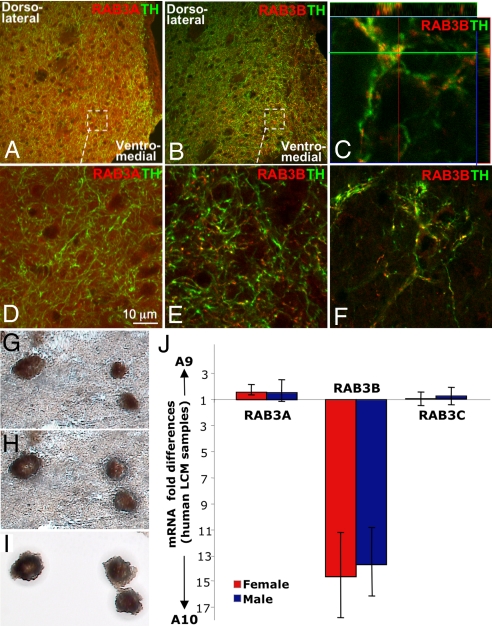
DA neurons in the SN (A9) mainly project to the dorsolateral striatum whereas DA neurons in the VTA (A10) send their projections to the ventromedial striatum, nucleus accumbens, lateral septum and prefrontal cortex. Since RAB3 proteins are mainly enriched in presynaptic terminals, sections containing rat striatum, lateral septum and nucleus accumbens were stained with RAB3A or RAB3B antibody. RAB3A was expressed evenly throughout the striatum and nucleus accumbens, and not limited to DA terminals marked by tyrosine hydroxylase (TH) staining (Fig. 1 A and D). In contrast, RAB3B was highly enriched in the ventromedial striatum, nucleus accumbens and lateral septum compared to the dorsolateral striatum (Fig. 1 B, C, E, and F). In these areas, it was found mainly in DA terminals in a punctate fashion (Fig. 1 C, E, and F). These findings demonstrate that RAB3B is elevated in projections from the resistant group of DA neurons in the VTA (A10) area in rat.
Fig. 1.
RAB3B expression is elevated in VTA (A10) DA terminals in rat and RAB3B mRNA is elevated in VTA (A10) DA neurons in human. In rat striatum, RAB3A (red) was evenly distributed throughout the striatum including TH (green) positive fibers (A and D). In contrast, RAB3B (red) was enriched in the VTA (A10) DA projection area, including the ventromedial striatum (B and E) and the septum (F). Unlike RAB3A, a RAB3B expression pattern was exclusive to TH-positive terminals in a punctate manner (B–F). Colocalization of RAB3B and TH was confirmed by the z-stack confocal image (C). (G–J) SN (A9) and VTA (A10) DA neurons were collected using LCM. DA neurons were labeled using the quick TH-staining method described (3) (G). The TH-positive cells were targeted for laser capture with a 7.5-μm laser diameter (H). Captured cells on the thermoplastic film were visualized before processing for RNA extraction (I). Quantitative PCR results using unamplified LCM RNA samples demonstrated that among isoforms of RAB3, only RAB3B mRNA was highly elevated in VTA (A10) VTA (A10) compared to SN (A9) DA neurons in human (J). Data are shown as mRNA ratios of A9/A10 DA neurons ± SEM (n = 4 for male human and n = 4 for female human).
We next investigated if the differential expression pattern observed in rat is conserved in human. To compare mRNA levels of various RAB3 isoforms in human A9 and A10 DA neurons without interference of other cell types, we used quick TH immunostaining and the laser capture microdissection (LCM) technique to selectively collect DA neurons from the SN (A9) and the VTA (A10) in fresh frozen human midbrain sections (Fig. 1 G–I). Quantitative PCR using unamplified RNA isolated from LCM samples revealed that among three isoforms of RAB3 (RAB3A, B, and C), only RAB3B showed approximately 14-fold higher mRNA levels in the VTA (A10) compared to the SN (A9), whereas RAB3A and RAB3C were not differentially expressed between A9 and A10 DA neurons (Fig. 1J).
RAB3B Overexpression in Vitro Increases [3H]-Dopamine Uptake, Neurotransmitter Content, and Levels of Presynaptic Proteins.
Since RAB3B levels were elevated in the resistant group of DA neurons, we next investigated the functional consequence of RAB3B overexpression in vitro. DA neuroblastoma cells, BE (2)-M17, were transduced with a lentivirus encoding GFP or RAB3B tagged with c-myc. Previous studies indicate that the c-myc tag does not interfere with the function of RAB3B (16). Since RAB3B is reported to increase [3H] noradrenaline uptake in PC12 cells (17), we determined if RAB3B increases [3H] dopamine uptake when overexpressed in BE (2)-M17 cells. c-myc-tagged RAB3B overexpression increased both nomifensine-sensitive and reserpine-sensitive uptake in BE (2)-M17 cells (Fig. S1 A and B), indicating that RAB3B increased DAT-sensitive and VMAT-sensitive uptake. In addition, RAB3B overexpression caused an increased in neurotransmitter content measured by HPLC, including dopamine, noradrenaline, and 5-HT (Fig. S1_C_).
We also investigated levels of presynaptic proteins after overexpressing RAB3B in BE (2)-M17 cells (Fig. S1 D and E). RAB3B overexpression was confirmed in these cells by the increased levels of c-myc. Among known RAB3B effector proteins such as rabphilin 3A, calmodulin, synapsin, and RIM1, only calmodulin levels were significantly increased after RAB3B overexpression (Fig. S1 D and E). Among other proteins localized in vesicles, levels of synaptophysin and SNAP-25 were significantly increased in these cells, whereas levels of synaptotagmin and RAB3A levels were reduced, suggesting a functional connection of these proteins to RAB3B. In the case of RAB3A, the reduction in its levels by RAB3B overexpression may reflect a known functional redundancy between these isoforms (7). Tyrosine hydroxylase (TH), dopamine transporter (DAT), and vesicular monoamine transporter 2 (VMAT2) levels remained unaltered.
RAB3B Overexpression in DA Neurons of the Rat SN Increases Dopamine Content, Number and Size of Synaptic Vesicles, and Levels of Presynaptic Proteins.
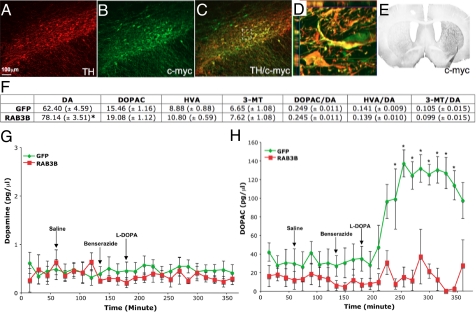
Since RAB3B overexpression produced these changes in dopamine handling in vitro, we next investigated the effect of RAB3B overexpression in vivo. AAV GFP or RAB3B tagged with c-myc under the neuron-specific synapsin promoter, was injected above the rat SN. By using an antibody against c-myc, we detected robust expression of RAB3B in the majority of DA neurons in the SN (Fig. 2 A–D) and their projection target, the striatum (Fig. 2E), indicating that RAB3B is properly transported to its endogenous location in presynaptic terminals. RAB3B expression levels measured by immunoflorescent staining of the striatum after AAV injection appeared to be comparable to endogenous RAB3B levels in A10 projection areas such as the nucleus accumbens.
Fig. 2.
RAB3B overexpression in vivo increases striatal dopamine tissue content and prevents an extracellular DOPAC surge after l-DOPA injection in the striatum. AAV2 RAB3Bc-myc injection into the SN resulted in very efficient transduction of DA neurons (A–C) and their projection target, striatum (E) detected by an antibody against c-myc. The z-stack image of the perforated square in (C) confirmed colocalization of TH/c-myc (D). Three weeks after injection, GFP or RAB3B overexpressing striata were dissected for HPLC analysis. A significant increase in dopamine content was measured in the RAB3B overexpressing striatum compared to the GFP expressing striatum. Ratios of dopamine metabolites to dopamine, however, remain unchanged in the RAB3B overexpressing striatum (F). Data are shown as means ± SEM (AAV GFP, n = 8; AAV RAB3Bc-myc, n = 8; *, P < 0.05 two-tailed t test). Extracellular dopamine and DOPAC levels were measured in the striatum of GFP or RAB3B overexpressing rats before and after 50 mg/kg l-DOPA administration using microdialysis. l-DOPA administration at this dose did not alter dopamine levels (G). DOPAC levels were dramatically increased after l-DOPA injection in GFP overexpressing striatum whereas they remain unaltered in the RAB3B overexpressing striatum (H). Data are shown as mean ± SEM (GFP, n = 6; RAB3B, n = 5; *, P < 0.05 two-tailed t test).
Overexpression of RAB3B resulted in a significant increase in striatal dopamine content without changing the dopamine turnover rate, determined by ratios of dopamine metabolites (DOPAC, HVA, and 3-MT) to dopamine (Fig. 2F). Since HPLC primarily measures vesicular dopamine, these results indicate that RAB3B overexpression led to increased vesicular dopamine content. These data are also consistent with findings in RAB3B-overexpressing cells in vitro (Fig. S1_C_).
In light of the known role of RAB3B in neurotransmitter exocytosis, we next measured extracellular dopamine and DOPAC levels using microdialysis in the striatum after overexpressing either GFP or RAB3B. We measured these levels before and after 50 mg/kg l-DOPA administration. l-DOPA was used to examine how DA terminals handled a transient increase in intracellular dopamine. Regardless of RAB3B overexpression, baseline extracellular dopamine levels were not altered in response to exogenous l-DOPA (Fig. 2G). However, whereas there was a marked increase in DOPAC levels in the control (GFP) condition (Fig. 2H), this surge was abolished in the RAB3B condition (Fig. 2H). One plausible explanation for the decrease in dopamine metabolism in the RAB3B condition is increased sequestration of dopamine into synaptic vesicles.
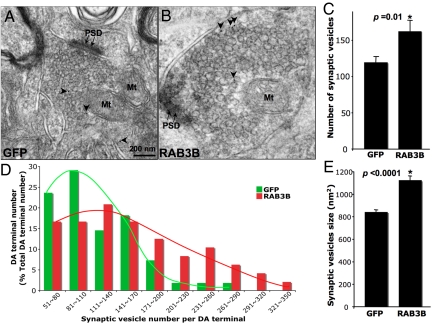
The RAB3B-mediated increase in the dopamine storage capacity supported by the increase in striatal dopamine content and the absence of the DOPAC surge after l-DOPA may plausibly related to increased number or size of synpatic vesicles at presynaptic terminals. To address this possibility, we quantified the number and size of synaptic vesicles in GFP- or c-myc (RAB3B)-positive presynaptic terminals using electron microscopy combined with ImmunoGold staining. RAB3B-positive terminals showed significantly higher number of synaptic vesicles per terminal than GFP-positive terminals (Fig. 3 A–C). When ImmunoGold-positive terminals were sorted based on the number of synaptic vesicles, more RAB3B-positive terminals fell into bins with higher number of synaptic vesicles, showing a right shifted curve (Fig. 3D). When the average vesicle size in a single presynaptic terminal was determined, RAB3B-positive terminals contained significantly larger vesicles than GFP-positive terminals (Fig. 3E). These results suggest that the increase in number and/or size of synaptic vesicles by RAB3B overexpression may, at least in part, explain the increase in striatal dopamine content and the absence of extracellular DOPAC surge in the striatum after l-DOPA injection (see Fig. 6).
Fig. 3.
RAB3B overexpression in vivo increases the number and size of synaptic vesicles in DA presynaptic terminals. The number and size of synaptic vesicles were quantifed in GFP or c-myc (RAB3B)-positive presynaptic terminals in the striatum identified by ImmunoGold technique (A and B). Vesicle number was determined by counting all vesicles contained in the individual presynaptic terminal (73 terminals for GFP and 85 terminals for RAB3B-expressing conditions). RAB3B-positive terminals possessed a greater number of synaptic vesicles than GFP-positive terminals when averaged (C). RAB3B-positive terminals had a tendency to contain a higher number of synaptic vesicles, when sorted by total number of synaptic veiscle per terminal (D). Average vesicle size of a single presynaptic terminal was determined using fractionator and nucleator function in Stereo Inveistigator software (Microbrightfield). RAB3B-positive terminals contained significantly larger vesicles compared to GFP-positive terminals (E).
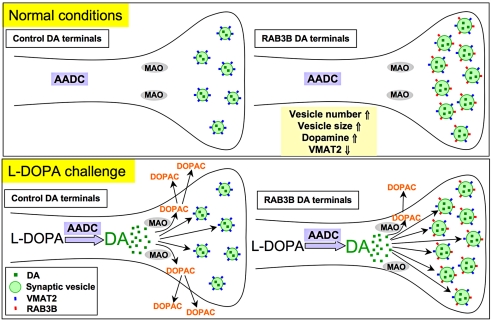
Fig. 6.
Putative synaptic vesicle dynamics in RAB3B overexpressing DA presynaptic terminals. Potential synaptic vesicle dynamics in DA terminals overexpressing GFP (control) or RAB3B were described. RAB3B overexpressing terminals contain more and larger synaptic vesicles, which increases vesicular dopamine content. These changes potentially provide improved dopamine handling and storage capacity to RAB3B overexpressing terminals. Due to the increase in the dopamine content in these terminals, synaptic vesicles contain less VMAT2 levels as a compensatory response. Following l-DOPA injection, in control DA terminals, l-DOPA is metabolized to dopamine by aromatic amino acid decarboxylase (AADC), the levels of which RAB3B did not alter. DOPAC levels were dramatically increased in the control GFP expressing striatum due to the instant metabolism of the increased cytosolic dopamine to DOPAC by monoamine oxidase (MAO) in the cells, which would diffuse out to the extracellular space and be detected by microdialysis probes. In contrast, the increase in DOPAC levels after l-DOPA injection is abolished in the RAB3B overexpressing striatum potentially because the increased cytosolic dopamine followed by l-DOPA injection was rapidly taken up in synaptic vesicles due to increased dopamine storage capacity (increased number and size of synaptic vesicles), avoiding metabolism by MAO.
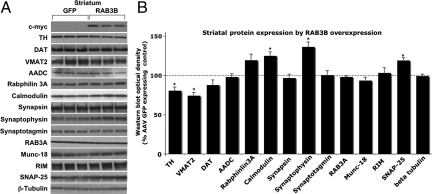
Presynaptic protein profiles determined by differential centrifugation and Western blot analysis revealed that, consistent with the in vitro findings (Fig. S1 D and E), levels of calmodulin, synaptophysin, and SNAP-25 were significantly increased by RAB3B overexpression in the striatum (Fig. 4 A and B). TH and VMAT2 levels, however, were significantly reduced in the rat striatum overexpressing RAB3B without any changes in the levels of aromatic amino acid decarboxylase (AADC), the enzyme that converts l-DOPA to dopamine (Fig. 4 A and B).
Fig. 4.
RAB3B overexpression in vivo increases levels of presynaptic proteins in the striatum. Western blot analysis revealed that TH and VAMT2 levels were significantly reduced whereas calmodulin, synaptophysin, and SNAP-25 levels were increased (A and B). Optical densities of the individual bands were quantified using NIH image. Optical densities of RAB3B-overexpressing conditions were normalized by the averaged value of GFP expressing condition. Data are shown as mean ± SEM (GFP, n = 4; RAB3B, n = 4; *, P < 0.05 two-tailed t test).
RAB3B Overexpression Protects BE (2)-M17 Cells from 6-Hydroxydopamine (6-OHDA) and MG-132 Toxicity in Vitro and A9 DA Neurons from a Retrograde 6-OHDA Lesion in Rat.
We next investigated the role of RAB3B in the protection of DA neurons in vitro using BE (2)-M17 cells expressing GFP, RAB3A-c-myc, or RAB3B-c-myc. Cell viability or cell death was measured after treating these cells with various doses of 6-OHDA, an oxidative stressor, and MG-132, a proteasome inhibitor. 6-OHDA and MG-132 were chosen because oxidative stress and impaired proteasomal function are two processes widely thought to be involved in PD pathogenesis (18, 19). Overexpression of RAB3B was protective against 6-OHDA and MG-132 toxicity, whereas RAB3A was protective only against MG-132 toxicity (Fig. S2). On the other hand, reduction of endogenous RAB3B using small interfering RNA (siRNA) increased the vulnerability of the cells to both toxins (Figs. S2 and S3), supporting the idea that endogenous RAB3B confers protection against these insults. Interestingly, protection against 6-OHDA toxicity appeared to be specific to RAB3B, and not RAB3A, suggesting distinct functions for the closely related RAB3 isoforms.
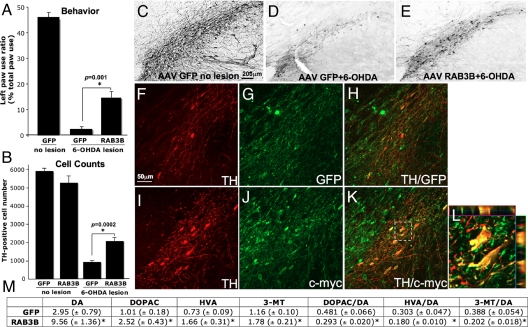
Since RAB3B was protective in in vitro models of PD, we next examined if RAB3B overexpression in vivo is protective against a retrograde 6-OHDA lesion in rat. In these experimenets, 6-OHDA was infused into the rat striatum 3 weeks after AAV GFP or RAB3B injection into the SN. As a behavioral assay we assessed paw reaching preference. Retrograde lesioning of the SN results in deficient paw reaching ipsilateral to the side of the lesion (right side lesion in our paradigm). Functional recovery is assessed by resolution of this asymmetry. RAB3B expression resulted in enhanced recovery with the left paw used more frequently than in the control condition. RAB3B thus rescues the 6-OHDA-induced functional deficit (Fig. 5A). In the postmortem analysis, stereological counting of TH-positive neurons showed that there were significantly more TH-positive neurons remaining in SN for the RAB3B compared to the GFP condition after the 6-OHDA lesion (Fig. 5 B–E). Most of the surviving TH-positive neurons were strongly c-myc (or RAB3B)-positive (Fig. 5 F–L), suggesting that RAB3B overexpression, not lesion variability, was the cause of their increased survival. Protection of SN (A9) DA neurons by RAB3B was also confirmed by increased striatal dopamine content and reduced dopamine turnover in the RAB3B condition (Fig. 5M).
Fig. 5.
RAB3B overexpression protects DA neurons from a retrograde 6-OHDA lesion in rat. 6-OHDA was injected into the striatum 3 weeks after AAV GFP or RAB3Bc-myc injection. Paw reaching test results showed that RAB3B overexpression improved the behavioral asymmetry caused by the 6-OHDA lesion (A). Data are shown as means ± SEM (*; two-tailed t test). TH-positive neurons in the SN were stained using DAB immunohistochemistry (C–E) and double immunofluorescent (GFP/TH or c-myc/TH; F–L) technique. Stereological counting demonstrated that more TH-positive neurons remained in the AAV RAB3Bc-myc injected SN compared to the AAV GFP injected SN (Data are shown as means ± SEM.*; two-tailed t test). Most of the remaining TH-positive neurons in AAV RAB3Bc-myc injected midbrain were strongly c-myc-positive (I–K). Colocalization was confirmed by a z-stack image of the perforated square in K (L). HPLC analysis in the striatum after the 6-OHDA lesion, demonstrated that a significant increase in DA tissue content was measured in the RAB3B-overexpressing striatum compared to the GFP expressing striatum (M). Ratios of dopamine metabolites to dopamine were reduced in the RAB3B overexpressing striatum (M). Data are shown as means ± SEM (AAV GFP, n = 12; AAV RAB3Bc-myc, n = 12; *, P < 0.05 two-tailed t test).
Discussion
Relative neuronal vulnerability is a common feature of neurodegenerative diseases. The different susceptibilities to degeneration of A9 and A10 DA neurons in PD are a striking example of this phenomenon. In this study, we demonstrate that RAB3B expression levels are elevated in the resistant group of DA neurons in rodent and human, and that increasing the expression level of this molecule confers protection to the vulnerable group of DA neurons in an in vivo model of PD. We also characterize the biological consequences of RAB3B elevation in DA neurons. Our data delineate a role for RAB3B in modulating synaptic vesicle dynamics at DA terminals, and suggest a potential mechanism of RAB3B-mediated protection. We suggest RAB3B is an intriguing neuroprotective candidate that may modify disease progression potentially by restoring neurotransmission and synaptic vesicle transport. More broadly, our findings illustrate that the analysis of proteins differentially expressed in subsets of DA neurons can give clues to pathophysiological processes for PD and provide strategies for developing neuroprotective therapies relevant to this disease.
RAB3B May Improve Dopamine Handling and Storage Capacity.
It has been shown that RAB3 acts as a modulator of neurotransmitter release, possibly acting on the recruitment or the tethering of synaptic vesicles at the active zone (20). Here, we have demonstrated aspects of RAB3B function as a regulator of synaptic vesicle dynamics in mammalian neurons both in vitro and in vivo.
Overexpression of RAB3B in DA neurons in vivo increases the number and size of synaptic vesicles, along with increasing vesicular dopamine content in the striatum. Such changes in synaptic vesicles appear to improve handling and storage capacity of dopamine at synaptic terminals. This notion is supported by the results from the l-DOPA challenge experiments. The infusion of l-DOPA is well known to increase cytosolic dopamine within midbrain DA neurons through conversion of l-DOPA to dopamine by AADC. We, and others, have observed a marked concomitant increase of the dopamine metabolite, DOPAC (21), putatively by rapid metabolism of increased cytosolic dopamine to DOPAC by monoamine oxidase (MAO). In this study we found this increase was abrogated by RAB3B overexpression without any change in AADC level. One possible explanation is that the increased cytosolic dopamine following l-DOPA injection was rapidly taken up in synaptic vesicles due to increased dopamine storage capacity (increased number and/or size of synaptic vesicles) caused by RAB3B overexpression (Fig. 6). That is, increased storage of cytosolic dopamine within synaptic vesicles may have prevented the metabolism of dopamine into DOPAC by MAO. Interestingly, extracellular dopamine after l-DOPA infusion was not increased at this dose of l-DOPA either in the presence or absence of RAB3B, suggesting tight regulation of neurotransmitter release. In fact, there is a precedent for this in the literature. A sudden increase in dopamine by l-DOPA injection as high as100 mg/kg was found in a previous study not to increase extracellular dopamine levels in the striatum, demonstrating that synaptic release of dopamine remain unchanged despite an increase in cytosolic dopamine by l-DOPA (21).
Improved synaptic vesicle handling by RAB3B has additional implications for DA neurons. In contrast to vesicular dopamine, free cytosolic dopamine appears to be an oxidative stressor for neurons (22). As shown in the l-DOPA challenge experiment, RAB3B overexpression appears to improve handling of a sudden increase of dopamine, plausibly by increased vesicular storage capability. DA neurons would be expected to derive benefit from such a reduction of cytosolic dopamine. In addition, after striatal infusion of 6-OHDA, this increased storage capability may have beneficially led to the sequestration of 6-OHDA in synaptic vesicles and subsequent protection.
Our data may appear surprising in view of previous investigations using RAB3 knockout mice. These studies have primarily illustrated the role of RAB3 on synaptic transmission, and data from these studies did not support its role in synaptic vesicle transport and/or biogenesis (7, 20). When hippocampal neurons from RAB3ABCD−/− quadruple knockout mice were compared with cultures from RAB3BCD−/− triple knockout mice, there was no change in synaptic morphology, vesicle size, biogenesis, delivery, docking, or priming but a reduced synaptic release probability (7). These data suggest that removing RAB3A, a major RAB3 isoform in the brain, did not alter the number or the size of synaptic vesicle in the hippocampal primary cultures. In these knockout mice, the possibility of compensatory mechanisms to restore vesicle biogenesis and transport during development cannot be disregarded. In addition, involvement of other RAB3 isoforms remained unanswered. Our findings clearly indicate a role for RAB3B in synaptic vesicle transport and/or biogenesis. It is therefore possible that overexpression of RAB3B in adult DA neurons in vivo using viral vectors may overcome potential developmental adaptations seen in transgenic mouse approaches and thus are able to delineate the specific role of RAB3B. It is worth noting that our data are corroborated by findings in the invertebrate and cell culture systems. First, elimination of RAB3 function caused depletion of synaptic vesicles at terminals of neuromuscular junction in C. elegans (23). Second, elimination of a RAB3 effector protein, RAB3 GDP/GTP exchange protein (GEP), caused a reduction in number and size of synaptic vesicles and in vesicular expression levels of RAB3A in cultured hippocampal neurons (11). In addition, a role for RAB3-GEP in synaptic vesicle transport to synaptic terminals was highlighted by experiments showing that RAB3-GEP mediates transport of vesicles by binding directly to two synaptic vesicle transport motor proteins, KIF1A and KIF1Bβ (10). These data provide a plausible mechanism for the increased number of synaptic vesicles we observed by RAB3B overexpression.
RAB3B-Mediated Changes in Levels of Proteins Involved in Neurotransmission.
Overexpression of RAB3B in vitro and in vivo altered levels of some synaptic proteins including SNAP-25, synaptophysin, and calmodulin. These data may give clues to functional partners of RAB3B. Among known RAB3 effector proteins including rabphilin 3, RIM1, calmodulin, and synapsin, only calmodulin expression levels were increased by RAB3B overexpression in our experiments. Calmodulin interacts with RAB3 to regulate synaptic vesicle recycling by controlling the SNARE mechanism (8). SNAP-25 is one of the SNARE proteins on the presynaptic plasma membrane that mediates membrane fusion during synaptic release (24). It has been shown that RAB3 interacts with SNAP-25 via rabphilin 3, an effector protein of RAB3 (25). Synaptophysin is also coimmunoprecipitated with RAB3 (10). It is possible that, as functional partners of RAB3B, expression levels of these proteins were increased by overexpression of RAB3B both in vivo and in vitro. In cell culture conditions, RAB3B overexpression led to a reduction of synaptotagmin and RAB3A levels. It is plausible that some functions of RAB3B are shared by those of RAB3A and synaptotagmin and that this redundancy led to down-regulation of their cellular levels after RAB3B overexpression. More dramatic changes in these protein levels were observed in in vitro cultures compared to striatal tissue preparation from rat. This might be because striatal tissue contains terminals other than DA terminals, which would “dilute out” any effect contributed by AAV-transduced DA terminals.
Dopamine is packaged into synaptic vesicles by VMAT2. The levels of VMAT2 were reduced along with TH expression levels in the rat striatum by RAB3B overexpression. This reduction in TH and VMAT2 may represent a compensatory response of DA terminals to the increased vesicular dopamine storage capacity mediated by RAB3B. In other words, we hypothesize this may be a physiological response to the increased vesicular dopamine content, to maintain homeostasis in synaptic dopamine release in the context of signal circuitry within the basal ganglia. Cells cultured in vitro, however, are not integrated in a circuit, and feedback mechanisms regulating dopamine release may be absent, potentially explaining why there was no VMAT2 or TH reduction in cell culture conditions in response to the increase neurotransmitter content. Although the absolute VMAT2 levels were reduced by RAB3B, the remaining VMAT2, coupled with increased size and number of vesicles, appears to be sufficient to cope with the sudden increase in dopamine accompanying l-DOPA infusion (Fig. 6 and preceding discussion).
Materials and Methods
Detailed materials and methods are described in the SI Text.
AAV2 Injection.
Female Sprague–Dawley rats weighing approximately 250 g received unilateral injection of AAV2 GFP (titer: 1.5 × 1012 genome copy/mL) or c-myc tagged RAB3B (titer: 2.8 × 1012 genome copy/mL) virus above the SN. Synapsin, a neuron specific promoter was used for AAV2. Two microliters of AAV2 was injected at two sites over the SN.
6-OHDA IntraStriatal Injection.
Three weeks following AAV2 injection, animals received three 2.5 μL striatal injections of 3.0 μg/μL 6-OHDA (total dose = 22.5 μg 6-OHDA). The lesion was allowed to progress for 3 weeks.
In Vivo Microdialysis.
Three weeks after nigral injection of AAV2 GFP or RAB3B, a dialysis probe was attached to the stereotaxic frame and implanted into the striatum. Samples were collected before and after i.p. injection of l-DOPA 50 mg/kg.
Supplementary Material
Supporting Information
Acknowledgments.
We thank Dr. Vikram Khurana for discussion of the manuscript and Casper Reske-Nielsen, Kari Ording, Alyssa Yow, Linda Hassinger, and Raymond Johnson for their excellent technical assistance. We also thank Dr. Pavel Oston for kindly providing AAV2 synapsin GFP construct. This study was conducted at McLean Hospital and was supported by funds to O.I. from the National Institutes of Health/National Institute of Neurological Diseases and Stroke (NIH/NINDS) P50 (NS39793) Parkinson's Disease Udall Research Centers of Excellence to McLean/Harvard Medical School, the Michael Stern Foundation for Parkinson's Disease Research, the Consolidated Anti-Aging Foundation, Harold and Ronna Cooper Family, and funds to C.Y.C. from the NIH/NINDS R03 (NS063083).
Footnotes
The authors declare no conflict of interest.
References
- 1.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 2.Grimm J, Mueller A, Hefti F, Rosenthal A. Molecular basis for catecholaminergic neuron diversity. Proc Natl Acad Sci USA. 2004;101:13891–13896. doi: 10.1073/pnas.0405340101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung CY, et al. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet. 2005;14:1709–1725. doi: 10.1093/hmg/ddi178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene JG, Dingledine R, Greenamyre JT. Gene expression profiling of rat midbrain dopamine neurons: Implications for selective vulnerability in parkinsonism. Neurobiol Dis. 2005;18:19–31. doi: 10.1016/j.nbd.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Isacson O. On neuronal health. Trends Neurosci. 1993;16:306–308. doi: 10.1016/0166-2236(93)90104-t. [DOI] [PubMed] [Google Scholar]
- 6.Chung CY, Koprich JB, Endo S, Isacson O. An endogenous serine/threonine protein phosphatase inhibitor, G-substrate, reduces vulnerability in models of Parkinson's disease. J Neurosci. 2007;27:8314–8323. doi: 10.1523/JNEUROSCI.1972-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schluter OM, Schmitz F, Jahn R, Rosenmund C, Sudhof TC. A complete genetic analysis of neuronal Rab3 function. J Neurosci. 2004;24:6629–6637. doi: 10.1523/JNEUROSCI.1610-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppola T, et al. Disruption of Rab3-calmodulin interaction, but not other effector interactions, prevents Rab3 inhibition of exocytosis. EMBO J. 1999;18:5885–5891. doi: 10.1093/emboj/18.21.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuda M. Distinct Rab binding specificity of Rim1, Rim2, rabphilin, and Noc2. Identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. J Biol Chem. 2003;278:15373–15380. doi: 10.1074/jbc.M212341200. [DOI] [PubMed] [Google Scholar]
- 10.Niwa S, Tanaka Y, Hirokawa N. KIF1Bbeta- and KIF1A-mediated axonal transport of presynaptic regulator Rab3 occurs in a GTP-dependent manner through DENN/MADD. Nat Cell Biol. 2008;10:1269–1279. doi: 10.1038/ncb1785. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi K, et al. A GDP/GTP exchange protein for the Rab3 small G protein family up-regulates a postdocking step of synaptic exocytosis in central synapses. Proc Natl Acad Sci USA. 2002;99:14536–14541. doi: 10.1073/pnas.212511399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci. 2009;29:3365–3373. doi: 10.1523/JNEUROSCI.5427-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper AA, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gitler AD, et al. The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci USA. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rupnik M, et al. Distinct role of Rab3A and Rab3B in secretory activity of rat melanotrophs. Am J Physiol. 2007;292:C98–105. doi: 10.1152/ajpcell.00005.2006. [DOI] [PubMed] [Google Scholar]
- 17.Weber E, Jilling T, Kirk KL. Distinct functional properties of Rab3A and Rab3B in PC12 neuroendocrine cells. J Biol Chem. 1996;271:6963–6971. doi: 10.1074/jbc.271.12.6963. [DOI] [PubMed] [Google Scholar]
- 18.McNaught KS, Belizaire R, Isacson O, Jenner P, Olanow CW. Altered proteasomal function in sporadic Parkinson's disease. Exp Neurol. 2003;179:38–46. doi: 10.1006/exnr.2002.8050. [DOI] [PubMed] [Google Scholar]
- 19.Zhou C, Huang Y, Przedborski S. Oxidative stress in Parkinson's disease: A mechanism of pathogenic and therapeutic significance. Ann N Y Acad Sci. 2008;1147:93–104. doi: 10.1196/annals.1427.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geppert M, et al. The role of Rab3A in neurotransmitter release. Nature. 1994;369:493–497. doi: 10.1038/369493a0. [DOI] [PubMed] [Google Scholar]
- 21.Miller DW, Abercrombie ED. Role of high-affinity dopamine uptake and impulse activity in the appearance of extracellular dopamine in striatum after administration of exogenous l-DOPA: Studies in intact and 6-hydroxydopamine-treated rats. J Neurochem. 1999;72:1516–1522. doi: 10.1046/j.1471-4159.1999.721516.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, et al. Unregulated cytosolic dopamine causes neurodegeneration associated with oxidative stress in mice. J Neurosci. 2008;28:425–433. doi: 10.1523/JNEUROSCI.3602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nonet ML, et al. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J Neurosci. 1997;17:8061–8073. doi: 10.1523/JNEUROSCI.17-21-08061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudhof TC. The synaptic vesicle cycle. Ann Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 25.Deak F, et al. Rabphilin regulates SNARE-dependent re-priming of synaptic vesicles for fusion. EMBO J. 2006;25:2856–2866. doi: 10.1038/sj.emboj.7601165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information