Glucosamine Protects Neonatal Cardiomyocytes from Ischemia-Reperfusion Injury via increased protein O-GlcNAc and increased mitochondrial Bcl-2 (original) (raw)
. Author manuscript; available in PMC: 2010 Jan 1.
Published in final edited form as: Am J Physiol Cell Physiol. 2008 Mar 26;294(6):C1509–C1520. doi: 10.1152/ajpcell.00456.2007
Abstract
We have previously reported that glucosamine protected neonatal rat ventricular myocytes (NRVMs) against ischemia/reperfusion (I/R) injury, and this was associated to an increase in protein O linked-N-acetylglucosamine (_O_-GlcNAc) levels. However, the protective effect of glucosamine could be mediated via pathways other that _O_-GlcNAc formation; thus, the initial goal of this study was to determine whether increasing _O_-GlcNAc transferase (OGT) expression, which catalyzes the formation of O-GlcNAc, had similar protective effect as glucosamine. To better understand the potential mechanism underlying O-GlcNAc-mediated cytoprotection we examined whether increased _O_-GlcNAc levels altered the expression and translocation of members of the Bcl-2 protein family. Both glucosamine (5mM) and OGT overexpression increased basal and I/R induced _O_-GlcNAc levels, significantly decreased cellular injury, and attenuated loss of cytochrome C. Both interventions also attenuated the loss of mitochondrial membrane potential induced by H2O2 and were also associated with an increase in mitochondrial Bcl-2 levels, but had no effect on Bad on Bax levels. Compared to glucosamine and OGT overexpression, NButGT (100 µM), an inhibitor of _O_-GlcNAcase, was less protective against I/R and H2O2 and did not affect Bcl-2 expression, despite a 5 to 10 fold greater increase in overall _O_-GlcNAc levels. Decreased OGT expression resulted in lower basal _O_-GlcNAc levels, prevented the I/R induced increase in O-GlcNAc and mitochondrial Bcl-2 and increased cellular injury. These results demonstrate that the protective effects of glucosamine are mediated via increased formation of _O_-GlcNAc and suggest that this is due in part to enhanced mitochondrial Bcl-2 translocation.
INTRODUCTION
In mammalian cells a variety of stress stimuli have been shown to increase the level of _O_-linked-N-acetylglucosamine (_O_-GlcNAc) on nuclear and cytoplasmic proteins (54). Inhibition of this response increased sensitivity to stress whereas augmentation of the _O_-GlcNAc levels increased tolerance to the same stress stimuli and improved cell survival (54). We have previously reported that ischemic stress in isolated cardiomyocytes and the intact heart also increases _O_-GlcNAc levels; furthermore, treatment with glucosamine augments this increase in _O_-GlcNAc level, improving the tolerance to ischemic injury (9, 15, 32, 33). These data are consistent with the notion that in the heart an increase in _O_-GlcNAc protein modification is an endogenous stress response and that the protection seen with glucosamine is mediated via this same pathway.
However, in addition to increasing _O_-GlcNAc levels, glucosamine also increases UDP-GlcNAc levels, glucosamine-6-phosphate levels and could potentially be metabolized to fructose-6-phosphate thereby increasing glycolytic flux. Thus, in addition to increasing _O_-GlcNAc levels, the protection associated with glucosamine treatment in cardiomyocytes and the intact heart could be mediated via a number of other pathways. Support for the hypothesis that the protective effect of glucosamine is mediated by _O_-GlcNAc levels, was provided by studies showing that increasing _O_-GlcNAc levels with PUGNAc an inhibitor of _O_-GlcNAcase had similar effects to glucosamine (9, 32). However, while the inhibition of _O_-GlcNAcase with PUGNAc is frequently used to increase cellular _O_-GlcNAc levels, it also inhibits other β-hexosamindases (23, 34, 42) and thus will alter processing of glycoconjugates in addition to _O_-GlcNAc. We have also found that the pattern of _O_-GlcNAc modified proteins is different in glucosamine and PUGNAc treated cardiomyocytes (9). This suggests that these two different methods for increasing cellular _O_-GlcNAc levels may not have equal effects on cell function, which may be a consequence of their actions on other pathways.
Importantly, the mechanisms underlying the protection associated with increased protein _O_-GlcNAc levels have yet to be determined. Zachara et al., (54) reported that increased survival seen with elevated _O_-GlcNAc levels was associated with increased expression of HSP70; in contrast Sohn et al., (47) reported improved survival associated with increased _O_-GlcNAc levels without any change in HSP70 levels, which suggests that other mechanism(s) may also contribute to the protection associated with increased _O_-GlcNAc levels. We found that hyperglycemia-mediated protection of cardiomyocytes against ischemic injury also appeared to be mediated, at least in part, by increased _O_-GlcNAc levels(9). It has also been reported that hyperglycemia-induced protection against hypoxic injury induced apoptosis and necrosis was associated with upregulation of the antiapoptotic factor Bcl-2 (45).
Apoptosis, a genetically programmed form of cell death, contributes to myocyte cell loss in a variety of cardiac pathologies, including cardiac failure and those related to ischemia/reperfusion injury. The apoptotic program is complex, involving both pro- and anti-apoptotic proteins, and apoptosis occurs when the equilibrium between these opposing factors is perturbed (44). The pro- and anti-apoptotic members of the Bcl-2 family are intrinsic to the apoptotic pathway; Bcl-2 and Bcl-xL protect cells from apoptosis, whereas Bax and Bad promote the response. It has been reported that increased Bcl-2 expression is significantly limits cell death after acute myocardial infarction (55) or during cardiac post-transplant ischemia/reperfusion injury (20). Several lines of evidence have also shown that members of the Bcl-2 protein family are associated with the loss of apoptogentic factors, including release of cytochrome c from the intermembrane space of the mitochondria into cytosol (1, 12, 28, 30). Many, if not all, apoptotic responses involve mitochondrial dysfunction and a loss of the mitochondrial membrane potential (19). Thus, anti-apoptotic Bcl-2 may function in the mitochondrial membrane to prevent loss of cytochrome c and thus inhibit apoptosis.
Therefore, the goals of this study were to determine, whether increased OGT expression had similar effects as glucosamine treatment on the response to ischemic injury and acute oxidative stress. Studies were also performed with NButGT, an inhibitor of _O_-GlcNAcase, which has ~1500-fold greater specificity for _O_-GlcNAcase over β-hexosaminidase than PUGNAc (34). To better understand the potential mechanism underlying O-GlcNAc-mediated cytoprotection we examined whether increased _O_-GlcNAc levels altered the expression and translocation of members of the Bcl-2 protein family. Finally, we also examined the consequences of decreased OGT expression on the response to ischemic injury.
Materials and Methods
Materials
Unless otherwise noted, except for glucosamine (Fluka) all chemicals were purchased from Sigma Chemical (St. Louis, MO). Culture medium products were purchased from GIBCO Invitrogen (Grand Island, NY). Adenovirus containing _O_-GlcNAc transferase was a kind gift from Dr. W.H. Dillmann (Department of Medicine, University of California, San Diego). 1,2-dideoxy-2'-methyl-alpha-d-glucopyranoso[2,1-d]-Delta2'-thiazoline (NButGT), an inhibitor of _O_-GlcNAcase was a kind gift from Dr. David Vocadlo (Simon Fraser University, Burnaby, BC, Canada) (34). NButGT has ~1500-fold greater specificity for _O_-GlcNAcase over β-hexosaminidase than PUGNAc (34).
Neonatal rat ventricular myocyte (NRVM) primary cultures
Animal experiments were approved by the University of Alabama Institutional Animal Care and Use Committee and conformed to the Guide for the Care and Usage of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, 1996). Primary cultures of neonatal rat ventricular myocytes (NRVMs) were obtained from 2–3 days old neonatal Sprague Dawley rats and cultured as described previously (9, 24). NRVMs were grown in collagen-coated plates in the culture growth medium containing 15% fetal bovine serum (FBS) on the first day. On the next day, medium was replaced and cells were grown in the culture growth medium without FBS. Within 1–2 days of isolation, a confluent monolayer of spontaneously beating NRVMs had formed and cells were used as described below.
Ischemia and reperfusion
Ischemia and reperfusion was induced as described previously (4, 5, 9). Briefly, following 2 days in culture, NRVMs were exposed to ischemia by adding a fresh Esumi modified ischemic medium (137 mM NaCl, 12 mM KCl, 0.49 mM MgCl2, 0.9 mM CaCl2.2H2O, 4 mM HEPES and 20 mM sodium lactate, pH 6.2) and then incubated in the chamber atmosphere of 95% argon and 5% CO2 for 4 hours. Following 4 hours of ischemia, cells were returned to the culture growth medium (serum free 4:1 (v/v) Dulbecco’s modified Eagle’s medium (DMEM) / Medium 199 with Hanks salts (M199), supplemented with 2% Nutridoma and 1% penicillin/streptomycin) and then incubated in an incubator atmosphere of 5% CO2 for 2 hours. In control normoxia experiments, cells were incubated with fresh culture growth medium in an incubator atmosphere of 5% CO2 for 6 hours.
Cell injury in response to ischemia/reperfusion was determined as previously described (9). Necrosis was assessed by measuring the release of lactate dehydrogenase (LDH) in culture medium and LDH in remaining attached cells using an LDH assay kit (Sigma). NRVMs (1×106 cells) were seed into a multi 12 well-plate (Falcon). The percent LDH release was calculated by the ratio of the released LDH into the media by the total LDH (release plus cellular content) at the end of treatment. Apoptosis was determined by using In Situ Cell Death Detection Kit (Roche). NRVMs (0.2–0.3×106 cells) were seed into a 4-chambered cover glass (Lab-Tek). At the end of treatments, permeabilized cells were exposed to the TUNEL reaction mixture for 1.5 hour and were counter stained with 0.1 mg/ml of Hoechst 33258 (Invitrogen). In each treatment, a total of at least 200 cells were counted through a 40× objective with excitation wavelength at 528 nm.
Assessment of Protein O-GlcNAc and cytochrome C levels by Immunofluorescence
At the end of treatment, NRVMs were fixed with 3.7% Formaldehyde in PBS for 30 min at room temperature (RT). After washing cells with 3 times PBS, cells were permeabilized with 0.1% Triton X-100 in PBS for 10 min on ice. Permeabilized cells were exposed to the O-GlcNAc antibody, CTD 110.6 (Covance) at 1:50 and cytochrome C (556432, BD Pharmingen) in 3%FBS/PBS for 1 hour at room temperature. After washing cells with 3 times of PBS, cells were incubated with secondary antibodies; Alexa-Flour(R) 594 goat anti-mouse IgM (Invitrogen) at 1:200 for CTD 110.6 and Alexa-Flour(R) 488 goat anti-mouse IgG (Invitrogen) at 1:200 for cytochrome C in 3%FBS/PBS for 1 hour at RT. After washing cells with PBS, cells were stained with 0.1 mg/ml of Hoechst 33258 (Invitrogen). Cells were visualized through a 20× objective with excitation wavelength at 528 nm for cytochrome C staining, 623 nm for _O_-GlcNAc staining, and 456 nm for Hoechst nuclear staining using an inverted fluorescent microscope (Olympus).
Immunoblot analysis
At the end of treatment, cells were lyzed with 1xRIPA (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% (v/v) NP-40, 0.5% (w/v) sodium deoxycholate, 1 mM EDTA and 0.1% SDS) containing 2% protease inhibitor cocktail (Sigma) on ice for 30 min. Lysed proteins were harvested and assayed for protein concentration using the Bio-Rad protein assay kit. Proteins (10 µg) were separated on 7.5% or 12% SDS-polyacrylamide gel electrophoresis and transferred onto a PVDF membrane (Milipore). After transferring, the blotted were soaked in 100% methanol and dried completely under the hood. Dried blots were probed with the following antibodies; anti-_O_-GlcNAc antibody CTD110.6 (Covance) at 1:2000, RL2 (Affinity Bioreagents) at 1:2000, OGT (Sigma) at 1:1000, β-actin (sigma) at 1:20000, Bcl-2 (Santa Cruz) at 1:200, Bax (Santa Cruz) at 1:200, Bad (Cell signaling) at 1:1000, COX4 (Abcam) at 1:20000, and GAPDH (Abcam) at 1:5000. Blots and antibodies were incubated in 1× PBS/casein blocker (Pierce) containing 0.01% Tween 20 for 2 hours at room temperature. After washing three times with PBS, the membrane was then incubated with an appropriate secondary antibody; goat anti-mouse IgM (Calbiochem) for anti-O-GlcNAc CTD110.6, goat anti-mouse IgG, or goat anti-rabbit IgG (Santa Cruz) at the same blocking buffer for 1 hour at room temperature. After further washing in PBS, the immunoblots were developed with enhanced chemiluminescence (Super Signal West Pico or Femto Maximum Sensitivity; Pierce) and visualization was performed using Bioimaging system of UVP (UVP, Inc., CA). Densitometric analysis was performed on the entire lane of each sample using LabWorks analysis software (UVP, Inc., CA) and the mean intensity normalized to the control group.
Mitochondrial fractionation
NRVMs (10×106 cells) were seeded into a 10 cm tissue culture dishes (Falcon) and mitochondria were prepared using a Mitochondrial/Cytosol Fractionation Kit (K256, BioVision) with slight modifications. Briefly, NRVMs were washed with PBS and resuspended in 200 µl of 1× Cytosol Extraction Buffer on ice for 10 minutes. Cells were homogenized and then centrifuged at 700× g for 10 minutes at 4°C and the supernatant centrifuged at 10,000× g for 30 minutes at 4°C. At the end of this second centrifugation, the supernatant (post-mitochondria), consisting of the cytosolic/microsomal fraction was collected for subsequent immunoblot analysis and the pellet (mitochondria) was resuspended in 50 µl of Mitochondrial Extraction Buffer. Lysed proteins were assayed for protein concentration using the Bio-Rad protein assay kit. Proteins (3–5 µg) were separated on 12% SDS-polyacrylamide gel electrophoresis and transferred onto a PVDF membrane (Milipore). Immunoblot analysis was performed as described above.
Electroporation of siRNA oligonucleotides
siRNA oligonucleotides, _O_-GlcNAc transferase (OGT) and negative control (Silencer® negative control 1) were purchased from Ambion Inc. (Austin, TX). The sense and antisense sequences of si-OGT were 5’-CCCUUGACCCAAUUUUCUtt-3’ and 5’-AGAAAUUUGGGUCAAGGGtg-3’, respectively. Transfection of the siRNA oligos into neonatal cardiomyocytes was carried out as described according to the manufacturer's instructions (Amaxa Inc.) with slight modification. Briefly, one day after cell isolation, attached cardiomyocytes (107 cells in a 10cm culture dish) were detached by trypsin-EDTA solution (Sigma) and resuspended in the transfection reagent Nucleofector kit (Amaxa Inc.). A total of 2 µg of siRNAs per 2 ×106 cells were transfected using the Nucleofector II Device (Amaxa Inc.) according to the manufacturer's instructions. After transfection, cells were grown in the culture medium containing 15% FBS and on the next day, medium was replaced and cells were grown in the culture growth medium without FBS. Approximately 2 days following transfection OGT levels were confirmed by western blotting as described above and cells were subject to ischemia/reperfusion.
Assessment of mitochondrial membrane potential
Mitochondrial function was assessed by using JC-1 reagent, Mitochondrial Membrane Potential detection Kit as described according to the manufacturer's instructions (Stratagene). After isolation NRVMs were seeded into a 4 chambered coverglass (Lab-Tek) and pre-treated with a unique fluorescent cationic dye, 5,5’,6,6’-terachloro-1,1,3,3’-tetraethyl-0-benzamidazolocarbocyanin iodide, commonly known as JC-1 for 15 minutes followed by washing with assay buffer to remove remaining reagent. 500 µl of the culture growth medium was added to the cells, which were then visualized through a 20× objective with excitation wavelength at 623 nm to detect Texas Red dye and at 528 nm to detect rhodamine using an inverted fluorescent microscope (Olympus). In living cells, JC-1 exists as a monomer in the cytosol, which exhibits green fluorescence, and also accumulates as aggregates in the mitochondria, where it exhibits red fluorescence. In apoptotic and dead cells, JC-1 exists in the monomeric form only, staining the cytosol green. Mitochondrial membrane potential (MMP) was monitored before and addition of hydrogen peroxide (1mM final concentration). A reduction in the ratio of red to green fluorescence indicated a fall in MMP. The ratio of red and green intensity of cells incorporating JC-1 dye was measured by using IPLab version3.6 analysis software (BD Biosciences).
Statistics
All data are presented as means±SEM. Unpaired T-tests, one-way and repeated measure ANOVA were used where appropriate followed by a Bonferroni’s multiple comparison test using Prism 4.0c (GraphPad Software Inc., San Diego CA). Statistically significant differences between groups were defined as P <0.05.
RESULTS
Glucosamine reduces cellular injury during ischemia/reperfusion and increases _O_-GlcNAc levels
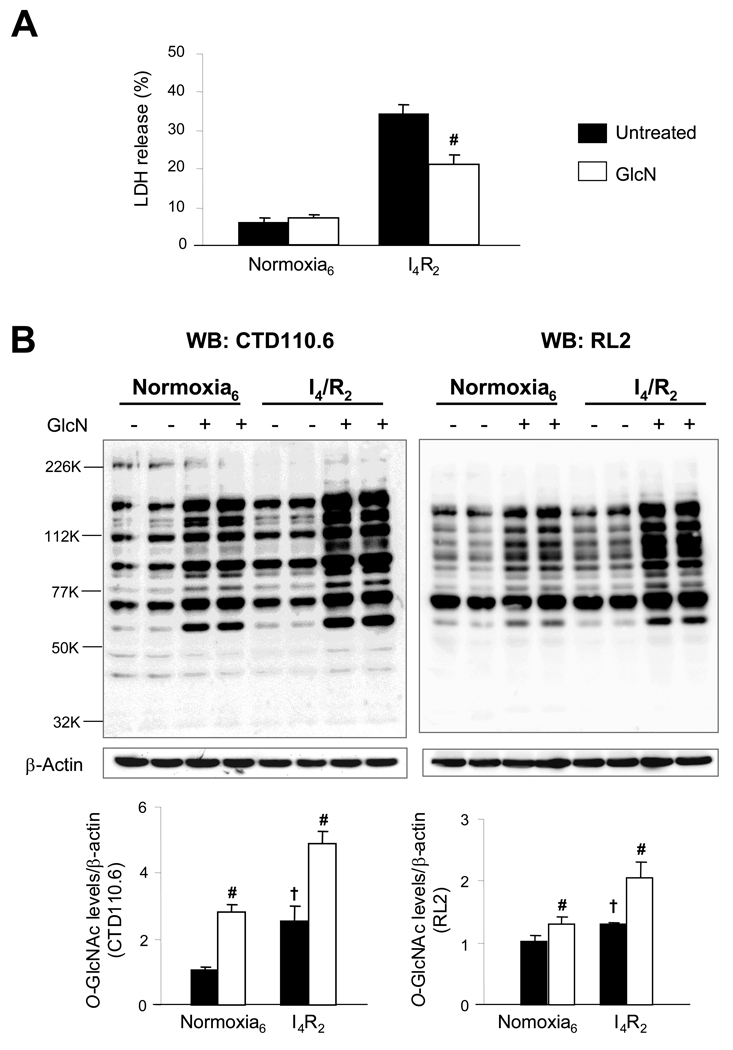
We have previously reported that in NRVMs glucosamine decreased both necrosis and apoptosis following ischemia/reperfusion and that the increase in _O_-GlcNAc levels was maximal at 2 hours of reperfusion following 4 hours of ischemia (9). Therefore, in this study we focused on the 2 hr reperfusion time point to investigate the effects of ischemia/reperfusion on protein and cellular function in this study. In Fig. 1A, we demonstrate that, consistent with our earlier study, glucosamine decreased cell injury following ischemia/reperfusion. Using two _O_-GlcNAc antibodies, which recognize different _O_-GlcNAc motifs (CTD110.6 and RL2) we confirm that ischemia/reperfusion alone significantly increases overall _O_-GlcNAc levels in the control, untreated group (Fig. 1B) (CTD: 2.55±0.47 Vs 1.07±0.06, P<0.05) and (RL2: 1.29±0.02 Vs 0.97±0.10, P<0.05). Consistent with our earlier study, glucosamine treatment markedly augmented the response to ischemia/reperfusion significantly compared to the untreated groups (CTD: 4.89±0.37 Vs 2.55±0.47 and RL2: 2.03±0.43 Vs 1.29±0.02). Equal protein loading was confirmed by densitometric analysis of β-actin levels.
Fig. 1.
Increasing global _O_-GlcNAc protein levels by glucosamine (GlcN) reduced cellular injury during ischemia and reperfusion injury. A) Cell injury assessed by determining lactate dehydrogenase (LDH) release as a percentage of total LDH; B) Representative CTD110.6 and RL2 immunoblot of _O_-linked N-acetylglucosamine (_O_-GlcNAc) proteins and mean intensity of all _O_-GlcNAc proteins determined by densitometric analysis. Equal protein loading was normalized by densitometric analysis of β-actin levels. _O_-GlcNAc levels are normalized to normoxic control untreated cells. Control untreated cells and glucosamine (5 mM)-treated cells were incubated under normoxic condition for 6 h or subjected to 4 h of ischemia followed by 2 h of reperfusion (I4/R2). Data are presented as means±SEM of more than 3 experiments. # = P< 0.05 Vs untreated, † P< 0.05 Vs normoxia.
The increase in _O_-GlcNAc in response to ischemia and glucosamine appears to be less robust when assessed with RL2 compared to CTD110. However, this is primarily due to the fact that, just below 77kD there is a very intense band in the RL2 immunoblots that is relatively constant across the different groups. Above 77kD the changes in intensity in the CTD110 and RL2 immunoblots are similar (data not shown).
Glucosamine mediates translocation of mitochondrial Bcl-2
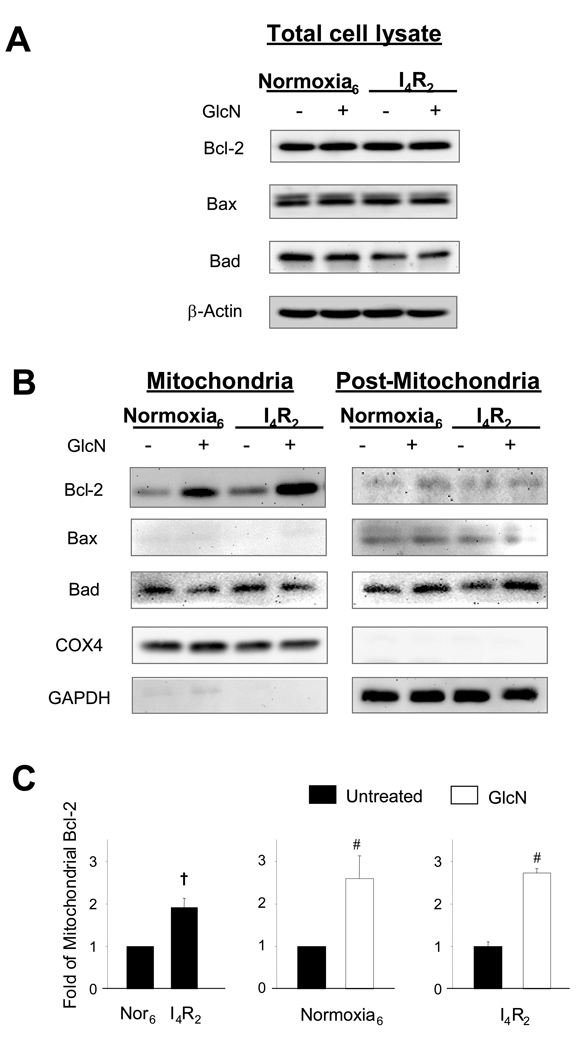
In Fig 2A it can be seen that glucosamine had little or no effect on whole cell expression of Bcl-2, Bad, or Bax levels either under normoxic conditions or following ischemia/reperfusion. Since mitochondria play a critical role in regulating cell death, we examined expression levels of the same proteins in mitochondrial and post-mitochondrial (cytosolic/microsomal) fractions. There was no effect of glucosamine or ischemia/reperfusion on levels of Bcl-2, Bad or Bax in the post-mitochondrial fraction; however, in the mitochondrial fraction, ischemia significantly increased Bcl-2 levels (Fig. 2C, 1.0±0.0 Vs 1.91±0.22, P<0.05). Glucosamine treatment significantly increased mitochondrial Bcl-2 levels under normoxic conditions (1.0±0.0 Vs 2.59±0.54) and augmented the response to ischemia/reperfusion (1.0±0.12 Vs 2.71±0.11) (Fig 2B, C).
Fig. 2.
Glucosamine had no effect on Bcl-2 family proteins, Bcl-2, Bax, and Bad, but enhanced mitochondrial Bcl-2 translocation. A) Representative Bcl-2, Bax, and Bad immunoblots of the whole cell lysate of NRVMs. Equal protein loading was confirmed by β-actin levels; B) Representative Bcl-2, Bax, and Bad of the mitochondrial and post-mitochondrial (cytosolic/microsomal) fraction of NRVMs. Purity of each fraction was assessed by GAPDH and COX4, respectively; C) Mean intensity of mitochondrial Bcl-2 determined by densitometric analysis. Levels are normalized to normoxic control untreated cells. Control untreated cells and glucosamine (5 mM)-treated cells were incubated under normoxic condition for 6 h or subjected to 4 h of ischemia followed by 2 h of reperfusion (I4/R2). Data are presented as means±SEM of more than 3 experiments. # P< 0.05 Vs untreated, † P< 0.05 Vs normoxia.
Following ischemia/reperfusion mitochondrial Bad levels appeared to be reduced in the glucosamine treated group (2.5±1.4 Vs 1.5±0.6); however, this difference was not significant, due to the high variance in the untreated group. In contrast to Bcl-2 and Bad, Bax was virtually undetectable in the mitochondrial fraction.
The effect of increased OGT expression and _O_-GlcNAcase inhibition on response of NRVMs to ischemia/reperfusion
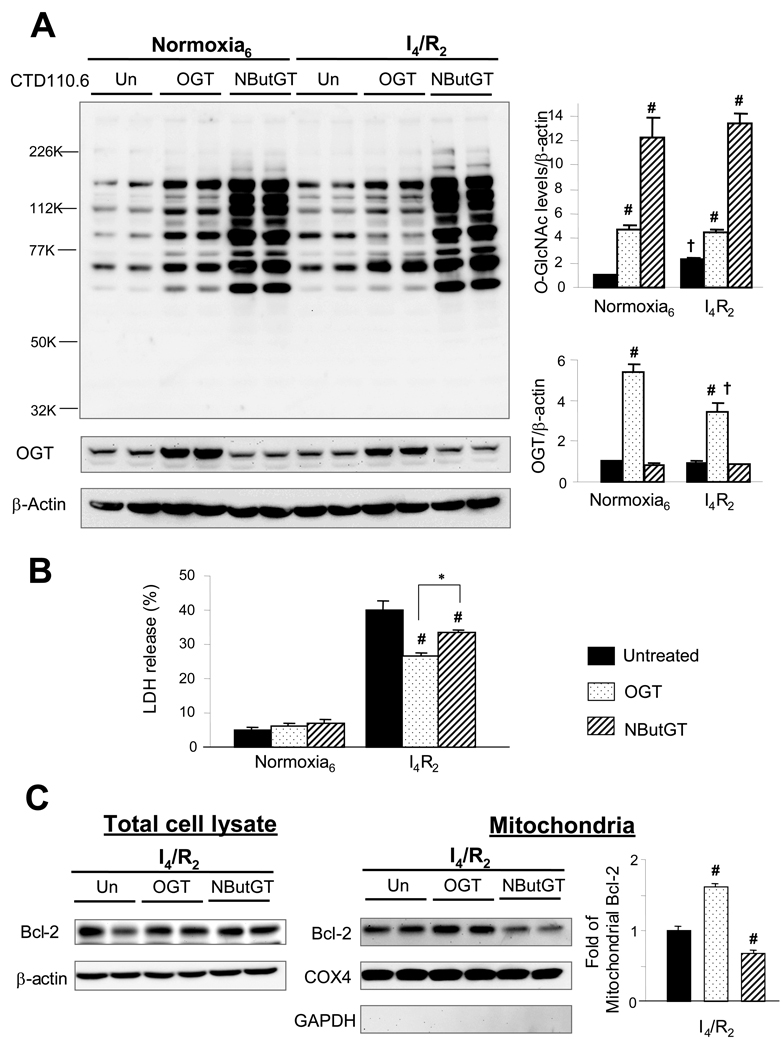
Since glucosamine may affect cell function independent of increased _O_-GlcNAc formation, we asked whether increasing OGT expression levels would also afford protection against ischemia/reperfusion injury and if so whether this also associated with increased mitochondrial Bcl-2. In Fig 3A it can be seen that transfection of NRVMs with the OGT adenovirus increased OGT expression levels 4–5 fold and this was associated with increased _O_-GlcNAc levels under normoxia and following ischemia/reperfusion. Unlike glucosamine treatment ischemia/reperfusion did not increase _O_-GlcNAc levels further in the OGT transfected group. Interestingly, however, the increase in _O_-GlcNAc achieved by OGT transfection was ~4–5 fold compared to non-transfected cells in both conditions, which was similar to the increase seen in the glucosamine treated group following ischemia/reperfusion (Fig 1A, Fig 3B). Increased OGT expression significantly attenuated cell injury following ischemia/reperfusion as indicated by reduced LDH release (Fig 3B). Consistent with glucosamine treatment, increased OGT expression was also associated with increased mitochondrial Bcl-2 expression (Fig 3C).
Fig. 3.
Increasing _O_-GlcNAc transferase (OGT) and blocking _O_-GlcNAcase by NButGT had different effects on _O_-GlcNAc protein modification, cellular survival and Bcl-2 translocation. A) Representative immunoblotting CTD110.6 and OGT and mean intensity of all _O_-GlcNAc proteins and OGT of the total whole cell lysate determined by densitometric analysis. Equal protein loading was normalized by densitometric analysis of β-actin levels. Proteins are normalized to normoxic control untreated cells; B) Cell injury assessed by determining lactate dehydrogenase (LDH) release as a percentage of total LDH; C) Representative immunoblotting of Bcl-2 expression levels in total cell lysate and mitochondrial fractions and mean densitometric analysis of mitochondrial Bcl-2. Purity of mitochondrial fraction was assessed by COX4 and GAPDH. Control untreated cells, _O_-GlcNAc transferase (OGT) adenovirus infected cells, NButGT (100 µM)-treated cells were incubated under normoxic condition for 6 h or subjected to 4 h of ischemia followed by 2 h of reperfusion (I4/R2). Data are presented as means±SEM of more than 3 experiments. # =P< 0.05 Vs untreated, † P< 0.05 Vs normoxia, * P< 0.05 Vs OGT overexpression.
Inhibition of _O_-GlcNAcase, which is responsible for removing _O_-GlcNAc from proteins, is another means by which cellular _O_-GlcNAc levels can be increased. We have previously shown that the _O_-GlcNAcase inhibitor PUGNAc increased _O_-GlcNAc levels to a much greater extent than glucosamine; however, it was less protective (9). While PUGNAc is typically used as an _O_-GlcNAcase inhibitor it also inhibits lysosomal β-hexosaminidases, which could also affect the response of cardiomyocytes to ischemia/reperfusion (34). Therefore, we examined the effect of NButGT a new _O_-GlcNAcase inhibitor, which shows ~1500-fold greater specificity for _O_-GlcNAcase over β-hexosaminidase than PUGNAc (34). NButGT treatment increased _O_-GlcNAc levels more than 10-fold compared to untreated cells (Fig 3A); however, while it decreased LDH release compared to untreated cells, this was effect was significantly attenuated compared to increased OGT expression (Fig 3B) and glucosamine treatment (Fig 1A). Interestingly, NButGT treatment did not increase mitochondrial Bcl-2 expression and blocked the ischemia/reperfusion induced increase in mitochondrial Bcl-2 seen in untreated cells (Fig 3C).
Attenuation of cytochrome C release by glucosamine and increased OGT expression
It has been reported that the balance of anti- and pro- apoptotic Bcl-2 family proteins that reside in the outer mitochondrial membrane play an important role in regulating mitochondria-mediated apoptosis (44). One characteristic of mitochondria-mediated apoptosis is the release of cytochrome C from the mitochondria into the cytosol. However, using cell fractionation and immunoblotting techniques, we observed very low cytosolic cytochrome C levels following ischemia/reperfusion. Since there is significant loss of cytosolic proteins in this model of ischemia/reperfusion, as indicated by LDH release (Fig 1A), the low levels of cytosolic cytochrome C could be due to loss of the protein from the cell. Indeed, we found appreciable levels of cytochrome C in the media following ischemia/reperfusion, which was attenuated by 50% in the glucosamine treated group (data not shown).
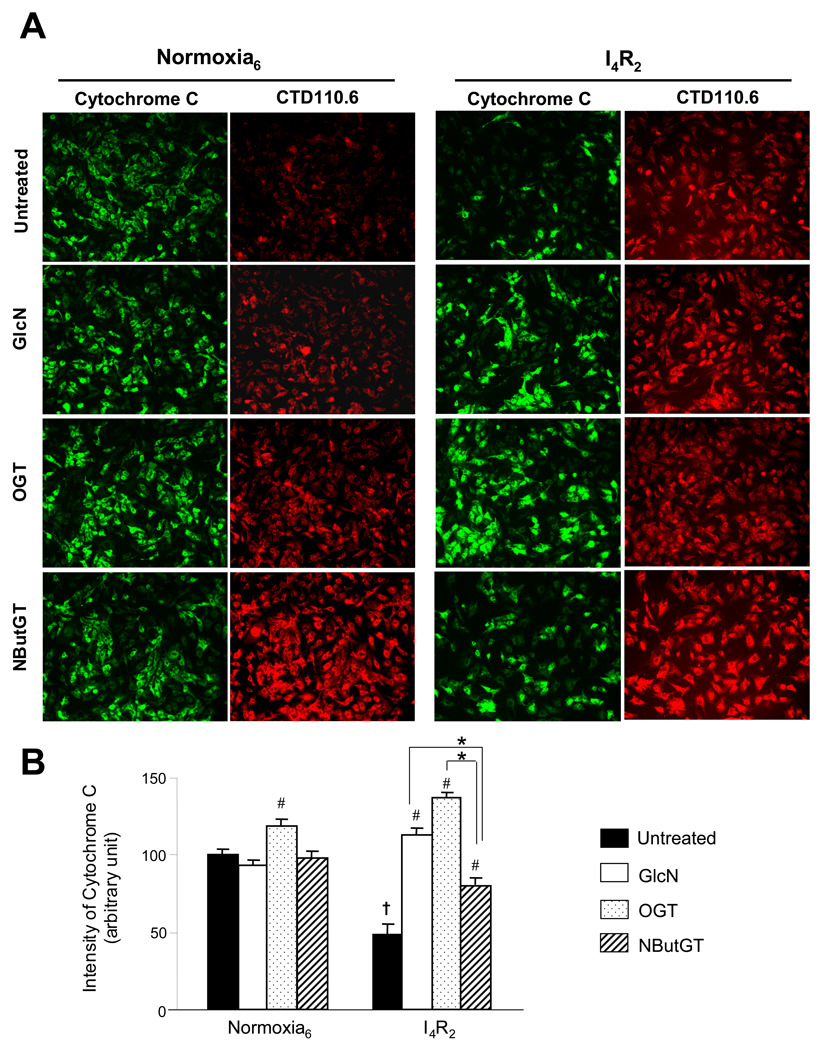
Therefore we used immunofluorescence to look at the relationship between _O_-GlcNAc and cytochrome C levels (Fig 4A). Consistent with immunoblot analysis of _O_-GlcNAc proteins (Fig. 1B and Fig. 3B), ischemia/reperfusion increased global _O_-GlcNAc levels in the untreated cells. Glucosamine, OGT overexpression and NButGT all increased _O_-GlcNAc levels under normoxic conditions as well as following ischemia/reperfusion and the increase in _O_-GlcNAc levels was clearly much greater in the NButGT treated cells compared to the other groups. Following ischemia/reperfusion there was a marked loss of cytochrome C staining in untreated cells, which was significantly attenuated by glucosamine, increased OGT expression and NButGT (Fig 4B). However, consistent with LDH release (Fig 3B) despite the marked increase in _O_-GlcNAc levels, the effect of NButGT in attenuating cytochrome C release was significantly less than either glucosamine or increased OGT expression.
Fig. 4.
Increasing global _O_-GlcNAc proteins protected the loss of cytochrome C. A) Representative immunofluorescence of cytochrome C and_O_-GlcNAc proteins (CTD 110.6) under normoxia and following ischemia/reperfusion; B) Mean intensity of cytochrome C determined by IPLab analysis software was normalized with the control untreated cells. Data are presented as means±SEM of 150–200 cells. Control untreated cells, glucosamine (5 mM)-treated cells, _O_-GlcNAc transferase (OGT) adenovirus infected cells, NButGT (100 µM)-treated cells were incubated under normoxic condition for 6 h or subjected to 4 h of ischemia followed by 2 h of reperfusion (I4/R2). # = P< 0.05 Vs untreated, * P< 0.05 Vs NButGT.
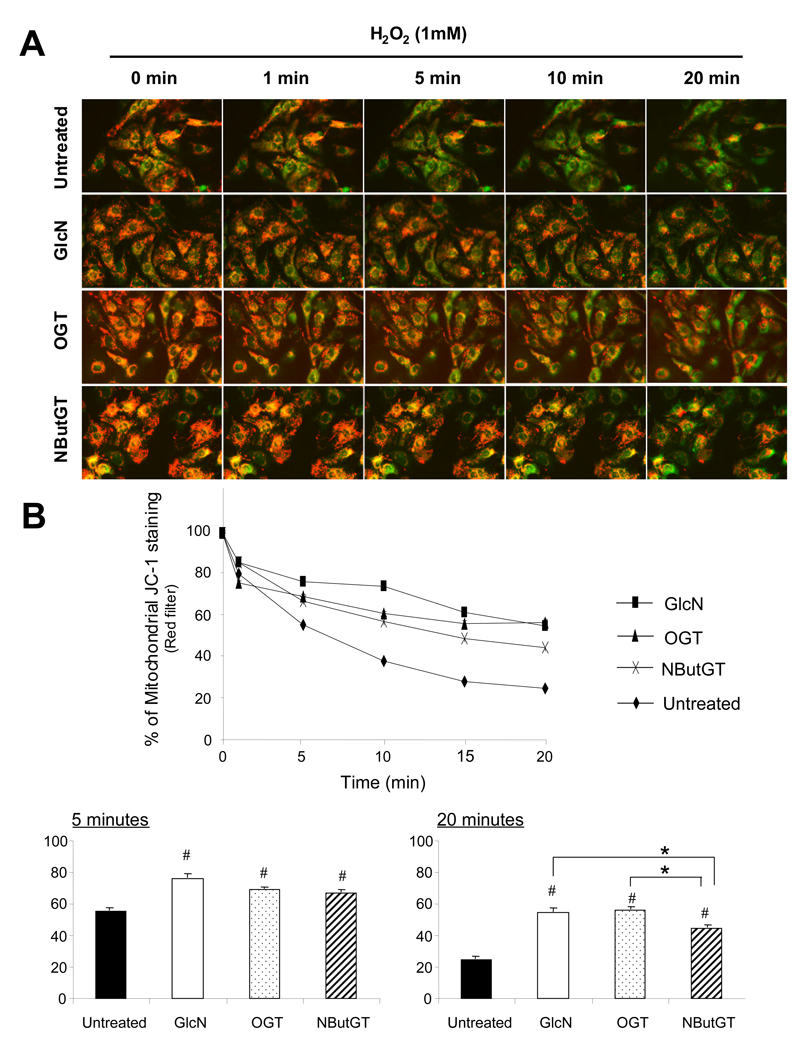
Increasing O-GlcNAc levels attenuates hydrogen peroxide-induced loss of mitochondrial membrane potential
Release of cytochrome C from the mitochondria is usually associated with opening of the mitochondrial membrane transition pore (mMTP), which is a critical step in mitochondria-mediated apoptosis (29). Opening of the mMTP is characterized by loss of mitochondrial membrane potential (29); therefore, we asked whether increasing _O_-GlcNAc levels attenuated the loss of mitochondrial membrane potential in response to transient exposure to hydrogen peroxide (H2O2). Cardiomyocytes were pre-treated with the fluorescent cationic dye, JC-1, which exhibits a red fluorescence in healthy cells with intact mitochondrial membrane potential. We used real-time immunofluorescence technique to monitor the mitochondrial membrane potential (MMP) of individual cardiomyocytes before and after H2O2 treatment.
As shown in Fig. 5 in untreated cardiomyocytes, H2O2 resulted in a rapid decrease of the ratio of red to green fluorescence intensity, indicating loss of mitochondrial membrane potential. However, glucosamine, increased OGT expression and NButGT all significantly attenuated the loss of mitochondrial membrane potential compared to untreated groups. This was apparent within 5 min of H2O2 treatment and was sustained for at least 20 mins (Fig 5B). Interestingly, after 20 minutes of H2O2 treatment, glucosamine and increased OGT expression were more effective in preventing the loss of mitochondrial membrane potential than NButGT treated cells.
Fig. 5.
Increased _O_-GlcNAc levels attenuated the loss of mitochondrial membrane potential (MMP). A) Representative fluorescent merged images with red and green filters of JC-1 staining NRVMs treated with hydrogen peroxide at 1 mM from 0 to 20 mins; B) Mean intensity of mitochondrial JC-1 staining (Red filter) determined by IPLab analysis software and normalized with cells at starting time. Data are presented as means±SEM of 30 cells. Control untreated cells, glucosamine (5 mM)-1 hour pretreated cells, _O_-GlcNAc transferase (OGT) adenovirus infected cells, NButGT (100 µM)-1 hour pretreated cells were incorporated with JC-1 to monitor MMP. # = P< 0.05 Vs untreated, * P< 0.05 Vs NButGT.
Decreased OGT expression decreases cardiomyocyte survival following ischemia/reperfusion
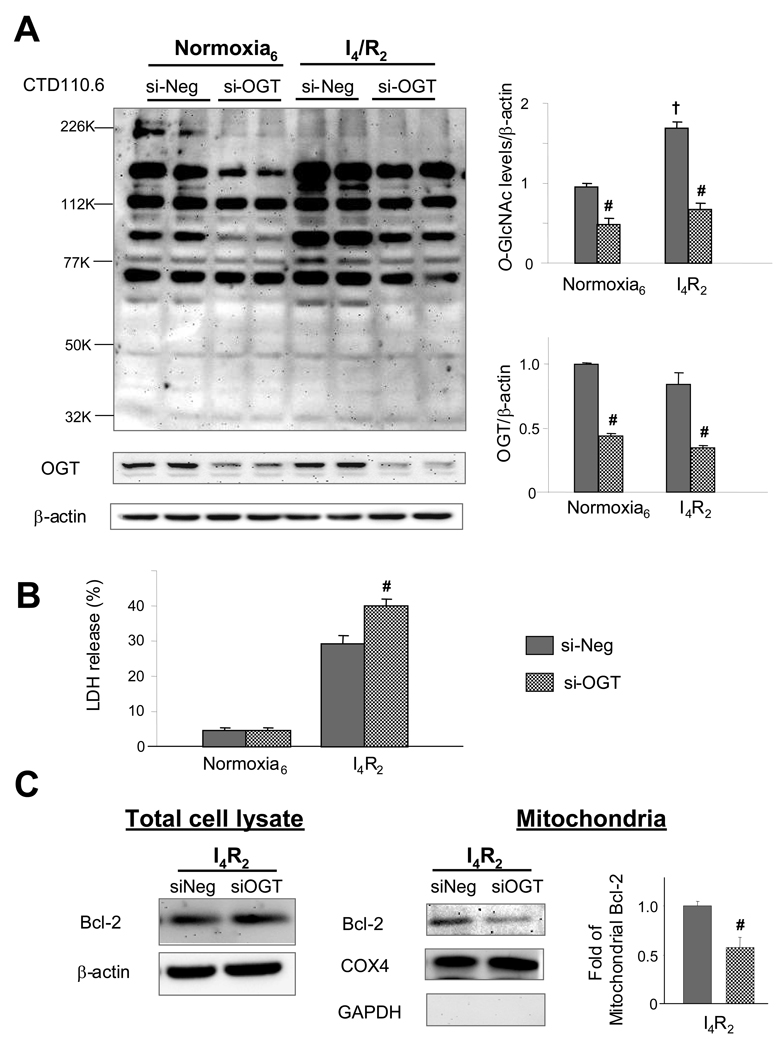
Zachara et al., (54) showed that OGT deletion using Cre-recombinase techniques in mouse embryonic fibroblasts significantly decreased survival following heat stress. Therefore, we asked whether decreased OGT expression in cardiomyocytes would also attenuate survival following ischemia/reperfusion injury. Since global OGT gene deletion is embryonically lethal (41) and even cell specific OGT gene deletion resulted in cell death during embryogenesis (41, 46), we used siRNA approach to decrease OGT expression in NRVMs.
Following electroporation and transfection with siRNA cell viability, measured by trypan blue exclusion, was ~80–90%.Two days after transfection, _O_-GlcNAc and OGT levels were significantly attenuated in the siOGT cells compared to si-Negative oligonucleotide (si-Neg) transfected controls (Fig 6A); furthermore, the ischemia induced increase in _O_-GlcNAc was completely blocked in the si-OGT cells. The decrease in OGT and _O_-GlcNAc was associated with a significant increase in LDH release following ischemia/reperfusion (Fig 6B). At the end of ischemia/reperfusion, there was little effect in total Bcl-2 expression between si-OGT and si-Neg groups. However, in si-OGT cells, ischemia/reperfusion significantly decreased Blc-2 levels compared to si-Neg cells (Fig 6C). These results are consistent with the notion that decreased flux through OGT attenuated the ischemia/reperfusion induced increase in mitochondrial Bcl-2 levels.
Fig. 6.
_O_-GlcNAc modification is essential for cellular survival and Bcl-2 translocation. A) Representative immunoblotting CTD110.6 and OGT and mean intensity of all _O_-GlcNAc proteins and OGT of the total whole cell lysate determined by densitometric analysis. Equal protein loading was normalized by densitometric analysis of β-actin levels. Proteins are normalized to normoxic control untreated cells; B) Cell injury assessed by determining lactate dehydrogenase (LDH) release as a percentage of total LDH; C) Representative immunoblotting of Bcl-2 expression levels in total cell lysate and mitochondrial fractions and mean densitometric analysis of mitochondrial Bcl-2. Purity of mitochondrial fraction was assessed by COX4 and GAPDH. Control, si-Negative oligonuleotides transfected cells (si-Neg) and si-OGT oligonucleotides transfected cells (si-OGT) cells were incubated under normoxic condition for 6 h or subjected to 4 h of ischemia followed by 2 h of reperfusion (I4/R2). Data are presented as means±SEM of more than 3 experiments. # =P< 0.05 Vs untreated, † P< 0.05 Vs normoxia.
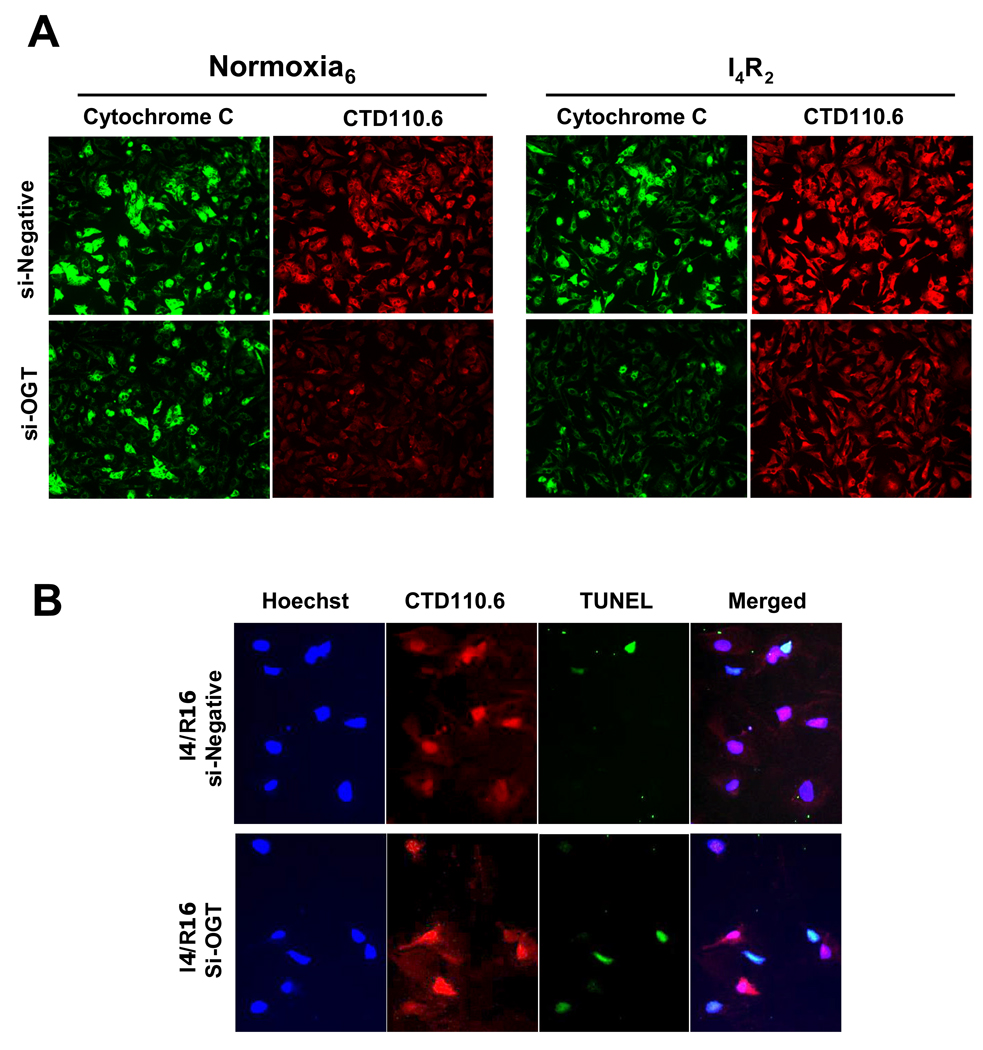
In Fig 7A cytochrome C levels are shown in si-Neg and si-OGT cells under normoxic conditions and following ischemia/reperfusion. Under normoxic conditions there was no difference between the two groups; however, compared to si-Neg cells there was much greater loss of cytochrome C following ischemia/reperfusion in si-OGT cells. Following ischemia/reperfusion the decrease in cytochrome C levels in the si-Neg cells appears to be attenuated compared to that seen in untreated cells (Fig 4 A). Comparison of immunofluorescence images between different sets of experiments must be made with caution; however, is possible that the process of electroporation used to transfect cells with the si-RNA induces some sort of protection against ischemia/reperfusion stress, akin to a preconditioning response. Nevertheless, the relevant comparison here is between si-Neg and si-OGT groups, both of which have undergone the same electroporation treatment; it is evident that the si-OGT cells have markedly lower cytochrome C levels following ischemia/reperfusion than si-Neg cells and this was associated with greater number of TUNEL positive cells following reperfusion of 16 hours (Fig 7B).
Fig. 7.
Decreasing global _O_-GlcNAc proteins exacerbated the loss of cytochrome C and apoptosis. A) Representative immunofluorescence of cytochrome C and _O_-GlcNAc proteins (CTD 110.6) under normoxia and following ischemia/reperfusion; B) Representative immunofluorescent of apoptosis (TUNEL), _O_-GlcNAc proteins (CTD 110.6), and nuclear staining (Hoechst). Control, si-Negative oligonuleotides transfected cells (si-Neg) and si-OGT oligonucleotides transfected cells (si-OGT) cells were incubated under normoxic condition for 6 h or subjected to 4 h of ischemia followed by 2 h or 16 h of reperfusion.
DISCCUSION
There is increasing appreciation of the role of _O_-linked GlcNAc modification of proteins in regulating cell function (22, 52). While much of the focus has been on the role of increased _O_-GlcNAc in mediating the adverse effects of diabetes (2, 8, 37), there is a growing body of data indicating that activation of pathways leading to _O_-GlcNAc formation is an endogenous stress response and that augmentation of this response improves tolerance to stress (31, 47, 54). We have previously demonstrated that glucosamine is remarkably protective against ischemic injury at the cellular (9), organ (15, 32, 33) and organismal level (40, 51) and that this protection is associated with increased _O_-GlcNAc levels. However, the protection associated with glucosamine treatment could be mediated via pathways other than _O_-GlcNAc such as gangliosides (36) or cell surface _N_-glycans (48). Although we have observed similar effects to glucosamine by increasing _O_-GlcNAc levels via inhibition of _O_-GlcNAcase with PUGNAc (32, 56), we have also observed some differences, in which PUGNAc appears to be less effective than glucosamine in attenuating cell death (9).
We show here for the first time that the effect of glucosamine is mimicked by increased OGT expression and that both interventions increase the tolerance of NRVMs to injury induced by either ischemia/reperfusion or H2O2. Both glucosamine and OGT overexpression augmented the ischemia/reperfusion-induced increase in mitochondrial Bcl-2. The marked similarity between glucosamine treatment and increased OGT expression, demonstrates that the protective effect of glucosamine is mediated primarily via increased flux through OGT. Conversely, we found that decreasing OGT expression decreased tolerance to ischemia/reperfusion injury and blunted the ischemia/reperfusion-induced increase in mitochondrial Bcl-2. We also found that while inhibition of _O_-GlcNAcase with NButGT resulted in a much greater increase in _O_-GlcNAc levels than glucosamine or OGT overexpression, it was significantly less protective and than either. This demonstrates that the similar observations previous reported with PUGNAc (9) were most likely not due to non-specific effects of PUGNAc, but rather suggests that there may be some threshold for _O_-GlcNAc in increasing cell survival, beyond which the detrimental effects of excessive _O_-GlcNAc levels outweigh the pro-survival mechanisms.
While it is becoming increasingly apparent that elevating _O_-GlcNAc levels improves the tolerance of cells to stress, the specific mechanisms underlying this protective response have not been fully defined. Zachara et al. demonstrated that _O_-GlcNAc-mediated tolerance to heat stress was due in part to increased transcription of heat shock proteins (54). Our studies have suggested that the protection associated with glucosamine treatment may be due at least in part to attenuation of calcium-mediated stress responses such as calpain activation (9, 32). We have also shown that glucosamine treatment significantly attenuated cardiomyocyte apoptosis (9). Given the importance of the Bcl-2 family of proteins in regulating apoptosis (11, 21) we examined the effect of increasing _O_-GlcNAc levels on Bcl-2, Bad and Bax. found that there was no effect of either glucosamine or OGT overexpression on whole cell levels of Bcl-2, Bad or Bax; however, both interventions specifically increased mitochondrial Bcl-2 levels. Surprisingly this was observed under normoxic conditions as well as following ischemia/reperfusion; ischemia/reperfusion increased mitochondrial Bcl-2 in untreated cells and this response was augmented by glucosamine and OGT overexpression.
Modulation of _O_-GlcNAc levels has been shown to modify sub-cellular localization of proteins (3, 17) and since there was no change in total Bcl-2, the increase in mitochondrial Bcl-2 in the glucosamine and OGT overexpression groups presumably reflects its redistribution from other cellular compartments such as the endoplasmic reticulum, and nuclear envelope (18, 43). We attempted to use immunofluorescence techniques to monitor Bcl-2 localization; however, all Bcl-2 antibodies we tested were ineffective (Santa Cruz, Abcam, and BioVision). Based on the prediction of potential _O_-GlcNAcylation sites by YinOYang 1.2 (http://www.cbu.dtu.dk/services/yinoyang), there are at least 4 potential sites for _O_-GlcNAc modification of rat Bcl-2 including Thr65, Ther69, Ser70, and Ser84. However, our attempts to determine whether the redistribution of Bcl-2 in these experiments could be due to _O_-GlcNAc modification of Bcl-2 were unsuccessful.
Despite the evidence demonstrating that anti-apoptotic Bcl-2 proteins may have therapeutic potential, the mechanism(s) by which they protect cells remains unclear. Bcl-2 has been shown to block p53-mediated apoptosis in cardiac myocytes (27) and overexpression of Bcl-2 suppressed both p53-dependent and p53-independent activation of the intrinsic death pathway (35). Transgenic mice overexpressing Bcl-2 decreased apoptosis, reduced infract size and improved cardiac function after ischemia/reperfusion (7, 10, 25). It has been suggested that Bcl-2 inhibits opening of mMTP, possibly by direct interaction with voltage-dependant anion channel (VDAC) (49, 50), thereby preventing the release of death factors from mitochondria. Consistent with the notion that Bcl-2 protection is mediated via attenuation of mMTP opening, both glucosamine and OGT overexpression attenuated loss of cytochrome C release following ischemia/reperfusion (Fig. 4) and slowed the H2O2-induced loss of mitochondrial membrane potential (Fig. 5). Thus, these data suggest that an increase in mitochondrial Bcl-2 may be an important contributing factor to the cellular protection associated with increased _O_-GlcNAc levels. Clearly, however, further studies are needed to delineate the mechanism(s) by which _O_-GlcNAc levels modulate the cellular distribution of Bcl-2.
We focused here on mitochondrial Bcl-2, because of the extensive data demonstrating its role in attenuating mitochondria-mediated apoptosis (38); however, Bcl-2 is also associated with other subcellular compartments such as the ER/SR and nuclear envelope (18, 43) and it has been suggested that Bcl-2 localized to the ER/SR may play a role in attenuating apoptosis possibly by mediating ER/SR calcium homeostasis (6, 13). There are also other antiapoptotic members of the Bcl-2 family of proteins, such as Bcl-XL and Bcl-w that are associated with the mitochondria (26), which were not examined here and could also contribute to the protection seen with increased O-GlcNAc levels. However, it should be noted that while there is considerable evidence demonstrating that mitochondrial Bcl-2 plays an important role in mediating apoptosis the precise mechanisms by which this occurs are still not well defined (38).
_O_-GlcNAc levels can be elevated not only by increasing the rate of synthesis, but also by decreasing the rate of removal via inhibition of _O_-GlcNAcase. PUGNAc is a widely used inhibitor of _O_-GlcNAcase, and we have previously reported that both glucosamine and PUGNAc are protective against ischemic injury (9, 32). However, despite the fact that PUGNAc resulted in markedly greater _O_-GlcNAc levels than glucosamine, it was somewhat less protective (9). Therefore, here we used a new _O_-GlcNAcase inhibitor, NButGT, which has ~1500-fold greater specificity for _O_-GlcNAcase over β-hexosaminidase than PUGNAc (34). NButGT increased _O_-GlcNAc levels 5–10 fold more than OGT overexpression; however, while compared to untreated cells NButGT attenuated necrosis following ischemia/reperfusion, this was effect was significantly less than that seen with OGT overexpression (Fig. 3B). The difference between NButGT treatment and both OGT overexpression and glucosamine expression was particularly apparent with regard to the loss of cytochrome C following ischemia/reperfusion (Fig 4). It is also noteworthy that in contrast to OGT overexpression and glucosamine treatment, NButGT had no effect on mitochondrial Bcl-2 levels, under either normoxia or following ischemia/reperfusion.
These observations are rather contradictory to the notion that increasing protein _O_-GlcNAc levels is cytoprotective, since NButGT was clearly less protective than either glucosamine or OGT overexpression despite ~10-fold higher _O_-GlcNAc levels. This could point to differences resulting from increasing _O_-GlcNAc levels via new synthesis versus inhibiting the removal. For example, proteins, which under steady state conditions contain _O_-GlcNAc that is constantly cycling between on and off states, will show an enhancement of _O_-GlcNAc levels with _O_-GlcNAcase inhibition. In contrast, some proteins may become _O_-GlcNAcylated only when UDP-GlcNAc or OGT levels increase. Thus, our results could be explained if some proteins important to mediating the protective response, including the increase in mitochondrial Bcl-2 levels, were in the latter class. If this is the case, it is possible that the combination of glucosamine plus NButGT could increase mitochondrial Bcl-2 levels and thus provide greater protection that NButGT alone. It should also be noted that OGT is predominantly localized to nucleus whereas O-GlcNAcase is found primarily in the cytosol (14); consequently, glucosamine and NButGT may lead to different spectrum of O-GlcNAcylated proteins in different cellular compartments, which could also account for their different levels of protection. However, it is also possible that there is a threshold effect with cellular O-GlcNAc levels, such that beyond a certain level increased O-GlcNAc levels will increase cell death. Indeed it has been proposed that high as well as low levels of _O_-GlcNAc may trigger apoptosis (53). If this is the case, then a combination of glucosamine and NButGT may lead to further loss of protection compared with either treatment alone.
The importance of OGT and _O_-GlcNAc in mediating cardiomyocyte survival was further supported by the fact that decreasing OGT expression by ~50%, using siRNA not only attenuated basal _O_-GlcNAc levels, but also prevented the increase induced by ischemia (Fig 6A, 7A). This was associated with an increase in necrosis (Fig 6B), greater loss of cytochrome C (Fig 7A) and increased apoptosis (Fig 7B) in OGT siRNA group. In contrast to OGT overexpression and glucosamine treatment, reduced OGT expression resulted in an increase in whole cell Bcl-2 levels, thereby complicating the interpretation of the Bcl-2 data. Nevertheless, the increase in cardiomyocyte injury in the OGT siRNA group was associated with attenuation of mitochondrial Bcl-2 following ischemia/reperfusion, which is in contrast to the increase seen in control si-Neg cells.
An alternative explanation for the improved survival associated with glucosamine and OGT overexpression could be that increased O-GlcNAc levels leads to decreased energy consumption as seen with β-blockers or calcium antagonists or by increasing energy production by stimulating glycolysis. In this study we did not assess cardiomyocyte contractility or energy metabolism; however, in an earlier study using the same NRVM model, we found that glucosamine had no effect on ATP levels under normoxic conditions, at the end of ischemia or the end of reperfusion (9). We have also shown that neither glucosamine nor PUGNAc affected the beating rate of neonatal cardiomyocytes (39) suggesting that increasing O-GlcNAc formation or inhibiting O-GlcNAc removal does not block Ca2+-handling pathways associated with contractility. In the intact perfused heart we have not observed any negative inotropic or chronotropic effect of glucosamine and the rate of ATP loss during ischemia was not reduced by glucosamine treatment even though functional recovery was improved (15). Preliminary studies on the effect of glucosamine on cardiac metabolism demonstrate that, at least under normoxic conditions, glucosamine has no effect on glycolytic flux (16). Thus, while we cannot entirely rule out an effect of increasing O-GlcNAc on improving cardiac energy metabolism, our data to date suggest this is not the case.
In conclusion we have shown that in NRVMs the glucosamine-mediated protection against both ischemia/reperfusion and H2O2 is mimicked by OGT overexpression, and that decreasing OGT expression significantly increased sensitivity of NRVMs to ischemia/reperfusion injury. These results provide strong evidence to support the notion that the protective effects of glucosamine seen at the cellular, organ and whole animal level are mediated via increased formation of _O_-GlcNAc. We have also demonstrated that one potential mechanism contributing to this protection is an increase in mitochondrial Bcl-2 levels, which was also associated with attenuation of H2O2-induced loss of mitochondrial membrane potential. However, further studies are clearly warranted to better understand the mechanism by which increased _O_-GlcNAc levels modulate the cellular distribution of Bcl-2 and whether this is a consequence of _O_-GlcNAc modification of Bcl-2 itself.
Acknowledgements
This work was supported by NIH grants HL076175 (RBM) and HL67464 (JC). We would like to thank Dr. W.H. Dillmann, Department of Medicine, University of California, San Diego for providing the OGT adenovirus. We are grateful to Dr. David Vocadlo, Simon Fraser University, Burnaby, BC, Canada for providing NButGT and for insightful comments on the manuscript.
REFERENCES
- 1.Adachi S, Cross AR, Babior BM, Gottlieb RA. Bcl-2 and the outer mitochondrial membrane in the inactivation of cytochrome c during Fas-mediated apoptosis. J Biol Chem. 1997;272:21878–21882. doi: 10.1074/jbc.272.35.21878. [DOI] [PubMed] [Google Scholar]
- 2.Akimoto Y, Hart GW, Wells L, Vosseller K, Yamamoto K, Munetomo E, Ohara-Imaizumi M, Nishiwaki C, Nagamatsu S, Hirano H, Kawakami H. Elevation of the post-translational modification of proteins by O-linked N-acetylglucosamine leads to deterioration of the glucose-stimulated insulin secretion in the pancreas of diabetic Goto-Kakizaki rats. Glycobiology. 2007;17:127–140. doi: 10.1093/glycob/cwl067. [DOI] [PubMed] [Google Scholar]
- 3.Andrali SS, Qian Q, Ozcan S. Glucose mediates the translocation of NeuroD1 by O-linked glycosylation. J Biol Chem. 2007;282:15589–15596. doi: 10.1074/jbc.M701762200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brar BK, Jonassen AK, Stephanou A, Santilli G, Railson J, Knight RA, Yellon DM, Latchman DS. Urocortin protects against ischemic and reperfusion injury via a MAPK-dependent pathway. J Biol Chem. 2000;275:8508–8514. doi: 10.1074/jbc.275.12.8508. [DOI] [PubMed] [Google Scholar]
- 5.Brar BK, Stephanou A, Wagstaff MJ, Coffin RS, Marber MS, Engelmann G, Latchman DS. Heat shock proteins delivered with a virus vector can protect cardiac cells against apoptosis as well as against thermal or hypoxic stress. J Mol Cell Cardiol. 1999;31:135–146. doi: 10.1006/jmcc.1998.0857. [DOI] [PubMed] [Google Scholar]
- 6.Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608–8618. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- 7.Brocheriou V, Hagege AA, Oubenaissa A, Lambert M, Mallet VO, Duriez M, Wassef M, Kahn A, Menasche P, Gilgenkrantz H. Cardiac functional improvement by a human Bcl-2 transgene in a mouse model of ischemia/reperfusion injury. J Gene Med. 2000;2:326–333. doi: 10.1002/1521-2254(200009/10)2:5<326::AID-JGM133>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 8.Buse MG. Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab. 2006;290:E1–E8. doi: 10.1152/ajpendo.00329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Champattanachai V, Marchase RB, Chatham JC. Glucosamine protects neonatal cardiomyocytes from ischemia-reperfusion injury via increased protein-associated O-GlcNAc. Am J Physiol Cell Physiol. 2007;292:C178–C187. doi: 10.1152/ajpcell.00162.2006. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Chua CC, Ho YS, Hamdy RC, Chua BH. Overexpression of Bcl-2 attenuates apoptosis and protects against myocardial I/R injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–H2320. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 11.Cook SA, Sugden PH, Clerk A. Regulation of bcl-2 family proteins during development and in response to oxidative stress in cardiac myocytes: association with changes in mitochondrial membrane potential. Circ Res. 1999;85:940–949. doi: 10.1161/01.res.85.10.940. [DOI] [PubMed] [Google Scholar]
- 12.Correa F, Soto V, Zazueta C. Mitochondrial permeability transition relevance for apoptotic triggering in the post-ischemic heart. Int J Biochem Cell Biol. 2007;39:787–798. doi: 10.1016/j.biocel.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Dremina ES, Sharov VS, Kumar K, Zaidi A, Michaelis EK, Schoneich C. Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) Biochem J. 2004;383:361–370. doi: 10.1042/BJ20040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulop N, Marchase RB, Chatham JC. Role of protein O-linked N-acetyl-glucosamine in mediating cell function and survival in the cardiovascular system. Cardiovasc Res. 2007;73:288–297. doi: 10.1016/j.cardiores.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulop N, Zhang Z, Marchase RB, Chatham JC. Glucosamine cardioprotection in perfused rat heart associated with increased O-Linked N-acetylglucosamine protein modification and altered p38 activation. Am J Physiol Heart Circ Physiol. 2007;292:H2227–H2236. doi: 10.1152/ajpheart.01091.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fülöp N, Onay-Besikci A, Marchase RB, Chatham JC. Regulation of cardiac substrate utilization by protein O-glycosylation. J Mol Cell Cardiol. 2007 [Google Scholar]
- 17.Gandy JC, Rountree AE, Bijur GN. Akt1 is dynamically modified with O-GlcNAc following treatments with PUGNAc and insulin-like growth factor-1. FEBS Lett. 2006;580:3051–3058. doi: 10.1016/j.febslet.2006.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotow T, Shibata M, Kanamori S, Tokuno O, Ohsawa Y, Sato N, Isahara K, Yayoi Y, Watanabe T, Leterrier JF, Linden M, Kominami E, Uchiyama Y. Selective localization of Bcl-2 to the inner mitochondrial and smooth endoplasmic reticulum membranes in mammalian cells. Cell Death Differ. 2000;7:666–674. doi: 10.1038/sj.cdd.4400694. [DOI] [PubMed] [Google Scholar]
- 19.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 20.Grunenfelder J, Miniati DN, Murata S, Falk V, Hoyt EG, Kown M, Koransky ML, Robbins RC. Upregulation of Bcl-2 through caspase-3 inhibition ameliorates ischemia/reperfusion injury in rat cardiac allografts. Circulation. 2001;104:I202–I206. doi: 10.1161/hc37t1.094833. [DOI] [PubMed] [Google Scholar]
- 21.Gustafsson AB, Gottlieb RA. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol. 2007;292:C45–C51. doi: 10.1152/ajpcell.00229.2006. [DOI] [PubMed] [Google Scholar]
- 22.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 23.Horsch M, Hoesch L, Vasella A, Rast DM. N-acetylglucosaminono-1,5-lactone oxime and the corresponding (phenylcarbamoyl)oxime. Novel and potent inhibitors of beta-N-acetylglucosaminidase. Eur J Biochem. 1991;197:815–818. doi: 10.1111/j.1432-1033.1991.tb15976.x. [DOI] [PubMed] [Google Scholar]
- 24.Hunton DL, Lucchesi PA, Pang Y, Cheng X, Dell'Italia LJ, Marchase RB. Capacitative calcium entry contributes to nuclear factor of activated T-cells nuclear translocation and hypertrophy in cardiomyocytes. J Biol Chem. 2002;277:14266–14273. doi: 10.1074/jbc.M107167200. [DOI] [PubMed] [Google Scholar]
- 25.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res. 2004;95:734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann T, Schinzel A, Borner C. Bcl-w(edding) with mitochondria. Trends Cell Biol. 2004;14:8–12. doi: 10.1016/j.tcb.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Kirshenbaum LA, de Moissac D. The bcl-2 gene product prevents programmed cell death of ventricular myocytes. Circulation. 1997;96:1580–1585. doi: 10.1161/01.cir.96.5.1580. [DOI] [PubMed] [Google Scholar]
- 28.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 29.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 30.Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15:691–699. doi: 10.1016/j.ceb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Lim KH, Chang HI. O-linked N-acetylglucosamine suppresses thermal aggregation of Sp1. FEBS Lett. 2006;580:4645–4652. doi: 10.1016/j.febslet.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Marchase RB, Chatham JC. Increased O-GlcNAc levels during reperfusion leads to improved functional recovery and reduced calpain-proteolysis. Am J Physiol Heart Circ Physiol. 2007 doi: 10.1152/ajpheart.00285.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol. 2006;40:303–312. doi: 10.1016/j.yjmcc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Macauley MS, Whitworth GE, Debowski AW, Chin D, Vocadlo DJ. O-GlcNAcase uses substrate-assisted catalysis: kinetic analysis and development of highly selective mechanism-inspired inhibitors. J Biol Chem. 2005;280:25313–25322. doi: 10.1074/jbc.M413819200. [DOI] [PubMed] [Google Scholar]
- 35.Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- 36.Masson E, Wiernsperger N, Lagarde M, El Bawab S. Glucosamine induces cell-cycle arrest and hypertrophy of mesangial cells: implication of gangliosides. Biochem J. 2005;388:537–544. doi: 10.1042/BJ20041506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNulty PH. Hexosamine biosynthetic pathway flux and cardiomyopathy in type 2 diabetes mellitus. Focus on "Impact of type 2 diabetes and aging on cardiomyocyte function and O-linke;d N-acetylglucosamine levels in the heart". Am J Physiol Cell Physiol. 2007;292:C1243–C1244. doi: 10.1152/ajpcell.00521.2006. [DOI] [PubMed] [Google Scholar]
- 38.Murphy E, Imahashi K, Steenbergen C. Bcl-2 regulation of mitochondrial energetics. Trends Cardiovasc Med. 2005;15:283–290. doi: 10.1016/j.tcm.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Nagy T, Champattanachai V, Marchase RB, Chatham JC. Glucosamine inhibits angiotensis II induced cytoplasmic Ca2+ elevation in neonatal cardiomyocytes via protein-associated O-GlcNAc. Am J Physiol Cell Physiol. 2006;290:C57–C65. doi: 10.1152/ajpcell.00263.2005. [DOI] [PubMed] [Google Scholar]
- 40.Nöt LG, Marchase RB, Fülöp N, Brocks CA, Chatham JC. Glucosamine treatment improves survival following trauma-hemorrhage without resuscitation. SHOCK. 2007;28:345–351. doi: 10.1097/shk.0b013e3180487ebb. [DOI] [PubMed] [Google Scholar]
- 41.O'Donnell N, Zachara NE, Hart GW, Marth JD. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol. 2004;24:1680–1690. doi: 10.1128/MCB.24.4.1680-1690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perreira M, Kim EJ, Thomas CJ, Hanover JA. Inhibition of O-GlcNAcase by PUGNAc is dependent upon the oxime stereochemistry. Bioorg Med Chem. 2006;14:837–846. doi: 10.1016/j.bmc.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Ryan JJ, Prochownik E, Gottlieb CA, Apel IJ, Merino R, Nunez G, Clarke MF. c-myc and bcl-2 modulate p53 function by altering p53 subcellular trafficking during the cell cycle. Proc Natl Acad Sci U S A. 1994;91:5878–5882. doi: 10.1073/pnas.91.13.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scarabelli TM, Knight R, Stephanou A, Townsend P, Chen-Scarabelli C, Lawrence K, Gottlieb R, Latchman D, Narula J. Clinical implications of apoptosis in ischemic myocardium. Curr Probl Cardiol. 2006;31:181–264. doi: 10.1016/j.cpcardiol.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Schaffer SW, Croft CB, Solodushko V. Cardioprotective effect of chronic hyperglycemia: effect on hypoxia-induced apoptosis and necrosis. Am J Physiol Heart Circ Physiol. 2000;278:H1948–H1954. doi: 10.1152/ajpheart.2000.278.6.H1948. [DOI] [PubMed] [Google Scholar]
- 46.Shafi R, Iyer SP, Ellies LG, O'Donnell N, Marek KW, Chui D, Hart GW, Marth JD. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci U S A. 2000;97:5735–5739. doi: 10.1073/pnas.100471497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sohn KC, Lee KY, Park JE, Do SI. OGT functions as a catalytic chaperone under heat stress response: a unique defense role of OGT in hyperthermia. Biochem Biophys Res Commun. 2004;322:1045–1051. doi: 10.1016/j.bbrc.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 48.Stanley P. A method to the madness of N-glycan complexity? Cell. 2007;129:27–29. doi: 10.1016/j.cell.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 49.Tsujimoto Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol. 2003;195:158–167. doi: 10.1002/jcp.10254. [DOI] [PubMed] [Google Scholar]
- 50.Tsujimoto Y, Nakagawa T, Shimizu S. Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta. 2006;1757:1297–1300. doi: 10.1016/j.bbabio.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 51.Yang S, Zou LY, Bounelis P, Chaudry I, Chatham JC, Marchase RB. Glucosamine administration during resuscitation improves organ function after trauma hemorrhage. Shock. 2006;25:600–607. doi: 10.1097/01.shk.0000209563.07693.db. [DOI] [PubMed] [Google Scholar]
- 52.Zachara NE, Hart GW. Cell signaling, the essential role of O-GlcNAc! Biochim Biophys Acta. 2006;1761:599–617. doi: 10.1016/j.bbalip.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 53.Zachara NE, Hart GW. O-GlcNAc a sensor of cellular state: the role of nucleocytoplasmic glycosylation in modulating cellular function in response to nutrition and stress. Biochim Biophys Acta. 2004;1673:13–28. doi: 10.1016/j.bbagen.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Zachara NE, O'Donnell N, Cheung WD, Mercer JJ, Marth JD, Hart GW. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress. A survival response of mammalian cells. J Biol Chem. 2004;279:30133–30142. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 55.Zhao W, Lu L, Chen SS, Sun Y. Temporal and spatial characteristics of apoptosis in the infarcted rat heart. Biochem Biophys Res Commun. 2004;325:605–611. doi: 10.1016/j.bbrc.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 56.Zou LY, Yang S, Chaudry IH, Marchase RB, Chatham JC. PUGNAc administration during resuscitation improves organ function following trauma-hemorrhage. Shock. 2007;27:402–408. doi: 10.1097/01.shk.0000209563.07693.db. [DOI] [PubMed] [Google Scholar]