Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade (original) (raw)
. Author manuscript; available in PMC: 2010 Feb 10.
Abstract
Currently available evidence strongly supports the position that the initiating event in Alzheimer’s disease (AD) is related to abnormal processing of β-amyloid (Aβ) peptide, ultimately leading to formation of Aβ plaques in the brain. This process occurs while individuals are still cognitively normal. Biomarkers of brain β-amyloidosis are reductions in CSF Aβ42 and increased amyloid PET tracer retention. After a lag period, which varies from patient to patient, neuronal dysfunction and neurodegeneration become the dominant pathological processes. Biomarkers of neuronal injury and neurodegeneration are increased CSF tau and structural MRI measures of cerebral atrophy. Neurodegeneration is accompanied by synaptic dysfunction, which is indicated by decreased fluorodeoxyglucose uptake on PET. We propose a model that relates disease stage to AD biomarkers in which Aβ biomarkers become abnormal first, before neurodegenerative biomarkers and cognitive symptoms, and neurodegenerative biomarkers become abnormal later, and correlate with clinical symptom severity.
Introduction
As recently as 2 to 3 decades ago, a compartmentalised model of Alzheimer’s disease (AD) was widely accepted. The view at that time was that people either had AD pathological changes, in which case they had dementia, or they did not have such changes and were cognitively normal. In the meantime, a revised view of the disease has been developed, in which both AD pathological processes and clinical decline occur gradually, with dementia representing the end stage of many years of accumulation of these pathological changes. An additional feature of the current view of AD is that these changes begin to develop decades before the earliest clinical symptoms occur.
Biomarkers, both chemical and imaging, are indicators of specific changes that characterise AD in vivo. Evidence suggests that these AD biomarkers do not reach abnormal levels or peak simultaneously but do so in an ordered manner. Measurement of these biomarkers in longitudinal observational studies is now commonplace, enabling investigators to establish the correct ordering of the relevant biomarkers and their relationships to clinical symptoms.
For biomarkers of AD to be used effectively for disease staging, the time-dependent ordering of biomarkers must be thoroughly understood. This is particularly true since the introduction of clinical trials of disease-modifying therapies in which disease biomarkers play an increasingly important part both as outcome measures and as inclusion criteria. We will review the five most well validated AD biomarkers. We then propose a hypothetical model of the time-dependent ordering of onset and maxima of these biomarkers. The purpose of this paper is to offer this model as a conceptual construct within which research studies from different disciplines can relate to one another through a common framework. The model suggests a series of testable hypotheses from which a clearer picture of the time-dependent trajectories of AD biomarkers relative to clinical disease stage and to each other can be derived.
AD clinical features and pathological changes
Dementia is the clinically observable result of the cumulative burden of multiple pathological insults in the brain. Most elderly patients with dementia have multiple pathological changes underlying their dementia; however, the most common pathological substrate is AD.1,2
The clinical disease stages of AD have been divided into three phases. First is a pre-symptomatic phase in which individuals are cognitively normal but some have AD pathological changes. To some extent, labelling these individuals as having pre-symptomatic AD is a hypothesis rather than a statement of fact, because some of these individuals will die without ever expressing clinical symptoms.3–5 The hypothetical assumption is that an asymptomatic individual with pathological changes that are indicative of AD would ultimately have become symptomatic if he or she lived long enough. Second is a prodromal phase of AD, commonly referred to as mild cognitive impairment (MCI),6 which is characterised by the onset of the earliest cognitive symptoms (typically deficits in episodic memory) that do not meet the criteria for dementia. The severity of cognitive impairment in the MCI phase of AD varies from the earliest appearance of memory dysfunction to more widespread dysfunction in other cognitive domains. The final phase in the evolution of AD is dementia, defined as impairments in multiple domains that are severe enough to produce loss of function.
Recent recommendations have suggested redefining research criteria for AD by labelling individuals with memory impairment plus accompanying biomarker evidence of AD as having early AD.7 These investigators propose eliminating the distinction between pre-dementia (ie, MCI) and dementia, but this is not uniformly accepted because the label “dementia” serves a practical role in clinical practice. A clinical diagnosis of dementia is a clear indication to both the patient and family that the patient has a disorder that precludes independent living and has a decidedly worse prognosis than do milder forms of cognitive impairment, and implies that he or she is on an inevitable course toward complete loss of independence.
The concept of using biomarkers for early diagnostic purposes has a long history, with many studies showing that AD biomarkers can be used to predict conversion from MCI to AD. These prediction studies show that individuals destined to develop AD can be identified earlier in the disease course by use of the MCI designation with the addition of imaging and CSF biomarkers to enhance diagnostic specificity.8–13 However, at present, the clinical diagnosis of AD requires the presence of dementia.14
A widely accepted assumption is that AD begins with abnormal processing of amyloid precursor protein (APP), which then leads to excess production or reduced clearance of β-amyloid (Aβ) in the cortex.15 All known forms of autosomal-dominant AD involve genes that either encode APP itself, or encode protease subunits (PS1 and PS2) that are involved in the cleavage of Aβ from APP to generate amyloidogenic Aβ peptides. By unknown mechanisms, but possibly as a result of the toxic effects of Aβ oligomers,16 one or more forms of Aβ leads to a cascade characterised by abnormal tau aggregation, synaptic dysfunction, cell death, and brain shrinkage.17
The abnormal protein deposits that characterise AD pathologically are well known: Aβ plaques and neurofibrillary tangles (NFTs) formed by hyperphosphorylated tau. Neurodegeneration is as important as these hallmark pathological lesions of AD, and manifests as atrophy, neuron loss, and gliosis, which are routinely noted in research post-mortem examinations. Although the loss of synapses also is highly significant for the clinical manifestations of AD, this is difficult to assess without the use of labour-intensive morphometric methods, so it is not routinely measured in most AD research centres. Neurodegeneration and NFT deposition are both neuronal processes and occur in roughly the same topographic distribution. Aβ plaques are extracellular and occur in a different, but to some degree overlapping, topographic distribution from NFT and neurodegenerative pathological changes.
Clinical symptoms are more closely related to NFTs than to plaque formation.18,19 However, the most direct pathological substrate of clinical symptoms is neurodegeneration, and most specifically synapse loss.20 Recent autopsy data have confirmed that gross cerebral atrophy (indicating the loss of synapses and neurons), and not Aβ or NFT burden, is the most proximate pathological substrate of cognitive impairment in AD.5
Panel: Imaging and CSF biomarker categories in Alzheimer’s disease
Brain Aβ-plaque deposition
- CSF Aβ1–42
- PET Aβ imaging
Neurodegeneration
- CSF tau
- Fluorodeoxyglucose-PET
- Structural MRI
Aβ=β-amyloid.
Biomarkers of AD
Biomarkers are variables (physiological, biochemical, anatomical) that can be measured in vivo and that indicate specific features of disease-related pathological changes. We have used the term “biomarker” to denote both imaging and biospecimen (ie, CSF) measures. We will focus on the five most widely studied biomarkers of AD pathology, based on the current literature: decreased CSF Aβ42, increased CSF tau, decreased fluorodeoxyglucose uptake on PET (FDG-PET), PET amyloid imaging, and structural MRI measures of cerebral atrophy. Each of these five biomarkers is well validated enough to be used in currently active therapeutic trials and large multisite observational studies. Other potential AD biomarkers are summarised elsewhere,21,22 and are not discussed here. We briefly review the evidence supporting the position that each of these biomarkers is an in vivo indicator of a specific aspect of AD pathology (panel).
Biomarkers of Aβ-plaque deposition
Both CSF Aβ42 and amyloid PET imaging are biomarkers of brain Aβ plaque deposition. Nearly all patients who have a clinical diagnosis of AD have positive amyloid imaging studies.23–25 Excellent correspondence has been seen between Pittsburgh compound B (PiB) binding and fibrillar Aβ deposition in the brain (or cerebral vasculature) in most,26,27 but not all,28 patients who have undergone ante-mortem PiB-PET imaging and autopsy. PiB specifically binds to fibrillar Aβ, and not to soluble Aβ or to diffuse plaques.26,27 Low concentrations of CSF Aβ42 correlate with both the clinical diagnosis of AD and Aβ neuropathology at autopsy.29–31 Nearly 100% concordance is present between abnormally low CSF Aβ42 and positive PiB amyloid imaging findings in patients who have undergone both tests.32–35 The evidence therefore strongly supports the notion that both amyloid imaging and low CSF Aβ42 are valid biomarkers of brain Aβ-plaque load.
Biomarkers of neurodegeneration
CSF tau is an indicator of tau pathological changes and associated neuronal injury. Although phosphotau might be a more specific indicator of AD, concentrations of both phosphotau and total tau increase in AD.36 Increased CSF tau is not specific for AD, but does correlate with clinical disease severity, with higher concentrations associated with greater cognitive impairment in individuals on the normal–MCI–AD cognitive spectrum.37 In general, increases in CSF tau seem to indicate neuronal damage, and are seen in ischaemic and traumatic brain injury.38,39 In AD, increased tau in the CSF is thought to occur as a direct result of tau accumulation in neurons, particularly axons; this disrupts neuronal activity and causes release into the extracellular space of cytoskeletal elements, including tau, which then appear in the CSF.40,41 Increased CSF tau correlates with the presence of NFTs at autopsy.42 Of note, for reasons that remain elusive, similar increases in CSF tau are not seen in pure tauopathies such as progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD), despite the fact that the brains of patients with CBD often show far more tau accumulation than do the brains of patients with AD at autopsy.43,44 This might suggest that extracellular Aβ plaques have an effect on the clearance of tau released from degenerating neurons in AD, that different species of pathological tau are involved in AD versus CBD and PSP, or that other factors render tau more readily diffusible and less degradable in AD versus CBD and PSP.45
FDG-PET is used to measure net brain metabolism, which, although including many neural and glial functions, largely indicates synaptic activity.46,47 Brain glucose metabolism measured with FDG-PET is highly correlated with post-mortem measures of the synaptic structural protein synaptophysin.48 In the context of AD, decreased FDG-PET uptake is an indicator of impaired synaptic function. FDG-PET studies in patients with AD show a specific topographic pattern of decreased glucose uptake in a lateral temporal-parietal and posterior cingulate, precuneus distribution.49 Correction for cortical atrophy in patients with AD leaves metabolism still diminished.50 Greater decreases in FDG uptake correlate with greater cognitive impairment along the continuum from normal cognitive status to MCI to AD dementia.51 Combined imaging and autopsy studies show good correlation between the ante-mortem FDG-PET diagnosis of AD and post-mortem confirmation.52 In cognitively normal elderly individuals, correlations are seen between decreased FDG-PET uptake and both low CSF Aβ and increased CSF tau.53 Together, these data indicate that FDG-PET uptake is a valid indicator of the synaptic dysfunction that accompanies neurodegeneration in AD.
Structural MRI can provide measures of cerebral atrophy, which is caused by dendritic pruning and loss of synapses and neurons.54 Volumetric or voxel-based measures of brain atrophy show a strong correlation between the severity of atrophy and the severity of cognitive impairment in patients along the continuum from normal cognitive status to AD dementia.55 Thus, rates of neuronal and synaptic loss indicated by the progressive rate of brain atrophy correlate with rates of cognitive decline.56 Atrophy on MRI is not specific for AD, but the degree of atrophy correlates well with Braak staging at autopsy.57–59 Additionally, the topographic distribution of atrophy on MRI maps well onto Braak’s staging of NFT pathology in patients who have undergone ante-mortem MRI and post-mortem AD staging.60
Temporal ordering of biomarker abnormalities
A crucial element of biomarker-based staging of AD is the notion of temporal ordering of different biomarkers. The model that we propose, which relates pathological stage to AD biomarkers, is based on a largely biphasic view of disease progression.61,62 In this model, biomarkers of Aβ deposition become abnormal early, before neurodegeneration and clinical symptoms occur. Biomarkers of neuronal injury, dysfunction, and neurodegeneration become abnormal later in the disease. Cognitive symptoms are directly related to biomarkers of neurodegeneration rather than biomarkers of Aβ deposition. We examine evidence to support these time-dependent assumptions, beginning with evidence that biomarker abnormalities typically precede clinical symptoms.
Biomarker abnormalities precede clinical symptoms
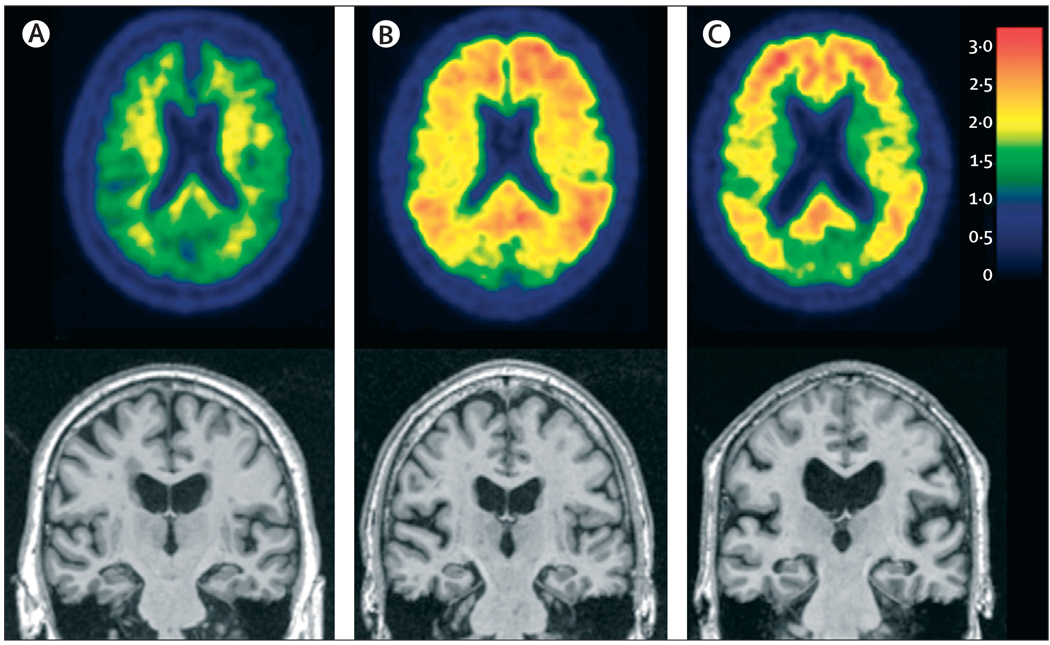
Approximately 20–40% of cognitively normal elderly people have evidence of significant brain Aβ-plaque deposition, either from amyloid imaging or CSF Aβ42 concentrations.37,63–66 These data on Aβ imaging and CSF biomarkers are in agreement with autopsy studies that also show an AD pathological burden sufficient to meet criteria for a diagnosis of AD in roughly the same proportion of cognitively normal elderly individuals.3–5 These data support the principle that the presence of Aβ-plaque deposition alone, even in substantial quantities, is not sufficient to produce dementia, and that abnormalities in biomarkers of Aβ deposition precede clinical/cognitive symptoms.67–71 This principle is clearly illustrated by data from the individual in figure 1B who was cognitively normal with no evidence of atrophy on MRI, but had a highly abnormal PiB study. Calculated rates from serial PiB imaging studies indicate that Aβ-plaque accumulation in individuals destined to become demented might begin as much as two decades before the manifestation of clinical symptoms.62 We note that both Aβ deposition and NFTs can be present in individuals with no symptoms. However, the presence of NFTs in asymptomatic individuals tends to be confined to the entorhinal cortex, Braak stage I–II, whereas NFTs in symptomatic individuals are far more widespread.3–5,72 By contrast, Aβ-plaque deposition can be widespread in clinically asymptomatic individuals.
Figure 1. Illustration of biomarker staging of Alzheimer’s disease.
Three elderly individuals are placed in order from left to right by use of our proposed biomarker staging scheme. (A) A cognitively normal individual with no evidence of Aβ on PET amyloid imaging with PiB and no evidence of atrophy on MRI. (B) A cognitively normal individual who has no evidence of neurodegenerative atrophy on MRI, but has significant Aβ deposition on PET amyloid imaging. (B) An individual who has dementia and a clinical diagnosis of Alzheimer’s disease, a positive PET amyloid imaging study, and neurodegenerative atrophy on MRI. Aβ=β-amyloid. PiB=Pittsburgh compound B.
There is strong evidence that MRI, FDG-PET, and CSF tau biomarkers are already abnormal in patients who are in the MCI phase of AD.37,51,73–75 Abnormalities in neurodegenerative AD biomarkers also precede the appearance of the first cognitive symptoms. Of the three neurodegenerative biomarkers, evidence that FDG-PET abnormalities precede any cognitive symptoms in individuals who later progress to AD is probably the strongest.76,77 However, rates of atrophy on MRI do become abnormal in cognitively normal individuals who later progress to AD.78–80 Thus, the available data strongly support the conclusion that abnormalities in both Aβ and neurodegenerative biomarkers precede clinical symptoms.
Aβ biomarker abnormalities precede neurodegenerative biomarker abnormalities
The rate of MRI atrophy on serial imaging studies is greatest in patients with a clinical diagnosis of AD, least in cognitively normal individuals, and intermediate in those with a clinical diagnosis of MCI. By contrast, rates of change in PiB retention over time are just slightly greater than zero for all these three clinical groups, and do not differ by clinical group.62 Thus, in patients who are rapidly declining clinically (ie, patients with AD) MRI rates map well onto simultaneous cognitive deterioration, whereas rates of change in PiB do not.62,81 Similarly, CSF Aβ does not change significantly over time in patients with AD. Rates of brain atrophy correlate well with pathological indices of NFTs and other neurodegenerative changes, but do not correlate with severity of Aβ deposition at autopsy.82 Cognitively normal individuals with positive Aβ imaging studies might have normal structural MRI studies, implying that a substantial Aβ load can accumulate with no immediate effect on gross brain structure or cognition (figure 1).83 In a modelling study inferring cause and effect, Mormino and colleagues84 found that the direct substrate of memory impairment was hippocampal atrophy on MRI, and not Aβ deposition as measured by PiB imaging. Frisoni and colleagues85 also placed amyloid deposition before MRI changes in the sequence of events. These findings support the conclusion that an abnormality in biomarkers of Aβ-plaque deposition is an early event that nears a plateau before the appearance of both atrophy on MRI and cognitive symptoms, and remains relatively static thereafter. By contrast, abnormalities in neurodegenerative biomarkers on MRI accelerate as symptoms appear, and then parallel cognitive decline.
Neurodegenerative biomarkers are temporally ordered
Available evidence suggests that FDG-PET changes might precede MRI changes.77,86,87 Up to this point, we have discussed the temporal ordering of AD biomarkers from the perspective of which biomarker becomes abnormal earlier during the progression of AD. However, the order in which the dynamic range of biomarkers approaches its maximum is also relevant to the discussion of biomarker ordering. MRI and CSF tau correlate well with cognition if individuals who span the entire cognitive spectrum (controls, MCI, and AD) are combined. However, among patients with MCI or AD alone, correlations with measures of general cognition are strong with structural MRI, but are not significant with CSF tau.88 These data are consistent with studies indicating that CSF tau does not change appreciably over time in cognitively impaired patients.89,90 Furthermore, although both MRI and CSF tau are predictive of future conversion from MCI to AD, the predictive power of structural MRI is greater.91 These findings imply that the correlations between cognition and CSF tau weaken as patients progress into the mid and late stages of the clinical AD spectrum. Conversely, structural MRI measures of atrophy retain a highly significant correlation with observed clinical impairment in both the MCI and dementia phases of AD. Moreover, rates of atrophy on MRI are significantly greater in patients with AD than in cognitively normal elderly individuals.92 This body of literature implies that MRI atrophy is a later event in AD progression, preceded by abnormalities in CSF tau and FDG-PET, and that MRI retains a closer correlation with cognitive symptoms later in disease progression than does CSF tau.
Use of biomarkers to stage AD in vivo
Autopsy studies have been, and will continue to be, essential in uncovering the biological basis of the clinical symptoms in AD. However, by definition, autopsy studies are unable to provide clinical–histological correlations during life, when pathological changes actually occur, resulting in an inability to isolate relationships between time-dependent histological changes and clinical/cognitive consequences. This point underlies the value of using biomarkers in the staging of disease.
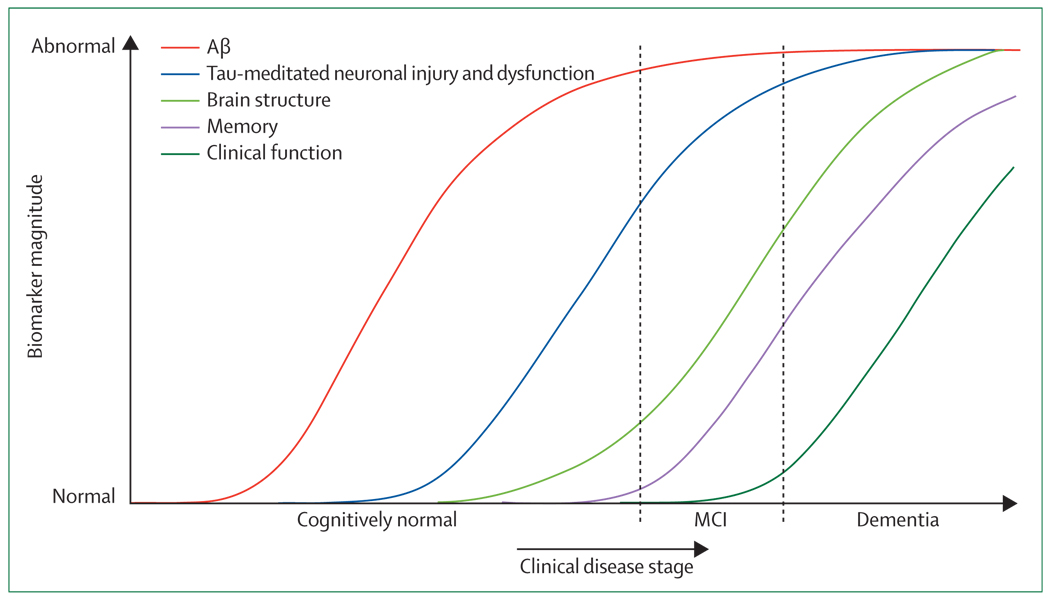
On the basis of the evidence presented above, we propose the use of specific AD biomarkers for disease staging in vivo. The disease model and biomarker staging are shown in figure 2, which embodies the following set of principles. First, the biomarkers become abnormal in a temporally ordered manner as the disease progresses. Second, Aβ-plaque biomarkers are dynamic early in the disease, before the appearance of clinical symptoms, and have largely reached a plateau by the time clinical symptoms appear. Third, biomarkers of neuronal injury, dysfunction, and degeneration are dynamic later in the disease and correlate with clinical symptom severity. Fourth, MRI is the last biomarker to become abnormal; however, MRI retains a closer relationship with cognitive performance later into the disease than other biomarkers. 88,91 Fifth, none of the biomarkers is static; rates of change in each biomarker change over time and follow a non-linear time course, which we hypothesise to be sigmoid shaped. Non-linearity has been clearly shown in MRI studies, in which atrophy rates accelerate as patients approach clinical dementia.93,94 A sigmoid shape as a function of time implies that the maximum effect of each biomarker varies over the course of disease progression.95 Comprehensive biomarker-based staging of disease in an individual at a given point in time should be possible from measures of the magnitude and slope (ie, rate of change) of several different biomarkers (figure 3). Sixth, anatomical information from imaging biomarkers provides crucial disease-staging information. Similar to NFT accumulation, cerebral atrophy, for example, begins in medial temporal limbic areas and spreads from there to adjacent limbic and paralimbic areas and later to the isocortical association cortex.96 Therefore, at a given timepoint, different brain areas will be at different stages. In any given area of an individual’s brain there might be an amyloid phase, a neuronal dysfunction phase, etc, and this will occur in an anatomical order (figure 4). This implies an advantage for imaging biomarkers over fluid biomarkers, because imaging can resolve the different phases of the disease both temporally and anatomically. Finally, a feature of this biomarker model of AD is the presence of a lag phase of unknown duration between Aβ-plaque formation and the neurodegenerative cascade. Between-patient variation in the lag period between Aβ deposition and the neurodegenerative cascade is probably an indication of differences in Aβ processing, in the effects of abnormal Aβ processing on neuronal injury, and differences in brain resilience or cognitive reserve.97 Other pathological changes, particularly cerebrovascular, α-synuclein, and TAR DNA-binding protein 43 proteinopathy mechanisms, also contribute significantly to between-individual variations in clinical disease expression.98
Figure 2. Dynamic biomarkers of the Alzheimer’s pathological cascade.
Aβ is identified by CSF Aβ42 or PET amyloid imaging. Tau-mediated neuronal injury and dysfunction is identified by CSF tau or fluorodeoxyglucose-PET. Brain structure is measured by use of structural MRI. Aβ=β-amyloid. MCI=mild cognitive impairment.
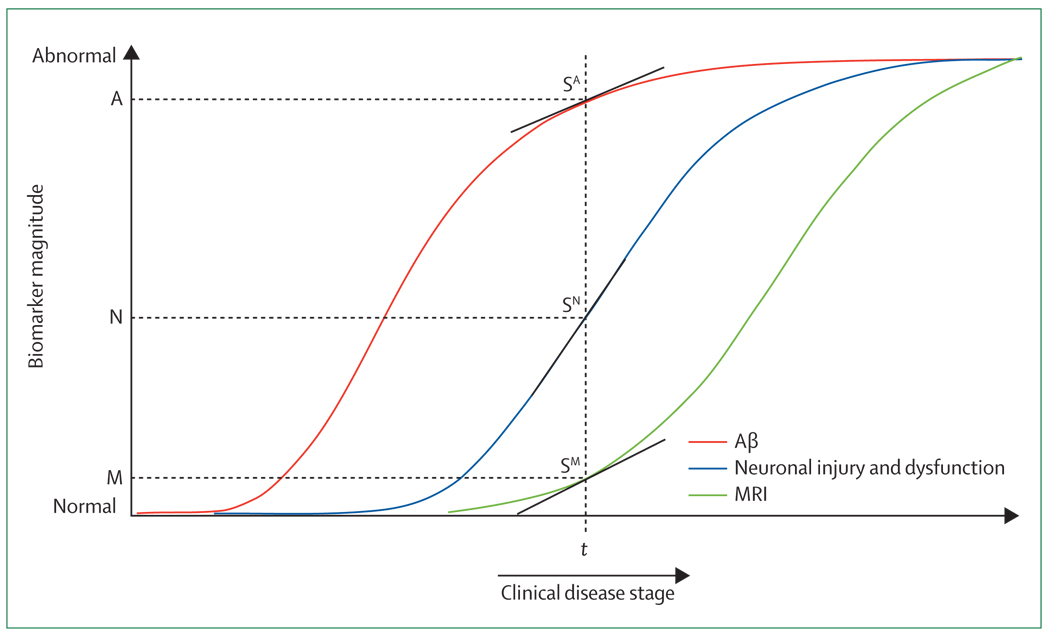
Figure 3. Staging Alzheimer’s disease with dynamic biomarkers.
Disease stage based on biomarkers is described by the magnitude of the biomarker abnormality and its rate of change at a given point in time (t). This is illustrated by the following terms: A(t)=magnitude of the Aβ plaque biomarker at time t; SA(t)=slope of the Aβ plaque function at time t; N(t)=tau-mediated neuron injury at time t; SN(t)=slope of N(t); M(t)=MRI at time t; SM(t)=slope of M(t). Aβ=β-amyloid.
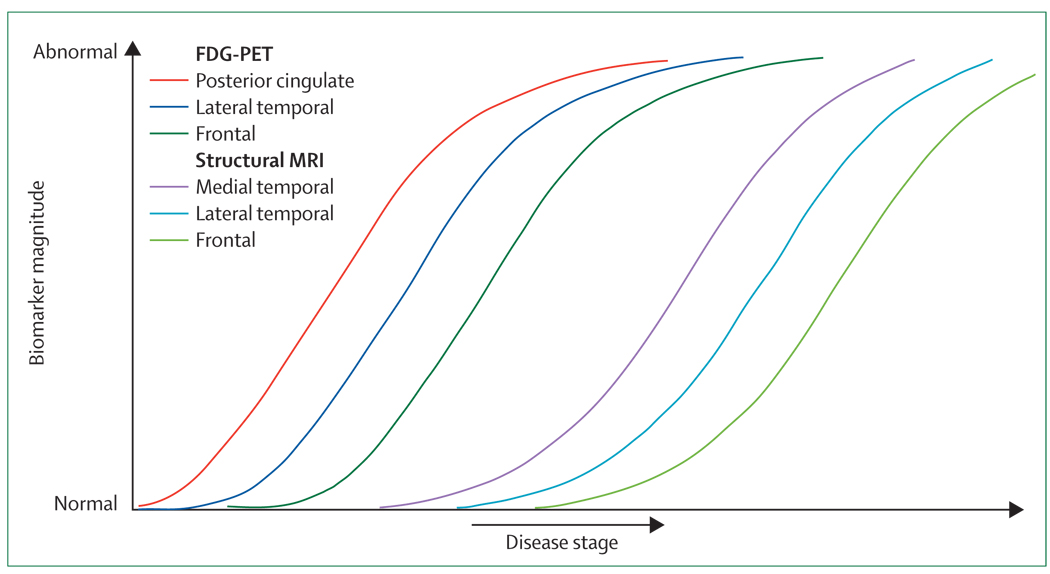
Figure 4. Anatomical imaging information.
For simplicity in other figures, imaging biomarkers have been shown as individual curves. However, anatomical variation exists in the time courses within each imaging mode. For example, in FDG-PET, one would expect abnormalities to appear in the following order: precuneus/posterior cingulate, lateral temporal, and frontal lobe much later. Similarly, in structural MRI, one would expect abnormalities to appear in the following order: medial temporal, lateral temporal, and frontal lobe later. FGD=fluorodeoxyglucose.
Clinical trials
Our proposed model has implications for clinical trials. For example, it is rational to select patients for inclusion in trials of anti-Aβ therapies on the basis of biomarker evidence of the presence of Aβ in the brain by use of either amyloid PET imaging or CSF Aβ42. Although biomarkers of neurodegeneration correlate with clinical and pathological severity, they are not specific for AD and thus should not take precedence over Aβ biomarkers as inclusion criteria for patients in anti-amyloid therapeutic trials (although an Aβ biomarker might be combined with MRI volumetrics to provide an indication of disease stage). Conversely, change in Aβ load over time has little relation to change in cognition in natural history studies. In addition, evidence of therapeutic plaque removal in patients who already have dementia does not seem to correlate with a change in the trajectory of cognitive deterioration (at least, not in every case examined).99 Measures of the neurodegenerative portion of the cascade (eg, CSF tau, FDG-PET, or structural MRI) should therefore be used in therapeutic trials as evidence of therapeutic modification of the neurodegenerative aspect of the AD pathological process. Therapeutic modification of the slopes of clinical outcome measures is a common outcome metric used in clinical trials. A consideration in the use of biomarkers as co-primary outcome measures is the fact that the slopes (rates of change over time) of different biomarkers vary over the course of the disease (figure 3). Ultimately, a combination of biomarkers might be needed in clinical trials to select appropriate participants and to follow disease progression.
Caveats
We have attempted to integrate the five most thoroughly validated biomarkers of AD pathology into a model that is supported by currently available data. We have used a best-fit approach, realising that every published observation cannot neatly fit into our (or any other) single idealised model of disease progression. The proposed biomarker model represents a model of typical disease progression. We certainly do not preclude the existence of individual deviations from this generic model.
Although we have used the available evidence to support our model relating biomarkers to disease stage in AD, we recognise that some of the proposed relationships take the form of hypotheses rather than statements of fact. For example, although Aβ imaging and CSF Aβ are denoted as occurring simultaneously (figure 2, figure 3, and figure 5), it might be that one precedes or plateaus earlier than the other. The same logic applies to the timing relationships between FDG-PET and CSF tau. We have superimposed these curves in the current model not because there is good evidence to suggest that the curves should be identical, but because there is not good evidence at present to show that they are different. We also acknowledge that temporal ordering among the neurodegenerative biomarkers might ultimately differ from that in our proposed model.100 For example, recent data from Fagan and colleagues68 suggest that MRI atrophy might precede increases in CSF tau.
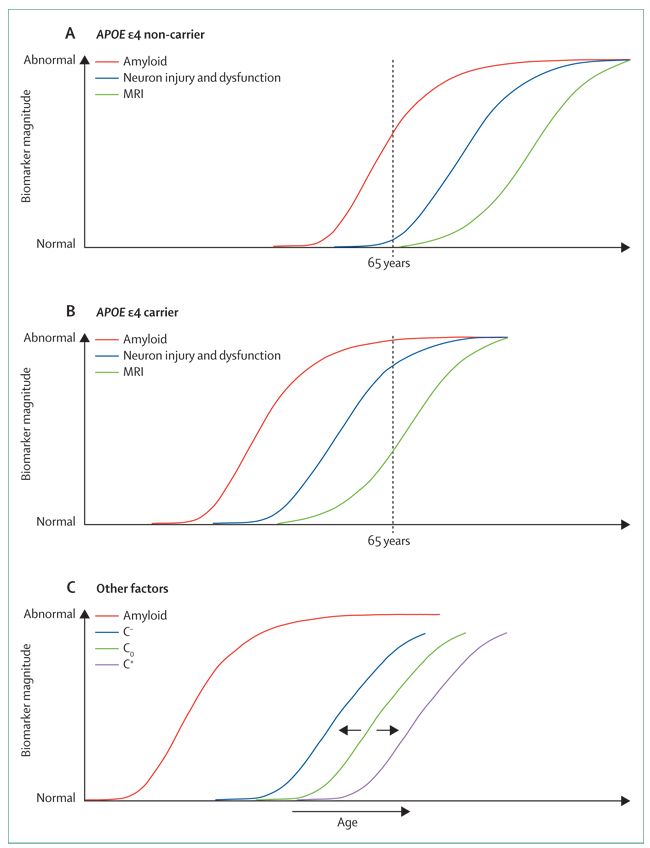
Figure 5. Modulators of biomarker temporal relationships.
(A,B) Relative to a fixed age (here, 65 years), the hypothesised effect of APOE ε4 is to shift β-amyloid plaque deposition and the neurodegenerative cascade both to an earlier age compared with ε4 non-carriers. (C) The hypothesised effect of the presence of different diseases and genes on cognition: C−=cognition in the presence of comorbidities (eg, Lewy bodies or vascular disease) or risk amplification genes; C+=cognition in patients with enhanced cognitive reserve or protective genes; Co=cognition in individuals without comorbidity or enhanced cognitive reserve.
We recognise that well-validated biomarkers do not currently exist for some important features of the disease. This includes reliable chemical biomarkers of specific toxic oligomeric forms of soluble Aβ and imaging measures of soluble Aβ or diffuse plaques. We are therefore not in a position to include in our model important mechanistic features such as the role of toxic Aβ oligomeric species and the timing of their appearance. The absence of PET ligands that specifically measure the burden of NFTs and other tau abnormalities also constitutes a serious gap in our current armamentarium of imaging biomarkers. Another shortcoming is the absence of a widely accepted biomarker for microglial activation. PET imaging ligands and the magnetic resonance spectroscopy metabolite myo-inositol have been proposed as potential biomarkers of glial activation;101,102 however, neither is widely used for this purpose at present. Thus, our biomarker model of disease is just that—a model of the stages of disease that can be assessed with currently validated biomarkers, and not a comprehensive model of all pathological processes in AD. In this context, we acknowledge that all biomarker information about disease is limited by the inevitable filter imposed by detection sensitivity and measurement precision. Clearly, an in vivo measure is unlikely to be as sensitive to the underlying pathology as a detailed autopsy examination would be.
An observation that does not fit our model is that of Braak and Braak,72 who concluded that stage I–II (entorhinal) NFT changes precede isocortical Aβ changes, leading to the conclusion that NFT accumulation is the initiating event in AD.103 However, the following observations contradict this conclusion: (1) the genetics data in early-onset AD, which implicate disordered Aβ metabolism as the initiating event; (2) the fact that the final pathological picture is identical between late-onset and early-onset AD cases; and (3) the fact that the major genetic risk factor of late-onset AD (APOE ε4) is implicated in disordered trafficking of Aβ peptide.104
Observations that will need to be incorporated into future revisions of this model relate to evidence of disordered glucose uptake in cognitively normal young and middle-aged APOE ε4 carriers up to decades before disease-related clinical symptoms would be expected to appear.105,106 No consensus exists on the interpretation of these observations at this point. These findings could be neurodevelopmental in origin or early indicators of AD.107,108
Conclusions
Our main objective was to provide a framework for hypothesis testing that relates temporal changes in AD biomarkers to clinical disease stage and to each other. The temporal relationships among the biomarkers and with clinical disease stage constitute an array of testable hypotheses. For example, carriers of APOE ε4 have an earlier age of onset of dementia than non-carriers,109 and we hypothesise that APOE ε4 carriers will have a leftward (earlier in time) shift of both the Aβ-plaque and neurodegenerative biomarker cascades relative to non-carriers (figure 5).110 We also hypothesise that modifiers of the relationship between Aβ pathological changes and their downstream effect on cognition might alter the lag time between Aβ-plaque deposition and cognitive decline (figure 5). For example, the cognitive decline curve might shift closer to the Aβ curve (to the left) in individuals with comorbidities (eg, vascular disease), whereas the cognitive decline curve would shift away from the amyloid curve (to the right) in individuals with enhanced cognitive reserve.97 Similarly, as yet undiscovered neuroprotective genes might shift the cognitive decline curve to the right, away from the amyloid curve, whereas genes that amplify the effect of Aβ dysmetabolism on the neurodegenerative cascade might shift the cognitive decline curve closer to the amyloid curve. For example, recent genome-wide association studies have identified new susceptibility loci involving CR1, CLU, and PICALM genes.111,112 Clusterin (apolipoprotein J) seems to regulate the toxicity and solubility of Aβ and might modify its clearance at the blood–brain barrier.111 CR1 might also modify Aβ clearance.112 PICALM might be related to AD through a role in altering synaptic vesicle cycling or APP endocytosis.111 Finally, we anticipate that other diagnostic modalities (eg, functional MRI connectivity)113–115 will be added to this biomarker staging model as they mature.
Search strategy and selection criteria
References for this paper were identified through searches of PubMed between 1984 and October, 2009, with combinations of the search terms “Alzheimer’s disease”, “dementia”, “MCI”, “imaging”, “PET”, “PiB”, “amyloid imaging”, “MRI”, and “biomarker”. Articles were also identified through searches of the authors’ own files. Only papers published in English were reviewed.
Acknowledgments
CRJ receives research support from the National Institute on Aging (US National Institutes of Health grant AG11378). DSK and RCP receive National Institute on Aging funding (AG16574 and MCSA AG06786). JQT receives funding from the Alzheimer’s Disease Center (AG10124). We thank Nick C Fox and Prashanthi Vemuri for advice, and Denise A Reyes for the figures.
Footnotes
Conflicts of interest
CRJ is an investigator in clinical trials sponsored by Pfizer and Baxter, and consults for Elan and Lilly. DSK has served on a data and safety monitoring board for Lilly, has been a consultant for GlaxoSmithKline, and receives grant funding for clinical trials by Baxter, Elan, and Forest. WJJ has consulted for Genentech, Synarc, Elan Pharmaceuticals, Ceregene, Schering Plough, Merck & Co, and Lilly. LMS has consulted for Pfizer. PSA has consulted for Elan, Wyeth, Eisai, Neurochem, Schering-Plough, Bristol-Myers-Squibb, Lilly, Neurophase, Merck, Roche, Amgen, Genentech, Abbott, Pfizer, Novartis, and Medivation, has stock options with Medivation and Neurophase, and has received grant support from Pfizer, Baxter, and Neuro-Hitech. MWW has been on the scientific advisory boards for the Alzheimer’s Study Group, Bayer Schering Pharma, Lilly, CoMentis, Neurochem, Eisai, Avid, Aegis, Genentech, Allergan, Bristol-Myers Squibb, Forest, Pfizer, McKinsey, Mitsubishi, and Novartis, has received travel funding from Nestlé, honoraria from BOLT International, commercial research support from Merck and Avid, and has stock options in Synarc and Elan. RCP has been a chair, member of the safety monitoring committee, and consultant for Elan, has chaired a data monitoring committee for Wyeth, and has been a consultant for GE Healthcare. JQT has received lecture honoraria from Wyeth, Pfizer, and Takeda.
References
- 1.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 2.White L, Small BJ, Petrovitch H, et al. Recent clinical-pathologic research on the causes of dementia in late life: update from the Honolulu-Asia Aging Study. J Geriatr Psychiatry Neurol. 2005;18:224–227. doi: 10.1177/0891988705281872. [DOI] [PubMed] [Google Scholar]
- 3.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 4.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 7.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 8.Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 9.Jack CR, Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan Y, Gu ZX, Wei WS. Fluorodeoxyglucose-positron-emission tomography, single-photon emission tomography, and structural MR imaging for prediction of rapid conversion to Alzheimer disease in patients with mild cognitive impairment; a meta-analysis. AJNR Am J Neuroradiol. 2009;30:404–410. doi: 10.3174/ajnr.A1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattsson N, Zetterberg H, Hansson O, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 12.Jagust WJ, Haan MN, Eberling JL, Wolfe N, Reed BR. Functional imaging predicts cognitive decline in Alzheimer’s disease. J Neuroimaging. 1996;6:156–160. doi: 10.1111/jon199663156. [DOI] [PubMed] [Google Scholar]
- 13.Visser PJ, Verhey F, Knol DL, et al. Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619–627. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 16.Klein WL, Stine WB, Jr, Teplow DB. Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer’s disease. Neurobiol Aging. 2004;25:569–580. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Oddo S, Caccamo A, Tran L, et al. Temporal profile of amyloid-β (Aβ) oligomerization in an in vivo model of Alzheimer disease. A link between Aβ and tau pathology. J Biol Chem. 2006;281:1599–1604. doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Isla T, Hollister R, West H, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- 19.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 20.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 21.Hampel H, Burger K, Teipel SJ, Bokde AL, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimers Dement. 2008;4:38–48. doi: 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007;6:295–303. doi: 10.1038/nrd2176. [DOI] [PubMed] [Google Scholar]
- 23.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 24.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 25.Edison P, Archer HA, Hinz R, et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68:501–508. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 26.Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer’s disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bacskai BJ, Frosch MP, Freeman SH, et al. Molecular imaging with Pittsburgh compound B confirmed at autopsy: a case report. Arch Neurol. 2007;64:431–434. doi: 10.1001/archneur.64.3.431. [DOI] [PubMed] [Google Scholar]
- 28.Rosen RF, Ciliax BJ, Wingo TS, et al. Deficient high-affinity binding of Pittsburgh compound B in a case of Alzheimer’s disease. Acta Neuropathol. 2009 doi: 10.1007/s00401-009-0583-3. published online Aug 19. DOI:10.1007/ s00401-009-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark CM, Xie S, Chittams J, et al. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol. 2003;60:1696–1702. doi: 10.1001/archneur.60.12.1696. [DOI] [PubMed] [Google Scholar]
- 30.Strozyk D, Blennow K, White LR, Launer LJ. CSF Aβ 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology. 2003;60:652–656. doi: 10.1212/01.wnl.0000046581.81650.d0. [DOI] [PubMed] [Google Scholar]
- 31.Schoonenboom NS, van der Flier WM, Blankenstein MA, et al. CSF and MRI markers independently contribute to the diagnosis of Alzheimer’s disease. Neurobiol Aging. 2008;29:669–675. doi: 10.1016/j.neurobiolaging.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Aβ42 in humans. Ann Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 33.Jagust WJ, Landau SM, Shaw LM, et al. Relationships between biomarkers in aging and dementia. Neurology. 2009;73:1193–1199. doi: 10.1212/WNL.0b013e3181bc010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimmer T, Riemenschneider M, Forstl H, et al. Beta amyloid in Alzheimer’s disease: increased deposition in brain is reflected in reduced concentration in cerebrospinal fluid. Biol Psychiatry. 2009;65:927–934. doi: 10.1016/j.biopsych.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tolboom N, van der Flier WM, Yaqub M, et al. Relationship of cerebrospinal fluid markers to 11C-PiB and 18F-FDDNP binding. J Nucl Med. 2009;50:1464–1470. doi: 10.2967/jnumed.109.064360. [DOI] [PubMed] [Google Scholar]
- 36.Buerger K, Ewers M, Pirttila T, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006;129:3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 37.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hesse C, Rosengren L, Andreasen N, et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci Lett. 2001;297:187–190. doi: 10.1016/s0304-3940(00)01697-9. [DOI] [PubMed] [Google Scholar]
- 39.Ost M, Nylen K, Csajbok L, et al. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology. 2006;67:1600–1604. doi: 10.1212/01.wnl.0000242732.06714.0f. [DOI] [PubMed] [Google Scholar]
- 40.Arai H, Terajima M, Miura M, et al. Tau in cerebrospinal fluid: a potential diagnostic marker in Alzheimer’s disease. Ann Neurol. 1995;38:649–652. doi: 10.1002/ana.410380414. [DOI] [PubMed] [Google Scholar]
- 41.Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26:231–245. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- 42.Tapiola T, Overmyer M, Lehtovirta M, et al. The level of cerebrospinal fluid tau correlates with neurofibrillary tangles in Alzheimer’s disease. Neuroreport. 1997;8:3961–3963. doi: 10.1097/00001756-199712220-00022. [DOI] [PubMed] [Google Scholar]
- 43.Bian H, Van Swieten JC, Leight S, et al. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology. 2008;70:1827–1835. doi: 10.1212/01.wnl.0000311445.21321.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grossman M, Farmer J, Leight S, et al. Cerebrospinal fluid profile in frontotemporal dementia and Alzheimer’s disease. Ann Neurol. 2005;57:721–729. doi: 10.1002/ana.20477. [DOI] [PubMed] [Google Scholar]
- 45.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz WJ, Smith CB, Davidsen L, et al. Metabolic mapping of functional activity in the hypothalamo-neurohypophysial system of the rat. Science. 1979;205:723–725. doi: 10.1126/science.462184. [DOI] [PubMed] [Google Scholar]
- 47.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 48.Rocher AB, Chapon F, Blaizot X, Baron JC, Chavoix C. Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: a study in baboons. Neuroimage. 2003;20:1894–1898. doi: 10.1016/j.neuroimage.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Jagust W, Reed B, Mungas D, Ellis W, Decarli C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology. 2007;69:871–877. doi: 10.1212/01.wnl.0000269790.05105.16. [DOI] [PubMed] [Google Scholar]
- 50.Ibanez V, Pietrini P, Alexander GE, et al. Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer’s disease. Neurology. 1998;50:1585–1593. doi: 10.1212/wnl.50.6.1585. [DOI] [PubMed] [Google Scholar]
- 51.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 52.Hoffman JM, Welsh-Bohmer KA, Hanson M, et al. FDG PET imaging in patients with pathologically verified dementia. J Nucl Med. 2000;41:1920–1928. [PubMed] [Google Scholar]
- 53.Petrie EC, Cross DJ, Galasko D, et al. Preclinical evidence of Alzheimer changes: convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol. 2009;66:632–637. doi: 10.1001/archneurol.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bobinski M, de Leon MJ, Wegiel J, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience. 2000;95:721–725. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- 55.Jack CR, Jr, Petersen RC, O’Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 56.Fox NC, Scahill RI, Crum WR, Rossor MN. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology. 1999;52:1687–1689. doi: 10.1212/wnl.52.8.1687. [DOI] [PubMed] [Google Scholar]
- 57.Jack CR, Jr, Dickson DW, Parisi JE, et al. Antemortem MRI findings correlate with hippocampal neuropathology in typical aging and dementia. Neurology. 2002;58:750–757. doi: 10.1212/wnl.58.5.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silbert LC, Quinn JF, Moore MM, et al. Changes in premorbid brain volume predict Alzheimer’s disease pathology. Neurology. 2003;61:487–492. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- 59.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 60.Whitwell JL, Josephs KA, Murray ME, et al. MRI correlates of neurofibrillary tangle pathology at autopsy: a voxel-based morphometry study. Neurology. 2008;71:743–749. doi: 10.1212/01.wnl.0000324924.91351.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ingelsson M, Fukumoto H, Newell KL, et al. Early Aβ accumulation and progressive synaptic loss, gliosis, and tangle formation in AD brain. Neurology. 2004;62:925–931. doi: 10.1212/01.wnl.0000115115.98960.37. [DOI] [PubMed] [Google Scholar]
- 62.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer’s disease: implications for sequence of pathological events in Alzheimer’s disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 64.Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peskind ER, Li G, Shofer J, et al. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid beta-amyloid 42 in adults with normal cognition. Arch Neurol. 2006;63:936–939. doi: 10.1001/archneur.63.7.936. [DOI] [PubMed] [Google Scholar]
- 66.Bouwman FH, Schoonenboom NS, Verwey NA, et al. CSF biomarker levels in early and late onset Alzheimer’s disease. Neurobiol Aging. 2009;30:1895–1901. doi: 10.1016/j.neurobiolaging.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 67.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 68.Fagan AM, Head D, Shah AR, et al. Decreased cerebrospinal fluid Aβ (42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li G, Sokal I, Quinn JF, et al. CSF tau/Aβ42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 70.Gustafson DR, Skoog I, Rosengren L, Zetterberg H, Blennow K. Cerebrospinal fluid beta-amyloid 1–42 concentration may predict cognitive decline in older women. J Neurol Neurosurg Psychiatry. 2007;78:461–464. doi: 10.1136/jnnp.2006.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stomrud E, Hansson O, Blennow K, Minthon L, Londos E. Cerebrospinal fluid biomarkers predict decline in subjective cognitive function over 3 years in healthy elderly. Dement Geriatr Cogn Disord. 2007;24:118–124. doi: 10.1159/000105017. [DOI] [PubMed] [Google Scholar]
- 72.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 73.Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport. 2002;13:1939–1943. doi: 10.1097/00001756-200210280-00022. [DOI] [PubMed] [Google Scholar]
- 74.Ewers M, Buerger K, Teipel SJ, et al. Multicenter assessment of CSF-phosphorylated tau for the prediction of conversion of MCI. Neurology. 2007;69:2205–2212. doi: 10.1212/01.wnl.0000286944.22262.ff. [DOI] [PubMed] [Google Scholar]
- 75.Sluimer JD, Bouwman FH, Vrenken H, et al. Whole-brain atrophy rate and CSF biomarker levels in MCI and AD: a longitudinal study. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.06.016. published online Aug 7. DOI:10.1016/j.neurobiolaging.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 76.de Leon MJ, Convit A, Wolf OT, et al. Prediction of cognitive decline in normal elderly subjects with 2-[18F]fluoro-2-deoxy-D-glucose/poitron-emission tomography (FDG/PET) Proc Natl Acad Sci USA. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jagust W, Gitcho A, Sun F, Kuczynski B, Mungas D, Haan M. Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann Neurol. 2006;59:673–681. doi: 10.1002/ana.20799. [DOI] [PubMed] [Google Scholar]
- 78.Fox NC, Warrington EK, Rossor MN. Serial magnetic resonance imaging of cerebral atrophy in preclinical Alzheimer’s disease. Lancet. 1999;353:2125. doi: 10.1016/S0140-6736(99)00496-1. [DOI] [PubMed] [Google Scholar]
- 79.Jack CR, Jr, Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaye JA, Swihart T, Howieson D, et al. Volume loss of the hippocampus and temporal lobe in healthy elderly persons destined to develop dementia. Neurology. 1997;48:1297–1304. doi: 10.1212/wnl.48.5.1297. [DOI] [PubMed] [Google Scholar]
- 81.Engler H, Forsberg A, Almkvist O, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129:2856–2866. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- 82.Josephs KA, Whitwell JL, Ahmed Z, et al. Beta-amyloid burden is not associated with rates of brain atrophy. Ann Neurol. 2008;63:204–212. doi: 10.1002/ana.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mormino EC, Kluth JT, Madison CM, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frisoni GB, Lorenzi M, Caroli A, Kemppainen N, Nagren K, Rinne JO. In vivo mapping of amyloid toxicity in Alzheimer disease. Neurology. 2009;72:1504–1511. doi: 10.1212/WNL.0b013e3181a2e896. [DOI] [PubMed] [Google Scholar]
- 86.De Santi S, de Leon MJ, Rusinek H, et al. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001;22:529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 87.Reiman EM, Uecker A, Caselli RJ, et al. Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer’s disease. Ann Neurol. 1998;44:288–291. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- 88.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology. 2009;73:287–293. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sunderland T, Wolozin B, Galasko D. Longitudinal stability of CSF tau levels in Alzheimer patients. Biol Psychiatry. 1999;46:750–755. doi: 10.1016/s0006-3223(99)00143-2. [DOI] [PubMed] [Google Scholar]
- 90.Bouwman FH, van der Flier WM, Schoonenboom NS, et al. Longitudinal changes of CSF biomarkers in memory clinic patients. Neurology. 2007;69:1006–1011. doi: 10.1212/01.wnl.0000271375.37131.04. [DOI] [PubMed] [Google Scholar]
- 91.Vemuri P, Wiste HJ, Weigand SD, et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology. 2009;73:294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fox NC, Freeborough PA. Brain atrophy progression measured from registered serial MRI: validation and application to Alzheimer’s disease. J Magn Reson Imaging. 1997;7:1069–1075. doi: 10.1002/jmri.1880070620. [DOI] [PubMed] [Google Scholar]
- 93.Chan D, Janssen JC, Whitwell JL, et al. Change in rates of cerebral atrophy over time in early-onset Alzheimer’s disease: longitudinal MRI study. Lancet. 2003;362:1121–1122. doi: 10.1016/S0140-6736(03)14469-8. [DOI] [PubMed] [Google Scholar]
- 94.Ridha BH, Barnes J, Bartlett JW, et al. Tracking atrophy progression in familial Alzheimer’s disease: a serial MRI study. Lancet Neurol. 2006;5:828–834. doi: 10.1016/S1474-4422(06)70550-6. [DOI] [PubMed] [Google Scholar]
- 95.Wahlund LO, Blennow K. Cerebrospinal fluid biomarkers for disease stage and intensity in cognitively impaired patients. Neurosci Lett. 2003;339:99–102. doi: 10.1016/s0304-3940(02)01483-0. [DOI] [PubMed] [Google Scholar]
- 96.Whitwell JL, Przybelski SA, Weigand SD, et al. 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer’s disease. Brain. 2007;130:1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20 suppl 2:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 98.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2008 doi: 10.1111/j.1750-3639.2008.00244.x. published online Nov 19. DOI:10.1111/ j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Holmes C, Boche D, Wilkinson D, et al. Long-term effects of Aβ42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 100.Petersen RC. Alzheimer’s disease: progress in prediction. Lancet Neurol. 2010;9:4–5. doi: 10.1016/S1474-4422(09)70330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Edison P, Archer HA, Gerhard A, et al. Microglia, amyloid, and cognition in Alzheimer’s disease: an [11C](R)PK11195-PET and [11C]PIB-PET study. Neurobiol Dis. 2008;32:412–419. doi: 10.1016/j.nbd.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 102.Ross BD, Bluml S, Cowan R, Danielsen E, Farrow N, Tan J. In vivo MR spectroscopy of human dementia. Neuroimaging Clin N Am. 1998;8:809–822. [PubMed] [Google Scholar]
- 103.Duyckaerts C, Hauw JJ. Prevalence, incidence and duration of Braak’s stages in the general population: can we know? Neurobiol Aging. 1997;18:362–369. doi: 10.1016/s0197-4580(97)00047-x. 89–92. [DOI] [PubMed] [Google Scholar]
- 104.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 105.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci USA. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scarmeas N, Habeck CG, Stern Y, Anderson KE. APOE genotype and cerebral blood flow in healthy young individuals. JAMA. 2003;290:1581–1582. doi: 10.1001/jama.290.12.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ghebremedhin E, Schultz C, Braak E, Braak H. High frequency of apolipoprotein E ε4 allele in young individuals with very mild Alzheimer’s disease-related neurofibrillary changes. Exp Neurol. 1998;153:152–155. doi: 10.1006/exnr.1998.6860. [DOI] [PubMed] [Google Scholar]
- 108.Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roses AD. Apolipoprotein E affects the rate of Alzheimer disease expression: beta-amyloid burden is a secondary consequence dependent on APOE genotype and duration of disease. J Neuropathol Exp Neurol. 1994;53:429–437. doi: 10.1097/00005072-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 110.Drzezga A, Grimmer T, Henriksen G, et al. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology. 2009;72:1487–1494. doi: 10.1212/WNL.0b013e3181a2e8d0. [DOI] [PubMed] [Google Scholar]
- 111.Harold D, Abraham R, Hollingworth P, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lambert JC, Heath S, Even G, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 113.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sperling RA, Laviolette PS, O’Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buckner RL, Sepulcre J, Talukdar T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]