The Reaction between Nitric Oxide, Glutathione and Oxygen in the Presence and Absence of Protein: How are S-Nitrosothiols Formed? (original) (raw)
. Author manuscript; available in PMC: 2011 Jan 1.
Abstract
The reaction between NO, thiols and oxygen has been studied in some detail in vitro due to its perceived importance in the mechanism of NO-dependent signal transduction. The formation of S-nitrosothiols and thiol disulfides from this chemistry has been suggested to be an important component of the biological chemistry of NO, and such subsequent thiol modifications may result in changes in cellular function and phenotype. In this study we have re-investigated this reaction using both experiment and simulation and conclude that: (i) S-Nitrosation through radical and non-radical pathways is occurring simultaneously (ii) S-Nitrosation through direct addition of NO to thiol does not occur to any meaningful extent and (iii) protein hydrophobic environments do not catalyze or enhance S-nitrosation of either themselves or of glutathione. We conclude that S-nitrosation and disulfide formation in this system occur only after the initial reaction between NO and oxygen to form nitrogen dioxide, and that hydrophobic protein environments are unlikely to play any role in enhancing and targeting S-nitrosothiol formation.
Keywords: Nitric oxide, glutathione, _S_-nitrosoglutathione, _S_-nitrosothiols, mechanisms
Introduction
The mechanism of formation of S-nitrosothiols (RSNO) in vivo is an important factor in understanding the biological actions of NO. RSNO have been implicated in the regulation of many cellular processes, including apoptosis [1], cell proliferation [2], and hypoxic vascular responsiveness [3]. While there are some reports of enzymatic involvement in S-nitrosothiol formation [4,5], thiol nitrosation is largely thought to be driven through the intrinsic biological chemistry of NO.
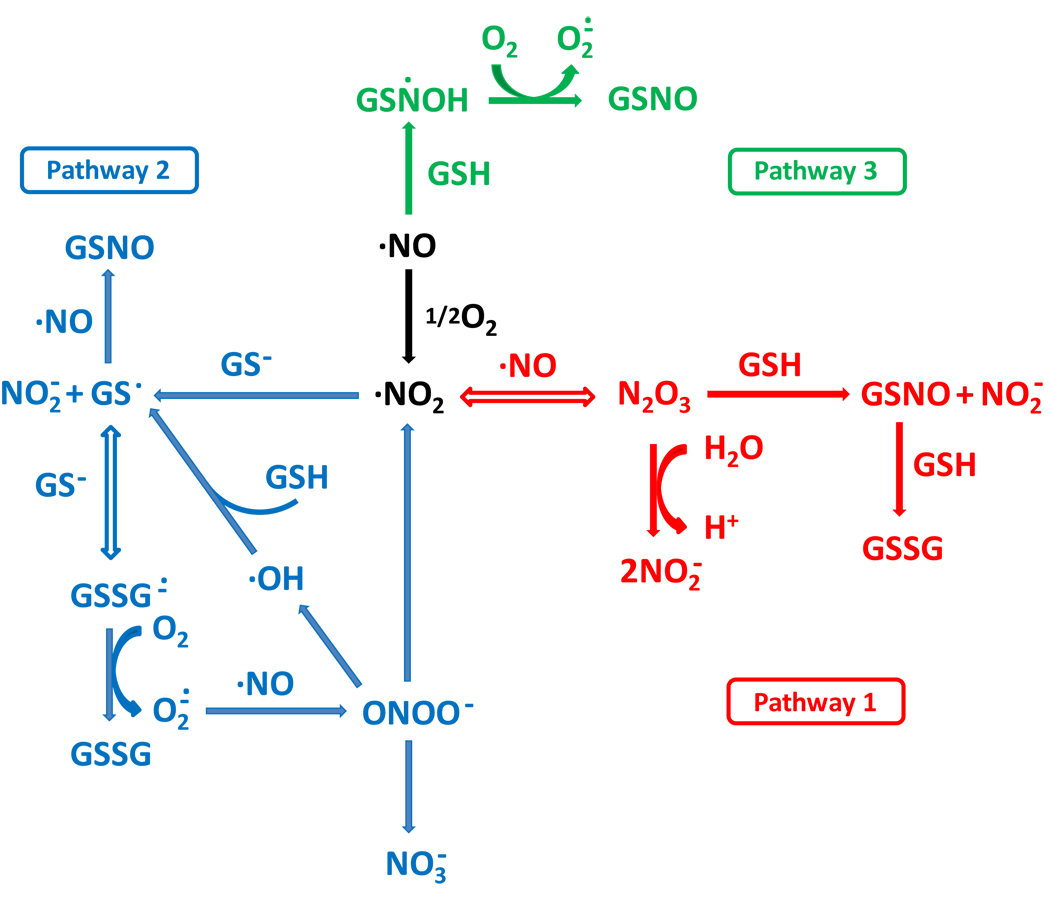
Three mechanisms have been proposed for the formation of S-nitrosothiols, in vitro, from a combination of nitric oxide, thiol and oxygen (the three pathways are illustrated in Scheme 1). The first (pathway 1 in scheme 1) involves the formation of an intermediary nitrosating agent, N2O3, from the reaction between NO and oxygen [6,7]. The second (pathway 2 in Scheme 1) involves the one electron oxidation of the thiol by NO2 followed by radical-radical combination of the thiyl radical and nitric oxide [7–9]. The third (pathway 3 in Scheme 1) involves the reduction of oxygen by a putative intermediate radical (RSNOH) formed from the direct combination of nitric oxide and thiol [10]. Experimental evidence exists in the literature for all three mechanisms based on kinetic, stoichiometric and scavenger studies. Both pathway 1 and pathway 3 are relatively simple with clearly defined stoichiometries, whereas pathway 2 is more complex due to the fact that the formation of thiyl radical as an intermediate opens up a plethora of additional reaction possibilities.
Scheme 1. Proposed pathways of the reaction between GSH, NO, and oxygen.
Pathways 1, 2 and 3 are indicated in red, blue and green respectively.
An additional factor that has been proposed to be involved in S-nitrosothiol synthesis is the presence of hydrophobic microenvironments [3]. As both NO and oxygen are hydrophobic it has been demonstrated that hydrophobic environments can enhance the rate of reaction between these species due to increases in their local concentration [11]. It has been suggested that this effect may enhance S-nitrosation in the vicinity of hydrophobic environments [12]. In extremity, it has been reported that NO oxidation and nitrosation can be localized internally to a single protein [13,14], such that the interaction of NO with a specific protein molecule can S-nitrosate a thiol within that same protein. Although this mechanism would allow for a very directed mechanism of S-nitrosthiol formation and NO signaling, it would requires significant curtailment of normal diffusivity of gas molecules, and also unprecedented stabilization of reaction intermediates.
In this study we have performed a series of experiments and kinetic simulations to attempt to differentiate between these various mechanisms of GSNO formation using glutathione (GSH) as the target thiol and to assess the importance of hydrophobic protein environements to the nitrosation process. We conclude that both thiyl-radical and N2O3-medaited nitrosation (pathways 1 and 2) occur in this system and that pathway 3 has negligible importance. In addition we show that thiyl radical scavengers have little effect on the formation of GSNO, but instead switch the mechanism of GSNO formation from pathway 2 to pathway 1. In addition the use of phosphate to inhibit pathway 1 also inhibits pathway 2. Consequently in such complex reaction networks, the use of inhibitors to probe specific pathways can affect more than one pathway. In addition we have re-examined the ability of hydrophobic environments to enhance S-nitrosothiol formation and conclude that proteins inhibit rather than enhance S-nitrosation. These results clarify potential mechanisms of S-nitrosothiol formation in biological systems.
Materials and Methods
Materials
1-[2-(Carboxylato)pyrrolidin-1-yl]diazen-1-ium-1,2diolate (PROLI/NO), (Z)-1-[N-(3-Ammoniopropyl)-N-[4-(3-aminopropylammonio) butyl]-amino]diazen-1-ium-1,2-diolate (SPER/NO) and 2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1H-imidazollyl-1-oxy-3-oxide (CPTIO) were purchased from Cayman Chemical Co., NO gas was obtained from Matheson Gas Co. Other materials were Sigma-Aldrich products unless otherwise noted.
Preparation of NO solution
NO gas, passed through a 1M NaOH solution to remove contaminating nitrogen oxides, was introduced into a degassed buffer solution (50mM potassium phosphate, 1mM DTPA, pH 7.4), which had been deoxygenated by vacuum and refilled with argon five times. The concentration of NO in solution was determined as previously described [15].
EPR spin-trapping
DMPO (100 mM) was added into the reaction mixture of GSH, NO or SPER/NO, and oxygen. The EPR spectrum was recorded immediately at room temperature with a Bruker EMX EPR spectrometer operating at 9.85 GHz microwave frequency, 10 mW microwave power, 100 kHz modulation frequency, and 1.0 G modulation amplitude.
S-Nitrosothiol detection using ozone-based chemiluminescence
_S_-nitrosothiol formation from the reaction of GSH and NO donors was determined by I3-dependent ozone-based chemiluminescence using a Sievers Model 280 NO analyzer [16–18]. Prior to injection, samples were incubated with excess sulfanilamide/2N HCl solution in order to remove nitrite. S-Nitrosothiol levels were quantified using a standard curve generated from known amounts of GSNO.
HPLC analysis
For the specific measurement of GSNO, samples (50µl), pretreated with access of NEM and acidified with 2% TFA were injected onto a 25 cm Kromasil C-18 column (0.46 mm ID and 0.5 µM particle size) and eluted with a mobile phase consisting of methanol and 0.05% TFA (6:94). The effluent was monitored by a diode array spectrophotometer at 336 nm [19]. For determination of GSH, nitrite, GSSG, nitrate, and GSNO in a single run, an ion-pair method using tetrabutylammonium hydrogensulfate (TBAHS) was performed [8]. Samples were injected onto a 25 cm Kromasil C-18 column (0.46mm ID and 0.5µM particle size) and eluted isocratically with 10mM K2HPO4 and 10mM TBAHS in acetonitrile:water (5–95 v/v%, pH 7.0). Effluents were detected at 210 nm with a diode array spectrophotometer.
Measurement of oxygen and NO consumption by polarography
Oxygen and NO consumption were measured respectively by an oxygen electrode (Yellow Spring Instrument Co.) or an NO electrode (World Precision Instrument), maintained at 37°C by a circulating water bath.
Protein experiments
For kinetic experiments, SPER/NO (200 µM) was introduced into the mixture of GSH (1mM) and BSA or HSA (400µM of either) in 50mM phosphate buffer containing 1 mM DTPA, pH 7.4, at room temperature. For GSH-dependence and protein-dependence experiments PROLI/NO (50 µM) was injected into the mixture at the indicated concentrations of GSH and BSA or HSA. Aliquots were removed and quenched with NEM (30 mM) in order to block all non-reacted thiols. The formed GSNO and total RSNO were quantified with HPLC, and ozone-based chemiluminescence, respectively.
BSA and HSA were used without further reduction or purification and contain approximately 0.5 thiol groups per protein. For certain experiments free thiol were blocked with excess of NEM (1 hour incubation at room temperature), then the protein was separated on G-25 size exclusion column.
Anaerobic experiments
Anaerobic experiments were performed using a Coy anaerobic chamber. All solutions were deoxygenated with alternating vacuum assisted removal of oxygen and introduction of argon gas prior to introduction into the anaerobic chamber.
Kinetic simulation
Kinetic simulations were performed using Gepasi 3.30, a freely downloadable program at www.gepasi.org [20]. The kinetic model was fitted to experimental data using the Simplex algorithm.
Statistics
Statistical analysis of data was made with a Student’s t test. Changes were considered statistically significant when P<0.05.
Results
Nitric Oxide and Oxygen Consumption in the presence of GSH
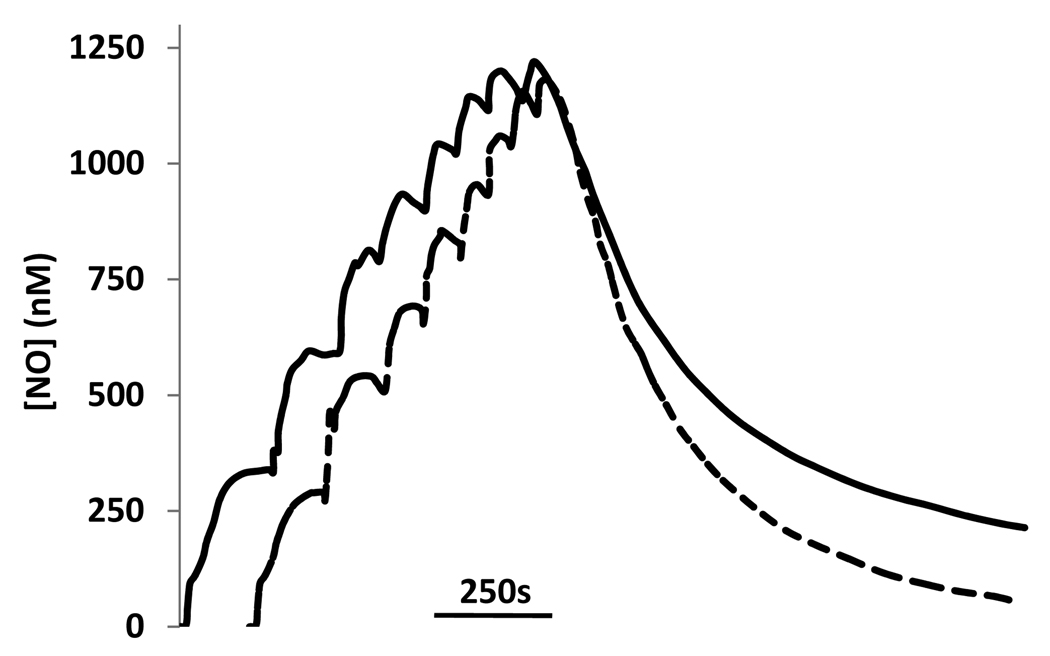
According to pathway 1 (in Scheme 1), the presence of GSH should not increase the rate of NO consumption because the reaction is rate-limited by the NO/oxygen reaction and the stoichiometry of NO consumption is identical for S-nitrosation and hydrolysis of N2O3. Although pathway 2 is also rate-limited by the NO/oxygen reaction, the stiochiometry of NO consumption is potentially affected by GSH due to potential radical-mediated chain reactions. In pathway 3 there is a direct effect of GSH on NO consumption. It has previously been shown that cysteine causes an enhanced oxygen-dependent NO consumption [10]. We examined this, using GSH rather than cysteine, by adding aliquots of NO solution into oxygenated buffer (50 mM phosphate containing 1 mM DTPA at 37 °C) in the presence and absence of GSH (Figure 1). In this experiment, eight sequential additions of nitric oxide were made, each calculated to give a final concentration of 350 nM. After the eighth addition, the accumulated nitric oxide was allowed to decay. As can be seen, the presence of GSH affected the electrode response in two distinct ways. In the presence of GSH, the maximum level of nitric oxide attained after addition was lower (although this effect was minimized at higher total NO concentrations), and the decay rate after the last addition was faster (particularly at low NO concentrations), when compared to an identical experiment in the absence of GSH. This is in qualitative agreement with Gow et al [10], who used cysteine, though cysteine appears to have a much larger effect than GSH. These data illustrate that NO-consuming reactions, over and above the reaction of NO with oxygen, occur in this reaction system and indicates that pathway 1 cannot be occurring in isolation.
Figure 1. The effect of GSH on NO consumption.
NO gas was sequentially added into a buffer solution with (dotted line) or without (solid line) GSH (1mM) at 37°C. Each addition was calculated to give a final concentration of 350nM of NO. The NO level was recorded by an NO electrode.
In an attempt to understand these additional reactions, we examined the stoichiometry of oxygen consumption upon addition of NO to a solution of either oxygen alone, or oxygen and GSH. Table 1 shows the amount of oxygen consumed upon sequential titration of oxygenated phosphate buffer (50 mM containing 1 mM DTPA, at 37 °C) with NO under various conditions. The stoichiometry of oxygen consumption by NO was calculated to be approximately 4.3:1 (NO:O2), close to the 4:1 ratio accepted for this reaction [21]. In the presence of GSH, however, this stoichiometry was closer to 3:1. This indicates that 25 % more oxygen is consumed in the presence of GSH than in its absence. To examine if the oxidation of NO to nitrogen dioxide resulted in the additional consumption of oxygen, we studied the effect of the NO oxidant, CPTIO. This compound has been shown to donate an oxygen atom to NO to generate nitrogen dioxide with a rate constant of 6000 M−1s−1[22,23]. As shown in Table 1, addition of NO to a solution of CPTIO completely inhibits the consumption of oxygen, indicating that the concentration of CPTIO used was sufficient to compete with the oxidation of NO by oxygen. However, in the presence of GSH and CPTIO oxygen was consumed with a stoichiometry of 5.5:1 (NO:O2). It should be noted that CPTIO also scavenges nitrogen dioxide with a rate constant of similar magnitude to the reaction of nitrogen dioxide with GSH [24]. It is likely that some of the NO2 generated is thus scavenged by remaining CPTIO leading to an underestimate of the NO2-dependent contribution to oxygen consumption. Regardless, these data indicate that additional oxygen-consuming reactions can occur, down-stream of the initial reaction of NO with oxygen, when NO autoxidation occurs in the presence of GSH, and that these reactions are likely due to the formation of NO2. These data support pathway 2 as an alternative to pathway 1 as a potential mechanism of thiol S-nitrosation.
Table 1.
The effect of GSH on the consumption of oxygen.
| Conditions | NO added(nmol) | O2 consumed(nmol) | NO/O2 |
|---|---|---|---|
| NO | 267 | 62.3 ± 3.0 (4) | 4.32 ± 0.21 |
| NO + GSH | 267 | 81.3 ± 2.4 (4) | 3.25 ± 0.05 |
| NO + CPTIO | 267 | 2.3 ± 3.3 (4) | 0 |
| NO + GSH + CPTIO | 267 | 48.6 ± 4.8 (4) | 5.54 ± 0.55 |
Formation of glutathionyl radicals from GSH during NO autoxidation
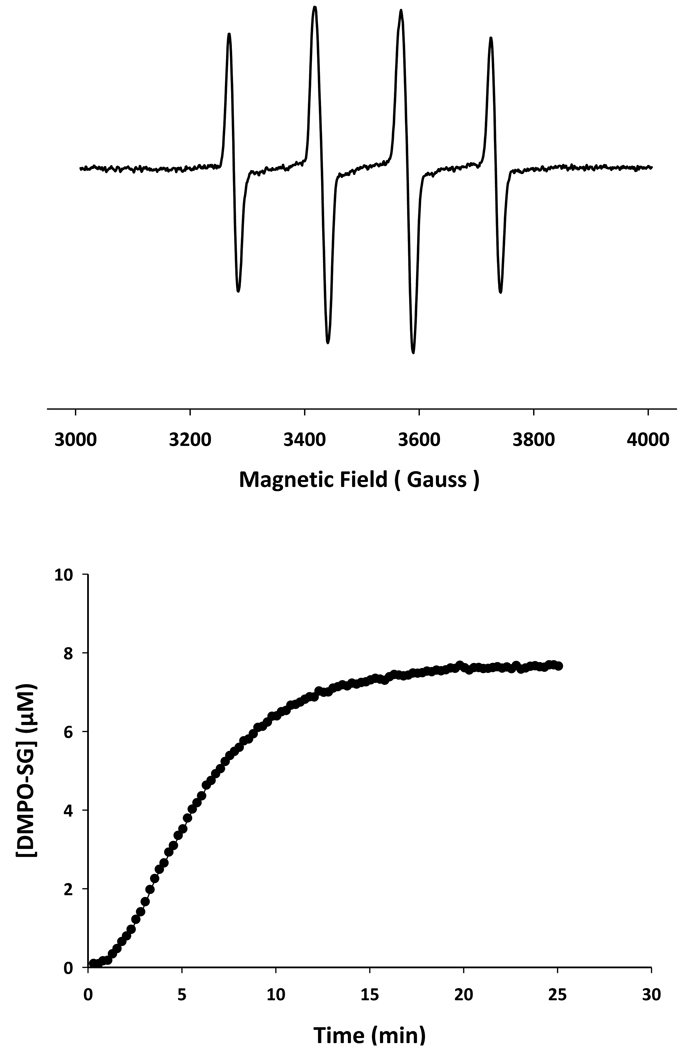
The most likely mechanism NO2-dependent oxygen consumption is the intermediate formation of glutathionyl radical, from the reaction between NO2 and GSH, which can then consume oxygen by a number of pathways. To examine the formation of thiyl radicals we used the EPR spin-trapping technique using DMPO as the spin trap. Both bolus addition of NO (Figure 2A) and slow generation of NO (Figure 2B), in the presence of GSH, resulted in the formation of the DMPO-SG adduct. In the case of slow nitric oxide generation, the formation of DMPO-SG exhibited a clear sigmoidal shape, suggesting an acceleration of NO2 formation during the initial period until the concentration of nitric oxide achieves steady-state. These data indicate that thiyl radicals are formed in the NO/oxygen/glutathione reaction system and are in agreement with Pou and Rosen [25] and Schrammel et al [9].
Figure 2. Reaction of NO and GSH in the presence of DMPO. Upper panel.
The EPR spectrum recorded after mixing GSH (5mM), DMPO (100mM) and NO (200µM). Lower panel: Formation of DMPO-SG adduct in the reaction of GSH (1mM) and SPER/NO (200µM) and DMPO (100mM).
Thiol oxidation vs. nitrosation by nitric oxide
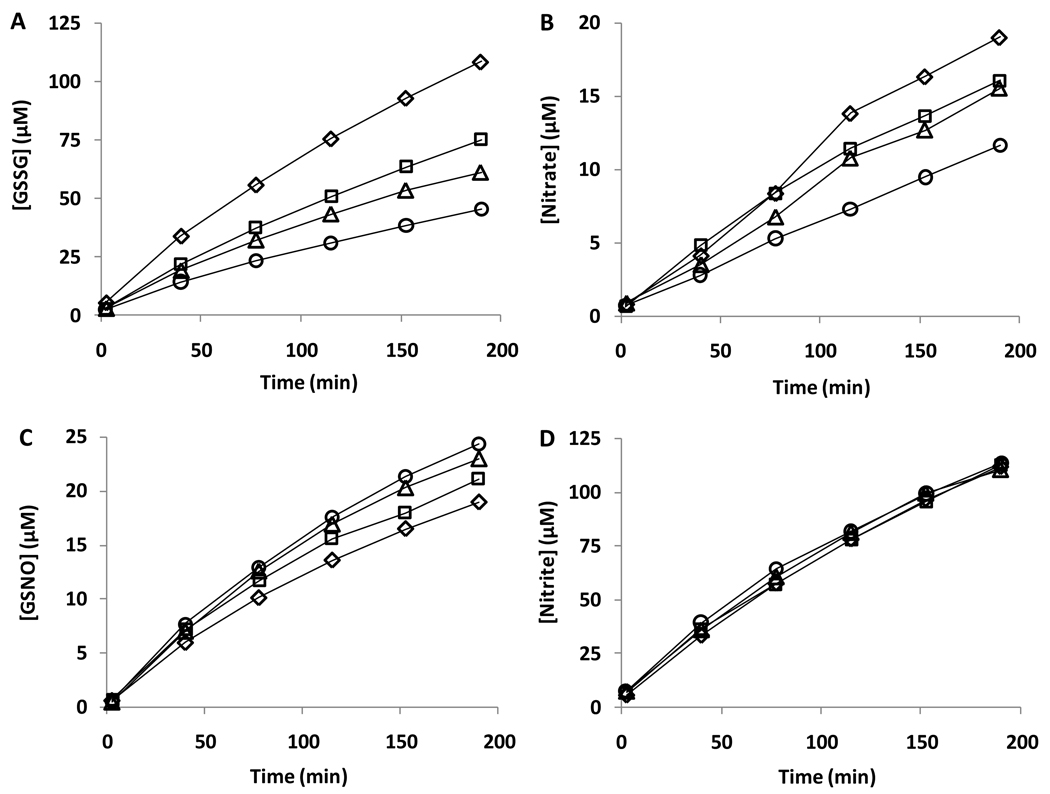
We have previously observed that DMPO did not affect the formation of GSNO in the reaction mixture of SPER/NO and GSH [26]. Jourdeuil et al [8] demonstrated that the release of NO into an oxygenated solution of GSH formed GSSG as the major product, and GSNO was formed at significantly lower levels. However, in contradiction to our earlier study [26], they also demonstrated that the formation of GSNO could be inhibited by the thiyl radical trap, DMPO, thus providing evidence that thiyl radical formation represented a major pathway for GSNO formation (Pathway 2). Schrammel et al [9] had earlier arrived at a similar conclusion using TEMPOL as a radical scavenging agent. In contrast Koshiishi et al reported that DMPO inhibited GSSG formation but enhanced GSNO formation [27] suggesting thiyl radicals were not involved in S-nitrosothiol formation. Figure 3A–D shows the formation of GSNO, GSSG, nitrite and nitrate measured using the ion-pair HPLC method described by Jourdeuil et al. [8]. In agreement with these authors, we observe the formation of both GSSG and GSNO in this reaction system, and GSSG is by far the major product by a ratio of approximately 5:1. In addition, both nitrite and nitrate are formed, with nitrite in large excess of nitrate. However, the introduction of DMPO causes a concentration-dependent decrease in GSSG and nitrate formation but a slight increase in the rate of formation of GSNO. We have examined the effect of temperature (24 °C and 37 °C), nitric oxide release rate (SPER/NO vs DEA/NO) and GSH concentration and in all cases DMPO slightly increased GSNO formation (data not shown). This is in agreement with our previous observations [26] and with the recent study of Koshiishi et al [27].
Figure 3. The effect of DMPO on GSNO, GSSG, nitrite, and nitrate formation in the reaction of SPER/NO with GSH.
SPER/NO (200µM) was incubated with GSH (1mM) at room temperature in the presence of various concentrations of DMPO. The GSNO, glutathione disulfide (GSSG), nitrite and nitrate concentrations at different time points were determined by HPLC (TBAHS method). Diamonds, squares, triangles, and circles refer to 0, 50, 100, and 150mM DMPO, respectively.
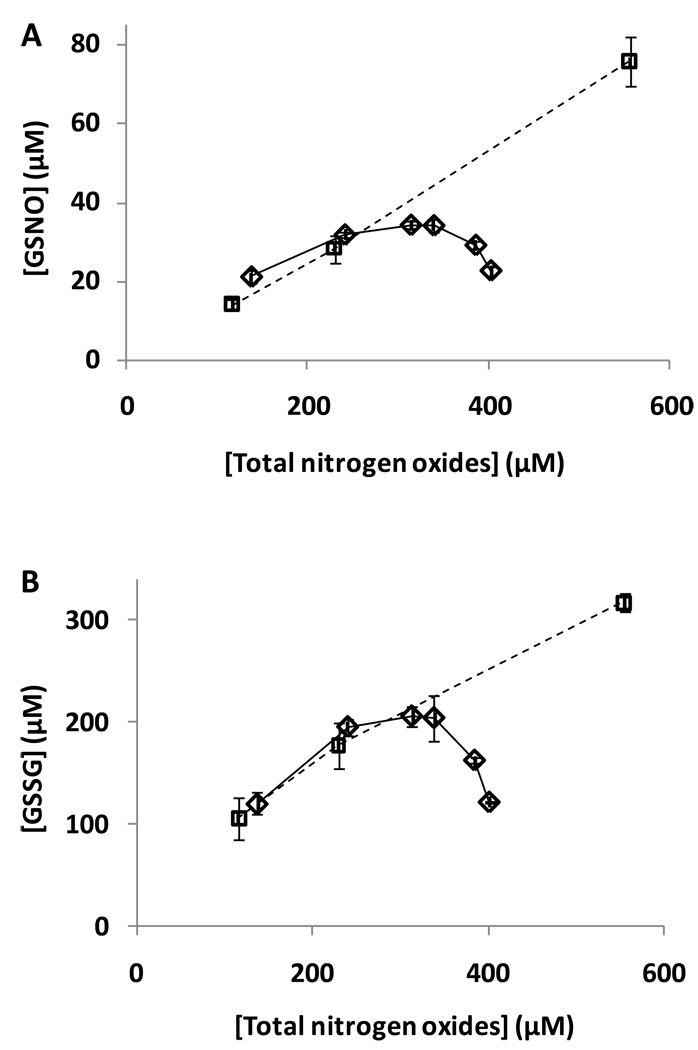
Our data, on the surface, appear to support a mechanism whereby GSSG is formed via thiyl radical mechanisms but GSNO is formed exclusively via the non-radical N2O3 formation (pathway 1 in scheme 1). To investigate this we examined the effect of phosphate concentration on the yield of GSNO and GSSG. Phosphate is known to accelerate N2O3 hydrolysis and therefore should diminish GSNO formation. Unfortunately the decay rate of SPER/NO is acutely sensitive to phosphate concentrations and so in order to examine the effect of phosphate independently of NO release rate, we conducted a series of experiments looking at the effect of NO release rate on the formation of GSSG and GSNO by using a range of concentrations of SPER/NO in 50 mM phosphate. As shown in Figure 4A., the formation of both GSSG and GSNO, as a function of the total nitrogen oxides formed (the sum of nitrite, nitrate and GSNO) was a relatively linear function of SPER/NO concentration at constant phosphate concentration. In the presence of increasing concentrations of phosphate, the amount of GSNO, as a function of total nitrogen oxides formed, decreased from linearity indicating that phosphate inhibited the formation of GSNO. Surprisingly, however, phosphate also decreased the formation of GSSG (Figure 4B). This indicates that phosphate inhibits not only the N2O3-mediated nitrosation, but also thiyl radical-mediated pathways. Consequently, phosphate cannot be used as a diagnostic agent to distinguish between pathways 1 and 2 in this system.
Figure 4. The effect of phosphate on GSNO and GSSG formation.
SPER/NO was incubated with GSH (1mM) at room temperature for 190 minutes. Various concentrations of SPER/NO (Squares: 100, 200, 500 µM) were used were incubated in 50 mM phosphate buffer containing 1 mM DTPA, pH 7.4, and HPLC analysis of the products was performed. SPER/NO (200 µM) was incubated in , various concentrations of phosphate (Diamonds: 10, 50, 100, 150, 250, or 500mM) containing 1 mM DTPA, pH 7.4, and HPLC analysis of the products was performed. The GSNO and GSSG concentrations at different time points were determined by HPLC (ion-pair method) and plotted vs the total released nitrogen oxide content (the sum of nitrite, nitrate and GSNO). Data represent mean ± SD (n = 3).
Anaerobic formation of GSNO from GSH and NO in the presence of NAD+
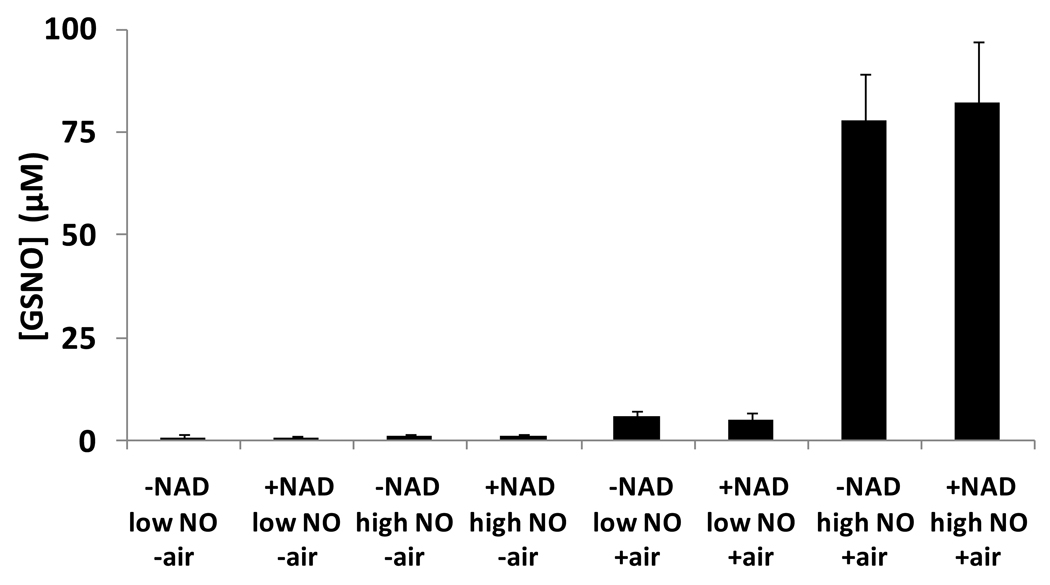
To investigate pathway 3, we examined the direct reaction between GSH and NO under anaerobic conditions in the presence of an electron acceptor NAD+. Gow et al [10] indicated that NAD+ could act as an electron acceptor to oxidize the hydroxylamino radical to the S-nitrosothiol, although only indirect methods were used to detect S-nitrosothiol formation. We reexamined this reaction by incubating GSH (750 µM) in phosphate buffer (50 mM, containing 100 µM DTPA, pH 7.4) deoxygenated with alternating vacuum assisted removal of oxygen and introduction of argon gas, and then equilibrated in an anaerobic chamber for an hour, where PROLI/NO (40 or 400µM, half-life of 1.8s) was added from a deoxygenated stock solution. The mixture was incubated for 10 minutes, and quenched with NEM (50 mM) in order to block non-reacted thiol. GSNO was detected using the ozone-based chemiluminescence method (see methods section). For comparison, separate control experiments were carried out in air. All results are summarized in Figure 5. It can be seen that S-nitrosation was much more efficient in the presence of air, where about 10% of liberated NO was converted into GSNO. However, much lower levels of GSNO were detected when oxygen was not present and there was no statistically significant variation among the levels of GSNO whether 40 or 400µM NO-donor was used, or if NAD+ was present or absent. Our results suggest that the NAD+-assisted direct formation of GSNO from NO and GSH (Pathway 3) represents a negligible pathway of GSNO formation.
Figure 5. Nitrosation of GSH with PROLI/NO in the presence of NAD.
GSNO generated in the interaction of 750µM GSH with 40 and 400µM PROLI/NO (referred as low NO and high NO, respectively), under anaerobic conditions, and in the presence of air. Values are means±SD (n=3). No significant differences were found between the pairs of experiments with and without NAD+. (P = 0.13, 0.16, 0.73, 0.78, respectively from left to right.)
Kinetic Simulations
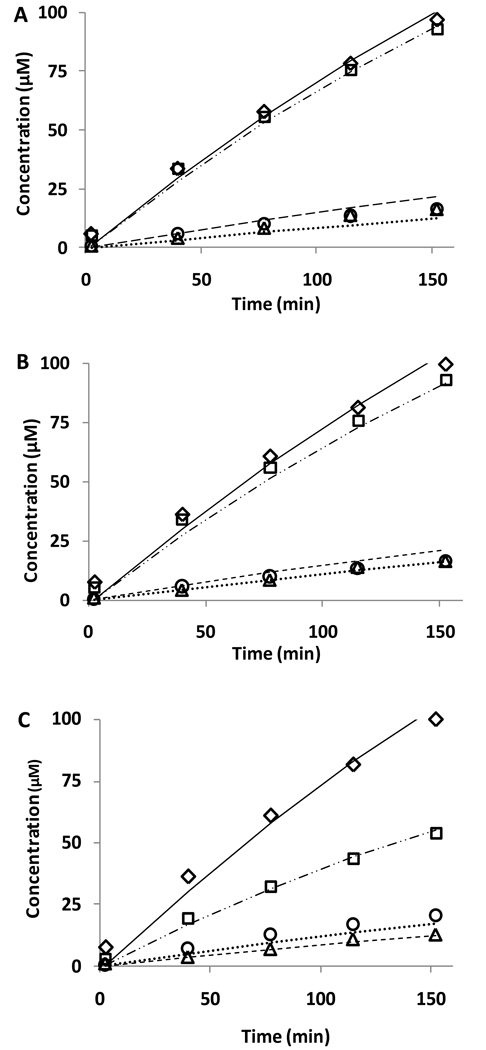
In total, the above experiments indicate that both pathway 1 and pathway 2 are operative in this reaction mixture, but also indicate that the complexities of this reaction system are such that interventions using scavengers have the potential to misdiagnose which pathways are involved. Therefore we have chosen to model this system by obtaining a global numerical kinetic solution to the differential equations derived from the reaction mechanism shown in scheme 1. The rate constants and equations used in the global simulation are shown in table 2 and give excellent agreement with the data (Figure 6). Using this model, the basic qualities of this reaction system can be understood and are as follows:
- The major reactions that occur are oxidative and not nitrosative. The major products are GSSG and nitrite with GSNO and nitrate being minor products. This conclusion was also drawn by Lancaster using a more complex kinetic scheme [28]
- If we increase the rate constants of the reaction for pathway 3 so that these reactions influence the rate of GSNO formation, the model is no longer able to fit the experimental data in terms of yields of nitrite, nitrate and GSSG.
- S-Nitrosothiols are formed by both radical and non-radical mechanisms. The importance of each pathway is dictated by the steady-state concentration of thiyl radical, but surprisingly, this has little effect on the total amount of RSNO formed.
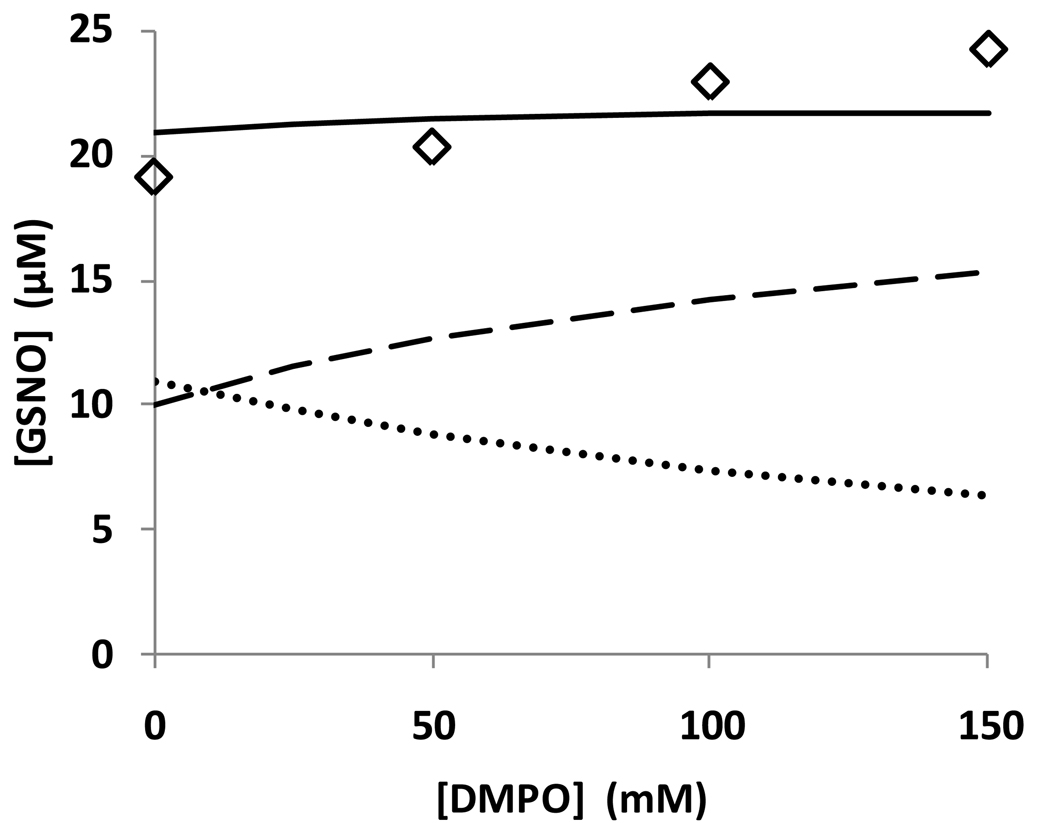
- The reversibility of the formation of N2O3 from NO and NO2 means that any intervention in one pathway of RSNO formation will have an effect on the other pathway. For example, if we simulate the presence of high phosphate by increasing the N2O3 hydrolysis rate, GSNO formation is reduced, but the rate of thiyl radical formation (and consequently GSSG formation) is also decreased due to the decrease in steady-state concentration of NO2. Conversely, if we simulate the trapping of thiyl radicals with DMPO, this has the effect of removing downstream reactions that scavenge NO, thus increasing the steady-state level of NO and favoring N2O3-mediated S-nitrosation. The switch between the N2O3-mediated S-nitrosation and thiyl radical-mediated S-nitrosation as a function of DMPO concentration is shown in Figure 7.
Table 2.
Rate constants used for kinetic simulations.
| # | Reaction | Published RateConstant | Ref | Rate constantused insimulations (ifdifferent) |
|---|---|---|---|---|
| 1 | Sper/NO → 2NO | 5 × 10−5 s−1 | a | 4.62 × 10−5 s−1 |
| 2 | 2NO + O2 → 2NO2 | 2 × 106 M−2 s−1 | [21] | |
| 3 | NO + NO2 ⇌ N2O3 | 1.1 × 109 M−1 s−1 | [29] | |
| 8.1 × 104 s−1 | [29] | |||
| 4 | N2O3 + GSH → GSNO(1) + HNO2 | 6.6 × 107 M−1 s−1 | [36] | 7 × 108 M−1 s−1 |
| 5 | N2O3→2HNO2 | 4.75 × 104 s−1 | [7]b | |
| 6 | NO2 + GSH → GS· + HNO2 | 2.2 × 107 M−1 s−1 | [37] | |
| 7 | GS· + NO → GSNO(2) | 3 × 109 M−1 s−1 | [38] | |
| 8 | GS· + GS· → GSSG | 1 × 109 M−1 s−1 | [38] | |
| 9 | GS· + GSH ⇌ GSSG·− | 7 × 107 M−1 s−1 | [39] | |
| 2.3 × 105 s−1 | [39] | |||
| 10 | GSSG·− + O2 → GSSG + O2− | 5 × 109 M−1 s−1 | [40] | |
| 11 | NO + O2−→ ONOO− | 1.9 × 1010 M−1 s−1 | [41] | |
| 12 | ONOO− +GSH →GSOH+HNO2 | 660 M−1 s−1 | [42] | |
| 13 | GSOH+GSH →GSSG | 1 × 105 M−1 s−1 | estimate | |
| 14 | ONOO− →OH· + NO2 | 0.35 s−1 | [43] | |
| 15 | OH· + GSH →GS· | 1 × 109 M−1 s−1 | fast | |
| 16 | ONOO− →NO3− | 0.9 s−1 | [43] | |
| 17 | OH· + NO →HNO2 | 1 × 109 M−1 s−1 | fast | |
| 18 | GS· + DMPO →GSDMPO | 6.4 × 105 M−1 | ||
| s−1 | ||||
| 19 | NO + GSH ⇌ GSNOH | 10 M−1 s−1 | ||
| 1000 s−1 | ||||
| 20 | GSNOH+O2 →GSNO(3) + O2− | 1 × 10−3 M−1 s−1 |
Figure 6. Kinetic simulation of the reaction between NO, GSH, and oxygen.
The model described in table 2 was simulated and fitted to a selection of the data shown in Figure 3. A: Fit with published rate constants. B: Fit with adjusted rate constants k4 (see table 2). C: Effect of DMPO (100 mM) using adjusted rate constant for reaction 4 (table 2). Diamonds, squares, circles, and triangles refer to nitrite, GSSG, GSNO, and nitrate, respectively.
Figure 7. Kinetic simulation of the different reaction routes of the reaction between NO, GSH, and oxygen.
Simulated GSNO yields from (i) dashed line, N2O3 pathway (pathway 1 in scheme 1); (ii) dotted line, thiyl radical pathway (pathway 2 in scheme 1); (iii) bold line, total GSNO formation; as a function of DMPO concentration. diamonds: experimental data from Figure 3C.
Reaction of GSH with NO in the presence of protein
It was established earlier [11] that the reaction between NO and oxygen occurs preferentially in hydrophobic sub-environments within aqueous solution due to the selective partitioning of both NO and oxygen in hydrophobic phases. This has led to the idea that the hydrophobic interior of proteins represents a locus of enhanced S-nitrosothiol formation [3]. Indeed serum albumin has been reported to be an avid catalyst of both protein and glutathione S-nitrosation [13,14].
To investigate potential autocatalysis of protein nitrosation by both HSA and BSA, DEA/NO (half-life of 2 min) was incubated with either cysteine, GSH, HSA or BSA (50 µM in each case in 50mM phosphate buffer/1mM DTPA pH 7.4) and absorbance changes recorded at 336 nm, indicative of the formation of an S-nitrosothiol. In contrast to Rafikova et al [14], no difference was observed between the kinetics of _S_-nitrosation of amino acid, peptide, or protein (Figure S1, supplementary data).
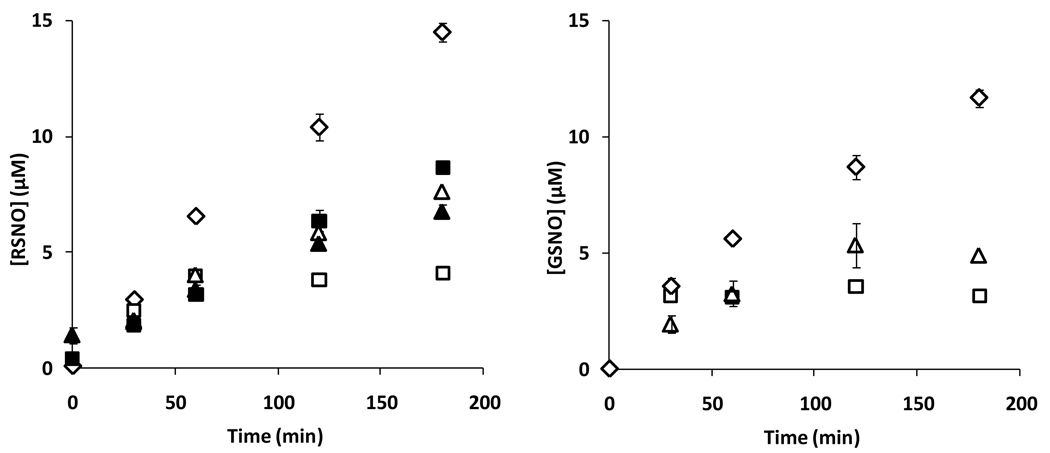
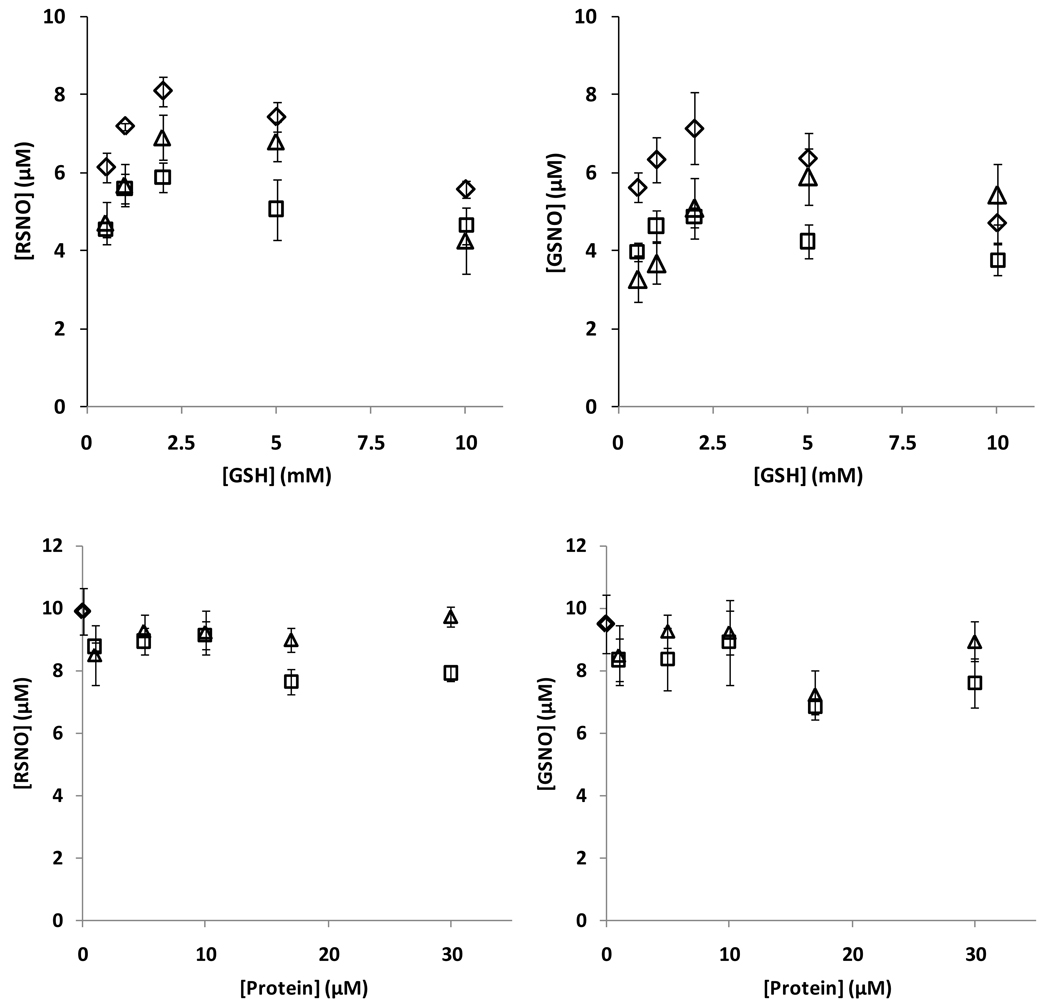
To further examine if albumin could influence the kinetics of GSNO formation from GSH and NO, we examined both overall RSNO content (using ozone-based chemiluminescence), and also GSNO formation by HPLC (TFA method) during incubations of albumin, GSH and SPER/NO. Figure 8 shows that the presence of both BSA and HSA decreased the total amount of RSNO detected. Pre-treatment of the proteins with NEM did not greatly affect the outcome. Although NEM-treated BSA did appear to support more RSNO formation than untreated BSA, this level was still significantly less that when GSH was used alone. In a separate control experiment it was determined that by the time of analysis the NO donor had completely decomposed, and did not yield an artificial peak during chemiluminescence detection (not shown). Addition of HgCl2 to the samples consistently abolished the chemiluminescence signal, indicating the exclusively formation of _S_-nitrosothiols (data not shown). The results in Figure 8 left panel were recapitulated when GSNO was examined directly (Figure 8 right panel), with both proteins inhibiting GSNO formation. The effect of both protein and glutathione concentration are shown in Figure 9, measuring either total RSNO or GSNO. In no case was a protein-dependent increase in GSNO yield observed. No protein covalent cross-linking or fragmentation was observed by reducing SDS-PAGE during incubation in these conditions (data not shown).
Figure 8. Nitrosation of GSH with SPER/NO in the presence of protein.
Kinetics of S-nitrosothiol formation from GSH (1mM) and SPER/NO (200µM) with and without addition of 400µM BSA, and HSA. Left panel: total RSNO measured by chemiluminescence. Right panel: GSNO measured with HPLC. Symbols: ◊: no protein, □: with BSA, ∆: with HSA, ■: with BSA without GSH, ▲: with HSA, without GSH. Values are means±SD (N=3).
Figure 9. Nitrosation of GSH with PROLI/NO.
Upper panel: levels of RSNO (left), and GSNO (right) as a function of GSH concentration with 17µM protein and 50µM PROLI/NO. Lower panel: levels of RSNO (left), and GSNO (right) as a function of protein concentration with 2mM GSH and 50µM PROLI/NO. Symbols: ◊: no protein, □: with BSA, ∆: with HSA. Values are means±SD (N=3).
Discussion
Kinetic Considerations
The formation of GSNO from the aerobic reaction of nitric oxide with oxygen has been shown to be second order in nitric oxide and first order in oxygen [7]. On the assumption that the initial reaction between NO and oxygen is the radical-radical combination of these two radicals, Czapski et al [29] analyzed the reaction between NO, oxygen and GSH according to the Equations 1 to 4. From this reaction sequence, if
steady-state approximations are made for the concentrations of ONOO•, •NO2 and N2O3, the following expression can be derived:
d[GSNO]dt=2k1k2[NO]2[O2]k2[NO]+k−1
This mechanism has some interesting features. Firstly, the stoichiometry of the reaction is 1 O2: 4 NO: 2 GSNO. Additionally, if k−1 >> k2[NO] (as is likely) the rate of GSNO formation can be approximated to:
d[GSNO]dt=2kα[NO]2[O2]wherekα=k1k2k−1
Consequently the rate of formation of RSNO has a first order dependence on the concentration of oxygen, and a second order dependence on NO concentration. Wink et al [6], Czapski et al [7] and Kharitonov et al [30] have confirmed this by experiment.
In addition to this mechanism it has been proposed that a competing pathway of nitrosation may involve the direct oxidation of GSH by nitrogen dioxide to form glutathionyl radical, and the subsequent addition of NO to this radical (Equations 1, 2 5 and 6). As written, this mechanism is stoichiometrically and kinetically indistinguishable
from the one described above, as it also is limited by the reaction between NO and oxygen and has a theoretical stoichiometery of 1 O2: 4 NO: 2 GSNO. However, this reaction involves a thiyl radical intermediate that may have many other competing reactions, including reaction with thiol, oxygen and RSNO itself, all of which will alter the reaction stoichiometry and are discussed in detail below.
Gow et al [10] proposed an alternative mechanism for the formation of RSNO from NO, oxygen and thiols. Their study exclusively examined the reaction of NO with cysteine, but extrapolated their findings to other S-nitrosothiols. This mechanism (illustrated using GSH) envisaged the direct reaction between NO and the thiol to generate a putative free radical intermediate (Equation 7) that then reduces oxygen to
form superoxide and RSNO (Equation 8). The superoxide then reacts with NO to form peroxynitrite (Equation 9). The putative GSNOH radical intermediate has previously been proposed as an intermediate in the thiol-mediated reduction of NO under anaerobic conditions [31].
Assuming GSNOH and superoxide are at steady-state, it can be shown that:
d[GSNO]dt=k7k8[•NO][GSH][O2]k−7+k8[O2]
The stoichiometry of this mechanism is 1 O2 : 1 GSNO : 2 •NO and, if it assumed that k−7 >> k8[O2] this equation collapses to:
d[GSNO]dt=kβ[NO][O2][GSH]wherekβ=k7k8k−7
This reaction is first order in NO, oxygen and GSH. The study of Gow et al [10] in support of pathway 3 reported the inherent contradiction of a second order dependence on NO. If it assumed that all mechanisms are in operation, and ignoring downstream reactions of the glutathionyl radical, then the formation of GSNO is given by the sum of these two processes:
d[GSNO]dt=2kα[NO]2[O2]+kβ[NO][O2][GSH]
The value of kα has been determined to be 2 × 106 M−2s−1[7], in very close agreement to the known rate constant for the oxidation of nitric oxide by oxygen [21,32,33]. The value of kβ is unknown. However, it can be calculated that for kβ to be the major route of GSNO formation then:
As the inclusion of the Pathway 3 does not improve the fit to the data we conclude that at a steady state NO concentration of 5 µM (the approximate situation in the experiments presented here), kβ << 400 M−2s−1 (i.e. much less than 10% of the reaction follows this pathway). Experimentally, we also did not observe GSNO formation from the anaerobic reaction between NO, GSH and NAD+ as reported by Gow et al [10] for cysteine. It is possible that the pathway 3 could play a role at lower steady state levels of NO, however, without knowledge of the value of kβ this remains to be proven and can at this stage only be regarded as speculative.
The role of thiyl radicals
In agreement with previous investigators [9,25], we have demonstrated a robust formation of glutathionyl radicals during the oxidation of NO in the presence of GSH. It is likely that such radicals are formed from reaction of NO2 with GSH and are responsible for the additional consumption of both NO and oxygen that occurs downstream of the reaction between NO and oxygen. Both kinetic and product analyses indicate that thiyl radical formation is the predominant pathway in this reaction system and that the major product is glutathione disulfide formed from the down-stream reactions of the glutathionyl radical. This agrees with Jour’dheuil et al [8], and also with cell culture experiments showing that NO exposure, from either endogenous or exogenous sources, results in very inefficient S-nitrosation [18,34].
How is GSNO formed?
The two major mechanisms of S-nitrosothiol formation that have been proposed are 1) that N2O3 nitrosates thiols or 2) that NO nitrosates thiyl radicals. Our data indicate that attempting to dissect these mechanisms by using either scavengers of thiyl radicals or by enhancing N2O3 hydrolysis has complex effects on the reaction system. We show that increasing phosphate concentration to enhance N2O3 hydrolysis decreases both GSNO and GSSG levels. In addition, DMPO, used as a thiyl radical trap, inhibits the formation of GSSG but does not inhibit formation of GSNO – in fact it slightly increases GSNO formation. Kinetic simulation of the reaction network indicates that phosphate lowers GSSG formation by reducing the steady-state level of NO2 and thereby preventing GS· formation. The effect of DMPO is to inhibit all thiyl radical-mediated reactions, which leads to an increase in the steady-state NO level (DMPO will inhibit both NO consumption by GS· and that which occurs of from the downstream formation of superoxide), consequently enhancing N2O3 formation. Thus DMPO inhibits one pathway while enhancing the other pathway resulting in very little change in the rate of GSNO formation. The effect of DMPO on GSNO formation depends on the ratio of the rate constants for reactions 4 and 6 in table 2. Using published values [7] this ratio is about 3. If this ratio is increased by increasing the rate constant for reaction 4, , the result is to both increase the yield of GSNO and switch the effect of DMPO from slightly inhibiting to slightly enhancing the rate of GSNO formation. Our data and simulations suggest that the ratio of these two rate constants should be closer to ~15 at 22 °C. The inhibition of GSNO formation by TEMPOL and ascorbate observed by Schrammel et al [9] probably speaks to the fact that these agents could inhibit the process at multiple levels, scavenging both thiyl radicals and nitrosating intermediates.
Using a more complex simulated network of reactions, including reactions of tyrosine and differential fluxes of superoxide, Lancaster [28] concluded that S-nitrosation in vivo is a minor reaction that occurs almost exclusively via radical recombination (pathway 2 in scheme 3).
As expected (from the kinetic discussion above) if the reactions for Pathway 3 in Scheme 1 (Equations 7–9) were included, and rate constants were set significantly high to influence the formation of GSSG and GSNO, then the data and the simulation were no longer compatible (not shown). In other words, if this pathway was allowed to influence the simulation, then the simulation could no longer model the data. This further indicates that in the reaction system under study, pathway 3 is not playing a major role.
S-Nitrosation and hydrophobic protein environments
We demonstrate that protein does not catalyze _S_-nitrosation of either low molecular mass thiols or protein sulfhydryls. Instead, BSA and HSA hinder _S_-nitrosothiol formation in a concentration dependent manner. These findings are in stark disagreement with the findings of Rafikova et al [14], according to which NO diffuses into the hydrophobic core of the protein, then after reacting with oxygen, remains trapped as a long-lived nitrosating species (BSAN2O3, t1/2~10min). The inhibitory effect of protein on S-nitrosation can be explained by hypothesizing that hydrophobic environments enhance the rate of the NO/oxygen reaction [11], thus lowering the steady-state NO level. Our results suggest that any NO oxidation product formed in the hydrophobic interior of protein, does not convert easily into an S-nitrosothiol. We can conclude that _S_-nitrosation does not appear to be enhanced by the presence of protein. Recently Zhang et al [35] reported similar negative results for S-nitrosation of thiols in the membrane interior by NO/oxygen.
Conclusions
Both radical and non-radical mechanisms of GSNO formation are occurring in the simplified system studied here, and the presence of thiyl radical scavengers that do not yield down-stream superoxide formation results in a switch from one mechanism to the other. In the presence of cellular SOD it is possible that the effect of superoxide will be minimized and so the presence of any reaction that scavenges thiyl radicals will favor N2O3-mediated nitrosation over GS·-mediated nitrosation. Consequently in cells, that contain significant levels of SOD, GSH, other thiols, oxygen, and any other targets for GS· may serve to favor GSH nitrosation by N2O3. Conversely, any mechanism that could enhance thiyl radical formation, such as increased superoxide formation or the action of peroxidases, should favor nitrosation via thiyl radicals. Whether such process can actually increase the level of nitrosation, or rather just divert nitrosation from one mechanism to another, remains to be seen. We find no support for the notion that hydrophobic protein environments enhance S-nitrosation of protein or low molecular mass thiol. This does not rule out the likelyhood that some individual proteins contain cystiene residues in microenvironments that poise their chemical reactivity with nitrogen oxides such to enhance their ability to be S-nitrosated.
Supplementary Material
01
Acknowledgements
This work was supported by National Institute of Health Grants GM55792 and HL063119. EPR experiments were conducted in the National Biomedical EPR Center, Medical College of Wisconsin (Grant P41-EB001980).
Abbreviations
GSH
glutathione
GSNO
_S_-nitroso glutathione
GSSG
glutathione disulfide
RSNO
_S_-nitroso protein
SNO
nitrosothiol
DTPA
diethilenetriamine pentaacetic acid
TBAHS
tetrabutyl ammonium hydrogen sulfate
CPTIO
2-(4-carboxyphenyl)-4,5-dihydro-4,4,5,5-tetramethyl-1_H_-imidazollyl-1-oxy-3-oxide
DMPO
5,5-dimethylpyrroline-_N_-oxide
NEM
_N_-ethyl maleimide
SPER/NO
N-[4-(3-aminopropyl)-2-hydroxy-2-nitrosohydrazino]butyl]-1,3-propanediamine
PROLI/NO
proline nitric oxide
DEA/NO
1,1-diethyl-2-hydroxy-2-nitrosohydrazine
BSA
bovine serum albumin
HSA
human serum albumin
BSA-NEM
NEM-blocked BSA
HSA-NEM
NEM-blocked HSA
DTT
dithiothreitol
TFA
trifluoroacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Mannick JB, Schonhoff C, Papeta N, Ghafourifar P, Szibor M, Fang K, Gaston B. S-Nitrosylation of mitochondrial caspases. J. Cell Biol. 2001;154:1111–1116. doi: 10.1083/jcb.200104008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lander HM, Hajjar DP, Hempstead BL, Mizra UA, Chait BT, Campbell S, Quilliam LA. A molecular redox switch on p21ras. Structural basis for the nitric oxide-p21ras interaction. J. Biol. Chem. 1997;272:4323–4326. doi: 10.1074/jbc.272.7.4323. [DOI] [PubMed] [Google Scholar]
- 3.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 4.Inoue K, Akaike T, Miyamoto Y, Okamoto T, Sawa T, Otagiri M, Suzuki S, Yoshimura T, Maeda H. Nitrosothiol Formation Catalyzed by Ceruloplasmin. Implication for cytoprotective mechanism in vivo. J. Biol. Chem. 1999;274:27069–27075. doi: 10.1074/jbc.274.38.27069. [DOI] [PubMed] [Google Scholar]
- 5.Nagababu E, Ramasamy S, Rifkind JM. S-Nitrosohemoglobin: A mechanism for its formation in conjunction with nitrite reduction by deoxyhemoglobin. Nitric Oxide. 2006;15:20–29. doi: 10.1016/j.niox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Wink DA, Nims RW, Darbyshire JF, Christodoulou D, Hanbauer I, Cox GW, Laval F, Laval J, Cook JA, Krishna MC. Reaction kinetics for nitrosation of cysteine and glutathione in aerobic nitric oxide solutions at neutral pH. Insights into the fate and physiological effects of intermediates generated in the NO/O2 reaction. Chem. Res. Toxicol. 1994;7:519–525. doi: 10.1021/tx00040a007. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein S, Czapski G. Mechanism of the nitrosation of thiols and amines by oxygenated NO solutions: the nature of the nitrosating intermediates. J. Am. Chem. Soc. 1996;118:3419–3425. [Google Scholar]
- 8.Jourd'heuil D, Jourd'heuil FL, Feelisch M. Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide. Evidence for a free radical mechanism. J. Biol. Chem. 2003;278:15720–15726. doi: 10.1074/jbc.M300203200. [DOI] [PubMed] [Google Scholar]
- 9.Schrammel A, Gorren ACF, Schmidt K, Pfeiffer S, Mayer B. S-nitrosation of glutathione by nitric oxide, peroxynitrite, and NO/O2. Free Radic. Biol. Med. 2003;34:1078–1088. doi: 10.1016/s0891-5849(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 10.Gow AJ, Buerk DG, Ischiropoulos H. A novel reaction mechanism for the formation of S-nitrosothiol in vivo. J. Biol. Chem. 1997;272:2841–2845. doi: 10.1074/jbc.272.5.2841. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Miller MJS, Joshi MS, Thomas DD, Lancaster JR., Jr Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc. Natl. Acad. Sci. USA. 1998;95:2175–2179. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moller MN, Li Q, Vitturi DA, Robinson JM, Lancaster JR, Denicola A. Membrane "Lens" Effect: Focusing the Formation of Reactive Nitrogen Oxides from the NO/O2 Reaction. Chem. Res. Toxicol. 2007;20:709–714. doi: 10.1021/tx700010h. [DOI] [PubMed] [Google Scholar]
- 13.Nedospasov A, Rafikov R, Beda N, Nudler E. An autocatalytic mechanism of protein nitrosylation. Proc. Natl. Acad. Sci. USA. 2000;97:13543–13548. doi: 10.1073/pnas.250398197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rafikova O, Rafikov R, Nudler E. Catalysis of S-nitrosothiols formation by serum albumin: the mechanism and implication in vascular control. Proc. Natl. Acad. Sci. USA. 2002;99:5913–5918. doi: 10.1073/pnas.092048999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogg N, Kalyanaraman B. The use of nitric oxide gas in biological systems. In: Titheradge MA, editor. Nitric oxide protocols. Totowa, New Jersey: Humana Press; 1997. pp. 233–238. [Google Scholar]
- 16.Samouilov A, Zweier JL. Development of chemiluminescence-based methods for specific quantitation of nitrosylated thiols. Anal. Biochem. 1998;258:322–330. doi: 10.1006/abio.1998.2609. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Bryan NS, MacArthur PH, Rodriguez J, Gladwin MT, Feelisch M. Measurement of Nitric Oxide Levels in the Red Cell: Validation of tri-iodide-based chemiluminescence with acid-sulfanilamide pretreatment. J. Biol. Chem. 2006;281:26994–27002. doi: 10.1074/jbc.M603953200. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Hogg N. The mechanism of transmembrane S-nitrosothiol transport. Proc. Natl. Acad. Sci. USA. 2004;101:7891–7896. doi: 10.1073/pnas.0401167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh RJ, Hogg N, Joseph J, Kalyanaraman B. Mechanism of nitric oxide release from S-nitrosothiols. J. Biol. Chem. 1996;271:18596–18603. doi: 10.1074/jbc.271.31.18596. [DOI] [PubMed] [Google Scholar]
- 20.Mendes P. Biochemistry by numbers: simulation of biochemical pathways with Gepasi 3. Trends Biochem. Sci. 1997;22:361–363. doi: 10.1016/s0968-0004(97)01103-1. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein S, Czapski G. Kinetics of nitric oxide autoxidation in aqueous solution in the absence and presence of various reductants. The nature of the oxidizing intermediates. J. Am. Chem. Soc. 1996;118:3419–3425. [Google Scholar]
- 22.Akaike T, Yoshida M, Miyamoto Y, Sato K, Kohno M, Sasamoto K, Miyazaki K, Ueda S, Maeda H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.NO through a radical reaction. Biochemistry. 1993;32:827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- 23.Hogg N, Singh RJ, Joseph J, Neese F, Kalyanaraman B. Reactions of nitric oxide with nitronyl nitroxides and oxygen: prediction of nitrite and nitrate formation by kinetic simulation. Free Radic. Res. 1995;22:47–56. doi: 10.3109/10715769509147527. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein S, Russo A, Samuni A. Reactions of PTIO and Carboxy-PTIO with {middle dot}NO, {middle dot}NO2, and O3- J. Biol. Chem. 2003;278:50949–50955. doi: 10.1074/jbc.M308317200. [DOI] [PubMed] [Google Scholar]
- 25.Pou S, Rosen GM. Generation of thiyl radical by nitric oxide: a spin trapping study. J. Chem. Soc. Perkin Trans. 1998;2:1507–1512. [Google Scholar]
- 26.Hogg N, Singh RJ, Kalyanaraman B. The role of glutathione in the transport and catabolism of nitric oxide. FEBS Lett. 1996;382:223–228. doi: 10.1016/0014-5793(96)00086-5. [DOI] [PubMed] [Google Scholar]
- 27.Koshiishi I, Takajo T, Tsuchida K. Regulation of S-thiolation and S-nitrosylation in the thiol/nitric oxide system by radical scavengers. Nitric Oxide. 2007;16:356–361. doi: 10.1016/j.niox.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Lancaster JR. Nitroxidative, Nitrosative, and Nitrative Stress: Kinetic Predictions of Reactive Nitrogen Species Chemistry Under Biological Conditions. Chem. Res. Toxicol. 2006;19:1160–1174. doi: 10.1021/tx060061w. [DOI] [PubMed] [Google Scholar]
- 29.Czapski G, Goldstein S. The role of the reactions of .NO with superoxide and oxygen in biological systems: a kinetic approach. Free Radic. Biol. Med. 1995;19:785–794. doi: 10.1016/0891-5849(95)00081-8. [DOI] [PubMed] [Google Scholar]
- 30.Kharitonov VG, Sundquist AR, Sharma VS. Kinetics of nitrosation of thiols by nitric oxide in the presence of oxygen. J. Biol. Chem. 1995;270:28158–28164. doi: 10.1074/jbc.270.47.28158. [DOI] [PubMed] [Google Scholar]
- 31.Pryor WA, Church DF, Govindan CK, Crank G. Oxidation of thiols by nitric oxide and nitrogen dioxide: Synthetic utility and toxicological implications. J. Org. Chem. 1982;47:159–161. [Google Scholar]
- 32.Kharitonov VG, Sundquist AR, Sharma VS. Kinetics of nitric oxide autoxidation in aqueous solution. J. Biol. Chem. 1994;269:5881–5883. [PubMed] [Google Scholar]
- 33.Wink DA, Beckman JS, Ford PC. Kinetics of Nitric Oxide Reaction in Liquid and Gas Phase. In: Feelisch M, Stamler JS, editors. Methods in Nitric Oxide Research. John Wiley & Sons Ltd; 1996. pp. 29–37. [Google Scholar]
- 34.Zhang Y, Hogg N. The Formation and Stability of S-Nitrosothiols in RAW 264.7 Cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L467–L474. doi: 10.1152/ajplung.00350.2003. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H, Andrekopoulos C, Xu Y, Joseph J, Hogg N, Feix J, Kalyanaraman B. Decreased S-Nitrosation of peptide thiols in the membrane interior. Free Radic. Biol. Med. 2009 doi: 10.1016/j.freeradbiomed.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keshive M, Singh S, Wishnok JS, Tannenbaum SR, Deen WM. Kinetics of S-nitrosation of thiols in nitric oxide solutions. Chem. Res. Toxicol. 1996;9:988–993. doi: 10.1021/tx960036y. [DOI] [PubMed] [Google Scholar]
- 37.Ford E, Hughes MN, Wardman P. Kinetics of the reactions of nitrogen dioxide with glutathione, cysteine, and uric acid at physiological pH. Free Radic. Biol. Med. 2002;32:1314–1323. doi: 10.1016/s0891-5849(02)00850-x. [DOI] [PubMed] [Google Scholar]
- 38.Madej E, Folkes LK, Wardman P, Czapski G, Goldstein S. Thiyl radicals react with nitric oxide to form S-nitrosothiols with rate constants near the diffusion-controlled limit. Free Radic. Biol. Med. 2008;44:2013–2018. doi: 10.1016/j.freeradbiomed.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Mezyk SP, Armstrong DA. Disulfide anoin radical equilibria: effects of NH3+, -CO2−, -NH(CO)- and -CH3 groups. J. Chem. Soc. Perkin Trans. 2:1411–1419. 199. [Google Scholar]
- 40.Wardman P, von SC. Kinetic factors that control the fate of thiyl radicals in cells. Methods Enzymol. 1995;251:31–45. doi: 10.1016/0076-6879(95)51108-3. [DOI] [PubMed] [Google Scholar]
- 41.Kissner R, Nauser T, Bugnon P, Lye PG, Koppenol WH. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem. Res. Toxicol. 1997;10:1285–1292. doi: 10.1021/tx970160x. [DOI] [PubMed] [Google Scholar]
- 42.Bonini MG, Augusto O. Carbon dioxide stimulates the production of thiyl, sulfinyl, and disulfide radical anion from thiol oxidation by peroxynitrite. J. Biol. Chem. 2001;276:9749–9754. doi: 10.1074/jbc.M008456200. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein S, Merenyi G. The chemistry of peroxynitrite: implications for biological activity. Methods Enzymol. 2008;436:49–61. doi: 10.1016/S0076-6879(08)36004-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01