PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM in human islets of Langerhans (original) (raw)
Abstract
Potential insulin secretagogue properties of an acetoxymethyl ester of a cAMP analog (8-pCPT-2′-_O_-Me-cAMP-AM) that activates the guanine nucleotide exchange factors Epac1 and Epac2 were assessed using isolated human islets of Langerhans. RT-QPCR demonstrated that the predominant variant of Epac expressed in human islets was Epac2, although Epac1 was detectable. Under conditions of islet perifusion, 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM) potentiated first- and second-phase 10 mM glucose-stimulated insulin secretion (GSIS) while failing to influence insulin secretion measured in the presence of 3 mM glucose. The insulin secretagogue action of 8-pCPT-2′-_O_-Me-cAMP-AM was associated with depolarization and an increase of [Ca2+]i that reflected both Ca2+ influx and intracellular Ca2+ mobilization in islet β-cells. As expected for an Epac-selective cAMP analog, 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM) failed to stimulate phosphorylation of PKA substrates CREB and Kemptide in human islets. Furthermore, 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM) had no significant ability to activate AKAR3, a PKA-regulated biosensor expressed in human islet cells by viral transduction. Unexpectedly, treatment of human islets with an inhibitor of PKA activity (H-89) or treatment with a cAMP antagonist that blocks PKA activation (Rp-8-CPT-cAMPS) nearly abolished the action of 8-pCPT-2′-_O_-Me-cAMP-AM to potentiate GSIS. It is concluded that there exists a permissive role for PKA activity in support of human islet insulin secretion that is both glucose dependent and Epac regulated. This permissive action of PKA may be operative at the insulin secretory granule recruitment, priming, and/or postpriming steps of Ca2+-dependent exocytosis.
Keywords: protein kinase A; exocytosis; adenosine-3′,5′-cyclic monophosphate; exchange protein directly activated by adenosine-3′,5′-cyclic monophosphate; calcium
adenosine-3′,5′-cyclic monophosphate (cAMP) is a cytosolic second messenger that potentiates the glucose metabolism-dependent secretion of insulin from pancreatic β-cells located within the islets of Langerhans (58). Although it is generally accepted that the insulin secretagogue action of cAMP results from its ability to activate protein kinase A (PKA), studies of rodent islets and insulin-secreting cell lines provide evidence for the existence of an alternative mechanism of cAMP signal transduction, one that is mediated by cAMP-regulated guanine nucleotide exchange factors known as the exchange proteins directly activated by cAMP (Epac) (26, 28, 62). Two variants of Epac exist (Epac1 and Epac2) (10, 40), both of which couple cAMP production to the activation of Rap1, a small GTPase of the Ras family (5). Using an Epac2-knockout mouse, Shibasaki et al. (63) provided evidence that the Epac2-mediated activation of Rap1 in β-cells plays an essential role in the cAMP-dependent potentiation of glucose-stimulated insulin secretion (GSIS). Thus, the existence of Epac2 in β-cells may explain prior reports that cAMP-elevating agents such as the blood glucose-lowering hormone glucagon-like peptide-1-(7–36) amide (GLP-1) exert novel PKA-independent actions to control rodent and possibly human islet function (3, 15, 20, 34, 39, 42, 44, 48, 51, 60, 64, 71).
The main objective of the present study was to extend on such prior investigations to determine what role Epac2 might play in human islet insulin secretion. To achieve this goal, we evaluated potential insulin secretagogue properties of a newly developed “prodrug,” acetoxymethyl (AM) ester of an Epac-selective cAMP analog (ESCA-AM). This compound is 8-pCPT-2′-_O_-Me-cAMP-AM, a cAMP analog first synthesized by Vliem et al. (69). 8-pCPT-2′-_O_-Me-cAMP-AM exhibits high lipophilicity and quickly crosses the plasma membrane of β-cells (7), thereby allowing it to be intracellularly metabolized and converted by cytosolic esterases into its active non-AM ester form (69). Importantly, intracellular 8-pCPT-2′-_O_-Me-cAMP generated in this manner retains its selectivity as an activator of Epac (7, 69). In fact, 8-pCPT-2′-_O_-Me-cAMP is established to be a “superactivator” of Epac, whereas it has little capacity to activate PKA when tested at low micromolar concentrations (9, 16, 29, 57).
We now report that, in isolated human islets, 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM) exerted a potent insulin secretagogue action to potentiate first- and second-phase GSIS. This action of 8-pCPT-2′-_O_-Me-cAMP-AM was associated with β-cell depolarization and an increase of intracellular Ca2+ concentration ([Ca2+]i) that reflected both Ca2+ influx and intracellular Ca2+ mobilization. Unexpectedly, and in contrast to what was reported previously in studies of mouse islets or rat INS-1 cells (7, 42), treatment of human islets with an inhibitor of PKA catalytic activity (H-89) or treatment with a cAMP antagonist that blocks PKA activation (Rp-8-CPT-cAMPS) abrogated the action of 8-pCPT-2′-_O_-Me-cAMP-AM to potentiate GSIS. Despite such observations, assays of cAMP response element-binding protein (CREB), Kemptide, or A-kinase activity reporter 3 (AKAR3) biosensor phosphorylation status demonstrated that 8-pCPT-2′-_O_-Me-cAMP-AM (1–10 μM) failed to activate PKA in human islets or human β-cells. Therefore, findings reported here demonstrate a previously unappreciated role for PKA in support of human islet insulin secretion that is both glucose dependent and Epac regulated. We propose that this permissive action of PKA is operative at the insulin secretory granule “recruitment, priming, and/or postpriming steps” of Ca2+-dependent exocytosis (49, 60, 66).
MATERIALS AND METHODS
Cell culture of human islets and β-cells.
Human islets of Langerhans obtained posthumously from anonymous donors were provided under the auspices of the National Institutes of Health, National Center for Research Resources, National Islet Cell Resource Centers. Primary cultures of human islets were maintained in a humidified incubator containing 95% air and 5% CO2 at 37°C in CMRL-1066 modified culture medium (Mediatech; cat. no. 99-603-CV) containing 10% (vol/vol) fetal bovine serum (34). For single-cell studies, suspensions of islet cells were prepared by digestion of the islets with trypsin-EDTA, and single cells were plated onto glass coverslips (25CIR-1; Fisher Scientific) coated with 1 mg/ml concanavalin A (type V; Sigma-Aldrich). β-Cells were identified by fluorescence microscopy after infection of cultures with adenovirus (Ad.RIP2-EYFP) directing expression of enhanced yellow fluorescent protein (EYFP) under the control of the rat insulin 2 (RIP2) gene promoter (34).
Islet perifusion assay for secreted insulin.
After overnight culture, islets were transferred to Millipore Swinnex 13-mm-diameter filter chamber holders (20 islets/chamber) for perifusion, as described previously (41). The perifusate was Krebs-Ringer buffer (KRB) that contained 24 mM sodium bicarbonate and which was gassed with 95% air and 5% CO2 (pH 7.4). Perifusion (1 ml/min) was initiated at 37°C in KRB containing 3 mM glucose. During the first 30 min of perifusion, the insulin secretory rate decreased to a stable level (41). Subsequently, 1-ml fractions of the perifusate were collected for a total of 15 min, after which the composition of the perifusate was switched to KRB containing 10 mM glucose. Again, 1-ml fractions of this 10 mM glucose KRB perifusate were collected, and insulin present within individual fractions of the 3 and 10 mM glucose KRB perifusates was measured by radioimmunoassay (Sensitive Rat Insulin RIA, cat. no. SRI-13K; Millipore).
Static incubation assay for secreted insulin.
After overnight culture in CMRL-1066 medium, human islets were transferred to MilliCell-PCF culture plate filter inserts (cat. no. PIXP01250; Millipore) at a density of ∼40 islets/insert. The inserts were mounted within individual wells of a 24-well cell culture plate (Falcon 353047), and each well of the plate was filled with 1 ml of KRB containing 24 mM sodium bicarbonate and the indicated concentrations of glucose (pH 7.4). Culture plates containing these islets were then placed within a cell culture incubator gassed with 95% air and 5% CO2 at 37°C. Prior to the start of an experiment, islets were exposed to 2.8 mM glucose KRB for 30 min. The inserts containing islets were then transferred between adjacent wells on the plate to measure basal and stimulated insulin secretion. For each test solution, the duration of exposure was 30 min. A 200-μl fraction of each solution to which the islets were exposed was then collected and assayed for insulin content by an enzyme-linked immunosorbent assay (Mercodia Ultrasensitive Rat Insulin ELISA, cat. no. 10-1137-01) after appropriate dilution of samples.
Ser133 CREB phosphorylation assay.
The Ser133 phosphorylated form of transcription factor CREB was assayed by Western blot analysis using an anti-phospho-Ser133 CREB polyclonal antiserum (cat. no. 9191; Cell Signaling Technology). Immunoreactivity corresponding to the phosphorylated and nonphosphorylated forms of CREB (i.e., total CREB) was measured using a monoclonal antibody (48H2, cat. no. 9197; Cell Signaling Technology). A horseradish peroxidase-conjugated secondary antiserum (cat. no. A6667; Sigma-Aldrich) was used in combination with these two primary antisera. Immunoreactivity was imaged using a SuperSignal West Pico ECL detection system (Thermo Scientific) and a ChemiDoc XRS gel documentation system (Bio-Rad).
PKA activation assay.
PKA activity in lysates of human islets was measured using a PepTag nonradioactive cAMP-dependent protein kinase assay (Promega). Prior to their lysis, islets were exposed to AM esters of PKA- or Epac-selective cAMP analogs for 30 min while being equilibrated at 37°C in CMRL-1066 medium containing 5.6 mM glucose and gassed with 95% air and 5% CO2. Islets were homogenized in a PKA extraction buffer, after which the crude homogenate was subjected to centrifugation (14,000 g) for 5 min at 4°C. The supernatant fraction was collected and assayed for PKA activity by addition of PepTag A1 peptide, which is a fluorescent Kemptide (LRRASLG) (43). The phosphorylated form of PeptTag A1 peptide was resolved by electrophoresis on a 0.8% agarose gel (100 V, 15 min) and quantified using a ChemiDoc XRS and Quantity One image analysis software (Bio-Rad).
Imaging of AKAR3.
Human β-cells adherent to glass coverslips were imaged using a Nikon Ti inverted microscope equipped with an NA 1.45 TIRF objective (×60), a Photometrics Cascade 512b EMCCD camera (Roper Scientific), and a chameleon-2 filter set (Chroma Technology) comprised of a D440/20 excitation filter, a 455DCLP dichroic, and D485/40 [cyan fluorescent protein (CFP)] or D535/30 [yellow fluorescent protein (YFP)] emission filters (7). Experiments were performed 36–42 h after transduction, with adenovirus directing expression of AKAR3. AKAR3 incorporates CFP at its NH2 terminus and YFP at its COOH terminus (1). A phospho-amino acid-binding domain and a PKA substrate motif (LRRATLVD) within AKAR3 together act as a linker so that AKAR3 undergoes a conformational change in response to its phosphorylation by PKA. Fluorescence resonance energy transfer (FRET) between CFP (donor) and YFP (acceptor) increases in response to PKA-dependent phosphorylation of AKAR3 so that the PKA-phosphorylated form of AKAR3 exhibits an increase of the YFP/CFP (535/485 nm) emission ratio. β-Cells expressing AKAR3 were bathed in a standard extracellular saline (SES) solution containing (in mM): 138 NaCl, 5.6 KCl, 2.6 CaCl2, 1.2 MgCl2, 11.1 glucose, and 10 HEPES (295 mOsm, pH 7.4). Ratiometric analysis of the emitted light corresponding to fluorescence originating within a defined region of the cytoplasm was performed, and images were acquired and processed using Metafluor version 7.5 (Molecular Devices) (7).
Measurement of [Ca2+]i.
Human islets or β-cells adherent to glass coverslips were equilibrated in SES supplemented with 2% FBS, 1 μM fura-2 AM, and 0.02% Pluronic F-127 (wt/vol) (Invitrogen). Exposure to fura-2 AM was for 20–30 min at 22°C, after which the loading solution was removed and the preparations were washed and equilibrated in fresh SES for 10 min at 22°C (34). Fura-2 spectrofluorimetry was performed ratiometrically at 1-s intervals at 32°C using a video imaging system (IonOptix) interfaced with a TE300 inverted microscope (Nikon) equipped with a temperature-controlled stage (Medical Systems) and ×100 or ×40 Nikon SuperFluor oil immersion objectives for the imaging of single β-cells or whole islets, respectively.
Simultaneous measurements of [Ca2+]i and membrane potential.
Human β-cells adherent to glass coverslips were loaded with fura-2, as described above. [Ca2+]i was calculated according to the method of Grynkiewicz et al. (18). Calibration of the raw fluorescence values was performed using fura-2 (K+)5 salt dissolved in calibration buffers obtained from Invitrogen (Calcium Calibration Kit 1 with Mg2+). For cells expressing EYFP, the filter sets chosen allowed accurate measurements of [Ca2+]i, as described previously (32, 34, 35). Current clamp measurements of the membrane potential were obtained from these same fura-2-loaded cells using the perforated patch technique and an EPC-9 amplifier under the control of PatchMaster software (HEKA Instruments). Patch pipettes pulled from borosilicate glass (Kimax-51, tip resistance 2–3 MΩ) were fire polished and tip-dipped in a pipette solution containing (in mM): 10 KCl, 10 NaCl, 70 K2SO4, 7 MgCl2, 10 HEPES, and 0.5 EGTA (300 mOsm, pH 7.35) and back filled with the same solution containing nystatin (240 μg/ml). The series resistance (Rs) was monitored following seal formation, and experiments were conducted when Rs declined to 12–25 MΩ (25).
UV flash photolysis for liberation of caged Ca2+.
Human β-cells were bathed for 60 min at 23°C in SES containing cell-permeable caged Ca2+ (NP-EGTA-AM, 5 μM; Invitrogen). The loading solution also contained 1 μM fura-2 AM, 2% FBS, and 0.02% Pluronic F-127. Uncaging of Ca2+ was achieved using a flash photolysis system (Model JML-C2; Rapp OptoElectronic), as described previously (35). The excitation light of 80-J intensity and 600-μs duration was filtered using a short-pass filter (cutoff 390 nm) and was delivered to the specimen by way of the microscope's objective. The intensity and duration of the flash were minimized so that no photobleaching of fura-2 was observed. The intensity of 340 and 380 nm excitation light for detection of fura-2 was also reduced to be so low as to produce no measurable uncaging of Ca2+.
Quantitative PCR for Epac.
RNA was isolated from human islets using RNEasy kits (Qiagen). RNA concentration and purity was assessed using a NanoDrop ND-1000 spectrofluorimeter. Quantitative PCR (QPCR) reactions were performed using QuantiTect SYBR green one-step kits (Qiagen) with ∼100 ng of template RNA. Reactions were performed using an MJ MiniOpticon cycler with 40 cycles of 94°C for 15 s, 60°C for 30 s, and 72°C for 30 s, followed by a melting curve analysis from 60 to 94°C. Products were then run on 2% agarose gels, products were gel extracted using QIAquick kits (Qiagen), and product identity was confirmed by sequencing. The PCR primers were Epac1 (sense, CATGGCAAGGGGCTGGTGAC; antisense, GTCCTGCTTGTCCACACGCAG) and Epac2 (sense, CGCCATGCAACCATCGTTACC; antisense, GAGCCCGTTTCCATAACACC). Ribosomal S18 subunit mRNA was used as the reference template with the following primers: sense, GCCATCACTGCCATTAAGGG; antisense, CCAGTCTGGGATCTTGTACTG. Primers were tested at different starting template concentrations to validate their equal efficiencies. Threshold crossing (CT) values were set manually, and the difference between the CT value for S18 and Epac mRNAs (ΔCT) was calculated for each reaction. ΔCT values were entered into Origin 8 software for analysis using ANOVA.
Analysis of insulin secretion data.
The repeatability of all findings was confirmed by performing each experiment a minimum of two times. Statistical analysis was performed using Student's paired _t_-test and SigmaStat (Systat Software). A P value of <0.05 was considered significant. Values for the area under the curve (AUC) describing first- and second-phase insulin secretion were calculated using Origin 8 software.
Sources of additional reagents.
cAMP analogs and phosphate-AM3 were synthesized by Biolog Life Science Institute. H-89, forskolin, IBMX, diazoxide, nifedipine, and ryanodine were from Sigma-Aldrich. AKAR3 in pcDNA 3.0 was obtained from J. Zhang and subcloned into a shuttle vector for production of adenovirus. The Ad.AKAR3 virus was used at multiplicities of infection equivalent to 100–200.
Administration of test substances to single cells.
Test substances dissolved in SES were applied to single cells under microscopic examination using borosilicate glass “puffer” micropipettes (cat. no. 1B150-6; World Precision Instruments). The pipettes were under the control of a Narishige International hydraulic micromanipulator, and they were pressurized with a PicoSpritzer II pneumatic pressure ejection system (General Valve). The rapid “on” and “off” kinetics of this drug delivery system are as described previously (25).
RESULTS
Potentiation of first- and second-phase GSIS by 8-pCPT-2′-O-Me-cAMP-AM.
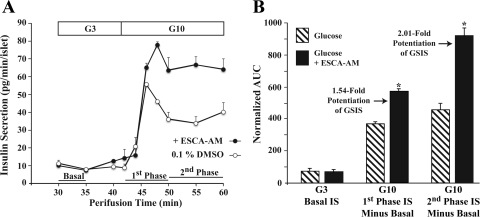
To evaluate potential insulin secretagogue properties of 8-pCPT-2′-_O_-Me-cAMP-AM, human islet insulin secretion was monitored under standard conditions of islet perifusion (41). When human islets were perifused with KRB containing 3 mM glucose, a basal rate of insulin secretion equivalent to ∼11 pg·min−1·islet−1 was measurable (Fig. 1_A_). If the glucose concentration was then increased to 10 mM, the rate of insulin secretion increased and then subsequently declined to a new plateau value (Fig. 1_A_). Consistent with prior studies (41), we refer to the initial peak of insulin secretion as first-phase GSIS, whereas the plateau is defined as second-phase GSIS.
Fig. 1.
8-pCPT-2′-_O_-Me-cAMP-AM potentiates 1st- and 2nd-phase glucose-stimulated insulin secretion (GSIS). A: secretion profile for GSIS under conditions of human islet perifusion. Prior to the 30-min time point, islets were perifused with Krebs-Ringer buffer (KRB) containing 3 mM glucose only. This allowed basal insulin secretion to decline to a stable level (data not shown). The glucose concentration was then raised to 10 mM to evoke 1st- and 2nd-phase insulin secretion. Administration of KRB containing 8-pCPT-2′-_O_-Me-cAMP-AM [10 μM; exchange protein directly activated by cAMP (Epac)-selective cAMP analog (ESCA-AM)] began at the 30-min time point and was continuous until the 60-min time point. ESCA-AM indicates where 8-pCPT-2’-_O_-Me-cAMP-AM was applied, here and in subsequent figures. Dimethylsulfoxide (DMSO; vehicle control) was included in the KRB for islets not treated with 8-pCPT-2′-_O_-Me-cAMP-AM. B: quantitative analysis of GSIS potentiated by 8-pCPT-2′-_O_-Me-cAMP-AM. Summarized are findings obtained using the experimental design illustrated in A, with 2 batches of islets obtained from 2 donors. Insulin secretion is expressed as the normalized area under the curve (AUC) for 1st- and 2nd-phase GSIS, as defined in A. For B, *P < 0.05, _t_-test.
If 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM) was included in the initial perifusate containing 3 mM glucose, the basal rate of insulin secretion was not significantly different from the control value measured in its absence (Fig. 1, A and B). However, a potentiation of first- and second-phase GSIS was measured when the perifusate was switched to KRB containing 10 mM glucose and 10 μM 8-pCPT-2′-_O_-Me-cAMP-AM (Fig. 1, A and B). By calculating the AUC for the insulin secretion rate vs. time relationship (Fig. 1_B_), it was determined that 8-pCPT-2′-_O_-Me-cAMP-AM potentiated first- and second-phase GSIS by 1.54- and 2.01-fold, respectively. No such potentiation of GSIS was measured when islets were instead treated with phosphate-AM3 (3.3 μM; data not shown), which served as a control for metabolites generated as a consequence of AM ester hydrolysis (phosphate-AM3 liberates 3-mol equivalents of acetic acid and formaldehyde per mol of phosphate when it is hydrolyzed by intracellular esterases). Thus, 8-pCPT-2′-_O_-Me-cAMP-AM acted in human islets to exert a glucose-dependent insulin secretagogue action measurable as the potentiation of first- and second-phase GSIS.
PKA activity supports stimulatory actions of 8-pCPT-2′-O-Me-cAMP-AM on human islet insulin secretion.
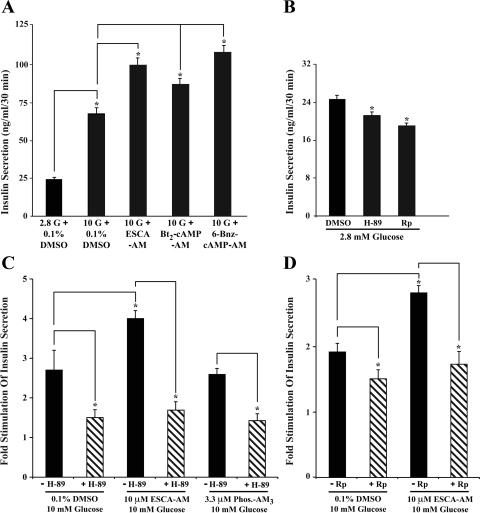
Under conditions of static incubation in which human islets were equilibrated in KRB containing 2.8 mM glucose, a subsequent elevation of the glucose concentration to 10 mM resulted in a 2.7-fold stimulation of insulin secretion (Fig. 2_A_). GSIS measured in this assay was potentiated an additional 1.5-fold by 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM). The prosecretagogue action of 8-pCPT-2′-_O_-Me-cAMP-AM was dose dependent over a concentration range of 1–10 μM (data not shown), and the magnitude of the effect of 10 μM 8-pCPT-2′-_O_-Me-cAMP-AM was similar to that which was measured when islets were instead treated with 10 μM of dibutyryl-cAMP-AM (Bt2-cAMP-AM; Fig. 2_A_), which is the AM ester of a selective PKA activator (61). Similarly, 10 μM of the AM ester of the PKA-selective cAMP analog _N_6-benzoyladenosine-cAMP (6-Bnz-cAMP) (9) also potentiated GSIS (Fig. 2_A_).
Fig. 2.
PKA-dependent potentiation of GSIS by 8-pCPT-2′-_O_-Me-cAMP-AM. A: human islets were equilibrated in KRB containing 2.8 or 10 mM glucose (2.8 G or 10 G, respectively) with or without added 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM; ESCA-AM), dibutyryl-cAMP-AM (Bt2-cAMP-AM; 10 μM), or 6-Bnz-cAMP-AM (10 μM). B: basal insulin secretion measured under conditions in which the KRB contained 2.8 mM glucose with or without added 0.1% DMSO, H-89 (10 μM), or Rp-8-CPT-cAMPS (200 μM). C and D: GSIS measured under conditions in which the KRB contained 10 mM glucose with or without added DMSO, ESCA-AM, phosphate-AM3 (Phos-AM3), H-89 (10 μM), or Rp-8-CPT-cAMPS (200 μM). Values of fold stimulation in C and D were calculated by measuring secreted insulin under conditions in which islets were exposed to KRB containing 2.8 or 10 mM glucose. Results obtained in 3 static incubation assays using islets from 3 donors are summarized in A_–_D. *P < 0.05, _t_-test. Error bars denote the mean ± SE for triplicate determinations.
Having established that AM esters of Epac- and PKA-selective cAMP analogs potentiate GSIS in this static incubation assay, we next sought to assess whether insulin secretion was modified by treatment of human islets with either H-89, an inhibitor of PKA catalytic activity (8), or membrane-permeable Rp-8-CPT-cAMPS, an inhibitor of PKA activation (12). In the absence of PKA inhibitors, the basal rate of insulin secretion was determined to be 24.6 ng·ml−1·30 min−1, and this value was reduced to 21.6 and 18.5 ng·ml−1·30 min−1 for islets treated with H-89 (10 μM) or Rp-8-CPT-cAMPS (200 μM), respectively (Fig. 2_B_). Thus, basal insulin secretion measured in this static incubation assay exhibited significant PKA dependence.
If the glucose concentration was then raised from 2.8 to 10 mM in the absence of PKA inhibitors, the rate of insulin secretion increased to 66.4 ng·ml−1·30 min−1, equivalent to a 2.7-fold increase over basal levels (Fig. 2_C_). Remarkably, this action of 10 mM glucose was reduced in a quantitatively identical manner upon treatment of islets with H-89 or Rp-8-CPT-cAMPS. Thus, a 1.5-fold stimulation of insulin secretion was measured in islets treated with either 10 μM H-89 (Fig. 2_C_) or 200 μM Rp-8-CPT-cAMPS (Fig. 2_D_). It may be concluded that GSIS measured in this manner was also significantly dependent on PKA activity.
We next sought to determine what effect PKA inhibitors might exert under conditions in which GSIS was potentiated by 8-pCPT-2′-_O_-Me-cAMP-AM. It was first determined that, in the absence of PKA inhibitors, combined administration of 10 mM glucose and 10 μM 8-pCPT-2′-_O_-Me-cAMP-AM resulted in a fourfold stimulation of insulin secretion (Fig. 2_C_). Furthermore, phosphate-AM3 failed to potentiate GSIS under these conditions (Fig. 2_C_). Unexpectedly, the action of 8-pCPT-2′-_O_-Me-cAMP-AM to potentiate GSIS was abrogated in a quantitatively identical manner upon treatment of islets with H-89 or Rp-8-CPT-cAMPS (Fig. 2, C and D). Thus, a 1.7-fold stimulation of insulin secretion was measured in islets treated with either 10 μM H-89 (Fig. 2_C_) or 200 μM Rp-8-CPT-cAMPS (Fig. 2_D_). It may be concluded that the action of 8-pCPT-2′-_O_-Me-cAMP-AM to potentiate GSIS was strongly dependent on PKA activity.
Evidence that 8-pCPT-2′-O-Me-cAMP-AM fails to activate PKA in human islets.
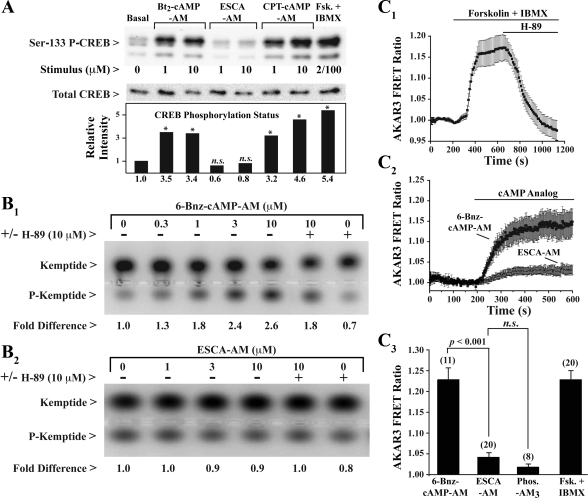
It was next determined whether an unexpected action of 8-pCPT-2′-_O_-Me-cAMP-AM to stimulate PKA might explain how it potentiated GSIS. To evaluate this possibility, assays of human islet PKA activity were performed under conditions identical to those used for assays of insulin secretion. We found that 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM) failed to promote Ser133 phosphorylation of CREB, a known substrate of PKA (Fig. 3_A_) (53). However, the Ser133 phosphorylation status of CREB was increased significantly upon treatment of islets with activators of PKA. These activators included dibutyryl-cAMP-AM, 8-CPT-cAMP-AM (46), or forskolin and IBMX (Fig. 3_A_).
Fig. 3.
8-pCPT-2′-_O_-Me-cAMP-AM fails to activate PKA. A: cAMP response element-binding protein (CREB) phosphorylation assay for human islets treated with Bt2-cAMP-AM, ESCA-AM, 8-CPT-cAMP-AM, forskolin (Fsk), and IBMX. Immunoblot analysis was performed to detect Ser133-phosphorylated CREB (P-CREB) or total CREB. The histogram is based on the average of 2 experiments using 2 islet preparations (*P < 0.05, _t_-test). B 1 and B 2: PKA activation assay for human islets treated with 6-Bnz-cAMP-AM or 8-pCPT-2′-_O_-Me-cAMP-AM. Electrophoresis was performed to resolve phosphorylated (P-Kemptide) and nonphosphorylated (Kemptide) forms of PepTag. C 1_–_C 3: AKAR3 assay for PKA activation in human β-cells. C 1: AKAR3 exhibited an increase of fluorescence resonance energy transfer (FRET) emission ratio in response to forskolin (2 μM) and IBMX (100 μM), and this increase was reversed by H-89 (10 μM). C 2: an increase of FRET emission ratio was also measured in response to 6-Bnz-cAMP-AM (10 μM) but not 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM). C 3: single-cell population study of AKAR3 responsiveness to cAMP analogs, Phos-AM3, or forskolin with IBMX using concentrations of test substances indicated for C 1 and C 2. Numbers above each histogram bar indicate the number of cells imaged. Statistical significance was evaluated using the _t_-test. n.s., Not significant. Findings are representative of results obtained in 2 experiments using islets obtained from 2 donors.
To obtain independent confirmation that 8-pCPT-2′-_O_-Me-cAMP-AM failed to activate PKA in human islets, an in vitro assay of PKA catalytic activity was performed. Islets were exposed to 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM) for 30 min, and lysates of these islets were then prepared. To each lysate a fluorescent PepTag derivative of the PKA substrate Kemptide [LRRASLG] was added (43). The unphosphorylated and phosphorylated forms of Kemptide were then resolved on the basis of their differential electrophoretic mobilities on an agarose gel (Fig. 3_B_). This analysis demonstrated that 0.3-10 μM of the AM ester of the PKA-selective cAMP analog 6-Bnz-cAMP (9) stimulated phosphorylation of Kemptide in a dose-dependent manner (Fig. 3_B_ 1). However, 8-pCPT-2′-_O_-Me-cAMP-AM (1–10 μM) was completely without effect (Fig. 3_B_ 2).
Since the limited temporal resolution of the above-summarized in vitro assays might preclude accurate detection of transient PKA activation within human islets, live-cell imaging was performed to determine whether 8-pCPT-2′-_O_-Me-cAMP-AM activated AKAR3, a biosensor that is a substrate for PKA (1) and that we expressed by viral transduction of human islets. Control experiments demonstrated that AKAR3 exhibited the expected increase of 535/485 nm FRET emission ratio when single human islet cells were treated with KRB containing 2 μM forskolin and 100 μM IBMX (Fig. 3_C_ 1). This increase of emission ratio was a consequence of PKA activation because it was reversed during treatment of cells with H-89 (Fig. 3_C_ 1). It was then demonstrated that AKAR3 reported PKA activation in response to the PKA-selective cAMP analog 6-Bnz-cAMP-AM (10 μM), whereas an equivalent concentration of 8-pCPT-2′-_O_-Me-cAMP-AM exerted little or no effect (Fig. 3, C 2 and C 3). Therefore, it may be concluded that the insulin secretagogue action of 8-pCPT-2′-_O_-Me-cAMP-AM reported here most likely did not result from an unexpected action of this cAMP analog to activate PKA in human islets.
8-pCPT-2′-O-Me-cAMP-AM stimulates an increase of [Ca2+]i in human islets and β-cells.
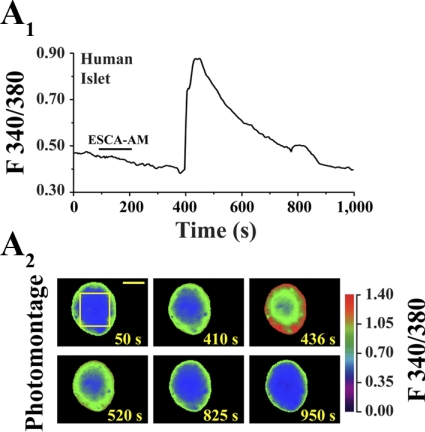
Since insulin secretion is Ca2+ dependent (23), we sought to establish whether 8-pCPT-2′-_O_-Me-cAMP-AM increased [Ca2+]i in human islets. Under conditions in which a human islet was equilibrated in SES containing 5.6 mM glucose, the extracellular application of 8-pCPT-2′-_O_-Me-cAMP-AM (1 μM) stimulated an increase of [Ca2+]i that appeared across the entire islet (Fig. 4, A 1 and A 2). Nearly identical findings were obtained in assays of five additional islets from three donors. No such effect of 8-pCPT-2′-_O_-Me-cAMP-AM was measured when islets were equilibrated in SES containing 2.8 mM glucose (5 islets tested).
Fig. 4.
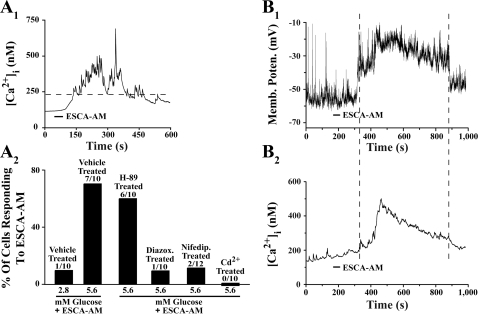
Effects of 8-pCPT-2′-_O_-Me-cAMP-AM on intracellular Ca2+ concentration ([Ca2+]i) in a human islet. A 1: ESCA-AM (1 μM; application indicated by the horizontal bar) stimulated an increase of [Ca2+]i in a human islet equilibrated in standard extracellular saline (SES) containing 5.6 mM glucose. A 2: the increase of [Ca2+]i imaged in this same islet at selected time points. Yellow bracket indicates the region from which ratiometric measurements of fura-2 fluorescence were obtained. Calibration bar indicates 65 μm. Findings are representative of results obtained in 3 experiments using 5 islets from 3 donors.
To determine whether 8-pCPT-2′-_O_-Me-cAMP-AM increased [Ca2+]i in β-cells of human islets, the islets were dispersed and single islet cells plated onto glass coverslips. The β-cells were identified on the basis of insulin gene promoter-directed EYFP expression (34). This approach was guided by our prior finding that the non-AM ester of 8-pCPT-2′-_O_-Me-cAMP (100 μM) stimulated a transient increase of [Ca2+]i accompanied by a brief burst of exocytosis in human β-cells (34). In the present study, lower concentrations of 8-pCPT-2′-_O_-Me-cAMP-AM stimulated an increase of [Ca2+]i, and this effect was manifest as an oscillatory, not transient, increase of [Ca2+]i. Thus, under conditions in which human β-cells were equilibrated in SES containing 5.6 mM glucose, oscillations of [Ca2+]i occurred 30–60 s after application of 8-pCPT-2′-_O_-Me-cAMP-AM (Fig. 5_A_ 1). Although the initial increase of [Ca2+]i exhibited a lag time, this delay was not explained by the kinetics of the drug delivery system (25). Instead, it reflected the fact that 8-pCPT-2′-_O_-Me-cAMP-AM must be metabolized to its non-AM ester form in order for it to activate Epac (69). The delay may also be explained by the latency of downstream signaling events that are Epac regulated (5, 26, 28).
Fig. 5.
8-pCPT-2′-_O_-Me-cAMP-AM increases [Ca2+]i and depolarizes human β-cells. A 1: 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM) stimulated an increase of [Ca2+]i in a single human β-cell equilibrated in SES containing 5.6 mM glucose. A 2: population study conducted at the single-cell level compares the action of 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM) to increase [Ca2+]i in human β-cells under conditions in which the SES contained 2.8 or 5.6 mM glucose or, alternatively, 5.6 mM glucose plus 10 μM H-89, 200 μM diazoxide, 10 μM nifedipine, or 100 μM CdCl2. Numbers of cells tested (denominator) and the number of cells responding (numerator) are indicated above each histogram bar. A positive response was assigned to cells in which the [Ca2+]i increased to a level >2-fold of the initial starting value (dashed horizontal bar in A 1). B 1 and B 2: simultaneous measurements of membrane potential (B 1) and [Ca2+]i (B 2) in a human β-cell equilibrated in SES containing 5.6 mM glucose and stimulated with 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM). B is representative of results obtained in 2 experiments using 5 β-cells from 2 donors.
When human β-cells were equilibrated in SES containing 5.6 mM glucose, the action of 8-pCPT-2′-_O_-Me-cAMP-AM to increase [Ca2+]i was dose dependent over a concentration range of 0.3-10 μM (data not shown). This action of 8-pCPT-2′-_O_-Me-cAMP-AM was also glucose dependent, because lowering the glucose concentration to 2.8 mM decreased the likelihood that individual cells would exhibit a greater than twofold increase of [Ca2+]i relative to the initial level of [Ca2+]i (Fig. 5_A_ 2). In contrast, when β-cells were exposed to 10 mM glucose, spontaneous oscillations of [Ca2+]i were observed, upon which an additional increase of [Ca2+]i was measured in response to 8-pCPT-2′-_O_-Me-cAMP-AM (data not shown).
Remarkably, under conditions in which human β-cells were equilibrated in SES containing 5.6 mM glucose, the action of 8-pCPT-2′-_O_-Me-cAMP-AM to increase [Ca2+]i was retained after treatment with 10 μM H-89, whereas it was nearly abolished after treatment with 200 μM of the KATP channel opener diazoxide (Fig. 5_A_ 2). Such findings are interpretable if Epac activation leads to KATP channel closure, depolarization, and Ca2+ entry through voltage-dependent Ca2+ channels (36, 37). Consistent with this concept, the action of 8-pCPT-2′-_O_-Me-cAMP-AM to increase [Ca2+]i was reduced after treatment with the L-type Ca2+ channel blocker nifedipine (10 μM) and abolished following exposure of cells to 100 μM of the Ca2+ channel blocker Cd2+ (Fig. 5_A_ 2). Thus, under conditions of diazoxide, nifedipine, or Cd2+ treatment, no statistically significant increase of [Ca2+]i was measured in response to the ESCA-AM.
As predicted, direct measurements of the membrane potential confirmed that 8-pCPT-2′-_O_-Me-cAMP-AM depolarized β-cells and increased [Ca2+]i (Fig. 5, B 1 and B 2). For cells equilibrated in SES containing 5.6 mM glucose, the mean resting potential was −58 ± 6 mV, and the mean change of membrane potential in response to 10 μM 8-pCPT-2′-_O_-Me-cAMP-AM was 37 ± 5 mV (5 cells tested). No such depolarizing action of 8-pCPT-2′-_O_-Me-cAMP-AM was measured when cells were equilibrated in SES containing 2.8 mM glucose (5 cells tested).
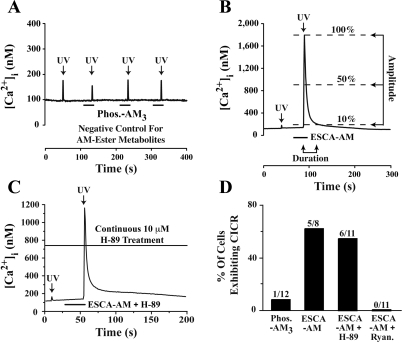
Depolarization-induced Ca2+ influx, as reported here for human β-cells, is unlikely to be the sole mechanism by which 8-pCPT-2′-_O_-Me-cAMP-AM increases [Ca2+]i. In fact, the non-AM ester of this ESCA was previously reported to facilitate Ca2+-induced Ca2+ release (CICR) from intracellular Ca2+ stores in human β-cells (34). The conclusions of this prior report were reached on the basis of indirect evidence in which ryanodine, a blocker of CICR (35), abolished the action of 8-pCPT-2′-_O_-Me-cAMP to stimulate a transient increase of [Ca2+]i (34). To test directly for CICR in the mechanism by which Epac activators increase [Ca2+]i, we used UV flash photolysis to uncage Ca2+, as studied in β-cells loaded with photolabile caged Ca2+ (NP-EGTA-AM) (35). Low-intensity UV excitation delivered to human β-cells stimulated a small (<100 nM) increase of [Ca2+]i that resulted not from CICR but simply from the uncaging of Ca2+ (Fig. 6_A_). However, if identical UV excitation was paired with application of 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM), the uncaging of Ca2+ resulted in a large transient increase of [Ca2+]i (Fig. 6_B_). This Ca2+ transient was defined as CICR on the basis of established criteria (35), and the mean amplitude of these Ca2+ transients was 1,640 ± 665 nM; n = 5 cells. Thus, Ca2+ uncaged from NP-EGTA-AM acted as a trigger for CICR, and this action of Ca2+ was facilitated by 8-pCPT-2′-_O_-Me-cAMP-AM.
Fig. 6.
8-pCPT-2′-_O_-Me-cAMP-AM facilitates Ca2+-induced Ca2+ release (CICR) in human β-cells. A: Ca2+ was uncaged by delivering UV excitation light (arrows) to a β-cell loaded with NP-EGTA and equilibrated in SES containing 5.6 mM glucose. UV light was delivered alone (1st flash) or in combination with Phos-AM3 (3.3 μM, repeated 30-s applications; note _y-_axis scaling), which failed to facilitate CICR. B: CICR was not observed in response to UV excitation alone (1st flash), whereas CICR was evoked when UV light was delivered in combination with 8-pCPT-2′-_O_-Me-cAMP-AM (10 μM, 30-s application, 2nd flash; note _y-_axis scaling). CICR was defined as an increase of [Ca2+]i, the duration of which did not exceed 30 s when measured at the 10% amplitude cutoff. The increase of [Ca2+]i also must have exceeded 300 nM when measured at the 50% amplitude cutoff. C: facilitation of CICR by 8-pCPT-2′-_O_-Me-cAMP-AM in a human β-cell treated with H-89 (10 μM). D: population study demonstrating the 10 μM H-89-resistant but the 10 μM ryanodine-sensitive action of 10 μM 8-pCPT-2′-_O_-Me-cAMP-AM to facilitate CICR.
As expected for a mechanism of β-cell CICR sensitized by Epac activators, the action of 8-pCPT-2′-_O_-Me-cAMP-AM to facilitate CICR was not blocked by H-89 (10 μM), but it was abolished by ryanodine (10 μM) (Fig. 6, C and D). Interestingly, we also found that in β-cells loaded with NP-EGTA-AM, 8-pCPT-2′-_O_-Me-cAMP-AM failed to exert any effect on [Ca2+]i in the absence of UV excitation. Although not investigated here, this finding is understandable if the Ca2+ buffering action of NP-EGTA abrogates depolarization-induced Ca2+ influx that normally occurs when β-cells are exposed to 8-pCPT-2′-_O_-Me-cAMP-AM. Thus, free cytosolic Ca2+ may play a significant role in support of CICR and also in support of Epac-regulated processes that control the β-cell membrane potential. In fact, it was reported previously that the action of cAMP-elevating agent GLP-1 to inhibit KATP channels and depolarize mouse β-cells was disrupted after treatment of these cells with agents that interfere with Ca2+ signaling (11).
RT-QPCR for Epac1 and Epac2 mRNA.
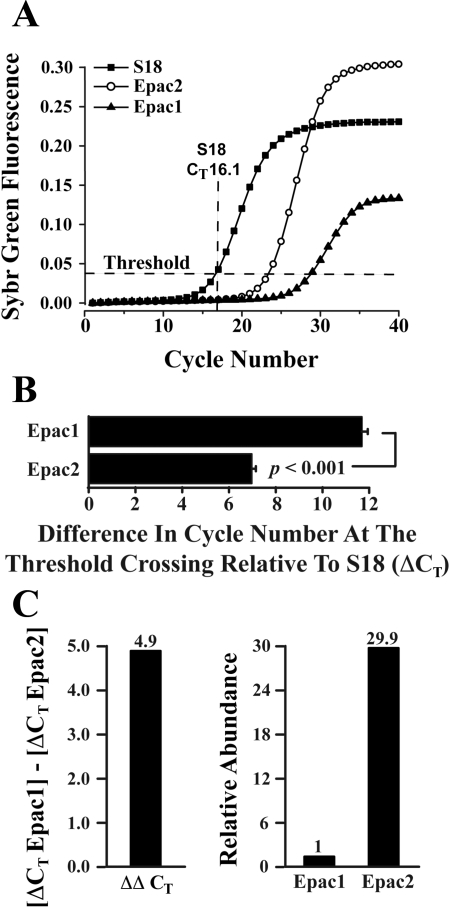
Finally, we determined the relative levels of expression of Epac1 and Epac2 mRNA in human islets. RT-QPCR was performed to determine the CT values for amplification of mRNAs corresponding to Epac1, Epac2, and the S18 ribosomal subunit mRNA serving as a reference standard (Fig. 7_A_). This analysis allowed a determination of the ΔCT values for Epac1 and Epac2 (Fig. 7_B_). By performing a subtraction of the Epac1 and Epac2 ΔCT values, it was possible to calculate ΔΔCT (Fig. 7_C_, left) and determine the relative abundance of Epac1 and Epac2 mRNAs (Fig. 7_C_, right) (52). This analysis revealed that the level of expression of Epac2 mRNA was 29.9-fold higher than that of Epac1 (Fig. 7_C_, right). Thus, Epac2 is likely to constitute the predominant intracellular receptor at which 8-pCPT-2′-_O_-Me-cAMP-AM exerts its stimulatory effect on human islet insulin secretion.
Fig. 7.
RT-quantitative PCR (QPCR) for Epac1 and Epac2. A: RT-QPCR fluorescence growth curves obtained using 100 ng of human islet RNA in a single PCR reaction from islets of a single donor. Ribosomal S18 mRNA was used as the reference target for quantification of Epac1 and Epac2 mRNA. The threshold crossing value (CT) for S18 mRNA (16.1) is indicated. B: comparison of ΔCT values obtained after averaging results of PCR reactions run for Epac1 (ΔCT 11.83 ± 0.31; 10 reactions) and Epac2 (ΔCT 6.93 + 0.39; 13 reactions). The ΔCT value for each Epac isoform was calculated as the difference in threshold cycle number relative to S18. C: the ΔΔCT value (4.9) for Epac1 relative to Epac2 was computed by subtraction of the ΔCT values for each isoform (left), and the relative abundance of Epac1 and Epac2 mRNA was then calculated to be 1:29.9 (right). Results in B and C are based on averaged data obtained using 2 batches of islets from 2 donors.
DISCUSSION
Here we report the unanticipated finding that, in human islets, GSIS potentiated by a selective activator of Epac (8-pCPT-2′-_O_-Me-cAMP-AM) was entirely dependent on permissive PKA activity. The permissive role for PKA activity in support of Epac-regulated human islet insulin secretion was established by demonstrating that, under conditions in which these islets were treated with PKA inhibitors H-89 or Rp-8-CPT-cAMPS, the action of 8-pCPT-2′-_O_-Me-cAMP-AM to potentiate GSIS was nearly abolished. Importantly, we also demonstrated that human islet GSIS was potentiated by selective activators of PKA (Bt2-cAMP-AM, 6-Bnz-cAMP-AM) under conditions in which Epac activation was unlikely to occur. Thus, it would appear that, for human islets, GSIS potentiated by Epac activators does not occur in the absence of permissive PKA activity, whereas Epac activation might be dispensable for PKA-dependent potentiation of GSIS. Such a conclusion is in agreement with the prior studies of Hatakeyama et al. (21, 22) that highlighted the critically important role PKA plays in support of mouse islet GSIS. However, it should be noted that contrary to the conclusions of Hatakeyama et al. (21, 22), new findings presented here provide clear evidence for an Epac-mediated stimulatory action of cAMP on human islet insulin secretion.
Surprisingly, we also found that human islet GSIS measured in the absence of 8-pCPT-2′-_O_-Me-cAMP-AM was reduced substantially under conditions of H-89 or Rp-8-CPT-cAMPS treatment. These are unexpected findings in view of the fact that mouse islet GSIS measured in the absence of cAMP-elevating agents was originally reported to be unaffected by PKA inhibitors (19, 56, 67). Furthermore, unlike human islets, mouse islet GSIS potentiated by 8-pCPT-2′-_O_-Me-cAMP-AM was reported to be reduced but not abrogated under conditions of H-89 treatment (42). Therefore, compared with mouse islets, human islet insulin secretion is critically dependent on PKA activity, so much so that PKA may be considered to be a permissive factor in support of insulin secretion that is both glucose stimulated and Epac regulated. This permissive PKA activity may reflect basal PKA activity or PKA activity stimulated by β-cell glucose metabolism (45). In fact, β-cell glucose metabolism is linked to increased production of ATP that serves as a precursor for cAMP (38, 66). It is also possible that glucose metabolism enhances β-cell cAMP production via its stimulatory effects on transmembrane adenylyl cyclases (14, 50) or soluble adenylyl cyclases (59). Thus, it will be of interest to determine whether the PKA-dependent and Epac-regulated insulin secretion reported here for human islets is supported by the activities of one or both of these two distinct classes of adenylyl cyclases.
It is also remarkable that we found that 8-pCPT-2′-_O_-Me-cAMP-AM potentiated first- and second-phase human islet GSIS measured in the presence of 10 mM glucose while failing to alter insulin secretion measured in the presence of 3 mM glucose. Furthermore, 8-pCPT-2′-_O_-Me-cAMP-AM exerted a glucose-dependent action to depolarize β-cells and to increase [Ca2+]i. Such actions of 8-pCPT-2′-_O_-Me-cAMP-AM in human islets resemble the previously described membrane-depolarizing (11, 25, 64), [Ca2+]i-elevating (3), and insulin secretagogue actions (17, 27) of cAMP-elevating hormone GLP-1 in rodent islets. However, despite the fact that Epac2 activation most likely subserves stimulatory effects of GLP-1 on islet insulin secretion (26, 39, 54), it is clear that GLP-1 also acts through PKA (13, 17, 24), and in fact the full insulinotropic action of GLP-1 may arise only under conditions in which there is dual activation of both Epac2 and PKA (47–49).
Given that 8-pCPT-2′-_O_-Me-cAMP-AM is established to be a selective activator of Epac proteins (7, 69), and in view of our finding that Epac2 mRNA was expressed at levels nearly 30-fold higher than that of Epac1 in human islets, it appears that Epac2 may in fact transduce the insulin secretagogue actions of cAMP and possibly GLP-1 in human β-cells. However, due to the fact that Epac1 is expressed at low levels in human islets, and because the knockout of Epac1 gene expression is linked to glucose intolerance in mice (31), the relative importance of Epac1 and Epac2 as determinants of human islet insulin secretion remains to be explored more fully.
How does one explain the PKA dependence with which 8-pCPT-2′-_O_-Me-cAMP-AM exerts its insulin secretagogue action in human islets? One explanation revolves around the role PKA plays as a determinant of secretory granule “recruitment” processes (60). Evidence exists that activated PKA recruits secretory granules into a readily releasable pool (RRP) located in close proximity to β-cell voltage-dependent Ca2+ channels (VDCCs). This RRP undergoes exocytosis in response to high concentrations (10–20 μM) of Ca2+ that exist in microdomains and which form at the inner mouths of VDCCs that open in response to depolarization (4, 6). In the model we propose, β-cell glucose metabolism generates membrane depolarization, and this action of glucose is reinforced by 8-pCPT-2′-_O_-Me-cAMP-AM as a consequence of its ability to enhance glucose-dependent KATP channel closure. Such a model is consistent with our finding that 8-pCPT-2′-_O_-Me-cAMP-AM depolarized human β-cells and with prior studies demonstrating inhibitory effects of Epac activators at the KATP channels of human β-cells (36, 37). This model is also consistent with our finding that the action of 8-pCPT-2′-_O_-Me-cAMP-AM to increase [Ca2+]i was nearly abolished by treatment of cells with diazoxide, an opener of KATP channels (23), or by nifedipine and Cd2+, which are blockers of VDCCs. Furthermore, we found that the disruption of intracellular Ca2+ mobilization by treatment of β-cells with thapsigargin or ryanodine reduced but failed to block the increase of [Ca2+]i measured in response to 8-pCPT-2′-_O_-Me-cAMP-AM (Holz GG, unpublished observations). Thus, we propose that under conditions in which Epac activators enhance glucose-dependent closure of KATP channels, resultant depolarization-induced Ca2+ influx triggers the release of secretory granules that must be recruited into the RRP as a consequence of basal PKA activity or possibly glucose metabolism-stimulated PKA activity. This model emphasizes the important role Epac2 plays in “proximal” or “early” events of β-cell stimulus-secretion coupling, and it predicts that, under conditions in which PKA activity is reduced, the action of 8-pCPT-2′-_O_-Me-cAMP-AM to potentiate GSIS will also be reduced, as is demonstrated here. One potential substrate mediating this action of PKA is Rip11, a Rab11-interacting protein (65).
Evidence also exists for an ability of PKA to recruit secretory granules into a highly Ca2+-sensitive pool (HCSP), one that is capable of undergoing exocytosis in response to low concentrations (0.5-1.0 μM) of Ca2+ (70, 72). It has been proposed that PKA activity increases the number of highly Ca2+-sensitive secretory granules located near the plasma membrane of β-cells and that these secretory granules need not be located in close proximity to VDCCs. Thus, exocytosis of the HCSP might occur in response to elevations of [Ca2+]i that are generated by the opening of intracellular Ca2+ release channels known to be expressed in β-cells (13, 30, 35, 44). Importantly, earlier studies demonstrated an ability of the non-AM ester of 8-pCPT-2′-_O_-Me-cAMP-AM to mobilize an intracellular source of Ca2+ in human β-cells (34). A role for CICR in this process was inferred on the basis of the ryanodine sensitivity of the Ca2+ release mechanism (34). However, formal proof that CICR exists in human β-cells, and that it is facilitated by Epac activators, was not provided. Thus, the present study is notable in that Epac-regulated CICR originating from ryanodine-sensitive Ca2+ stores is validated to exist in human β-cells, as demonstrated through the use of an experimental protocol in which Ca2+ released from NP-EGTA acts as a direct “trigger” for CICR. Evidently, 8-pCPT-2′-_O_-Me-cAMP-AM sensitizes β-cell intracellular Ca2+ release channels to the stimulatory action of cytosolic Ca2+, thereby promoting CICR and a rise of [Ca2+]i that is positively coupled to exocytosis (33). Similar findings have been obtained in prior studies of mouse β-cells and rat INS-1 cells in which ryanodine, heparin, and the sarcoendoplasmic reticulum Ca2+ ATPase inhibitor thapsigargin were demonstrated to disrupt intracellular Ca2+ mobilization by the non-AM ester form of 8-pCPT-2′-_O_-Me-cAMP-AM (35). On the basis of such observations, we propose that, in human β-cells, basal PKA activity or possibly PKA activity stimulated by glucose metabolism may allow the HCSP to undergo exocytosis in response to CICR that is initiated by VDCC-mediated Ca2+ influx, and that is facilitated by Epac activators. When β-cells are treated with PKA inhibitors, this CICR-induced exocytosis of the HCSP is expected to be disrupted.
Less well understood is what role PKA may play in support of secretory granule “priming,” an ATP hydrolysis-dependent step that renders secretory granules competent to undergo exocytosis (2, 49). One mechanism of priming in β-cells involves Munc13-1-assisted unfolding of syntaxin (49), a soluble _N_-ethylmaleimide-sensitive factor attachment protein receptor (SNARE). Importantly, evidence exists that this action of Munc13-1 is facilitated by Rim2 (49), a substrate of PKA. It may be concluded that the insulin secretagogue actions of Epac activators in β-cells might be contingent on PKA activity that supports secretory granule priming. Given that Rim2 is reported to interact with Epac2 (55), and that Epac2 interacts with the SNARE protein synaptosome-associated protein of 25 kDa to promote exocytosis (68), it is apparent that significant cross-talk may exist between the PKA- and Epac2-dependent signal transduction pathways that regulate human islet insulin secretion.
Finally, the insulin secretagogue action of 8-pCPT-2′-_O_-Me-cAMP-AM reported here might also be contingent on PKA activity that facilitates a “postpriming” step of exocytosis (38, 66). Although the molecular nature of the postpriming step remains unknown, it may reflect the action of PKA to phosphorylate SNARE apparatus-associated proteins that directly regulate the Ca2+-dependent fusion of secretory granules with the plasma membrane. Since the postpriming step is proposed to be under the control of PKA activity stimulated by glucose metabolism in β-cells (21, 22, 38, 66), a downregulation of the postpriming step might explain our finding that PKA inhibitors reduced human islet GSIS measured in the absence of cyclic nucleotides while also reducing the action of 8-pCPT-2′-_O_-Me-cAMP-AM to potentiate GSIS.
In conclusion, Epac-regulated exocytosis of insulin from human islets, as reported here, is demonstrated to be stimulated by the concerted action of glucose metabolism and 8-pCPT-2′-_O_-Me-cAMP-AM but blocked by inhibitors of PKA.
GRANTS
Funding was provided by the National Institute of Diabetes and Digestive and Kidney Diseases (DK-045817 and DK-069575 to G. G. Holz, DK-074966 to M. W. Roe) and the American Diabetes Association (Research Award to C. A. Leech).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Allen MD, Zhang J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem Biophys Res Commun 348: 716–721, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Barg S, Huang P, Eliasson L, Nelson DJ, Obermüller S, Rorsman P, Thévenod F, Renström E. Priming of insulin granules for exocytosis by granular Cl− uptake and acidification. J Cell Sci 114: 2145–2154, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bode HP, Moormann B, Dabew R, Göke B. Glucagon-like peptide 1 elevates cytosolic calcium in pancreatic beta-cells independently of protein kinase A. Endocrinology 140: 3919–3927, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Barg S, Rorsman P. Insulin secretion: a high-affinity Ca2+ sensor after all? J Gen Physiol 124: 623–625, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci 31: 680–686, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, Johnson PR, Rorsman P. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes 57: 1618–1628, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Chepurny OG, Leech CA, Kelley GG, Dzhura I, Dzhura E, Li X, Rindler MJ, Schwede F, Genieser HG, Holz GG. Enhanced Rap1 activation and insulin secretagogue properties of an acetoxymethyl ester of an Epac-selective cyclic AMP analog in rat INS-1 cells: studies with 8-pCPT-2′-O-Me-cAMP-AM. J Biol Chem 284: 10728–10736, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, _N_-[2-(_p_-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem 265: 5267–5272, 1990 [PubMed] [Google Scholar]
- 9.Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, Døskeland SO. cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem 278: 35394–35402, 2003 [DOI] [PubMed] [Google Scholar]
- 10.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396: 474–477, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Ding WG, Kitasato H, Matsuura H. Involvement of calmodulin in glucagon-like peptide 1(7-36) amide-induced inhibition of the ATP-sensitive K+ channel in mouse pancreatic beta-cells. Exp Physiol 86: 331–339, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Dostmann WR, Taylor SS, Genieser HG, Jastorff B, Døskeland SO, Ogreid D. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinases I and II with analogs of adenosine 3′,5′-cyclic phosphorothioates. J Biol Chem 265: 10484–10491, 1990 [PubMed] [Google Scholar]
- 13.Dyachok O, Gylfe E. Ca2+-induced Ca2+ release via inositol 1,4,5-trisphosphate receptors is amplified by protein kinase A and triggers exocytosis in pancreatic beta-cells. J Biol Chem 279: 45455–45461, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Dyachok O, Idevall-Hagren O, Sågetorp J, Tian G, Wuttke A, Arrieumerlou C, Akusjärvi G, Gylfe E, Tengholm A. Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metab 8: 26–37, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Eliasson L, Ma X, Renström E, Barg S, Berggren PO, Galvanovskis J, Gromada J, Jing X, Lundquist I, Salehi A, Sewing S, Rorsman P. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol 121: 181–197, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enserink JM, Christensen AE, de Rooij J, Triest MV, Schwede F, Genieser HG, Døskeland SO, Blank JL, Bos JL. A novel Epac-selective cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4: 901–906, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Gromada J, Brock B, Schmitz O, Rorsman P. Glucagon-like peptide-1: regulation of insulin secretion and therapeutic potential. Basic Clin Pharmacol Toxicol 95: 252–262, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 19.Harris TE, Persaud SJ, Saermark T, Jones PM. A myristoylated pseudosubstrate peptide inhibitor of protein kinase C: effects on glucose- and carbachol-induced insulin secretion. Mol Cell Endocrinol 121: 133–141, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Hashiguchi H, Nakazaki M, Koriyama N, Fukudome M, Aso K, Tei C. Cyclic AMP/cAMP-GEF pathway amplifies insulin exocytosis induced by Ca2+ and ATP in rat islet beta-cells. Diabetes Metab Res Rev 22: 64–71, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Hatakeyama H, Kishimoto T, Nemoto T, Kasai H, Takahashi N. Rapid glucose sensing by protein kinase A for insulin exocytosis in mouse pancreatic islets. J Physiol 570: 271–282, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatakeyama H, Takahashi N, Kishimoto T, Nemoto T, Kasai H. Two cAMP-dependent pathways differentially regulate exocytosis of large dense-core and small vesicles in mouse beta-cells. J Physiol 582: 1087–1098, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49: 1751–1760, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Holz GG, Habener JF. Signal transduction crosstalk in the endocrine system: pancreatic beta cells and the glucose competence concept. Trends Biochem Sci 17: 388–393, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holz GG, 4th, Kühtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37). Nature 361: 362–365, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holz GG. Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes 53: 5–13, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holz GG. New insights concerning the glucose-dependent insulin secretagogue action of glucagon-like peptide-1 in pancreatic beta-cells. Horm Metab Res 36: 787–794, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol 577: 5–15, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holz GG, Chepurny OG, Schwede F. Epac-selective cAMP analogs: new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell Signal 20: 10–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson JD, Kuang S, Misler S, Polonsky KS. Ryanodine receptors in human pancreatic β-cells: localization and effects on insulin secretion. FASEB J 8: 878–880, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Kai AK, Lam AK, Zhang X, Lai AK, Xu Z, Vanhoutte PM, Lam KS, Chung SS, Chung SK. Targeted disruption of exchange protein directly activated by cyclic AMP in mice leads to altered islet architecture and reduced beta cell distribution of GLUT-2. American Diabetes Association 69th Scientific Sessions, New Orleans, LA, Late-Breaking Abstract, 81-LB, 2009 [Google Scholar]
- 32.Kang G, Chepurny OG, Holz GG. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta-cells. J Physiol 536: 375–385, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang G, Holz GG. Amplification of exocytosis by Ca2+-induced Ca2+ release in INS-1 pancreatic beta cells. J Physiol 546: 175–189, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-_O_-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta cells. J Biol Chem 278: 8279–8285, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li WH, Harbeck M, Roe MW, Holz GG. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic beta cells. J Physiol 566: 173–188, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang G, Chepurny OG, Malester B, Rindler MJ, Rehmann H, Bos JL, Schwede F, Coetzee WA, Holz GG. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic beta cells and rat INS-1 cells. J Physiol 573: 595–609, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang G, Leech CA, Chepurny OG, Coetzee WA, Holz GG. Role of cAMP sensor Epac as a determinant of K-ATP channel ATP-sensitivity in human pancreatic beta cells and rat INS-1 cells. J Physiol 586: 1307–1319, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kasai H, Suzuki T, Liu TT, Kishimoto T, Takahashi N. Fast and cAMP-sensitive mode of Ca2+-dependent exocytosis in pancreatic beta-cells. Diabetes 51, Suppl 1: S19–S24, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S. Critical role of cAMP-GEFII-Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem 276: 46046–46053, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science 282: 2275–2279, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Kelley GG, Zawalich KC, Zawalich WS. Synergistic interaction of glucose and neurohumoral agonists to stimulate islet phosphoinositide hydrolysis. Am J Physiol Endocrinol Metab 269: E575–E582, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Kelley GG, Chepurny OG, Schwede F, Genieser HG, Leech CA, Roe MW, Li X, Dzhura I, Dzhura E, Afshari P, Holz GG. Glucose-dependent potentiation of mouse islet insulin secretion by Epac activator 8-pCPT-2′-_O_-Me-cAMP-AM. Islets 1: 1–6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kemp BE, Graves DJ, Benjamini E, Krebs EG. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem 252: 4888–4894, 1977 [PubMed] [Google Scholar]
- 44.Kim BJ, Park KH, Yim CY, Takasawa S, Okamoto H, Im MJ, Kim UH. Generation of nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose by glucagon-like peptide-1 evokes Ca2+ signal that is essential for insulin secretion in mouse pancreatic islets. Diabetes 57: 868–878, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Kim JW, Roberts CD, Berg SA, Caicedo A, Roper SD, Chaudhari N. Imaging cyclic AMP changes in pancreatic islets of transgenic reporter mice. PLoS One 3: e2127, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kruppa J, Keely S, Schwede F, Schultz C, Barrett K, Jastorff B. Bioactivatable derivatives of 8-substituted cAMP-analogues. Bioorg Med Chem Lett 7: 945–948, 1997 [Google Scholar]
- 47.Kwan EP, Gaisano HY. Glucagon-like peptide 1 regulates sequential and compound exocytosis in pancreatic islet beta-cells. Diabetes 54: 2734–2743, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Kwan EP, Gao X, Leung YM, Gaisano HY. Activation of exchange protein directly activated by cyclic adenosine monophosphate and protein kinase A regulate common and distinct steps in promoting plasma membrane exocytic and granule-to-granule fusions in rat islet beta cells. Pancreas 35: e45–e54, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Kwan EP, Xie L, Sheu L, Ohtsuka T, Gaisano HY. Interaction between Munc13-1 and RIM is critical for glucagon-like peptide-1 mediated rescue of exocytotic defects in Munc13-1 deficient pancreatic beta-cells. Diabetes 56: 2579–2588, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Landa LR, Jr, Harbeck M, Kaihara K, Chepurny O, Kitiphongspattana K, Graf O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta-cell line. J Biol Chem 280: 31294–31302, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leech CA, Dzhura I, Chepurny OG, Schwede F, Genieser HG, Holz GG. Facilitation of β-cell KATP channel sulfonylurea sensitivity by a cAMP analog selective for the cAMP-regulated guanine nucleotide exchange factor Epac. Islets 2: 7–17, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2[−Delta Delta C(T)] method. Methods 25: 402–408, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem 66: 807–822, 1997 [DOI] [PubMed] [Google Scholar]
- 54.Nakazaki M, Crane A, Hu M, Seghers V, Ullrich S, Aguilar-Bryan L, Bryan J. cAMP-activated protein kinase-independent potentiation of insulin secretion by cAMP is impaired in SUR1 null islets. Diabetes 51: 3440–3449, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, Seino S. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol 11: 805–811, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Persaud SJ, Jones PM, Howell SL. Glucose-stimulated insulin secretion is not dependent on activation of protein kinase A. Biochem Biophys Res Commun 173: 833–839, 1990 [DOI] [PubMed] [Google Scholar]
- 57.Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, Schwede F, Genieser HG, Bos JL, Doskeland SO, Beavo JA, Butt E. Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods 5: 277–288, 2008 [DOI] [PubMed] [Google Scholar]
- 58.Prentki M, Matschinsky FM. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev 67: 1185–1248, 1987 [DOI] [PubMed] [Google Scholar]
- 59.Ramos LS, Zippin JH, Kamenetsky M, Buck J, Levin LR. Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J Gen Physiol 132: 329–338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Renström E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol 502: 105–118, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schultz C, Vajanaphanich M, Harootunian AT, Sammak PJ, Barrett KE, Tsien RY. Acetoxymethyl esters of phosphates, enhancement of the permeability and potency of cAMP. J Biol Chem 268: 6316–6322, 1993 [PubMed] [Google Scholar]
- 62.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev 85: 1303–1342, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J, Seino S. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA 104: 19333–19338, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suga S, Kanno T, Ogawa Y, Takeo T, Kamimura N, Wakui M. cAMP-independent decrease of ATP-sensitive K+ channel activity by GLP-1 in rat pancreatic beta-cells. Pflugers Arch 440: 566–572, 2000 [DOI] [PubMed] [Google Scholar]
- 65.Sugawara K, Shibasaki T, Mizoguchi A, Saito T, Seino S. Rab11 and its effector Rip11 participate in regulation of insulin granule exocytosis. Genes Cells 14: 445–456, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Takahashi N, Kadowaki T, Yazaki Y, Ellis-Davies GC, Miyashita Y, Kasai H. Post-priming actions of ATP on Ca2+-dependent exocytosis in pancreatic beta-cells. Proc Natl Acad Sci USA 96: 760–765, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thams P, Anwar MR, Capito K. Glucose triggers protein kinase A-dependent insulin secretion in mouse pancreatic islets through activation of the K-ATP channel-dependent pathway. Eur J Endocrinol 152: 671–677, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Vikman J, Svensson H, Huang YC, Kang Y, Andersson SA, Gaisano HY, Eliasson L. Truncation of SNAP-25 reduces the stimulatory action of cAMP on rapid exocytosis in insulin-secreting cells. Am J Physiol Endocrinol Metab 297: E452–E461, 2009 [DOI] [PubMed] [Google Scholar]
- 69.Vliem MJ, Ponsioen B, Schwede F, Pannekoek WJ, Riedl J, Kooistra MR, Jalink K, Genieser HG, Bos JL, Rehmann H. 8-pCPT-2′-_O_-Me-cAMP-AM: an improved Epac-selective cAMP analogue. Chembiochem 9: 2052–2054, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Wan QF, Dong Y, Yang H, Lou X, Ding J, Xu T. Protein kinase activation increases insulin secretion by sensitizing the secretory machinery to Ca2+. J Gen Physiol 24: 653–662, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamada S, Komatsu M, Sato Y, Yamauchi K, Kojima I, Aizawa T, Hashizume K. Time-dependent stimulation of insulin exocytosis by 3′,5′-cyclic adenosine monophosphate in the rat islet beta-cell. Endocrinology 143: 4203–4209, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Yang Y, Gillis KD. A highly Ca2+-sensitive pool of granules is regulated by glucose and protein kinases in insulin-secreting INS-1 cells. J Gen Physiol 124: 641–651, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]