Gene Therapy for Leber's Congenital Amaurosis is Safe and Effective Through 1.5 Years After Vector Administration (original) (raw)
Abstract
The safety and efficacy of gene therapy for inherited retinal diseases is being tested in humans affected with Leber's congenital amaurosis (LCA), an autosomal recessive blinding disease. Three independent studies have provided evidence that the subretinal administration of adeno-associated viral (AAV) vectors encoding RPE65 in patients affected with LCA2 due to mutations in the RPE65 gene, is safe and, in some cases, results in efficacy. We evaluated the long-term safety and efficacy (global effects on retinal/visual function) resulting from subretinal administration of AAV2-hRPE65v2. Both the safety and the efficacy noted at early timepoints persist through at least 1.5 years after injection in the three LCA2 patients enrolled in the low dose cohort of our trial. A transient rise in neutralizing antibodies to AAV capsid was observed but there was no humoral response to RPE65 protein. The persistence of functional amelioration suggests that AAV-mediated gene transfer to the human retina does not elicit immunological responses which cause significant loss of transduced cells. The persistence of physiologic effect supports the possibility that gene therapy may influence LCA2 disease progression. The safety of the intervention and the stability of the improvement in visual and retinal function in these subjects support the use of AAV-mediated gene augmentation therapy for treatment of inherited retinal diseases.
Introduction
Leber's congenital amaurosis (LCA) is a group of hereditary retinal dystrophies characterized by severe loss of retinal and visual functions early in life with progressive degeneration of the cellular structure of the retina.1,2 The clinical and ophthalmic features include severely reduced electroretinograms and pupillary light reflexes (PLRs), nystagmus (rhythmic, involuntary eye fixation instability), and fundus abnormalities on ophthalmoscopy.3,4
LCA is usually inherited as an autosomal recessive trait, and mutations in 15 different genes have been reported so far.5,6 LCA2, accounting for 10% of LCA,1,4 is caused by mutations in the RPE65 gene. The biochemical blockade of the visual cycle resulting from the all-trans retinyl esters isomerase RPE65 deficiency causes a profound impairment in visual function with delayed histological degeneration of retinal cells.7 Clinical assessment of LCA2 patients reveals, in most cases, the presence of retained visual capabilities in the first decade of life associated with nearly normal macular thickness.4
RPE65 gene replacement has been considered as a potential therapeutic strategy for LCA2, and successful proof-of-principle studies in LCA2 animal models using a replication-defective adeno-associated viral vector (AAV)8,9,10 provided a strong basis for three independent phase 1 dose escalation studies of gene therapy in patients with LCA2.11,12,13,14 Preliminary results from the lowest dose cohort in our trial (three subjects) and from subjects treated in the low dose cohorts of two other trials (six subjects) show no evidence of serious adverse events, systemic dissemination of vector or harmful immunological responses to vector or transgene. We observed a macular hole following subretinal administration of vector in one subject,14 and a foveal thinning has been reported in a subject enrolled in a different trial.13
Bainbridge et al.11 reported no improvements in visual acuity, peripheral visual fields, or electroretinographic responses in the three subjects treated. One subject showed significantly increased visual function as judged by microperimetry and dark adapted perimetry at 6 months after injection. Hauswirth et al. and Cideciyan et al. reported significantly higher dark adapted sensitivity 3 months after vector delivery compared to baseline in two out of three subjects, whereas no significant changes were reported in central visual acuity.12,13
We reported, at short-term follow-up, amelioration in retinal and visual function in all three subjects treated with a low dose (1.5 × 1010 particles/eye) of AAV2-h_RPE65_v2.14 Improvement in objective measures including PLR and reduction in nystagmus were accompanied by increased central visual acuity in three out of three subjects and ability to navigate an obstacle course in one out of three subjects.14 The significant improvement in retinal and visual function we observed as early as 1 month after treatment14 and through 1.5 years after treatment allows us to: (i) quantitatively assess whether visual function improves, deteriorates, or remains stable over time after gene transfer, and (ii) compare the influence of the treatment with the untreated condition: i.e., evaluate the possibility that natural history of this disease has been altered.7 We have extended the follow-up in our cohort of subjects treated with the low-vector dose through 1.5 years after vector administration. Here, we show the safety and tolerability of the gene therapy approach as well as the stability of its therapeutic efficacy.
Results
Safety profile
In the follow-up visits through 1.5 years after surgery, immunologic responses were benign and no serious adverse events occurred. Serum antibodies to the RPE65 transgene product were not detected after vector administration. There was a mild increase in serum neutralizing antibodies to AAV2 at day 90 in subject 1 and at day 14 in subject 2 (Supplementary Table S1). Levels diminished thereafter and had returned to baseline levels by day 365 after vector administration.
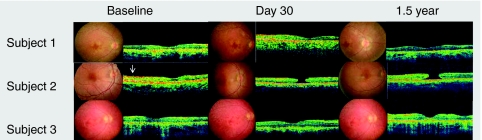
In subjects 1 and 3, fundus photography and optical coherence tomography performed on the treated eye at 1.5 years after surgery show an unchanged macular profile and thickness compared to baseline. In subject 2, a stage 4 macular hole developed 14 days after surgery. There was no expansion of this hole over the 1.5-year follow-up period (Figure 1). The macular hole did not prevent the vision improvement observed as early as 30 days after injection. Visual acuity (VA) in all three subjects was stable for up to 1.5 years, the last time point of observation.
Figure 1.
Fundus photographs and corresponding optical coherence tomography images through the fovea are shown for all three subjects at baseline, day 30, and 1.5 years after gene delivery. In subject 2, the epiretinal membrane is visible at baseline (arrow). A full-thickness macular hole is apparent on day 30 and 1.5 years. The adjacent retina remains attached.
Efficacy profile
All three subjects reported that the improvement in vision perception in dimly lit environments they noticed starting 2 weeks after therapy was maintained through 1.5 years, the latest time point for the analysis reported here.
Objective evaluation of visual function
Analysis of the PLR. In normal-sighted individuals, a stimulus delivered to either eye alone will cause both pupils to constrict equally—i.e., the PLR response is consensual (Figure 2, controls). When the light is removed, both pupils normally expand. Successive illumination of right and left eyes thus leads to oscillations in pupil diameters of both eyes (Figure 2, controls). PLRs are significantly impaired in subjects with LCA. We showed previously that subretinal delivery of AAV2.hRPE65v2 resulted in improvement in PLRs only for stimuli in the injected eye. This results in the appearance of a relative afferent pupillary defect of the untreated eye; i.e., the injected eye has better retinal function/PLR sensitivity than the untreated eye.14 The differences can be quantitated through measures of the velocity and the amplitude of the PLR (VPLR and amplitude of pupillary constriction APC, respectively).
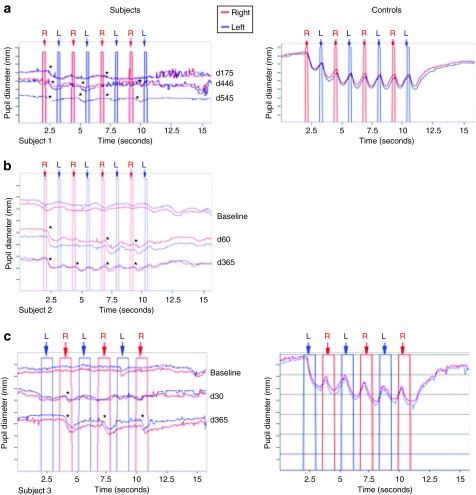
Figure 2.
Representative long-term follow-up pupillometry results in the three subjects. The pupillary light reflexes after dark adaptation are shown as a response to alternating stimulation of the right eye and the left eye. The curves represent the diameter of the pupils; the right pupil is slightly higher than the left pupil in each trace where two pupils are shown. The following pupil traces were displaced vertically to facilitate comparisons: subject 1 day 545, subject 2 baseline, day 365, day 545; subject 3 baseline, subject 3 day 365, subject 3 day 545. The average pupil diameters immediately prior to the first light exposure in the series were as follows: subject 1, 6.4 mm for both pupils at day 175; 5.6 (right), 5.28 (left) at day 446, and 4.9 (right) at day 545; subject 2: 7.1 for both pupils at baseline, 7.8 mm (right) and 7.1 mm (left) pupil at day 60; 4.7 mm for both pupils at day 365 and 4.96 mm (left) pupil at day 545; subject 3: 6.6 mm for both pupils at baseline; 6.3 (right) and 6.1 (left) at day 30; 5.8 (right), 5.4 at day 305 and 4.56 mm at day 545. Alternating stimulation with light of 10.0 lux, 10.0 lux, and 0.04 lux, respectively for subjects 1, 2, and 3. Timepoints (days) with respect to injection are indicated to the right of the traces. Results for test 1 [exposure of eyes to alternating brief (0.2 seconds) flashes of light] are shown for subjects 1 and 2; results for test 2 [exposure of eyes to alternating long (1.0 seconds) flashes of light] are shown for subject 3. Alternating stimuli were presented 2 seconds after the recording was initiated.
Measurement of PLR in the study subjects provided an objective evaluation of the transmission of impulses initiated in photoreceptors through the retinal ganglion cells toward pupillomotor centers in the central nervous system. Baseline testing of PLR showed no detectable difference in VPLR and APC between the two eyes in all three subjects. The PLR of the three subjects were much less sensitive to light than those of control subjects.14 As soon as 1 month after injection, when eyes were exposed to alternating brief (0.2 seconds) flashes of light (test 1), both VPLR and APC increased in the injected eye relative to that of the uninjected eye. The differences between the PLRs and APCs in the injected and the uninjected eyes persisted through 1.5 years (day 545) for all subjects, the latest timepoint tested (Figure 2). Representative PLRs in Figure 2 show presence of a PLR in the injected right eyes (asterisks). The responses in the uninjected left eyes are unchanged from baseline—i.e., responses are flat or are significantly reduced compared to the injected right eyes.14 The asymmetries are similar to those observed at the previous timepoints: day 446 for subject 1 (VPLR: P < 0.0001; APC: P = 0.001) (Figure 2a), day 365 for subject 2 (VPLR: P = 0.03; APC: P = 0.007) (Figure 2b) and day 305 for subject 3 (VPLR: P = 0.004; APC: P = 0.001) (Figure 2c; see also Supplementary Appendix). Whereas most of the improvement occurred in the first 1–4 months after injection in all three subjects, there was evidence of further improvement in velocity between day 150 and 415 timepoints (and then with this improvement persisting through day 545) in subject 1 (P = 0.007; also see changes in downward slopes over time after stimulation of the right eye in Figure 2a).
Additional testing was performed by exposing the eyes to alternating longer duration (1.0 second) flashes (test 2). Similar to the results using the short flashes, both the velocity and the constriction amplitudes of the pupillary light response were increased in the injected eyes relative to the uninjected eyes at ~1–1.5 years after injection. The P values comparing the velocities at day 415, day 365, and day 305 after injection of subject 1, subject 2, and subject 3, are P = 0.01, 0.09, and 0.001, respectively. The P values comparing the constriction amplitudes at those same long-term timepoints are P = 0.04, 0.32, and 0.02, respectively for subject 1, subject 2, and subject 3; also note the changes in constriction amplitudes over time after stimulation of the right eye in Figure 2a–c (also see Supplementary Appendix). Overall these results suggest that the improvement in PLR observed in subjects 1–3 is stable for at least 1 year after vector administration.
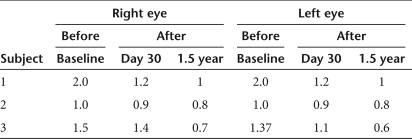
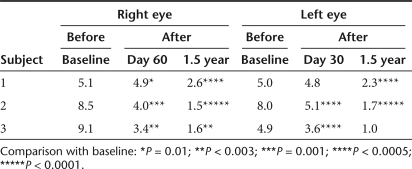
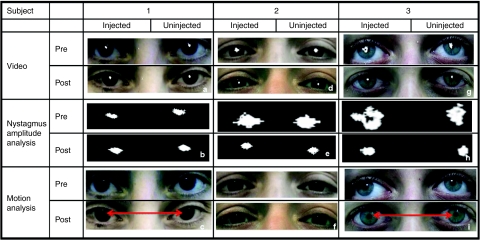
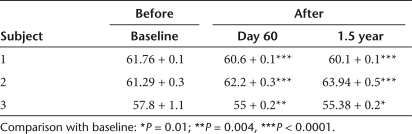
Ocular motility testing. Digital eye-movement videos showed at baseline that subject 1 had exotropia (outward deviation of the visual axis in one or both eyes) and multiplanar, vertical-greater-than horizontal, moderate-amplitude and moderate-frequency ocular movements; subject 2 had largely oblique, jerk, low-amplitude, and moderate-frequency ocular movements; subject 3 had exotropia of the right eye and a largely vertical, pendular, low-amplitude, low-frequency oscillation. After treatment in all three subjects the eye movements were constant, and symmetric, with frequency oscillation that increased in intensity in eccentric gaze and with monocular cover. At follow-up from day 60 on, subjects 1 and 3 had at least a 50% decrease in both monocular and binocular nystagmus frequency in primary position (Table 1). All subjects also show a significant reduction in binocular nystagmus amplitude in primary gaze at follow-up (Table 2). Interestingly, we also noticed a decrease in exotropia in subjects 1 and 3, as evident from the decreased interpupillary distance (Figure 3; Table 3). In patient 3, we also observed a change from exotropia in the right eye to alternating exotropia with a slight predominance of the right eye. These results show that following RPE65 gene delivery to one eye of the LCA2 subjects there is a stable reduction in ocular movements of both eyes accompanied by an improved ocular motility.
Table 1.
Nystagmus frequency measured in primary gaze as a function of time after injection
Table 2.
Amplitude of nystagmus measured in primary gaze before and after injection (degrees)
Figure 3.
Pre- and postinjection nystagmus analysis. (a,b) Frames from pre- and postinjection video recordings in subject 1 with motion paths of the pupils superimposed. Motion of each eye is shown by indicating the location of the center of the pupil over 100 sequential frames (total of 4 seconds) isolated from the video recordings. In b, the motion path is isolated. In c, pre- and postinjection measurements in subject 1 show reduction in interpupillary distance with concomitant changes in the corneal light reflexes (arrowheads). Similar analyses are shown for (d–f) subject 2 and (g–i). Analyses show a significant decrease in binocular amplitude of nystagmus in primary position in subjects 2 and 3 after injection and a smaller decrease in amplitude in subject 1 after injection. There is also a reduction in interpupillary distance in (i) subject 3.
Table 3.
Maximum interpupillary distance (mm) measured in primary gaze
Electroretinographic testing. Full-field electroretinography (ERG) performed before and after surgery and up to 1.5 years in all three subjects shows no change in retinal responses to flash.
Subjective evaluation of visual function
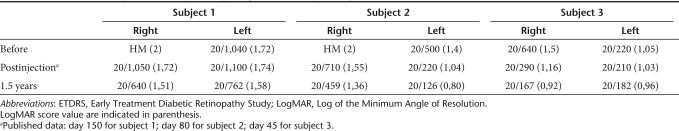
Acuity testing. Visual acuity had significantly improved in the injected eye in all three subjects as early as 1 month after injection as previously reported.14 Here, at the 1.5-year timepoint, visual acuity has continued to improve compared to previously reported values assessed short-term after treatment.14 LogMAR (Logarithm of the Minimum Angle of Resolution) score improved by 0.21 for subject 1 (from 3 to 5 lines on the eye chart at 50 cm), by 0.19 for subject 2 (from 4.5 to 6.2 lines on the eye chart at 50 cm) and by 0.24 for subject 3 (from 8 to 10.4 lines of letters at 50 cm) (Table 4).
Table 4.
ETDRS visual acuity results in injected eye (right) and uninjected eye (left)
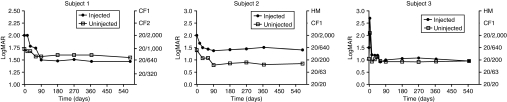
The VA data (Figure 4) show that the greatest gain in the injected eye was obtained in the first few months following injection. However, interestingly, there was an additional improvement thereafter, although the low number of timepoints, and thus tests, allows us to present descriptive statistical data only. It is worth emphasizing that in both subjects 1 and 3, the treated eyes, the worst eyes before injection, improved and have become the eyes with the better VA. Furthermore, improved, albeit not statistically significant, VA was evident in subject 2's uninjected eye as early as the first few days after gene delivery (going from an average LogMAR of 1.40 at baseline to an average LogMAR of 1.14 in the first 2 weeks after injection (P = 0.34) and this improvement has continued up to 1.5 years after injection. In subjects 1 and 3 there was no statistically significant improvement in the untreated eyes between baseline and short-term follow-up14 whereas a slight improvement was observed in subject 1 between short term (day 150) and 1.5-year follow-up (Figure 4). In subject 3 no improvement was observed between short term (day 45) and 1.5 year follow-up (Figure 4).
Figure 4.
Visual acuity (LogMAR on the left y axis and Snellen value on the right y axis) over time after gene delivery. Baseline is indicated as day 0; gene delivery (no visual acuity measurement) is on day 1.
Goldmann visual field tests. Goldmann visual field tests carried out 1.5 years after injection did not show a significant change in the total measured area seen by the injected eye (or the uninjected eye) compared to early postinjection changes which were significant compared to baseline (data not shown). The small, enlargement of the peripheral isopters observed in subject 2 may reflect the extreme variability of perimetric measurements in low-vision subjects.15
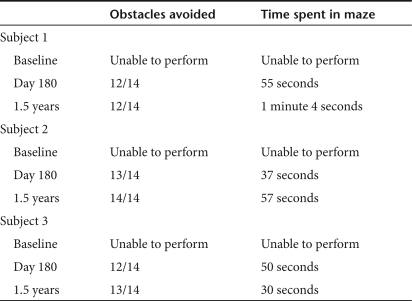
Mobility testing. In the ability to navigate an obstacle course, all three subjects, compared to day 30,14 showed a slight continuous improvement up to 1.5 years after surgery in terms of both time necessary to complete the test and number of obstacles avoided (see Table 5 and subject 2; Supplementary Video S1). A learning effect in this subjective test cannot be excluded, although the course configuration was changed for each test.14 Subject 2 appearing in the Supplementary Video S1 signed media consents.
Table 5.
Performance of the patients in the mobility test
Discussion
In LCA, vision deteriorates over time bilaterally and patients usually reach total blindness by the third or fourth decade of life. Our results show that improvement in both subjective and objective measurements of visual/retinal function observed as early as 1 month after subretinal administration of a low dose of AAV2-h_RPE65_v2 in three subjects persists through the 1.5-year postinjection timepoint.14 The improvement in both velocity and amplitude of PLR is particularly relevant considering the objectivity of this test which is used to probe transmission of retinal signals to higher nervous centers.3 This test also revealed a sustained improvement in light sensitivity in each of the subjects. In addition, a significant reduction in nystagmus was observed in all subjects continuing through 1.5 years after injection. Even though the subjects received uniocular treatment, the nystagmus showed binocular damping. There is evidence that both animals16 and humans17,18 with nystagmus due to vitreous opacity or cataract, show improvement of ocular motility in both eyes when receiving uniocular treatment that restores visual function. This could be due to a net improved visual acuity that allows a more stable fixation and hence better ocular-motor response in both eyes. Alternatively, it may also reflect the combining of the visual signals from each eye in the lateral geniculate nucleus as suggested by Jacobs et al.16 Alternatively, it may result from increased visual input which initiates and maintains the calibration of the ocular motor system.17,18 Indeed, results from canine and humans studies suggest that late plasticity enables the self-recalibration of the ocular motor system once sufficient visual input is available.16
We have additionally observed persistence over time of a significant improvement in VA in the injected eyes. Notably, in subjects 1 and 3, the injected eye, the worst eye before treatment, improved. Even though a placebo effect cannot be excluded because the subjects were aware of which eye was injected, the differences in the number of letters read at baseline versus after injection timepoints are significant using conservative analytic criteria. Together, the improvement in VA, the reduction in nystagmus and strabismus, and the PLR improvement suggest that significant amelioration of visual function occurs after treatment. No change was observed compared to baseline in the full-field ERG but the test was likely not informative due to: the limited area of retina treated; the low-vector dose injected; the minimal amount of photoreceptors remaining at this advanced stage of disease; the capability of retinal pigment epithelial (RPE) cells to synthesize 11-_cis_-retinal despite restoration of RPE65 expression; and the capability of photoreceptors to use 11-_cis_-retinal.
In contrast to this study, Bainbridge et al.11 and Cideciyan et al.12 did not report improved acuity in their study subjects despite evidence for improved retinal function especially for rod photoreceptors. Several factors could account for this difference. First, the subjects in their studies had substantially better acuity at baseline than those described here and it is unlikely that they would manifest improvement even with restoration of rod function in the paramacular area where resolution is limited. In addition, in the present study, the subretinal injection was delivered close to the central macula where both rod and cone density and, thereby, resolution is greater. It is possible that some of the improvement in VA measured in our study results from neuroanatomical and physiologic changes in the brain secondary to enhanced stimulation from the AAV-transduced retinal neurons. This possibility will be explored in future studies. Nonetheless, improvements in the PLR correlated in time with improvements in VA in the current study, providing objective confirmation of the subjective measures of efficacy. Interestingly, we observed improvement in VA of the untreated eye in subject 2 that was not associated with an improvement in the PLR. This result may be due to the reduction in nystagmus amplitude of both eyes, resulting in more stable fixation.
An analysis of the mobility tests performed at various timepoints after injection shows a gradual and more confident behavior of the subjects when navigating the obstacle course. A better perception of the course and its obstacles was evident.
Finally, the long-lasting improvements in retinal/visual function in the three subjects were achieved safely and with minimal immune response and no serious adverse events. Although low levels of neutralizing antibodies to AAV2 did develop in two of the three subjects, those responses were transient and levels returned to baseline between 3 and 12 months after administration. Interestingly, neutralizing antibody levels plateaued at different timepoints following surgery for the two responding subjects. The significance of the differences in timecourses is unknown. The macular hole that developed in one patient 14 days after injection did not show signs of evolution at long term. In accordance with our previous hypothesis,11 the macular hole can be considered a result of the constriction of a pre-existing membrane stimulated by the surgical procedure.
In all three trials testing the safety and efficacy of AAV2 in the retina of LCA2 subjects, no serious adverse events have been reported.11,13,14 In recent reports from Bainbridge et al.11 and Cideciyan et al.12,19 subjects were followed-up to 1 year. Neither group showed significant improvements in objective test results, although Cideciyan et al. used subjective testing (dark adaptometry) to show persistence of enhanced light sensitivity.12,19 Use of the PLR response allows an objective comparison between retinal function in treated and control eyes that provides a robust basis to determine any rescue effect of the intervention. Cideciyan et al. also used subjective testing to show improved fixation in one of three subjects.20 Here, we show improved fixation in three out of three subjects, evident much sooner than 1–1.5 years, using objective criteria.
The data from the 1.5-year follow-up in the LCA2 subjects treated in this trial allow us to draw some important conclusions: (i) transgene expression resulting from AAV delivery is stable over time, thus ruling out the possibility that the improvement in visual function observed is the result of a transient neurotrophic effect induced by the surgical procedure; (ii) the kinetics of the improvement with early amelioration which stabilizes over time are consistent with AAV2-hRPE65v2-mediated correction of a visual cycle enzymatic defect: indeed AAV2-mediated RPE65 gene expression occurring in animal models a few weeks after gene transfer is expected to rapidly “unlock” the visual cycle blocked by RPE65 deficiency; (iii) although longer term follow-up is required, efficacy of gene transfer seems not to be affected by the progressive degenerative nature of LCA2; and (iv) AAV2-mediated gene transfer to the human retina, similarly to what was observed in muscle21 and central nervous system,22 does not elicit the cytotoxic T-lymphocyte responses to AAV capsids observed in human liver.23 The safety and efficacy results reported here suggest that the retina is an amenable target for stable AAV2-mediated gene transfer in humans.
Materials and Methods
All details of the design, consent, and vector administration in this phase 1 clinical trial have previously been reported.14 Briefly, three LCA subjects, (referred to here as subjects 1, 2, and 3) aged 19–26 years old were first evaluated at the Second University of Napoli (Napoli, Italy) and received their diagnosis based on visual and retinal function studies.4 They then underwent mutation screening for LCA genes and were assigned a molecular diagnosis of LCA2 at the Telethon Institute of Genetics and Medicine. After informed consent and confirmation of trial eligibility criteria, including legal blindness based on visual acuity and visual field testing as well as independent evaluation of the likelihood that the mutations were disease-causing (Carver Lab, Iowa City, IA), the eye with worse visual function was selected for delivery of AAV2-hRPE65v2. The vector was manufactured by the Center for Cellular and Molecular Therapeutics at The Children's Hospital of Philadelphia (Philadelphia, PA) and delivered subretinally at The Children's Hospital of Philadelphia as described.14
Subjects were evaluated before and after surgery at designated follow-up visits (1, 2, 3, 14, 30, 60, 90, 180, 270, 365 days, and 1.5 years) by complete ophthalmic examination, a general physical examination, and clinical and laboratory tests, including an assessment of vector biodistribution (shedding) and immune response. Baseline tests and follow-up visits up to day 30 were performed at both The Children's Hospital of Philadelphia and Second University of Napoli while the follow-up visits from day 60 to 1.5 years were performed at Second University of Napoli.
Efficacy for each subject was monitored with objective and subjective measures of vision, as described previously.14 Objective measures included evaluation of the PLR, ERG, and nystagmus testing. Bilateral full-field ERGs were recorded using LACE Elettronica Electrophysiology system (Pisa, Italy), with ERG-jet contact lens electrodes following International Society for Clinical Electrophysiology of Vision standard guideline.24
Pupil responses were recorded simultaneously in both eyes with a Procyon P2000 pupillometer and PupilFit4 software (Monmouthshire, UK) (N.J. Volpe, L. Dadvand, S.K. Kim, M.G. Maguire, G.-S. Ying, M.L. Moster et al., manuscript submitted). Pupillary responses to light were recorded with variants of the basic protocol. Test 1 presented the light stimulus for 0.2 seconds followed by a 1 second dark interval. Test 2 used a longer stimulus cycle with light stimulus presented for 1 second followed by a 0.6 seconds dark interval.25 Nystagmus was characterized qualitatively and quantitatively by analysis of motion paths in videos taken at baseline and postinjection timepoints. Interpupillary distances were measured directly from video frames.
Subjective measures included standard tests of VA, kinetic visual field measured using Goldmann perimetry (Haag Streit Perimeter 940; Haag Streit, Mason, OH),26 and mobility testing to assess the ability of the subjects to navigate an obstacle course. For mobility testing different mazes were used each time the test was performed and number of obstacles avoided or hit, number of landmarks identified and time spent in the maze were assessed.
Statistical analyses. As described in Maguire et al.,14 VA was measured with Early Treatment Diabetic Retinopathy Study (ETDRS) charts at 0.5 m and at 2.0 m and letter counts were recorded. These were then converted into the correct LogMAR score. For statistical purposes, the VA scores at baseline, day 30, and later were calculated using the average of at least four measurements at each time point. Comparisons were made between the VA results recorded in follow-up with the baseline and early postinjection measurements. Pupillometry data were quantified through comparison of VPLRs and APCs obtained in a series of recordings obtained using different intensities of light stimuli (0.04, 0.4, and 10 lux). Recordings were also obtained after stimulating the right eye first or the left eye first. With the exception of subject 1, in whom only one set of baseline recordings was obtained, baseline and postinjection recordings were obtained on at least two different days after 40 minutes of dark adaptation. Significance of changes in amplitudes and velocities of pupil constriction in the pupillometry studies were evaluated using two different methods. The paired _t_-test was used to determine whether there were differences in response resulting from light stimulation of the injected (right) eyes and the control (left) eyes. Analysis of variance and post hoc pairwise comparisons were used to determine whether there were differences in response at baseline and follow-up timepoints. Significance of changes in interpupillary distances an nystagmus amplitudes were evaluated with the paired _t_-test.
SUPPLEMENTARY MATERIALTable S1. Serum neutralizing antibodies directed against AAV2.Appendix. Summary of data analyzed for statistics of pupillometry responses in subjects 1, 2, 3 (pupillometry response summary).Video S1. Subject 2 navigates the obstacle course with few errors at 1.5 years after injection.
Supplementary Material
Table S1.
Serum neutralizing antibodies directed against AAV2.
Appendix.
Summary of data analyzed for statistics of pupillometry responses in subjects 1, 2, 3 (pupillometry response summary).
Video S1.
Subject 2 navigates the obstacle course with few errors at 1.5 years after injection.
Acknowledgments
This work was supported by the Center for Cellular and Molecular Therapeutics at the Children's Hospital of Philadelphia, the Foundation Fighting Blindness which sponsors the CHOP-Penn Pediatric Center for Retinal Degenerations, Research to Prevent Blindness senior scientist award (J.B.) and departmental award, the Macula Vision Foundation, the Paul and Evanina Mackall Foundation Trust, the Scheie Eye Institute, the F.M. Kirby Foundation, grants (TIGEM-P21, to A.A., E.M.S., and S.B., and GGP07180, to F.S.) from the Italian Telethon Foundation, Regione Campania Convenzione, grants 018933 Clinigene and 223445 AAVEYE from the European Community to A.A., a grant (UL1-RR-024134) from the Institutional Clinical and Translational Science Award Research Center, the Rosanne H. Silbermann Foundation, the Associazione Italiana Amaurosi Congenita di Leber, and the Howard Hughes Medical Institute (to K.A.H.). We thank the subjects and their families for their continuous support of the study; the medical, operating-room, anesthesia, and nursing staff at Children's Hospital of Philadelphia and the physicians and staff in the Division of Ophthalmology; Barbara Konkle and Giovanni Cucchiaro for lending their clinical expertise; the members of the hospital's institutional review board and its chair, Mark Schreiner, along with David Brint, Richard Hurwitz, Mark Blumenkranz, and David Birch, for their invaluable guidance; Robert Nelson, Andrea Ballabio, Alfredo Ciccodicola, Ernesto Rinaldi, and Valder Arruda for their helpful discussions; Stuart Fine and Monte Mills for their support; Daniel Chung, Fred Letterio, Randall Toy, Matthew Canver, Sohani Amarasekera, Mohammed Toure, Nicholas Volpe, Jr., Katherine H. Maguire, Armida Faella, Anna Nesti, Angelo Torre, Valentina Di Iorio, Vito de Novellis and Ida Marabese for their technical and clinical assistance.
REFERENCES
- Cremers FP, van den Hurk JA., and , den Hollander AI. Molecular genetics of Leber congenital amaurosis. Hum Mol Genet. 2002;11:1169–1176. doi: 10.1093/hmg/11.10.1169. [DOI] [PubMed] [Google Scholar]
- Fulton AB, Hansen RM., and , Mayer DL. Vision in Leber congenital amaurosis. Arch Ophthalmol. 1996;114:698–703. doi: 10.1001/archopht.1996.01100130690009. [DOI] [PubMed] [Google Scholar]
- Aleman TS, Jacobson SG, Chico JD, Scott ML, Cheung AY, Windsor EA, et al. Impairment of the transient pupillary light reflex in Rpe65(−/−) mice and humans with leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2004;45:1259–1271. doi: 10.1167/iovs.03-1230. [DOI] [PubMed] [Google Scholar]
- Simonelli F, Ziviello C, Testa F, Rossi S, Fazzi E, Bianchi PE, et al. Clinical and molecular genetics of Leber's congenital amaurosis: a multicenter study of Italian patients. Invest Ophthalmol Vis Sci. 2007;48:4284–4290. doi: 10.1167/iovs.07-0068. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Roepman R, Koenekoop RK., and , Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Wang H, den Hollander AI, Moayedi Y, Abulimiti A, Li Y, Collin RW, et al. Mutations in SPATA7 cause Leber congenital amaurosis and juvenile retinitis pigmentosa. Am J Hum Genet. 2009;84:380–387. doi: 10.1016/j.ajhg.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SG, Aleman TS, Cideciyan AV, Sumaroka A, Schwartz SB, Windsor EA, et al. Identifying photoreceptors in blind eyes caused by RPE65 mutations: Prerequisite for human gene therapy success. Proc Natl Acad Sci USA. 2005;102:6177–6182. doi: 10.1073/pnas.0500646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acland GM, Aguirre GD, Bennett J, Aleman TS, Cideciyan AV, Bennicelli J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- Bennicelli J, Wright JF, Komaromy A, Jacobs JB, Hauck B, Zelenaia O, et al. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther. 2008;16:458–465. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG., and , Carr RE. Stargardt's disease and fundus flavimaculatus. Arch Ophthalmol. 1979;97:1281–1285. doi: 10.1001/archopht.1979.01020020023005. [DOI] [PubMed] [Google Scholar]
- Jacobs JB, Dell'Osso LF, Hertle RW, Acland GM., and , Bennett J. Eye movement recordings as an effectiveness indicator of gene therapy in RPE65-deficient canines: implications for the ocular motor system. Invest Ophthalmol Vis Sci. 2006;47:2865–2875. doi: 10.1167/iovs.05-1233. [DOI] [PubMed] [Google Scholar]
- Cross SA, Smith JL., and , Norton EW. Periodic alternating nystagmus clearing after vitrectomy. J Clin Neuroophthalmol. 1982;2:5–11. [PubMed] [Google Scholar]
- Yagasaki T, Sato M, Awaya S., and , Nakamura N. Changes in nystagmus after simultaneous surgery for bilateral congenital cataracts. Jpn J Ophthalmol. 1993;37:330–338. [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, et al. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 2009;20:999–1004. doi: 10.1089/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, et al. Vision 1 year after gene therapy for Leber's congenital amaurosis. N Engl J Med. 2009;361:725–727. doi: 10.1056/NEJMc0903652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Pierce GF, Ozelo MC, de Paula EV, Vargas JA, Smith P, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. 2006;14:452–455. doi: 10.1016/j.ymthe.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Marmor MF, Holder GE, Seeliger MW., and , Yamamoto S.International Society for Clinical Electrophysiology of Vision (2004Standard for clinical electroretinography (2004 update) Doc Ophthalmol 108107–114. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Moore P., and , Kardon RH. Variability of the relative afferent pupillary defect. Am J Ophthalmol. 1995;120:622–633. doi: 10.1016/s0002-9394(14)72209-3. [DOI] [PubMed] [Google Scholar]
- Ross DF, Fishman GA, Gilbert LD., and , Anderson RJ. Variability of visual field measurements in normal subjects and patients with retinitis pigmentosa. Arch Ophthalmol. 1984;102:1004–1010. doi: 10.1001/archopht.1984.01040030806021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Serum neutralizing antibodies directed against AAV2.
Appendix.
Summary of data analyzed for statistics of pupillometry responses in subjects 1, 2, 3 (pupillometry response summary).
Video S1.
Subject 2 navigates the obstacle course with few errors at 1.5 years after injection.