Regulation of synaptic vesicle recycling by complex formation between intersectin 1 and the clathrin adaptor complex AP2 (original) (raw)
Abstract
Clathrin-mediated synaptic vesicle (SV) recycling involves the spatiotemporally controlled assembly of clathrin coat components at phosphatidylinositiol (4, 5)-bisphosphate [PI(4,5)P2]-enriched membrane sites within the periactive zone. Such spatiotemporal control is needed to coordinate SV cargo sorting with clathrin/AP2 recruitment and to restrain membrane fission and synaptojanin-mediated uncoating until membrane deformation and clathrin coat assembly are completed. The molecular events underlying these control mechanisms are unknown. Here we show that the endocytic SH3 domain-containing accessory protein intersectin 1 scaffolds the endocytic process by directly associating with the clathrin adaptor AP2. Acute perturbation of the intersectin 1-AP2 interaction in lamprey synapses in situ inhibits the onset of SV recycling. Structurally, complex formation can be attributed to the direct association of hydrophobic peptides within the intersectin 1 SH3A-B linker region with the “side sites” of the AP2 α- and β-appendage domains. AP2 appendage association of the SH3A-B linker region inhibits binding of the inositol phosphatase synaptojanin 1 to intersectin 1. These data identify the intersectin-AP2 complex as an important regulator of clathrin-mediated SV recycling in synapses.
Keywords: endocytosis, synapse, scaffolding proteins, appendage, synaptojanin
Synaptic vesicles (SVs), following their activity-dependent exocytic fusion with the presynaptic plasma membrane, are recycled by compensatory endocytosis at the periactive zone (1–3), largely via clathrin-mediated reinternalization of fully fused SV membrane (4). Clathrin-coated pit (CCP) formation (5) proceeds through the assembly of endocytic proteins at phosphatidylinositiol (4, 5)-bisphosphate [PI(4,5)P2]-enriched membrane sites (6, 7). A key factor in the assembly pathway is the heterotetrameric adaptor complex AP2, whose α- and β2-appendage domains act as major recruitment platforms for accessory proteins (6, 7), regulating distinct steps within the pathway. Despite our extensive knowledge regarding the endocytic interactome, we know comparably little about the structural components within the periactive zone that scaffold the endocytic process, thereby allowing the high fidelity of SV recycling. Such spatiotemporal control is needed to coordinate SV cargo protein sorting with coat recruitment (8) and to restrain membrane fission and uncoating until membrane deformation and CCP assembly are completed. Moreover, stabilizing scaffolds may aid coupling of SV exo- and endocytosis (1, 3). The Drosophila multidomain protein Dap160, an ortholog of mammalian intersectin, has been postulated to act as an endocytic scaffold of the periactive zone (9–11), although its precise role in SV recycling in mammalian nerve terminals remains largely unclear (12).
Here we show that intersectin 1 scaffolds the endocytic process by directly associating with AP2. Acute perturbation of intersectin-AP2 complex formation blocks the onset of SV recycling. Moreover, association of the SH3A-B linker region of intersectin with AP2 inhibits binding of the inositol phosphatase synaptojanin 1. These data identify the intersectin-AP2 complex as an important regulator of clathrin-mediated SV recycling in synapses.
Results
Antibodies Targeting the Linker Region Between SH3 Domains A and B of Lamprey Intersectin 1 Disrupt SV Endocytosis at Early Stages.
Acute perurbation of intersectin interactions in living lamprey synapses with antibodies raised against the SH3 domain-containing module revealed multiple effects on SV recycling (13). Endocytosis was inhibited at the stage of constricted CCPs, consistent with a defect in dynamin-mediated fission. However, microinjection of antibodies targeting SH3 domains A to C, including the connecting linker regions of intersectin 1, also led to an accumulation of large membrane expansions, indicative of endocytosis inhibition at early stages (13). To further explore the underlying mechanisms of the early endocytic phenotype associated with intersectin perturbation at vertebrate synapses, we raised antibodies against different parts of the SH3 module, including the linker region between SH3 domains A and B of lamprey intersectin (LIS-linker). Affinity-purified LIS-linker IgG specifically recognized two bands of expected molecular weights of about 200 and 170 kDa (13) (lamprey intersectin 1 short and long isoforms) in detergent-extracted lamprey brain homogenates, and this reactivity was lost upon preincubation with the antigenic peptide (Fig. S1_A_). Similar results were seen if antibody specificity was assessed by immunoprecipitation of native endogenous intersectin 1 (Fig. S1_D_). LIS-linker IgG also recognized endogenous intersectin 1 accumulated within SV clusters in reticulospinal axons, as seen previously (13); again, this labeling was completely abolished by preincubation with the antigen (Fig. S1 B and C). A similar accumulation of intersectin 1 at SV clusters is also observed in mammalian synapses of hippocampal mossy fibers from the CA3 region and synapses established by Schaffer recurrent colaterals from CA1 and at corresponding postsynaptic elements (Fig. 1_A_ and Fig. S2 A–E). Thus, intersectin 1 is accumulated at presynaptic sites both in lamprey and in mammals.
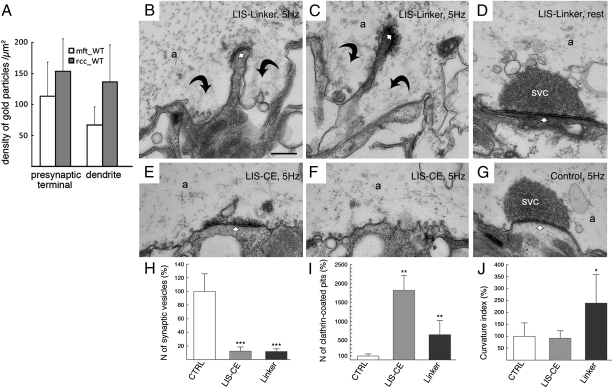
Fig. 1.
Antibodies targeting the linker region between SH3 domains A and B of intersectin 1, a pre- and postsynaptic endocytic protein, inhibit SV recycling. (A) Intersectin 1 accumulates at pre- and postsynaptic membrane sites. Depicted are quantifications of immunogold particles accumulated over nerve terminals and dendrites in mossy fiber terminals and recurrent Schaffer collateral synapses within the mouse hippocampus labeled with intersectin 1-specific antibodies. The difference between densities of gold particles in each synaptic compartment is not significantly different for both types of synapses (P > 0.05; n = 20 in each case; Student's t test). Bars depict mean densities of gold particles ± SEM. Background is below two particles per square micrometers in both cases. (B and C) Electron micrographs of reticulospinal synapses in an axon stimulated at 5 Hz for 20 min after injection of antibodies against the linker region between SH3 domains A and B of lamprey intersectin (LIS-linker). The vesicle cluster (SVC) is depleted, large membrane invaginations (curved arrows) and clathrin-coated intermediates are present. Small arrow, active zone. a, axoplasm. (D) Electron micrograph of an intact reticulospinal synapse after injection of LIS-linker antibodies kept at rest. Note the presence of a densely packed SV cluster at the active zone. (E) Electron micrograph of a reticulospinal synapse in an axon stimulated at 5 Hz for 20 min after injection of antibodies against the SH3 domains C to E of lamprey intersectin (LIS-CE). Note the accumulation of CCPs and the absence of membrane invaginations. (F) Electron micrograph of an intact reticulospinal synapse stimulated at 5 Hz for 20 min from a reticulospinal axon from the same preparation as in E. (G) A synapse from an axon microinjected with nonspecific IgG and stimulated at 5 Hz. (H) Bar graph showing a reduction in the number of SVs in LIS antibody injected synapses. LIS-CE antibodies cause a strong increase in the number of CCPs (I), whereas LIS-linker antibody-injected synapses display numerous membrane pockets and invaginations (see curvature index, CI) (J). Nonspecific IgGs did not induce significant changes in synaptic morphology when compared to noninjected control synapses (H–J). Depicted are the means ± SD (*, P < 0.05; **, P < 0.01; ***, P < 0.001; Student's t test). (Scale bar, A and B, 1 μm.)
Having established that LIS-linker IgGs specifically recognize intersectin 1 in vitro and in situ, we microinjected these IgGs into lamprey reticulospinal axons and stimulated at 5 Hz for 20 min to induce SV recycling at a physiological rate. Analysis of ultrathin sections revealed a profound loss of SVs at active zones in synapses from these axons (Fig. 1 B, C, and H), but not from control axons microinjected with nonspecific IgGs (Fig. 1 G and H) (13). No morphological alterations were observed in axons microinjected with LIS-linker IgGs kept at rest (Fig. 1_D_). This change was accompanied by an accumulation of large membrane pockets and invaginations and a concomitant increase in the number of CCPs, predominantly at early stages of endocytosis (Fig. 1 B, C, H–J), indicative of an inhibition of clathrin-mediated SV recycling. This phenotype was clearly distinct from that observed following microinjection of antibodies against SH3 domains C to E of intersectin, which did not induce large membrane expansions around the active zone (Fig. 1 E, F, and J). A significant accumulation of coated intermediates in the periactive zones was observed instead in these synapses (Fig. 1 E, F, and I). These functional data indicate that the linker region between SH3 domains A and B of intersectin plays an important role at early stages of clathrin-mediated SV endocytosis, perhaps via direct interactions with one of the major coat components.
Intersectin 1 via its SH3A-B Linker Region Directly Associates with the α- and β-Appendage Domains of AP2.
To directly address the possibility that intersectin 1 interacts with clathrin/AP2, we performed immunoprecipitations from rat (Fig. 2_A_) and lamprey brain extracts (Fig. 2_B_). Antibodies specific for intersectin 1 (Fig. S1_E_) efficiently coprecipitated not only the established intersectin 1 binding partner dynamin 1, but also the plasmalemmal clathrin adaptor AP2 (Fig. 2 A and B). Hsc70 or AP1 were absent from the immunoprecipitates. Conversely, intersectin was found in immunoprecipitated material using monoclonal antibodies against AP2 (see below), indicating that intersectin and AP2 are present in a tight complex in vivo in both lamprey and in mammals. This conclusion is supported by the near perfect colocalization of intersectin 1 with AP2, but not with AP1 in astrocytes (Fig. S2 F and G) or primary neurons in culture (Fig. S2_H_). We also confirmed that intersectin 1 associates with CCPs in synapses by immuno-electron microscopy. In lamprey reticulospinal synapses stimulated at 5 Hz for 30 min, intersectin immunogold labeling on clathrin/AP2-coated intermediates at sites of endocytosis was predominantly associated with coats (Fig. 2_C_).
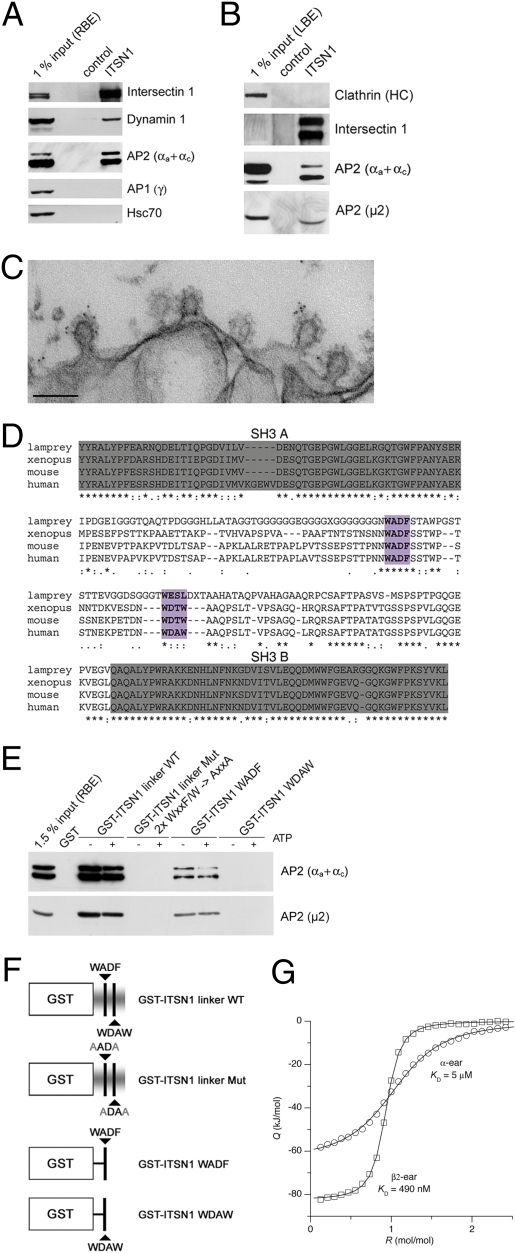
Fig. 2.
Intersectin associates with AP2 via direct binding to the α- and β2-appendage domains of AP2. (A) Immunoprecipitation from detergent-extracted rat brain lysates using control or anti-intersectin 1 (ITSN1) antibodies coupled to beads. Samples were analyzed by SDS/PAGE and immunoblotting. 1% input, 1% of the total amount of rat brain extract (RBE) added to the assay. (B) Same as in A but using detergent extracted lamprey brains instead. 1% input, 1% of the total amount of lamprey brain extract (LBE) added to the assay. (C) Electron micrograph of CCPs from the periactive zone of a lamprey reticulospinal synapse stimulated at 5 Hz for 30 min and labeled with intersectin LIS-AC antibodies. Note the accumulation of gold particles on CCPs. (Scale bar, 0.2 μm.) (D) Multiple sequence alignment of intersectin 1 SH3A-SH3B. Putative AP2-binding peptides are highlighted in purple. (E) GST or GST-intersectin 1 fusion proteins were immobilized and incubated with RBE in the presence or absence of ATP. Samples were analyzed by SDS/PAGE and immunoblotting. (F) Schematic representation of the proteins used for pull downs. Putative AP2 binding motifs and corresponding mutants are labeled (compare also to D). (G) High-affinity binding of intersectin 1 linker to the AP2β appendage as determined by isothermal titration calorimetry (ITC) analysis. Intersectin 1-derived linker peptide was injected into α- or β2-appendage. Depicted are integrated, normalized, and dilution-corrected heats of reaction, Q, versus molar peptide/protein ratio, R. Heats of reaction obtained by injecting peptide into α- (circles) or β2-appendage (squares) were fitted by a one-site binding model (solid lines), yielding the given _K_D values.
Given the strong early endocytic phenotype elicited by microinjection of LIS-linker IgG, we suspected that the intersectin 1 SH3A-B linker region is involved in complex formation with AP2. Analysis of the primary sequences of the linker region from various species indeed revealed the presence of two evolutionary conserved sequences that resemble AP2 binding motifs found in other endocytic proteins. Although none of the two strictly conforms to any of the known AP2 binding consensus sequences, the strong conservation of large aromatic residues (W, F) flanked by negative charges suggested that the WADF and WDxW peptides might bind to AP2 (Fig. 2_D_). To directly test this possibility, we prepared GST fusion proteins comprising the WT linker or mutants, in which one or both putative AP2-binding peptides had been mutationally inactivated or deleted (Fig. 2_F_). Native AP2 was efficiently retained by immobilized GST-intersectin 1 linker but not by a mutant lacking the conserved aromatic residues within the putative AP2 binding motifs. A GST-fused WADF peptide also retained AP2 from brain extracts, albeit less efficiently, whereas GST-WDAW was ineffective (Fig. 2_E_). As most other endocytic proteins target the appendage domains of AP2 (6, 7), we incubated GST-linker region fusion proteins with purified His6-tagged α- or β2 appendage domains. We observed potent binding of GST-linker to both ear domains and this was dependent on conserved aromatic amino acids within both peptides. Under these conditions, avid binding of GST-WADF and a much weaker association of GST-WDAW with both appendages was observed (Fig. S3). Isothermal titration calorimetry revealed a thermodynamic preference of the intersectin 1 linker for the AP2-β- over the AP2-α-appendage domain with binding constants of 490 nM and 5 μM, respectively (Fig. 2_G_).
To verify that the intersectin 1 SH3A-B linker region indeed can associate functionally with AP2, we coexpressed intersectin 1-mCer linker (WT or AxxA mutant) with the pH-sensitive reporter synaptopHluorin (14) in primary cultures of cerebellar granule neurons (CGNs). CGNs showed a robust increase in fluorescence after stimulation by either KCl or a train of action potentials, reflecting fusion of SVs with the cell surface (Fig. S4 A and B). This fluorescence remained elevated in neurons expressing intersectin 1-mCer (WT) after stimulation, indicative of a block in SV endocytosis. By contrast, fluorescence in neurons expressing the AP2 binding-defective mutant decreased back to its initial value (Fig. S4 A and B). The slight difference in fluorescence increase in neurons expressing intersectin 1-mCer (WT) compared to mutant-expressing cells during the late phase of stimulation likely reflects inhibition of compensatory SV endocytosis accompanying exocytic fusion events. In agreement with this, kinetic rise times of pHluorin de-quenching are identical in neurons expressing intersectin 1-mCer (WT) or mutant (Fig. S5_A_). This interpretation is corroborated by experiments using FM1-43, which reveal a selective inhibition of SV recycling, but not exocytosis (Fig. S5 B–D). A similar block of SV endocytosis at early stages was observed in reticulospinal axons of the lamprey following microinjection of a lamprey intersectin-1 linker region-derived AP2 binding peptide. Analysis of ultrathin sections revealed a partial loss of SVs at active zones, an accumulation of large membrane pockets and invaginations, and a concomitant increase in the number of CCPs in intersectin 1-peptide (Fig. S4 C, D, and F–H), but not in non-injected control synapses (Fig. S4 E–H). Thus, overexpression or microinjection of an intersectin 1-derived AP2 binding peptide impairs SV endocytosis, likely via sequestration of endogenous AP2, confirming our biochemical data.
Structural Basis for the Association of Intersectin 1 with the Sandwich Subdomains of AP2α and β2.
The observation that antibody-mediated perturbation of intersectin 1 association with AP2 potently inhibits SV endocytosis at early stages (Fig. 1) prompted us to investigate the structural basis of complex formation. We synthesized the major AP2 binding peptide (compare Fig. 2; PNNWADFSSTWP) and used it for cocrystallization experiments with purified α- or β2-appendage. We obtained crystals for both α- and β2-appendages that diffracted to a maximal resolution of 1.9 Å and 2.15 Å, respectively. The structure model refined with excellent B-factors for α-appendage and peptide ligand. Somewhat higher B-factors were obtained for the β2-appendage complex (Table S1). The intersectin 1 WADF peptide binds to an extensive surface on the β-sandwich subdomain of the α-appendage (also termed the side site) (Fig. 3_A_). W843 and F846 (within mouse intersectin 1L), which form the core consensus motif, are seen in shallow pockets. F846 is π-stacked to F740 of AP2α and W843 is hydrogen-bonded to G780. Additional hydrogen bonds involve E702, E718, Q723, and Q782, which interact with the peptide backbone or with the side chain of S848 (Fig. 3_B_).
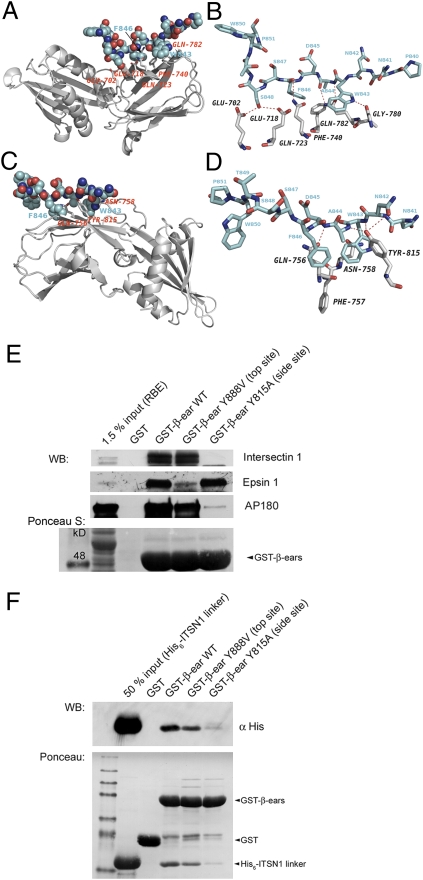
Fig. 3.
The intersectin 1-derived WADF motif peptide accommodates the sandwich subdomain of the AP2α or AP2β appendages. (A) Ribbon diagram showing the binding site for the mouse intersectin 1-derived peptide (amino acids 840–851, blue) in complex with the α-appendage (gray). The peptide binds to an elongated pocket at the side site of the α-appendage sandwich subdomain. (B) Close-up view showing direct molecular contacts between the intersectin 1-derived peptide (blue) and the side site of the α-appendage. See text for details. (C) Ribbon diagram showing the binding site for the mouse intersectin 1-derived peptide (blue) in complex with the β2-appendage (gray). The peptide binds to an elongated pocket at the side site of the β2-appendage sandwich subdomain. (D) Close-up view showing direct molecular contacts between the intersectin 1-derived peptide (blue) and the side site of the β2-appendage. See text for details. (E) One-hundred microgram GST-fusion protein or GST immobilized on glutathione beads were incubated with 2 mg RBE. Samples were analyzed by SDS/PAGE and immunoblotting. ITSN1 binding was abolished in the side site mutant Y815A but unaffected by mutation of the top site of the β2-appendage. (F) In vitro binding assay: 50 μg GST-fusion protein or GST immobilized on glutathione beads were incubated with 10 μg His6-tagged ITSN1 linker. A side site mutant (Y815A) of the β2-appendage fails to bind to the ITSN1 linker.
The same intersectin-1 peptide can also accommodate the sandwich subdomain of the β2-appendage (Fig. 4_C_), a rare, if not unique feature among the known endocytic proteins. Of note, the side site of the β2-sandwich lies on the opposite face of the subdomain compared to the α-appendage (compare Fig. 3 A and C). The WADF peptide largely binds via aromatic amino acids W843, and F846. W843 is surrounded by Y815 and N758. F846 projects into a shallow pocket formed by F757 and Q756 (Fig. 3_D_). Additional major contacts involve hydrogen bonds between Y815 and Q756 with the peptide backbone (Fig. 3_D_ and Table S2). The conformation of the WADF peptide is similar to that previously reported for a short peptide fragment derived from Eps15 (Table S3) (15, 16). Intersectin 1 binding to the side site of the β2-appendage was confirmed by site-directed mutagenesis. GST-fused β2-appendage avidly bound to intersectin 1, the FxDxF motif-containing endocytic protein AP180, and epsin 1, an endocytic adaptor that predominantly associates with the top site of β2 via DPF motifs. Mutation of Y815 to A815 selectively abolished association with intersectin 1 and AP180, identifying both proteins as β2 side-site specific ligands, whereas binding to epsin 1 was unaffected. Conversely, the GST-β2 appendage Y888V mutant had selectively lost the ability to pulldown epsin 1 although retaining complex formation with intersectin 1 and AP180 (Fig. 3_E_). Selective association of intersectin 1 with the crystallographically determined side site of the β2-appendage was finally demonstrated using purified His6-tagged intersectin 1 linker (Fig. 3_F_). We conclude that intersectin 1 contacts the side sites of both AP2 appendage domains, a feature that may allow the protein privileged access to the AP2 hub during early stages of CCP formation. These structural and biochemical data fit well with the early endocytic defects observed upon perturbation of intersectin-AP2 complex formation in situ.
Fig. 4.
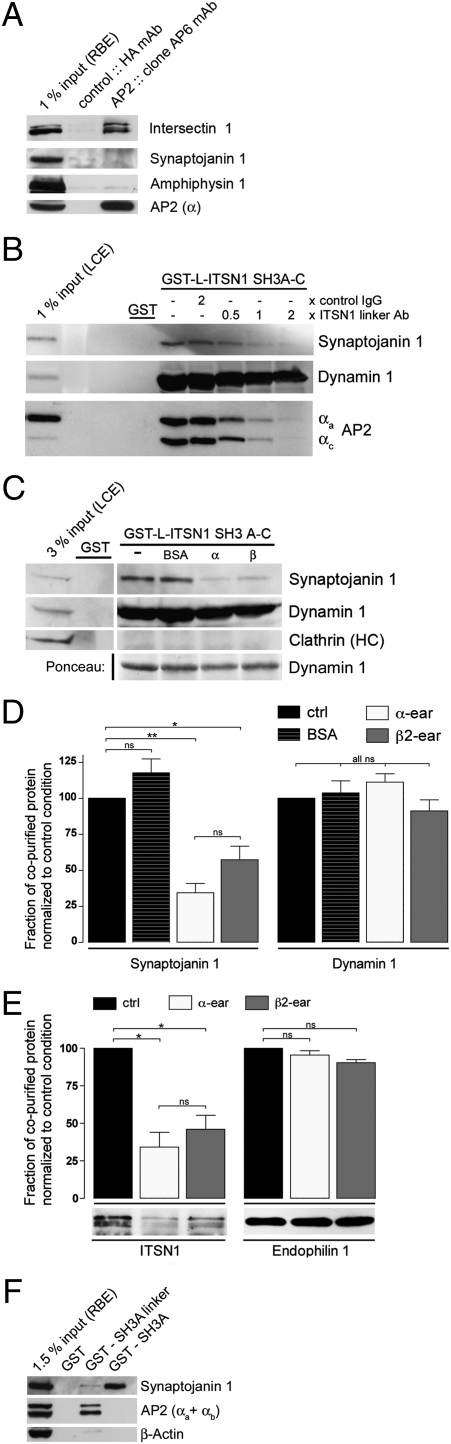
Association of intersectin 1 with AP2 impairs its binding to synaptojanin 1. (A) Top site-selective AP2 α-appendage antibodies (AP.6) coimmunoprecipitate intersectin but not synaptojanin, AP180, or amphiphysin from RBE. Samples were analyzed by SDS/PAGE and immunoblotting. 1% input, 1% of the total amount of RBE added to the assay. (B) Anti-intersectin linker domain antibodies inhibit complex formation of intersectin SH3A-C with AP2 and synaptojanin 1, but not with dynamin. GST-SH3A-C (LIS) or GST (100 μg) were used for affinity chromatography from lamprey spinal cord extracts (LCE) in the presence of the indicated molar ratios of IgGs targeting the intersectin 1 linker region or control IgGs. Samples were analyzed by SDS/PAGE and immunoblotting. 1% input, 1% of the total amount of LCE added to the assay. (C) Same as in B except that 1.5 mg purified α- or β2-appendage domains were used as competitors. Samples were analyzed by SDS/PAGE, staining with Ponceaus S (to visually illustrate dynamin 1 association), and immunoblotting for synaptojanin 1, dynamin 1, and clathrin heavy chain as a control. 3% input, 3% of the total amount of LCE added to the assay. (D) Quantification of the immunoblot signals for synaptojanin 1 and dynamin 1 seen in C (mean ± SEM; n = 3). (E) AP2 α- or β2-appendage domains inhibit complex formation between synaptojanin 1 and intersectin 1, but not endophilin A1. RBEs were subjected to immunoprecipitations with anti-synaptojanin 1-p145 antibodies in the presence or absence of AP2 α- or β2-appendage domains (500 μg). Depicted are the relative levels of intersectin 1 or endophilin A1 found in the immunoprecipitate normalized to the control condition (mean ± SEM; n = 2). Representative immunoblots for intersectin and endophilin are shown below the bar diagram. (F) GST-SH3A-linker, GST-SH3A, or GST (50 μg) were used for affinity chromatography from RBEs. Samples were analyzed by SDS/PAGE and immunoblotting for synaptojanin 1, AP2α, or β-actin. 1.5% input, 1.5% of the total amount of RBE added to the assay.
Association of AP2 with Intersectin 1 Prevents Synaptojanin 1 Binding.
Based on our data reported here and on previous studies, intersectin 1 acts as a major endocytic scaffold and recruitment platform that may coordinate AP2-based CCP assembly with dynamin-dependent membrane fission and synaptojanin 1-mediated PI(4,5)P2-hydrolysis. Intersectin 1 association with dynamin 1 has been shown to involve its SH3 domains A, C, and E, whereas synaptojanin 1 preferentially binds to SH3A (13). To obtain insights into possible mechanisms for coordinating these various activities of intersectin 1, we tested whether intersectin 1 can simultaneously bind to dynamin 1, synaptojanin 1, and AP2. To this aim, we first employed mouse monoclonal antibodies that selectively associate with the platform subdomain (the so-called top site) of the AP2 α-appendage. When immobilized on beads, these antibodies precipitated native AP2 from rat brain together with substantial amounts of intersectin 1. Synaptojanin 1 and amphiphysin, an endocytic protein that associates predominantly with the platform subdomain of AP2α, were both absent from these immunoprecipitates (Fig. 4_A_). To further explore the relationship between recruitment of AP2 and synaptojanin 1 to intersectin 1, we carried out affinity chromatography experiments from lamprey spinal cord extracts using immobilized GST-SH3A-C of lamprey intersectin 1 in the presence of IgG against the AP2-binding linker region (LIS-linker IgG). LIS-linker IgGs effectively competed recruitment not only of native AP2, but also of synaptojanin 1-p145 (the brain-specific short isoform of the enzyme). By contrast, GST-SH3A-C binding to dynamin 1 was much less affected (Fig. 4_B_). Similar results were obtained if the purified AP2α or β2 appendage domains were taken as competitors (Fig. 4 C and D). Conversely, purified recombinant synaptojanin 1-p145 tail domain did not interfere with AP2 binding to GST-SH3A-C (Fig. S6_A_), suggesting that synaptojanin 1 is unable to disrupt intersectin 1-AP2 complex formation directly. Collectively, our data suggest that association of intersectin 1 with AP2 may prevent it from binding to synaptojanin 1-p145. To test this prediction more directly, we carried out affinity chromatography experiments using GST-tagged SH3A domain fusion proteins either containing or lacking the AP2-binding linker peptide. As seen in Fig. 4_F_, GST-SH3A, a fusion protein lacking the ability to associate with AP2, displayed a greatly improved ability to pull down native endogenous synaptojanin 1-p145 from rat brain extracts when compared to GST-SH3A-linker. By contrast, both versions of GST-tagged SH3A avidly bound to purified synaptojanin 1-tail domains in the absence of AP2 (Fig. S6_B_).
To investigate whether native endogenous endocytic protein complexes may be subject to a similar regulatory mechanism, we carried out immunoprecipation experiments from detergent-extracted rat brains using mouse monoclonal antibodies directed against synaptojanin 1. In agreement with previous data (13, 17), synaptojanin 1-p145 was found to be associated with the SH3 domain-containing proteins endophilin A1 and intersectin 1. Addition of AP2α- or β2-appendage domains to these extracts selectively impaired association of synaptojanin 1-p145 with endogenous intersectin 1, whereas complex formation with endophilin A1 was unaffected (Fig. 4_E_). These data further support our conclusion that intersectin complex formation with AP2 impairs its association with synaptojanin 1.
Discussion
In this work we show that the endocytic scaffold intersectin 1 regulates early steps in clathrin-mediated SV recycling by directly associating with the clathrin adaptor complex AP2. Biochemical and crystallographic analyses identify an evolutionary conserved peptide motif that accommodates the side sites of the sandwich subdomains of the AP2 α- and β-appendages by a two-pin-plug mechanism. Blocking complex formation between AP2 and intersectin 1 by specific antibodies functionally arrests SV recycling at early stages, resulting in the accumulation of numerous plasma membrane-derived membrane pockes and invaginations. Thus, our data argue in favor of intersectin-1 complex formation with AP2 during early steps of CCP assembly. We predict similar phenotypes to be observed in mammalian neurons derived from knockout mice lacking both intersectins 1 and 2. In this context we note the presence of putative binding motifs for both AP2α (i.e., 1690WVxFD) and clathrin in intersectin 2, an intersectin paralog expressed in the brain of intersectin-1 knockout mice (18). It is likely that the scaffolding function of intersectin is aided by its association with the AP2-binding endocytic protein Eps15/Eps15R. The resulting multiprotein complex might then serve as a template to scaffold assembly of AP2-containing coat complexes (19) at PI(4,5)P2-enriched membrane sites within the periactive zone.
Our data also indicate that AP2 binding to intersectin 1 might occlude recruitment of the PI(4,5)P2-phosphatase synaptojanin 1. These results suggest a potential, although at this stage clearly hypothetical, mechanism whereby direct complex formation between AP2 and intersectin inhibits premature recruitment of synaptojanin 1 to CCPs at early stages, thereby preventing premature PI(4,5)P2-hydrolysis. How can such competition be explained in molecular terms? Earlier data have shown that synaptojanin 1 preferentially associates with the SH3A domain of intersectin 1 (13), a site in close proximity to the major AP2 binding sequence within the SH3A-B linker region. It is thus possible that intersectin 1-bound AP2 by steric hindrance selectively impairs synaptojanin 1-p145 recruitment to intersectin 1-containing endocytic sites during the initial stages of CCP assembly. As clathrin recruitment and assembly proceeds, engagement of the clathrin terminal domain with the side site of the AP2 β-appendage (20) might release the AP2 clamp from the intersectin linker, allowing for the recruitment of synaptojanin 1 to intersectin 1 within the assembled coat. Likely, other endocytic SH3-domain containing proteins, most notably endophilin, are involved in the stable tethering of synaptojanin 1 to late-stage endocytic intermediates (17), thereby initiating PI(4,5)P2 hydrolysis and uncoating (19). This hypothetical scenario fits well with recent TIRF-based imaging data showing that neuronal synaptojanin 1-p145 is recruited to CCPs at late stages preceding membrane fission, but is excluded from early endocytic intermediates (21) and is compatible with the metastability of newly assembling CCPs observed in living cells (22). Future studies will need to address these possibilities further.
Materials and Methods
Affinity Chromatography and Immunoprecipitation Experiments.
Detergent extracts were prepared from rat or lamprey brain using established procedures (13, 23). For immunoprecipitation experiments, antibodies were coupled to protein A/G PLUS Agarose (Santa Cruz Biotechnology) and incubated with 4 mg rat or lamprey brain Triton X-100 extract in buffer A (20 mM Hepes buffer, pH 7.4, containing 50 mM KCl, 2 mM MgCl2, and 1% Triton X-100) plus protease inhibitors (Sigma) in a total volume of 1 mL for 2 h at 4 °C. Following extensive washes, samples were eluted with sample buffer and analyzed by SDS/PAGE and immunoblotting. For affinity chormatography or direct binding experiments, 100 or 50 μg GST-fusion proteins were coupled to GST-Bind resin (Novagen) and incubated with 2 mg rat brain extract or 10 μg His6-tagged recombinant protein, respectively, in a total volume of 1 mL for 1 h at 4 °C on a rotating wheel. Following extensive washes, samples were eluted with sample buffer and analyzed by SDS/PAGE and immunoblotting.
Microinjection of Compounds into Lamprey Reticulospinal Axons and Analysis by Electron Microscopy.
Spinal cord preparations, microinjection experiments, and electron microscopic analysis were essentially done as described before (13). See SI Materials and Methods for details.
Protein Crystallography.
Detailed procedures of protein crystallography are available as SI Materials and Methods. Briefly, crystals were grown at 18 °C using the sitting-drop vapor-diffusion method. X-ray data were collected at beamline BL2 at BESSY-II, Berlin and processed using HKL2000 and scalepack. The phase problem was solved by molecular replacement with CCP4 program molrep using the 1.60-Å resolution structures of the α- and β2-appendage domains (PDB code 1KYF) as models. The statistics of the structures are reported in Table S1.
Supplementary Material
Supporting Information
Acknowledgments
We thank Drs. Thomas Südhof (Stanford University, CA), Pietro De Camilli (Yale University, CT), and Gero Miesenbock (Oxford University) for the kind gift of reagents. This work was supported by the Deutsche Forschungsgemeinschaft Grant HA2686/ 3-1/ FOR 806 (to V.H.), Swedish Research Council (Projects 13473, 20587), the Wallenbergs Foundation and Linné Center DBRM (O.S.), Grant SFB 449/ Z3 (to W.S.), (Exc 257-Neurocure), and the European Science Foundation (O.S. and V.H.). A.P. and J.B. are the recipients of scholarships from the Deutsche Forschungsgemeinschaft (GRK 1123).
Footnotes
The authors declare no conflict of interest.
Data deposition: Protein structures are accessible under PDB ID codes 3HS8 and 3HS9.
References
- 1.Murthy VN, De Camilli P. Cell biology of the presynaptic terminal. Annu Rev Neurosci. 2003;26:701–728. doi: 10.1146/annurev.neuro.26.041002.131445. [DOI] [PubMed] [Google Scholar]
- 2.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 3.Shupliakov O. The synaptic vesicle cluster: a source of endocytic proteins during neurotransmitter release. Neuroscience. 2009;158:204–210. doi: 10.1016/j.neuroscience.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 4.Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 5.Bonifacino JS, Lippincott-Schwartz J. Coat proteins: shaping membrane transport. Nat Rev Mol Cell Biol. 2003;4:409–414. doi: 10.1038/nrm1099. [DOI] [PubMed] [Google Scholar]
- 6.Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. doi: 10.1038/nature06031. [DOI] [PubMed] [Google Scholar]
- 7.Wieffer M, Maritzen T, Haucke V. SnapShot: endocytic trafficking. Cell. 2009;137:382.e1–3. doi: 10.1016/j.cell.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Jung N, Haucke V. Clathrin-mediated endocytosis at synapses. Traffic. 2007;8:1129–1136. doi: 10.1111/j.1600-0854.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 9.Koh TW, et al. Eps15 and Dap160 control synaptic vesicle membrane retrieval and synapse development. J Cell Biol. 2007;178:309–322. doi: 10.1083/jcb.200701030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43:193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 11.Marie B, et al. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004;43:207–219. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Thomas S, et al. Intersectin regulates dendritic spine development and somatodendritic endocytosis but not synaptic vesicle recycling in hippocampal neurons. J Biol Chem. 2009;284:12410–12419. doi: 10.1074/jbc.M809746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evergren E, et al. Intersectin is a negative regulator of dynamin recruitment to the synaptic endocytic zone in the central synapse. J Neurosci. 2007;27:379–390. doi: 10.1523/JNEUROSCI.4683-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anggono V, et al. Syndapin I is the phosphorylation-regulated dynamin I partner in synaptic vesicle endocytosis. Nat Neurosci. 2006;9:752–760. doi: 10.1038/nn1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edeling MA, et al. Molecular switches involving the AP-2 beta2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev Cell. 2006;10:329–342. doi: 10.1016/j.devcel.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Schmid EM, et al. Role of the AP2 beta-appendage hub in recruiting partners for clathrin-coated vesicle assembly. PLoS Biol. 2006;4:e262. doi: 10.1371/journal.pbio.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ringstad N, et al. Endophilin/SH3p4 is required for the transition from early to late stages in clathrin-mediated synaptic vesicle endocytosis. Neuron. 1999;24:143–154. doi: 10.1016/s0896-6273(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 18.Yu Y, et al. Mice deficient for the chromosome 21 ortholog Itsn1 exhibit vesicle-trafficking abnormalities. Hum Mol Genet. 2008;17:3281–3290. doi: 10.1093/hmg/ddn224. [DOI] [PubMed] [Google Scholar]
- 19.Cremona O, et al. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- 20.Thieman JR, et al. Clathrin regulates the association of PIPKIgamma661 with the AP-2 adaptor beta2 appendage. J Biol Chem. 2009;284:13924–13939. doi: 10.1074/jbc.M901017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perera RM, Zoncu R, Lucast L, De Camilli P, Toomre D. Two synaptojanin 1 isoforms are recruited to clathrin-coated pits at different stages. Proc Natl Acad Sci USA. 2006;103:19332–19337. doi: 10.1073/pnas.0609795104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loerke D, et al. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 2009;7:e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diril MK, Wienisch M, Jung N, Klingauf J, Haucke V. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev Cell. 2006;10:233–244. doi: 10.1016/j.devcel.2005.12.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information