Repetitive behavior and increased activity in mice with Purkinje cell loss: a model for understanding the role of cerebellar pathology in autism (original) (raw)
. Author manuscript; available in PMC: 2010 Mar 29.
Abstract
Repetitive behaviors and hyperactivity are common features of developmental disorders, including autism. Neuropathology of the cerebellum is also a frequent occurrence in autism and other developmental disorders. Recent studies have indicated that cerebellar pathology may play a causal role in the generation of repetitive and hyperactive behaviors. In this study, we examined the relationship between cerebellar pathology and these behaviors in a mouse model of Purkinje cell loss. Specifically, we made aggregation chimeras between Lc/+ mutant embryos and +/+ embryos. Lc/+ mice lose 100% of their Purkinje cells postnatally due to a cell-intrinsic gain-of-function mutation. Through our histological examination, we demonstrated that Lc/+↔+/+ chimeric mice have Purkinje cells ranging from zero to normal numbers. Our analysis of these chimeric cerebella confirmed previous studies on Purkinje cell lineage. The results of both open-field activity and hole-board exploration testing indicated negative relationships between Purkinje cell number and measures of activity and investigatory nose-poking. Additionally, in a progressive-ratio operant paradigm, we found that Lc/+ mice lever-pressed significantly less than +/+ controls, which led to significantly lower breakpoints in this group. In contrast, chimeric mice lever-pressed significantly more than controls and this repetitive lever-pressing behavior was significantly and negatively correlated with total Purkinje cell numbers. Although the performance of Lc/+ mice is probably related to their motor deficits, the significant relationships between Purkinje cell number and repetitive lever-pressing behavior as well as open-field activity measures provide support for a role of cerebellar pathology in generating repetitive behavior and increased activity in chimeric mice.
Keywords: chimera, lineage, lurcher, progressive-ratio, stereotypy
Introduction
Repetitive and restrictive behaviors are a major behavioral component of many psychiatric and neurological conditions as well as a variety of developmental disorders, including autism spectrum disorder (ASD; e.g. Bleuler, 1950; Hutt & Hutt, 1970; Bodfish et al., 2000; Militerni et al., 2002). In autism, repetitive behavior may function to control behavior–environment relations such as access to reinforcers, or to gain access to or eliminate particular types of stimulation such as hyperarousal (Ridley, 1994; Kennedy et al., 2000). Hyperactivity often accompanies repetitive behavior in developmental disorders, with approximately 30% of ASD children comorbid for attention deficit/hyperactivity disorder (ADHD; Simonoff et al., 2008).
Although developmental disorders are associated with varied neuropathology, an abnormal cerebellum has been commonly reported. In autism, substantial reductions in the number of cerebellar Purkinje cells have been the most consistent finding in post-mortem brains (Palmen et al., 2004). Pierce & Courchesne (2001) reported that the rate of repetitive behavior in a group of ASD children was negatively correlated with the area of cerebellar vermal lobules VI–VII. They also reported that the ASD children demonstrated increased non-exploratory activity over controls, although a relationship between activity level and cerebellar size was not apparent. However, both functional and morphometric cerebellar abnormalities have been consistently demonstrated in ADHD (Soliva et al., 2009).
We used an animal model to investigate the role of the cerebellum in the occurrence of both repetitive behavior and increased activity by varying the number of Purkinje cells. This was accomplished by making aggregation chimeras between heterozygous lurcher (Lc/+) and wildtype (+/+) embryos. The lurcher mutant mouse is a semi-dominant mutation in the _δ_2 glutamate receptor (GRID2) gene that results in the loss of all Purkinje cells between the second and fourth weeks of life (Caddy & Biscoe, 1979; Zuo et al., 1997). The use of the lurcher mutation in mouse chimeras provides a reasonable model system to study cerebellar function (Martin et al., 2003, 2004, 2006).
In this study we tested lurcher, chimeric and wild-type mice in behavioral paradigms designed to assess activity, exploration and repetitive behaviors. Activity was measured as ambulation in an open-field, exploratory behavior was measured by quantifying nose-pokes through a hole-board and repetitive behavior was measured as patterns of learned behavior in the progressive-ratio (PR) task.
The PR task is a commonly used lever-press task that programs a systematic increase in the value of the fixed-ratio (FR) requirement following reinforcer delivery. Subjects are free to lever-press as frequently or infrequently as they choose, but must exert progressively more lever-presses to obtain successive reinforcers. As repetitive behavior can take any form and occur in virtually any environment (Kennedy et al., 2000), we reasoned that this task would allow us to explore the role of cerebellar pathology in repetitive behaviors similar to those observed in autism.
We found that open-field activity was negatively correlated with total Purkinje cell number. Similarly, repetitive lever-pressing was negatively related to total Purkinje cell number. These results provide support for a role of cerebellar pathology in generating increased activity and repetitive behaviors.
Methods
Subjects
The original stock of lurcher mice, B6CBACa_Aw_−J/A-Grid2Lc, was obtained from the Jackson Laboratory (Bar Harbor, ME, USA) and was maintained at the University of Tennessee Animal Care Facility and the University of Memphis Animal Care Facility. From these mice, three groups of animals were formed: lurcher (gene symbol Lc/+), control (gene symbol +/+) and chimeric mice with varying proportions of cells from the +/+ and Lc/+ genotypes.
Mouse chimeras were made by the standard method of fusing two-four- to eight-cell embryos (Goldowitz et al., 1992). Female +/+ and Lc/+ mice were superovulated with pregnant mare serum (4–5 IU; Sigma, St Louis, MO, USA) followed by human chorionic gonadotropin (Sigma; 4–5 IU) 44 h later. Then, +/+ females were mated with Lc/+ males and Lc/+ females were mated with +/+ males. The following morning, females were checked for the presence of a vaginal plug. All females found positive for a vaginal plug were distinguished as donor females. Two days after the appearance of the vaginal plug, embryos were harvested from the oviducts of the donor females who were overclosed with anesthesia (Averlin, Sigma-Aldrich, St. Louis, MO, USA). The harvested embryos were then subjected to a light pronase treatment to remove the zonae pellucidae, aggregated in pairs, and cultured overnight (37°C, 5% CO2) in drops of medium (Mullen & Whitten, 1971) covered with paraffin oil. The following afternoon, successfully fused embryos were transplanted into the uterine horns of pseudo-pregnant ICR females. Avertin was administered intraperitoneally as the general anesthetic for the ICR host females prior to transplantation. All surgical procedures and animal care were in accordance with National Institutes of Health guidelines for the use of animals in research and were approved by the institutional animal care and use committees of the University of Memphis and the University of Tennessee Health Science Center. Because the genotype of the embryos at the time of chimera production was necessarily unknown, only half of the progeny were expected to have a truly chimeric, or mixed, genotype (Lc/+↔+/+), while the remaining animals were expected to be entirely composed of Lc/+ or +/+ cells. Of the 81 mice generated, 36 were determined to be truly chimeric in genotype, consistent with these expectations.
In addition to mice generated as chimeras, another group of nine +/+ and nine Lc/+ mice were derived from the breeding colony. Mice in all groups were housed with siblings of the same sex until they reached maturity (< 3 months of age), with food and water available ad libitum. They were then transferred to the University of Memphis Animal care facility, individually housed, and then tested following a 2-week period of acclimatization. The animals were maintained under a 12/12-h light–dark cycle (lights on at 06:00 h).
Apparatus
The open-field activity and exploration assessments were done in eight identical test environments (Model ENV-515; Med Associates, St. Albans, VT, USA) interfaced to a computer. The open field of each test environment measured 27.9 × 27.9 cm and was enclosed by vertical polycarbonate walls. Three 16-beam infrared arrays detected movement in the x, y and z axes. For the exploration task, a stainless steel insert with four rows of four evenly spaced holes on 10.6-cm centers was placed in each test environment (Model ENV-515HBM). Each hole measured 2.2 cm in diameter with a depth of 1.8 cm.
The progressive-ratio task was carried out in four identical operant chambers (Model ENV-307; 15.9 cm long, 14 cm wide, 12.7 cm tall; Med Associates). Each was equipped with a retractable lever (Model E23-07) positioned on the short wall of the chamber such that the lever, when extended, was 1.0 cm from the wall and 2.5 cm above the grid floor. A liquid dipper presented reinforcement consisting of 0.02 mL of evaporated milk sweetened with 0.2% sucrose solution into a food magazine centered on the short wall and adjacent to the lever. The duration of dipper presentation was 7 s throughout training and testing. An infrared photobeam positioned inside the food magazine was used to detect entries into the magazine. A white light located within the food receptacle was used to signal reinforcement delivery. An additional infrared photobeam, used to detect locomotor activity in the rear of the chambers, was located 2 cm above the cage floor and 2 cm from the rear of the chamber (opposite to the wall housing the food receptacle). The chambers were equipped with a house light, located on the rear wall opposite the food magazine and lever, which served as a stimulus to signal when the experimental contingencies were in effect. Each experimental chamber was enclosed within a sound-attenuating Melamine cubicle with a small exhaust fan to provide continuous airflow and background noise. Data were collected with a microcomputer (Spider; Paul Fray Ltd, Cambridge, UK) which controlled the delivery of the liquid reinforcer, and recorded lever-pressing, entries into the food magazine and locomotor activity in the test chamber with a resolution of 0.01 s.
Open field monitoring
Mice were individually placed into the center of the open field and allowed to explore the quadrant freely for 60 min. The movements of each mouse were detected by 16 photobeams on both the x and the y axes of the floor grid positioned 2.2 cm above the floor creating 17 activity quadrants. In addition, vertical movements were detected by an additional set of 16 photobeams positioned in the z axis 7 cm above the floor. Med Associates software was then used to analyse the data and generate counts of photocell beam breaks, vertical jumps, clockwise and counterclockwise rotations, in addition to total jumping time, ambulatory time, resting time, distance traveled and average velocity.
Exploration task
One week after the completion of the open field monitoring, mice were individually placed into each test environment with the hole-board insert covering the open field and allowed to explore freely for 60 min. Exploration of each hole was detected by the x and y photobeams and recorded as a nose-poke. Data from six mice simultaneously tested in this task were lost due to a computer malfunction.
Assessment of hunger motivation
Because PR tasks are often used to assess differences in motivation, we tested mice in a probe aimed at determining the presence of any difference between groups in their hunger motivation. In this probe, mice were gradually food-deprived until they weighed between 85 and 90% of their baseline body weight and then allowed to free-feed on the 0.2% sucrose/evaporated milk solution for 40 min in order to familiarize them with the food and weighing procedures, and to prevent food neophobia and reduce handling stress. Each mouse was weighed immediately prior to the beginning of the free-feed session and again after 20 and 40 min. Mice were then tested on two separate days (Probes 1 and 2). Increases in the body weight of each mouse after feeding were used as an index of hunger motivation.
Progressive ratio procedure
Food deprivation was maintained throughout PR testing with a restricted diet of Purina mouse chow (approximately 3 g daily) given after each daily test occurred. Using procedures described in Skjoldager et al. (1993), all mice were trained to lever-press. Once all animals reliably obtained 100 reinforcers during 15 min, FR 1 sessions, they began PR training. During each daily PR session, subjects were reinforced for lever-pressing under an arithmetically increasing FR 5 schedule of reinforcement (i.e. 5, 10, 15, 20, etc., lever-presses were necessary to obtain reinforcement), using a procedure similar to that described by Hodos & Kalman (1963). An experimental session terminated when the subject failed to lever-press for five consecutive minutes. The lever-press that completed each FR within the PR schedule was reinforced at lever release. Thus, upon activation of the liquid dipper, the house light was extinguished and the light within the food magazine was illuminated for 1 s. During this 1-s time out, lever-press responses were counted but had no scheduled consequences. All subjects were tested for 17 daily sessions, a time frame in which asymptotic levels of performance were achieved in our own pilot studies.
Dependent measures
In addition to total Purkinje cell numbers, a total of eight behavior-dependent measures were compared among wild-type control, chimeric and lurcher (Lc/+) mice. Breakpoint was defined as the value of the last completed (reinforced) ratio. Breakpoints are directly related to the total number of lever-presses, although there are only minimal temporal constraints on responding (Hodos, 1961; Hodos & Kalman, 1963; Mello et al., 1984; Winger & Woods, 1985; Roberts et al., 1989a,b). Several measures of the temporal characteristics of lever-pressing were also obtained for each completed ratio. A post-reinforcement pause (PRP) was defined as the time interval between the end of the 1-s reinforcer delivery period and the first lever-press following reinforcement. Mean inter-response times (IRTs) were computed by summing for each ratio the time from the offset of a lever-press to the onset of the subsequent lever-press for all responses within that ratio. The summed IRTs were then divided by the response requirement minus 1 to obtain the mean IRT for each ratio. Average response durations were computed for each ratio by summing the time the lever was held in the downward position (switch closure) and dividing by the number of responses in the ratio.
Several measures of task-related behavior were also obtained for each completed ratio. A food entry was counted whenever a mouse blocked the photocell at the opening of the food magazine. In addition, food entry latencies were obtained following each reinforcer delivery. Time-out responses were those lever-presses that occurred during the 1-s time-out period that followed activation of the liquid dipper. An activity ‘count’ was recorded when the photocell beam at the rear of the operant chambers was broken. Food entries, time-out responses and activity counts were recorded for each completed ratio.
Mean IRTs, response durations and food entry latencies obtained for each ratio were then rank ordered and the median values were computed. For these measures, the median, instead of the mean, was used as a measure of central tendency for these values because the median was less affected by relatively extreme values.
Histology
Following the completion of behavioral testing, all chimeric mice were overdosed with anesthesia (Avertin) and transcardially perfused with physiologic saline followed by acetic acid/95% ethanol fix (1: 3) for 20 min. Heads were removed at the cervical vertebrae and immersion-fixed overnight in the same fixative. Heads were then placed in 70% ethanol before the brains were dissected out and dehydrated with increasing concentrations of ethanol. Mid-sagittal sectioned brains were then cleared with a series of xylenes and infiltrated with paraffin, followed by embedding in paraffin blocks. Paraffin-embedded brains were sectioned in the sagittal plane using a microtome set for 8 _μ_m thickness. A set of eight chimeric brains was sectioned in the coronal plane at the same thickness. Every 25th section was then mounted on Superfrost+ slides. Immunocytochemistry using an anti-Calbindin antibody (Chemicon, Bellerica, MA, USA) was then performed on all slides to enable the identification of Purkinje cells. After incubation in primary antisera, slides were exposed to an anti-rabbit biotinylated secondary antibody and subsequently visualized using the ABC reaction (Vectastain kit; Vector Labs, Burlingame, CA, USA). Purkinje cells were identified by the brown reaction product of diaminobenzidine. Slides were then counterstained with cresyl violet and dehydrated through ascending alcohol concentrations before being cleared with xylenes. Glass coverslips were applied with Permount.
Purkinje cell counts
Purkinje cell nuclei were identified with the aid of a standard brightfield microscope equipped with 10× eyepieces and a 25× objective. The nuclei are easily identifiable at this magnification due to their light appearance in comparison with the surrounding darkly stained cytoplasm. Purkinje cell nuclei in every 25th section throughout the entire cerebellum were counted with the exception of the parafloccular lobe. Total numbers of Purkinje cells for the entire cerebellum were then estimated from this sampling and corrected for split nuclei using the Abercrombie correction factor (Abercrombie, 1946).
Data analysis
For the open-field monitoring tasks, each dependent measure was analysed using one-way ANOVA with group (control, chimeric or lurcher) as the between-subjects factor. In order to evaluate group differences in the behaviors emitted by the PR test subjects, each behavioral dependent measure was subjected to a two-factor ANOVA (Winer, 1971). In each of these analyses the between-subjects factor was group (control, chimeric or lurcher) and the within-subjects factor was day (1–17). When appropriate, Dunnett’s ‘_t_’ tests were used to further analyse group differences in behavior.
A second set of analyses was conducted to determine potential relationships between the total number of cerebellar Purkinje cells and all behavior-dependent measures in the open-field, exploration and PR tasks. Additionally, the relationship between PR breakpoint and the other seven variables on the PR task was also evaluated. Both analyses involved the calculation of Pearson product-moment correlation coefficients using a two-tailed alpha level set to P < 0.05. Although the same mice were used in both the open-field and the exploration tasks, data from six mice in the exploration task were unavailable for analysis due to mechanical error of the testing equipment. The PR data analyses included behavioral measures averaged over the final four test days. Log values were used for the temporal measures of IRT and response duration in order to normalize these data. Twenty-four Lc/+↔+/+ chimeras and eight +/+↔+/+ wild-type control animals that underwent histological assessment were included in this analysis. One +/+↔+/+ wild-type control was excluded from this analysis because it was found to have a mean breakpoint more than three standard deviations above the control group mean.
Results
Two different sets of mice were used. The first set consisted of a total of 35 mice which were used in the open-field monitoring experiments. The second set of mice consisted of 64 mice which were trained and tested in the PR task; a subgroup of 22 of these mice was assessed for hunger motivation. Following histology, the mice generated as chimeras were assigned to either the chimeric, lurcher or wild-type groups based upon Purkinje cell number. Additional lurcher and wild-type mice were generated from the breeding colony. Of the 35 mice in the first set, 10 were found to be composed of both mutant and wild-type neurons (Lc/+↔+/+) in that they possessed cerebellar Purkinje cell numbers intermediate between those of +/+ and Lc/+ animals. Of the remaining mice, histology revealed that 12 had no cerebellar Purkinje cells (Lc/+ lineage), while 13 had normal numbers of cells (+/+ lineage). For the set of 64 mice used in the PR task, 26 were determined to be Lc/+↔+/+ chimeras, 20 were Lc/+ and 18 were +/+ (including nine Lc/+ and nine +/+ mice from the breeding colony). It should be noted that two chimeric mice with very few Purkinje cells (fewer than 1000) were dropped from the PR experiment (and all analyses) because they demonstrated gross motor impairment (i.e. ataxia) indistinguishable from that of the Lc/+ mice.
Histological analysis
Figure 1 shows the differences in cerebellar Purkinje cells amongst Lc/+, chimeric and control mice. Purkinje cell numbers of the mice used in the PR task were compared across three cerebellar lobule groupings (I–V, VI–VII, VIII–X) and between right and left sides of the cerebellar cortex. Purkinje cells were found to have an apparently equal distribution pattern across all three lobular groupings (data not shown), but differences in Purkinje cell number were evident between hemicerebella of a single animal, most notably in low-percentage chimeras (Table 1).
Fig. 1.
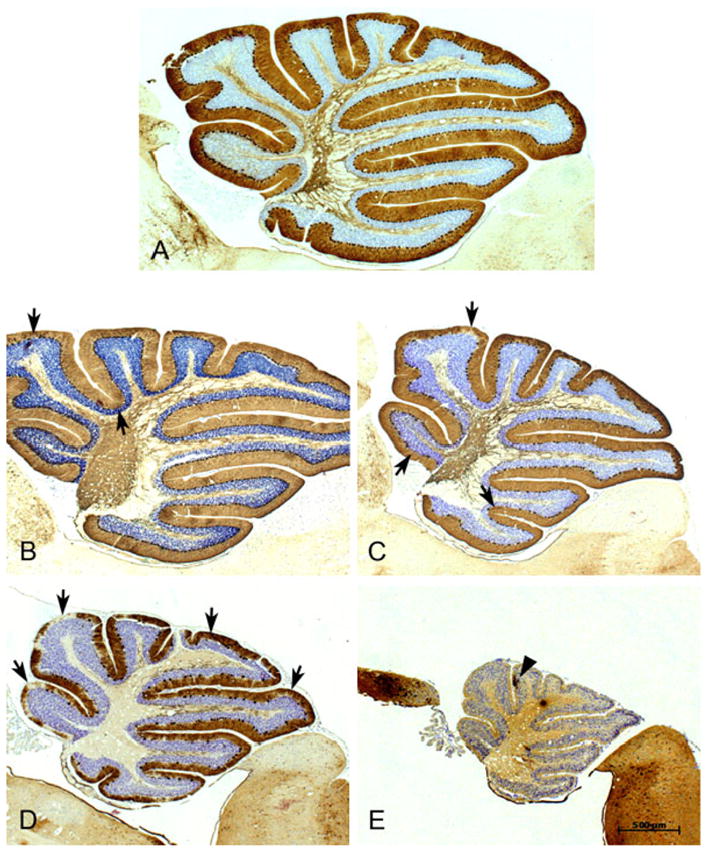
Examples of the cerebellum from the spectrum of chimeras that were studied. Material is stained for anti-Calbindin to highlight Purkinje cell bodies, their dendritic arborizations into the molecular layer and their axons which pass out through the white matter en route to the cerebellar nuclei. (A) This midline section is from a cerebellum that had normal numbers of Purkinje cells and is thus considered a 100% +/+ chimera. Note the large size of the cerebellum as well as the full complement of Purkinje cells aligned without interruptions along the internal granule cells. The arbors of the dendritic trees highlight the molecular layer. (B) The cerebellum from this chimera was estimated to be about 85% wild-type and the relative normality of the cerebellum is indicated by a total size that is only slightly smaller than the 100% wild-type cerebellum and has only a few gaps in Purkinje cells (arrows). (C) This cerebellum is from a chimera that was estimated to be a 50% chimera. Note the smaller size of the cerebellum and the larger gaps in the Purkinje cell layer (PCL) that are void of Purkinje cells (arrows show a few examples). (D) Although over-stained for immunocytochemistry this cerebellum is obviously smaller than the cerebella in A–C and there are large gaps of Purkinje-cell-free regions in the PCL. One can also see a dramatic reduction in the Calbindin-positive axons in the white matter. This cerebellum is from a 80% lurcher chimera. (E) From a virtually 100% lurcher chimera showing the diminished size of the cerebellum and the virtual lack of anti-Calbindin staining within the cerebellum except for one Purkinje cell (arrowhead) found in this midline section. The rest of the brain stains normally for the antibody, indicating the specific loss of Purkinje cells. The scale bar applies to all images.
Table 1.
Numbers of Purkinje cells in the cerebella of all Lc/+↔+/+ and +/+↔+/+ chimeras used in the PR study, and in four +/+ mice
| Numbers of Purkinje cells in the cerebellum | |||
|---|---|---|---|
| Brain number | Left | Right | Left + right |
| Lc/+↔+/+ chimeras | |||
| 10 | 460 | 224 | 684 |
| 25 | 804 | 0 | 804 |
| 80 | 6615 | 8851 | 15 466 |
| 29 | 6268 | 12 418 | 18 686 |
| 26 | 9446 | 11 303 | 20 749 |
| 86 | 16 095 | 14 545 | 30 640 |
| 53 | * | * | 33 422 |
| 110 | 21 042 | 19 093 | 40 135 |
| 82 | 18 246 | 22 548 | 40 794 |
| 104 | 26 438 | 24 616 | 51 054 |
| 60 | * | * | 51 970 |
| 126 | 32 041 | 23 150 | 55 190 |
| 24 | 28 886 | 26 432 | 55 317 |
| 8 | 34 999 | 23 166 | 58 165 |
| 108 | 25 033 | 33 893 | 58 926 |
| 106 | 28 751 | 31 501 | 60 252 |
| 58 | * | * | 66 189 |
| 57 | * | * | 70 096 |
| 11 | * | * | 81 122 |
| 52 | * | * | 87 043 |
| 59 | * | * | 96 094 |
| 27 | 51 946 | 47 529 | 99 475 |
| 56 | * | * | 106 164 |
| 23 | 50 965 | 66 224 | 117 189 |
| 65 | 47 144 | 70 619 | 117 763 |
| 7 | 65 549 | 64 606 | 130 155 |
| +/+↔+/+ Chimeras | |||
| 113 | 73 161 | 64 018 | 137 180 |
| 5 | 72 238 | 65 601 | 137 838 |
| 6 | 68 038 | 71 191 | 139 229 |
| 68 | 68 961 | 74 228 | 143 189 |
| 4 | 63 802 | 81 657 | 145 459 |
| 84 | 73 694 | 77 981 | 151 676 |
| 69 | 75 063 | 82 488 | 157 552 |
| 36 | 83 291 | 75 414 | 158 705 |
| 61 | 87 534 | 74 980 | 162 515 |
| +/+ Mice | |||
| 206 | † | 68 390 | 136 780* |
| 201 | 77 093 | 68 649 | 145 742 |
| 205 | 72 440 | 76 947 | 149 386 |
| 204 | 78 586 | 84 856 | 163 442 |
Open-field monitoring and hole-board exploration
Of the nine dependent measures obtained through the open field monitoring, ANOVA only revealed significant group differences in clockwise (Group; F = 6.085, d.f. = 2.32, P = 0.006) and counterclockwise rotations (Group; F = 3.192, d.f. = 2.32, P = 0.05). Dunnett’s _t_-tests showed that Lc/+ mice had significantly more clockwise rotations than chimeric and wild-type mice, and significantly more counterclockwise rotations than wild-type mice. ANOVA of the number of nose-pokes during the hole-board exploration task revealed a significant difference between groups (Group; F = 8.137, d.f. = 2.26, P = 0.002). Dunnett’s _t_-tests showed that chimeric mice (mean = 156) had more nose-pokes than both control (mean = 105) and lurcher (mean = 76) mice.
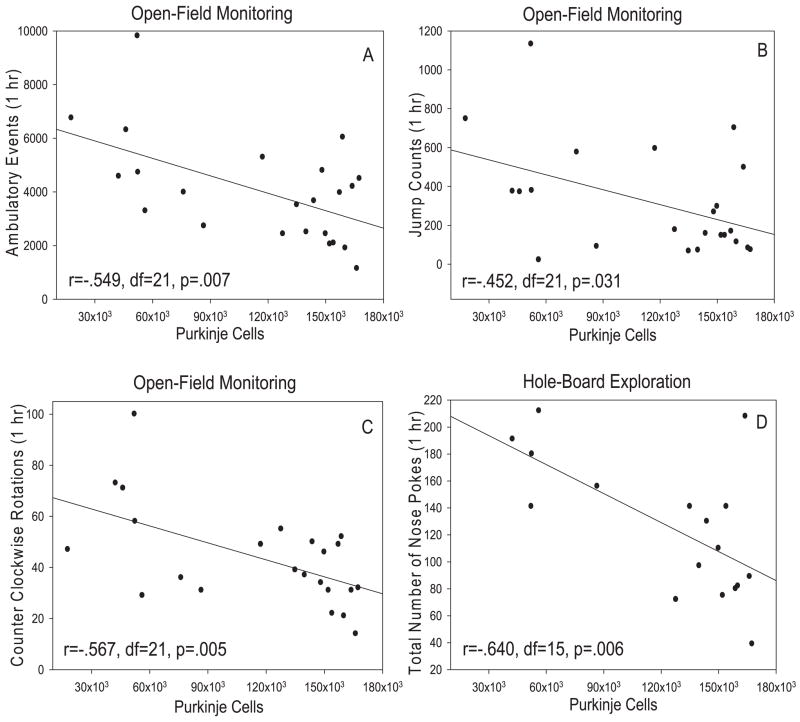
Relationships between total Purkinje cell number in chimeric and wild-type mice and each dependent measure of the open-field and hole-board exploration experiments were examined via Pearson correlations. Negative relationships were found between total Purkinje cell number and photocell beam breaks (r = −0.549, d.f. = 21, P = 0.007; Fig. 2A), ambulatory time (r = −0.527, d.f. = 21, P = 0.010), distance traveled (r = −0.543, d.f. = 21, P = 0.007), jump counts (r = −0.452, d.f. = 21, P = 0.031; Fig. 2B) and counterclockwise rotations (r = −0.567, d.f. = 21, P = 0.005; Fig. 2C) during open-field monitoring. Probably related to the negative relationship between Purkinje cell number and measures of ambulation, there was also a positive relationship between total Purkinje cells and resting time (r = 0.478, d.f. = 21, P = 0.021). Pearson correlation also demonstrated a negative relationship between total Purkinje cell number and the number of nose-pokes (r = −0.640, d.f. = 15, P = 0.006; Fig. 2D) during the 60-min testing session using the hole-board. An analysis of each 10-min testing block revealed that this relationship was strongest during the first 10 min of the task (r = −0.722, d.f. = 15, P < 0.001).
Fig. 2.
Scatterplots showing the relationship between total cerebellar Purkinje cell number and some of the measures of ambulation during open-field monitoring and the total number of hole pokes during hole-board exploration. Total Purkinje cell number was negatively related to ambulatory events (A; r = −0.549, d.f. = 21, P = 0.007), total jump counts (B; r = −452, d.f. = 21, P = 0.031) and counter clockwise rotations (C; r = −0.567, d.f. = 21, P = 0.005) as determined by Pearson correlations. (D) Total Purkinje cell number was also negatively related to the total number of nose-pokes during the 60-min session of hole-board exploration (r = −0.640, d.f. = 15, P = 0.006). Data from six mice tested in this task were unavailable due to computer malfunction.
Assessment of hunger motivation
All groups of mice demonstrated significant gains in body weight after both 20 and 40 min in the free-feeding probe sessions. Group differences were found in the amount of weight gain following each 40-min session (Group; F = 5.34, d.f. = 2.19, P < 0.05; Fig. 3). Dunnett’s _t_-tests revealed that Lc/+ mice gained significantly more weight than control and chimeric mice following this probe.
Fig. 3.
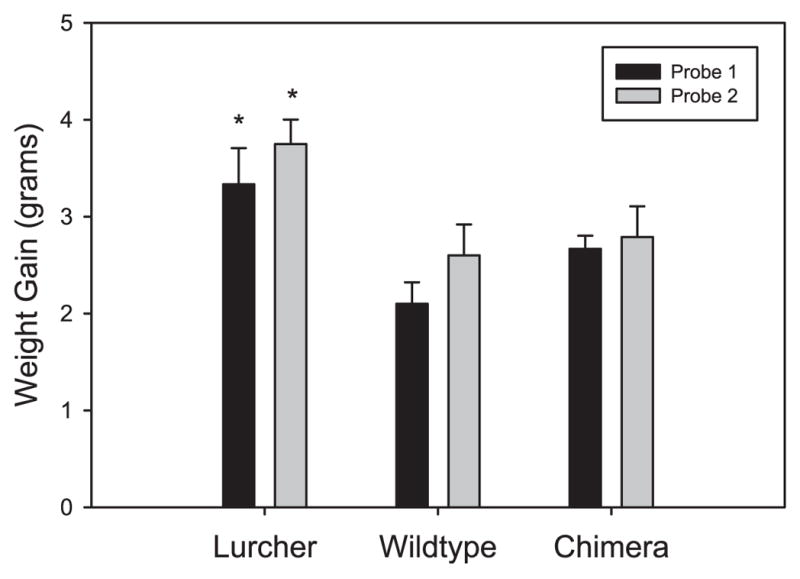
Mean weight gain of Lc/+, +/+ and Lc/+↔+/+ chimeras in each of two 40-min sessions of the hunger motivation task. Dunnett’s _t_-tests revealed that Lc/+ mice gained significantly more weight than control and chimeric mice following these probes (*P < 0.01).
Progressive-ratio analyses
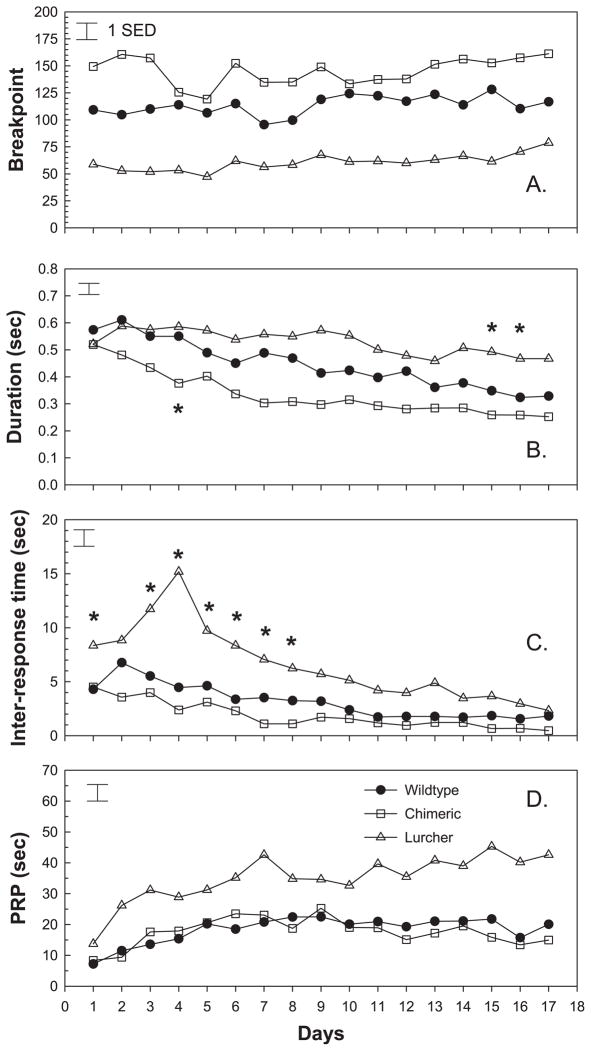
All groups of mice rapidly acquired PR responding (Fig. 4). As shown in Fig. 4A, average breakpoint increased with repeated testing in all the groups (Day; F = 2.98, d.f. = 16.944, P < 0.001). There were, however, consistent group differences in breakpoint when considered over the 17 test days (Group; F = 20.61, d.f. = 2.59, P < 0.001). Dunnett’s _t_-tests revealed that chimeric mice lever-pressed significantly more than controls, with an average breakpoint of 145.5 in comparison with the mean control breakpoint of 113.5. Lc/+ animals, in comparison, averaged significantly lower breakpoints (mean = 60.7) than wild-type controls.
Fig. 4.
Mean breakpoint, lever-press duration, inter-response time and post-reinforcement pause of Lc/+, +/+ and Lc/+↔+/+ chimeras in each trial session of the PR task. *Individual trial sessions in which there were significant differences (P < 0.05) between Lc/+ and wildtype control mice or chemeric and control mice (Panel B session 4 only). (A) Mean breakpoint increased with repeated testing in all groups (Day; F = 2.98, d.f. = 16.944, P < 0.001). In addition, analysis of mean breakpoint across all test days revealed that chimeric mice (145.5) lever-pressed significantly more than controls (113.5) while Lc/+ mice (60.7) lever-pressed significantly less (Group; F = 20.61, d.f. = 2.59, P < 0.001). (B) Mean lever-press duration decreased with repeated testing in all three groups (Day; F = 17.84, d.f. = 16.944, P < 0.001), but the groups differed in how much they changed over the course of testing (Group × Day; F = 2.04, d.f. = 32.944, P < 0.001). Lc/+ mice had significantly longer durations during test days 15 and 16, while chimeras had a significantly shorter duration on day 4. (C) Mean inter-response time across all test days differed between groups (Group; F = 17.72, d.f. = 2.59, P < 0.001). Lc/+ mice (6.57 s) had significantly longer inter-response intervals than control mice (3.15 s), but chimeric mice did not differ significantly from controls. In addition, Lc/+ mice displayed a different pattern of change across test days (Group × Day; F = 3.55, d.f. = 32.944, P < 0.001). IRTs lengthened during the first four test days and then began to decline with repeated testing, resulting in significantly longer IRTs during most of the first eight test days. (D) Mean post-reinforcement pause differed between groups across all test days (Group; F = 22.99, d.f. = 2.59, P < 0.001). Lc/+ mice maintained consistently longer PRPs over the 17 test days than controls.
Related to these group differences in breakpoint, there were group differences in the topography of lever-pressing. All groups learned to press the lever more efficiently with repeated testing (Day; F = 17.84, d.f. = 16.944, P < 0.001). The duration that the response lever was held in the depressed (closed) state declined from 0.54 s on the first day of testing to 0.34 s during the final session (Fig. 4B). Groups differed in how much they changed over testing (Group × Day; F = 2.04, d.f. = 32.944, P < 0.001). Durations declined the least in Lc/+ mice and declined the most in chimeric animals, in comparison with controls. This pattern resulted in lurchers showing significantly longer durations during test days 15 and 16, while chimeras had a significantly shorter duration on day 4 (Dunnett’s _t_-tests).
Associated with the relatively inefficient pattern of lever-pressing in Lc/+ mice, they also exhibited significantly longer inter-response intervals, when considered over the course of testing (Fig. 4C). In comparison with mean control IRTs of 3.15 s, Lc/+ mice averaged 6.57 s between lever-presses (Group; F = 17.72, d.f. = 2.59, P < 0.001). Lurchers also showed a different pattern of change across the test days (Group × Day; F = 3.55, d.f. = 32.944, P < 0.001). IRTs lengthened during the first four test days and then began to decline with repeated testing. This resulted in significantly longer IRTs in Lc/+ mice during most of the first eight test days, in comparison with controls that showed a steady decline in IRTs over test days. Although IRTs were shorter in chimeric mice, they did not differ significantly from controls on this measure.
As shown in Fig. 4D, there were also significant group differences in PRP time (Group; 22.99 s, d.f. = 2.59, P < 0.001) that remained constant with repeated testing (Group × Day; F = 1.39, d.f. = 32.944, P = n.s.). Dunnett’s _t_-tests indicated that, in comparison with controls (average PRP = 18.4 s), Lc/+ mice maintained consistently longer PRPs over the 17 test days (average PRP = 34.89 s).
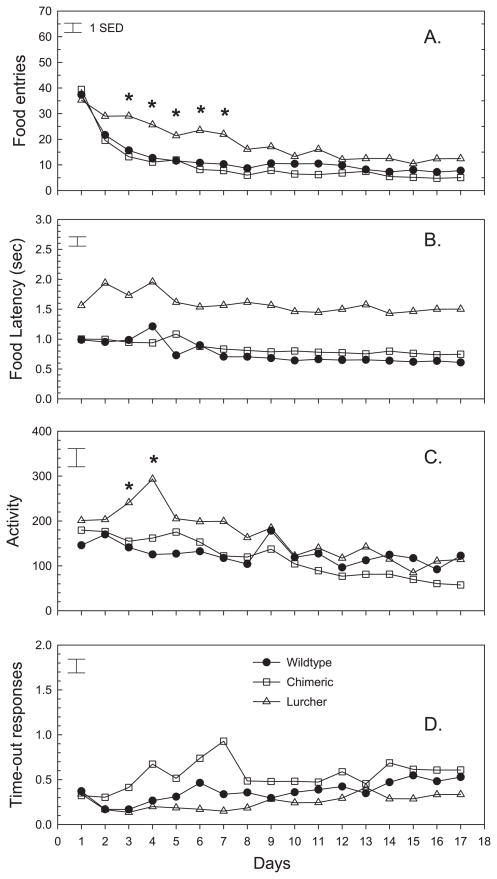
Figure 5 depicts other behaviors that occurred during the acquisition of PR responding. As mice in all groups learned the PR task, the number of times per trial that they entered into the food magazine declined significantly (Day; F = 41.47, d.f. = 16.944, P < 0.001). In comparison with controls, Lc/+ animals showed a significantly slower rate of decline in entries (Group × Day, F = 1.82, d.f. = 32.944, P < 0.005), which accounted for significant group differences between test days 3 and 7 (Fig. 5A). The performance of chimera and control mice was very similar. Associated with the elevated number of food entries in lurchers, these mice were consistently slower to enter the food magazine once reinforcement had been delivered (Group; F = 51.74, d.f. = 2.59, P < 0.001). Thus, over the 17 test days entry latencies averaged 0.76, 0.84 and 1.59 s, respectively, in control, chimeric and Lc/+ mice (Fig. 5B). As shown in Fig. 5C, activity in the rear of the test chamber declined significantly in all groups with repeated testing (Day; F = 11.21, d.f. = 16.944, P < 0.001). There were relatively small group differences in activity over the test days (Group × Day; F = 1.84, d.f. = 32.944, P < 0.005). Specifically, Lc/+ mice had higher levels of activity on test days 3 and 4, in comparison with controls. Unlike the decline in activity, time-out responses (lever-presses that occurred in the 1 s following reinforcement) remained relatively constant over testing (Fig. 5D) although there was a consistent group difference (Group; F = 5.49, d.f. = 2.59, P = 0.007). When considered over the 17 test days, lurchers exhibited fewer time-out responses than chimeric animals, although neither group differed significantly from wild-type control animals.
Fig. 5.
Mean number of food entries, food latency, off-task activity and number of time-out responses of Lc/+, +/+ and Lc/+↔+/+ chimeras in each trial session of the PR task. *Individual trial sessions in which there were significant differences (P < 0.05) between Lc/+ and wildtype control mice. (A) The number of times per trial that each group entered into the food magazine declined significantly across test days (Day; F = 41.47, d.f. = 16.944, P < 0.001). Lc/+ animals showed a significantly slower rate of decline in entries than control mice (Group × Day, F = 1.82, d.f. = 32.944, P < 0.005), which accounted for significant group differences between test days 3 and 7. (B) The latency to reach the food magazine once reinforcement had been delivered differed between groups (Group; F = 51.74, d.f. = 2.59, P < 0.001). Lc/+ mice (1.59 s) demonstrated significantly longer food latencies than control mice (0.76) across all test days. (C) Activity in the rear of the test chamber declined significantly in all groups with repeated testing (Day; F = 11.21, d.f. = 16.944, P < 0.001). There were relatively small group differences in activity over the test days (Group × Day; F = 1.84, d.f. = 32.944, P < 0.005) in that Lc/+ mice had higher levels of activity on test days 3 and 4 in comparison with controls. (D) Mean time-out responses differed between groups but remained relatively constant over all test days (Group; F = 5.49, d.f. = 2.59, P = 0.007). Lc/+ mice exhibited fewer time-out responses than chimeric mice over the 17 test days, although neither group differed significantly from controls.
The relationship of Purkinje cell number and PR measures
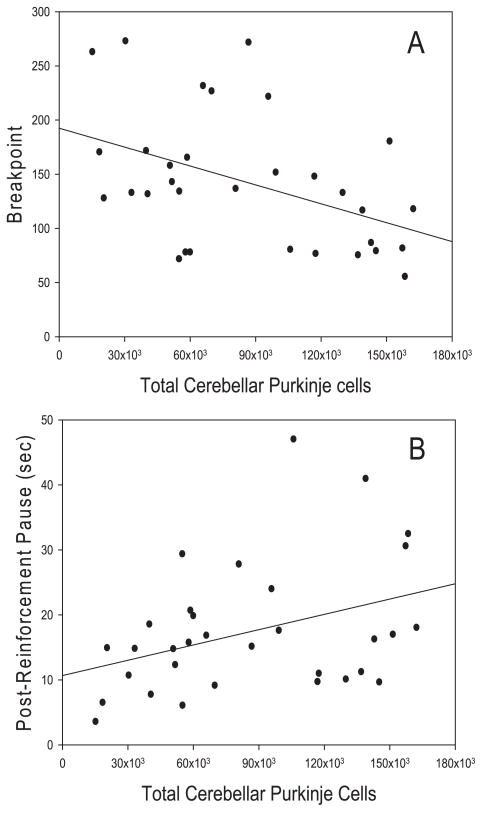
Purkinje cell number in chimeric and wild-type mice was related to two of the eight behavioral measures assessed in the PR task. Figure 6A shows the negative relationship between breakpoint and Purkinje cell number. Pearson correlations indicated that this relationship was significant (r = −0.439, d.f. = 31, P = 0.012). Thus, as the total number of Purkinje cells increased, breakpoint declined. As indicated in Fig. 6B, post-reinforcement pause and Purkinje cell number were significantly related (r = 0.369, d.f. = 31, P = 0.038) in that mice with lower numbers of Purkinje cells had shorter PRPs.
Fig. 6.
Scatterplots showing the relationships between total cerebellar Purkinje cell number and both breakpoint and post-reinforcement pause. (A) Total Purkinje cell number was negatively related to breakpoint as revealed by Pearson correlations (r = −0.439, d.f. = 31, P = 0.01). Breakpoint declined as total Purkinje cell number decreased. (B) Total Purkinje cell number was positively related to post-reinforcement pause (r = 0.369, d.f. = 31, P < 0.05) in that mice with lower numbers of Purkinje cells had shorter PRPs.
Discussion
Recent evidence suggests a role for the cerebellum in both repetitive and hyperactive behavior. This evidence includes reports of stereotyped repetitive behaviors in children with prenatal cerebellar malformations (Boltshauser et al., 1996; Hottinger-Blanc et al., 2002), structural and functional magnetic resonance imaging data linking cerebellar abnormalities with ADHD (Soliva et al., 2009), as well as a study demonstrating an inverse relationship between the area of the cerebellar vermis in ASD children and rates of repetitive behavior (Pierce & Courchesne, 2001). Repetitive or stereotyped patterns of behavior and interests are one of the core symptoms of ASDs. Hyperactivity, although not required for diagnosis, is also often observed in ASD children. Thus, given that the most consistent finding in post-mortem autistic brains is pathology of the cerebellum (Palmen et al., 2004) we investigated the role of the cerebellum in hyperactivity and repetitive behavior. To this end, we compared Lc/+↔+/+ chimeric mice and +/+ control mice in open-field monitoring, hole-board exploration and the PR task.
Developmental aspects of Purkinje cell lineage
Through our quantitative analysis of Purkinje cell number, we found that the group of 26 chimeras in this study involved the full range of Purkinje cell number from about 1000 total Purkinje cells in our lowest-percentage chimeras to near normal numbers of Purkinje cells (130 000) in our highest-percentage chimeras.
The cerebella of Lc/+↔+/+ chimeric mice have been studied previously in order to gain insight into the development of the cerebellum and determine the progenitor pool size that gives rise to the Purkinje cell population (Wetts & Herrup, 1982a,b). Our comparisons of Purkinje cell number across lobule groupings (lobules I–V, VI–VII, VIII–X) have confirmed previous reports of a random dispersal of Purkinje cell clones throughout the cerebellar cortex (data not shown; Baader et al., 1996; Hawkes et al., 1998). Even in our low-percentage chimeras, Purkinje cells were found in all three lobule groupings; however, differences were evident between left and right hemicerebella, consistent with previous findings (Table 1; Baader et al., 1996; Hawkes et al., 1998). Purkinje cell numbers of our two lowest-percentage chimeras also provide confirmation of the estimated clonal size for a Purkinje cell progenitor and the number of progenitors in the Purkinje cell pool, around 1000 and 100–150, respectively (Baader et al., 1996; Mathis et al., 1997; Hawkes et al., 1998).
Increased activity during open-field and hole-board testing
The results from chimeric and wild-type mice during open-field monitoring demonstrated significant negative relationships between Purkinje cell number and ambulatory events, ambulatory time, distance traveled, jump counts and counterclockwise rotations. These results are probably explained by an inverse relationship between Purkinje cell number and general activity. The positive relationship between Purkinje cell number and resting time supports this conclusion. A significant negative relationship between Purkinje cell number and nose-pokes was also found during the hole-board exploration and, as a group, chimeric mice had significantly more nose-pokes than +/+ mice. Although it is possible that this relationship is also caused by an overall increase in general activity level in mice with fewer Purkinje cells, Pearson correlations failed to demonstrate significant relationships between the number of nose-pokes and any of the dependent measures from the open-field assessment (data not shown). Thus, the increase in nose-poking behavior with decreasing Purkinje cell number may be indicative of increased motivation to explore in these mice. Alternatively, the repetitive nose-poking behavior can be viewed as a repetitive behavior similar to that which was demonstrated by these mice in the PR task.
The role of the cerebellum in exploratory behavior revisited
Previous research has shown that Lc/+ mice and other mice with cerebellar deficits show a significant decrease in nose-poking behavior on a hole-board matrix compared with controls (Lalonde & Botez, 1985; Lalonde et al., 1993; Caston et al., 1998). We also found that Lc/+ mice had fewer nose-pokes than +/+ controls, although this difference was not significant. In contrast, our chimeric mice demonstrated significantly more nose-pokes than controls during this task and the number of Purkinje cells in these mice was negatively correlated with nose-poking behavior (Fig. 2D). These findings cast doubt on previous conclusions suggesting reduced motivation to explore in cerebellar deficient mice and rather suggest that previous results were caused by the motor impairments of the mice (Caston et al., 1998).
Repetitive pattern of responding in the PR task
From the results of Lc/+↔+/+ chimeras, we demonstrated a relationship between Purkinje cells and lever-pressing behavior in the PR task. Breakpoint was significantly higher for the chimeric group than controls and within the group of chimeric mice was significantly and negatively correlated with the number of Purkinje cells in the cerebellum (Figs 4A and 6A).
We consider it unlikely that the higher breakpoint in Lc/+↔+/+ chimeras results from enhanced food motivation. Not only were the chimeric and wild-type mice food-deprived to a similar degree, but they also did not show any group difference in weight gain when allowed to free-feed for a set amount of time (Fig. 3). Instead, we believe that the increases in lever-pressing that resulted in higher breakpoint for mice with reduced Purkinje cell numbers may be related to the repetitive nature of this task. The significant positive relationship between Purkinje cell number and PRP helps to demonstrate the increased focus of these mice on repetitive lever-pressing behavior, as those chimeras with the fewest Purkinje cells, and therefore the highest breakpoints, paused for the shortest amount of time following reinforcement (Fig. 6).
Although chimeric mice demonstrate improved performance in this repetitive task based upon their degree of Purkinje cell loss, it is interesting that Lc/+ mice with complete Purkinje cell loss do not. One possible explanation for this apparent contradiction is the ataxia of the Lc/+ mice. It may be that this PR task is too demanding for these motor-deficient mice to exhibit the same repetitive pattern of responding as chimeric mice. The presence of repetitive behaviors in these mice may only be detected through ethological observation. Indeed, Lc/+ mice do exhibit a repetitive ‘lurching’ gait as part of their ataxia.
Influence of motor deficits on the performance of lurcher mice in the behavioral tasks
Lc/+ mice are ataxic, but chimeric mice with Purkinje cell numbers above a threshold of about 1000 cells show no outward signs of motor impairment (Martin et al., 2003, 2004). It is therefore prudent to interpret the behavioral results of Lc/+ mice independently of chimeras. During open-field monitoring, Lc/+ mice had significantly more rotations than chimeric and wild-type mice. This is probably related to the ataxia of Lc/+ mice, as they are often observed moving in circular patterns as they stumble around. In the PR task, Lc/+ mice had more head entries and a slower rate of decline in head entry number over trials compared with controls. These results can again be explained by the ataxia of these mice, particularly with their characteristic ‘lurching’ movements. The lurching movement involves a forward and backward head motion that may result in increased head entries into the food chamber. Lc/+ mice were also consistently slower to enter the food magazine once reinforcement was delivered. This finding is probably caused by a greater difficulty in moving between the lever and food magazine (Martin et al., 2004).
For the PR task-related behaviors, Lc/+ mice demonstrated a significantly lower breakpoint than control mice. Breakpoint has previously been used as an index of motivation (Schmelzeis & Mittleman, 1996). However, because the motivating factor of hunger seemed to be higher for Lc/+ mice (Fig. 3), and the reward was consistent in each trial (0.02 mL of evaporated milk/sucrose solution), the increase in task demand due to motor impairment is most likely responsible for their lower breakpoint. The higher amount of weight gain observed in Lc/+ mice in the hunger motivation probe may be due to the fact that Lc/+ mice are significantly smaller than controls at baseline. The food deprivation may therefore have a greater impact on these mice.
Lc/+ mice also demonstrated significantly higher post-reinforcement pause times and less efficient lever-pressing than control mice. The increase in rest between trials is another indication of the increased effort demanded from Lc/+ mice by this task. Although the less efficient lever-pressing may be partially explained by the motor impairment of these mice, this deficit may also be related to practice. Lever-press duration and IRT are both measures that improve with practice. This is clear by the gradual decline in latencies of both measures for all groups over the 17 test sessions (Fig. 4) and significant negative correlations between breakpoint and these temporal characteristics of lever-pressing (data not shown). Because of their lower breakpoint, Lc/+ mice were exposed to fewer trials in a session, and therefore received less practice than control and chimeric mice.
The role of the prefrontal cortex in the observed Purkinje cell relationships
We propose that the consistent negative correlations between Purkinje cell number and activity, nose-poking and repetitive lever-pressing demonstrated in this study are driven by a disruption of prefrontal dopamine neurotransmission. Specifically, we have previously reported that brief electrical stimulation of the cerebellar Purkinje cell layer resulted in stimulation time-locked increases in extracellular dopamine levels in the medial prefrontal cortex (MPFC). This increase was absent in Lc/+ mice with a complete loss of cerebellar Purkinje cells, suggesting that Purkinje cell outputs are a critical component of this modulatory cerebellar–MPFC circuit (Mittleman et al., 2008). Although chimeric mice have not yet been investigated in this paradigm, it is reasonable to predict that the magnitude of influence of this circuitry on cortical dopamine transmission will be dependent on the number of cerebellar Purkinje cells. In this vein it is interesting to note that similar to the results of the present study, rats with small neonatal, excitotoxic lesions of the MPFC show increased lever-pressing and significantly increased breakpoints in a food-rewarded PR task (Schwabe et al., 2006). Additionally, both monkeys and humans with lesions within the ventral regions of the frontal cortex in a variety of paradigms display deficits in inhibitory response control that have been described as, ‘increased control over behavior by pre-potent stimuli’, where ‘pre-potent’ is defined as stimuli with previous motivational significance (Jentsch & Taylor, 2001). In the PR task the response lever is a pre-potent stimulus as it has acquired motivational significance by contingent association with the liquid food reward. Thus, it appears probable that in the current study reductions in the number of Purkinje cells in chimeric mice leads to a disruption in dopamine transmission in the MPFC, and this in turn leads to increased and repetitive lever-pressing that could be interpreted as a deficit in inhibitory response control. Interestingly, an early study on cats with bilateral cerebellar cortical ablation demonstrated an impaired ability to inhibit bar-pressing during periods of non-reward in an operant task, a structure–function relationship perhaps similar to our findings in cerebellar deficient chimeric mice (Davis et al., 1970).
Conclusion
Stereotypy has been defined by Ridley (1994) as ‘the excessive production of one type of motor act, or mental state, which necessarily results in repetition’. The excessive production of lever-pressing by Purkinje cell-deficient chimeric mice in this study seems to fit this definition. However, it is premature to conclude that the Lc/+↔+/+ chimeric mice in this study demonstrated ‘stereotypies’ analogous to those found in children with ASD. Nonetheless, the superior performance of these mice in a rewarded task demanding repetitive behavior, together with the increased activity and repetitive behavior patterns shown by these mice in the open-field observations, make an intriguing comparison with potential clinical correlates in ASD. Indeed, a previous study found that children with ASD demonstrated a significant increase in activity over controls and also had a significant negative relationship between the rate of repetitive behavior and the size of their cerebellar vermis (Pierce & Courchesne, 2001). Also, one of the proposed causes of ASD is self-generated or maternally derived pathogenic antibodies and several recent studies have shown that antibodies from children with ASD or their mothers target cell populations within the cerebellum including Purkinje cells (Dalton et al., 2003; Singer et al., 2006; Wills et al., 2009). It is therefore of interest that a recent study demonstrated increased activity and repetitive behavior in rhesus monkeys exposed to IgG antibodies from mothers of children with ASD (Martin et al., 2008). Thus, the above findings, coupled with the consistent pathology of the cerebellum demonstrated in ASD, suggest that cerebellar impairment may lead to these maladaptive behaviors in children with ASD.
Acknowledgments
This research was supported by a UTHSC, Center for Neuroscience Predoctoral Fellowship (L.A.M.), a grant from Cure Autism Now (CAN), and NIH grant 1R01NS063009 to G.M. and D.G. We thank Richard Cushing, Meifen Lu and John Roberts for technical support, and Charles Blaha for comments on a draft of this manuscript.
Abbreviations
ADHD
attention deficit/hyperactivity disorder
ASD
autism spectrum disorder
FR
fixed-ratio
IRT
inter-response time
MPFC
medial prefrontal cortex
PR
progressive-ratio
PRP
post-reinforcement pause
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Baader SL, Schilling ML, Rosengarten B, Pretsch W, Teutsch HF, Oberdick J, Schilling K. Purkinje cell lineage and the topographic organization of the cerebellar cortex: a view from X inactivation mosaics. Dev Biol. 1996;174:393–406. doi: 10.1006/dbio.1996.0083. [DOI] [PubMed] [Google Scholar]
- Bleuler EP. Dementia Praecox or the Group of Schizophrenias. International University Press; New York: 1950. [Google Scholar]
- Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. J Autism Dev Disord. 2000;30:237–243. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- Boltshauser E, Steinlin M, Martin E, Deonna T. Unilateral cerebellar aplasia. Neuropediatrics. 1996;27:50–53. doi: 10.1055/s-2007-973748. [DOI] [PubMed] [Google Scholar]
- Caddy KW, Biscoe TJ. Structural and quantitative studies on the normal C3H and Lurcher mutant mouse. Philos Trans R Soc Lond B Biol Sci. 1979;287:167–201. doi: 10.1098/rstb.1979.0055. [DOI] [PubMed] [Google Scholar]
- Caston J, Chianale C, Delhaye-Bouchaud N, Mariani J. Role of the cerebellum in exploration behavior. Brain Res. 1998;808:232–237. doi: 10.1016/s0006-8993(98)00847-6. [DOI] [PubMed] [Google Scholar]
- Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, Stein J, Styles P, Vincent A. Maternal neuronal antibodies associated with autism and a language disorder. Ann Neurol. 2003;53:533–537. doi: 10.1002/ana.10557. [DOI] [PubMed] [Google Scholar]
- Davis HN, Watkins GM, Angermeier WF, Rubia FJ. The role of the cortical parts of the cerebellar hemispheres in discrimination learning of cats. Pflugers Arch. 1970;318:346–352. doi: 10.1007/BF00586974. [DOI] [PubMed] [Google Scholar]
- Goldowitz D, Moran H, Wetts R. Mouse chimeras in the study of genetic and structural determinants of behavior. In: Goldowitz D, Wahlsten D, Wimer RE, editors. Techniques for the Genetic Analysis of Brain and Behavior: Focus on the Mouse. Elsevier; Amsterdam: 1992. pp. 271–290. [Google Scholar]
- Hawkes R, Faulkner-Jones B, Tam P, Tan SS. Pattern formation in the cerebellum of murine embryonic stem cell chimeras. Eur J Neurosci. 1998;10:790–793. doi: 10.1046/j.1460-9568.1998.00085.x. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hodos W, Kalman G. Effects of increment size and reinforcer volume on progressive ratio performance. J Exp Anal Behav. 1963;6:387–392. doi: 10.1901/jeab.1963.6-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottinger-Blanc PM, Ziegler AL, Deonna T. A special type of head stereotypies in children with developmental (? cerebellar) disorder: description of 8 cases and literature review. Eur J Paediatr Neurol. 2002;6:143–152. doi: 10.1053/ejpn.2002.0582. [DOI] [PubMed] [Google Scholar]
- Hutt C, Hutt SJ. Effect of environmental complexity upon stereotyped behavior in children. Anim Behav. 1970;13:1–4. [Google Scholar]
- Jentsch JD, Taylor JR. Impaired inhibition of conditioned responses produced by subchronic administration of phencyclidine to rats. Neuropsychopharmacology. 2001;24:66–74. doi: 10.1016/S0893-133X(00)00174-3. [DOI] [PubMed] [Google Scholar]
- Kennedy CH, Meyer KA, Knowles T, Shukla S. Analyzing the multiple functions of stereotypical behavior for students with autism: implications for assessment and treatment. J Appl Behav Anal. 2000;33:559–571. doi: 10.1901/jaba.2000.33-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R, Botez MI. Exploration of a hole-board matrix in nervous mutant mice. Brain Res. 1985;343:356–359. doi: 10.1016/0006-8993(85)90755-3. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Joyal CC, Guastavino JM, Botez MI. Hole poking and motor coordination in lurcher mutant mice. Physiol Behav. 1993;54:41–44. doi: 10.1016/0031-9384(93)90041-d. [DOI] [PubMed] [Google Scholar]
- Martin LA, Goldowitz D, Mittleman G. The cerebellum and spatial ability: dissection of motor and cognitive components with a mouse model system. Eur J Neurosci. 2003;18:2002–2010. doi: 10.1046/j.1460-9568.2003.02921.x. [DOI] [PubMed] [Google Scholar]
- Martin LA, Escher T, Goldowitz D, Mittleman G. A relationship between cerebellar Purkinje cells and spatial working memory demonstrated in a lurcher/chimera mouse model system. Genes Brain Behav. 2004;3:158–166. doi: 10.1111/j.1601-183x.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- Martin LA, Goldowitz D, Mittleman G. Sustained attention in the mouse: a study of the relationship with the cerebellum. Behav Neurosci. 2006;120:477–481. doi: 10.1037/0735-7044.120.2.477. [DOI] [PubMed] [Google Scholar]
- Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22:806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis L, Bonnerot C, Puelles L, Nicolas JF. Retrospective clonal analysis of the cerebellum using genetic laacZ/lacZ mouse mosaics. Development. 1997;124:4089–4104. doi: 10.1242/dev.124.20.4089. [DOI] [PubMed] [Google Scholar]
- Mello NK, Bree MP, Mendelson JH. Buprenorphine, heroin, and methadone: comparison of relative reinforcing properties. NIDA Res Monogr. 1984;49:172–178. [PubMed] [Google Scholar]
- Militerni R, Bravaccio C, Falco C, Fico C, Palermo MT. Repetitive behaviors in autistic disorder. Eur Child Adolesc Psychiatry. 2002;11:210–218. doi: 10.1007/s00787-002-0279-x. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Goldowitz D, Heck DH, Blaha CD. Cerebellar modulation of frontal cortex dopamine efflux in mice: relevance to autism and schizophrenia. Synapse. 2008;62:544–550. doi: 10.1002/syn.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Whitten WK. Relationship of genotype and degree of chimerism in coat color to sex ratios and gametogenesis in chimeric mice. J Exp Zool. 1971;178:165–176. doi: 10.1002/jez.1401780203. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127:2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry. 2001;49:655–664. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- Ridley RM. The psychology of perserverative and stereotyped behaviour. Prog Neurobiol. 1994;44:221–231. doi: 10.1016/0301-0082(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989a;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology (Berl) 1989b;97:535–538. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Schmelzeis MC, Mittleman G. The hippocampus and reward: effects of hippocampal lesions on progressive-ratio responding. Behav Neurosci. 1996;110:1049–1066. doi: 10.1037//0735-7044.110.5.1049. [DOI] [PubMed] [Google Scholar]
- Schwabe K, Klein S, Koch M. Behavioural effects of neonatal lesions of the medial prefrontal cortex and subchronic pubertal treatment with phencyclidine of adult rats. Behav Brain Res. 2006;168:150–160. doi: 10.1016/j.bbr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry. 2008;47:921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol. 2006;178:149–155. doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Skjoldager P, Pierre PJ, Mittleman G. Reinforcer magnitude and progressive ratio responding in the rat: effects of increased effort, prefeeding, and extinction. Learn Motiv. 1993;24:303–343. [Google Scholar]
- Soliva JC, Carmona S, Fauquet J, Hoekzema E, Bulbena A, Hilferty J, Vilarroya O. Neurobiological substrates of social cognition impairment in attention-deficit hyperactivity disorder: gathering insights from seven structural and functional magnetic resonance imaging studies. Ann N Y Acad Sci. 2009;1167:212–220. doi: 10.1111/j.1749-6632.2009.04604.x. [DOI] [PubMed] [Google Scholar]
- Wetts R, Herrup K. Interaction of granule, Purkinje and inferior olivary neurons in lurcher chimaeric mice. I. Qualitative studies. J Embryol Exp Morphol. 1982a;68:87–98. [PubMed] [Google Scholar]
- Wetts R, Herrup K. Interaction of granule, Purkinje and inferior olivary neurons in lurcher chimeric mice. II. Granule cell death. Brain Res. 1982b;250:358–362. doi: 10.1016/0006-8993(82)90431-0. [DOI] [PubMed] [Google Scholar]
- Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral D, Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun. 2009;23:64–74. doi: 10.1016/j.bbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. McGraw-Hill; New York: 1971. [Google Scholar]
- Winger G, Woods JH. Comparison of fixed-ratio and progressive-ratio schedules of maintenance of stimulant drug-reinforced responding. Drug Alcohol Depend. 1985;15:123–130. doi: 10.1016/0376-8716(85)90036-5. [DOI] [PubMed] [Google Scholar]
- Zuo J, De Jager PL, Takahashi KA, Jiang W, Linden DJ, Heintz N. Neurodegeneration in Lurcher mice caused by mutation in delta2 glutamate receptor gene. Nature. 1997;388:769–773. doi: 10.1038/42009. [DOI] [PubMed] [Google Scholar]