Infection Associated with Prosthetic Joints (original) (raw)
. Author manuscript; available in PMC: 2010 Apr 6.
Published in final edited form as: N Engl J Med. 2009 Aug 20;361(8):787–794. doi: 10.1056/NEJMcp0905029
Abstract
A 62-year-old woman with osteoarthritis presents with a 7-month history of progressively worsening left hip pain radiating to the groin, 8 months after undergoing total left-hip arthroplasty. The pain has not responded to nonsteroidal antiinflammatory drugs. Physical examination reveals a sinus tract overlying her left hip. Her leukocyte count is 8000 per cubic millimeter, and the C-reactive protein (CRP) level is 15.5 mg per liter. A radiograph shows loosening of the prosthesis at the bone–cement interface. Synovial-fluid aspirate shows 15×103 cells per cubic millimeter (89% neutrophils); cultures of an aspirate from the hip grow Staphylococcus epidermidis. How should her case be managed?
THE CLINICAL PROBLEM
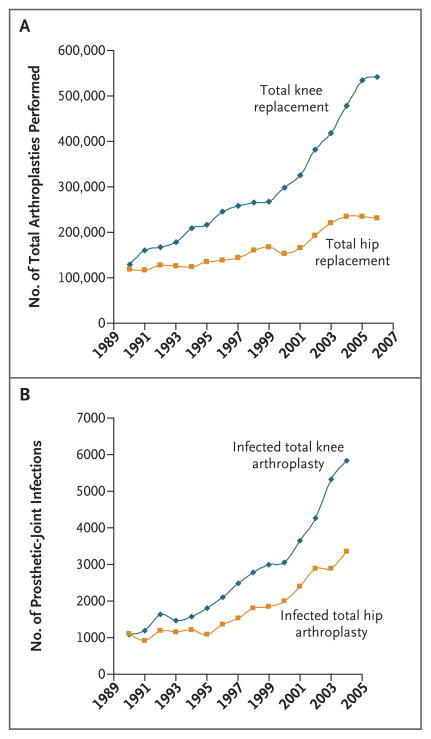
The numbers of primary total hip and total knee arthroplasties have been increasing over the past decade, with nearly 800,000 such procedures performed in the United States in 2006 (Fig. 1A).1 Procedures to replace the shoulder, elbow, wrist, ankle, temporomandibular, metacarpophalangeal, and interphalangeal joints are less commonly performed.
Figure 1. Total Arthroplasties Performed and Prosthetic Infections, According to Procedure.
Panel A shows the number of total arthroplasties performed from 1990 through 2006. Data are from the Centers for Disease Control and Prevention.1 Panel B shows the number of prosthetic-joint infections from 1990 through 2004. Data are from Kurtz et al.2
Prosthetic joints improve the quality of life, but they may fail, necessitating revision or resection arthroplasty. Causes of failure include aseptic loosening, infection, dislocation, and fracture of the prosthesis or bone. Infection, although uncommon, is the most serious complication, occurring in 0.8 to 1.9% of knee arthroplasties3–5 and 0.3 to 1.7% of hip arthroplasties.5–7 The frequency of infection is increasing as the number of primary arthroplasties increases (Fig. 1B).2 Patient-related risk factors for infection include previous revision arthroplasty or previous infection associated with a prosthetic joint at the same site, tobacco abuse, obesity, rheumatoid arthritis, a neoplasm, immunosuppression, and diabetes mellitus. Surgical risk factors include simultaneous bilateral arthroplasty, a long operative time (>2.5 hours), and allogeneic blood transfusion, and postoperative risk factors include wound-healing complications (e.g., superficial infection, hematoma, delayed healing, wound necrosis, and dehiscence), atrial fibrillation, myocardial infarction, urinary tract infection, prolonged hospital stay, and S. aureus bacteremia.3–6,8–11
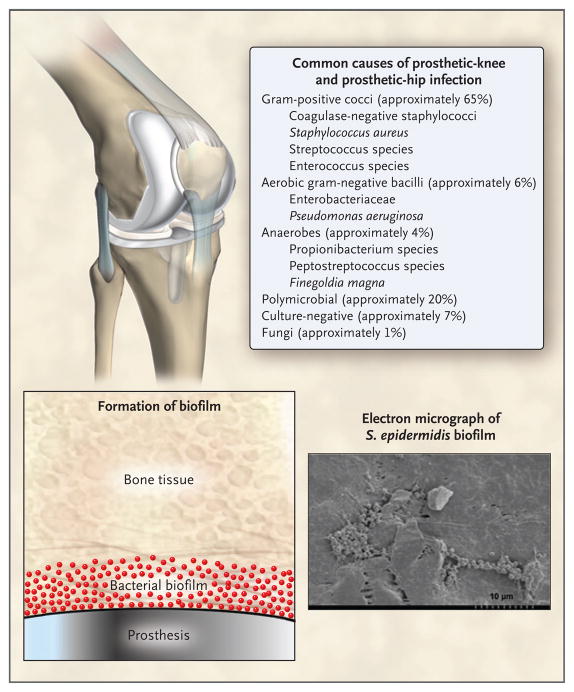
Staphylococci (S. aureus and coagulase-negative staphylococcus species) account for more than half of cases of prosthetic-hip and prosthetic-knee infection12 (Fig. 2). S. aureus infection is particularly common in patients with rheumatoid arthritis.13 Other bacteria and fungi cause the remainder of cases.14,15 Propionibacterium acnes is a common cause of infection associated with shoulder arthroplasty.16 Up to 20% of cases are polymicrobial, most commonly involving methicillin-resistant S. aureus (MRSA) or anaerobes.17 Approximately 7% of cases are culture-negative, often in the context of previous antimicrobial therapy.18
Figure 2. Causes of Infection Associated with Prosthetic Joints.
A small number of often otherwise nonvirulent bacteria contaminate the implant during surgery and persist as a biofilm despite a functional immune system and antimicrobial treatment. Commonly isolated microorganisms are shown. Unusual organisms that can also cause infection include (but are not limited to) Actinomyces israelii, Aspergillus fumigatus, Histoplasma capsulatum, Sporothrix schenckii, Mycoplasma hominis, Tropheryma whipplei, and mycobacterium (including tuberculosis), brucella, candida, corynebacterium, granulicatella, and abiotrophia species.
The pathogenesis of infection associated with a prosthetic joint involves interactions among the implant, the host’s immune system, and the involved microorganism or microorganisms. Only a small number of microorganisms is needed to seed the implant; such organisms adhere to the implant and form a biofilm in which they are protected from conventional antimicrobial agents and the host immune system.19 Associated microorganisms are often skin bacteria that are inoculated at joint implantation. In some cases, organisms seed the implant hematogenously or through compromised local tissues.
Infection with virulent organisms (e.g., S. aureus and gram-negative bacilli) inoculated at implantation is typically manifested as acute infection in the first 3 months (or, with hematogenous seeding of the implant, at any time) after surgery, whereas infection with less virulent organisms (e.g., coagulase-negative staphylococci and P. acnes) is more often manifested as chronic infection several months (or years) postoperatively. The most common symptom of infection associated with a prosthetic joint is pain. In acute infection, local signs and symptoms (e.g., severe pain, swelling, erythema, and warmth at the infected joint) and fever are common. Chronic infection generally has a more subtle presentation, with pain alone, and it is often accompanied by loosening of the prosthesis at the bone–cement interface and sometimes by sinus tract formation with discharge.
STRATEGIES AND EVIDENCE
DIAGNOSTIC APPROACH
It is important to accurately diagnose prosthetic-joint–associated infection because its management differs from that of other causes of arthroplasty failure. Although there is no universally accepted definition of this type of infection, the criteria listed in Table 1 have been applied in a number of studies.9,12,16,18,20,21
Table 1.
Criteria for the Diagnosis of a Prosthetic-Joint Infection.*
The presence of at least one of the following findings:
- Acute inflammation detected on histopathological examination of periprosthetic tissue
- Sinus tract communicating with the prosthesis
- Gross purulence in the joint space
- Isolation of the same microorganism from two or more cultures of joint aspirates or intraoperative periprosthetic-tissue specimens, isolation of the organism in substantial amounts (e.g., ≥20 CFU per 10 ml from the implant in a total volume of 400 ml of sonicate fluid), or both
Establishing the presence of acute infection or, in the presence of a draining sinus, chronic infection, is uncomplicated. In these situations, testing may be limited to that needed to establish the microbiologic diagnosis. Chronic infection manifested as localized joint pain alone poses more diagnostic difficulty, warranting additional testing. The criteria for interpreting laboratory and imaging findings in patients with a prosthetic joint are distinct from those applied in patients with a native joint. In addition to establishing the diagnosis, the identification of the involved organism or organisms and their antimicrobial susceptibility (i.e., on the basis of cultures of synovial fluid, periprosthetic tissue, the implant, or a combination of such cultures) is important in order to guide antimicrobial therapy.
C-Reactive Protein
In the absence of underlying inflammatory conditions, CRP measurement is the most useful preoperative blood test for detecting infection associated with a prosthetic joint. CRP testing has a sensitivity of 73 to 91% and a specificity of 81 to 86% for the diagnosis of prosthetic-knee infection with the use of a cutoff point of 13.5 mg per liter or more.22,23 It has a sensitivity of 95% and a specificity of 62% for the diagnosis of prosthetic-hip infection with the use of a cutoff point of more than 5 mg per liter.24 Although the CRP level and erythrocyte sedimentation rate are elevated after uncomplicated arthroplasty, the CRP level returns to the preoperative level within 2 months, whereas the erythrocyte sedimentation rate may remain elevated for several months.25 A normal CRP level generally indicates an absence of infection, although false negative results may occur in patients who have been treated with antimicrobial agents or who have infection that is caused by low-virulence organisms such as P. acnes. Elevations in the peripheral-blood leukocyte count and levels of procalcitonin have low sensitivity for detecting infection.
Imaging
Plain radiography has low sensitivity and low specificity for detecting infection associated with a prosthetic joint.26 Periprosthetic radiolucency, osteolysis, migration, or all of these features may be present on radiographs in patients with either infection or aseptic loosening of the prosthesis. Diagnostic studies with the use of computed tomography (CT) or magnetic resonance imaging (MRI) are hampered by artifacts produced by prostheses, although implants that are not ferromagnetic (i.e., titanium or tantalum) are associated with minimal MRI artifacts, and MRI scans of such implants provide good resolution for detecting soft-tissue abnormalities. Bone scans obtained after the administration of technetium-99m–labeled methylene diphosphonate are sensitive for detecting failed implants but nonspecific for detecting infection, and they may remain abnormal for more than a year after implantation. Some studies suggest that combined bone and gallium-67 scans are more specific than bone scans alone. However, labeled-leukocyte imaging (e.g., leukocytes labeled with indium-111) combined with bone marrow imaging with the use of technetium-99m–labeled sulfur colloid is more accurate than bone imaging alone, combined bone and gallium-67 imaging, or labeled-leukocyte and bone imaging when compared head to head, and it is considered the imaging test of choice when imaging is required.26 18F-fluorodeoxyglucose positron-emission tomography (PET) has a sensitivity of 82% and a specificity of 87% for the detection of prosthetic-knee or prosthetic-hip infection, on the basis of pooled data from several studies, but it is not widely available.27 Newer imaging strategies such as scintigraphy with antigranulocyte monoclonal antibodies and hybrid imaging (e.g., combined PET and CT) (see Fig. 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org) are under investigation.
Synovial-Fluid Studies
If there is uncertainty about the diagnosis, the most useful preoperative diagnostic test is aspiration of joint synovial fluid for a total and differential cell count and culture. Aspiration should not be performed through overlying cellulitis. Hip aspiration may require imaging guidance. A synovial-fluid leukocyte count of more than 1.7×103 per cubic millimeter or a differential count with more than 65% neutrophils is consistent with prosthetic-knee infection.28 A synovial-fluid leukocyte count of more than 4.2×103 per cubic millimeter or more than 80% neutrophils is consistent with prosthetic-hip infection.29 The leukocyte count cutoffs are dramatically lower than those used to diagnose native-joint infection. Synovial-fluid culture has a sensitivity of 56 to 75% and a specificity of 95 to 100%,12,22,30 and to achieve optimal sensitivity and specificity, it should be performed by means of inoculation into a blood-culture bottle.31 If an organism of questionable clinical significance is isolated, repeat synovial-fluid aspiration for culture should be considered. Previous antimicrobial treatment reduces the sensitivity.
Histopathological Examination of Periprosthetic Tissue
In patients in whom the diagnosis of prosthetic-joint–associated infection has not been established preoperatively, an intraoperative frozen section may be obtained to look for evidence of acute inflammation. In studies that used a polymorphonuclear-cell count ranging from more than 5 to 10 or more cells per high-power field as a positive test, sensitivity for infection ranged from 50 to 93% and specificity ranged from 77 to 100%32–35; the rate of interobserver agreement was 86%.36
Intraoperative Microbiologic Testing
Identification of the pathogen or pathogens is critical for choosing the antimicrobial regimen; if microbiologic testing has not been done preoperatively, specimens should be collected for microbiologic study at the time of surgery. Antimicrobial therapy should be discontinued at least 2 weeks before surgery, and perioperative antimicrobial coverage should be deferred until culture specimens have been collected. Cultures of sinus tract exudates should be avoided; these are often positive because of microbial skin colonization and correlate poorly with cultures of surgical specimens.
If periprosthetic tissue is obtained, collection of multiple periprosthetic-tissue specimens for aerobic and anaerobic bacterial culture is imperative because of the poor sensitivity of a single culture and to distinguish contaminants from pathogens. A study that used mathematical modeling to estimate yield based on the number of cultures concluded that to maximize accuracy, five or six specimens should be submitted for culture, and two or three culture-positive samples would be considered to be diagnostic.37
Periprosthetic-tissue cultures may be falsely negative because of previous antimicrobial therapy, leaching of antimicrobial agents from antimicrobial-impregnated cement, biofilm growth on the surface of the prosthesis (but not in the surrounding tissue), a low number of organisms in tissue, an inappropriate culture medium, an inadequate culture incubation time, or a prolonged time to transport the specimen to the laboratory. Because of poor sensitivity, neither intraoperative swab cultures38 nor Gram’s staining of the periprosthetic tissue37 is recommended. Fungal cultures, mycobacterial cultures, or both may be considered (e.g., if bacterial cultures are negative in a patient with apparent infection), but they are not routinely recommended.
Microorganisms form a biofilm on the prosthesis; therefore, if the prosthesis is removed, obtaining a sample from its surface is useful for microbiologic diagnosis.12 The implant is removed and transported to the laboratory in a sterile jar. After the addition of Ringer’s solution, the container is vortexed and sonicated (frequency, 40 kHz; power density, 0.22 W per square centimeter) for 5 minutes in a bath sonicator, and the resultant fluid is cultured. This technique is more sensitive than and as specific as multiple periprosthetic-tissue cultures for diagnosing infection of a prosthetic hip, knee, or shoulder, provided that an appropriate cutoff for significant results is applied (Table 1).12,16 This technique is particularly helpful in patients who have received previous antimicrobial therapy. In a study involving patients receiving antimicrobial agents within 2 weeks before surgery, the sensitivity of periprosthetic-tissue culture was 45%, whereas the sensitivity of sonicate-fluid culture was 75% (P<0.001).12 Sonication in bags is not recommended because of the potential for contamination.39,40
TREATMENT
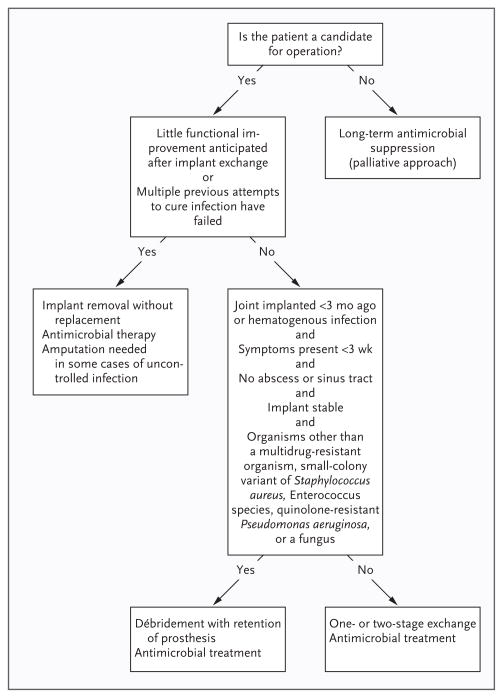
The goal of treatment is to cure the infection, prevent its recurrence, and ensure a pain-free, functional joint. This goal can best be achieved by a multidisciplinary team consisting of an orthopedic surgeon, an infectious-disease specialist, and a clinical microbiologist. On the basis of clinical experience, the use of antimicrobial agents alone, without surgical intervention, ultimately fails in most cases. Careful surgical débridement is critical. A general approach to surgical management is outlined in Figure 3 21,38,41; different centers and surgeons may use slightly different strategies. Chronic infections require resection arthroplasty either as a one-stage exchange (i.e., removal of the infected prosthesis and reimplantation of a new prosthesis during the same surgical procedure) or a two-stage exchange (i.e., removal of the infected prosthesis and administration of systemic antimicrobial agents with subsequent implantation of a new prosthesis, usually between 6 weeks and 3 months after the first stage). Case series have suggested improved outcomes with a one-stage exchange when polymethylmethacrylate impregnated with one or more antimicrobial agents is used. A spacer impregnated with one or more antimicrobial agents may be used to maintain the leg at its correct length and to control infection during the prosthesis-free interval of a two-stage exchange. In a randomized trial involving patients with infection associated with hip arthroplasty, the use of a vancomycin-loaded spacer (as compared with no spacer) resulted in a lower rate of recurrent infection (11% vs. 33%, P = 0.002).42
Figure 3. Algorithm for the Treatment of Infection Associated with a Prosthetic joint.
Patients who have had symptoms of infection for fewer than 3 weeks, who present with infection within 3 months after implantation or who have hematogenous infection, and who have a well-fixed, functioning prosthesis, without a sinus tract, and with an appropriate microbiologic diagnosis (Fig. 3) may be candidates for débridement and retention of the prosthesis.43 The addition of rifampin is recommended in cases of rifampin-susceptible staphylococcal infection. In a small, randomized trial comparing different antibiotic regimens in patients with staphylococcal infection of prosthetic knees or hips or osteosynthetic implants, salvage of the implant was successful in all 12 patients treated for 3 to 6 months with rifampin and ciprofloxacin, as compared with successful salvage in 7 of 12 patients treated with ciprofloxacin alone for 3 to 6 months (P = 0.02).43
When unacceptable joint function is anticipated after surgery or the infection has been refractory to multiple surgical attempts at cure, resection arthroplasty with creation of a pseudarthrosis for hips (Girdlestone resection) or arthrodesis for knees may be considered. If the patient is not a candidate for surgery, antimicrobial suppression may be attempted; this approach is unlikely to cure infection, so the use of antimicrobial agents is often continued indefinitely.
A detailed discussion of antimicrobial therapy for infection associated with prosthetic joints is beyond the scope of this article. In brief, information about antimicrobial susceptibility should be used to confirm the activity of any antimicrobial agent used for therapy. Data from randomized trials on the optimal duration of treatment are lacking. In patients undergoing débridement with retention of the prosthesis, 3-month courses of treatment for infection associated with hip prostheses and 6-month courses for infection associated with knee prostheses are often used. Oral therapy can be used if the agent has good oral bioavailability (e.g., quinolones, trimethoprim–sulfamethoxazole, and tetracyclines). In patients undergoing a two-stage exchange, systemic antimicrobial therapy is often administered for 4 to 6 weeks. Commercially available, preblended, polymethylmethacrylate impregnated with an antimicrobial agent is indicated for use in the second stage of a two-stage revision after elimination of active infection.44 Although it is not standard clinical practice, two studies involving a long period between the initial and second stages suggest that when a polymethylmethacrylate spacer impregnated with one or more antimicrobial agents or impregnated beads are used, the administration of systemic antimicrobial therapy for 2 weeks may be sufficient or systemic therapy may even be unnecessary.45, 46
PROPHYLAXIS
In addition to good aseptic technique and procedures in the operating room, the administration of intravenous antimicrobial agents immediately before surgery minimizes the risk of infection. Cefazolin at a dose of 1 g (2 g if the patient weighs ≥80 kg) every 8 hours or cefuroxime at a dose of 1.5 g, followed by 750 mg every 8 hours is recommended routinely; vancomycin at a dose of 15 mg per kilogram every 12 hours (assuming normal renal function) is used in patients with a _β_-lactam allergy or MRSA colonization. Prophylaxis should begin within 60 minutes before surgical incision (within 120 minutes if vancomycin is used) and should be completed within 24 hours after the end of surgery.47 The entire antimicrobial dose should be infused before inflation of a tourniquet.47
AREAS OF UNCERTAINTY
Although surgical intervention is generally recommended, the optimal surgical strategy in a given patient remains controversial. Likewise, the optimal antimicrobial regimen and its duration are incompletely defined. The optimal care for patients who are initially thought to have aseptic failure but who have intraoperative culture results that suggest infection is also uncertain; although a variety of medical treatments have been successful, further studies are needed to identify patients who can be treated with oral antimicrobial agents alone and those who may not need medical treatment.48
Polymerase-chain-reaction (PCR) assays may provide a more rapid diagnosis than culture and may facilitate diagnosis in patients with culture-negative infection (e.g., as a result of antimicrobial therapy),49 but their use in diagnosing infection associated with a prosthetic joint remains investigational. Some studies show that a broad-spectrum PCR assay (alone or combined with a specific PCR assay for staphylococcus) may be useful in establishing the diagnosis,49,50 but other studies indicate that it has low specificity for testing synovial fluid51 and low sensitivity for testing synovial fluid, tissue, or both52,53 and thus adds little value to cultures.54
GUIDELINES
Guidelines for the management of infection associated with prosthetic joints are expected from the Infectious Diseases Society of America later this year.
CONCLUSIONS AND RECOMMENDATIONS
The woman in the vignette has a sinus tract, which indicates that she has an infection associated with a prosthetic joint. Although additional testing is not needed to support the diagnosis, the elevated CRP level, the elevated synovial-fluid leukocyte count, and the percentage of neutrophils are consistent with infection. S. epidermidis is the likely pathogen. However, since this organism may be a contaminant, additional culture specimens should be obtained for confirmation: synovial fluid (obtained by reaspiration), five or six tissue specimens, or the implant (subjected to vortexing and sonication, with culture of the sonicate fluid). Given the presence of the sinus tract in this case and the duration of the patient’s symptoms, she is not a candidate for débridement and retention of the prosthesis. Instead, arthroplasty with a two-stage exchange plus antibiotics (according to the culture results) administered for 4 weeks would be appropriate.
Supplementary Material
Supplementary Data
Acknowledgments
Supported by grants from the National Center for Research Resources of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research (1 UL1 RR024150), and by a grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR056647).
Dr. Patel reports having an unlicensed U.S. patent pending for a method and an apparatus for sonication (and forgoing her right to receive royalties in the event that the patent is licensed) and receiving research funding from Pfizer, Cubist, Arobella Medical, Bard Medical, and SUBC/Zybac. No other potential conflict of interest relevant to this article was reported.
The views expressed in this article are solely those of the authors and do not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Center for Research Resources, or the NIH.
We thank James M. Steckelberg, M.D., for his thoughtful comments on an earlier version of the manuscript and Brian P. Mullan, M.D., for his assistance with the section on imaging.
References
- 1.National Hospital Discharge Survey: survey results and products. Atlanta: Centers for Disease Control and Prevention; 2009. [Accessed July 24, 2009]. http://www.cdc.gov/nchs/nhds/nhds_products.htm. [Google Scholar]
- 2.Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–91. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Jämsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty: a register-based analysis of 43,149 cases. J Bone Joint Surg Am. 2009;91:38–47. doi: 10.2106/JBJS.G.01686. [DOI] [PubMed] [Google Scholar]
- 4.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. [PubMed] [Google Scholar]
- 5.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–5. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choong PF, Dowsey MM, Carr D, Daffy J, Stanley P. Risk factors associated with acute hip prosthetic joint infections and outcome of treatment with a rifampin-based regimen. Acta Orthop. 2007;78:755–65. doi: 10.1080/17453670710014527. [DOI] [PubMed] [Google Scholar]
- 7.Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943–8. doi: 10.1302/0301-620X.88B7.17150. [DOI] [PubMed] [Google Scholar]
- 8.Murdoch DR, Roberts SA, Fowler VG, Jr, et al. Infection of orthopedic prostheses after Staphylococcus aureus bacteremia. Clin Infect Dis. 2001;32:647–9. doi: 10.1086/318704. [DOI] [PubMed] [Google Scholar]
- 9.Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27:1247–54. doi: 10.1086/514991. [DOI] [PubMed] [Google Scholar]
- 10.Bongartz T, Halligan CS, Osmon DR, et al. Incidence and risk factors of prosthetic joint infection after total hip or knee replacement in patients with rheumatoid arthritis. Arthritis Rheum. 2008;59:1713–20. doi: 10.1002/art.24060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowsey MM, Choong PF. Obesity is a major risk factor for prosthetic infection after primary hip arthroplasty. Clin Orthop Relat Res. 2008;466:153–8. doi: 10.1007/s11999-007-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trampuz A, Piper KE, Jacobson MJ, et al. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J Med. 2007;357:654–63. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- 13.Berbari EF, Osmon DR, Duffy MC, et al. Outcome of prosthetic joint infection in patients with rheumatoid arthritis: the impact of medical and surgical therapy in 200 episodes. Clin Infect Dis. 2006;42:216–23. doi: 10.1086/498507. [DOI] [PubMed] [Google Scholar]
- 14.Marculescu CE, Berbari EF, Cockerill FR, III, Osmon DR. Fungi, mycobacteria, zoonotic and other organisms in prosthetic joint infection. Clin Orthop Relat Res. 2006;451:64–72. doi: 10.1097/01.blo.0000229337.21653.f2. [DOI] [PubMed] [Google Scholar]
- 15.Unusual aerobic and anaerobic bacteria associated with prosthetic joint infections. Clin Orthop Relat Res. 2006;451:55–63. doi: 10.1097/01.blo.0000229317.43631.81. Idem. [DOI] [PubMed] [Google Scholar]
- 16.Piper KE, Jacobson MJ, Cofield RH, et al. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol. 2009;47:1878–84. doi: 10.1128/JCM.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marculescu CE, Cantey JR. Polymicrobial prosthetic joint infections: risk factors and outcome. Clin Orthop Relat Res. 2008;466:1397–404. doi: 10.1007/s11999-008-0230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berbari EF, Marculescu C, Sia I, et al. Culture-negative prosthetic joint infection. Clin Infect Dis. 2007;45:1113–9. doi: 10.1086/522184. [DOI] [PubMed] [Google Scholar]
- 19.del Pozo JL, Patel R. The challenge of treating biofilm-associated bacterial infections. Clin Pharmacol Ther. 2007;82:204–9. doi: 10.1038/sj.clpt.6100247. [DOI] [PubMed] [Google Scholar]
- 20.Marculescu CE, Berbari EF, Hanssen AD, et al. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis. 2006;42:471–8. doi: 10.1086/499234. [DOI] [PubMed] [Google Scholar]
- 21.Betsch BY, Eggli S, Siebenrock KA, Tauber MG, Mühlemann K. Treatment of joint prosthesis infection in accordance with current recommendations improves outcome. Clin Infect Dis. 2008;46:1221–6. doi: 10.1086/529436. [DOI] [PubMed] [Google Scholar]
- 22.Fink B, Makowiak C, Fuerst M, Berger I, Schäfer P, Frommelt L. The value of synovial biopsy, joint aspiration and C-reactive protein in the diagnosis of late periprosthetic infection of total knee replacements. J Bone Joint Surg Br. 2008;90:874–8. doi: 10.1302/0301-620X.90B7.20417. [DOI] [PubMed] [Google Scholar]
- 23.Greidanus NV, Masri BA, Garbuz DS, et al. Use of erythrocyte sedimentation rate and C-reactive protein level to diagnose infection before revision total knee arthroplasty: a prospective evaluation. J Bone Joint Surg Am. 2007;89:1409–16. doi: 10.2106/JBJS.D.02602. [DOI] [PubMed] [Google Scholar]
- 24.Müller M, Morawietz L, Hasart O, Strube P, Perka C, Tohtz S. Diagnosis of periprosthetic infection following total hip arthroplasty — evaluation of the diagnostic values of pre- and intraoperative parameters and the associated strategy to preoperatively select patients with a high probability of joint infection. J Orthop Surg. 2008;3:31. doi: 10.1186/1749-799X-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bilgen O, Atici T, Durak K, Karaeminoğullari O, Bilgen MS. C-reactive protein values and erythrocyte sedimentation rates after total hip and total knee arthroplasty. J Int Med Res. 2001;29:7–12. doi: 10.1177/147323000102900102. [DOI] [PubMed] [Google Scholar]
- 26.Love C, Marwin SE, Palestro CJ. Nuclear medicine and the infected joint replacement. Semin Nucl Med. 2009;39:66–78. doi: 10.1053/j.semnuclmed.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Kwee TC, Kwee RM, Alavi A. FDG-PET for diagnosing prosthetic joint infection: systematic review and metaanalysis. Eur J Nucl Med Mol Imaging. 2008;35:2122–32. doi: 10.1007/s00259-008-0887-x. [DOI] [PubMed] [Google Scholar]
- 28.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for diagnosis of prosthetic knee infection. Am J Med. 2004;117:556–62. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008;90:1869–75. doi: 10.2106/JBJS.G.01255. [DOI] [PubMed] [Google Scholar]
- 30.Virolainen P, Lähteenmäki H, Hiltunen A, Sipola E, Meurman O, Nelimarkka O. The reliability of diagnosis of infection during revision arthroplasties. Scand J Surg. 2002;91:178–81. doi: 10.1177/145749690209100208. [DOI] [PubMed] [Google Scholar]
- 31.Hughes JG, Vetter EA, Patel R, et al. Culture with BACTEC Peds Plus/F bottle compared with conventional methods for detection of bacteria in synovial fluid. J Clin Microbiol. 2001;39:4468–71. doi: 10.1128/JCM.39.12.4468-4471.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ko PS, Ip D, Chow KP, Cheung F, Lee OB, Lam JJ. The role of intraoperative frozen section in decision making in revision hip and knee arthroplasties in a local community hospital. J Arthroplasty. 2005;20:189–95. doi: 10.1016/j.arth.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 33.Wong YC, Lee QJ, Wai YL, Ng WF. Intraoperative frozen section for detecting active infection in failed hip and knee arthroplasties. J Arthroplasty. 2005;20:1015–20. doi: 10.1016/j.arth.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Francés Borrego A, Martinez FM, Cebrian Parra JL, Grañeda DS, Crespo RG, López-Durán Stern L. Diagnosis of infection in hip and knee revision surgery: intraoperative frozen section analysis. Int Orthop. 2007;31:33–7. doi: 10.1007/s00264-005-0069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nuñez LV, Buttaro MA, Morandi A, Pusso R, Piccaluga F. Frozen sections of samples taken intraoperatively for diagnosis of infection in revision hip surgery. Acta Orthop. 2007;78:226–30. doi: 10.1080/17453670710013726. [DOI] [PubMed] [Google Scholar]
- 36.Morawietz L, Classen RA, Schröder JH, et al. Proposal for a histopathological consensus classification of the periprosthetic interface membrane. J Clin Pathol. 2006;59:591–7. doi: 10.1136/jcp.2005.027458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atkins BL, Athanasou N, Deeks JJ, et al. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. J Clin Microbiol. 1998;36:2932–9. doi: 10.1128/jcm.36.10.2932-2939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–54. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 39.Trampuz A, Piper KE, Hanssen AD, et al. Sonication of explanted prosthetic components in bags for diagnosis of prosthetic joint infection is associated with risk of contamination. J Clin Microbiol. 2006;44:628–31. doi: 10.1128/JCM.44.2.628-631.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esteban J, Gomez-Barrena E, Cordero J, Martín-de-Hijas NZ, Kinnari TJ, Fernandez-Roblas R. Evaluation of quantitative analysis of cultures from sonicated retrieved orthopedic implants in diagnosis of orthopedic infection. J Clin Microbiol. 2008;46:488–92. doi: 10.1128/JCM.01762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sendi P, Rohrbach M, Graber P, Frei R, Ochsner PE, Zimmerli W. Staphylococcus aureus small colony variants in prosthetic joint infection. Clin Infect Dis. 2006;43:961–7. doi: 10.1086/507633. [DOI] [PubMed] [Google Scholar]
- 42.Cabrita H, Croci A, De Camargo O, De Lima A. Prospective study of the treatment of infected hip arthroplasties with or without the use of an antibiotic-loaded spacer. Clinics (Sao Paulo) 2007;62:99–108. doi: 10.1590/s1807-59322007000200002. [DOI] [PubMed] [Google Scholar]
- 43.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE Foreign-Body Infection (FBI) Study Group. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA. 1998;279:1537–41. doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]
- 44.Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg Am. 2006;88:2487–500. doi: 10.2106/JBJS.E.01126. [DOI] [PubMed] [Google Scholar]
- 45.Whittaker JP, Warren RE, Jones RS, Gregson PA. Is prolonged systemic antibiotic treatment essential in two-stage revision hip replacement for chronic Gram-positive infection? J Bone Joint Surg Br. 2009;91:44–51. doi: 10.1302/0301-620X.91B1.20930. [Erratum, J Bone Joint Surg Br 2009;91:700.] [DOI] [PubMed] [Google Scholar]
- 46.Stockley I, Mockford BJ, Hoad-Red-dick A, Norman P. The use of two-stage exchange arthroplasty with depot antibiotics in the absence of long-term antibiotic therapy in infected total hip replacement. J Bone Joint Surg Br. 2008;90:145–8. doi: 10.1302/0301-620X.90B2.19855. [DOI] [PubMed] [Google Scholar]
- 47.Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189:395–404. doi: 10.1016/j.amjsurg.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 48.Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Osmon DR. Prosthetic joint infection diagnosed postoperatively by intraoperative culture. Clin Orthop Relat Res. 2005;439:38–42. doi: 10.1097/01.blo.0000183091.83509.d8. [DOI] [PubMed] [Google Scholar]
- 49.Vandercam B, Jeumont S, Cornu O, et al. Amplification-based DNA analysis in the diagnosis of prosthetic joint infection. J Mol Diagn. 2008;10:537–43. doi: 10.2353/jmoldx.2008.070137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallo J, Kolar M, Dendis M, et al. Culture and PCR analysis of joint fluid in the diagnosis of prosthetic joint infection. New Microbiol. 2008;31:97–104. [PubMed] [Google Scholar]
- 51.Panousis K, Grigoris P, Butcher I, Rana B, Reilly JH, Hamblen DL. Poor predictive value of broad-range PCR for the detection of arthroplasty infection in 92 cases. Acta Orthop. 2005;76:341–6. [PubMed] [Google Scholar]
- 52.De Man FH, Graber P, Lüem M, Zimmerli W, Ochsner PE, Sendi P. Broad-range PCR in selected episodes of prosthetic joint infection. Infection. 2009;37:292–4. doi: 10.1007/s15010-008-8246-1. [DOI] [PubMed] [Google Scholar]
- 53.Fihman V, Hannouche D, Bousson V, et al. Improved diagnosis specificity in bone and joint infections using molecular techniques. J Infect. 2007;55:510–7. doi: 10.1016/j.jinf.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Dora C, Altwegg M, Gerber C, Böttger EC, Zbinden R. Evaluation of conventional microbiological procedures and molecular genetic techniques for diagnosis of infections in patients with implanted orthopedic devices. J Clin Microbiol. 2008;46:824–5. doi: 10.1128/JCM.01227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data