Parallel Actin Bundles and Their Multiple Actin-bundling Proteins (original) (raw)
. Author manuscript; available in PMC: 2010 Apr 13.
Abstract
Parallel actin bundles are present in a diverse array of structures, where they are critical determinants of cellular shape and physiology. In the last 18 months, new findings have solidified the concept that parallel actin bundles are assembled in cells through the sequential action of multiple actin-bundling proteins and have begun to shed light on the roles played by the individual actin-bundling proteins.
Introduction
Cells display an ability to organize their actin filaments into higher-order, cross-linked structures that have a profound influence on cellular shape, division, adhesion, motility and/or signaling. One type of cross-linked structure is the parallel actin bundle, in which the actin filaments are aligned axially, packed relatively tightly together and of the same polarity [1,2]. Parallel actin bundles can be found in a variety of complex structures (Figure 1), where they appear to function in part as scaffolds that help support or stabilize cellular protrusions, invaginations and/or domains of the plasma membrane. The cross-linking of actin filaments to form parallel actin bundles is accomplished by actin-bundling proteins. Of modular organization, actin-bundling proteins contain multiple actin-binding sites per monomer and can include regulatory domains that make them sensitive to specific stimuli, such as changes in the concentration of calcium ion [1--3]. In recent years, it has become evident that the cellular structures that contain parallel actin bundles make use of their own specific complements of multiple actin-bundling proteins. This review highlights the results of recent research on some relatively long-lived cellular specializations that contain parallel actin bundles.
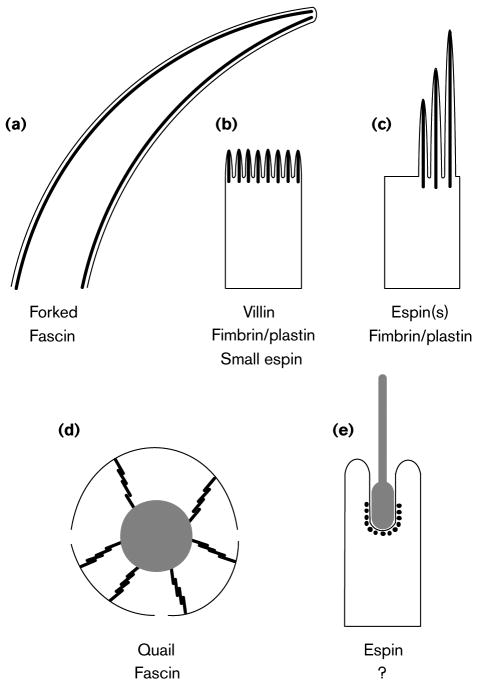
Figure 1. Schematic depictions of some cellular structures that contain parallel actin bundles in sectional view (not to scale) and a listing of their actin-bundling proteins.
The parallel actin bundles are shown as thick black lines when revealed in longitudinal section (a--d) or as black dots when revealed in transverse section (e). The plasma membrane is shown as a thin black line. (a) Neurosensory bristle of Drosophila. These actin bundles can be up to 70 μm (microchaete) or 400 μm (macrochaete) in length. Although bristles and their actin bundles taper, microchaetes contain >500 filaments per bundle at their base [4,5,9**]. (b) Brush border microvilli. Depending on location, these actin bundles are typically 1--5 μm in length and contain 20--25 actin filaments per bundle [21]. (c) Hair cell stereocilia. Depending on location, these actin bundles vary in length from 1--10 μm and can contain up to 900 actin filaments per bundle [34,35]. (d) Cytoplasmic actin bundles of Drosophila nurse cell. These actin bundles are composed of a linear series of relatively uniform bundle modules that are ∼3 μm in length, contain ∼25 actin filaments and appear to overlap like the units of an extension ladder [8,29-31]. The nurse cell nucleus is shown in gray, and the openings in the plasma membrane represent the ring canals. (e) Sertoli cell-spermatid ectoplasmic specialization. These actin bundles form a layer that is only 5-7 actin filaments in width that appears to wrap around the invagination of the Sertoli cell plasma membrane that is in contact with the acrosomal region of the head of an elongating spermatid [23,44,45]. The spermatid is shown in gray.
Neurosensory bristles of Drosophila
These long (up to 400 μm), curved protrusions found on the thorax of a Drosophila pupa contain multiple parallel actin bundles that run the length of the bristle beneath the plasma membrane [4] (Figure 1a). Perhaps because they are so long, these actin bundles are constructed via a novel mechanism that involves the end-to-end joining of preformed bundle modules [5]. Genetic analyses have indicated that the appearance of normal actin bundles and bristles requires the sequential action of at least two different actin-bundling proteins during bristle formation: forked and fascin, encoded by the forked and singed genes, respectively [4--8,9**,10**]. Forked acts first and appears to control many of the initial aspects of bundle assembly: the formation of small, disordered bundles beneath the plasma membrane; the cross-linking of these small bundles into larger bundles; and then finally the facilitation of the binding of fascin [4,9**,10**]. Fascin acts second and is the source of the regular cross-bridges noted at 12-nm intervals by electron microscopy that are believed to make the bundles/bristles straight and stiff [4,8,9**,10**]. Although bacterially expressed forked constructs are largely insoluble and, therefore, difficult to examine in vitro, one of the forked isoforms, forked A (Figure 2), has been found to elicit the formation of coarse actin fiber bundles and the accumulation of actin filaments when expressed in transfected mammalian cells [11*]. These activities require the presence of the peptides encoded by exons A5 and A3 (Figure 2). The peptide encoded by exon A5, which contains a 66-amino acid peptide that is 39% identical to an actin-binding peptide present within the C-terminal actin-bundling module of the espins [ 12*] (Figure 2 and see below), was found to co-localize with actin stress fibers in the transfected cells [11*]. The peptide encoded by exon A3 may also bind to actin, but appears to form aggregates both in vitro and in transfected cells ([11*], NS Petersen, H Qin, Mol Biol Cell 1996, 7 Suppl:514a). Both natural and recombinant fascin appear to bind to actin filaments with relatively low affinity [8,13,14], thereby making fascin well suited for the reversible making and breaking of cross-links that is believed to be necessary to form a straight, maximally cross-linked bundle from a smaller, disordered bundle nucleus [15]. The suggestion that the forked protein somehow facilitates fascin binding stems in part from the observation that, unlike forked, fascin appears to be in excess in the bristle compartment and cytoplasm of bristle cells before showing significant accumulation in the actin bundles [9**,10**]. How this facilitation might be accomplished in presently unclear. It is possible that the binding of forked might avail additional binding sites on the actin filament for fascin, perhaps by changing the conformation of the actin subunits (e.g., like the N-terminal 375-amino acid fragment of fimbrin [16]) and/or the twist on the actin filament (e.g., like cofilin [17]). The further application of cryoelectron microscopy [16] and the development of a new technique involving the examination of Fourier transforms of electron micrographs of two-dimensional bundles (“rafts”) [18*] may ultimately allow the effects of different actin-bundling proteins on filament and bundle structure to be discerned. Alternatively, the regulation of fascin binding could be more indirect, perhaps involving the dephosphorylation of fascin or the removal of interfering actin-binding proteins. The actin-binding/bundling activities of fascin in vitro have been found to be inhibited by phosphorylation on Ser-39 by protein kinase C [19] or by the inclusion of caldesmon and tropomyosin [20*]. Paradoxically, however, the addition of the broad-spectrum protein kinase inhibitor staurosporine to cultures of pupal thoracic epithelium appears to block or reverse fascin-mediated crosslinking in the actin bundles of bristles (LG Tilney, PS Connelly, KA Vranich, MK Shaw, GM Guild, personal communication). The observation of small actin bundles [10**] or monolayers of actin filaments [4] in close association with the plasma membrane in forked/singed double mutants suggest that an actin filament-membrane connector and possibly even a third actin-bundling protein remain to be identified in this system.
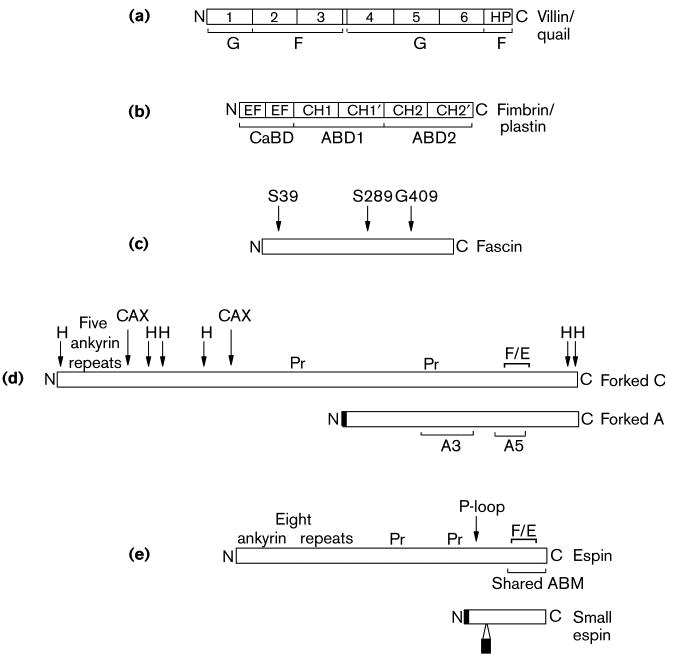
Figure 2. Schematic stick-figure diagrams of the actin-bundling proteins implicated in the assembly of the cellular structures shown in Figure 1, highlighting the relative positions and sizes of selected elements of primary sequence with structural or functional significance (roughly to scale).
Villin/Quail: Villin and quail are composed of six copies of an ∼15-kDa repeat characteristic of the members of the gelsolin family of actin-binding proteins (numbered 1-6), which account for the severing activity of villin observed in the presence of relatively high concentrations of calcium ion, and an ∼9-kDa actin-binding “headpiece” domain (HP), which is required for actin-bundling activity noted at lower concentrations of calcium ion. The actin monomer (G) or actin filament (F) binding portions of the molecule are shown. Fimbrin/Plastin: The fimbrins/plastins are members of the calponin-homology superfamily of actin-binding proteins and contains two ∼27-kD actin-binding domains (ABD1 and ABD2), each of which in turn contains two calponin homology domains (CH1,CH1′, CH2 and CH2′, respectively), and an N-terminal calcium ion-binding domain (CaBD) composed of two EF hand motifs. There are three highly related isoforms of fimbrin/plastin in mammals that are expressed in a cell-type specific manner. The I-isoform is present in the mature brush border microvillus. (Those interested in learning about the three-dimensional structures of the domains of villin and fimbrin/plastin should consult [3].) Fascin: Homologs of fascin have been detected in species ranging from echinoderms to humans. The positions of two point mutations that reduce the actin-bundling activity of Drosophila fascin (S289 and G409) and the position of the protein kinase C-mediated phosphorylation that inhibits the actin-bundling activity of human fascin (S39) are shown. Forked C and Forked A: The forked gene encodes at least 6 different transcripts, the products of different sites of transcriptional initiation and differential splicing. Shown here are the proteins encoded by one of the major transcripts, forked A, the ∼70-kDa isoform that has been examined in cell transfection experiments, and forked C, one of the largest transcripts, which is predicted to encode an ∼155-kDa protein that contains the maximum number of N-terminal ankyrin repeats and is included for the sake of comparion with espin (see below). An N-terminal peptide that is specific to forked A has been shaded, but regions of alignment between forked C and forked A that have not been shaded are identical. Also noted for the forked proteins are: the 66-amino acid forked/espin homology domain (F/E), proline-rich peptides (Pr), peptides containing an unusually high percentage of hydrophobic amino acids (H), peptides rich in Gln and His encoded by CAX trinucleotide repeats (CAX), and the peptides encoded by exons A3 and A5. Espin and Small Espin: The ∼110-kDa espin and the ∼30-kDa small espin are splice-isoforms that are expressed in a cell type-specific fashion. Peptides that are unique to small espin have been shaded, but regions of alignment between espin and small espin that are not shaded are identical. Also noted for the espins are: their shared 116-amino acid C-terminal actin-bundling module (shared ABM), which includes the 66-amino acid forked/espin homology domain (F/E); a potential P-loop, which is present in both isoforms; and the two proline-rich peptides (Pr) and 8 N-terminal ankyrin repeats that are present in espin, but not in small espin.
Brush border microvilli
These numerous and highly regular, finger-like projections dramatically increase the apical plasma membrane of absorptive epithelial cells [21] (Figure 1b). It was through the study of the stepwise assembly of the brush border microvilli that a role for multiple actin-bundling proteins in the construction of parallel actin bundles was first recognized [22]. As if two actin-bundling proteins were not enough, the parallel actin bundle at the core of the brush border microvillus has recently been found to contain yet a third actin-bundling protein, small espin [12*]. Small espin is a member of what appears to be a new family of high affinity actin-bundling proteins, the espins [12*,23] (Figure 2). The espins display some intriguing similarities to the forked proteins of Drosophila, that include a 66-amino acid actin-binding peptide that is 39% identical, centrally placed proline-rich peptides, and N-terminal ankyrin-like repeats in the larger isoforms [6,12*,23] (Figure 2). Recombinant small espin elicits the formation of parallel actin bundles in vitro under physiological conditions and, when expressed ectopically in transiently transfected cells, small espin decorates actin stress fiber-like structures and appears to cause their bundling and/or accumulation [12*]. Small espin is minor compared to villin and fimbrin/plastin, at least in isolated brush borders, and appears to accumulate in the brush border later than these other two actin-bundling proteins during brush border assembly [12*]. Thus, small espin may be added to a largely already constructed core bundle, perhaps to lock the structure into place with a stable cross-link that is of higher affinity and, unlike the cross-links provided by villin and fimbrin/plastin, is insensitive to changes in the concentration of calcium ion.
The presence of a third actin-bundling protein in brush border microvilli might help explain why villin-knockout mice appear to have relatively normal brush border microvilli and core actin bundles, at least under normal conditions [24*,25**]. Recent experiments suggest that there is a phenotype for the villin-knockout mouse after all, but that the system must be stressed in order to see it. Specifically, the disruption of the brush border actin cytoskeleton observed in response to treatments that either raise the intracellular concentrations of calcium ion, such as serosal administration of carbachol and mucosal administration of A23187 in isolated intestinal loops, or otherwise injure the small intestinal epithelium, such as fasting/refeeding, has been found to be significantly reduced in villin-knockout mice [25**]. In addition, the extent of mucosal damage and mortality resulting from oral administration of the colitis-inducing compound dextran sulfate sodium was also found to be increased in the villin-knockout mice [25**]. These results suggest that villin may not be required as an actin-bundling protein per se in brush border microvilli, but that it may actually be involved in mediating calcium ion-induced rearrangements of the microvillar core actin bundle in response to cellular injury, presumably in part via its long-appreciated calcium ion-activated actin filament-severing activity [25**]. Some rather dramatic rearrangements of brush border microvilli and their actin cytoskeleton have been found to take place under a variety of other conditions, such as during infection with enteropathogenic E. coli [26] and during renal ischemia or ATP depletion/repletion [27].
The actin-bundling activity of the other major actin-bundling protein of brush border microvilli, fimbrin/plastin, is also inhibited by calcium ion, and an atomic model of its binding to F-actin, made by fitting the crystal structure of the N-terminal actin-binding domain of fimbrin to helical reconstructions of fimbrin-decorated actin filaments, suggests a reason why [28**]. In the model, the N- and C-termini of the two calponin-homology domains that make up actin-binding domain 1 (Figure 2) point in opposite directions [28**]. This arrangement would place actin-binding domain 2 (Figure 2) away from the filament and in position to bind a second filament with the correct interfilament spacing. The N-terminal 100-amino acid calcium ion-binding domain, made up of two EF hand domains (Figure 2), appears to occupy a crevice between the two calponin homology domains of actin-binding domain 1, where it could change their relative positions in response to binding of calcium ion [28**].
Cytoplasmic actin bundles in Drosophila nurse cells
These parallel actin bundles extend from the nurse cell plasma membrane to form a cage around the nucleus that prevents lobes of the nucleus from clogging the ring canals during the delivery of cytoplasmic components from nurse cells to oocytes [8,29] (Figure 1 d). The assembly of these bundles, which appears to involve the joining together of multiple microvillus-derived core bundles like the units of an extension ladder [29], requires two actin cross-linking proteins, quail (a villin homolog) followed by fascin, which are encoded by the quail and singed genes, respectively [8,30]. Mutants in either gene do not form nurse cell cytoplasmic actin bundles, and female sterility results [8,30]. In contrast to the situation described above for villin [25**], quail appears to act as a bonafide actin-bundling protein in this system. Unlike villin, recombinant quail does not sever or cap actin filaments or nucleate actin filament assembly in the presence of relatively high concentrations of calcium ion, but instead appears to bundle actin filaments irrespective of calcium ion concentration (Matova N, Mahajan-Miklos S, Mooseker MS, Cooley L, personal communication). In fact, there is an indication that quail can at least partially substitute for fascin in this system, because over-expression of quail in singed mutants initially appears as a wild-type, but the cytoplasmic actin bundles become disorganized in the final stages [31*]. The issue of partial functional redundancy among the multiple actin-crosslinking proteins has also been addressed in Dictyostelium, where eliminating actin cross-linking proteins, either singly or in pairs, has been found to give rise to a complicated mixture of additive and synthetic phenotypes [32*].
Hair cell stereocilia
These highly specialized microvillus-like projections found in organized arrays on the apical surfaces of hair cells in the inner ear are intimately involved in the mechanical-electrical signal transduction of sound and motion [33] (Figure 1c). The assembly of these collections of stereocilia is a stepwise process punctuated by changes in the location, number, length and width of the parallel actin bundles found at the core the stereocilium [34]. Although fimbrin/plastin is one of the major actin cross-linking proteins of the hair cell stereocilium [34,35], the retention of the core actin bundle after the extraction of fimbrin/plastin [35] suggest that there are other actin cross-linking proteins at work. Villin is not present [35], but recently, some potentially novel isoforms of espin have been identified in hair cell stereocilia (JR Bartles, G Sekerkova, A Li, B Chen, K Vranich, E Mugnaini, LG Tilney, Mol Biol Cell 1998, 9 Suppl: 135a). Deafness mutations that give rise to defects in the organization or dimensions of the hair cell stereocilia have been mapped to genes that encode unconventional myosins VIIA [36,37], VI [38] and XV [39*,40]. Both myosins VIIA and VI have been localized to the cuticular plate, the specialized actin filament gel-containing counterpart to the brush border terminal web that is situated beneath hair cell stereocilia [34], and myosin VIIA has also been detected in the cross-links between adjacent stereocilia [41]. Another unconventional myosin, myosin Iβ [42], and a novel actin-associated protein, 2E4 (kaptin) [43] have been localized at the tips of hair cell stereocilia.
Sertoli cell ectoplasmic specializations
These intercellular junctions, which are believed to anchor and position spermatids throughout much of spermiogenesis [44,45], are perhaps the most neglected of all the parallel actin bundle containing structures. Found where Sertoli cells contact the head of an elongating spermatid, the ectoplasmic specialization is characterized ultrastructurally by its junctional plaque, which contains a thin layer of parallel actin bundles with hexagonally packed filaments sandwiched between the Sertoli cell plasma membrane and an affiliated flattened cistern of endoplasmic reticulum [44,45] (Figure 1e). The parallel actin bundles of the ectoplasmic specialization junctional plaque are believed to function in part as a scaffold that supports and stabilizes an adhesive domain in the Sertoli cell plasma membrane [44,45]. They are also believed to act indirectly, via their connection to the cistern of endoplasmic reticulum, as a link to an underlying network of microtubules that may be responsible for changes in the depth of the ectoplasmic specialization-spermatid complex within the seminiferous epithelium [45,46]. Espin, the founding member of the espin family of actin-bundling proteins, was identified as a component of the parallel actin bundles in the ectoplasmic specialization junctional plaque [23]. Espin shares a 116-amino acid C-terminal actin-bundling module with small espin of brush border microvilli, but it has a longer N-terminus with multiple motifs for protein-protein interaction, 8 ankyrin-like repeats and two proline-rich peptides and an extra actin-binding site ([12*, 23], B Chen, A Li, D Wang, M Wang, L Zheng, JR Bartles, unpublished data) (Figure 2). Unlike the situation for small espin in brush border microvilli [12*], espin is a major actin-bundling protein of the ectoplasmic specialization junctional plaque, and it accumulates in the plaque coincident with the formation of parallel actin bundles during spermiogenesis (B Chen, A Li, D Wang, M Wang, L Zheng, JR Bartles, unpublished data).
Conclusions
The findings reviewed above indicate that the use of different complements of multiple actin-bundling proteins is the rule, rather than the exception, when it comes to forming parallel actin bundles in cells. At first glance this may seem inconceivable, or at least uneconomical. But it is important to remember that the actin bundles in question are present in complex cellular structures with highly specific functions; the bundles exhibit different dimensions, are assembled using different multiple-step pathways, and presumably exhibit different properties as well. Accordingly, it is perhaps not so surprising after all that cells employ different combinations of actin-bundling proteins with different structures, different binding affinities and different modes of regulation to assemble these fascinating permuations on the parallel actin bundle theme. By far, the clearest demonstation that the different actin-bundling proteins are not functionally redundant in vivo has come through the analysis of neurosensory bristles and nurse cell cytoplasmic bundles in Drosophila, where leaving out one or the other actin-bundling protein causes profound defects. Comfortingly, the villin-knockout mouse has also been found to display a phenotype, albeit more subtle, emphasizing the importance of applying multiple assays and stresses. The new ideas about the role of villin in brush border microvilli suggests that we must consider the possibility that proteins that display actin-bundling activity in vitro or in transfected cells display alternative functions in situ. The presence of the unaccounted-for domains in proteins like forked and the espins may provide some clues about these alternative functions, or at least provide some insight into possible modes of regulation. The application of new methods of structural analysis in conjunction with more conventional biochemical approaches should ultimately reveal how the different actin-bundling proteins might compete or cooperate within the bundle, but we still know comparatively little about how the different actin-bundling proteins are distributed within the bundles and how their association with the bundle is regulated. In spite the progress over the last 18 months, it is clear that we still have a great deal more to learn before we will understand how the utilization of a particular set of actin-bundling proteins -- in a particular order -- influences the properties of an actin bundle and facilitates the proper assembly of the bundle and the cellular specialization with which it is associated.
Acknowledgments
I gratefully acknowledge influential discussions with Drs. Lew Tilney, Nancy Petersen, Greg Guild, David DeRosier, Paul Matsudaira, and Dorit Hanein. I thank Drs. Tilney, Petersen, and Guild and Drs. Sylvie Robine, Daniel Louvard, Marcus Fechheimer, and Elizabeth Luna for generously conveying their results prior to publication. Work in my lab on the espins is supported by NIH grant R01 HD35280, NIH Independent Scientist Award K02 HD 01210 and American Cancer Society grant #RPG-96-094-04-CSM.
References and recommended reading
- 1.Matsudaira P. Modular organization of actin crosslinking proteins. Trends Biochem Sci. 1991;16:87–92. doi: 10.1016/0968-0004(91)90039-x. [DOI] [PubMed] [Google Scholar]
- 2.Furukawa R, Fechheimer M. The structure, function, and assembly of actin filament bundles. Int Rev Cytol. 1997;175:29–90. doi: 10.1016/s0074-7696(08)62125-7. [DOI] [PubMed] [Google Scholar]
- 3.Puius YA, Mahoney NM, Almo SC. The modular structure of actin-regulatory proteins. Curr Opin Cell Biol. 1998;10:23–34. doi: 10.1016/s0955-0674(98)80083-5. [DOI] [PubMed] [Google Scholar]
- 4.Tilney LG, Tilney MS, Guild GM. F-Actin bundles in Drosophila bristles. I. Two filament cross-links are involved in bundling. J Cell Biol. 1995;130:629–638. doi: 10.1083/jcb.130.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilney LG, Connelly P, Smith S, Guild GM. F-Actin bundles in Drosophila bristles are assembled from modules composed of short filaments. J Cell Biol. 1996;135:1291–1308. doi: 10.1083/jcb.135.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoover KK, Chien AJ, Corces VJ. Effect of transposable elements on the expression of the forked gene of Drosophila melanogaster. Genetics. 1993;135:507–526. doi: 10.1093/genetics/135.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen NS, Lankenau DH, Mitchell HK, Young P, Corces VG. Forked proteins are components of fiber bundles present in developing bristles of Drosophila melanogaster. Genetics. 1994;136:173–182. doi: 10.1093/genetics/136.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cant K, Knowles BA, Mooseker MS, Cooley L. Drosophila singed, a fascin homolog, is required for actin bundle formation during oogenesis and bristle extension. J Cell Biol. 1994;125:369–380. doi: 10.1083/jcb.125.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **9.Tilney LG, Connelly PS, Vranich KA, Shaw MK, Guild GM. Why are two different crosslinkers necessary for actin bundle formation in vivo and what does each crosslink contribute? J Cell Biol. 1998;143:121–133. doi: 10.1083/jcb.143.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]; By carefully examining the consequences on actin bundle ultrastructure of providing too little or too much forked, of providing too little fascin, or of differentially removing the fascin by extraction with KI, the authors extend the conclusions of earlier studies [4--8] to implicate highly specific and sequential roles for forked and fascin in the construction of the parallel actin bundles in the neurosensory bristles of Drosophila pupae (see also [10**] below).
- **10.Wulfkuhle JD, Petersen NS, Otto JJ. Changes in the F-actin cytoskeleton during neurosensory bristle development in Drosophila: the role of singed and forked proteins. Cell Motil Cytoskel. 1998;40:119–132. doi: 10.1002/(SICI)1097-0169(1998)40:2<119::AID-CM2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]; This study provides a light-microscopic view of bristle actin bundle assembly, comparing wild-type to singed and forked mutants, that is in many ways complementary to that described in [9**]. In addition, by localizing forked and fascin at selected times during development, these authors confirm the prediction that forked appears in bristle actin bundles before fascin and provide some of the first evidence that fascin is present in excess within the bristle cytoplasm before being incorporated into the actin bundles.
- *11.Grieshaber S, Petersen NS. The Drosophila forked protein induces the formation of actin fiber bundles in vertebrate cells. J Cell Sci. 1999;112:2203–2211. doi: 10.1242/jcs.112.13.2203. [DOI] [PubMed] [Google Scholar]; Although forked proteins expressed in bacteria have proved to be difficult to work with, the authors of this article do the next best thing: they show that forked can induce the accumulation of actin filaments and the formation of actin bundles when expressed ectopically in transfected mammalian cells. In addition, they map one domain required for these two activities to a peptide that shows homology to one of the actin-binding peptides of the espins [12*].
- *12.Bartles JR, Zheng L, Li A, Wierda A, Chen B. Small espin: a third actin-bundling protein and potential forked protein ortholog in brush border microvilli. J Cell Biol. 1998;143:107–119. doi: 10.1083/jcb.143.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes the identification and characterization of an ∼30-kDa splice-isoform in the espin family of actin-binding/bundling proteins that shows the properties expected for a third actin-bundling protein of brush border microvilli.
- 13.Bryan J, Kane RE. Separation of interaction of the major components of sea urchin actin gel. J Mol Biol. 1978;125:207–224. doi: 10.1016/0022-2836(78)90345-5. [DOI] [PubMed] [Google Scholar]
- 14.Edwards RA, Herrera-Sosa H, Otto J, Bryan J. Cloning and expression of a murine fascin homolog from mouse brain. J Biol Chem. 1995;270:10764–10770. doi: 10.1074/jbc.270.18.10764. [DOI] [PubMed] [Google Scholar]
- 15.Stokes DL, DeRosier DJ. Growth conditions control the size and order of actin bundles in vitro. Biophys J. 1991;59:456–465. doi: 10.1016/S0006-3495(91)82239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanein D, Matsudaira P, DeRosier DJ. Evidence for a conformational change in actin induced by fimbrin (N375) binding. J Cell Biol. 1997;139:387–396. doi: 10.1083/jcb.139.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGough A, Pope B, Chiu W, Weeds A. Cofilin changes the twist of F-actin: implications for actin filament dynamics and cellular function. J Cell Biol. 1997;138:771–781. doi: 10.1083/jcb.138.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *18.Sukow C, DeRosier D. How to analyze electron micrographs of rafts of actin filaments crosslinked by actin-binding proteins. J Mol Biol. 1998;284:1039–1050. doi: 10.1006/jmbi.1998.2211. [DOI] [PubMed] [Google Scholar]
- 19.Ono S, Yamakita Y, Yamashiro S, Matsudaira PT, Gnarra JR, Obinata T, Matsumura F. Identification of an actin binding region and a protein kinase C phosphorylation site on human fascin. J Biol Chem. 1997;272:2527–2533. doi: 10.1074/jbc.272.4.2527. [DOI] [PubMed] [Google Scholar]
- *20.Ishikawa R, Yamashiro S, Kohama K, Matsumura F. Regulation of actin binding and actin bundling activities of fascin by caldesmon coupled with tropomyosin. J Biol Chem. 1998;273:26991–26997. doi: 10.1074/jbc.273.41.26991. [DOI] [PubMed] [Google Scholar]
- 21.Heintzelman MB, Mooseker MS. Assembly of the intestinal brush border cytoskeleton. Curr Top Dev Biol. 1992;26:93–122. doi: 10.1016/s0070-2153(08)60442-1. [DOI] [PubMed] [Google Scholar]
- 22.Shibayama T, Carboni JM, Mooseker MS. Assembly of the intestinal brush border: appearance and redistribution of microvillar core proteins in developing chick enterocytes. J Cell Biol. 1987;105:335–344. doi: 10.1083/jcb.105.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartles JR, Wierda A, Zheng L. Identification and characterization of espin, an actin-binding protein localized to the F-actin-rich junctional plaques of Sertoli cell ectoplasmic specializations. J Cell Sci. 1996;109:1229–1239. doi: 10.1242/jcs.109.6.1229. [DOI] [PubMed] [Google Scholar]
- **24.Pinson KI, Dunbar L, Samuelson L, Gumucio DL. Targeted disruption of the mouse villin gene does not impair the morphogenesis of microvilli. Dev Dyn. 1998;211:109–121. doi: 10.1002/(SICI)1097-0177(199801)211:1<109::AID-AJA10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]; This is the first article to report that targeted disruption of the villin gene in the mouse has little or no effect on brush border microvilli, at least under normal conditions (see also [25**]).
- **25.Ferrary E, Cohen-Tannoudji M, Pehau-Arnaudet G, Lapillonne A, Athman R, Ruiz T, Boulouha L, El Marjou F, Doye A, Fontaine JJ, Antony C, Babinet C, Louvard D, Jaisser F, Robine S. In vivo, villin is required for Ca2+-dependent F-actin disruption in intestinal brush-borders. J Cell Biol. 1999;146:819–830. doi: 10.1083/jcb.146.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]; These authors independently prepared villin-knockout mice and found no noticeable changes in the ultrastructure of microvilli or in the localization and expression of actin-binding and membrane proteins in the intestine under normal conditions (see also [24*]). However, they did notice some striking differences between villin-knockout mice and the wild-type controls when the system was stressed by agents that either raised the intracellular concentration of calcium ion or otherwise damaged the intestinal epithelium. These observations led the authors to the unexpected conclusion that villin is not necessary to the bundling of actin filaments in brush border microvilli, but for the reorganization of the microvillus actin cytoskeleton in response to injury or specific signals.
- 26.Goosney DL, de Grado M, Finlay BB. Putting E. coli on a pedestal: a unique system to study signal transduction and the cytoskeleton. Trends Cell Biol. 1999;9:11–14. doi: 10.1016/s0962-8924(98)01418-4. [DOI] [PubMed] [Google Scholar]
- 27.Raman N, Atkinson SJ. Rho controls actin cytoskeletal assembly in renal epithelial cells during ATP depletion and recovery. Am J Physiol. 1999;276:C1312–C1324. doi: 10.1152/ajpcell.1999.276.6.C1312. [DOI] [PubMed] [Google Scholar]
- **28.Hanein D, Volkmann N, Goldsmith S, Michon AM, Lehman W, Craig R, DeRosier D, Almo S, Matsudaira P. An atomic model of fimbrin binding to F-actin and its implications for filament crosslinking and regulation. Nat Struct Biol. 1998;5:787–792. doi: 10.1038/1828. [DOI] [PubMed] [Google Scholar]; By fitting the crystal structure of the N-terminal actin-binding domain of human T-fimbrin to helical reconstructions of fimbrin-decorated actin filaments, the authors propose the first atomic working model of a fimbrin cross-link and its regulation by calcium ion.
- 29.Guild GM, Connelly PS, Shaw MK, Tilney LG. Actin filament cables in Drosophila nurse cells are composed of modules that slide passively past one another during dumping. J Cell Biol. 1997;138:783–797. doi: 10.1083/jcb.138.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahajan-Miklos S, Cooley L. The villin-like protein encoded by the Drosophila quail gene is required for actin bundle assembly during oogenesis. Cell. 1994;78:291–301. doi: 10.1016/0092-8674(94)90298-4. [DOI] [PubMed] [Google Scholar]
- *31.Cant K, Knowles BA, Mahajan-Miklos S, Heintzelman M, Cooley L. Drosophila fascin mutants are rescued by overexpression of the villin-like protein, quail. J Cell Sci. 1998;111:213–221. doi: 10.1242/jcs.111.2.213. [DOI] [PubMed] [Google Scholar]; The authors over-express quail in singed mutants using P-element germline transformation, find that excess quail can rescue the formation of cytoplasmic actin bundles in nurse cells and conclude that quail fascin is partially redundant with quail in the Drosophila germline.
- *32.Rivero F, Furukawa R, Fechheimer M, Noegel AA. Three actin cross-linking proteins, the 34 kDa actin-bundling protein α-actinin and gelation factor (ABP-120), have both unique and redundant roles in the growth and development of Dictyostelium. J Cell Sci. 1999;112:2737–2751. doi: 10.1242/jcs.112.16.2737. [DOI] [PubMed] [Google Scholar]
- 33.Roberts WM, Howard J, Hudspeth AJ. Hair cells: transduction, tuning, and transmission in the inner ear. Ann Rev Cell Biol. 1988;4:63–92. doi: 10.1146/annurev.cb.04.110188.000431. [DOI] [PubMed] [Google Scholar]
- 34.Tilney LG, Tilney MS, DeRosier DJ. Actin filaments, stereocilia and hair cells: how cells count and measure. Ann Rev Cell Biol. 1992;8:257–274. doi: 10.1146/annurev.cb.08.110192.001353. [DOI] [PubMed] [Google Scholar]
- 35.Tilney MS, Tilney LG, Stephens RE, Merte C, Drenckhahn D, Cotanche DA, Bretscher A. Preliminary characterization of the stereocilia and cuticular plate of hair cells in the chick cochlea. J Cell Biol. 1989;109:1711–1723. doi: 10.1083/jcb.109.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibson F, Walsh J, Mburu P, Varela A, Brown KA, Antonio M, Beisel KW, Steel KP, Brown SD. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature. 1995;374:62–64. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- 37.Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, Mburu P, Varela A, Levilliers J, Weston MD, et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- 38.Avraham KB, Hasson T, Steel KP, Kingsley DM, Russell LB, Mooseker MS, Copeland NG, Jenkins NA. The mouse Snell's waltzer deafness gene encodes an unconventional myosin required for structural integrity of the inner ear hair cells. Nat Genet. 1995;11:369–375. doi: 10.1038/ng1295-369. [DOI] [PubMed] [Google Scholar]
- *39.Probst FJ, Fridell RA, Raphael Y, Sauders TL, Wang A, Liang Y, Moreel RJ, Touchman JW, Lyons RH, Noben-Trauth K, et al. Correction of deafness in shaker-2 mice by an unconventional myosin in a BAC transgene. Science. 1998;280:1444–1447. doi: 10.1126/science.280.5368.1444. [DOI] [PubMed] [Google Scholar]
- 40.Wang A, Liang Y, Fridell RA, Probst FJ, Wilcox ER, Touchman JW, Morton CC, Morell RJ, Noben-Trauth K, Camper SA, Friedman TB. Association of unconventional myosin MYO15 mutations with human nonsyndromic deafness DFNB3. Science. 1998;280:1447–1451. doi: 10.1126/science.280.5368.1447. [DOI] [PubMed] [Google Scholar]
- 41.Hasson T, Gillespie PG, Garcia JA, MacDonald RB, Zhao Y, Yee AG, Mooseker MS, Corey DP. Unconventional myosins in inner-ear sensory epithelia. J Cell Biol. 1997;137:1287–307. doi: 10.1083/jcb.137.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Metcalf AB. Immunolocalization of myosin Iβ in the hair cell's hair bundle. Cell Motil Cytoskel. 1998;39:159–165. doi: 10.1002/(SICI)1097-0169(1998)39:2<159::AID-CM6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 43.Bearer EL, Abraham MT. 2E4 (kaptin): a novel actin-associated protein from human blood platelets found in lamellipodia and the tips of the stereocilia of the inner ear. Eur J Cell Biol. 1999;78:117–126. doi: 10.1016/S0171-9335(99)80013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- 45.Vogl AW, Pfeiffer DC, Redenbach DM. Ectoplasmic (“junctional”) specializations in mammalian Sertoli cells: influence on spermatogenic cells. Ann NY Acad Sci. 1991;637:175–202. doi: 10.1111/j.1749-6632.1991.tb27310.x. [DOI] [PubMed] [Google Scholar]
- 46.Beach SF, Vogl AW. Spermatid translocation in the rat seminiferous epithelium: coupling membrane trafficking machinery to a junction plaque. Biol Reprod. 1999;60:1036–1046. doi: 10.1095/biolreprod60.4.1036. [DOI] [PubMed] [Google Scholar]