Differential Activation and Regulation of CXCR1 and CXCR2 by CXCL8 monomer and dimer (original) (raw)
. Author manuscript; available in PMC: 2010 Sep 1.
Published in final edited form as: J Immunol. 2009 Aug 10;183(5):3425–3432. doi: 10.4049/jimmunol.0900305
SUMMARY
CXCL8 (also known as interleukin-8) activates CXCR1 and CXCR2 to mediate neutrophil recruitment and trigger cytotoxic effect at sites of infection. Under physiological conditions, CXCL8 could exist as monomers, dimers, or a mixture of monomers and dimers. Therefore, both forms of CXCL8 could interact with CXCR1 and CXCR2 with different affinities and potencies to mediate different cellular responses. In the present study, we have used a “trapped” non-associating monomer (L25NMe), and a non-dissociating dimer (R26C), to investigate their activities for human neutrophils which express both receptors, and for RBL-2H3 cells stably expressing either CXCR1(RBL-CXCR1) or CXCR2 (RBL-CXCR2). The monomer was more active than the dimer for activities such as intracellular Ca2+ mobilization, phosphoinositide hydrolysis, chemotaxis and exocytosis. Receptor regulation, however, is distinct for each receptor. The rate of monomer-mediated regulation of CXCR1 is greater for activities such as phosphorylation, desensitization, βarrestin translocation and internalization. In contrast, for CXCR2, both monomeric and dimeric CXCL8 mediate these activities to similar extent. Interestingly, receptor-mediated signal-regulated kinases (ERK) phosphorylation in response to all three CXCL8 variants was more sustained for CXCR2 relative to CXCR1. Taken together, the results indicate that the CXCL8 monomer and dimer differentially activate and regulate CXCR1 and CXCR2 receptors. These distinct properties of the ligand and the receptors play a critical role in orchestrating neutrophil recruitment and eliciting cytotoxic activity during an inflammatory response
INTRODUCTION
CXCL8 (also known as interleukin-8, IL-8) belongs to the CXC subfamily of chemokines that mediates neutrophil accumulation and activation at site of inflammation and infection. These functions are mediated by binding to two cell surface receptors, CXCR1 and CXCR2 (1, 2). CXCR1 is specific for CXCL8, whereas CXCR2 is promiscuous and also interacts with CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, and CXCL7 (3). Upon activation, both receptors couple to pertussis toxin (Ptx)-sensitive G protein to mediate phosphoinositide (PI) hydrolysis, intracellular Ca2+ mobilization, chemotaxis, and exocytosis(4, 5).
A characteristic feature of chemokines, including CXCL8, is their ability to reversibly exist as both monomers and dimers (6). During active neutrophil recruitment, CXCL8 concentrations could vary spatially and temporally and reach high levels so that both monomers and dimers exist at different locations and time points, suggesting both forms play an active role in recruitment and inflammatory response. Previous studies using a non-associating ‘trapped’ monomer (L25NMe) and a non-dissociating ‘trapped’ dimer (R26C) of CXCL8 have shown that the monomer is the high affinity ligand, and the dimer is the low affinity ligand for both receptors(7–9). However, a knowledge of the ligand binding affinities alone is not sufficient to understand receptor-mediated cellular function. Function is a downstream event that is a consequence of receptor binding, and differences in binding affinities could be attenuated or amplified.
Like many G-protein coupled receptors (GPCRs) on leukocytes, CXCR1 and CXCR2 become phosphorylated, desensitized and internalized upon activation by CXCL8. Previous studies using wild type (WT) CXCL8 have shown that CXCR2, compared to CXCR1, internalizes more rapidly and recovers more slowly (4, 5, 10–13). These differences in receptor trafficking appear to be important factors that influence how and to what extent and how the individual receptors mediate neutrophil recruitment and activation (4, 5, 14).
To date, little is known about how monomeric and dimeric forms of CXCL8 regulate CXCR1 and CXCR2 functions. In the present work, we sought to determine the role of the two forms of CXCL8 in mediating various receptor activities and their downregulation and trafficking. To that end, rat basophilic leukemia (RBL-2H3) cells stably expressing CXCR1 or CXCR2 were generated. The ability of the monomer and dimer forms to mediated different receptor activities such as phosphorylation, desensitization, arrestin translocation and internalization were compared to WT CXCL8. The data herein indicate that the monomer and dimer modulate differently the desensitization and trafficking of CXCR1, but not of CXCR2. We propose that these distinct properties of the monomer and dimer forms of the two receptors play an essential role in mediating neutrophil trafficking and eliciting cytotoxic activities at the site of inflammation.
MATERIALS AND METHODS
Materials
[32P]Orthophosphate (8500–9120 Ci/mmol), myo-[2-3H]inositol (24.4 Ci/mmol) and [125I]-CXCL8 were purchased from Perkin Elmer. Geneticin (G418) and all tissue culture reagents were purchased from Invitrogen, Inc. Human microvascular endothelial cells (HMECs) were either purchased from 3H Biomedical AB, Sweden, or obtained from Dr. Rakesh Singh’s laboratory(University of Nebraska Medical Center, Omaha, NE). Monoclonal 12CA5 antibody, protein G-agarose and protease inhibitors were purchased from Roche Diagnostics. Anti-Human IL-8RA (CXCR1) and IL-8RB (CXCR2) antibodies were purchased from BD Pharmingen. Phorbol 12-myristate 13-acetate (PMA) was purchased from Sigma. All other reagents are from commercial sources.
Ligand synthesis
The trapped R26C disulfide-linked dimer, and the trapped L25NMe monomer were synthesized using solid-phase peptide synthesis (SPPS) and purified by reversed-phase HPLC, and the WT was produced using recombinant methods as described previously(15, 16).
Cell culture and transfection
RBL-2H3 cells were maintained as monolayer cultures in Dulbacco’s modified Eagle’s medium (DMEM) supplemented with 15% heat inactivated fetal bovine serum, 2 mM glutamine, penicillin (100 units/ml), and streptomycin (100 mg/ml) (17). RBL-2H3 cells (1×107 cells) were transfected by electroporation with 20 μg of pcDNA3 containing the receptor cDNAs. Geneticin-resistant cells were selected by fluorescence-activated cell sorter (FACS) analysis and cloned into single cells. HMECs were grown in endothelial cell medium supplemented with 10% heat inactivated fetal bovine serum. Levels of receptor expression were monitored by fluorescence-activated cell sorting (FACS) analysis.
FACS analysis
For flow cytometry, RBL cells were detached by Versene treatment, washed with HEPES buffered Hanks’ balanced salt solution (HHBSS) and resuspended in the same medium. Cells (1–5×106 cells) were incubated with anti-CXCR1 or anti-CXCR2 antibodies (1 μg/ml) in a total volume of 400 μl of HHBSS for 60 min at 4 °C. The cells were then washed and incubated with fluorescein (FITC)-antimouse IgG for 60 min at 4 °C, and then analyzed for cell surface expression of the receptor on a Beckton Dickenson FACScan cytometer(5).
Radioligand binding assays and receptor internalization
Radioligand binding assays were carried out as described previously (5). Briefly, RBL-2H3 cells were subcultured overnight in 24-well plates (0.5×106 cells/well) in growth medium. Cells were then rinsed with Dulbecco’s modified Eagles medium supplemented with 20 mM Hepes, pH 7.4 and 10 mg/ml BSA and incubated on ice for 2–4 hours in the same medium (250 μl) containing [125I]-CXCL8 (0.1–1 nM). Reactions were stopped with 1 ml of ice-cold PBS containing 10 mg/ml BSA, and washed 3 times with the same buffer. The cells were then solubilized with 200 μl of radioimmunoprecipitation assay (RIPA) buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 0.1% SDS, and dried under vacuum and bound radioactivity was counted(18). Nonspecific radioactivity bound was determined in the presence of 500 nM unlabelled WT CXCL8. Kd and Bmax were determined using the GraphPad radioligand binding data analysis. For competition binding, [125I]-CXCL8 binding were carried in the presence of different concentrations of CXCL8 ligands (0–1 μM). For receptor internalization, cells were incubated with ligand for 0–60min at 37 °C. The cells were then washed with ice-cold PBS, and 125I-CXCL8 binding (0.1–1 nM) was carried out as described above.
Phosphoinositide hydrolysis, β-hexosaminidase release, and intracellular calcium measurement
RBL-2H3 cells were subcultured overnight in 96-well culture plates (50,000 cells/well) in inositol-free medium supplemented with 10% dialyzed fetal bovine serum and 1 μCi/ml [3H]inositol. The generation of inositol phosphates and β-hexosaminidase were determined as previously reported (5, 17, 19, 20). For calcium mobilization, 5×106 cells were washed with HEPES buffered saline and loaded with 1 μM Indo I-AM in the presence of 1 μM pluronic acid for 30 min at room temperature. The cells were then washed with HEPES and resuspended in 1.5 ml of Siriganian buffer, and intracellular calcium levels for CXCL8 variants were measured as described previously(20).
Chemotaxis
RBL-2H3 cells or HMECs (50,000) were incubated at 37 °C with different concentration of ligands. Chemotaxis was assessed in 48 well microchemotaxis chambers, using polyvinylpyrrolidone-free 8μm pore size membranes. Migration was allowed to continue for 3 hours at 37 °C in humidified air containing 5% CO2. The membrane was removed, the upper surface washed with PBS and scraped, fixed and stained. For antibody pretreatment, HMECs were incubated with 10 μg/ml of anti-CXCR1, anti-CXCR2, or mouse IgG for 30 min as described previously (21, 22). The results are represented as chemotactic index (mean number of cells per high power field for chemokine dilution/mean number of cells per high power field for medium (5). The results are representative of three separate experiments.
Phosphorylation of receptors
Phosphorylation of receptors was performed as described previously (5, 18, 23). RBL-2H3 cells (5×106) expressing the receptors were incubated with [32P]orthophosphate (150 μCi/dish) for 90 min. The labeled cells were then stimulated with the CXCL8 variants for 5 minutes at 37 °C. Cells were then washed with ice-cold PBS and solubilized in 1 ml of RIPA. Cell lysates were immunoprecipitated with specific antibodies against either the N-terminus of CXCR1 or CXCR2 and analyzed by SDS electrophoresis and visualized by autoradiography.
βarrestin translocation
RBL-2H3 cells stably expressing CXCR1 and CXCR2 were transfected with βarrestin1-GFP by electroporation and plated on sterile 0.17 mm glass bottom dishes and grown overnight. The cells were washed twice with 2 ml of warm RPMI 1640 media without phenol red containing 10 mM HEPES. The experiments were carried in the same media. The cells were observed under the microscope using oil immersion 60X objective lens. The fluorescence images of the live cells were collected using TE-FM Epi-Fluorescence system attached to Nikon Inverted Microscope Eclipse TE300 at 37 °C at different time intervals (30 seconds for 1 hr) in the presence or absence of ligand. All the fluorescence images were captured by cool snap HQ digital B/W CCD (Roper Scientific) camera. The fluorescence of GFP was detected with filter set S480/20x, S525/40m (#86007, EGFP/DsRed, Chroma Technology Corp.). All the data were analyzed and images were pseudocolored using Metamorph 4.6r5 software (Universal Imaging Corp) (23).
Inhibition of GTPase activity
For desensitization, RBL-CXCR1 and RBL-CXCR2 (1 × 107 cells/ml) were washed twice with PBS and treated with 100 nM of either CXCL8, L25NM2 or R26C for 5 min at 37°C. The reaction was stopped by the addition of 10 ml of ice-cold PBS, cells were pelleted by centrifugation and membranes were prepared as previously described (17, 18). GTPase assays using 10–20 μg of membranes was measured at 30 °C in buffer that contained 20 mM NaHepes (pH 8.0), 25 mM NaCl, 0.1 mM EDTA, 1.0 mM MgCl2, 20 μg/ml bovine albumin, 50 nM GDP, 100 nM [γ-32P]GTP, and 100nm CXCL8 (17, 18). Basal activity (32Pi released in the absence of CXCL8, 6–10 pmol/mg of protein) has been subtracted from CXCL8-stimulated GTPase activity (15–20 pmol/mg of protein).
ERK Phosphorylation
RBL-2H3 cells (5 × 106) expressing the receptors were washed three times with PBS and then resuspended in PBS containing CXCL8 variants (100 nM) for different time intervals at 37 °C. The reactions were stopped with ice-cold PBS, and the centrifuged cells were lysed and assayed for protein concentration as described previously (23). Proteins (~50 μg) were resolved by 10% SDS-PAGE, transferred to a nitrocellulose membrane, and probed with antibody against either ERK1/2 or phospho-ERK1/2 (23). Detection was carried out with horseradish peroxidase-conjugatedsheep anti-mouse antibody and by ECL.
RESULTS
Function of CXCL8 monomer and dimer for human neutrophils
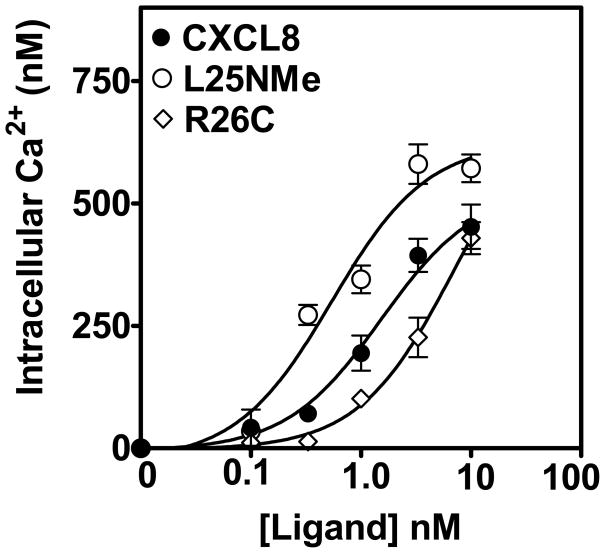
To assess the ability of the monomeric and dimeric variants of CXCL8 to mediate cellular responses in human neutrophils, intracellular calcium mobilization was measured. As shown in Fig. 1, the trapped monomer mediated greater intracellular Ca2+ mobilization (EC50= 0.5 ±0.2 nM) compared to the trapped dimer (EC50= 7.1 ±0.1 nM). The WT CXCL8 showed an activity similar to the monomeric CXCL8 (EC50= 1.5 ±0.1 nM).
Figure 1. Monomer, dimer or native CXCL8 induced intracellular Ca2+ mobilization in human neutrophils.
Human neutrophils (5 × 106) were loaded with indo-1AM and stimulated with different concentration of monomer, dimer, and WT CXCL8. The results are representative of one of three experiments performed in triplicate.
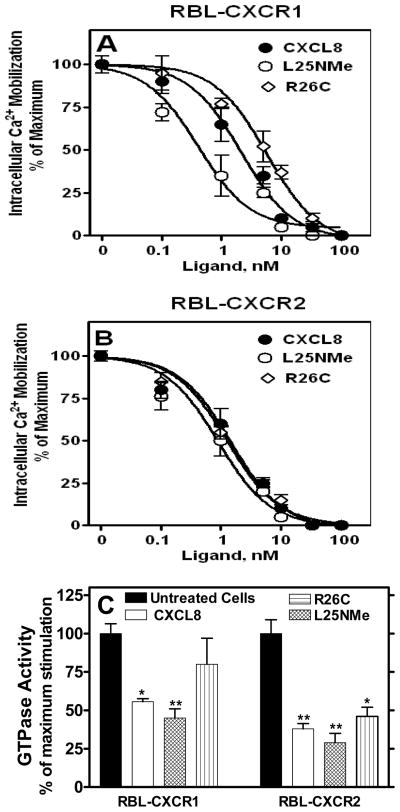
CXCL8 monomer and dimer-mediated CXCR1 and CXCR2 activation in RBL-2H3 cells
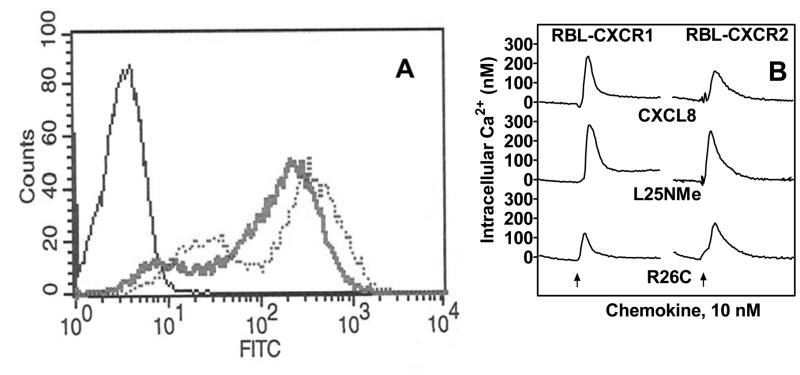
Since neutrophils express both CXCR1 and CXCR2, we sought to determine the effect of the trapped monomer and dimer ligands in RBL-2H3 cells stably expressing either CXCR1 (RBL-CXCR1) or CXCR2 (RBL-CXCR2). FACS analysis (Fig 2A) and ligand binding studies indicate that the cell lines express similar number of receptors (Bmax: 13,564±679 and 14,092±527 receptors/cell for RBL-CXCR1 and RBL-CXCR2, respectively). The receptors were previously shown to bind WT CXCL8 with similar affinities (Kd: 4.7±0.1 and 2.5±0.1 nM for RBL-CXCR1 and RBL-CXCR2, respectively) to that of the native receptors in neutrophils (Kd: 1–2 nM) (24). Competitive binding studies with RBL-CXCR1 and RBL-CXCR2 showed that the receptors bound the monomer (Kd: 2.9±0.1 and 1.7±0.1 nM for CXCR1 and CXCR2, respectively) with affinities similar to that of WT CXCL8, and bound the dimer (Kd: 17.1±0.7 and 9.0±0.2 nM for CXCR1 and CXCR2, respectively) with lower affinities (Table 1). These results are consistent with those previously reported (9).
Figure 2. Expression and characterization of WT CXCR1 and CXCR2.
A) A representative histogram of FACS analysis showing surface expression of CXCR1 and CXCR2 in RBL-2H3 cells after staining with CXCR1 (dotted line) or CXCR2 (broad line) specific antibodies. B) For intracellular Ca2+ mobilization, RBL cells (5 × 106) expressing human CXCR1 (RBL-CXCR1) or CXCR2 (RBL-CXCR2) were loaded with Indo-1AM and stimulated with monomer, dimer, or WT CXCL8. The representative tracings are one of the three experiments.
TABLE I. Binding affinities of monomer, dimer, and WT CXCL8 to CXCR1 and CXCR2 expressed in RBL-2H3 cells.
Apparent affinity binding (Kd) values determined by competitive displacement of 125I-CXCL8. Values are averaged of triplicate experiments.
| Ligand | Kd, nM | |
|---|---|---|
| RBL-CXCR1 | RBL-CXCR2 | |
| Monomer | 2.9±0.1 | 1.7±0.1 |
| Dimer | 17.1±0.7 | 9.0±0.2 |
| WT | 4.7±0.095 | 2.5±0.13 |
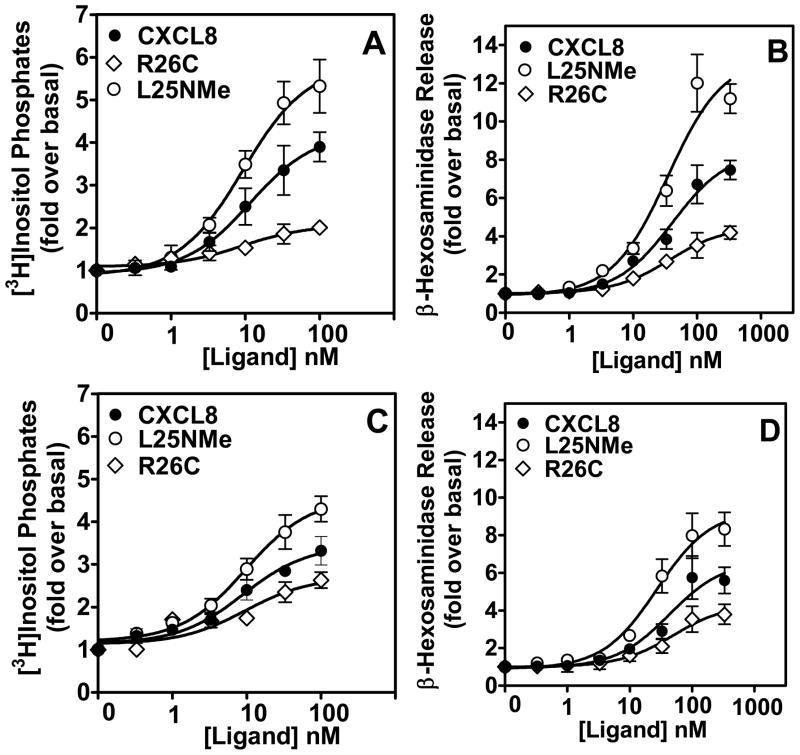
We next measured the ability of the CXCL8 variants to mediate cellular responses in RBL-2H3 cells. As shown for the neutrophils, the trapped monomer mediated greater intracellular Ca2+ mobilization in both RBL-CXCR1 and RBL-CXCR2 cells compared to the trapped dimer (Fig. 2B). The CXCL8 variants also displayed dose-dependent phosphoinositide (PI) hydrolysis and secretion of β-hexosaminidase. The potencies of the monomer and dimer were similar (EC50: ~20 and ~40 nM for PI hydrolysis and secretion of β-hexosaminidase, respectively), whereas the efficacies were different. The monomer, compared to the dimer, was more efficacious in eliciting both PI hydrolysis and β-hexosaminidase release, and the differences were observed to be more profound for the CXCR1 compared to the CXCR2 receptor. The relative efficacies of the monomer and dimer for PI hydrolysis are 5.9±0.4 and 2.0±0.4, respectively in RBL-CXCR1, and 4.7±0.2 and 2.7±0.2, respectively in RBL-CXCR2 cells (Figs 3A and 3C). The relative efficacies of the monomer and dimer for secretion of β-hexosaminidase are 13.5±0.8 and 4.5±0.2, respectively in RBL-CXCR1, and 9.4±0.4 vs. 4.3±0.4, respectively in RBL-CXCR2 cells (Figs. 3B and 3D). WT CXCL8 showed intermediate efficacies in both assays.
Figure 3. Phosphoinositide hydrolysis (PI), secretion of β-hexosaminidase release and Chemotaxis.
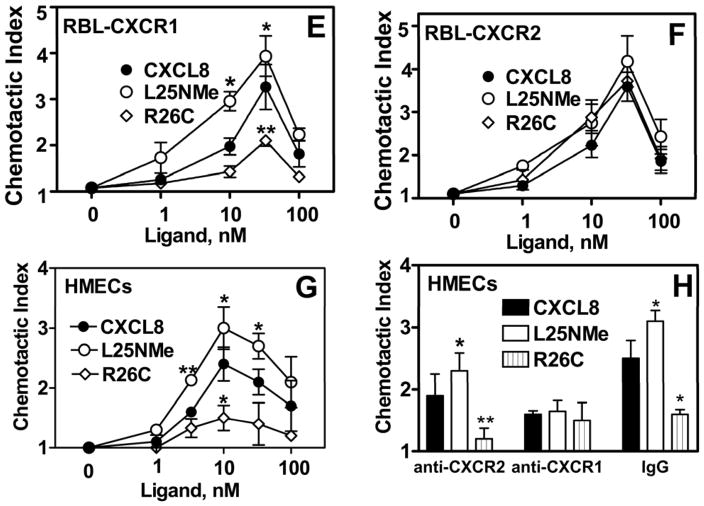
For the generation of inositol phosphates (A and C), cells were cultured overnight in the presence of [3H]inositol (1 μC/ml). Cells were preincubated for 10 min at 37°C with HEPES-buffered saline containing 10 mM LiCl in a total volume of 200 μl and stimulated with different concentrations of monomer, dimer, or WT CXCL8 for 10 min. The supernatant was used to determine the release of inositol phosphates. Data are represented as the fold stimulation over basal. For secretion of β-hexosaminidase (B and D), cells were seeded as indicated above and stimulated with different concentrations of CXCL8 variants for 10 min. The supernatant (15 μl) was removed and β-hexosaminidase release was measured. Data are represented as fold stimulation over basal. Both experiments were repeated three times with similar results. Chemotactic response to CXCL8 variants using RBL-CXCR1 and RBL-CXCR2 (E and F) or HMECs (G and H) were measured as described in Materials and Methods. The results are representative of one of four experiments performed in triplicate. *, P< 0.05 and ** P< 0.01.
We next measured the ability of the CXCL8 variants to induce chemotaxis in RBL-2H3 cells. Maximum chemotactic index was obtained at ~33 nM for both RBL-CXCR1 and RBL-CXCR2 (Figs 3E and 3F). The monomer was more potent in mediating chemotaxis to CXCR1 (3.9±1.0), as compared to the dimer (2±0.4) and WT CXCL8 (3.1±1.1). In contrast, chemotactic response to CXCR2 was similar for all three CXCL8 variants (4.1±0.7, 3.8±0.2, and 3.6±0.4 for monomer, WT, and dimer, respectively; Fig 3F).
We also assessed the ability of CXCL8 variants to induce chemotaxis in HMEC cells that express both CXCR1 and CXCR2 receptors(22). As shown in Figure 3G, the potency of the monomer was greater than that of the dimer. HMECs pretreated with anti-CXCR2 or or mouse IgG also displayed greater chemotaxis in response to the monomer than that of the dimer or WT CXCL8 (Fig 3H). HMECs pretreated with anti-CXCR1 however,, displayed similar chemotactic index (~1.5) for all three CXCL8 variants (Fig 3H). These data mirrored the ones obtained with RBL-CXCR2 and further indicate that the dimer is as potent as the monomer in mediating chemotactic response to CXCR2.
CXCL8-induced receptor phosphorylation
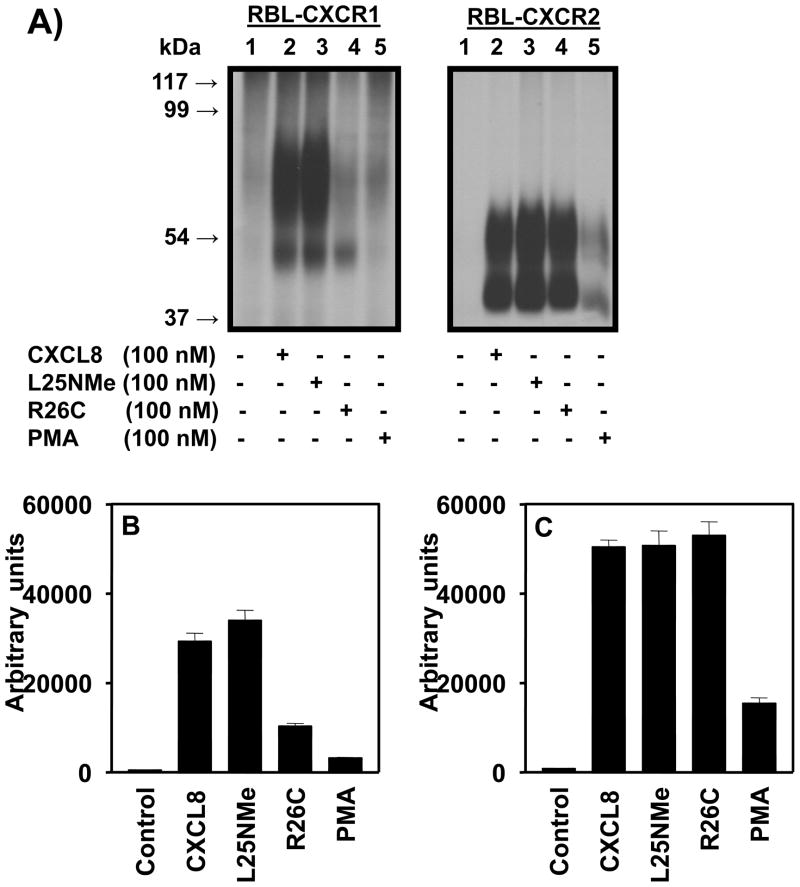
To assess the ability of the CXCL8 variants to induce receptor phosphorylation, 32P-labeled RBL-CXCR1 and RBL-CXCR2 cells were stimulated with CXCL8 variants and PMA as control. Both CXCL8 monomers and dimers induced phosphorylation of both CXCR1 and CXCR2 (Fig 4A). The receptors migrated as two forms: a high molecular mass form (~70 and ~55 kDa for CXCR1 and CXCR2, respectively) and a low molecular mass form (~50 and ~40 kDa for CXCR1 and CXCR2, respectively). The high molecular mass corresponds to the dimer and the low molecular mass to the monomer (25). For CXCR1, the monomer induced greater phosphorylation than the dimer, whereas for CXCR2, both monomers and dimers induced receptor phosphorylation to similar extent (Figures 4B and 4C). PMA-induced heterologous phosphorylation of CXCR1 and CXCR2 was significantly lower than the CXCL8 variants.
Figure 4. Phosphorylation of CXCR1 and CXCR2.
A) 32P-labeled RBL-2H3 cells expressing CXCR1 or CXCR2were incubated for 5 min with or without stimulants as shown. Cells were lysed, immunoprecipitated with specific antibodies against CXCR1 or CXCR2 and then analyzed by SDS-PAGE and autoradiography. The results are from a representative experiment that was repeated three times. B and C, The amount of radioactivity per lane was determined by counting excised phosphorylated bands.
Receptor internalization
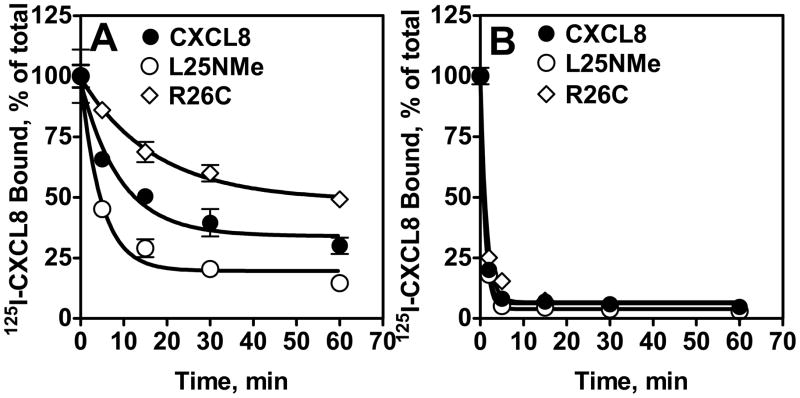
Both CXCL8 monomer and dimer induced internalization of CXCR1 and CXCR2 in a time-dependent manner (Fig. 5). Monomer-induced CXCR1 internalization was faster than that of the dimer (~70% vs. 30% after 15 min; Fig 5A), whereas both forms of CXCL8 induced rapid CXCR2 internalization (~95% after 5 min; Fig 5B). The WT showed intermediate activity for CXCR1 and the same activity as monomer and dimer for CXCR2.
Figure 5. Internalization CXCR1 and CXCR2.
RBL-CXCR1 (A) or RBL-CXCR2 (B) cells were treated with 100 nM of monomer, dimer, or WT CXCL8 for different times, washed and assayed for 125I-WT CXCL8 (0.1nM) binding. The value is presented as a percentage of totals, which is defined as the total amount of 125I-WT CXCL8 bound to control (untreated) cells. The experiment was repeated five times with similar results.
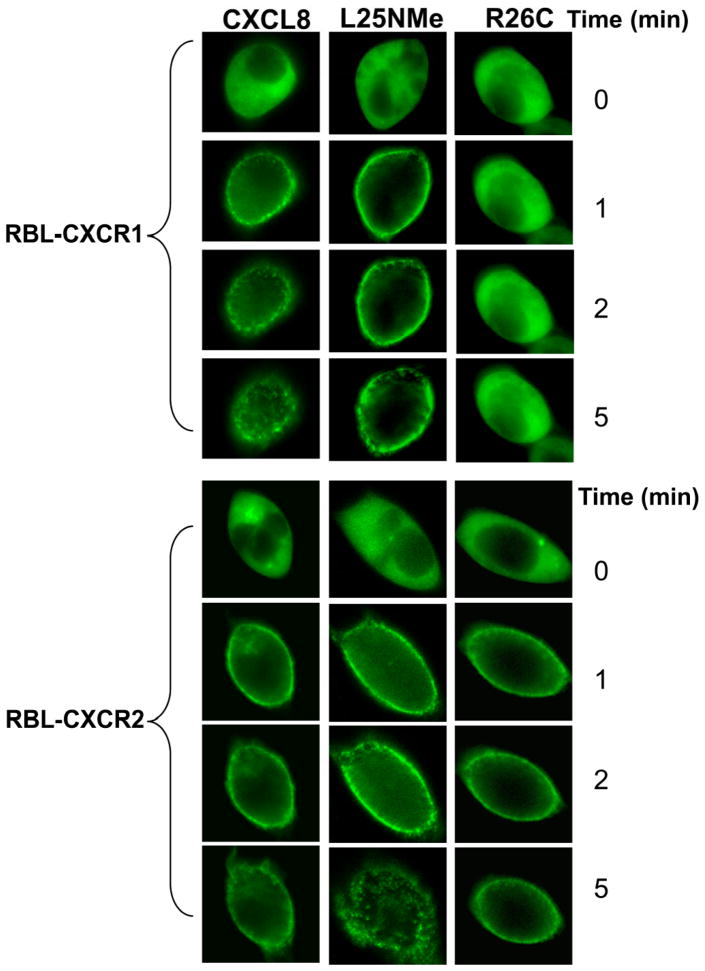
Both receptors have been shown to internalize via an arrestin-dependent mechanism (5, 10). In order to assess the time-dependency of CXCR1 and CXCR2-mediated arrestin recruitment, a GFP-tagged βarrestin-1 (βarr-1) was transiently expressed in RBL-CXCR1 and RBL-CXCR2 cells. Fluorescence microscopy was used to study the time-course of arrestin translocation to the cell surface and internalization. The monomer, but not the dimer, induced βarr-1 translocation to the cell membrane in RBL-CXCR1 cells. In contrast, both monomer and dimer induced βarr-1 translocation in RBL-CXCR2 cells (Fig. 6). The WT behaved like the monomer in RBL-CXCR1 cells, and like both monomer and dimer in RBL-CXCR2 cells.
Figure 6. CXCR1 and CXCR2-mediated βarrestin 1 translocation to the cell membrane.
RBL-CXCR1 and RBL-CXCR2 were transfected with β-arrestin1-GFP. The fluorescence images of GFP were captured upon addition of 100 nM of monomer, dimer, or WT CXCL8 to live cells as described in the method section.
CXCL8-mediated extracellular signal-regulated kinase (ERK) activation
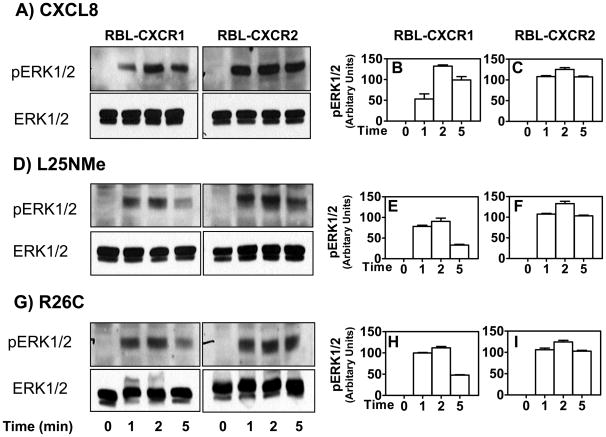
To determine the ability of the CXCL8 variants to activate downstream signals in RBL-2H3 cells, we measured ERK1/2 phosphorylation. Both monomer and dimer activate CXCR1 and CXCR2 to induce time-dependent phosphorylation of ERK1/2 (Fig 7, panels A, D and G). Maximum responses were obtained at 1–2 min. CXCR2-induced ERK1/2 phosphorylation (Fig 7, panels C, F and I), however was more sustained than that of CXCR1 (Fig 7, panels B, E and H).
Figure 7. CXCR1 and CXCR2-mediated ERK phosphorylation.
RBL-2H3 cells stably expressing CXCR1 or CXCR2 were stimulated with monomer (D), dimmer(G), or WT CXCL8(A) (100 nM) for 0–5 min. ERK1/2 phosphorylation and total ERK were determined by Western blotting using anti-phospho-ERK1/2 (pERK1/2) and anti-total ERK1/2 (ERK1/2) antibodies, respectively. The experiments were repeated three times with similar results(B, C, E, F, H and I). Band density from radiogram was calculated by Image-Pro Plus software (Media Cybernetics) and is represented as relative phospho-ERK1/2 expression (arbitrary units). Data shown are averages of three experiments.
Receptor desensitization
Intracellular Ca2+ mobilization in intact cells was measured to assess receptor desensitization. Cells were first exposed to different concentrations of CXCL8 variants (0–100 nM), and rechallenged 3 min later with 10 nM of WT CXCL8. As shown in Figure 8, all forms of CXCL8 desensitized intracellular Ca2+ mobilization to WT CXCL8 in a dose-dependent fashion. Monomer-mediated desensitization of CXCR1 was more potent than that of dimer (EC50 0.9 vs. 5.6 nM) (Figure 8A). In contrast, both monomer and dimer desensitized CXCR2 with similar potencies (EC50 ~1 nM) (Fig 8B). The WT CXCL8 showed intermediate activity for CXCR1 and the same activity as monomer and dimer for CXCR2.
Figure 8. Desensitization of CXCR1 and CXCR2-mediated intracellular calcium mobilization and GTPase activity.
RBL-2H3 cells (5×106 cells) expressing (A) CXCR1 or (B) CXCR2 were loaded with Indo-1 AM and exposed to a first dose of either monomer, dimer, or WT CXCL8 (0–100 nM). Cells were rechallenged 3 min later with a second dose of CXCL8 (10 nM) and intracellular Ca2+ mobilization was measured. Data are represented as percentage of maximum which represents intracellular Ca2+ mobilization-induced by CXCL8 (10 nM) in the absence of pretreatment (320±49 and 227±28 for RBL-CXCR1 and RBL-CXCR2, respectively). C) For GTPase activity, the cells were treated with or without ligand (100 nM) for 5 min. Membranes were prepared and assayed for WT CXCL8 (100 nM) stimulated GTP hydrolysis. The data are presented as percentage of maximum which is the net maximal stimulation obtained with untreated cells. Data shown are representative of three experiments performed in triplicate. *, P< 0.05 and ** P< 0.01.
To further assess the ability of the monomeric and dimeric forms of CXCL8 to mediate receptor desensitization, CXCL8-induced G protein activation in membrane preparations was measured. Pretreatment of RBL-CXCR1 cells with the monomer caused significantly higher inhibition of GTPase activity than the dimer (~60% vs. ~22%) (Fig 8C). In contrast, both monomer and dimer were equally potent in inhibiting GTPase activity in RBL-CXCR2 (~65%). Similar to desensitizing Ca2+ release activity, the WT showed intermediate GTPase inhibition for CXCR1 and the same as monomer and dimer for CXCR2.
Phosphorylation and internalization of the CXCR2 mutant BD199VA
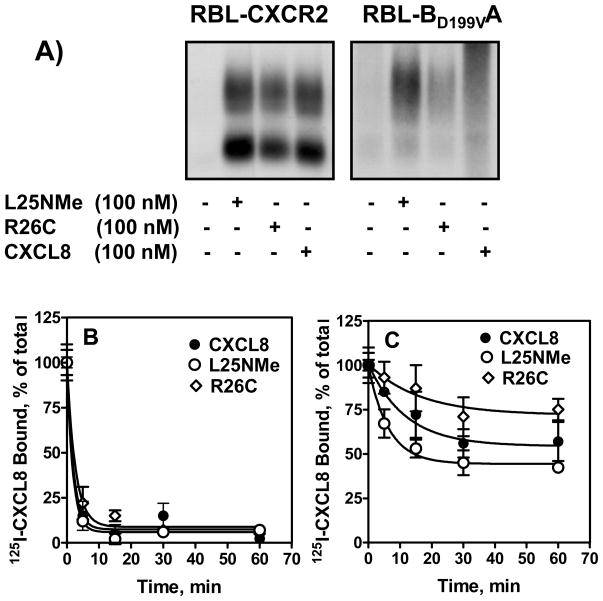
The CXCR2 mutant BD199VA, in which aspartate 199 of CXCR2 was substituted for its valine counterpart of CXCR1, was shown to internalize like CXCR1 upon exposure to WT CXCL8 (24). To further assess the role of the monomeric and dimeric forms of CXCL8 in receptor functions, we measured BD199VA phosphorylation. 32P-labeled RBL-CXCR2 and RBL-BD199VA cells were stimulated with monomer, dimer, and WT. As expected, all three variants induced phosphorylation of CXCR2 to similar extent (Fig 9A, left panel). For BD199VA, however, the monomer induced greater phosphorylation than the dimer, and WT (Fig 9A, right panel).
Figure 9. Phosphorylation and internalization of BD199VA.
32P-labeled RBL-2H3 cells (5 × 106 cells/60 mm plate) expressing CXCR2 (A, left panel) or BD199VA (A, right panel)were incubated for 5 min with or without stimulants as shown. Cells were lysed, immunoprecipitated with specific antibody against CXCR2 and then analyzed by SDS-PAGE and autoradiography. The results are from a representative experiment that was repeated twice. For internalization (panels B and C), cells were treated with 100 nM of ligands for different times, washed and assayed for 125I-WT CXCL8 (0.1nM) binding. The values are presented as a percentage of totals, which is defined as the total amount of 125I-WT CXCL8 bound to control (untreated) cells. The experiment was repeated three times with similar results.
To correlate receptor phosphorylation with internalization, we measured 125I-CXCL8 binding in cells pretreated with monomer, dimer or WT CXCL8. All three ligands induced rapid CXCR2 internalization (~95% after 5 min) (Fig. 9B). Monomer-induced BD199VA internalization, however, was faster than that of the dimer (~ 60% vs. 25% % after 15 min). The WT showed intermediate activity (Fig. 9C).
DISCUSSION
CXCL8 is a member of the ELR (Glu-Leu-Arg) subfamily of chemokines that forms non-covalent associating dimers under physiological conditions. Neutrophils express two CXCL8 receptors, CXCR1 and CXCR2, at similar levels (26, 27). Previous studies using trapped monomer and dimer have indicated that the monomer is the high affinity ligand and the dimer is the low affinity ligand for both receptors (9). CXCL8 binding elicits Ca2+ release and chemotaxis for both receptors but only CXCR1 mediates phospholipase D activation and respiratory burst (26, 28). These observations indicate that the two receptors could have unique roles for in vivo fine-tuning of CXCL8-mediated neutrophil recruitment and inflammatory response. To date, little is known concerning the role of the CXCL8 monomer and dimer in regulating neutrophil responses to CXCR1 and CXCR2. The data herein, using a “trapped” monomer and a trapped dimer, demonstrate that while the monomer/dimer equilibrium modulates CXCR1 and CXCR2 activation in a similar fashion (i.e. monomer>WT>dimer), it differs in its ability to mediate receptor desensitization and down-regulation. This contention is supported by the following observation. First, the monomer, compared to the dimer, is more efficacious for CXCR1 and CXCR2 receptors in mediating PI hydrolysis, secretion of β-hexosaminidase and intracellular Ca2+ mobilization, (Figs. 1–3). Second, the dimer is as active as the monomer in inducing CXCR2, but not CXCR1, phosphorylation, desensitization and internalization (Figs. 4–6 and 8). Similar CXCR2-related activities of the monomer and dimer, despite reduced binding affinities, indicate that the binding affinities are not correlated to function. Binding affinity is an equilibrium measurement (Kd = koff/kon), and it is very possible that function is determined not by Kd but by the lifetime (1/koff) of the ligand-bound receptor complex. We propose that the monomer and dimer have similar koff values, and therefore similar activities.
CXCR1 is a high affinity receptor only for CXCL8, whereas CXCR2 binds seven different chemokines (CXCL1-3, 5–8) with similar high affinity to mediate neutrophil responses. It is well established that CXCR2 is a promiscuous receptor and is less sensitive to mutations, and therefore it is not surprising that binding of both monomer and dimer induces the conformational change necessary for the receptor to be fully recognized by G protein coupled receptor kinases (GRKs) and undergo phosphorylation, desensitization, and internalization. Supporting this contention is that the CXCR2 mutant BD199VA in which aspartic-199 was exchanged for the valine counterpart of CXCR1 and was shown to desensitize and internalize like CXCR1(24), displayed partial dimer-mediated phosphorylation and internalization similar to that of CXCR1 (Fig. 9).
In addition to termination of GPCR mediated signaling, βarrestins also act as scaffold proteins by forming complexes with other proteins, which upon receptor activation and/or internalization activate downstream effector systems such as MAP kinase, tyrosine kinase and growth factors (29–32). Upon CXCR2 activation, all CXCL8 variants induced rapid βarr-1 translocation to the cell membrane and receptor internalization. These data likely indicate a greater ability of the CXCR2 to activate post-endocytic signals in response to CXCL8. Indeed, CXCR2-mediated ERK1/2 activation was sustained for both monomer and dimer whereas responses to CXCR1 were transient (Fig 7). Interestingly, despite the inability of the dimer to induce βarr-1 translocation to CXCR1 (Fig. 6), it mediates ERK1/2 activation to the same extent as the monomer. RBL-2H3 cells express βarr-1 and βarr-2 (33). CXCR1 and CXCR2 couple to both isoforms to internalize and regulate cellular responses (10, 20, 29, 31, 34). One explanation could be that the lower phosphorylated form of CXCR1 in response to the dimer, couples to βarr-2 to activate ERK1/2. However, expression of βarr-2-GFP in RBL-CXCR1 showed no GFP translocation to the cell membrane in response to dimer activation (data not shown). Another explanation could be that CXCR1 couples to a βarrestin-independent mechanism to mediate ERK1/2 activation.
Previous studies from our laboratory and others have shown that receptor phosphorylation and internalization play an important role in CXCL8-mediated chemotactic and cytotoxic activities (5, 35–38). CXCR1 and CXCR2 mutants deficient in receptor phosphorylation and internalization displayed impaired chemotaxis but greater cellular responses (i.e. PI hydrolysis, intracellular Ca2+ mobilization and exocytosis) compared to WT CXCR2 (5). Thus, the ability of the CXCL8 variants to induce receptor phosphorylation and internalization should correlate with their ability to mediate chemotaxis. Indeed, both CXCL8 monomer and dimer induced RBL-CXCR2 chemotaxis to similar extent whereas the monomer was significantly more potent in inducing RBL-CXCR1 chemotaxis as compared to the dimer (Fig. 3E and F). Furthermore, HMECs pretreated with anti-CXCR1 or anti-CXCR2 displayed similar results (Fig 3H).
A large number of GPCRs, including CXCR1 and CXCR2, exist as both monomer and dimer in the cell membrane (39). Oligomerization of GPCR can be ligand-dependent or ligand-independent (25). The CXCL8 receptor dimerization is ligand-independent and occurs in the endoplasmic reticulum (25, 39). However, both forms of the receptor seem to be capable of interacting with ligand to induce cellular responses (39). A question that remains to be addressed is whether the CXCL8 monomer and dimer have a binding preference. For instance, does the monomeric ligand preferentially interact with the monomeric form and the dimer with the dimeric form of the receptors? Does the equilibrium monomer-dimer modulate receptor functions by temporally activating a specific form of the receptor vs. the others? Our attempt to stably co-express receptor mutants deficient in receptor dimerization along with WT CXCR2 in RBL-2H3 cells to address these questions was not successful. The receptor mutants were either expressed very poorly (Y49-CXCR2), failed to express (D143-CXCR2) or displayed decreased affinity for ligand (A315-CXCR2) as compared to WT receptors. In addition, we reasoned that the changes in the structural and pharmacological properties of the receptors will likely complicate the interpretation of the results in term of monomer-dimer equilibrium. In addition to CXCL8 homodimerization, recent studies have also shown that CXCL8 can form heterodimers with other chemokines such as platelet factor 4 or CXCL4 (40). Whether or not CXCL8 heterodimerization has any physiological consequences or plays a role in CXCR1 and CXCR2 activation and regulation remains unclear. CXCL8 function also involves binding to glycosaminoglycans (GAGs) on endothelial cells and extracellular matrix, and it is believed such binding promotes formation of a concentration gradient for neutrophil migration to site of inflammation (41, 42). CXCL8 dimers bind GAGs with higher affinity, and we have recently observed in a mouse model that the CXCL8 dimer is more active in recruiting neutrophils at relatively high concentrations (Das et al., manuscript in preparation). These observations further emphasize that the ability to reversibly exist as monomers and dimers play a critical role in regulating in vivo neutrophil function.
On the basis of our data and from previous studies, we now propose a model as to how differential interactions of CXCL8 monomers and dimers for CXCR1 and CXCR2 receptors mediate neutrophil function. Neutrophil recruitment involves continuous engagement with both receptors, for multiple functions from cell shape change, chemotaxis, to cytotoxic events such as release of proteases and superoxide. These events have to be spatially and temporally coordinated as initial receptor engagement should lead to recruitment and chemotaxis, and receptor engagement leading to cytotoxic event should not be triggered until late at the site of infection. Therefore, recruitment and activation of neutrophils should be highly coordinated for successful resolution of inflammation. It is critical that conditions that favor triggering cytotoxic effects are disfavored during early stages and favored at the final stages of recruitment. During early stages of recruitment, CXCR2 plays a more active role, due its intrinsic higher activity, and once CXCR2 is depleted due to exocytosis, CXCR1 could become engaged and/or remain relatively inactive during the early stages of trafficking. At the site of infection, it is very likely that only CXCR1 is available on the cell surface and its activation results in cytotoxic events such as release of proteases and superoxide. The lower activity of the dimer for these activities also indicate that dimerization negatively regulates these function to destroy bacteria and minimize any collateral tissue damage. In summary, we propose that the ability of CXCL8 to reversibly exist as monomers and dimers and their differential activities and regulation for the CXCR1 and CXCR2 receptors play an essential role in all aspects of inflammatory response from recruiting neutrophils to the site of infection to trigger cytotoxic activities such as release of protease and oxygen radicals to eliminate infection.
Acknowledgments
We thank Dr. Rakesh K. Singh for the generous gift of HMECs. We thank Angela Isley and Hatajai Lassiter for their technical support.
The abbreviations used are
IL-8 or CXCL8
interleukin-8
CXCR1
IL-8 receptor A
CXCR2
IL-8 receptor B
PMA
phorbol 12-myristate 13-acetate
IP
inositol phosphate
G protein
GTP-regulatory protein
FITC
fluorescein isothiocyanate, βarr, βarrestin
Footnotes
1
This work was supported by National Institutes of Health Grants (AI38910 and 056-CA92077 to R.M.R., and AI069152 to K.R), and the U.S. Army MedicalResearch and Materiel Command (07-1-0418 to R.M.R.).
References
- 1.Baggiolini M. Reflections on chemokines. Immunol Rev. 2000;177:5–7. doi: 10.1034/j.1600-065x.2000.17722.x. [DOI] [PubMed] [Google Scholar]
- 2.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- 3.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 4.Richardson RM, Pridgen BC, Haribabu B, Ali H, Snyderman R. Differential cross-regulation of the human chemokine receptors CXCR1 and CXCR2. Evidence for time-dependent signal generation. J Biol Chem. 1998;273:23830–23836. doi: 10.1074/jbc.273.37.23830. [DOI] [PubMed] [Google Scholar]
- 5.Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol. 2003;170:2904–2911. doi: 10.4049/jimmunol.170.6.2904. [DOI] [PubMed] [Google Scholar]
- 6.Burrows SD, Doyle ML, Murphy KP, Franklin SG, White JR, Brooks I, McNulty DE, Scott MO, Knutson JR, Porter D, et al. Determination of the monomer-dimer equilibrium of interleukin-8 reveals it is a monomer at physiological concentrations. Biochemistry. 1994;33:12741–12745. doi: 10.1021/bi00209a002. [DOI] [PubMed] [Google Scholar]
- 7.Rajarathnam K, Sykes BD, Kay CM, Dewald B, Geiser T, Baggiolini M, Clark-Lewis I. Neutrophil activation by monomeric interleukin-8. Science. 1994;264:90–92. doi: 10.1126/science.8140420. [DOI] [PubMed] [Google Scholar]
- 8.Rajarathnam K, Clark-Lewis I, Sykes BD. 1H NMR solution structure of an active monomeric interleukin-8. Biochemistry. 1995;34:12983–12990. doi: 10.1021/bi00040a008. [DOI] [PubMed] [Google Scholar]
- 9.Rajarathnam K, Prado GN, Fernando H, Clark-Lewis I, Navarro J. Probing receptor binding activity of interleukin-8 dimer using a disulfide trap. Biochemistry. 2006;45:7882–7888. doi: 10.1021/bi0605944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barlic J, Khandaker MH, Mahon E, Andrews J, DeVries ME, Mitchell GB, Rahimpour R, Tan CM, Ferguson SS, Kelvin DJ. beta-arrestins regulate interleukin-8-induced CXCR1 internalization. J Biol Chem. 1999;274:16287–16294. doi: 10.1074/jbc.274.23.16287. [DOI] [PubMed] [Google Scholar]
- 11.Feniger-Barish R, Ran M, Zaslaver A, Ben-Baruch A. Differential modes of regulation of cxc chemokine-induced internalization and recycling of human CXCR1 and CXCR2. Cytokine. 1999;11:996–1009. doi: 10.1006/cyto.1999.0510. [DOI] [PubMed] [Google Scholar]
- 12.Chuntharapai A, Kim KJ. Regulation of the expression of IL-8 receptor A/B by IL-8: possible functions of each receptor. J Immunol. 1995;155:2587–2594. [PubMed] [Google Scholar]
- 13.Prado GN, Suzuki H, Wilkinson N, Cousins B, Navarro J. Role of the C terminus of the interleukin 8 receptor in signal transduction and internalization. J Biol Chem. 1996;271:19186–19190. doi: 10.1074/jbc.271.32.19186. [DOI] [PubMed] [Google Scholar]
- 14.Richardson RM, DuBose RA, Ali H, Tomhave ED, Haribabu B, Snyderman R. Regulation of human interleukin-8 receptor A: identification of a phosphorylation site involved in modulating receptor functions. Biochemistry. 1995;34:14193–14201. doi: 10.1021/bi00043a025. [DOI] [PubMed] [Google Scholar]
- 15.Clark-Lewis I, Vo L, Owen P, Anderson J. Chemical synthesis, purification, and folding of C-X-C and C-C chemokines. Methods Enzymol. 1997;287:233–250. doi: 10.1016/s0076-6879(97)87018-8. [DOI] [PubMed] [Google Scholar]
- 16.Fernando H, Nagle GT, Rajarathnam K. Thermodynamic characterization of interleukin-8 monomer binding to CXCR1 receptor N-terminal domain. Febs J. 2007;274:241–251. doi: 10.1111/j.1742-4658.2006.05579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali H, Richardson RM, Tomhave ED, Didsbury JR, Snyderman R. Differences in phosphorylation of formylpeptide and C5a chemoattractant receptors correlate with differences in desensitization. J Biol Chem. 1993;268:24247–24254. [PubMed] [Google Scholar]
- 18.Richardson RM, Pridgen BC, Haribabu B, Snyderman R. Regulation of the human chemokine receptor CCR1. Cross-regulation by CXCR1 and CXCR2. J Biol Chem. 2000;275:9201–9208. doi: 10.1074/jbc.275.13.9201. [DOI] [PubMed] [Google Scholar]
- 19.Ali H, Tomhave ED, Richardson RM, Haribabu B, Snyderman R. Thrombin primes responsiveness of selective chemoattractant receptors at a site distal to G protein activation. J Biol Chem. 1996;271:3200–3206. doi: 10.1074/jbc.271.6.3200. [DOI] [PubMed] [Google Scholar]
- 20.Nasser MW, Marjoram RJ, Brown SL, Richardson RM. Cross-desensitization among CXCR1, CXCR2, and CCR5: role of protein kinase C-epsilon. J Immunol. 2005;174:6927–6933. doi: 10.4049/jimmunol.174.11.6927. [DOI] [PubMed] [Google Scholar]
- 21.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 22.Salcedo R, Resau JH, Halverson D, Hudson EA, Dambach M, Powell D, Wasserman K, Oppenheim JJ. Differential expression and responsiveness of chemokine receptors (CXCR1-3) by human microvascular endothelial cells and umbilical vein endothelial cells. Faseb J. 2000;14:2055–2064. doi: 10.1096/fj.99-0963com. [DOI] [PubMed] [Google Scholar]
- 23.Brown SL, V, Jala R, Raghuwanshi SK, Nasser MW, Haribabu B, Richardson RM. Activation and regulation of platelet-activating factor receptor: role of G(i) and G(q) in receptor-mediated chemotactic, cytotoxic, and cross-regulatory signals. J Immunol. 2006;177:3242–3249. doi: 10.4049/jimmunol.177.5.3242. [DOI] [PubMed] [Google Scholar]
- 24.Nasser MW, Raghuwanshi SK, Malloy KM, Gangavarapu P, Shim JY, Rajarathnam K, Richardson RM. CXCR1 and CXCR2 activation and regulation. Role of aspartate 199 of the second extracellular loop of CXCR2 in CXCL8-mediated rapid receptor internalization. J Biol Chem. 2007;282:6906–6915. doi: 10.1074/jbc.M610289200. [DOI] [PubMed] [Google Scholar]
- 25.Trettel F, Di Bartolomeo S, Lauro C, Catalano M, Ciotti MT, Limatola C. Ligand-independent CXCR2 dimerization. J Biol Chem. 2003;278:40980–40988. doi: 10.1074/jbc.M306815200. [DOI] [PubMed] [Google Scholar]
- 26.Jones SA, Wolf M, Qin S, Mackay CR, Baggiolini M. Different functions for the interleukin 8 receptors (IL-8R) of human neutrophil leukocytes: NADPH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proc Natl Acad Sci U S A. 1996;93:6682–6686. doi: 10.1073/pnas.93.13.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy PM. Neutrophil receptors for interleukin-8 and related CXC chemokines. Semin Hematol. 1997;34:311–318. [PubMed] [Google Scholar]
- 28.Lee J, Horuk R, Rice GC, Bennett GL, Camerato T, Wood WI. Characterization of two high affinity human interleukin-8 receptors. J Biol Chem. 1992;267:16283–16287. [PubMed] [Google Scholar]
- 29.Barlic J, Andrews JD, Kelvin AA, Bosinger SE, DeVries ME, Xu L, Dobransky T, Feldman RD, Ferguson SS, Kelvin DJ. Regulation of tyrosine kinase activation and granule release through beta-arrestin by CXCRI. Nat Immunol. 2000;1:227–233. doi: 10.1038/79767. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 31.Zhao M, Wimmer A, Trieu K, Discipio RG, Schraufstatter IU. Arrestin regulates MAPK activation and prevents NADPH oxidase-dependent death of cells expressing CXCR2. J Biol Chem. 2004;279:49259–49267. doi: 10.1074/jbc.M405118200. [DOI] [PubMed] [Google Scholar]
- 32.Hupfeld CJ, Olefsky JM. Regulation of receptor tyrosine kinase signaling by GRKs and beta-arrestins. Annu Rev Physiol. 2007;69:561–577. doi: 10.1146/annurev.physiol.69.022405.154626. [DOI] [PubMed] [Google Scholar]
- 33.Huttenrauch F, Nitzki A, Lin FT, Honing S, Oppermann M. Beta-arrestin binding to CC chemokine receptor 5 requires multiple C-terminal receptor phosphorylation sites and involves a conserved Asp-Arg-Tyr sequence motif. J Biol Chem. 2002;277:30769–30777. doi: 10.1074/jbc.M204033200. [DOI] [PubMed] [Google Scholar]
- 34.Revankar CM, Vines CM, Cimino DF, Prossnitz ER. Arrestins block G protein-coupled receptor-mediated apoptosis. J Biol Chem. 2004;279:24578–24584. doi: 10.1074/jbc.M402121200. [DOI] [PubMed] [Google Scholar]
- 35.Su Y, Raghuwanshi SK, Yu Y, Nanney LB, Richardson RM, Richmond A. Altered CXCR2 signaling in beta-arrestin-2-deficient mouse models. J Immunol. 2005;175:5396–5402. doi: 10.4049/jimmunol.175.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang W, Wang D, Richmond A. Role of clathrin-mediated endocytosis in CXCR2 sequestration, resensitization, and signal transduction. J Biol Chem. 1999;274:11328–11333. doi: 10.1074/jbc.274.16.11328. [DOI] [PubMed] [Google Scholar]
- 37.Fan GH, Yang W, Wang XJ, Qian Q, Richmond A. Identification of a motif in the carboxyl terminus of CXCR2 that is involved in adaptin 2 binding and receptor internalization. Biochemistry. 2001;40:791–800. doi: 10.1021/bi001661b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan GH, Yang W, Sai J, Richmond A. Phosphorylation-independent association of CXCR2 with the protein phosphatase 2A core enzyme. J Biol Chem. 2001;276:16960–16968. doi: 10.1074/jbc.M009292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson S, Wilkinson G, Milligan G. The CXCR1 and CXCR2 receptors form constitutive homo- and heterodimers selectively and with equal apparent affinities. J Biol Chem. 2005;280:28663–28674. doi: 10.1074/jbc.M413475200. [DOI] [PubMed] [Google Scholar]
- 40.Nesmelova IV, Sham Y, Dudek AZ, van Eijk LI, Wu G, Slungaard A, Mortari F, Griffioen AW, Mayo KH. Platelet factor 4 and interleukin-8 CXC chemokine heterodimer formation modulates function at the quaternary structural level. J Biol Chem. 2005;280:4948–4958. doi: 10.1074/jbc.M405364200. [DOI] [PubMed] [Google Scholar]
- 41.Kuschert GS, Coulin F, Power CA, Proudfoot AE, Hubbard RE, Hoogewerf AJ, Wells TN. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38:12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- 42.Clark-Lewis I, Dewald B, Loetscher M, Moser B, Baggiolini M. Structural requirements for interleukin-8 function identified by design of analogs and CXC chemokine hybrids. J Biol Chem. 1994;269:16075–16081. [PubMed] [Google Scholar]