Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity (original) (raw)
. Author manuscript; available in PMC: 2010 Oct 29.
Published in final edited form as: Nature. 2010 Mar 3;464(7293):1367–1370. doi: 10.1038/nature08900
Abstract
Innate immunity provides the first line of defence against invading pathogens and provides important cues for the development of adaptive immunity. Type-2 immunity – responsible for protective immune responses to helminth parasites1,2 and the underlying cause of the pathogenesis of allergic asthma3,4 – consists of responses dominated by the cardinal type-2 cytokines interleukin (IL)-4, IL-5 and IL-13 (ref. 5). T cells are an important source of these cytokines in adaptive immune responses, but the innate cell sources remain to be comprehensively elucidated. Here, through the use of novel Il13eGFP reporter mice, we present the identification and functional characterisation of a new innate type-2 immune effector leukocyte that we have named the nuocyte. Nuocytes expand in vivo in response to the type 2-inducing cytokines IL-25 and IL-33, and represent the predominant early source of IL-13 during helminth infection with Nippostrongylus brasiliensis. In the combined absence of IL-25 and IL-33 signalling, nuocytes fail to expand, resulting in a severe defect in worm expulsion that is rescued by the adoptive transfer of in vitro cultured wildtype, but not IL-13-deficient, nuocytes. Thus, nuocytes represent a critically important innate effector cell in type-2 immunity.
Type-2 immunity evolved to respond to parasitic helminth infections, with type-2 cytokines orchestrating eosinophilia, goblet cell hyperplasia, mucus secretion, and IgE production5-7. These highly complex host responses involve the co-ordination of innate and adaptive immune cell types. Of the defined innate immune cells, basophils, eosinophils and mast cells are known sources of type-2 cytokines, but it is not clear that they are essential for N. brasiliensis expulsion5,8-12.
To identify new cell types that may mediate type-2 immunity we investigated the cellular sources of IL-13, a critical cytokine in the host response to helminth infection7,13 and allergy6,14. To allow live imaging of enhanced green fluorescent protein (eGFP) as a surrogate for IL-13 gene expression during the induction of type-2 responses we generated Il13eGFP mice (Supplementary Fig. 1). Analysis of these mice revealed very few constitutive eGFP+ cells in naïve mice (Supplementary Fig. 1). Administration of IL-25 or IL-33 to Il13eGFP mice resulted in the detection of ~3% eGFP+ cells in the mesenteric lymph nodes (mLN) (Fig. 1a), at least 80% of which could not be assigned to known cell lineages (including T cells, B cells, natural killer (NK) T cells, natural killer (NK) cells, dendritic cells, neutrophils, eosinophils, mast cells, basophils or macrophages) using a spectrum of cell surface markers (Fig. 1a and b, and Supplementary Fig. 2a). Immunofluorescence revealed highly increased numbers of eGFP+ cells in the intestines (Fig. 1c) and spleens (Supplementary Fig. 2b) of both IL-25 and IL-33-treated Il13eGFP mice, and these were confirmed to be non-T cells. The lineage−eGFP+ cells were T1/ST2+ (IL-33R) and IL-17BR+ (IL-25R) (Fig. 1b), suggesting that they respond directly to IL-33 and IL-25. Analysis of _il17br_−/− mice (Supplementary Fig. 3) and _Il1rl1_−/− mice demonstrated that exogenous IL-25 and IL-33 act redundantly to induce these cells in vivo (Fig. 1d). These lineage−eGFP+T1/ST2+IL-17BR+ cells represent a novel IL-13-producing leukocyte population that we have named nuocytes due to their high level expression of IL-13, and with nu being the 13th letter of the Greek alphabet. As discussed below nuocytes can additionally be defined as lineage− cells expressing ICOS, T1/ST2, IL-17BR and IL-7Rα.
Figure 1. IL-25 and IL-33 induce IL-13-producing nuocytes.
a, Detection of Il13eGFP+ NBNT cells in mLN of IL-25 or IL-33-treated mice. b, Cell surface marker expression of Il13eGFP+ NBNT cells in mLN following IL-25 administration. c, Immunofluorescence detection of Il13eGFP+ cells in small intestine of IL-25 and IL-33 treated mice. a – c, Data representative of 5 experiments with >3 mice per group. d, Nuocyte number. Data representative of two independent experiments with >4 mice per group. e, Cluster analysis for freshly isolated nuocytes (ex vivo) or day 9 in vitro expanded nuocytes (single data sets are shown for clarity).
Though present in the spleen, mesenteric lymph node and bone marrow of naïve mice, nuocytes represent less than 0.2% of cells in each tissue, but increase significantly in these tissues (Supplementary Fig. 4), with the exception of bone marrow (data not shown), following intra-peritoneal administration of IL-25. In contrast, basophil numbers did not increase in the blood or spleen, and their IL-4 production was unaffected, by IL-25 treatment (data not shown). Confirming that nuocytes were not T or B cells, mast cells, NKT cells or lymphoid tissue inducer (LTi) cells, we detected IL-25-driven nuocyte induction in _Rag2_−/− mice and nude mice, _Kit_W-sh/W-sh mice, _Ja18_−/− mice and _Rorg_−/− mice, respectively (Supplementary Fig. 5a-d and data not shown). Furthermore, microarray analysis of highly purified nuocytes failed to show any significant gene expression similarity to known leukocyte lineages (Fig. 1e). However, it did reveal a number of cell surface markers (Supplementary Table 1), including the receptor for IL-7 and the co-stimulatory molecule ICOS and MHC class II, that were subsequently confirmed by flow cytometry as being expressed on nuocytes (Supplementary Fig. 2c).
Importantly, nuocytes represent the predominant cell type expressing Il13eGFP at day 5 post-infection (p.i.) with the helminth parasite N. brasiliensis (Fig. 2a and Supplementary Fig. 2d). To investigate the potential roles of IL-25 and IL-33 in regulating nuocytes during helminth infection we infected _Il17br_−/−, _Il1rl1_−/− and combined _Il17br_−/−_Il1rl1_−/− mice with N. brasiliensis. _Il17br_−/− mice expelled their worms more slowly than wildtypes, but by day 14 even _Il17br_−/− mice had relatively few worms and a complete absence of worms by day 20 p.i. (Fig. 2b). By contrast, _Il1rl1_−/− mice efficiently expelled their worm burden with very few worms present at day 11 and complete absence by day 14 (Fig. 2b). Strikingly, the absence of both IL-25 and IL-33 signalling severely impaired worm expulsion from the _Il17br_−/−_Il1rl1_−/− mice, with significant worm burden persisting to day 20 post-infection (Fig. 2b). Using Il17br_−/−_Il13eGFP mice and Il1rl1_−/−_Il13eGFP mice we found that the loss of either IL-17BR or T1/ST2 resulted in a substantial fall in the numbers of eGFP+ cells early in the response (Fig. 2c). Notably, the expansion of nuocytes in the various mouse strains correlated faithfully with the onset of worm expulsion. Thus, nuocytes arose more rapidly in _Il1rl1_−/− mice (though more slowly than in wildtype controls) than in _Il17br_−/− mice. Nuocytes failed to expand in the combined _Il17br_−/−_Il1rl1_−/− mice in either the MLN (Fig. 2d) or peritoneal lavage (Supplementary Fig. 6a), even at day 20 p.i. Although eosinophils and IgE levels were also reduced in the _Il17br_−/− mice, _Il1rl1_−/− mice and _Il17br_−/−_Il1rl1_−/− mice (Supplementary Fig. 6b and c), these have been shown by others not to be essential for worm expulsion5,15. In addition, we found no defect in basophil expansion in the _Il17br_−/− mice (data not shown and Supplementary Fig. 7b).
Figure 2. IL-25 and IL-33 play partially redundant roles for nuocyte induction and worm expulsion.
a, Quantification of Il13eGFP+ cells five days p.i. with N. brasiliensis. Data are representative of two independent experiments with >5 mice per group. b, Intestinal worm burden of _N. brasiliensis_-infected mice. c, Quantification of Il13eGFP+ cells in _N. brasiliensis_-infected mice at day 5 p.i. Data are representative of two independent experiments with >5 mice per group. d, Quantification of nuocytes in _N. brasiliensis_-infected mice. * = p < 0.05, ** = _p_ < 0.01. Data are representative of two independent experiments with >5 mice per group.
To address the functional importance of nuocytes in the immune response to helminth infection, we purified nuocytes to homogeneity (Fig. 3a) and determined conditions for their expansion in vitro. Nuocytes did not grow or differentiate in culture with a cytokine cocktail (CC) used previously to differentiate a basophil/mast cell progenitor in vitro16 (Fig. 3b), or under conditions that readily generate mast cells from total bone marrow17 (data not shown). By contrast, inclusion of IL-33 and IL-7 into the cultures induced substantial expansion of nuocytes (Fig. 3b). Addition of IL-25 to IL-33 + IL-7 culture conditions did not change the growth rate of nuocytes (Fig. 3b).
Figure 3. Adoptive transfer of cultured nuocytes into _il17br_−/- mice restores an IL-25-responsive phenotype.
a, Morphology of Giemsa-stained nuocytes. b, Quantification of nuocyte growth in vitro. CC, cytokine cocktail. c, Flow cytometric analysis of interferon (IFN)-γ, IL-4, IL-5 and IL-13 intracellular staining of nuocytes after 7 days culture with IL-7 and IL-33. a – c, Data are representative of three independent experiments. d, Quantification of eosinophil infiltration of the peritoneal cavity following nuocyte (nuo) transfer. e, Transverse histological jejunum sections stained with Periodic acid-Schiff (PAS) for goblet cells. Data are representative of two independent experiments with >5 mice per group.
The cultured nuocytes maintained the expression of the majority of cell surface markers, including high levels of CD45 (data not shown) and ICOS, T1/ST2, IL-17BR and IL-7Rα (Supplementary Fig. 8), and did not differentiate into any of the currently known leukocyte lineages even after 15 days in culture (Fig. 1e and data not shown). All nuocytes expressed IL-13, with more than 70% also secreting IL-5, though less than 5% produced IL-4 (Fig. 3c). Analysis of nuocyte culture supernatants revealed the additional substantial secretion of IL-6, IL-10, and GM-CSF (Supplementary Fig. 9).
Strikingly, adoptive transfer of nuocytes into _Il17br_−/− mice re-established many of the features of IL-25-evoked type-2 immune responses (Fig. 3d and e) that are normally absent in these mice (Supplementary Fig. 3e-g). Crucially, the adoptive transfer of nuocytes did not induce any spontaneous inflammation in _Il17br_−/− recipients but restored their ability to respond to subsequent IL-25 administration (Fig. 3d and e). Cellular infiltrate of the peritoneal lavage, characterised by eosinophilia (Fig. 3d), and intestinal goblet cell hypertrophy and hyperplasia, were restored in _Il17br_−/− mice that received nuocytes, as compared to controls (Fig. 3e).
To investigate whether nuocytes play a critical role in co-ordinating immunity to helminth infection, we sought to restore protective immunity to _Il17br_−/− mice in response to N. brasiliensis through the adoptive transfer of wildtype (IL-25 responsive) nuocytes. Four days p.i., all infected animals had equivalent intestinal worm burdens (Fig. 4a), demonstrating that the transfer of nuocytes did not prevent establishment of infection. Strikingly, the majority of the infected _Il17br_−/− animals that received nuocytes had completely expelled their worms by day 11 post-infection, similar to wildtype controls, (Fig. 4a). This contrasted with the _Il17br_−/− mice that had not received nuocytes, and had burdens of greater than 50 worms at the same time point (Fig. 4a). The transfer of nuocytes also restored the numbers of nuocytes in the tissues at day 11 p.i. (Fig. 4b), and restored the early eosinophil response at day 6 p.i., but did not appreciably alter the levels of basophils at this time point (Supplementary Fig. 7a and b). Furthermore, adoptive transfer of wildtype nuocytes into the combined _Il17br_−/−_Il1rl1_−/− mice also resulted in the restoration of N. brasiliensis expulsion (Fig. 4c).
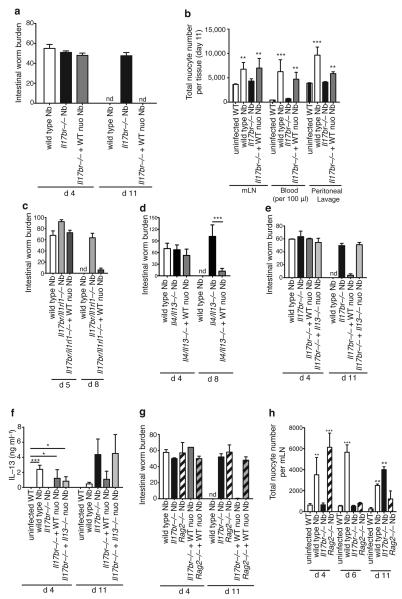
Figure 4. Adoptive transfer of wildtype nuocytes, but not IL-13-deficient nuocytes, restores rapid worm expulsion in N.brasiliensis infected _il17br_−/− mice.
a, Intestinal worm burdens. b, Quantification of nuocyte numbers in tissues. Data are representative of three independent experiments with >6 mice per group. c - d, Intestinal worm burdens. Data are from single experiments with 6 - 7 mice per group. e, Intestinal worm burdens. f, N. brasiliensis antigen-specific IL-13 production. g, Intestinal worm burden. h, Quantification of nuocyte numbers. e – h, Data are representative of two independent experiments with >6 mice per group. * = p < 0.05, ** = p < 0.01, *** = p < 0.005.
We have shown previously that IL-13, amongst the type-2 cytokines, is essential for the rapid eradication of N. brasiliensis13,18. We now demonstrate by transferring wildtype nuocytes into _Il4−/−Il13_−/− mice that, even when nuocytes are the only IL-13-secreting cells, they are capable of inducing worm expulsion (Fig. 4d). To further test the importance of nuocyte-derived IL-13 on the kinetics of N. brasiliensis expulsion, nuocytes were prepared from _Il13_−/− animals and transferred into _N. brasiliensis_-infected _Il17br_−/− animals. In contrast to the wildtype nuocytes, IL-13-deficient nuocytes failed to mediate worm expulsion, with mice continuing to harbour high numbers of intestinal worms at day 11 p.i. (Fig. 4e).
In vitro antigen re-stimulation of mLN cells from _N. brasiliensis_-infected _Il17br_−/− mice revealed delayed T cell-derived IL-13 secretion that was absent at day 4 p.i. However, the continuing presence of intestinal worms and the resulting antigen burden led to a robust cytokine response by day 11 p.i. (Fig. 4f), as reported previously19. We also observed fewer IL-13eGFP+ T cells in both _Il1rl1_−/− mice and _Il17br_−/− mice at five days p.i (data not shown). However, following adoptive transfer of wildtype or _Il13_−/− nuocytes into _Il17br_−/− mice, antigen-specific T cell production of IL-13 was restored (Fig. 4f). As expected, since _Il13_−/− nuocytes failed to induce worm expulsion, the duration of antigen-specific T cell responses was prolonged (Fig. 4f). Thus, nuocytes are capable of enhancing T cell cytokine production, and nuocytes augment T cell responses independently of IL-13.
Expulsion of N. brasiliensis is a T cell-dependent process, but neither T cell-derived IL-4 or IL-13 are necessary for worm expulsion15, indicating that an alternative cell source is responsible for the production of the IL-13 necessary for worm expulsion. It was not surprising then that nuocytes were unable to induce worm expulsion in _Rag2_−/− mice (Fig 4g). However, analysis of nuocyte numbers in the mLN of _N. brasiliensis_-infected _Rag2_−/− mice revealed that, despite rapid early nuocyte expansion by day 4 p.i. (Fig. 4h), nuocyte numbers were not maintained in the absence of T cells, falling to uninfected levels as assessed on days 6 and 11 p.i. (Fig. 4h), despite the continued presence of intestinal worms (Fig. 4g). This suggests that T cells (or possibly B cells, though B cells have been shown to be dispensable for worm expulsion15) mediate prolonged nuocyte expansion, migration or survival through an as yet unknown mechanism that requires further investigation. Our data demonstrate that a dialogue exists between T cells and nuocytes, and that this is necessary for robust N. brasiliensis expulsion.
Thus, nuocytes clearly provide a critical effector mechanism, through their provision of the IL-13, required to induce helminth expulsion. The ability of nuocytes to expand rapidly in response to two potent initiators of type-2 immunity (IL-25 and IL-33), and in response to helminth infection, their presence at multiple immuno-surveillance sites around the body, their capacity to secrete high levels of IL-13 and IL-5, and their ability to enhance T cell responses, shows them to be highly specialised type-2 regulatory cells. The characterisation of nuocytes will now allow assessment of their roles in other immune responses and disease pathologies including allergic asthma.
METHODS SUMMARY
Adoptive Transfers
Purified nuocytes were cultured for 2 - 5 days in RPMI supplemented with 10 ng/ml IL-33 and 10 ng/ml IL-7. Cells were washed and injected intravenously in PBS via the tail vein at 0.5 × 106 cells/mouse. Adoptive transfer was performed four hours after infection with N. brasiliensis, or 12 hours before the first cytokine administration of IL-25 i.p.
ONLINE METHODS
Animals
BALB/c mice were purchased from Charles River Laboratories (Margate, Kent, UK) as required. _Rag2_−/− mice20, on a BALB/c background, were provided by Jean Langhorne (NIMR, London). _Il13_−/− mice13 and _Il4−/−Il13_−/− mice18 were on a BALB/c background. _Jα18_−/− mice21, on a C57BL/6 background. _Kit_Wsh/Wsh mice22, on a C57BL/6 background, were provided by Catherine Lawrence (University of Strathclyde). In individual experiments all mice were matched for age, gender and background strain. Mice were maintained in specific pathogen-free conditions. All animal experiments undertaken in this study were done so with the approval of the UK Home Office.
Il13eGFP mice
The Il13eGFP mice were generated by recombineering23,24 (Supplementary Information). Neomycin negative, Cre-recombinase negative mice were backcrossed onto the BALB/c and C57BL/6 backgrounds. Genotyping of Il13eGFP mice used PCR primers ASEQ3755 (Forward, 5′-tcaacaggctaaggccacaagcc-3′), ASEQ4030 (Forward, 5′-CATGGTCCTGCTGGAGTTCGTG-3′) and ASEQ3958 (Reverse, 5′-GCTTCGTCTGTCACTCACACAGG-3′), giving a wildtype product of 300 bp and a targeted product of 522 bp.
_Il17br_−/− mice
The replacement vector was designed to replace exons 2 and 3 of the il17br gene (a region encoding 56 amino acids of IL-17BR) with a neomycin resistance gene (Supplementary Information). Targeted BALB/c embryonic stem (ES) cell clones were used to generate the line on a BALB/c background. Genotyping was performed by PCR using primers (5′-TTGCTGATCTTGGCTGCATCGTGC-3′), (5′-AGCAGGGCTTGCATCTGAATGCCT-3′) and (5′-CTATCAGGACATAGCGTTGGCTACC-3′) that give a product of 600 bp for the wild-type allele and 400 bp for the targeted allele.
Generation of monoclonal anti-IL-17BR antibody (Clone D9.2)
_Il17br_−/− mice were immunised intraperitioneally with murine IL-17Br/Fc fusion protein (R&D Systems) and monoclonal anti-IL-17BR antibodies generated by standard protocols (Supplementary Information).
IL-25 and IL-33 administration
0.4 _μ_g per dose of recombinant mouse IL-25 or recombinant mouse IL-33 (R&D Systems) in PBS was administered daily for 3 days intraperitoneally. Mice were sacrificed 24 hours later and tissues harvested for analysis. Control animals received PBS only.
Fluorescence-activated cell analysis
Mouse tissue cell suspensions at 2 × 108 cells/ml were incubated with purified anti-Fc receptor blocking antibody (anti-CD16/CD32) before addition of the specific antibodies. Cell surface markers were stained using a combination of FITC-, PE-, PE-Cy7-, APC-conjugated and biotin-conjugated monoclonal antibodies (see Supplementary Information). In each experiment the appropriate isotype control monoclonal antibodies and single conjugate controls were also included. Samples were analysed using a Becton Dickinson FACScalibur flow cytometer running CellQuest acquisition and analysed using FlowJo software (version 8.8.3, Tree Star Inc. OR, USA).
Fluorescence-activated cell sorting of nuocytes
Spleen cells prepared from IL-25-treated mice were depleted of lineage+ cells prior to cell sorting by incubation with biotin-conjugated anti-CD3, anti-CD19, anti-CD11b and anti-FcεRI antibodies before removal of antibody-bound cells by magnetic separation using Dynabeads (Invitrogen). Lineage-depleted cells were stained with PE-conjugated antibodies against CD4, CD8, B220, TER-119 and CD11b, a FITC-conjugated antibody against CD45 and an APC-conjugated antibody against ICOS. PE−, FITC+, APC+ cells were collected using a Mo-flo cell sorter, and purity checked by staining with lineage antibodies and antibodies against IL-17BR and T1/ST2.
Nuocyte cytokine/chemokine profile analysis
Supernatants collected from day 7 cultured nuocytes (~2 × 106 cells/ml) were analyzed by bioplex assay (Milliplex MAP mouse cytokine/chemokine 22-plex, Millipore). Supernatants were assayed according to manufacturer's instructions. Data were collected with the Bio-Plex 200 system, analyzed in Excel and graphed with GraphPad Prism 4.0 software. Statistical significance was determined by one-way ANOVA with Tukey Post Test. p < 0.05 was considered significant.
Helminth infection and antigen restimulation
Mice were inoculated subcutaneously with 300 viable third-stage N. brasiliensis larvae. mLN cells were stimulated in vitro at 2 × 106 cells/ml with 50 _μ_g/ml of parasite antigens (N. brasiliensis excretory/secretory antigen) for 72 hours. Supernatants were collected and analyzed for IL-13 by Quantikine ELISA (R&D Systems).
Immunofluorescence on cryosections and confocal microscopy
Cryosections were prepared (Supplementary Information) and incubated with conjugated antibodies: anti-mouse CD11b-Pacific Blue (clone M1/70, eBioscience), anti-mouse B220-Pacific Blue (clone RA3-6B2, eBioscience), anti-mouse CD4-biotin (clone RM4-5, Biolegend), anti-mouse CD3-Pacific Blue (clone eBio500A2, eBioscience), anti-mouse SIGN-R1-AlexaFluor 647 (clone eBio22D1, eBioscience), anti-GFP (rabbit IgG, Invitrogen). Sections were then rinsed and incubated with streptavidin conjugated with AlexaFluor 546 (Invitrogen) and/or anti-rabbit-AlexaFluor 488 (goat IgG, Invitrogen). 7-amino-actinomycin (7-AAD, eBioscience) was included in the last incubation to stain nuclei. Finally, samples were mounted with Vectashield (Vector Labs). Images were taken on a Carl-Zeiss inverted microscope (LSM 710) and processed with ZEN 2008 (Carl-Zeiss).
Microarray data analysis
Mouse Genome 430 2.0 GeneChips were used to analyse the gene expression of freshly isolated or 9 days in vitro expanded nuocytes from wildtype mice. Raw expression data were initially imported into R and Bioconductor software for quality assessment using the affyQCReport package. Nuocyte data were combined with publically available immune cells datasets with the same GeneChips. These include mouse B cells (n=3), CD11b+ dendritic cells (DCs) (n=2), CD8+ DCs (n=2), pDC (n=2), CD8+ cells (n=2), NK cells (n=2) (all from Gene Expression Omnibus (GEO), (http://www.ncbi.nlm.nih.gov/projects/geo), under accession ID GSE9810), CD4+ cells (n=2, GEO accession GSM44979 and GSM44982), mast cells (n=2, GEO accessions GSM258711 and 258712) and granulocytes (n=2, GEO accessions GSM149595 and GSM149596). Datasets for macrophages (n=3) were obtained from the National Cancer Institute caArray (http://caarray.nci.hih.gov/). All data were imported into R and Bioconductor software using affy package. Background correction, normalization, PM correction and summarisation of the data were performed with the methods: rma, quantiles, pmonly and medianpolish respectively, using the affy function expresso. The statistical properties of all the arrays after the pre-processing step were examined and confirmed to be very similar. Cluster analysis was then performed using the clustering algorithm divisive analysis clustering (Diana) which is a function in the cluster package from bioconductor.
Statistical Analysis
Graph Pad Prism was used to calculate the Standard Deviation (SD) between experimental samples when each experimental group contained an equal number of data sets. In the case where different numbers of data sets existed in each experimental group the Standard Error of the Mean (SEM) was used. When data were normally distributed and when two independent variables were being analysed, 2-way ANOVA with Bonferroni post-analysis was performed. In all other instances statistical differences between groups were calculated using Student's t test where a p value of < 0.05 was considered significant.
Supplementary Material
1
2
3
Acknowledgements
Thanks go to members of the McKenzie lab for their comments on the manuscript. We thank David Cousins (Kings College, London) for assistance with preliminary microarray analysis. R. Flynn was supported by Asthma UK. P. Fallon is supported by Science Foundation Ireland.
Footnotes
The authors declare competing financial interests.
References
- 1.Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J Exp Med. 2009;206:2059–2066. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perrigoue JG, Marshall FA, Artis D. On the hunt for helminths: innate immune cells in the recognition and response to helminth parasites. Cell Microbiol. 2008;10:1757–1764. doi: 10.1111/j.1462-5822.2008.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–230. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 4.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111:450–63. doi: 10.1067/mai.2003.169. quiz 464. [DOI] [PubMed] [Google Scholar]
- 5.Fallon PG, et al. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity. 2002;17:7–17. doi: 10.1016/s1074-7613(02)00332-1. [DOI] [PubMed] [Google Scholar]
- 6.Grunig G, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urban JFJ, et al. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 8.Min B, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009;113:2816–2825. doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 10.Perrigoue JG, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voehringer D. The role of basophils in helminth infection. Trends Parasitol. 2009 doi: 10.1016/j.pt.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimoto T, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 13.McKenzie GJ, Bancroft A, Grencis RK, McKenzie AN. A distinct role for interleukin-13 in Th2-cell-mediated immune responses. Curr Biol. 1998;8:339–342. doi: 10.1016/s0960-9822(98)70134-4. [DOI] [PubMed] [Google Scholar]
- 14.Walter DM, et al. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167:4668–4675. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]
- 15.Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity is controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells of the innate immune system. J Exp Med. 2006;203:1435–1446. doi: 10.1084/jem.20052448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arinobu Y, et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci U S A. 2005;102:18105–18110. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haig DM, et al. Effects of stem cell factor (kit-ligand) and interleukin-3 on the growth and serine proteinase expression of rat bone-marrow-derived or serosal mast cells. Blood. 1994;83:72–83. [PubMed] [Google Scholar]
- 18.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med. 1999;189:1565–1572. doi: 10.1084/jem.189.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fallon PG, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 21.Cui J, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 22.Tono T, et al. c-kit Gene was not transcribed in cultured mast cells of mast cell-deficient Wsh/Wsh mice that have a normal number of erythrocytes and a normal c-kit coding region. Blood. 1992;80:1448–1453. [PubMed] [Google Scholar]
- 23.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3