A Phase I Trial of a Human Papillomavirus (HPV) DNA Vaccine for HPV16+ Cervical Intraepithelial Neoplasia 2/3 (original) (raw)
. Author manuscript; available in PMC: 2010 May 7.
Abstract
Purpose:
To evaluate the safety and immunogenicity of a therapeutic HPV16 DNA vaccine administered to women with HPV16+CIN2/3.
Experimental Design:
This phase I trial incorporated the standard ‘3+3” dose escalation design with an additional 6 patients allocated to the maximally tolerated dose (MTD). Healthy adult women with colposcopically-directed biopsy-proven HPV16+ CIN2/3 received three intramuscular (IM) vaccinations (0.5 mg, 1 mg, or 3mg) of a plasmid expressing a Sig-E7(detox)-HSP70 fusion protein on days 0, 28 and 56, and underwent standard therapeutic resection of the cervical squamocolumnar junction at day 105 (week 15). Safety and immunogenicity of the vaccine and histologic outcome based on resection at week 15 were assessed.
Results:
Fifteen patients were evaluable (3 each at 0.5 mg and 1mg, 9 at 3mg). The vaccine was well tolerated: most adverse events were mild transient injection-site discomfort; no dose-limiting toxicities were observed. Although HPVE7-specific T-cell responses to E7 detected by enzyme-linked immunospot assays (IFNγ) were of low frequency and magnitude, detectable increases in response subsequent to vaccination were identified in subjects in the second and third cohorts. Complete histologic regression occurred in 3/9 (33%, CI: 7%-70%)) individuals in the highest dose cohort, Although the difference is not significant, it is slightly higher than would be expected in an unvaccinated cohort (25%).
Conclusions:
This HPV16 DNA vaccine was safe and well tolerated. While it appears possible to elicit HPV-specific T cell responses in patients with established dysplastic lesions, other factors are likely to play a role in lesion regression.
INTRODUCTION
Cervical cancer remains the second leading cause of cancer death in women worldwide despite the fact that, for over five decades, it has been possible to screen for and treat early stage disease. Even though a highly efficacious prophylactic vaccine against the causative agent, human papillomavirus (HPV) has been approved by the FDA, lack of access to health care in resource-poor settings is likely to limit the public health impact of the vaccine, as it has that of screening and treatment of early stage disease in the same environments. In the absence of a broadly-based preventative program, there will continue to be a need for effective therapeutic interventions for early and late stage cervical cancer.
HPV-associated neoplasia of the cervix presents a compelling opportunity to test immunotherapies after disease has been detected, because expression of two non-self, viral antigens, E6 and E7, is functionally required to initiate and maintain neoplastic lesions. If left undetected and/or untreated, a subset of CIN2/3 lesions will progress over a timeframe of years to invasive squamous cell carcinomas (SCCs). Both high grade dysplasia and SCC are associated with integration of the HPV genome into the host genome, with subsequent constitutive and functionally obligator expression of E6 and E7. However, we and others have found that between 20-25% of HPV16-associated CIN2/3 lesions undergo complete spontaneous regression within 15 weeks of diagnostic biopsy.(1) Since conventional histopathologic assessment of tissue at time of diagnosis does not predict either spontaneous regression or lesion persistence, all CIN2/3 lesions are treated by either surgical resection or ablation. However, because a fraction of established high grade dysplasias regress, and because lesions are accessible in a relatively noninvasive fashion, this patient population is a potentially informative cohort in which to test proof of principle for immune therapies.
Globally, HPV16 is the genotype most commonly associated with disease. Analyses of peripheral blood from patients with preinvasive dysplastic lesions have detected weak T cell responses to E6 and E7, suggesting that these antigens are indeed presented to and recognized by the immune system.(2) However, the consistently marginal or undetectable antibody responses to HPV16 proteins also suggest that genital/mucosal HPV infection does not commonly have a substantial viremic phase.
To determine if providing E7 in a potentially more immunogenic context might be beneficial for generating responses that could contribute to elimination of lesions, we developed a DNA vaccine targeting the HPV16 E7 oncoprotein, pNGVL4a-Sig/E7(detox)-HSP70, which was designed to elicit a CD8 T cell response to a mutated form of E7. This construct encodes a mutated non-functional E7 incapable of binding pRb, denoted E7(detox), thereby abrogating the transforming activity of the protein. DNA vaccines have generally exhibited low immunogenicity in humans, and we therefore linked HSP70, a chaperonin, to the E7(detox) sequence based on the notion that linkage of antigen to a heatshock protetin might enhance uptake by antigen presenting cells (APC) and MHC class I processing and presentation. We also attached a signal sequence to the hybrid antigen (E7 detox HSP70), which results in secretion of the linked E7 antigen, based on the reasoning that a secreted antigen would be more likely to gain access to professional APC than one that was intracellular. In preclinical experiments, homologous prime-boost vaccination with this construct in C57/BL6 mice elicited significantly more vigorous E7-specific CD8+ T cell responses than wildtype E7-containing DNA vaccines. These responses had therapeutic activity against established tumors expressing WTE7 (TC-1).(3) Here we report the clinical and immunologic characteristics of a phase I clinical trial testing safety, immunogenicity, and histologic outcomes in women with colposcopically-directed, biopsy-proven CIN2/3 associated with HPV16.
MATERIALS AND METHODS
2.1 Vaccine
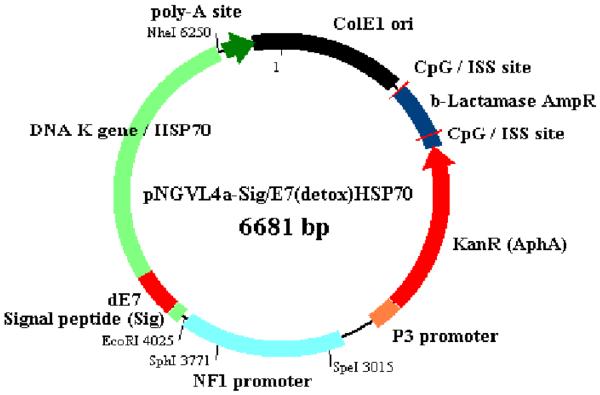
The study vaccine, pNGVL4a-Sig/E7(detox)/HSP70, was manufactured by the National Cancer Institute's Rapid Access to Interventional Development (RAID) program. It is composed of a closed circular DNA plasmid expressing HPV16E7 mutated at aa 24 and 26, linked to sequences coding for Sig and for HSP70. (Figure 1) Both bulk and formulated vaccine showed expression of E7(detox) in transfected HEK cells, confirming that clinical material had the capacity to support translation of the vaccine antigen.
Figure 1.
Schematic of pNGVL4a-Sig/E7(detox)/HSP70.
2.2 Study design
This Phase I dose-escalation study, J0323, was conducted as an open-label, single site study at the Johns Hopkins Medical Institutions. This trial incorporated the standard ‘3+3’ dose escalation design with an additional 6 patients allocated to the maximally tolerated dose (MTD). Human experimental guidelines of DHHS were followed in the conduct of clinical research, and the protocol was reviewed and approved by the Johns Hopkins Institutional Review Board. The primary objective was safety and tolerability of the study vaccine. Secondary endpoints included histology at week 15 (7 weeks post-vaccination), and systemic T-cell response measured by γ IFN enzyme-linked immunospot assay (Elispot).
Patients referred for colposcopic evaluation of high grade squamous intraepithelial lesions (HSIL) pap smears were approached for participation. Eligibility criteria included ability to give informed consent, immune competence, HIV-seronegativity, non-pregnant status, and presence of residual, visible lesion after a colposcopically directed biopsy-confirmed diagnosis of CIN2/3. Patients included in this analysis had lesions associated with HPV16, the genotype most commonly associated with cervical cancer and its precursor lesions. Patients underwent standard colposcopic evaluation and diagnosis at study entry (t=0), an interval colposcopic exam at week 8, standard cone or LEEP resection of the squamocolumnar junction at week 15, and had a postoperative exam at week 19. At each study visit, colpographs, cervical swabs, and peripheral blood were obtained.
The study protocol included 3 vaccinations with one of three doses of study vaccine, 0.5mg, 1.0mg, or 3.0 mg by intramuscular injection in alternating deltoid muscles. Immunizations were administered on day of enrollment (day 0), week 4 and 8. At the highest dose, 3mg, the vaccination was split equally between the left and right deltoids. Subjects were monitored throughout the study with physical assessments including serial colposcopic exams, and subject self-assessment for local and systemic symptoms by diary cards for 7 days after each vaccination. Adverse events were assessed for severity by criteria in National Cancer Institute CTCAE v.3.0. HIV antibody testing was assessed by standard institutional protocol. Complete HLA typing was carried out by the Hopkins Immunogenetics Core Laboratory. HPV testing was carried out real-time by the Hopkins Molecular Pathology Core Lab, using the HPV16-specific TaqMan real-time PCR method developed by Gravitt et al.(4) Histology at diagnosis and at therapeutic resection at week 15 was read first as part of routine care, and subsequently reviewed by the study pathologists.
2.3 Endpoint evaluations
2.3.1 Colposcopic evaluation
Using DenVu digital imaging software we obtained serial colposcopic images over the 15-week interval.
2.3.2 Cell preparation
Peripheral blood mononuclear cells (PBMCs) were obtained at study entry (screening/week 0), and at weeks 8, 15, and 19. Cells were prepared by density centrifugation with Lymphoprep™ (Axis-Shield PoC AS, Oslo, Norway), resuspended in freezing medium (90% heat-inactivated FBS+10% DMSO) and cryopreserved. Elispot assays were performed on thawed specimens. Average viability was 68.77%.
2.2.3 Enzyme-linked immunospot (ELISpot) assays
MultiScreen-IP plates (PVDF, Millipore) were coated overnight at 4°C with 1 □g per well of anti-human IFN-γ antibody 1-D1K (Mabtech). The plates were washed three times and blocked with culture medium (IMDM with 10% human AB serum; Invitrogen and Gemini Bio-Products, respectively) for 2 hrs at 37°C. Cryopreserved PBMCs were thawed, washed, and resuspended (5-10 × 106 cells) in 5ml culture medium in 50 ml tubes. Cells were incubated for 2 hrs at 37°C in a CO2 incubator, filtered through a nylon cell strainer to remove debris, and resuspended at a final concentration of 4 × 106 cells/ml in culture medium. PBMCs were plated in triplicates at 2 × 105 cells/well/0.1 ml with peptide antigens at 2 μg/ml or medium alone. The following antigens were used: HPV-16 E6- or E7-overlapping 15-mer peptides (synthesized at 60% purity, confirmed by high performance liquid chromatography,Sigma-Genesis, The Woodlands, Texas) combined in pools and CEF peptide pool (Mabtech). After 20-hr incubation at 37°C, cells were removed by 6 washes with PBS/0.05% Tween 20 followed by 2 washes with PBS alone. Captured IFN-γ was detected by the addition of 200 ng/well of biotinylated anti-human IFN-γ antibody 7-B6-1 (Mabtech) followed by 2-hr incubation at 37°C. After washing, as above, avidin-peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories) was added for 1 hr at room temperature. Plates were washed again 8 times as above, and peroxidase staining was performed with AEC (Sigma) substrate in acetate buffer for about 5 min. The reaction was stopped by rinsing the plates under running distilled water. After drying overnight, spot numbers were evaluated on KS ELISPOT Automated Reader System with KS ELISPOT software (Zeiss). Mean numbers of spot forming cells (SFC) from triplicate wells were calculated and expressed as spots per 1 × 106 PBMC. Means spot numbers from wells with PBMCs incubated with medium alone (background) were subtracted from means of PBMCs stimulated with peptides. Values ≥ 20 spots/1 × 106 PBMC after background subtraction, and ≥ 2 SD above background, were considered as positive.
A second protocol involving a single cycle of in vitro stimulation was used to carry out a separate set of analyses of peripheral blood responses. This protocol differed from the above in the following ways: On day 3, 1 ml of R10-AB medium containing 50 IU/ml recombinant human IL-2 (PeproTech, NJ) was added into each well. On day 5 and 7, half of the culture medium was replaced with fresh R10-AB containing 50 IU/ml recombinant human IL-2. On day 9, the cells were harvested, washed and resuspended with R10-AB medium and plated into 24-well plate overnight. ELISpot assays were performed on day 10, using a commercially available ELISpot kit (MABTECH, Inc). A response for this set of assays was defined as positive if the number of spots minus the background was twofold above background, at least 50 spots/105 PBMC. For individual subjects for whom the background was less than ten spots, background was defined as 25.
2.2.4 Measurement of antibody responses
Antibodies to HPV 16 E6 and E7 were measured by ELISA using the glutathione S-transferase (GST) capture method of Sehr et al(5) with minor modifications.
2.3 Statistical methods
The sample size was based upon the standard 3+3 dose finding design with three dosing levels and an additional 6 individuals at the MTD. Given the low toxicity levels observed in prior DNA vaccine studies, the expected sample size was 15 patients, 9 for dose escalation and 6 additional for the MTD. The analyses were primarily descriptive in nature. Summary statistics (means, standard deviations, proportions and confidence intervals) were used to descriptively characterize the cohorts numerically. Plots of the outcome variables including T cell response to the vaccine antigen, E7, over time, were used to describe the change in markers pre- and post-vaccination. Comparisons between groups were made using t-test or Wilcoxon rank-sum tests.
3. RESULTS
3.1 Study population
A total of seventy patients were screened and 16 participants enrolled for vaccination between December 10, 2003 and December 4, 2006. The mean age of study participants was 29.3 years (S.D. 8.97). (Table 1) Reasons for screening failure included no visible ectocervical high grade lesion, and not HPV16. The first patient enrolled was taken off study for noncompliance after she had undergone vaccination twice. All of the subsequently enrolled subjects received all three injections, and completed 41 weeks of clinical observation. No patient had progression of disease during the study window. A total of three subjects (3/9) (33%) had complete histologic regression of disease at week 15. All of the regressors were in the highest dose cohort (3mg). Colposcopic regression did correlate with histology at week 15.
Table 1.
Demographics of study cohort
| Age | |
|---|---|
| 18-20 | 3 (20%) |
| 21-30 | 7 (47%) |
| 31-40 | 3 (20%) |
| 41-50 | 2 (13%) |
| Race | |
| American Indian/Alaskan | 0 |
| Asian | 1 (7%) |
| Black or African American | 3 (20%) |
| White | 9 (60%) |
| Hispanic | 2 (13%) |
3.2 Vaccine safety and tolerability
No dose-limiting toxicities were observed. (Table 2) Transient local reactogenicity was reported in 5/15 (33 %), with the worst severity being mild. Systemic symptoms (malaise, myalgia, headache) after vaccination were also reported by 5 of 15 subjects. However, the worst severity in each case was mild. Two patients in the highest dose cohort reported transient, mild systemic symptoms after one or more vaccinations, suggestive of a dose effect.
Table 2.
Adverse events reported by study cohort
| Toxicity | Number reporting by grade | |||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Vaccine site tenderness | 4 | 0 | 0 | 0 |
| Malaise/flu-like symptoms | 3 | 0 | 0 | 0 |
| Fatigue | 5 | 0 | 0 | 0 |
| Vaginal discomfort/discharge | 2 | 0 | 0 | 0 |
3.3 Vaccine-induced antibodies
Vaccination did not elicit antibody responses. We identified measureable titers at study entry of anti-E6 IgG antibody in 3/15 (20%) and anti-E7 IgG antibody in 2/15 (13.3%). The antibody titers to E7 were not boosted following vaccination with the E7 DNA construct in any dose cohort. (data not shown) Measureable antibody titers to E7 at study entry did not correlate with γ-IFN immune response measured in PBMC.
3.4 HPV-specific T cell responses in PBMC
Direct ex vivo Elispot
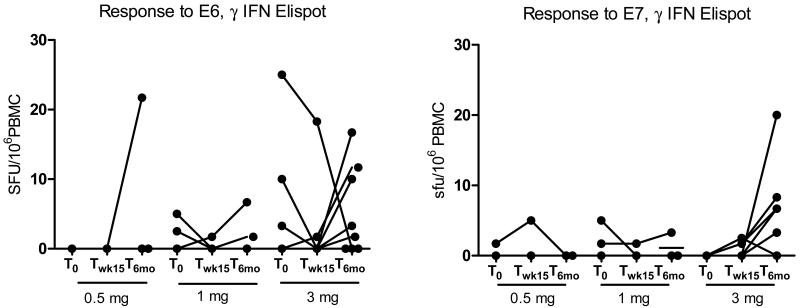
The frequency of T cell responses to pooled E7 overlapping peptides was measured in longitudinally obtained peripheral blood using γ-IFN Elispot assays. Using the overnight assay, HPV16 E7-specific T cell responses were identified at baseline in 3/15 subjects. E7 –specific response was increased in one of these patients subsequent to vaccination at week 15, and was stable in one other subject with a pre-existing response. In the third patient, response to E7 declined. Four patients in whom no detectable E7 response was found pre-vaccination had measureable responses post-vaccination.
Five patients had measureable responses to E6 at study entry. None of those subjects demonstrated an increase in either E6 or E7 responses subsequent to vaccination. Two of the individuals in the highest dose cohort, both regressors, had measureable responses to E6. (Figure 2) Overall, responses to E6 were not of greater absolute magnitude in regressors compared to non-regressors at either of the two time points. (p=0.4228 and 0.4964, respectively). However, it should be noted that one of the regressors was the only individual to maintain a high E6 response before and after the vaccine.
Figure 2.
T cell responses to HPV16 E6 and E7 overlapping peptides, pre- and post-vaccination, using direct ex vivo Elispot on unfractionated PBMCs.
We measured responses to E7 at six months, and, unexpectedly, detected responses to E7 in 5/9(55.6%) of subjects in the highest dose cohort. No new responses to E7 were detected in subjects in the lower dose cohorts.
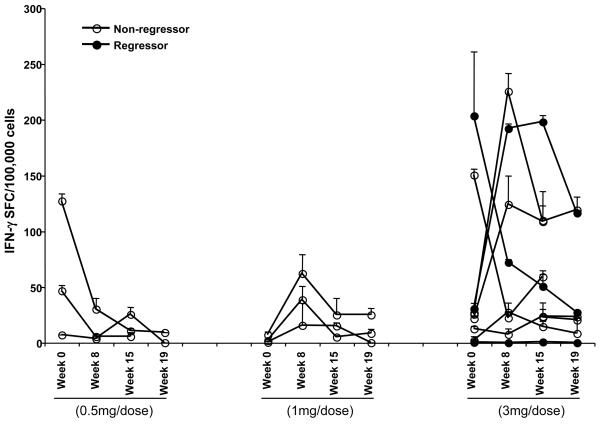
In vitro stimulation followed by Elispot
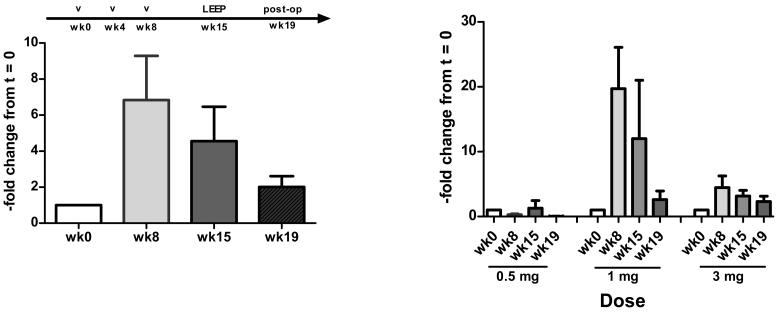
Because the detectable immune responses to E7 were low in frequency, in a second round of testing, we performed a single cycle of in vitro stimulation and performed Elispot assays subsequently. (Figure 3a) Using this method, overall, 5/15 patients had detectable responses pre-vaccination, and eight patients (8/12) (53%) demonstrated an increase in frequency of responses post-vaccination in the second and third dose cohorts (Figure 3b). Peripheral blood immune responses to the vaccine antigen measured using either method, although detectable, and suggestive of a dose-response, were not sustained.
Figure 3a.
T cell responses to HPV16 E7 overlapping peptides after a single cycle of in vitro stimulation, using Elispot on unfractionated PBMCs.
Figure 3b.
Fold change in T cell response to E7 post-vaccination, using Elispot on unfractionated PBMCs
DISCUSSION
In the United States, CIN2/3 is common, with an estimated age-adjusted incidence ranging from 30-60 per 100,000 women.(6) If left undetected and/or untreated, a subset of CIN2/3 lesions will progress to invasive squamous cell carcinomas. Conversely, complete histologic regression does occur spontaneously in up to 25% of HPV16-associated high grade dysplasia.(1, 7) Since conventional histopathological assessment of biopsy tissue at the time of diagnosis does not predict regression, all CIN2/3 lesions are treated by either surgical resection or ablation. Surgical resection of cervical dysplasias has been associated with subsequent risk of preterm delivery in several large studies.(8-10) The development of immunotherapeutic strategies would prevent morbidity even in high-resource settings. Moreover, because a subset of CIN2/3 lesions do indeed regress, this patient population is a potentially informative cohort in which to identify immune correlates of lesion regression versus persistence.
This single-plasmid HPV16 DNA vaccine construct was well tolerated in healthy patients with biopsy-confirmed CIN2/3 associated with HPV16. In addition, three of nine patients in the highest dose cohort had complete histologic regression of established CIN2/3 in the study window. Using an overnight Elispot assay, no patients had significant changes in recognition of E7 in the initial 19 weeks. However, we did detect new responses in 5/9(55.6%) of subjects in the highest dose cohort. Using an assay which included a cycle of in vitro stimulation, T cell responses to E7 were increased subsequent to vaccination in 8/15 patients. However, overall, HPV-specific T-cell responses were not of greater magnitude than those we have identified in unvaccinated subjects with HPV16+ CIN2/3. Vaccination was not associated with changes in serum IgG antibody titers to the vaccine antigen, within the study window.
The long interval before detection of responses was unexpected. However, maturing clinical data from other investigators have also identified new responses to DNA vaccine antigens after a protracted interval.(11) Other investigators assessing IFNγ responses to heterologous DNA prime-viral vector-based boost vaccination regimens have also uncovered different kinetics of response to vaccine antigens in direct ex vivo assays compared to cultured assays of patient-matched specimens.(12) Responses identified using cultured assays correlated with long-lasting T cell responses. The identification of the kinetics of response to vaccination will have obvious implication in the design of boosting strategies in humans.
Many investigators have demonstrated the safety, tolerability, and feasibility of DNA vaccination, in cohorts encompassing a spectrum of health, from healthy naïve cohorts to patients with end-stage disease. While DNA vaccine constructs are relatively simple to engineer and to produce, in humans, the potency of DNA vaccination alone is limited. In fact, the immunogenicity of the DNA vaccines in humans becomes obvious only after heterologous boosting. Clinical experience with DNA vaccination using other antigenic targets, notably malaria and HIV, have uncovered greater efficacy of priming when DNA vaccination is used as a priming vaccination in a heterologous prime-boost regimen, compared to homologous vaccination strategies. (13-17) In our preclinical model, heterologous E7-targeted vaccination using DNA priming and vaccinia-based boosting is considerably more immunogenic than homologous vaccination with either DNA DNA or vaccinia-vaccinia, or vaccinia-DNA regimens.(18) Because our target patient population is essentially healthy, our first trial needed to demonstrate safety of the vaccine construct used alone. It does appear possible to elicit measureable changes in HPV-specific T cell responses in patients with established preinvasive HPV disease.
Pre-existing adaptive immune responses to E6, presumably reflecting previous exposure and immune recognition, did not appear to be associated with ability to respond to vaccination with this E7-targeted construct. In this cohort, we identified endogenous responses to E6 more frequently than responses to E7, and were overall, of greater magnitude. Several lines of evidence suggest that the E6 oncoprotein may well be more immunogenic than E7. While functional T cell recognition of both antigens have been measured in peripheral blood in unvaccinated clinical cohorts, memory CD4+ T cell responses against HPV16 E6 are found frequently in healthy subjects without disease,(19, 20) and conversely, the absence of CD4 recognition of E6 correlates with higher incidence of disease. (21) In a recently published phase I trial assessing vaccination with E6 and E7, long peptides in subjects with late-stage cervical cancer, subjects who underwent vaccination with both E6 and E7 in the same limb displayed dominant responses to E6; vaccination with E6 peptides and E7 peptides targeting separate sets of draining lymph nodes, in contrast, increased the magnitude of response to E7, and did not affect the magnitude of responses to E6.(22) Therefore, in our next clinical trial, we will assess the effect of heterologous, viral-based boost vaccination on immunogenicity, and will also include E6 as an immunotherapeutic target.
Finally, another, plausible reason that HPV-specific immune responses measured in the peripheral blood do not correlate completely with regression of CIN2/3 is that relevant immune variables are likely to be compartmentalized at the site of the lesion. Since high grade dysplastic lesions develop only in the clinical setting of a chronic, mucosally-sequestered viral infection in the context of many co-existing commensal organisms, the immunologic ‘default’ set-point is likely one of localized immune ‘privilege’, i.e., one in which immunogenicity is suppressed.(23) Although localized immune suppression is widely observed in the clinical setting of many frankly invasive diseases, it is likely to play a role well before transition to malignancy in the case of HPV-associated dysplasia. Indeed, Frazer et al indicate a critical role for the generation of local lesional inflammation for effective tumor immunotherapy.(24) In an animal model that used HPV16 E6 and E7 as nominal antigens, even in the face of circulating antigen-specific effector T cells, local acute inflammation was necessary to allow antigen-specific effector T cells to eliminate epithelium expressing these antigens, even in primed hosts. Early clinical data suggests an increased likelihood of response to local immune modulation in the presence of pre-existing measureable HPV-specific T cell responses(25) Based on these data, our next clinical trial of sequential heterologous vaccination will also assess the effect of topical imiquimod on access to lesions.
Statement of Clinical Relevance.
Human papillomavirus (HPV) causes 10% of malignancies in women. Despite the availability of prophylactic vaccines, because barriers to obtaining prophylaxis are significant, the need to pursue therapeutic strategies remains real. Because the viral oncoproteins (E6 and E7) are functionally required for disease initiation and persistence, they present compelling immunotherapeutic targets.
In the US, high grade cervical dysplasia, the immediate precursor to cervical cancer is common. We developed an HPV16 E7 targeted vaccine which had considerable therapeutic effect in the preclinical TC-1 model. Here we report clinical, safety, and immunologic outcomes of a single-institution, investigator-initiated phase I dose-escalation trial in women with HPV16+ high grade dysplasia.
ACKNOWLEDGEMENTS
The authors thank the study volunteers, and the Johns Hopkins Hospital Outpatient Center, Johns Hopkins Bayview Medical Center, and Johns Hopkins at Green Spring Station staff.
GMP reagent was manufactured, and underwent toxicology and release testing by NCI RAID (Barry Kobrin, Jason Yovandich, Stephen Creekmore, Sheryl Ruppel, Karen Schweikart, Joseph Tomasciewski, Douglas Gaum, Steven Giardina).
The following reagents were generously provided by Michael Pawlita: glutathione casein, cleared lysate from E. coli ovrexpressing GST-HPV 16 E6, GST-HPV 16 E7 and GST alone.
Funding: NCI K23CA85437 (CLT), NCI Cervical Cancer SPORE Project 5 P50CA098252 (TCW), NCI RAID Project 048 (DMP), NCI QuickTrials R21 CA105696 (CLT), NCI Cancer Center Core Grant NCI 3 P30 CA006973-45, Maryland State Cigarette Restitution Funding (CLT), Dana Foundation (CLT)
Footnotes
References
- 1.Trimble CL, Piantadosi S, Gravitt P, Ronnett B, Pizer E, Elko A, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clin Cancer Res. 2005;11(13):4717–23. doi: 10.1158/1078-0432.CCR-04-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol. 2001;8(2):209–20. doi: 10.1128/CDLI.8.2.209-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CH, Wang TL, Hung CF, Yang Y, Young RA, Pardoll DM, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60(4):1035–42. [PubMed] [Google Scholar]
- 4.Gravitt PE. Reproducibility of HPV16 and HPV18 viral load quantitation using TaqMan real-time PCR assays. J Virol Methods. 2003;112(1-2):23–33. doi: 10.1016/s0166-0934(03)00186-1. [DOI] [PubMed] [Google Scholar]
- 5.Sehr P, Zumbach K, Pawlita M. A generic capture ELISA for recombinant proteins fused to glutathione S-transferase: validation for HPV serology. J Immunol Methods. 2001;253(1-2):153–62. doi: 10.1016/s0022-1759(01)00376-3. [DOI] [PubMed] [Google Scholar]
- 6.Galloway DA. Papillomavirus vaccines in clinical trials. Lancet Infect Dis. 2003;3(8):469–75. doi: 10.1016/s1473-3099(03)00720-5. [DOI] [PubMed] [Google Scholar]
- 7.Holowaty P, Miller AB, Rohan T, To T. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst. 1999;91(3):252–8. doi: 10.1093/jnci/91.3.252. [DOI] [PubMed] [Google Scholar]
- 8.Nohr B, Tabor A, Frederiksen K, Kjaer SK. Loop electrosurgical excision of the cervix and the subsequent risk of preterm delivery. Acta Obstet Gynecol Scand. 2007;86(5):596–603. doi: 10.1080/00016340701279145. [DOI] [PubMed] [Google Scholar]
- 9.Samson SL, Bentley JR, Fahey TJ, McKay DJ, Gill GH. The effect of loop electrosurgical excision procedure on future pregnancy outcome. Obstet Gynecol. 2005;105(2):325–32. doi: 10.1097/01.AOG.0000151991.09124.bb. [DOI] [PubMed] [Google Scholar]
- 10.Sjoborg KD, Vistad I, Myhr SS, Svenningsen R, Herzog C, Kloster-Jensen A, et al. Pregnancy outcome after cervical cone excision: a case-control study. Acta Obstet Gynecol Scand. 2007;86(4):423–8. doi: 10.1080/11038120701208158. [DOI] [PubMed] [Google Scholar]
- 11.Wloch MK, Smith LR, Boutsaboualoy S, Reyes L, Han C, Kehler J, et al. Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J Infect Dis. 2008;197(12):1634–42. doi: 10.1086/588385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keating SM, Bejon P, Berthoud T, Vuola JM, Todryk S, Webster DP, et al. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J Immunol. 2005;175(9):5675–80. doi: 10.4049/jimmunol.175.9.5675. [DOI] [PubMed] [Google Scholar]
- 13.McConkey SJ, Reece WH, Moorthy VS, Webster D, Dunachie S, Butcher G, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med. 2003;9(6):729–35. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- 14.Vuola JM, Keating S, Webster DP, Berthoud T, Dunachie S, Gilbert SC, et al. Differential immunogenicity of various heterologous prime-boost vaccine regimens using DNA and viral vectors in healthy volunteers. J Immunol. 2005;174(1):449–55. doi: 10.4049/jimmunol.174.1.449. [DOI] [PubMed] [Google Scholar]
- 15.Goonetilleke N, Moore S, Dally L, Winstone N, Cebere I, Mahmoud A, et al. Induction of multifunctional human immunodeficiency virus type 1 (HIV-1)-specific T cells capable of proliferation in healthy subjects by using a prime-boost regimen of DNA- and modified vaccinia virus Ankara-vectored vaccines expressing HIV-1 Gag coupled to CD8+ T-cell epitopes. J Virol. 2006;80(10):4717–28. doi: 10.1128/JVI.80.10.4717-4728.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mwau M, Cebere I, Sutton J, Chikoti P, Winstone N, Wee EG, et al. A human immunodeficiency virus 1 (HIV-1) clade A vaccine in clinical trials: stimulation of HIV-specific T-cell responses by DNA and recombinant modified vaccinia virus Ankara (MVA) vaccines in humans. J Gen Virol. 2004;85(Pt 4):911–9. doi: 10.1099/vir.0.19701-0. [DOI] [PubMed] [Google Scholar]
- 17.Harari A, Bart PA, Stohr W, Tapia G, Garcia M, Medjitna-Rais E, et al. An HIV-1 clade C DNA prime, NYVAC boost vaccine regimen induces reliable, polyfunctional, and long-lasting T cell responses. J Exp Med. 2008;205(1):63–77. doi: 10.1084/jem.20071331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen C, Wang T, Hung C, Pardoll DM, Wu T. Boosting with recombinant vaccinia increases HPV-16 E7-specific T cell precursor frequencies of HPV-16 E7-expressing DNA vaccines [In Process Citation] Vaccine. 2000;18(19):2015–22. doi: 10.1016/s0264-410x(99)00528-9. [DOI] [PubMed] [Google Scholar]
- 19.Welters MJ, de Jong A, van den Eeden SJ, van der Hulst JM, Kwappenberg KM, Hassane S, et al. Frequent display of human papillomavirus type 16 E6-specific memory t-Helper cells in the healthy population as witness of previous viral encounter. Cancer Res. 2003;63(3):636–41. [PubMed] [Google Scholar]
- 20.Gallagher KM, Man S. Identification of HLA-DR1- and HLA-DR15-restricted human papillomavirus type 16 (HPV16) and HPV18 E6 epitopes recognized by CD4+ T cells from healthy young women. J Gen Virol. 2007;88(Pt 5):1470–8. doi: 10.1099/vir.0.82558-0. [DOI] [PubMed] [Google Scholar]
- 21.de Jong A, van Poelgeest MI, van der Hulst JM, Drijfhout JW, Fleuren GJ, Melief CJ, et al. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 2004;64(15):5449–55. doi: 10.1158/0008-5472.CAN-04-0831. [DOI] [PubMed] [Google Scholar]
- 22.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res. 2008;14(1):169–77. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 23.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8(1):74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto K, Leggatt GR, Zhong J, Liu X, de Kluyver RL, Peters T, et al. Impaired antigen presentation and effectiveness of combined active/passive immunotherapy for epithelial tumors. J Natl Cancer Inst. 2004;96(21):1611–9. doi: 10.1093/jnci/djh301. [DOI] [PubMed] [Google Scholar]
- 25.van Poelgeest MI, van Seters M, van Beurden M, Kwappenberg KM, Heijmans-Antonissen C, Drijfhout JW, et al. Detection of human papillomavirus (HPV) 16-specific CD4+ T-cell immunity in patients with persistent HPV16-induced vulvar intraepithelial neoplasia in relation to clinical impact of imiquimod treatment. Clin Cancer Res. 2005;11(14):5273–80. doi: 10.1158/1078-0432.CCR-05-0616. [DOI] [PubMed] [Google Scholar]