Emergence and pandemic potential of swine-origin H1N1 influenza virus (original) (raw)
. Author manuscript; available in PMC: 2010 May 20.
Published in final edited form as: Nature. 2009 Jun 18;459(7249):931–939. doi: 10.1038/nature08157
Abstract
Influenza viruses cause annual epidemics and occasional pandemics that have claimed the lives of millions. The emergence of novel strains will continue to pose challenges to the public health and scientific communities. A prime example is the recent emergence of swine-origin H1N1 viruses that have transmitted to and spread among humans, resulting in outbreaks internationally. Efforts to control these outbreaks and real-time monitoring of the evolution of this virus should provide us with invaluable information to direct infectious disease control programs and to improve understanding of the factors that determine viral pathogenicity and/or transmissibility.
The genomes of influenza viruses are plastic, due to point mutations and reassortment events that contribute to the emergence of new variants or strains with epidemic or pandemic potential. Inasmuch, influenza A viruses have caused several pandemics during the last century, and continue to cause annual epidemics. Both epidemics and pandemics have substantial economic impact due to the costs of prevention and treatment, work absenteeism, physician visits, and excess hospitalizations. Therefore, a detailed understanding of the mechanisms that determine pathogenicity and interspecies transmission, combined with the availability of effective preventative and therapeutic measures, is therefore critical to the control of influenza virus infections.
Influenza A viruses
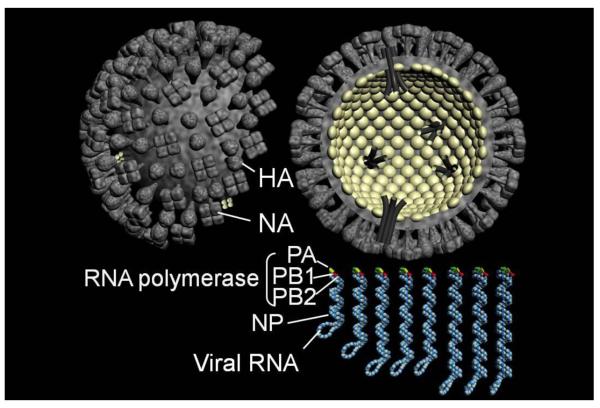
Influenza A viruses belong to the family Orthomyxoviridae. Based on the antigenicity of their hemagglutinin (HA) and neuraminidase (NA) molecules, they are classified into 16 HA subtypes (H1-H16) and 9 NA subtypes (N1-N9). Influenza A viruses contain a genome composed of eight segments of single-stranded, negative-sense RNA that each encodes one or two proteins (Fig. 1). The HA protein is critical for binding to cellular receptors and fusion of the viral and endosomal membranes (Fig. 2). Replication and transcription of viral RNAs (vRNAs) are carried out by the three polymerase subunits PB2, PB1, and PA, and the nucleoprotein NP. Newly synthesized viral ribonucleoprotein (vRNP) complexes are exported from the nucleus to the cytoplasm by the nuclear export protein (NEP, formerly called NS2) and the matrix protein M1, and are assembled into virions at the plasma membrane. The NA protein facilitates virus release from infected cells by removing sialic acids from cellular and viral HA and NA proteins. The functions of the ion channel protein M2, the interferon antagonist NS1, and the PB1-F2 protein are discussed in more detail below.
Fig. 1. Schematic diagram of influenza A viruses.
Virions are decorated with two surface glycoproteins, HA and NA. The genome is composed of eight segments of single-stranded RNA that interact with the nucleoprotein and the components of polymerase complex (PB2, PB1, PA).
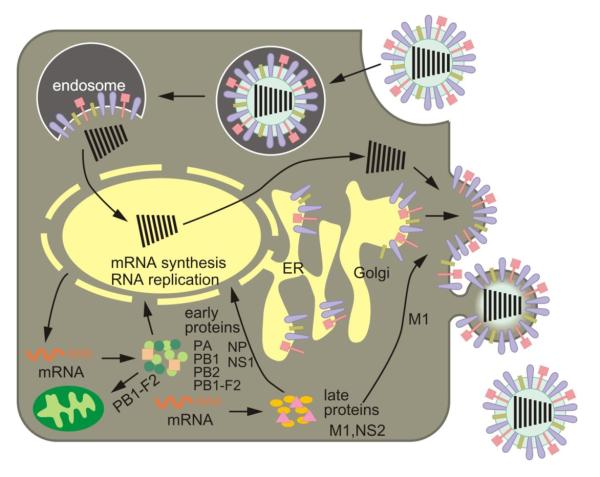
Fig. 2. Schematic diagram of the influenza viral life cycle.
Following receptor-mediated endocytosis, the viral ribonucleoprotein (vRNP) complexes are released into the cytoplasm and subsequently transported to the nucleus, where replication and transcription take place. mRNAs are exported to the cytoplasm for translation. Early viral proteins, i.e., those required for replication and transcription, are transported back to the nucleus. Late in the infection cycle, the M1 and NS2(=NEP) proteins facilitate the nuclear export of newly synthesized vRNPs. PB1-F2 associates with mitochondria. The assembly and budding of progeny virions occurs at the plasma membrane.
Influenza pandemics
Influenza A viruses cause recurrent epidemics and global pandemics. Pandemics are typically caused by the introduction of a virus with an HA subtype new to human populations. Two mechanisms that are not mutually exclusive, reassortment and inter-species transmission, result in the introduction of viruses with new HA subtypes into human populations (Fig. 3). The recently emerged swine-origin H1N1 influenza viruses (S-OIV) are being detected in an increasing number of countries and their global spread would undoubtedly result in a significant number of infected individuals. Currently, the mortality rate associated with S-OIV infections appears to be comparable to that of seasonal influenza virus outbreaks; however, increased surveillance activities could reveal a higher rate.
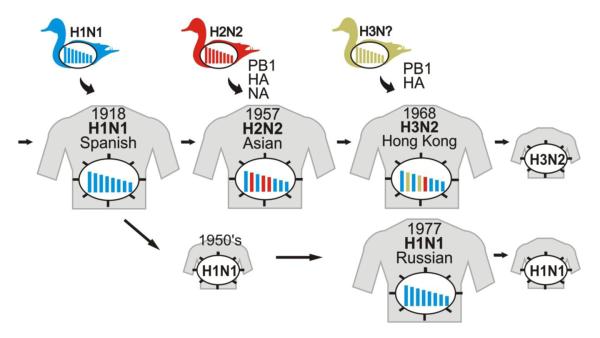
Fig. 3. Emergence of pandemic influenza viruses.
The ‘Spanish influenza’ was most likely caused by the transmission of an avian influenza virus to humans. In 1957, the introduction of avian virus H2 HA, N2 NA, and PB1 genes into human populations resulted in the ‘Asian influenza’. Similarly, the introduction of avian virus H3 HA and PB1 genes into human populations led to the ‘Hong Kong influenza’ in 1968. In 1977, H1N1 viruses reappeared which closely resembled strains that had been circulating in the mid 1950's.
‘Spanish influenza’ (H1N1)
The pandemic of 1918/1919 killed as many as 50 million people worldwide and remains unprecedented in its severity. A first, mild wave in the spring of 1918 was replaced by a second wave in September to November of 1918 that resulted in mortality rates of over 2.5%, compared to less than 0.1% as typically recorded for influenza outbreaks. A third wave with equally high mortality rates swept around the world in 1919.
The mortality pattern of the ‘Spanish influenza’ was unusual with high mortality rates for young adults. The atypical mortality pattern observed with the ‘Spanish influenza’ remains unexplained to this day. By contrast, the morbidity pattern was similar to other pandemics, that is, children under the age of 15 experienced the highest attack rates.
The ‘Spanish influenza’ virus was restricted to the respiratory tract; lack of systemic infection has also been observed in nonhuman primates experimentally infected with reconstituted 1918 virus1. Most patients died of bacterial pneumonia2, which may be attributed to the lack of antibiotics in 1918/1919; however, many others died due to viral pneumonia.
Although the ‘Spanish influenza’ virus was not isolated during the outbreak in 1918/1919, the genomic sequences of this virus were determined3,4 and revealed an avian-like H1N1 virus that contains human-like signature amino acids in several proteins. The ‘Spanish influenza’ virus lacks a multibasic HA cleavage site4, a hallmark of highly pathogenic avian influenza viruses (see ‘Role of HA in viral pathogenicity’).
Reverse genetics5 allowed the re-creation of the ‘Spanish influenza’ virus6 and its characterization. The ‘Spanish influenza’ virus elicits aberrant innate immune responses in mice7 and in nonhuman primates1, a feature that it shares with highly pathogenic H5N1 viruses8 and that likely contributes to pathogenicity and mortality in humans. Further studies revealed that the HA protein9-11, the replication complex6,12,13, the NS1 protein14, and the PB1-F2 protein15 contributed to its virulence, and that the HA and PB2 proteins were critical to its transmissibility13.
‘Asian influenza’ (H2N2)
The ‘Asian influenza’ originated in Southern China in February 1957. From there, it spread to Singapore (March 1957), Hong Kong (April 1957), Japan (May 1957), and the United States and the United Kingdom (October 1957). A second wave was detected in January 1958. In the United States, excess mortality was estimated to be 70,000. The pandemic was caused by a human/avian reassortant that introduced avian virus H2 HA and N2 NA genes into human populations (Fig. 3). In addition, the ‘Asian influenza’ virus also possessed a PB1 gene of avian virus origin.
‘Hong Kong influenza’ (H3N2)
In 1968, viruses of the H2N2 subtype were replaced by another human/avian reassortant that possessed an H3 HA gene of avian virus origin (Fig. 3). Again, the PB1 gene of the pandemic virus was derived from an avian virus. The virus was first isolated in Hong Kong in July 1968 and caused a pandemic in the winter of 1968-1969 and 1969-1970. In the United States, an estimated 33,800 people died from the ‘Hong Kong influenza’.
‘Russian influenza’ (H1N1)
In May 1977, an influenza virus outbreak was reported in China that affected young adults in the northern hemisphere in the winter of 1977/1978. The outbreak was caused by influenza viruses of the H1N1 subtype that closely resembled viruses that had circulated in the early 1950s16, suggesting accidental release of this virus. The re-emerging H1N1 virus did not replace the H3N2 viruses circulating at the time and both subtypes are cocirculating in humans to this day. Reassortment between viruses of these subtypes resulted in the emergence of H1N2 viruses in human populations in 2001. However, these H1N2 viruses have since disappeared.
Highly pathogenic H5N1 influenza viruses
The infection of 18 individuals in Hong Kong in 1997 with highly pathogenic avian influenza viruses of the H5N1 subtype, which resulted in 6 fatalities17,18, marked the first reported fatal infections of humans with avian influenza viruses. This outbreak was brought under control with the depopulation of live birds in poultry markets in Hong Kong. After a period of local and sporadic outbreaks, a new outbreak started in 2003. H5N1 viruses have since reassorted frequently19-22 and have spread to Europe and Africa and/or become enzootic in poultry populations in many Southeast Asian countries23.
The highly pathogenic H5N1 viruses have several remarkable features. First, they are not only lethal in chickens, but some highly pathogenic H5N1 viruses also kill waterfowl, the natural reservoir of influenza A viruses. Second, they replicate and cause lethal infection in mice without prior adaptation. Third, they have fatally infected a number of mammalian species. Non-lethal pig infections have been detected at low rates. Fourth, their pathogenicity in ferrets has increased over the years, indicating the acquisition of mutations that increase pathogenicity in mammalian species. Fifth, and of most concern, is their continued transmission to humans, resulting in severe respiratory infection with high mortality rates.
As of May 15, 2009, 424 human infections with H5N1 have been confirmed, resulting in 261 deaths (http://www.who.int/csr/disease/avian_influenza/country/cases_table_2009_05_15/en/index.html). Although several family clusters of H5N1 virus infection have been described, sustained human-to-human infection has not occurred. Hence, these H5N1 viruses are characterized by a high mortality rate but inefficient spread among humans; by contrast, S-OIV appear to spread efficiently among humans but have caused a limited number of fatal infections.
Several reports indicate that highly pathogenic avian H5N1 viruses replicate mainly in the lower respiratory tract of humans8,24, and that the virus load correlates with the outcome of the infection8. Human H5N1 infections cause severe pneumonia and lymphopenia24-26, and are characterized by high levels of cytokines and chemokines8,27,28, a finding that was also confirmed in in vitro studies29,30. The induction of hypercytokinemia and hyperchemokinemia may thus be associated with the level of virus replication8.
Outbreak of swine-origin H1N1 viruses
Epidemiological data now indicate that an outbreak of influenza-like respiratory illness started in the Mexican town of La Gloria, Veracruz, in mid February of 200931 (Table 1). In early April, public health authorities in Mexico began investigating high numbers of pneumonia/influenza-like illness, and informed the Pan American Health Organization (PAHO), the regional office of the World Health Organization (WHO), of a possible outbreak. In the United States, the Centers for Disease Control (CDC) identified S-OIV in two specimens independently collected in Southern California in mid April. On April 23, the Public Health Agency of Canada also detected S-OIV in specimens received from Mexico. Further cases and the finding that the Mexican and Californian cases were caused by similar viruses triggered alerts by the CDC and WHO on April 24. By the end of April, international spread and clusters of human-to-human transmission prompted the WHO to elevate the pandemic alert from phase 3 to phase 4, and shortly after, to phase 5 (human-to-human spread in at least two countries, and signs of an imminent pandemic). In Mexico, substantial social distancing measures were implemented. Moreover, massive campaigns were undertaken to educate the public about precautionary hygiene measures. As of May 21, 2009, 41 countries have reported 11,034 cases, including 85 deaths. Most cases outside Mexico and the US have been caused by travelers from Mexico. The majority of infections seem to be mild and do not require hospitalization32. Careful monitoring will be necessary during the following months (i.e., the during the winter season in the southern hemisphere) to be prepared for the potential emergence of more virulent variants, as observed with the 1918 pandemic.
Table 1.
Timeline of swine-origin H1N1 virus outbreak (see also 32)
| Mid-February | Outbreak of respiratory illness in La Gloria, Veracruz, Mexico31 |
|---|---|
| April 12 | Mexican public health authorities report outbreak in Veracruz to the PAHO |
| April 15 | CDC identifies S-OIV in the specimen of a boy from San Diego, California |
| April 17 | CDC identifies S-OIV in the specimen of a girl from Imperial, California |
| April 21 | CDC alerts doctors to a novel strain of H1N1 influenza virus |
| April 23 | The Public Health Agency of Canada identifies S-OIV in specimens from Mexico |
| April 24 | WHO issues Disease Outbreak Notice |
| April 27 | International spread and clusters of human-to-human transmission prompt WHO to raise the pandemic alert from phase 3 to 4 |
| April 29 | WHO raises the pandemic alert from phase 4 to 5 (human-to-human spread in at least two countries in one WHO region) |
| May 21 | 41 countries report 11,034 cases, including 85 deaths |
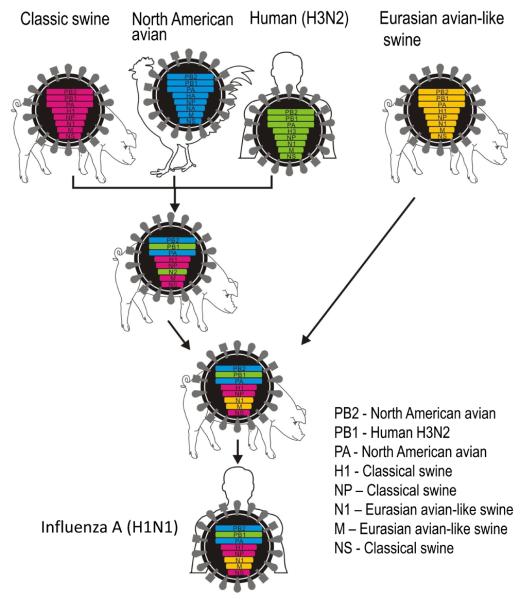
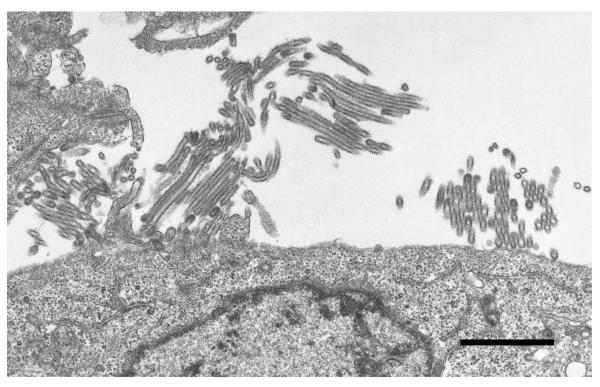
Data on the genetic composition of the virus became available soon after viral isolation from the initial cases32. The S-OIV likely resulted from the reassortment of recent North American H3N2 and H1N2 swine viruses (i.e., avian/human/swine ‘triple’ reassortant viruses) with Eurasian avian-like swine viruses (32; Fig. 4). As a result, these viruses possess PB2 and PA genes of North American avian virus origin, a PB1 gene of human H3N2 virus origin, HA (H1), NP, and NS genes of classical swine virus origin, and NA (N1) and M genes of Eurasian avian-like swine virus origin (hence their original description as ‘quadruple’ reassortants). However, the human-like PB1 gene and the avian-like PB2 and PA genes have been circulating in pigs since 1997/1998 (when triple reassortant swine viruses were first isolated), and have likely undergone adaptation to pigs. These viruses do not possess markers associated with high pathogenicity (see the following sections on the role of viral proteins in pathogenicity for more details). Unlike negatively stained S-OIV virions that appeared spherical (http://www.cdc.gov/h1n1flu/images.htm), our transmission electron microscopic analysis of cells infected with S-OIV revealed virions of a distinctively filamentous shape (Fig. 5).
Fig. 4. Genesis of swine-origin H1N1 influenza viruses.
In the late 1990's, reassortment between human H3N2, North American avian, and classical swine viruses resulted in triple reassortant H3N2 and H1N2 swine viruses that have since circulated in North American pig populations. A triple reassortant swine virus reassorted with a Eurasian avian-like swine virus, resulting in the S-OIV that are now circulating in humans.
Fig. 5. Electron microscopic picture of recently emerged swine-origin H1N1 viruses.
Madin-Darby canine kidney cells were infected with A/California/04/09 (H1N1) virus and observed by thin section electron microscopy 24 hours later. Most virus particles showed a filamentous shape of more than 1 μm in length. Bar, 1 μm.
Role of HA in viral pathogenicity
Influenza virus pathogenicity is multigenic, and the determinants of pathogenicity may differ among animal species. However, the HA protein plays an important role in expressing high pathogenicity in many animal species. It mediates the binding of the virus to host cells and the subsequent fusion of the viral and endosomal membranes for vRNP release into the cytoplasm. These functions assign a critical role to HA in the viral life cycle.
Receptor distribution on host cells
Influenza virus host specificity can be explained in part by the difference in receptor binding specificity for human and avian influenza viruses. Human influenza viruses preferentially bind to sialic acid that is linked to galactose by an α2,6-linkage (SAα2,6Gal)33. This preference is matched by SAα2,6Gal on epithelial cells in the human trachea. By contrast, avian influenza viruses preferentially recognize SAα2,3Gal which is matched by SAα2,3Gal on epithelial cells in the intestinal tract of waterfowl34 (the main replication site of avian influenza viruses).
The receptor-binding specificity of human and avian influenza viruses suggests that avian influenza viruses need to acquire the ability to recognize human-type receptors in order to cause a pandemic. Indeed, the earliest isolates of the 1918, 1957, and 1968 pandemics possessed HA that, although of avian origin, recognized human-type receptors. In light of these findings, the infection of humans with highly pathogenic avian H5N1 viruses seemed to be surprising, particularly since the H5N1 viruses isolated from infected individuals in Hong Kong in 1997 preferentially recognized SAα2,3Gal35. However, studies revealed avian-type receptors on human epithelial cells that line the respiratory bronchiole and the alveolar walls, but human-type receptors on human epithelial cells in nasal mucosa, paranasal sinuses, pharynx, trachea, and bronchi36,37. Another study, however, showed the ex vivo infection of upper respiratory organs with an H5N1 avian virus38. Still, the finding of avian-type receptors in human lungs explains the severe pneumonia seen in humans with highly pathogenic avian H5N1 viruses.
HA receptor specificity
The differences in receptor-binding specificity of human and avian viruses are determined by the amino acid residues in the HA receptor-binding pocket. Gln at position 226 and Gly at position 228 of H2 and H3 HAs confer binding to avian-type receptors, while Leu and Ser at these positions determine binding to human-type receptors. For H1 HAs, amino acids at position 190 and 225 (H3 numbering) determine receptor binding specificity. HA-190Asp and HA-225Asp (found in human H1 HAs) confer binding to human-type receptors, whereas HA-190Glu and HA-225Gly (found in avian H1 HAs) confer binding to avian-type receptors39. Two viruses that differ in receptor recognition were circulating during the 1918 pandemic: one recognizing only human-type receptors that transmits efficiently among ferrets, and one recognizing both avian- and human-type receptors that transmits inefficiently in this animal40. The recently emerged S-OIV possess the ‘human virus’-type amino acid at positions 190 and 225, likely supporting efficient transmissibility of these viruses in humans. Interestingly, some S-OIV isolates possess an amino acid substitution at position 135 or 226 that has been found in H5N1 viruses isolated from humans and has been shown to affect receptor binding (our unpublished data). These mutations may thus reflect viral adaptation in humans, an assumption that needs to be tested.
For H5N1 viruses, amino acid changes at positions 133, 138, 186, 192, and 227 (H3 numbering) have been identified in human isolates and confer human-type receptor recognition41-43. Experimental changes at positions 226 and 228 (Gln-to-Leu and Gly-to-Ser, respectively), but not at position 190 (Glu-to-Asp), resulted in the recognition of human-type receptors in addition to avian-type receptors44; however, the respective amino acid changes at positions 226 and 228 have not been detected in human H5N1 virus isolates.
HA cleavage
HA cleavage is essential for viral infectivity since exposure of the N-terminus of HA2 (the so-called ‘fusion peptide’) mediates fusion between the viral envelope and the endosomal membrane, an essential step for vRNP release to the cytoplasm. HA cleavability is determined by the amino acid sequence at the cleavage site. Low pathogenic avian viruses and non-avian influenza viruses (with the exception of H7N7 equine influenza viruses) possess a single Arg residue at the cleavage site which is cleaved by proteases in the respiratory and/or intestinal organs and hence restricts viral replication locally. By contrast, highly pathogenic H5 and H7 viruses possess multiple basic amino acids at the HA cleavage site45. This motif is recognized by ubiquitous proteases, such as furin and PC6, and leads to systemic infections. For several outbreaks in poultry, increased pathogenicity of avian influenza viruses has been linked to the acquisition of multibasic HA cleavage sites, a finding that underscores the significance of the HA cleavage motif for virulence.
Role of PB2 in pathogenicity and host specificity
Recently, the viral replication complex has been recognized as an important contributor to viral pathogenicity, likely by affecting viral growth. The amino acid at position 627 of the PB2 protein was first described by Subbarao et al. as a host range determinant, based on cell culture studies46. Hatta et al. demonstrated that the respective amino acid change determined the pathogenicity of H5N1 influenza viruses in mice47. Viruses with lysine at this position were pathogenic in mice, whereas those with glutamic acid were nonpathogenic in these animals47. Notably, almost all human influenza viruses possess lysine at this position, while most avian viruses (with the exception of the ‘Qinghai Lake’ lineage of H5N1 viruses and their descendants) possess glutamic acid at PB2-627. Lysine at position 627 of PB2 is now recognized as a determinant of viral pathogenicity in several mammalian species.
Several studies have addressed the mechanism by which PB2-627Lys affects virulence. The amino acid change does not affect tissue tropism in mice but viral replicative ability. This may result from an inhibitory activity in mammalian cells that prevents efficient replication by polymerase complexes possessing PB2-627Glu48, and/or from inefficient interaction of PB2-627Glu with mammalian-type NP protein49. Viruses possessing PB2-627Lys, but not those possessing PB2-627Glu, grow efficiently in the upper respiratory tract of mammals50, which may be explained by the fact that PB2-627Lys confers efficient replication at 33°C (the temperature of the upper airway in humans) while PB2-627Glu does not51. By contrast, both variants mediate efficient replication at 37°C50,51. Collectively, these findings suggest that PB2-627Lys allows efficient replication not only in the lower, but also in the upper respiratory tract of mammals, a feature that may facilitate transmission. In fact, replacement of PB2-627Lys with Glu reduced the transmissibility of human influenza viruses in a guinea pig model52.
The amino acid at position 701 of PB2 has also emerged as a determinant of virulence 53,54, a role likely related to its facilitation of binding of PB2 to importin α (a cellular nuclear import factor) in mammalian cells55. The recently emerged S-OIV possess the ‘low pathogenic’-type amino acids at positions 627 and 701 (i.e., Glu and Asp).
Recently, the PB2 and HA proteins of the ‘Spanish influenza’ virus were shown to be critical for droplet transmission13. The underlying mechanism and the amino acids in PB2 that are critical for this function remain to be determined.
In addition to PB2, other components of the replication complex may contribute to viral pathogenicity as well56. A recent study also suggested that the replication complex, particularly the PB1 protein, contributes to the virulence of the 1918 pandemic virus in ferrets12.
Structural data are now becoming available for the viral polymerase complex which may help in the interpretation of mutational analyses; in fact, two studies57,58 showed that PB2-627Lys is part of a basic groove that is disrupted upon replacement with Glu.
Role of NS1 in viral pathogenicity
The NS1 protein is an interferon antagonist59,60 that blocks the activation of transcription factors and IFN-β-stimulated gene products, and binds to double-stranded RNA (dsRNA) to prevent the dsRNA-dependent activation of 2′-5′ oligo(A) synthetase (OAS) and the subsequent activation of RNase L, a key player in the innate immune response. Recently obtained structural data are expected to help in the identification of domains that are critical for the biological functions of NS1.
Innate immune responses are stimulated upon the recognition of a pathogen by a pathogen recognition receptor (PRR). Several classes of PRRs have now been described, including retinoic acid inducible gene-I (RIG-I) and melanoma differentiation antigen 5 (MDA5), and Toll-like receptors (TLRs) 3, 7 and 8. Influenza virus infections activate RIG-I signaling, which is counteracted by the viral NS1 protein61, possibly by forming a complex with RIG-I. In addition, influenza virus infection affects TLR762,63- and TLR464-signaling, and TLR4-deficient mice do not develop acute lung injury upon infection with H5N1 viruses64. The direct contribution of NS1 to these signaling events is currently not known.
The NS1 proteins of H5N1 viruses confer resistance to the antiviral effects of IFN and are associated with high levels of proinflammatory cytokines27,28,30,65,66; the resulting cytokine imbalance likely contributes to the high mortality of H5N1 virus infections in humans. Several amino acids in NS1 have now been shown to affect virulence65,67,68. The S-OIV possess the ‘low pathogenic’-type amino acid at these positions. However, available data suggest that these amino acid changes affect virulence in a strain-specific manner, while a multibasic HA cleavage sequence and PB2-627Lys seem to be universal determinants of viral pathogenicity.
The four C-terminal amino acids of NS1 form a PDZ ligand domain motif that was identified by large-scale sequence analysis69. Introduction of the PDZ ligand domains of highly pathogenic H5N1 viruses or the pandemic 1918 virus into an otherwise human virus conferred slightly increased virulence in mice70. This increase in virulence was not paralleled by increased IFN production. The S-OIV lack the 11 C-terminal amino acids of NS1 and hence, the PDZ domain motif. The biological significance of this finding is currently unknown.
Role of PB1-F2 in viral pathogenicity
The PB1 gene of most avian and human influenza A viruses encodes a second protein, PB1-F2, that is expressed from the +1 reading frame71. The length of PB1-F2 of swine influenza viruses differs depending on their origin; classical swine viruses possess truncated PB1-F2 proteins of 8-11 amino acids, while Eurasian avian-like swine viruses possess full length PB1-F2 proteins (87-89 amino acids). The S-OIV encode a truncated PB1-F2 protein of 11 amino acids. PB1-F2 induces apoptosis, likely by interaction with two mitochondrial proteins71,72, enhances inflammation in mice, and increases the frequency and severity of secondary bacterial infections15. It may also affect virulence by interacting with the PB1 protein to retain it in the nucleus for efficient viral replication73. A recent study demonstrated that the amino acid at position 66 of PB1-F2 affects the pathogenicity of an H5N1 virus in mice74. This finding is of great interest since the pandemic 1918 Spanish influenza virus possessed the “high pathogenic”-type amino acid, and its replacement attenuated this virus74.
Interspecies transmission
Wild waterfowl are the natural reservoir of influenza A viruses. Besides the ongoing transmission of highly pathogenic avian H5N1 influenza viruses to humans, avian influenza A viruses were transmitted to pigs in Europe in 1979, to horses in China, and to seals. Moreover, an avian influenza virus reassorted with a human virus in pigs and transmitted from there to humans.
Human infections with avian or swine influenza viruses have been reported but have typically been self-limiting. It is in this context that the recently emerged H1N1 viruses, which are of swine origin and transmit among humans, raise great concern over an imminent pandemic.
Pigs can be naturally and experimentally infected with avian viruses and express both human- and avian-type influenza virus receptors on epithelial cells in trachea34, supporting the concept of a role as “mixing vessel” in which human and avian viruses reassort. In North American pig populations, classical swine H1N1 virus dominated for nearly six decades. In 1997/1998, however, H3N2 triple human/avian/swine reassortant viruses emerged that have spread widely within North American pig populations. The emergence of human/avian/swine triple reassortant viruses in pigs further indicates that this species can be infected with human and avian influenza viruses and may provide a platform for reassortment. The triple reassortant viruses that emerged in North American pigs in 1997/1998 are the progenitors of the S-OIV (Fig. 4).
Prevention and control
For the prevention and control of influenza virus infections, both vaccines and antiviral drugs are available. Nonetheless, the global community is likely not well prepared for a pandemic: antiviral drugs may not be in sufficient supply and the virus may acquire resistance to the available antiviral drugs. On the other hand, the production of a vaccine to a newly emerging strain would take 3-6 months – during which time a virus could spread globally and substantially strain health care systems and the global economy.
Antiviral drugs
Two classes of antiviral drugs – ion channel inhibitors and neuraminidase inhibitors – are currently licensed for use against influenza A viruses.
Adamantanes (i.e., amantadine hydrochloride and rimantadine) block the ion channel formed by the M2 protein, which is critical in the release of vRNPs into the cytoplasm. Resistance to adamantanes arises quickly and frequently, and most circulating human H1N1 and H3N2 viruses, some H5N1 viruses, and most European porcine H1N1, H1N2, and H3N2 viruses are resistant to adamantanes. The S-OIV are also resistant to ion channel inhibitors32.
Two neuraminidase inhibitors – oseltamivir and zanamivir – are currently licensed. Neuraminidase inhibitors interfere with the enzymatic activity of the NA protein, which is critical for the efficient release of newly synthesized viruses from infected cells.
In clinical trials, the emergence of resistance to NA inhibitors was rare, and oseltamivir-resistant influenza viruses were attenuated in vitro and in vivo. Hence, the dissemination of these viruses was not considered a major issue, despite the frequent use of the drug in some countries. However, a study in clinical settings indicated higher (18%) than expected rates of oseltamivir-resistance in children treated with this drug75. Recently, the rate of oseltamivir-resistant H1N1 influenza viruses in the US has increased from 0.7% in the 2006-2007 influenza season to 98.5% in the 2008-2009 influenza season76. Similar numbers have been reported for other countries. Equally alarming, oseltamivir-resistant H5N1 viruses have been reported77,78. Oseltamivir-resistant human H1N1 viruses may have emerged in immunocompromised patients where prolonged replication79-81 may have resulted in the selection of mutations that increase the fitness of oseltamivir-resistant viruses. The S-OIV are sensitive to neuraminidase inhibitors when tested in vitro in enzymatic assays32. Recent structural data provide an explanation for oseltamivir-resistance82 and suggest strategies for the design of improved compounds. In clinical settings, resistance to zanamivir has been reported only once for an influenza B virus isolated from an immunocompromised child83.
Several experimental antiviral drugs that target the NA or polymerase proteins are currently in different stages of development.
Peramivir, an NA inhibitor that was developed through structure-based design84, is active in vitro tests against viruses of all 9 NA subtypes including highly pathogenic H5N1 viruses. Phase II clinical trials are currently underway to assess the efficacy of intramuscularly administered peramivir against seasonal influenza.
CS-8958, a pro-drug of the novel NA inhibitor R-12548985, is a long-acting neuraminidase inhibitor that was found to be effective in phase II clinical trials against seasonal influenza. We also found that CS-8958 has prophylactic and therapeutic efficacy against highly pathogenic H5N1 influenza viruses (unpublished).
T-705 acts as a nucleoside analogue that interferes with the polymerase activity of influenza A, B, and C viruses, but also other RNA viruses86. It protected mice against infection with highly pathogenic H5N1 viruses (our unpublished findings). Phase II clinical trials for use of T-705 against seasonal influenza viruses have been completed in Japan, and phase III clinical trials are scheduled.
In addition, monoclonal antibodies to the HA are being developed for treatment of influenza virus infections. In mice, some antibodies demonstrated prophylactic and therapeutic efficacy against lethal challenge with H5N1 virus87, suggesting monoclonal antibody treatment as an alternative strategy to treat influenza virus infections.
Vaccines
Seasonal influenza vaccines include human influenza A viruses of the H1N1 and H3N2 subtypes, and an influenza B virus. These vaccines need to be updated every 1-3 years to account for mutations in the HA and NA proteins of circulating viruses (antigen drift).
Inactivated vaccines have been used for many decades. Typically, reassortment is used to generate a seed virus that possesses the HA and NA segments of the circulating virus and a variable number of segments from A/Puerto Rico/8/34 (H1N1) virus that confer efficient growth in embryonated chicken eggs. The allantoic fluid of embryonated, virus-infected chicken eggs is purified and concentrated by zonal centrifugation or column chromatography and inactivated with formalin or beta-propiolactone. Treatment with detergents or ether and removal of vRNP complexes leads to split or subunit vaccines that are administered intramuscularly or subcutaneously.
In children and young adults, inactivated influenza vaccines show a 60-80% efficacy rate against laboratory/culture confirmed influenza illness; this rate is lower in persons over the age of 60, that is, the group of people that is most likely to die from influenza virus infections or associated complications. The development of improved influenza virus vaccines is thus clearly warranted.
A live attenuated influenza virus vaccine is now licensed in the US for healthy individuals age 2-to-49. Briefly, serial passage of A/Ann Arbor/6/60 (H2N2) virus or B/Ann Arbor/1/66 at low temperatures resulted in viruses that are temperature-sensitive (ts), cold-adapted (ca), and attenuated (att). These viruses are then reassorted with currently circulating wild-type strains to generate seed viruses that possess the HA and NA genes of the circulating wild-type viruses in the background of the ts, ca, and att phenotypes.
Live attenuated vaccines elicit both humoral and cellular immune responses, and are therefore believed to be superior to inactivated vaccines. In fact, in infants and young children, live attenuated influenza vaccine provides better protection than inactivated vaccine88.
Currently, only egg-based vaccines are licensed for use in the US. In case of a pandemic, however, eggs may be in short supply. By contrast, cell cultures are highly controllable systems that can be scaled-by up for the mass production of vaccines, including those to highly pathogenic H5N1 viruses. The purity and immunogenicity of influenza vaccines produced in Madin-Darby canine kidney (MDCK) or African green monkey kidney (Vero) cells match those of vaccines produced in embryonated chicken eggs. Cell culture-based influenza vaccines have been approved for use in humans in Europe.
Recently, particular emphasis has been given to the development of vaccines to highly pathogenic H5N1 viruses. These viruses kill chicken embryos. Propagation of these viruses in eggs therefore results in low yields. To modify these viruses for efficient growth in embryonated chicken eggs and safe handling by production staff, reverse genetics technologies5 were used to replace the multibasic HA cleavage site with an avirulent-type HA cleavage site45, a known virulence factor. Reverse genetics then allowed the generation of a virus possessing the modified HA gene and the NA gene derived from the H5N1 virus and the remaining genes derived from A/Puerto Rico/8/34 virus. Clinical testing of an H5N1 vaccine candidate suggested low immunogenicity89, prompting the addition of adjuvants to vaccine candidates. In fact, aluminum hydroxide90,91 or oil-in-water emulsions such as MF5992,93 or AS0394 resulted in significant antigen-sparing effects. Adjuvanted vaccines also seem to induce broader immune responses, which may be a critical advantage with the emergence of new clades and subclades of H5N1 viruses.
Similarly, the NA and modified HA genes of highly pathogenic avian H5N1 viruses were combined with the remaining genes derived from the live attenuated influenza A virus95. To avoid the introduction of H5N1 HA and NA genes into human populations, live attenuated H5N1 vaccines should not be used prior to a pandemic. However, once H5N1 viruses are widespread in human populations, use of a live attenuated H5N1 vaccine, which is efficacious in nonhuman primates96, may be considered to overcome the low immunogenicity of inactivated vaccines.
Several novel vaccine approaches are now in various stages of development. A ‘universal’ vaccine based on the conserved ectodomain of the M2 protein confers protection against influenza virus infection in animals (reviewed in 97). Various virus-like particles (VLPs) expressing the HA and NA proteins, some in combination with the M1 and M2 proteins, have been tested for their antigenicity and protective efficacy. A recent study demonstrated protection of ferrets against H5N1 virus infection by a VLP expressing the HA, NA, and M1 proteins of a heterologous virus98. Also, vector approaches have been pursued. In one example, replication-incompetent adenoviruses expressing an H5 HA protected mice against challenge with homologous and heterologous H5N1 viruses99,100. In alternative approaches, Newcastle disease and fowlpox viruses have been explored as vector systems; however, these systems have not been approved for use in humans.
The Future
Although much has been learned about influenza viruses, key questions still remain unanswered: what factors determine interspecies transmission, reassortment, and human-to-human transmission – factors that have accounted for past pandemics and will be critical in the emergence of new pandemic viruses. The first wave of pandemic ‘Spanish influenza’ was characterized by relatively low pathogenicity in humans, but the virus presumably mutated into a more virulent form within a few months. Thus, careful monitoring of the S-OIV during the upcoming winter season in the southern hemisphere is of critical importance to detect more virulent variants, should they arise. From a scientific perspective, the opportunity to watch virus evolution in real time may provide us with invaluable information on the factors that determine pathogenicity, and/or transmissibility. In addition, large-scale sequencing efforts, bioinformatics analyses, and the ability to experimentally test predictions with recombinant viruses will eventually provide insight into key features for the emergence of pandemic viruses. In the meantime, the development of improved and novel antiviral drugs and vaccines will be critical to control influenza virus outbreaks.
Table 2.
Determinants of viral pathogenicity
| Protein | Position | Pathogenicity | Swine-origin H1N1 viruses | Function | Ref. | |
|---|---|---|---|---|---|---|
| Low | High | |||||
| PB2 | 627 | Glu | Lys | Glu | Replicative ability in some mammals including humans | 46,47 |
| 701 | Asp | Asn | Asp | Nuclear import kinetics affecting replicative ability in mice | 53,55 | |
| PB1-F2 | 66 | Asn | Ser | Truncated (11 a.a.) | Induction of apoptosis | 74 |
| HA | Single basic a.a. | Multiple basic a.a. | Single basic a.a. | HA cleavage | 45 | |
| NS1 | 92 | Asp | Glu | Asp | Unknown (Interferon response?) | 65 |
| C-term. | RSEV, deletion | ESEV | 11 C-term. a.a. truncated | Unknown | 69,70 |
Acknowledgements
We apologize to our colleagues whose critical contributions to influenza virus research could not be cited due to the number of references permitted.
We thank Krisna Wells for editing the manuscript. We also thank Makoto Ozawa and others in our laboratories who contributed to the data cited in this review. Our original research was supported by National Institute of Allergy and Infectious Diseases Public Health Service research grants; by Grant-in-Aid for Specially Promoted Research, by a contract research fund for the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases from the Ministry of Education, Culture, Sports, Science, and Technology, by grants-in-aid from the Ministry of Health and by ERATO (Japan Science and Technology Agency).
Reference List
- 1.Kobasa D, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 2.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taubenberger JK, Reid AH, Krafft AE, Bijwaard KE, Fanning TG. Initial genetic characterization of the 1918 “Spanish” influenza virus. Science. 1997;275:1793–6. doi: 10.1126/science.275.5307.1793. [DOI] [PubMed] [Google Scholar]
- 4.Reid AH, Fanning TG, Hultin JV, Taubenberger JK. Origin and evolution of the 1918 “Spanish” influenza virus hemagglutinin gene. Proc Natl Acad Sci U S A. 1999;96:1651–6. doi: 10.1073/pnas.96.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neumann G, et al. Generation of influenza A viruses entirely from cloned cDNA. Proc Natl Acad Sci U S A. 1999;96:9345–50. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tumpey TM, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 7.Kash JC, et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006;443:578–581. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong MD, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobasa D, et al. Enhanced virulence of influenza A viruses with the haemagglutinin of the 1918 pandemic virus. Nature. 2004;431:703–707. doi: 10.1038/nature02951. [DOI] [PubMed] [Google Scholar]
- 10.Tumpey TM, et al. Existing antivirals are effective against influenza viruses with genes from the 1918 pandemic virus. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13849–13854. doi: 10.1073/pnas.212519699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tumpey TM, et al. Pathogenicity and immunogenicity of influenza viruses with genes from the 1918 pandemic virus. Proc. Natl. Acad. Sci. U. S. A. 2004;101:3166–3171. doi: 10.1073/pnas.0308391100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe T, et al. Viral RNA polymerase complex promotes optimal growth of 1918 virus in the lower respiratory tract of ferrets. Proc. Natl. Acad. Sci U. S. A. 2009;106:588–592. doi: 10.1073/pnas.0806959106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Hoeven N, et al. Human HA and polymerase subunit PB2 proteins confer transmission of an avian influenza virus through the air. Proc. Natl. Acad. Sci U. S. A. 2009;106:3366–3371. doi: 10.1073/pnas.0813172106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geiss GK, et al. Cellular transcriptional profiling in influenza A virus-infected lung epithelial cells: the role of the nonstructural NS1 protein in the evasion of the host innate defense and its potential contribution to pandemic influenza. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10736–10741. doi: 10.1073/pnas.112338099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McAuley JL, et al. Expression of the 1918 influenza A virus PB1-F2 enhances the pathogenesis of viral and secondary bacterial pneumonia. Cell Host. Microbe. 2007;2:240–249. doi: 10.1016/j.chom.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakajima K, Desselberger U, Palese P. Recent human influenza A (H1N1) viruses are closely related genetically to strains isolated in 1950. Nature. 1978;274:334–339. doi: 10.1038/274334a0. [DOI] [PubMed] [Google Scholar]
- 17.Subbarao K, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–6. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 18.Claas EC, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–7. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 19.Smith GJ, et al. Emergence and predominance of an H5N1 influenza variant in China. Proc. Natl. Acad. Sci U. S. A. 2006;103:16936–16941. doi: 10.1073/pnas.0608157103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: Implications for pandemic control. Proc. Natl. Acad. Sci. U. S. A. 2006 doi: 10.1073/pnas.0511120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan Y, et al. Emergence of multiple genotypes of H5N1 avian influenza viruses in Hong Kong SAR. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8950–8955. doi: 10.1073/pnas.132268999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li KS, et al. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–213. doi: 10.1038/nature02746. [DOI] [PubMed] [Google Scholar]
- 23.Ducatez MF, et al. Avian flu: multiple introductions of H5N1 in Nigeria. Nature. 2006;442:37. doi: 10.1038/442037a. [DOI] [PubMed] [Google Scholar]
- 24.Tran TH, et al. Avian influenza A (H5N1) in 10 patients in Vietnam. N. Engl. J. Med. 2004;350:1179–1188. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 25.Beigel JH, et al. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 26.Chotpitayasunondh T, et al. Human disease from influenza A (H5N1), Thailand, 2004. Emerg Infect Dis. 2005;11:201–209. doi: 10.3201/eid1102.041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peiris JS, et al. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363:617–619. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.To KF, et al. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63:242–6. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 29.Chan MC, et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung CY, et al. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- 31.Fraser C, et al. Pandemic Potential of a Strain of Influenza A (H1N1): Early Findings. Science. 2009 May 14; doi: 10.1126/science.1176062. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novel Swine-Origin Influenza A (H1N1) Investigation Team Emergence of a Novel Swine-Origin Influenza A (H1N1) Virus in Humans. N. Engl. J Med. 2009 May 7; Epub ahead of print. [Google Scholar]
- 33.Rogers GN, Paulson JC. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 34.Ito T, et al. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol. 1998;72:7367–73. doi: 10.1128/jvi.72.9.7367-7373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shinya K, et al. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 37.van Riel D, et al. H5N1 Virus Attachment to Lower Respiratory Tract. Science. 2006;312:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- 38.Nicholls JM, et al. Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nat. Med. 2007;13:147–149. doi: 10.1038/nm1529. [DOI] [PubMed] [Google Scholar]
- 39.Stevens J, et al. Structure of the uncleaved human H1 hemagglutinin from the extinct 1918 influenza virus. Science. 2004;303:1866–1870. doi: 10.1126/science.1093373. [DOI] [PubMed] [Google Scholar]
- 40.Tumpey TM, et al. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science. 2007;315:655–659. doi: 10.1126/science.1136212. [DOI] [PubMed] [Google Scholar]
- 41.Gambaryan A, et al. Evolution of the receptor binding phenotype of influenza A (H5) viruses. Virology. 2006;344:432–438. doi: 10.1016/j.virol.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 42.Yamada S, et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature. 2006;444:378–382. doi: 10.1038/nature05264. [DOI] [PubMed] [Google Scholar]
- 43.Auewarakul P, et al. An avian influenza H5N1 virus that binds to a human-type receptor. J Virol. 2007;81:9950–9955. doi: 10.1128/JVI.00468-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevens J, et al. Structure and Receptor Specificity of the Hemagglutinin from an H5N1 Influenza Virus. Science. 2006;312:404–410. doi: 10.1126/science.1124513. [DOI] [PubMed] [Google Scholar]
- 45.Kawaoka Y, Webster RG. Sequence requirements for cleavage activation of influenza virus hemagglutinin expressed in mammalian cells. Proc Natl Acad Sci U S A. 1988;85:324–8. doi: 10.1073/pnas.85.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subbarao EK, London W, Murphy BR. A single amino acid in the PB2 gene of influenza A virus is a determinant of host range. J. Virol. 1993;67:1761–1764. doi: 10.1128/jvi.67.4.1761-1764.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hatta M, Gao P, Halfmann P, Kawaoka Y. Molecular Basis for High Virulence of Hong Kong H5N1 Influenza A Viruses. Science. 2001;293:1840–1842. doi: 10.1126/science.1062882. [DOI] [PubMed] [Google Scholar]
- 48.Mehle A, Doudna JA. An inhibitory activity in human cells restricts the function of an avian-like influenza virus polymerase. Cell Host. Microbe. 2008;4:111–122. doi: 10.1016/j.chom.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rameix-Welti MA, Tomoiu A, Dos Santos AE, van der WS, Naffakh N. Avian Influenza A virus polymerase association with nucleoprotein, but not polymerase assembly, is impaired in human cells during the course of infection. J Virol. 2009;83:1320–1331. doi: 10.1128/JVI.00977-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hatta M, et al. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS. Pathog. 2007;3:1374–1379. doi: 10.1371/journal.ppat.0030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Massin P, van der Werf S, Naffakh N. Residue 627 of PB2 is a determinant of cold sensitivity in RNA replication of avian influenza viruses. J Virol. 2001;75:5398–404. doi: 10.1128/JVI.75.11.5398-5404.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steel J, Lowen AC, Mubareka S, Palese P. Transmission of influenza virus in a mammalian host is increased by PB2 amino acids 627K or 627E/701N. PLoS. Pathog. 2009;5:e1000252. doi: 10.1371/journal.ppat.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Z, et al. Molecular basis of replication of duck H5N1 influenza viruses in a mammalian mouse model. J Virol. 2005;79:12058–12064. doi: 10.1128/JVI.79.18.12058-12064.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gabriel G, et al. Differential polymerase activity in avian and mammalian cells determines host range of influenza virus. J Virol. 2007;81:9601–9604. doi: 10.1128/JVI.00666-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gabriel G, Herwig A, Klenk HD. Interaction of polymerase subunit PB2 and NP with importin alpha1 is a determinant of host range of influenza A virus. PLoS. Pathog. 2008;4:e11. doi: 10.1371/journal.ppat.0040011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salomon R, et al. The polymerase complex genes contribute to the high virulence of the human H5N1 influenza virus isolate A/Vietnam/1203/04. J Exp. Med. 2006;203:689–697. doi: 10.1084/jem.20051938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tarendeau F, et al. Host determinant residue lysine 627 lies on the surface of a discrete, folded domain of influenza virus polymerase PB2 subunit. PLoS. Pathog. 2008;4:e1000136. doi: 10.1371/journal.ppat.1000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuzuhara T, et al. Structural basis of the influenza A virus RNA polymerase PB2 RNA-binding domain containing the pathogenicity-determinant lysine 627 residue. J Biol Chem. 2009;284:6855–6860. doi: 10.1074/jbc.C800224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Sastre A. Inhibition of interferon-mediated antiviral responses by influenza A viruses and other negative-strand RNA viruses. Virology. 2001;279:375–84. doi: 10.1006/viro.2000.0756. [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Sastre A, et al. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology. 1998;252:324–30. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 61.Pichlmair A, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 62.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 63.Lund JM, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imai Y, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seo SH, Hoffmann E, Webster RG. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat. Med. 2002;8:950–954. doi: 10.1038/nm757. [DOI] [PubMed] [Google Scholar]
- 66.Guan Y, et al. H5N1 influenza: a protean pandemic threat. Proc. Natl. Acad. Sci U. S. A. 2004;101:8156–8161. doi: 10.1073/pnas.0402443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiao P, et al. A single-amino-acid substitution in the NS1 protein changes the pathogenicity of H5N1 avian influenza viruses in mice. J Virol. 2008;82:1146–1154. doi: 10.1128/JVI.01698-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li Z, et al. The NS1 gene contributes to the virulence of H5N1 avian influenza viruses. J Virol. 2006;80:11115–11123. doi: 10.1128/JVI.00993-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Obenauer JC, et al. Large-Scale Sequence Analysis of Avian Influenza Isolates. Science. 2006;311:1576–1580. doi: 10.1126/science.1121586. [DOI] [PubMed] [Google Scholar]
- 70.Jackson D, Hossain MJ, Hickman D, Perez DR, Lamb RA. A new influenza virus virulence determinant: the NS1 protein four C-terminal residues modulate pathogenicity. Proc. Natl. Acad. Sci U. S. A. 2008;105:4381–4386. doi: 10.1073/pnas.0800482105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen W, et al. A novel influenza A virus mitochondrial protein that induces cell death. Nat Med. 2001;7:1306–12. doi: 10.1038/nm1201-1306. [DOI] [PubMed] [Google Scholar]
- 72.Zamarin D, Garcia-Sastre A, Xiao X, Wang R, Palese P. Influenza virus PB1-F2 protein induces cell death through mitochondrial ANT3 and VDAC1. PLoS. Pathog. 2005;1:e4. doi: 10.1371/journal.ppat.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mazur I, et al. The proapoptotic influenza A virus protein PB1-F2 regulates viral polymerase activity by interaction with the PB1 protein. Cell Microbiol. 2008;10:1140–1152. doi: 10.1111/j.1462-5822.2008.01116.x. [DOI] [PubMed] [Google Scholar]
- 74.Conenello GM, Zamarin D, Perrone LA, Tumpey T, Palese P. A single mutation in the PB1-F2 of H5N1 (HK/97) and 1918 influenza A viruses contributes to increased virulence. PLoS. Pathog. 2007;3:1414–1421. doi: 10.1371/journal.ppat.0030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kiso M, et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004;364:759–765. doi: 10.1016/S0140-6736(04)16934-1. [DOI] [PubMed] [Google Scholar]
- 76.Poland GA, Jacobson RM, Ovsyannikova IG. Influenza virus resistance to antiviral agents: a plea for rational use. Clin Infect Dis. 2009;48:1254–1256. doi: 10.1086/598989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Le QM, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437:1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- 78.de Jong MD, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N. Engl. J. Med. 2005;353:2667–2672. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- 79.Weinstock DM, Gubareva LV, Zuccotti G. Prolonged shedding of multidrug-resistant influenza A virus in an immunocompromised patient. N. Engl. J Med. 2003;348:867–868. doi: 10.1056/NEJM200302273480923. [DOI] [PubMed] [Google Scholar]
- 80.Baz M, Abed Y, McDonald J, Boivin G. Characterization of multidrug-resistant influenza A/H3N2 viruses shed during 1 year by an immunocompromised child. Clin Infect Dis. 2006;43:1555–1561. doi: 10.1086/508777. [DOI] [PubMed] [Google Scholar]
- 81.Ison MG, Gubareva LV, Atmar RL, Treanor J, Hayden FG. Recovery of drug-resistant influenza virus from immunocompromised patients: a case series. J Infect Dis. 2006;193:760–764. doi: 10.1086/500465. [DOI] [PubMed] [Google Scholar]
- 82.Collins PJ, et al. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature. 2008;453:1258–1261. doi: 10.1038/nature06956. [DOI] [PubMed] [Google Scholar]
- 83.Gubareva LV, Matrosovich MN, Brenner MK, Bethell RC, Webster RG. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J. Infect. Dis. 1998;178:1257–1262. doi: 10.1086/314440. [DOI] [PubMed] [Google Scholar]
- 84.Babu YS, et al. BCX-1812 (RWJ-270201): discovery of a novel, highly potent, orally active, and selective influenza neuraminidase inhibitor through structure-based drug design. J Med Chem. 2000;43:3482–3486. doi: 10.1021/jm0002679. [DOI] [PubMed] [Google Scholar]
- 85.Yamashita M, et al. CS-8958, a prodrug of the new neuraminidase inhibitor R-125489, shows long-acting anti-influenza virus activity. Antimicrob. Agents Chemother. 2009;53:186–192. doi: 10.1128/AAC.00333-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Furuta Y, et al. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 2002;46:977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sui J, et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 2009;16:265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Belshe RB, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N. Engl. J Med. 2007;356:685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 89.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J Med. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 90.Bresson JL, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367:1657–1664. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 91.Lin J, et al. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet. 2006;368:991–997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 92.Bernstein DI, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis. 2008;197:667–675. doi: 10.1086/527489. [DOI] [PubMed] [Google Scholar]
- 93.Stephenson I, et al. Antigenically distinct MF59-adjuvanted vaccine to boost immunity to H5N1. N. Engl. J Med. 2008;359:1631–1633. doi: 10.1056/NEJMc0805274. [DOI] [PubMed] [Google Scholar]
- 94.Levie K, et al. An adjuvanted, low-dose, pandemic influenza A (H5N1) vaccine candidate is safe, immunogenic, and induces cross-reactive immune responses in healthy adults. J Infect Dis. 2008;198:642–649. doi: 10.1086/590913. [DOI] [PubMed] [Google Scholar]
- 95.Suguitan AL, Jr., et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS. Med. 2006;3:e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fan S, et al. Immunogenicity and protective efficacy of a live attenuated H5N1 vaccine in nonhuman primates. PLoS. Pathog. 2009;5:e1000409. doi: 10.1371/journal.ppat.1000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schotsaert M, De FM, Fiers W, Saelens X. Universal M2 ectodomain-based influenza A vaccines: preclinical and clinical developments. Expert. Rev Vaccines. 2009;8:499–508. doi: 10.1586/erv.09.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mahmood K, et al. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine. 2008;26:5393–5399. doi: 10.1016/j.vaccine.2008.07.084. [DOI] [PubMed] [Google Scholar]
- 99.Gao W, et al. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J Virol. 2006;80:1959–1964. doi: 10.1128/JVI.80.4.1959-1964.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hoelscher MA, et al. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet. 2006;367:475–481. doi: 10.1016/S0140-6736(06)68076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]