Increased Microparticle Tissue Factor Activity in Cancer Patients with Venous Thromboembolism (original) (raw)
. Author manuscript; available in PMC: 2011 Jun 1.
Venous thromboembolism (VTE) is the second leading cause of cancer-associated mortality (1). However, cancer patients, particularly in the ambulatory setting, do not routinely receive thromboprophylaxis. This is because there are no validated biomarkers to identify patients at high risk of VTE. A number of candidate biomarkers of thrombotic risk have been proposed, including D-dimer, soluble P-selectin, C-reactive protein, and tissue factor (TF) (2–5). TF is the transmembrane receptor for factor VII/VIIa and functions as the primary initiator of blood coagulation (6). Patients with various diseases have elevated levels of TF in their plasma in the form of membrane-associated microparticles (MPs) as well as a soluble form (7, 8). MPs are submicron membrane vesicles generated from activated or apoptotic cells. Their procoagulant activity is increased by the presence of TF and the anionic phospholipid phosphatidylserine.
TF-positive MPs are highly procoagulant and they have been linked to thrombosis in a variety of diseases, such as cancer, sickle cell disease, and endotoxemia (9, 10). Several studies have measured the levels of MP TF antigen and activity in cancer patients (11–13). There have been two retrospective studies investigating MP TF activity in cancer patients. Tesselaar and colleagues found increased levels of MP TF activity compared with controls in pancreatic and breast adenocarcinoma patients. In addition, Hron and colleagues reported a two-fold higher level of TF-positive MPs in patients with advanced colorectal cancer compared to controls (11, 12). A prospective study by Khorana and colleagues reported that MP TF activity may be predictive of VTE in patients with pancreatic cancer (13).
In this study, we analyzed MP TF activity in patients with a variety of different cancers with or without acute VTE. We hypothesized that increased MP TF activity would be present in patients with VTE, irrespective of the type of cancer. Cancer patients (n=66) were recruited for this study at the University of Southern California in Los Angeles. Patients were enrolled in an ongoing IRB approved treatment trial for the management of malignancy-related VTE. Blood was drawn from cancer patients without VTE (n = 13) and from cancer patients with VTE within 24 hours of diagnosis (n = 53). All patients had to have either deep vein thrombosis confirmed by compression ultrasound and/or pulmonary embolism confirmed by computed tomography (CT) angiography utilizing a 16 slice-multi-detector CT. The distribution of cancer types in the 66 patients recruited for this study were as follows: 14 colon (10 VTE), 10 lung (7 VTE), 6 bladder (6 VTE), 5 pancreatic (3 VTE), 3 prostate (3 VTE), 3 rectal (2 VTE), 2 bile duct (2 VTE), 2 brain (2 VTE), 2 cholangio (2 VTE), 2 liver (2 VTE), 2 lymphoma (2 VTE), 2 renal cell (1 VTE), 2 testis (2 VTE), and 11 other types of cancer (9 VTE). All 66 subjects who participated in this study gave informed consent.
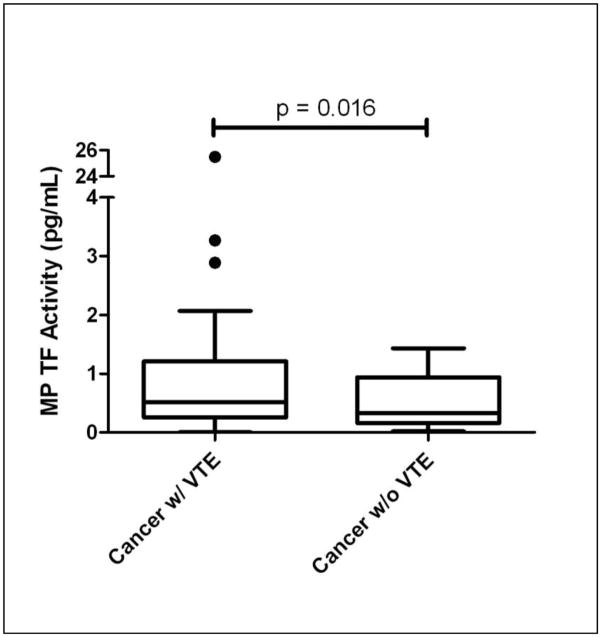
We have recently developed an assay to measure levels of TF activity on MPs isolated from plasma (13). Importantly, healthy individuals have very low levels of MP TF activity (0.21 ± 0.11 pg/mL) (13). In this study, we found a statistically significant increase in MP TF activity in cancer patients with VTE compared to cancer patients without VTE (1.7 ± 3.8 pg/mL vs. 0.5 ± 0.5 pg/mL, p < 0.05, Figure). Data is represented as mean ± standard deviation and statistical analysis was performed using a heteroscedastic Student’s T-test. After the completion of our study, Tesselaar and colleagues (14) reported an increase in MP TF activity in cancer patients with VTE (n=51) compared with cancer patients without VTE (n=49). The two groups were matched for age, sex, type of cancer, stage of disease and type of cancer treatment.
Figure.
Comparison of MP TF activity between cancer patients with VTE (n=53) and cancer patients without VTE (n=13) (Median, Inter-quartile Range, 2 Standard Deviations). Dots represent MP TF activity levels beyond 2 standard deviations.
Next, we compared levels of MP TF activity in pancreatic, lung, and colon cancer patients. One limitation of the study is that we had relatively small numbers in the three groups. Pancreatic cancer patients had the highest MP TF activity (6.6 ± 10.8 pg/mL), followed by lung (2.4 ± 2.5 pg/mL) and colon cancer (0.7 ± 0.8 pg/mL). This finding is consistent with the respective thrombosis rates in these types of cancer; it has been reported that 28.3% of pancreatic cancer patients develop VTE within a year of metastatic malignancy, compared to 7.4% for lung, and 5.7% for colon (15).
Plasma D-dimer and interleukin-6 (IL-6) levels were measured using commercial enzyme-linked immunoassays (IMUCLONE D-Dimer ELISA, American Diagnostica, Stamford, CT.; Human IL-6 Quantikine HS ELISA, R & D Systems Inc, Minneapolis, MN.). Cancer patients with VTE had a significant increase in D-dimer levels compared to cancer patients without VTE (4500 ± 6200 ng/mL vs. 670 ± 1800 ng/mL, p < 0.05). A recent study showed that elevated levels of D-dimer predict VTE in patients with cancer (16). IL-6 levels were also significantly elevated in cancer patients with VTE compared to those without VTE (9.1 ± 4.9 pg/mL vs. 6.6 ± 5.3 pg/mL, p < 0.05). Recent studies indicate a link between inflammation and cancer development (17). Inflammation may also induce TF expression within the vasculature and this would increase the risk of thrombosis in cancer patients. Interestingly, there was only a weak correlation between D-dimer and IL-6 compared to MP TF activity (r = 0.145 and r = 0.283, respectively). Tesselaar and colleagues (14) also found a weak correlation between MP TF activity and levels of thrombin-antithrombin complex in cancer patients.
The results of this study add to the growing body of evidence that MP TF activity may play an important role in the development of VTE in cancer patients. Our data show that MP TF activity is significantly increased in a broad population of cancer patients with acute VTE compared to cancer patients without VTE. However, as this was a cross sectional study, we are unable to conclude that MP TF activity can be used a biomarker of risk in these patients. Prospective studies are underway to test the predictive power of MP TF activity as a novel candidate biomarker for assessing the risk of VTE in cancer patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. Thromb Haemost. 2007;5(3):632–4. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 2.Kroger K, Weiland D, Ose C, et al. Risk factors for venous thromboembolic events in cancer patients. Ann Oncol. 2006 Feb;17(2):297–303. doi: 10.1093/annonc/mdj068. [DOI] [PubMed] [Google Scholar]
- 3.Ay C, Simanek R, Vormittag R, et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS) Blood. 2008 Oct 1;112(7):2703–8. doi: 10.1182/blood-2008-02-142422. [DOI] [PubMed] [Google Scholar]
- 4.Cosmi B, Legnani C, Cini M, et al. The role of D-dimer and residual venous obstruction in recurrence of venous thromboembolism after anticoagulation withdrawal in cancer patients. Haematologica. 2005 May;90(5):713–5. [PubMed] [Google Scholar]
- 5.Uno K, Homma S, Satoh T, et al. Tissue factor expression as a possible determinant of thromboembolism in ovarian cancer. Br J Cancer. 2007 Jan 29;96(2):290–5. doi: 10.1038/sj.bjc.6603552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:1687–93. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 7.Bogdanov VY, Balasubramanian V, Hathcock J, et al. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nature Med. 2003;9(4):458–62. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 8.Morel O, Toti F, Hugel B, et al. Procoagulant microparticles: disrupting the vascular homeostasis equation? Arterioscler Thromb Vasc Biol. 2006;26(12):2594–604. doi: 10.1161/01.ATV.0000246775.14471.26. [DOI] [PubMed] [Google Scholar]
- 9.Shet AS, Aras O, Gupta K, et al. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102(7):2678–83. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- 10.Aras O, Shet A, Bach RR, et al. Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood. 2004;103:4545–53. doi: 10.1182/blood-2003-03-0713. [DOI] [PubMed] [Google Scholar]
- 11.Hron G, Kollars M, Weber H, et al. Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost. 2007;97(1):119–23. [PubMed] [Google Scholar]
- 12.Tesselaar ME, Romijn FP, van dLI, et al. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007 Mar;5(3):520–7. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 13.Khorana AA, Francis CW, Menzies KE, et al. Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008 Nov;6(11):1983–5. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tesselaar MET, Romijn FP, Van Der Linden IK, et al. Microparticle-associated tissue factor activity in cancer patients with or without thrombosis. J Throm Haemost. 2009;7:1421–1423. doi: 10.1111/j.1538-7836.2009.03504.x. [DOI] [PubMed] [Google Scholar]
- 15.White RH, Chew H, Wun T. Targeting patients for anticoagulant prophylaxis trials in patients with cancer: who is at highest risk? Thromb Res. 2007;120 (Suppl 2):S29–40. doi: 10.1016/S0049-3848(07)70128-7. [DOI] [PubMed] [Google Scholar]
- 16.Ay C, Vormittag R, Dunkler D, et al. D-dimer and prothrombin fragment 1+2 predict venous thromboembolism in patients with cancer: results from the Vienna cancer and thrombosis study. J Clin Oncol. 2009;27:4124–4129. doi: 10.1200/JCO.2008.21.7752. [DOI] [PubMed] [Google Scholar]
- 17.Berasain C, Castillo J, Perugorria MJ, et al. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–221. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]