Barrett’s oesophagus and oesophageal adenocarcinoma: time for a new synthesis (original) (raw)
. Author manuscript; available in PMC: 2011 Feb 1.
Published in final edited form as: Nat Rev Cancer. 2010 Feb;10(2):87–101. doi: 10.1038/nrc2773
Abstract
The public health importance of Barrett’s oesophagus lies in its association with oesophageal adenocarcinoma. The incidence of oesophageal adenocarcinoma has risen at an alarming rate over the past four decades in many regions of the Western world and there are indications that the incidence of this disease is on the rise in Asian populations where it has been rare. Much has been learned of host and environmental risk factors that affect the incidence of oesophageal adenocarcinoma and data indicate that patients with Barrett’s oesophagus rarely develop oesophageal adenocarcinoma. Given that 95% of oesophageal adenocarcinoma arise in individuals without a prior diagnosis of Barrett’s oesophagus, what strategies can be used to reduce late diagnosis of oesophageal adenocarcinoma?
Barrett’s oesophagus has been defined as a condition in which the normal stratified squamous epithelium of the esophagus is replaced by metaplastic columnar epithelium, although no universally accepted definition currently exists1,2. The columnar-lined esophagus was described by Norman Barrett in 19503, reported to be associated with gastroesophageal reflux disease in 19534 and convincingly linked with oesophageal adenocarcinoma in 19755. Unless detected early oesophageal adenocarcinoma is a lethal cancer with mortality greater than 85% and for the past four decades its incidence has been increasing at an alarming rate in many regions of the Western world6. The paradigm is that Barrett’s oesophagus arises as a complication of symptomatic gastroesophageal reflux disease and predisposes to oesophageal adenocarcinoma.
Treatment of Barrett’s oesophagus has been based on this paradigm. Clinical guidelines initially endorsed endoscopic screening of individuals with symptomatic gastroesophageal reflux disease for Barrett’s oesophagus and endoscopic biopsy surveillance of Barrett’s oesophagus7,8. Increased endoscopic detection and surveillance of Barrett’s oesophagus have provided valuable insights into the natural history of this condition, and research has identified challenges to reducing the incidence and mortality of oesophageal adenocarcinoma when clinical decisions are made based on this paradigm. Here, we examine new data on the epidemiology of Barrett’s oesophagus and oesophageal adenocarcinoma, the global distribution of these conditions, the biology of [G]oesophageal specialized intestinal metaplasia, and somatic genomic alterations and evolutionary dynamics that predispose to oesophageal adenocarcinoma. A synthesis of these population, clinical, computational and laboratory advances can guide future research for prevention and early detection of oesophageal adenocarcinoma.
Barrett’s specialized intestinal metaplasia
The columnar epithelium of Barrett’s oesophagus has a crypt architecture similar to that of the intestine, and it has been described as a specialized intestinal metaplasia1,2 (Figure 1). Recently it has been proposed that Barrett’s specialized intestinal metaplasia represents a successful adaptation to the harsh intraesophageal environment of chronic gastroesophageal reflux disease because it has acquired a number of functions not present in the normal oesophageal squamous epithelium9. Several studies are consistent with this hypothesis and indicate that the intestinal metaplasia is a well differentiated epithelium with a number of acquired functions that participate in mucosal defence (Figure 1)10-15.
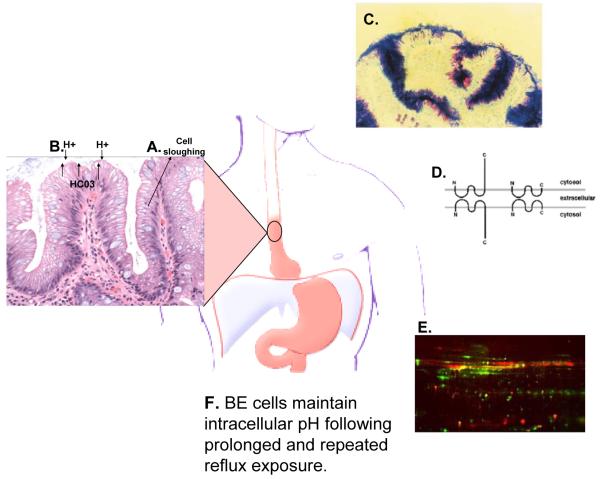
Figure 1. Barrett’s specialized intestinal metaplasia and mucosal defence.
(A) Specialized intestinal metaplasia is a well differentiated epithelium with crypt architecture in which putative stem cells residing at the base give rise to proliferating transient amplifying cells and differentiated cells that are sloughed into the lumen. This architecture has been proposed to be tumor suppressive because mutations occurring in transient amplifying or differentiated non-stem cells would be shed from the body before they could accumulate the serial mutations leading to cancer10. (B) The intestinal metaplasia also secretes anions, including bicarbonate, at levels more than fivefold greater than oesophageal squamous epithelium11. (C) Specialized intestinal metaplasia also secretes thick adherent mucus not present in normal squamous oesophageal cells12. Ultrastructural studies have shown that mucus secretion can be disrupted in Barrett’s oesophagus at increased risk of progression to oesophageal adenocarcinoma, including those with evidence of chromosomal instability and aneuploidy16. (D) Barrett’s oesophagus has claudin-18 tight junctions that provide greater protection against acid permeation than the claudin-18 deficient tight junctions of the oesophageal squamous epithelium13. (E) Barrett’s oesophagus also overexpresses genes involved in mucosal defence and repair14, and (F) Barrett’s oesophageal cells maintain physiologic intracellular pH following prolonged and repeated reflux exposure15. Abbreviation: Barrett’s oesophagus (BE).
The natural history of Barrett’s oesophagus
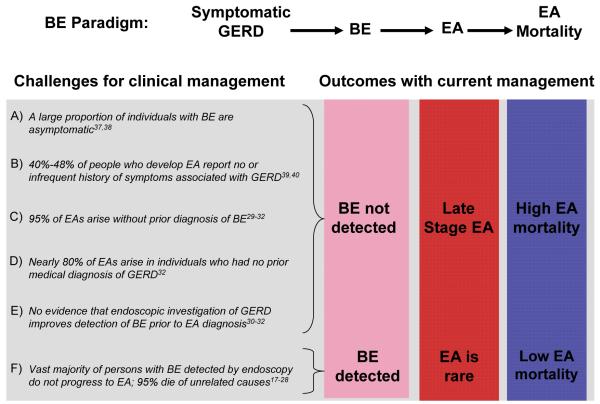
Results from surveillance cohorts indicate that the majority of individuals with Barrett’s oesophagus do not develop oesophageal adenocarcinoma during endoscopic follow up17-22. Meta-analyses estimate the incidence of oesophageal adenocarcinoma among individuals with Barrett’s oesophagus to be 6-7/1000 [G]person-years23,24, and oesophageal adenocarcinoma is an uncommon cause of death in persons with Barrett’s oesophagus25-28. Further, despite endoscopic detection and surveillance of Barrett’s oesophagus, the vast majority of oesophageal adenocarcinomas arise in patients who have no prior diagnosis of Barrett’s oesophagus29-32. Thus, the paradox of current clinical management of Barrett’s oesophagus – underdiagnosis of life threatening early disease, and [G]overdiagnosis of early benign changes that will not affect the lifespan of the individual (Figure 2) – is similar to many other premalignant or malignant diagnoses that follow indolent courses, including those of the prostate, lung, thyroid, breast and kidney33-36.
Figure 2. The paradox of Barrett’s oesophagus.
Recent research has identified multiple factors that contribute to underdiagnosis of life threatening early oesophageal adenocarcinoma (A-E) and overdiagnosis of benign Barrett’s oesophagus that will follow an indolent course for the lifetime of the individual (F). Abbreviations: Gastroesophageal reflux disease (GERD), Barrett’s oesophagus (BE), oesophageal adenocarcinoma (EA).
Epidemiology and etiology
Oesophageal adenocarcinoma
The ultimate public health importance of Barrett’s oesophagus lies in its association with oesophageal adenocarcinoma, a cancer whose incidence has risen substantially in the US, Western Europe, Australia, and in other developed countries over the past four decades, with little sign of abating6,41,42. There is disquieting evidence for an increasing incidence of oesophageal adenocarcinoma in some Asian populations, such as those residing in Singapore43, Japan44 and Iran45, where the disease has previously been uncommon, although this trend is not evident in other countries46,47. In the US, incidence is highest in Caucasian men where it is about eight times greater than Caucasian women and five times greater than African-American men. However, substantial increases have been recorded for every group, with the result that in the US oesophageal adenocarcinoma became the most common histological type of oesophageal cancer in the late 1990s6. Mortality remains high, and most with oesophageal adenocarcinoma survive less than one year after diagnosis48.
Much has been learned about the etiology of oesophageal adenocarcinoma from epidemiological studies over the past two decades. Symptomatic gastroesophageal reflux disease is the strongest and best understood risk factor. The largest population-based case-control studies have all observed four-fold or higher reported relative risks for those with the most frequent symptoms39,40,49-51. It is important to note, however, that symptomatic gastroesophageal reflux disease is infrequent or absent in 40% - 48% of persons who develop oesophageal adenocarcinoma39,40.
Obesity, as measured by body mass index (BMI), also clearly increases risk of oesophageal adenocarcinoma. This has been observed in both case-control and cohort studies6,49-60. Two recent meta-analyses have estimated relative risks for developing cancer of between 2.4 and 2.8 for those with BMI>30 kg/m2 (obese) and between 1.5 and 1.8 for those considered overweight (BMI=25.0-29.9 kg/m2)61,62. The importance of this relationship is magnified by the alarming increase in obesity observed in many developed countries63. For example, based on 2003-2004 National Health And Nutrition Examination Survey (NHANES) data, over 32% of adults in the US are obese, along with 17% of children and adolescents64. These figures represent substantial increases over a six-year period. Similar prevalence and trends in obesity have been observed in Australia and elsewhere65. Cancer incidence modeling has confirmed the importance of [G]period effects in the epidemiology of oesophageal adenocarcinoma and suggests that they are consistent with obesity trends66. Preliminary evidence suggests a pattern of interaction between gastroesophageal reflux disease and obesity, such that obese people with frequent symptoms of gastroesophageal reflux disease had substantially higher oesophageal adenocarcinoma risk (odds ratio (OR)=16.5, 95% CI=8.9-30.6) than people with obesity but no reflux (OR=2.2, 95% CI=1.1-4.3) or reflux but no obesity (OR=5.6, 95% CI=2.8-11.3) compared to people with healthy BMI and no reflux symptoms)49.
Additional but more modest risk factors for oesophageal adenocarcinoma include cigarette smoking, which approximately doubles oesophageal adenocarcinoma risk49,50,52,60,67, and a diet low in fruits and vegetables50,68-70. Alcohol does not appear to have an important role in oesophageal adenocarcinoma71,72. Infection with H. pylori has been linked with reduced oesophageal adenocarcinoma risk in many studies73-75; the underlying mechanisms are not clear, although reduction in acid reflux in association with gastric atrophy has been suggested to have a role76.
Based on data from a large multi-center U.S. study, it is estimated that the four major risk factors – obesity (as measured by BMI), cigarette smoking, gastroesophageal reflux disease and diet low in fruits and vegetables – individually account for 41%, 40%, 30% and 15% of cases in the US population, respectively, and collectively account for 79% (95% CI=66-87%) of cases77.
Barrett’s oesophagus
In contrast to oesophageal adenocarcinoma, the incidence and prevalence of Barrett’s oesophagus are not known with precision. Probably the most accurate population estimates of the prevalence of Barrett’s oesophagus in developed countries come from a random sample of 3,000 adults in two communities in Sweden who underwent endoscopy with biopsy: Barrett’s oesophagus was detected in 1.6%37. Importantly, the prevalence of Barrett’s oesophagus among persons reporting reflux symptoms (2.3%) was only modestly and non-significantly greater than those without such symptoms (1.2%). Remarkably similar findings were reported from an endoscopic study of 1,033 adults from two Italian villages, in whom 1.3% were found to have Barrett’s oesophagus38. Again, reflux symptoms were a poor predictor of Barrett’s oesophagus, as 46.2% of Barrett’s oesophagus cases did not report such symptoms.
Even in countries in which increases in oesophageal adenocarcinoma incidence have not (yet) been documented, such as Korea, it appears that Barrett’s oesophagus may be increasingly common78. For example, among 992 consecutive upper endoscopies at four university hospitals in Korea, 3.6% of individuals had histologically-proven Barrett’s oesophagus78. Prevalence of risk factors for Barrett’s oesophagus, such as gastroesophageal reflux disease and obesity, also appear to be increasing in some Asian countries43,79,80.
Further understanding of obesity’s effects on oesophageal adenocarcinoma must rely largely on studies of precursors, such as Barrett’s oesophagus, as cancer case-control studies and retrospective cohort studies typically are unable to accurately assess characteristics such as percent body fat and fat deposition. A cross-sectional analysis of baseline data from a cohort study of Barrett’s oesophagus was among the first to suggest that location of fat deposition was more important than weight in predicting risk81. Recent results from case-control studies of incident Barrett’s oesophagus strongly support the concept that abdominal adiposity, rather than BMI, may be the defining characteristic which places persons at increased risk of Barrett’s oesophagus, and presumably oesophageal adenocarcinoma82,83. For example, in a community-clinic-based case-control study of persons with incident Barrett’s oesophagus compared to a matched sample from the general population, persons in high categories of waist-to-hip ratio (0.90 or greater for men, 0.85 or greater for women) experienced a 4.1-fold increase in risk (95%CI = 1.7-10.0; [G]p-trend=0.003), whereas no increase was observed for increasing BMI after mutual adjustment. Similar observations were reported from a population-based case-control study of Barrett’s oesophagus83 and a case-control study nested in a large cohort in which abdominal diameter data were available59. Supportive findings were observed in a small clinical study (n=36 cases), in which visceral fat was assessed using CT scans; in models that included data for both visceral fat levels and BMI, visceral fat levels explained most of the association with risk of Barrett’s oesophagus84. As overweight men tend to have more visceral fat than overweight women, these studies suggest a possible explanation for the marked preponderance of men with oesophageal adenocarcinoma and Barrett’s oesophagus.
It has been hypothesized that abdominal obesity may increase risk of Barrett’s oesophagus and oesophageal adenocarcinoma primarily by promoting reflux via increasing intragastric pressure85. However, direct evidence for this pathway is surprisingly weak. For example, a cross-sectional hospital study using [G]manometry observed a correlation coefficient of only 0.11 (p=0.05) relating gastric pressure to BMI or waist circumference86. Other observations suggest moderate correlations between gastroesophageal reflux disease symptoms and BMI in the U.S. but not in Europe87. In one of the first studies investigating possible mediators of the obesity–Barrett’s oesophagus–oesophageal adenocarcinoma relationship, Kendall et al. reported that high serum leptin, a hormone produced by visceral fat which may promote carcinogenesis by mitogenic and angiogenic means, was associated with increased risk of Barrett’s oesophagus, particularly among males88. In addition to altering levels of adipokines such as leptin and adiponectin, obesity can increase concentrations of bioavailable IGF-1 and insulin, growth factors which can directly promote cellular proliferation and reduce apoptosis, as well as affect downstream signaling pathways involved in cell growth and proliferation89.
The strength of the relationship between cigarette smoking and Barrett’s oesophagus is less clear than for oesophageal adenocarcinoma, with most90,91 but not all50,92 studies observing a modest increase in risk among current smokers. Similar to oesophageal adenocarcinoma, risk of Barrett’s oesophagus appears to be moderately decreased with increasing intake of fruits and vegetables93,94.
Chronic inflammation
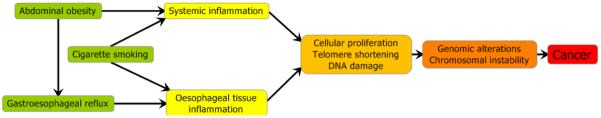
As described in Box 1, one aspect in common among the major risk factors for Barrett’s oesophagus and oesophageal adenocarcinoma is the promotion of chronic inflammation, both in the oesophageal epithelium and systemically. It has been hypothesized that telomere length in leukocytes of individuals with Barrett’s oesophagus might serve as an integrative measure of a person’s long-term history of inflammation and oxidative damage, since factors such as insulin resistance, obesity and smoking have been shown to reduce telomere length95-97. Longitudinal analysis of baseline blood samples in a Barrett’s oesophagus cohort revealed shorter telomere length to be associated with increased risk of progression to oesophageal adenocarcinoma (adjusted hazard ratio comparing extreme quartiles, 3.45, 95% CI=1.35-8.78)95. These observations were replicated in a case-control study that found overall telomere length, as well as 17p and 12q telomere lengths but not 11q and 2p telomere lengths, were associated with increased oesophageal adenocarcinoma risk98. These results suggest the importance of chronic systemic inflammation in the development of Barrett’s oesophagus and oesophageal adenocarcinoma and raise the possibility that telomere length may be a useful component to a biomarker panel designed to stratify risk in persons with Barrett’s oesophagus.
Box 1. Inflammation and oesophageal adenocarcinoma.
Chronic inflammation appears to play a central role in the development of oesophageal adenocarcinoma and its precursor lesions. Epidemiologic studies have identified three major risk factors – abdominal obesity (visceral fat), gastroesophageal reflux and cigarette smoking – as key driving forces for this cancer77. The refluxate contains numerous substances in addition to gastric acid, including bile salts, pancreatic enzymes, and ingested foods and their metabolites, which can cause acute and chronic inflammation of the oesophageal epithelium with resulting oxidative stress99-101. Abdominal obesity, in addition to promoting gastroesophageal reflux, is increasingly being recognized as causing a state of low-level systemic inflammation, characterized by increased plasma levels of pro-inflammatory cytokines and receptors, such as IL-6, TNF-alpha and sTNF-alpha receptor 2, C-reactive protein, and leptin63,102. In addition, cigarette smoking can cause inflammation both systemically and within the oesophageal epithelium in response to swallowed smoking products. In turn, a chronic state of systemic and localized inflammation and oxidative stress promotes DNA damage, cellular proliferation and telomere shortening, which can increase the risk of developing clones containing small and large-scale genomic alterations, eventually leading to widespread chromosomal instability and oesophageal adenocarcinoma103,104.
Host susceptibility
A genetic component to the development of gastroesophageal reflux disease, Barrett’s oesophagus and oesophageal adenocarcinoma has long been suspected based on case reports, familial clusters and clinical series105,106. For example, a family from the UK has been described which includes a male index case with oesophageal adenocarcinoma, three brothers with oesophageal adenocarcinoma or high-grade dysplasia in Barrett’s oesophagus, and six children with Barrett’s oesophagus107. Similarly, a three-generation family of 24 in Spain has been described, in which six developed oesophageal adenocarcinoma, four Barrett’s oesophagus and six gastroesophageal reflux disease108. Two well-designed twin studies of gastroesophageal reflux disease also indicated a heritability of 30-40%, lending further support for genetic susceptibility in the oesophageal adenocarcinoma disease process109,110.
Larger studies also suggest a genetic component to oesophageal adenocarcinoma and its precursors111,112. For example, familial Barrett’s oesophagus was confirmed in 7.3% of persons presenting with Barrett’s oesophagus or oesophageal adenocarcinoma113, which is several-fold higher than would be expected based on population surveys37,38. A higher frequency of a positive family history of Barrett’s oesophagus or oesophageal adenocarcinoma among cases with these conditions (24%) compared to gastroesophageal reflux disease cases without Barrett’s oesophagus (5%) has also been observed114. In clinical practice, a complete family history is now recommended for physicians seeing patients with Barrett’s oesophagus and oesophageal adenocarcinoma115, while linkage studies are being undertaken to better understand the inheritance of these conditions115,116.
An increasing number of studies have used a candidate gene approach to identify gene variants in pathways such as DNA repair, xenobiotic metabolism and inflammation that might alter the risk of developing Barrett’s oesophagus or oesophageal adenocarcinoma117-125. For example, a population-based study found that population heterogeneity for alcohol metabolism may have masked an increased risk with increased alcohol intake118. Among drinkers, intermediate metabolizers had a two-fold increase in risk of oesophageal adenocarcinoma and gastric cardia adenocarcinomas, while fast metabolizers (homozygous for variant ADH3) had a four-fold increased risk (OR=4.3; 95%CI=1.1-11.2). In another population-based study, relative risk of oesophageal adenocarcinoma was examined in relation to five single nucleotide polymorphisms in the DNA repair gene, MGMT. Among persons reporting frequent episodes of gastroesophageal reflux disease, a substantially increased relative risk was observed for those homozygous for the minor allele at the intronic locus rs12268840 (OR=15.5, 95%CI=5.8-42), although the association of the variant with altered expression or enzyme activity is unclear122. Another study examined variants in the NAD(P)H:quinone oxidoreductase 1(NQO1) gene, which codes for a detoxifying enzyme of common dietary compounds. Those with the TT genotype were observed to be less common than expected in Barrett’s oesophagus and oesophageal adenocarcinoma cases, yielding a 4.5-fold decreased risk of developing Barrett’s oesophagus (p=0.01) and a 6.2-fold decreased risk of oesophageal adenocarcinoma (p=0.04), and suggesting that the NQO1 TT genotype may offer protection from reflux complications126.
The COX-2 gene is of particular interest as it codes for an inducible form of cyclooxygenase observed to be expressed at increased levels in Barrett’s oesophagus, oesophageal adenocarcinoma, and a number of other cancers and their precursors. Cyclooxygenase has a central role in inflammation and potentially carcinogenesis via production of prostaglandins, which have a number of neoplastic properties127. Variants in the promoter region of the COX-2 gene have been observed to significantly increase risk of oesophageal adenocarcinoma128,129; this is intriguing, given the number of observational studies indicating a preventive effect of NSAIDs in the development of oesophageal adenocarcinoma130-134 (see below). Finally, in a cohort study of Barrett’s oesophagus, bleomycin sensitivity was assessed in baseline peripheral blood lymphocytes. Bleomycin-sensitive patients were at increased risk of developing aneuploid cells (adjusted HR 3.71, 95% CI 1.44-9.53) and non-significantly greater risk of oesophageal adenocarcinoma (adjusted HR 1.63, 95% CI 0.71-3.75)135. Trends for both oesophageal adenocarcinoma (p<0.001) and aneuploidy (p<0.005) were particularly strong among patients with 17p LOH involving TP53.
Together, the above results suggest the importance of taking into account genetic background when evaluating risk and preventive factors in the development of Barrett’s oesophagus and oesophageal adenocarcinoma and vice-versa. However, they all require replication and further functional studies before this information can be used in a clinical setting. Results from ongoing genome-wide association studies of Barrett’s oesophagus and oesophageal adenocarcinoma will likely add new loci of interest for more directed study.
Neoplastic progression in Barrett’s oesophagus
One of the fundamental goals of translational research in Barrett’s oesophagus is to distinguish the small number of individuals who progress to oesophageal adenocarcinoma from the majority who do not. Currently, periodic endoscopic biopsies with histological assessment of dysplasia are used to assess the risk of progression to oesophageal adenocarcinoma in patients with Barrett’s oesophagus. Dysplasia is also frequently used as a surrogate endpoint for oesophageal adenocarcinoma in research studies. However, this approach poses substantial challenges for both patient care and research (Box 2). Formal statistical criteria for evaluating surrogate biomarkers were developed two decades ago136. Although some surrogates with lower standards may be used for intermediate studies or biological pathway analysis137, surrogate markers for studies that intend to contribute to the evidence base for clinical policy need to accurately represent the true endpoint, oesophageal adenocarcinoma. Such markers need to be in key causal pathway(s) to oesophageal adenocarcinoma, have substantial predictive power to distinguish between those who will and will not develop oesophageal adenocarcinoma, and be easily and objectively measured. Since neither high-grade dysplasia nor any other grade of dysplasia in Barrett’s oesophagus has been demonstrated to be a valid surrogate for oesophageal adenocarcinoma, this review will focus on well designed longitudinal studies of neoplastic progression that have a definitive oesophageal adenocarcinoma endpoint.
Box 2. Challenges for histology-guided oesophageal adenocarcinoma risk assessment in individuals with Barrett’s oesophagus.
- Assessment of dysplasia is subjective with substantial observer variation in diagnosis between pathologists138,139.
- Large numbers of biopsies are required to reduce sampling error140,141.
- High-grade dysplasia is highly heterogeneous with regard to progression to oesophageal adenocarcinoma, and rates of progression vary substantially in different studies with reported five-year cumulative incidences of oesophageal adenocarcinoma ranging from less than 10% to 59%18,21.
- Low-grade dysplasia has a low rate of progression to oesophageal adenocarcinoma, non-robust reproducibility and frequently is not detected in subsequent endoscopies17-19,21,139,142-144.
- Reports of increased progression from low-grade dysplasia to high-grade dysplasia as a surrogate endpoint for oesophageal adenocarcinoma145 may be confounded by diagnostic misclassification138,139, sampling141, biological heterogeneity, or combinations of these factors.
- The lack of reproducible diagnostic classification138,139 confounds comparison of results from different centres.
- Use of dysplasia as a surrogate marker for oesophageal adenocarcinoma in molecular or imaging research for improved risk stratification can hardwire the limitations of the dysplasia classification system into the molecular and imaging markers146,147.
- Treatment of surrogate endpoints for oesophageal adenocarcinoma, such as low- or high-grade dysplasia, may not be associated with decreased incidence of advanced oesophageal adenocarcinomas or reduction in mortality148,149.
- Research on quantitative assessment of dysplasia150,151 and consensus interpretations152 is being carried out to improve histological classification, but some results are inconsistent and no studies have yet demonstrated the sensitivity and specificity expected of a practical diagnostic test.
In 1976, Nowell advanced the hypothesis that “Acquired genetic lability permits stepwise selection of variant sublines and underlies tumor progression”153. Data from genomic154-156, transcriptomic157-160 and proteomic161-163 studies have revealed the complexity of changes that develop during neoplastic evolution to oesophageal adenocarcinoma, including genome-wide chromosomal instability, disruption of regulatory pathways, and dynamic clonal evolution (Box 3).
Box 3. Opportunities for risk stratification, prevention and early detection.
The complex patterns of chromosome instability and mutations, combined with disruption of regulatory pathways, clonal evolution and generation of variants create challenges for treatment of advanced oesophageal adenocarcinoma (see the table). Rapidly advancing technology creates opportunities to measure fundamental, widely generalizable biomarkers of progression for risk stratification, early detection and prevention. For example, the presence or rate of chromosome instability can be measured on high-density, genome wide platforms and may be a fundamental biomarker that captures the complexity of neoplastic progression in Barrett’s oesophagus and many other conditions. Assessment of disruption of regulatory pathways at the expression or protein levels could integrate genomic, epigenetic and environmental influences on progression, and expression profiles have received regulatory approval for selection of patients for specific therapies as well as identification of carcinomas of unknown primary origin180,181. Evolutionary measures, including clonal expansion and generation of diversity, may also be fundamental biomarkers of progression that could be applicable to many conditions in addition to Barrett’s oesophagus. The complexity of these abnormalities appears to be lower in premalignant stages of Barrett’s oesophagus than in oesophageal adenocarcinoma, which could facilitate development of diagnostic tests, although it is likely that no single measurement will prove sufficient for cancer control. Early events found in high frequency in Barrett’s oesophagus are unlikely to be useful as biomarkers of risk of progression to oesophageal adenocarcinoma because the natural history of Barrett’s oesophagus indicates that progression to and death from oesophageal adenocarcinoma are rare events. High frequency early events in Barrett’s oesophagus could either be (1) part of the mucosal defence of Barrett’s oesophagus as an adaptation to chronic gastroesophageal reflux disease (Figure 1), (2) neutral alterations in regions susceptible to chromosome damage that undergo expansion as hitchhikers (“passengers”) on early selected genetic or epigenetic “drivers” or (3) necessary, but not sufficient for progression to oesophageal adenocarcinoma. Abbreviation: loss of heterozygosity (LOH).
| Fundamental propertiesof neoplastic progression | Measures of alterations | Challenges fortranslation |
|---|---|---|
| Genomic instability,chromosomal alterations,chromosome instability,microsatellite instability,mutations | Aneuploidy156,164,165, copynumber and LOH154,155,166,microsatellitealterations164 | High dimensionalcomplexity of genomicalterations and random,neutral events |
| Disruption of regulatorypathways | Transcription profiles157-160, methylationpatterns146,167-171,proteomics161-163, cellularproliferation172, cell-cycleabnormalities173-175 | Redundancy anddynamic adaptation ofnetworks |
| Changes in clonalevolutionary dynamics | Clonal expansion176,clone size177, geneticdivergence178, diversityand generation ofvariants178,179 | Heterogeneity, changesin rates, selection ofvariants |
Genomic instability
Genomic instability appears to be a fundamental property of neoplastic progression that develops before the onset of cancer. [G]Chromosome instability is the most common proven source of genomic instability in human cancers, and it has been best evaluated in colon cancer, where it constitutes about 85% of the genetic instability leading to cancer compared to microsatellite instability, which comprises the remaining 15%182. A large body of evidence now suggests that most oesophageal adenocarcinomas arise in association with a process of gain or loss of whole chromosomes or large portions of chromosomes, as detected by DNA content flow cytometry, cytogenetics, loss of heterozygosity (LOH), comparative genomic hybridization (CGH), array CGH, and SNP arrays154-156,164-166. A recent 317K SNP array study of 23 oesophageal adenocarcinomas reported an average of 97 copy number changes (range 23-208) per cancer that ranged in size from small homozygous deletions to large chromosome regions154. Copy gain, loss and copy neutral LOH averaged 13, 18 and 23MB, respectively. All tumors had LOH involving most of chromosome 17p, and alterations were identified in established tumor suppressor genes and oncogenes such as CDKN2A, TP53, FHIT and MYC, as well as novel candidate gene regions. These results indicate the complexity of genomic changes in oesophageal adenocarcinoma and suggest there will be both opportunities and challenges for risk stratification, cancer prevention and early detection.
Chromosome abnormalities have been detected in Barrett’s oesophagus epithelium adjacent to oesophageal adenocarcinomas, and distributions of cell populations with chromosome abnormalities have been reported at the scales of individual cells, crypts, and biopsies within Barrett’s oesophagus epithelia176,179,183,184. Spatial data at the level of biopsies in the Barrett’s oesophagus epithelia led to the hypothesis that 9p LOH (as well as methylation and mutation of CDKN2A) were early events in Barrett’s oesophagus that preceded 17p LOH and TP53 mutation, and later DNA content tetraploidy and aneuploidy176,183. In a long-term prospective study of 243 Barrett’s oesophagus patients using oesophageal adenocarcinoma as an outcome130, baseline biopsies were evaluated for the presence of 9p LOH, 17p LOH, DNA content abnormalities (tetraploidy and aneuploidy), TP53 mutation and CDKN2A mutation and methylation. After 10 years of follow up, all biomarkers contributed significantly to risk of oesophageal adenocarcinoma in univariate analysis with the exceptions of CDKN2A methylation and mutation. The chromosome instability panel of 9p LOH, 17p LOH and DNA content abnormalities was the best predictor of oesophageal adenocarcinoma (relative risk (RR)=38.7; 95% CI=10.8-138.5; p<0.001). The five-year cumulative incidence of oesophageal adenocarcinoma was 79.1% in individuals with 9p LOH, 17p LOH and a DNA content abnormality at baseline, whereas those with neither LOH nor DNA content abnormalities at baseline had a zero percent cumulative incidence of oesophageal adenocarcinoma almost eight years after the baseline endoscopy.
Although this study established that measures of chromosome instability can distinguish individuals at high and low risk for progression to oesophageal adenocarcinoma it used a constellation of technologies that are difficult to perform outside of research centres. Two recent studies have reported that SNP and BAC arrays have high sensitivity and specificity to detect DNA content aneuploidy, and SNP arrays provide a single platform to assess chromosome instability, including copy change and LOH155,185. Patients whose Barrett’s oesophagus biopsies contained copy number alterations involving more than 70 MB of the genome also had an increased risk of progressing to DNA content abnormalities or oesophageal adenocarcinoma during follow up185.
Thus, substantial evidence indicates that chromosome instability is strongly associated with progression from Barrett’s oesophagus to oesophageal adenocarcinoma. Rapid advances in DNA technology provide opportunities for translation of 9p, 17p, and DNA content abnormalities using clinically compatible platforms such as Pyrosequencing for LOH and fluorescent in situ hybridization for copy number alterations184,186. SNP arrays permit assessment of LOH, copy number and aneuploidy on a common platform in Barrett’s oesophagus and oesophageal adenocarcinoma, demonstrating that chromosome instability was common in persons with Barrett’s oesophagus that had progressed to oesophageal adenocarcinoma as well as in advanced oesophageal adenocarcinomas155. Small [G]interstitial deletions are observed frequently in persons with early stages of Barrett’s oesophagus who did not undergo progression to oesophageal adenocarcinoma155,166. These small deletions do not meet the definition of chromosomal instability155,182, and their roles in Barrett’s oesophagus are not yet clear. They might be selected during the adaptation for mucosal defence in gastroesophageal reflux disease (Figure 1), neutral alterations in regions susceptible to chromosome damage that expand as hitchhikers (passengers), or necessary but not sufficient for oesophageal adenocarcinoma (Box 3)155. Regardless, alterations in these small regions are far too common in early stages to be sufficient for development of oesophageal adenocarcinoma as evidence by the low rate of progression from Barrett’s oesophagus to oesophageal adenocarcinoma23,24. Microsatellite instability is another potential source of genome-wide instability in the development of oesophageal adenocarcinoma although it appears to be much less common than chromosome instability perhaps accounting for 5% of oesophageal adenocarcinomas164.
Epigenetic changes in Barrett’s oesophagus and oesophageal adenocarcinoma
There has been recent interest in epigenetic mechanisms, especially DNA methylation, in development of oesophageal adenocarcinoma, and the promoter regions of several dozen genes have been evaluated using candidate genes identified in other cancers167. A few [G]longitudinal studies of epigenetic abnormalities also have been reported, using a mixture of surrogate dysplasia and oesophageal adenocarcinoma endpoints and based on promoter regions of a small number of genes146,147. Recent studies have used unbiased scans of the genome to investigate DNA methylation in different tissue types and in cancers169,170, with one study of colon cancer reporting that most methylation changes were not in promoters or CpG islands171. Combining recent advances in genome-wide screens with spatial scale experiments will likely lead to better understanding of the roles of methylation in tissue maintenance and neoplasia in Barrett’s oesophagus and oesophageal adenocarcinoma169-171.
Clonal evolution and neoplastic progression in Barrett’s oesophagus
Although Nowell’s theory of clonal evolution is generally accepted153,187, few studies have addressed clonal evolutionary dynamics, which may be fundamental biomarkers of cancer risk applicable to a large number of neoplasms. Three studies carried out on overlapping cohort sets have evaluated evolutionary parameters in neoplastic progression in Barrett’s oesophagus. A spatial study reported that CDKN2A mutation and methylation, 9p LOH, TP53 mutations and 17p LOH were all highly selected (drivers) for clonal expansion176. In contrast, all microsatellite shifts and other LOH events behaved as neutral mutations. In some cases, neutral mutations underwent large clonal expansions, but these expansions could typically be explained by co-expansion as hitchhikers (passengers) on a clonal expansion driven by a known selective mutation. A second study evaluated the relative importance of clonal expansion and genetic instability and reported that the sizes of clones with 17p LOH or DNA content tetraploidy and aneuploidy increased the risk of progression from Barrett’s oesophagus to oesophageal adenocarcinoma177. Sizes of clones with CDKN2A abnormalities were not significant oesophageal adenocarcinoma risk factors when 17p LOH was included in the model, suggesting that expansion of a genetically unstable clone increases risk of progression of Barrett’s oesophagus to oesophageal adenocarcinoma. In a third study, increased clonal diversity, assessed by number of clones, [G]Shannon Index and mean pairwise genetic divergence between flow cytometry enriched fractions of Barrett’s oesophagus biopsies was associated with increased risk of progression to oesophageal adenocarcinoma even when 17p LOH and DNA content abnormalities were included in the model178. It is not yet clear whether measures of diversity in crypts or single cells are associated with an increased risk of progression to oesophageal adenocarcinoma.
Another interesting study observed marked genetic diversity at the crypt level in Barrett’s oesophagus after dissecting individual crypts and evaluating them for LOH involving APC (5q), CDKN2A (9p) and TP53 (17p) as well as mutations in CDKN2A and TP53179. In one patient, a non-coding CDKN2A mutation was present in both a squamous oesophageal duct and metaplastic Barrett’s oesophagus, suggesting a ductal origin of Barrett’s oesophagus. Such careful attention to spatial scale advances our understanding of levels of diversity in Barrett’s oesophagus that may be important in evolution of oesophageal adenocarcinoma or the development of treatment resistance.
Cellular proliferation
Abnormal proliferation and cell cycle intervals have long been known to be associated with Barrett’s oesophagus, and increased proliferative indices appear to be a physiological adaptation to reflux in some studies173. In a small study, expression of minichromosome maintenance proteins was reported to be associated with an increased risk of progression to oesophageal adenocarcinoma174. In a recent case-control study of 29 patients who progressed to oesophageal adenocarcinoma and six who progressed to the surrogate endpoint high-grade dysplasia, p53 expression (as assessed by immunohistochemisty) was associated with an increased risk of progression (OR = 11.7; 95% CI= 1.93-71.4), but expression of cyclin D1, COX-2 and beta-catenin was not175. However, an earlier nested case-control study of 12 individuals who progressed to oesophageal adenocarcinoma from a cohort of 307 persons with Barrett’s oesophagus reported that p53 immunopositivity was not associated with a significant risk of progression (OR = 2.99; 95%CI = 0.57 – 15.76) and that cyclin D1 expression was associated with progression (OR = 6.85; 95% CI = 1.57-29.91)188. The reasons for the discrepancies are unknown and population differences, sample size and, in the case of p53, clone size, type of TP53 mutation and other somatic genetic changes in the evolving Barrett’s segment may all contribute177,189.
A cohort study of 362 patients with mean follow up of 6.3 years and 1,752 person years follow up evaluated diploid cell proliferation and cell cycle intervals fractions (G1, S, 4N) assessed at the baseline endoscopy as predictors of progression to oesophageal adenocarcinoma172. Higher total proliferative or G1 fractions were not associated with progression to oesophageal adenocarcinoma; increased S phase fractions were marginally associated with progression (p=0.03); and increased 4N fractions, which were highly associated with biallelic inactivation of TP53, were quite significantly associated with progression (p<0.0001). Thus, some proliferative changes appear to be adaptive changes to reflux, whereas others are the consequence of inactivation of tumor suppressors. Those that are highly associated with inactivation of TP53, such as 4N fractions, are strong and significant predictors of progression to oesophageal adenocarcinoma.
Oesophageal adenocarcinoma prevention and early detection
The challenge remains to reduce the incidence and mortality of oesophageal adenocarcinoma. No prevention or early detection strategy has yet been conclusively proven to reduce oesophageal adenocarcinoma or all cause mortality in individuals with Barrett’s oesophagus. Current approaches to oesophageal adenocarcinoma control are based largely on the symptomatic gastroesophageal reflux disease-Barrett’s oesophagus-oesophageal adenocarcinoma paradigm, but emerging data challenge many underlying assumptions (Figures 1 and 2).
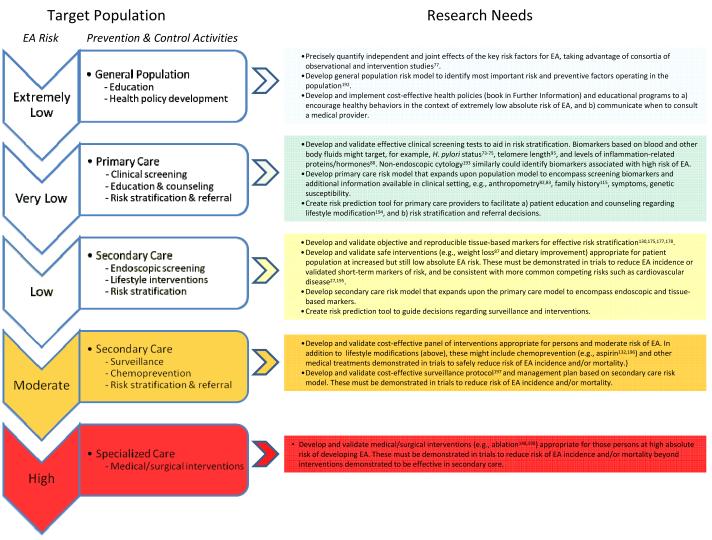
The usefulness of endoscopic screening for Barrett’s oesophagus and oesophageal adenocarcinoma has come into question190. In 2008, the American College of Gastroenterology Guidelines withdrew recommendations for endoscopic screening of patients with gastroesophageal reflux disease140, and an American Gastroenterological Association Institute technical review concluded there was no direct evidence supporting endoscopic screening for either Barrett’s oesophagus or oesophageal adenocarcinoma in individuals with gastroesophageal reflux disease191. An alternative research approach would be to develop a general population risk model taking advantage of existing data from consortia of observational and intervention studies as suggested previously for oesophageal adenocarcinoma (Figure 3)192. Such a model could be used to guide health policy and provide education on when to consult a medical provider (book in Other Information). Other measures derived from consortia data, such as H. pylori status, anthropometric measures, and family history, could be used to develop a primary care risk model to facilitate risk stratification and guide referral (Figure 3). Recent research has also identified promising leads for assessing biomarkers in the primary care setting, including blood tests95 and non-endoscopic oesophageal cytology193, which could include biomarkers identifying persons with Barrett’s oesophagus who are at high risk for progression to oesophageal adenocarcinoma. High sensitivity and especially specificity of the primary care risk model, perhaps as afforded by such biomarkers, will be key in developing programs of prevention and early detection that have a significant impact on oesophageal adenocarcinoma incidence and mortality.
Figure 3. Prevention and control of oesophageal adenocarcinoma.
A new strategy is proposed to build on research advances and overcome the limitations inherent in current approaches to controlling EA incidence and mortality (see Figure 2 and Box 2). A key goal is to cost-effectively classify persons into increasingly high-risk target populations (left side of figure), based on comprehensive risk models using the increasing amount and sophistication of information available in each setting. Each stratum then can be offered programs of prevention and early detection appropriate for their absolute risk of developing EA. A key to success of such an approach is substantial improvement of specificity at each stratum, most likely aided by blood and tissue-based biomarkers of risk, which will allow identification of the large fraction of persons who are unlikely to develop EA, allowing them to avoid or minimize worrisome, costly and risky endoscopic surveillance and interventions. At each level of risk, research needed to create effective prevention programs is listed on the right side of the figure. As suggested by Khoury, et al.199, such translational research typically involves developing and validating tests, risk models and prediction tools, and implementing corresponding preventive interventions in the target population/setting, followed by an evaluation component (not shown) to identify tools and interventions in need of improvement. Abbreviations: EA, oesophageal adenocarcinoma; BE, Barrett’s oesophagus.
There are data to support the effectiveness of endoscopic biopsy surveillance for early detection of oesophageal adenocarcinoma. Several retrospective studies have compared oesophageal adenocarcinomas arising in individuals who have been in a surveillance program for Barrett’s oesophagus to those with newly diagnosed oesophageal adenocarcinomas who had not been in endoscopic surveillance30,32,140,200-206. Oesophageal adenocarcinomas were detected at earlier stages in the surveillance populations compared to those not in surveillance, and patients in surveillance generally, but not always, also had significantly improved survival. However, most of these studies had small sample sizes, some had short follow-up intervals and none were randomized control trials.
The leading chemoprevention candidate for oesophageal adenocarcinoma is currently aspirin, as protective associations have been reported consistently in population-based case-control and cohort studies as well as in meta-analyses130,132-134,196. Inhibition of COX-2 has also been reported to decrease the incidence of oesophageal adenocarcinoma in an animal model of Barrett’s oesophagus207. In Ireland, a population-based study of persons with reflux oesophagitis, Barrett’s oesophagus, oesophageal adenocarcinoma and population controls observed that use of aspirin and other non steroidal anti-inflammatory drugs (NSAIDs) was associated with a significantly reduced risk of Barrett’s oesophagus and oesophageal adenocarcinoma131. Other population-based case-control studies have observed regular aspirin or other NSAID use to be associated with similar reductions in oesophageal adenocarcinoma incidence208,209. A prospective cohort study of individuals with Barrett’s oesophagus reported that current users of aspirin and other NSAIDs had a reduced rate for progression to oesophageal adenocarcinoma compared with never users134. Current users also had reduced progression to DNA content aneuploidy and tetraploidy compared with never users. Current use of aspirin and other NSAIDs has also been associated with a marked risk reduction in patients with multiple chromosome instability abnormalities at baseline with NSAID non-users having a 79% 10-year cumulative incidence of oesophageal adenocarcinoma compared to 30% for current NSAID users (p<0.001)130. It should be noted that one small trial of the COX-2 inhibitor, celecoxib, evaluated changes in a number of surrogate endpoints after 48 weeks of treatment, initially reporting no difference in the proportion of biopsies with dysplasia, total surface area of Barrett’s oesophagus, prostaglandin levels, cyclooxygenase-1/2 mRNA levls or methylation of several tumor suppressor genes210. However, a subsequent analysis using more detailed data available on a subset of the trial participants found a significant decrease in total Barrett’s area among those taking celecoxib211. Taken together, these results suggest that the anti-inflammatory effects of aspirin and other NSAIDs may exert both early and late effects on neoplastic progression.
Proton pump inhibitors, a class of medications that substantially reduces gastric acid production, came into widespread use in the early to mid-1990s for treatment of symptoms of gastroesophageal reflux, among other indications. Several observational studies have examined the association between use of these drugs and surrogate endpoints for oesophageal adenocarcinoma, but with conflicting results. One recent retrospective cohort study examined pharmacy records to estimate use of proton pump inhibitors in 344 individuals without any dysplasia at initial endoscopy, reporting no association with the development of any dysplasia, but a statistically significant reduction in risk of high grade dysplasia and/or oesophageal adenocarcinoma212. A potential limitation of the study, beyond the use of non-cancer endpoints, is the fact that more than 40% of the cohort were initially seen before proton pump inhibitors were generally available (1982-1992); thus any difference in risk of progression over time experienced by the cohort would bias the observed association with use of proton pump inhibitors. Another study examined the occurrence of regression of Barrett’s oesophagus among 188 persons taking proton pump inhibitors213. They found no evidence of reduction in lenth of the Barrett’s segment after a mean of 5.1 years of treatment. As in vitro studies suggest a possible antiproliferative effect of acid exposure in Barrett’s cell lines, mediated through p53, clinical trials are clearly needed to address the long-term effects of proton pump inhibitors on risk of oesophageal adenocarcinoma214.
A randomized trial of aspirin and two doses of proton pump inhibitors for Barrett’s oesophagus without high-grade dysplasia is currently underway in the UK that includes all cause mortality outcome and may shed additional light on the effectiveness of aspirin and proton pump inhibitors as chemopreventive agents in persons with Barrett’s oesophagus without high-grade dysplasia215. A randomized trial of high-risk individuals might also be considered in light of evidence that aspirin and other NSAIDs also act at an advanced stage of neoplastic progression130. Additional candidate preventive measures, including weight loss, increased physical activity, smoking cessation, and increased intake of plant-based foods, may help reduce the incidence of oesophageal adenocarcinoma in the general population, and in high-risk persons defined by genetics, lifestyle or biomarkers. However, all remain to be demonstrated as effective in a prevention trial.
More aggressive approaches to prevention, including treating patients with Barrett’s oesophagus with photodynamic therapy (PDT) and radiofrequency ablation (RFA) have been evaluated in multicenter randomized trials with incomplete blinding and surrogate dysplasia primary endpoints148,149,198. The PDT trial reported a decreased incidence of oesophageal adenocarcinoma as a secondary endpoint, with a non-significant increase in T2 and T3 oesophageal adenocarcinomas in the PDT arm, but patients who developed advanced cancers were excluded as treatment failures and oesophageal adenocarcinoma mortality may have been underestimated148,149. Adverse events, such as photosensitivity, strictures, nausea/vomiting and pain, were also quite common (94%). The RFA trial had only surrogate primary and secondary endpoints, small sample size and short post-ablation follow up of only a few months in many patients. Although there was a decrease of borderline significance in the incidence of oesophageal adenocarcinoma among patients with high-grade dysplasia in the treatment arm during the short follow-up period (p=0.04), a trial with substantially larger sample size, longer follow up and primary endpoints of oesophageal adenocarcinoma incidence and mortality is needed to validate the effect. No patient with low-grade dysplasia developed oesophageal adenocarcinoma, consistent with the known low risk, transient nature and lack of robust reproducibility of this diagnosis (Box 2). In addition, approximately 10% of patients receiving RFA for non-nodular dysplasia had adverse events requiring additional medical care including upper gastrointestinal bleeding, chest pain requiring hospitalization, and strictures requiring dilation, compared to none in the control arm. Endoscopic mucosal resection (EMR) is frequently performed in the setting of nodular dysplasia for effective selection of patients for endoscopic therapy prior to RFA, and the combination of EMR and RFA can result in a constellation of adverse events affecting more than 20% of patients, including bleeding, oesophageal laceration, oesophageal perforation, oesophageal stricture requiring dilatation, fever and chest pain requiring hospitalization216. Although the length of follow up in the RFA trial was insufficient to assess recurrence of Barrett’s oesophagus after therapy, the neosquamous epithelium after ablation is prone to undergo the fate of its precursor, the native oesophageal squamous epithelium, which lacks the mucosal defences of specialized intestinal metaplasia (Figure 1) and recurrence of Barrett’s oesophagus has been reported in up to two-thirds of patients217.
Conclusions and perspective
The incidence of oesophageal adenocarcinoma has risen more rapidly than any other cancer in Western countries, and there is evidence for increasing incidence in regions of Asia where the diagnosis was previously almost unknown. Current approaches for controlling oesophageal adenocarcinoma incidence and mortality largely based on endoscopic investigation of symptomatic gastroesophageal reflux disease and histology-guided surveillance and treatment of persons with Barrett’s oesophagus have significant limitations (Figure 2, Box 2). New oesophageal adenocarcinoma prevention strategies will be needed to overcome these limitations and decrease the current high mortality associated with oesophageal adenocarcinoma (Figure 3).
Advances have been made over the past decade in our understanding of host and environmental factors associated with oesophageal adenocarcinoma, including the role of obesity as well as the protective associations of aspirin and other NSAIDs. These and other factors can guide development of population risk models192. Advances have also been made that can assist development of primary care risk models, including family history, H. pylori testing, non-endoscopic cytology, and blood tests. With rapid advances in DNA array technology, more precise and higher resolution measurements of both the constitutive genome and the evolving neoplastic genome are now possible with platforms that can be translated into the clinic setting. However, the complexity of the process of neoplastic progression suggests that no single measure will likely be sufficient for practical clinical oesophageal adenocarcinoma risk stratification over a person’s lifetime (Box 3).
A significant remaining challenge is that no intervention, including lifestyle modification, chemoprevention, or medical or surgical treatments, has yet been convincingly shown to reduce oesophageal adenocarcinoma incidence and/or mortality. Consortia with multidisciplinary expertise in population, genomic, computational, clinical and other sciences will be required to effectively address these challenges with the goals of developing personal risk stratification based on interactions among environmental factors, the constitutive genome and the evolving neoplastic genome and delivering personalized care in the form of interventions tailored to an individual’s oesophageal adenocarcinoma risk.
At a glance
The paradigm that Barrett’s oesophagus develops as a consequence of symptomatic gastroesophageal reflux disease and predisposes to oesophageal adenocarcinoma has dominated clinical thought for more than three decades. However, current approaches for controlling the incidence and mortality of oesophageal adenocarcinoma largely based on endoscopic investigation of individuals with symptomatic gastroesophageal reflux disease, and histology-guided surveillance and treatment of individuals with Barrett’s oesophagus have significant limitations.
Barrett’s oesophagus rarely progresses to oesophageal adenocarcinoma, and a theory has recently been proposed that mucosal defences in most patients with Barrett’s oesophagus represent successful adaptations to the harsh intra-oesophageal environment of chronic gastroesophageal reflux disease. Several mucosal defences that arise in Barrett’s oesophagus have been identified, including secretion of bicarbonate and mucous, expression of claudin-18 tight junctions, overexpression of defence and repair genes, and resistance to prolonged and repeated acid exposure.
The incidence of oesophageal adenocarcinoma has been rising at an alarming rate in the US, Western Europe, Australia, and in other developed countries over the past four decades, and there is disquieting evidence of increased incidence of oesophageal adenocarcinoma in some Asian populations.
Four risk factors, gastroesophageal reflux disease, obesity, cigarette smoking and poor diet, account for the majority of oesophageal adenocarcinomas. Obesity may act at early and late stages of progression and interact biologically with gastroesophageal reflux disease, although a substantial proportion of the effect of obesity is likely to be through other pathways.
Neoplastic progression to oesophageal adenocarcinoma is characterized by genomic instability, including chromosome instability in most cases, disruption of regulatory pathways and temporal evolution of clones that may be modulated by host and environmental risk and protective factors. Proper measurement and quantification of the complexity of these alterations creates opportunities and challenges for improved risk stratification, prevention and early detection.
Aspirin and other non steroidal anti-inflammatory drugs have been consistently reported to have a protective association with oesophageal adenocarcinoma in case-control and cohort studies as well as meta-analyses; they may be useful in patients at both early and late stages of progression.
No intervention, whether based on lifestyle modification, chemoprevention, or medical or surgical treatments, has yet been convincingly demonstrated in a randomized trial to reduce incidence and/or mortality of oesophageal adenocarcinoma; this remains a particularly crucial area of unmet research need. New oesophageal adenocarcinoma prevention strategies are proposed to overcome these limitations.
Acknowledgements
This work was funded by National Institutes of Health grants NIH P01CA091955 and NIH K05CA124911.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Glossary terms
Oesophageal specialized intestinal metaplasia
Specialized intestinal metaplasia is a differentiated epithelium with crypt architecture that resembles the epithelium of the intestine, rather than that of the oesophagus.
Person-years
The denominator used in calculation of an incidence rate. It takes into account both the number of persons being observed and the period of observation. For example, 1,000 persons observed for 4 years would yield 4,000 person-years.
Overdiagnosis
Diagnosis of a disease or condition by screening that would not have been detected during the lifespan of the individual without screening.
Period effects
In statistical modeling of temporal trends of a disease, period effects are attributed to causes linked to calendar year, as opposed to age or year of birth.
p-trend
A statistical test to determine whether an association between an exposure and a disease is consistent with a monotonic relationship.
Gastric manometry
A test to measure electrical and motor activity in the stomach.
Chromosomal instability
An increased rate of gain or loss of whole chromosomes or large fractions of chromosomes182.
Interstitial deletion
A deletion of variable size that does not involve the terminal parts of a chromosome.
Longitudinal studies
Observational studies in which the disease (and perhaps exposure) experience of a group of individuals is observed over multiple time points.
Shannon Index
combines both the number and relative abundance of clones. It is also known as the information content or entropy and is calculated as
where pi is the relative frequency of clone i.
Footnotes
“This paper concerns a condition whose existence is denied by some, misunderstood by others, and ignored by the majority of surgeons. It has been called a variety of names which have confused the story because they have suggested incorrect etiological explanations…” Norman Barrett, 1957
References
- 1.Sharma P, et al. A critical review of the diagnosis and management of Barrett’s esophagus: the AGA Chicago Workshop. Gastroenterology. 2004;127:310–30. doi: 10.1053/j.gastro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20. doi: 10.1111/j.1572-0241.2006.00630.x. quiz 1943. [DOI] [PubMed] [Google Scholar]
- 3.Barrett N. Chronic peptic ulcer of the oesophagus and ‘oesophagitis’. Br J Surg. 1950;38:175–182. doi: 10.1002/bjs.18003815005. [DOI] [PubMed] [Google Scholar]
- 4.Allison PR, Johnstone AS. The oesophagus lined with gastric mucous membrane. Thorax. 1953;8:87–101. doi: 10.1136/thx.8.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naef AP, Savary M, Ozzello L. Columnar-lined lower esophagus: an acquired lesion with malignant predisposition. Report on 140 cases of Barrett’s esophagus with 12 adenocarcinomas. Journal of Thoracic and Cardiovascular Surgery. 1975;70:826–35. [PubMed] [Google Scholar]
- 6.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Sem Rad Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Sampliner RE. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol. 1998;93:1028–32. doi: 10.1111/j.1572-0241.1998.00362.x. [DOI] [PubMed] [Google Scholar]
- 8.Hirota WK, et al. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570–80. doi: 10.1016/j.gie.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Orlando RC. Mucosal Defense in Barrett’s Esophagus. In: S R, Sharma P, editors. Barrett’s Esophagus and Esophageal Adenocarcinoma. Blackwell Publishing, Ltd; Oxford, UK: 2006. pp. 60–72. [Google Scholar]
- 10.Cairns J. Mutation Selection and the Natural History of Cancer. Nature. 1975;255:197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- 11.Tobey NA, Argote CM, Vanegas XC, Barlow W, Orlando RC. Electrical parameters and ion species for active transport in human esophageal stratified squamous epithelium and Barrett’s specialized columnar epithelium. Am J Physiol Gastrointest Liver Physiol. 2007;293:G264–70. doi: 10.1152/ajpgi.00047.2007. [DOI] [PubMed] [Google Scholar]
- 12.Dixon J, et al. Esophageal mucin: an adherent mucus gel barrier is absent in the normal esophagus but present in columnar-lined Barrett’s esophagus. Am J Gastroenterol. 2001;96:2575–83. doi: 10.1111/j.1572-0241.2001.04159.x. [DOI] [PubMed] [Google Scholar]
- 13.Jovov B, et al. Claudin-18: a dominant tight junction protein in Barrett’s esophagus and likely contributor to its acid resistance. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1106–13. doi: 10.1152/ajpgi.00158.2007. [DOI] [PubMed] [Google Scholar]
- 14.Ostrowski J, et al. Molecular defense mechanisms of Barrett’s metaplasia estimated by an integrative genomics. J Mol Med. 2007;85:733–43. doi: 10.1007/s00109-007-0176-3. [DOI] [PubMed] [Google Scholar]
- 15.Lao-Sirieix P, et al. Physiological and molecular analysis of acid loading mechanisms in squamous and columnar-lined esophagus. Dis Esophagus. 2008;21:529–38. doi: 10.1111/j.1442-2050.2007.00807.x. [DOI] [PubMed] [Google Scholar]
- 16.Levine DS, Reid BJ, Haggitt RC, Rubin CE, Rabinovitch PS. Correlation of ultrastructural aberrations with dysplasia and flow cytometric abnormalities in Barrett’s epithelium. Gastroenterology. 1989;96:355–67. doi: 10.1016/s0016-5085(89)91559-x. [DOI] [PubMed] [Google Scholar]
- 17.Sharma P, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:566–72. doi: 10.1016/j.cgh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Schnell TG, et al. Long-term nonsurgical management of Barrett’s esophagus with high-grade dysplasia. Gastroenterology. 2001;120:1607–19. doi: 10.1053/gast.2001.25065. [DOI] [PubMed] [Google Scholar]
- 19.Conio M, et al. Long-term endoscopic surveillance of patients with Barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol. 2003;98:1931–9. doi: 10.1111/j.1572-0241.2003.07666.x. [DOI] [PubMed] [Google Scholar]
- 20.Macdonald CE, Wicks AC, Playford RJ. Ten years’ experience of screening patients with Barrett’s oesophagus in a university teaching hospital. Gut. 1997;41:303–7. doi: 10.1136/gut.41.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid BJ, Levine DS, Longton G, Blount PL, Rabinovitch PS. Predictors of progression to cancer in Barrett’s esophagus: baseline histology and flow cytometry identify low- and high-risk patient subsets. American Journal of Gastroenterology. 2000;95:1669–76. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hage M, et al. Oesophageal cancer incidence and mortality in patients with long-segment Barrett’s oesophagus after a mean follow-up of 12.7 years. Scand J Gastroenterol. 2004;39:1175–9. doi: 10.1080/00365520410003524. [DOI] [PubMed] [Google Scholar]
- 23.Thomas T, Abrams KR, De Caestecker JS, Robinson RJ. Meta analysis: Cancer risk in Barrett’s oesophagus. Aliment Pharmacol Ther. 2007;26:1465–77. doi: 10.1111/j.1365-2036.2007.03528.x. [DOI] [PubMed] [Google Scholar]
- 24.Yousef F, et al. The incidence of esophageal cancer and high-grade dysplasia in Barrett’s esophagus: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:237–49. doi: 10.1093/aje/kwn121. [DOI] [PubMed] [Google Scholar]
- 25.Anderson LA, et al. Mortality in Barrett’s oesophagus: results from a population based study. Gut. 2003;52:1081–4. doi: 10.1136/gut.52.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conio M, et al. Secular trends in the epidemiology and outcome of Barrett’s oesophagus in Olmsted County, Minnesota. Gut. 2001;48:304–9. doi: 10.1136/gut.48.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moayyedi P, et al. Mortality rates in patients with Barrett’s oesophagus. Aliment Pharmacol Ther. 2008;27:316–20. doi: 10.1111/j.1365-2036.2007.03582.x. [DOI] [PubMed] [Google Scholar]
- 28.Solaymani-Dodaran M, Logan RF, West J, Card T. Mortality associated with Barrett’s esophagus and gastroesophageal reflux disease diagnoses-a population-based cohort study. Am J Gastroenterol. 2005;100:2616–21. doi: 10.1111/j.1572-0241.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- 29.Dulai GS, Guha S, Kahn KL, Gornbein J, Weinstein WM. Preoperative prevalence of Barrett’s esophagus in esophageal adenocarcinoma: a systematic review. Gastroenterology. 2002;122:26–33. doi: 10.1053/gast.2002.30297. [DOI] [PubMed] [Google Scholar]
- 30.Corley DA, Levin TR, Habel LA, Weiss NS, Buffler PA. Surveillance and survival in Barrett’s adenocarcinomas: a population-based study. Gastroenterology. 2002;122:633–40. doi: 10.1053/gast.2002.31879. [DOI] [PubMed] [Google Scholar]
- 31.Bytzer P, Christensen PB, Damkier P, Vinding K, Seersholm N. Adenocarcinoma of the esophagus and Barrett’s esophagus: a population-based study. Am J Gastroenterol. 1999;94:86–91. doi: 10.1111/j.1572-0241.1999.00776.x. [DOI] [PubMed] [Google Scholar]
- 32.Cooper GS, Kou TD, Chak A. Receipt of previous diagnoses and endoscopy and outcome from esophageal adenocarcinoma: a population-based study with temporal trends. Am J Gastroenterol. 2009;104:1356–62. doi: 10.1038/ajg.2009.159. [DOI] [PubMed] [Google Scholar]
- 33.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. Jama. 2005;293:2095–101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 34.Marcus PM, et al. Extended lung cancer incidence follow-up in the Mayo Lung Project and overdiagnosis. J Natl Cancer Inst. 2006;98:748–56. doi: 10.1093/jnci/djj207. [DOI] [PubMed] [Google Scholar]
- 35.Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787. doi: 10.1038/427787a. [DOI] [PubMed] [Google Scholar]
- 36.Chawla SN, et al. The natural history of observed enhancing renal masses: meta-analysis and review of the world literature. J Urol. 2006;175:425–31. doi: 10.1016/S0022-5347(05)00148-5. [DOI] [PubMed] [Google Scholar]
- 37.Ronkainen J, et al. Prevalence of Barrett’s Esophagus in the General Population: An Endoscopic Study. Gastroenterol. 2005;129:1828–1831. doi: 10.1053/j.gastro.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 38.Zagari RM, et al. Gastro-oesophageal reflux symptoms, oesophagitis and Barrett’s oesophagus in the general population: the Loiano-Monghidoro study. Gut. 2008;57:1354–9. doi: 10.1136/gut.2007.145177. [DOI] [PubMed] [Google Scholar]
- 39.Farrow DC, et al. Gastroesophageal reflux disease, use of H2 receptor antagonists, and risk of esophageal and gastric cancer. Cancer Causes Control. 2000;11:231–8. doi: 10.1023/a:1008913828105. [DOI] [PubMed] [Google Scholar]
- 40.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. New England Journal of Medicine. 1999b;340:825–31. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 41.Brown LM, Devesa SS, Chow WH. Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst. 2008;100:1184–7. doi: 10.1093/jnci/djn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bollschweiler E, Wolfgarten E, Gutschow C, Holscher AH. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer. 2001;92:549–55. doi: 10.1002/1097-0142(20010801)92:3<549::aid-cncr1354>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 43.Fernandes ML, Seow A, Chan YH, Ho KY. Opposing trends in incidence of esophageal squamous cell carcinoma and adenocarcinoma in a multi-ethnic Asian country. Am J Gastroenterol. 2006;101:1430–6. doi: 10.1111/j.1572-0241.2006.00570.x. [DOI] [PubMed] [Google Scholar]
- 44.Shibata A, Matsuda T, Ajiki W, Sobue T. Trend in incidence of adenocarcinoma of the esophagus in Japan, 1993-2001. Jpn J Clin Oncol. 2008;38:464–8. doi: 10.1093/jjco/hyn064. [DOI] [PubMed] [Google Scholar]
- 45.Haghdoost AA, et al. Rising incidence of adenocarcinoma of the esophagus in Kerman, Iran. Arch Iran Med. 2008;11:364–70. [PubMed] [Google Scholar]
- 46.Yee YK, Cheung TK, Chan AO, Yuen MF, Wong BC. Decreasing trend of esophageal adenocarcinoma in Hong Kong. Cancer Epidemiol Biomarkers Prev. 2007;16:2637–40. doi: 10.1158/1055-9965.EPI-07-0421. [DOI] [PubMed] [Google Scholar]
- 47.Chung JW, et al. Unchanging trend of esophagogastric junction adenocarcinoma in Korea: experience at a single institution based on Siewert’s classification. Dis Esophagus. 2009 doi: 10.1111/j.1442-2050.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 48.Polednak AP. Trends in survival for both histologic types of esophageal cancer in US surveillance, epidemiology and end results areas. Int J Cancer. 2003;105:98–100. doi: 10.1002/ijc.11029. [DOI] [PubMed] [Google Scholar]
- 49.Whiteman DC, et al. Combined effects of obesity, acid reflux and smoking on the risk of adenocarcinomas of the oesophagus. Gut. 2008;57:173–80. doi: 10.1136/gut.2007.131375. [DOI] [PubMed] [Google Scholar]
- 50.Anderson LA, et al. Risk factors for Barrett’s oesophagus and oesophageal adenocarcinoma: results from the FINBAR study. World J Gastroenterol. 2007;13:1585–94. doi: 10.3748/wjg.v13.i10.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu AH, Tseng CC, Bernstein L. Hiatal hernia, reflux symptoms, body size, and risk of esophageal and gastric adenocarcinoma. Cancer. 2003;98:940–8. doi: 10.1002/cncr.11568. [DOI] [PubMed] [Google Scholar]
- 52.Vaughan TL, Davis S, Kristal A, Thomas DB. Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 1995;4:85–92. [PubMed] [Google Scholar]
- 53.Chow WH, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150–5. doi: 10.1093/jnci/90.2.150. [DOI] [PubMed] [Google Scholar]
- 54.Samanic C, Chow WH, Gridley G, Jarvholm B, Fraumeni JF., Jr. Relation of body mass index to cancer risk in 362,552 Swedish men. Cancer Causes Control. 2006;17:901–9. doi: 10.1007/s10552-006-0023-9. [DOI] [PubMed] [Google Scholar]
- 55.Lindblad M, Rodriguez LA, Lagergren J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Causes Control. 2005;16:285–94. doi: 10.1007/s10552-004-3485-7. [DOI] [PubMed] [Google Scholar]
- 56.Engeland A, Tretli S, Bjorge T. Height and body mass index in relation to esophageal cancer: 23-year follow-up of two million Norwegian men and women. Cancer Causes and Control. 2004;15:837–843. doi: 10.1023/B:CACO.0000043434.21558.ea. [DOI] [PubMed] [Google Scholar]
- 57.MacInnis RJ, English DR, Hopper JL, Giles GG. Body size and composition and the risk of gastric and oesophageal adenocarcinoma. Int J Cancer. 2006;118:2628–31. doi: 10.1002/ijc.21638. [DOI] [PubMed] [Google Scholar]
- 58.Steffen A, et al. Anthropometry and Esophageal Cancer Risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2009 doi: 10.1158/1055-9965.EPI-09-0265. [DOI] [PubMed] [Google Scholar]
- 59.Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:352–8. doi: 10.1158/1055-9965.EPI-07-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu A, Wan P, Bernstein L. A multiethnic population-based study of smoking, alcohol and body size and risk of adenocarcinomas of the stomach and esophagus (United States) Cancer Causes and Control. 2001;12:721–732. doi: 10.1023/a:1011290704728. [DOI] [PubMed] [Google Scholar]
- 61.Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:872–8. doi: 10.1158/1055-9965.EPI-05-0860. [DOI] [PubMed] [Google Scholar]
- 62.Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005;143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006. [DOI] [PubMed] [Google Scholar]
- 63.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 64.Ogden CL, et al. Prevalence of overweight and obesity in the United States, 1999-2004. Jama. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 65.Dollman J, Olds TS. Secular changes in fatness and fat distribution in Australian children matched for body size. Int J Pediatr Obes. 2006;1:109–13. doi: 10.1080/17477160600684260. [DOI] [PubMed] [Google Scholar]
- 66.Jeon J, Luebeck EG, Moolgavkar SH. Age effects and temporal trends in adenocarcinoma of the esophagus and gastric cardia (United States) Cancer Causes Control. 2006;17:971–81. doi: 10.1007/s10552-006-0037-3. [DOI] [PubMed] [Google Scholar]
- 67.Gammon MD, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277–84. doi: 10.1093/jnci/89.17.1277. [DOI] [PubMed] [Google Scholar]
- 68.Mulholland HG, et al. Glycemic index, carbohydrate and fiber intakes and risk of reflux esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma. Cancer Causes Control. 2009;20:279–88. doi: 10.1007/s10552-008-9242-6. [DOI] [PubMed] [Google Scholar]
- 69.Wu AH, Tseng CC, Hankin J, Bernstein L. Fiber intake and risk of adenocarcinomas of the esophagus and stomach. Cancer Causes Control. 2007;18:713–22. doi: 10.1007/s10552-007-9014-8. [DOI] [PubMed] [Google Scholar]
- 70.Mayne ST, et al. Nutrient Intake and Risk of Subtypes of Esophageal and Gastric Cancer. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:1055–1062. [PubMed] [Google Scholar]
- 71.Anderson LA, et al. The association between alcohol and reflux esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma. Gastroenterology. 2009;136:799–805. doi: 10.1053/j.gastro.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 72.Pandeya N, Williams G, Green AC, Webb PM, Whiteman DC. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology. 2009;136:1215–24. e1–2. doi: 10.1053/j.gastro.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 73.de Martel C, et al. Helicobacter pylori infection and the risk of development of esophageal adenocarcinoma. J Infect Dis. 2005;191:761–7. doi: 10.1086/427659. [DOI] [PubMed] [Google Scholar]
- 74.Chow WH, et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of esophageal and gastric cardia adenocarcinoma. Cancer Res. 1998;58:588–90. [PubMed] [Google Scholar]
- 75.Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res (Phila Pa) 2008;1:329–38. doi: 10.1158/1940-6207.CAPR-08-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blaser MJ. Disappearing microbiota: Helicobacter pylori protection against esophageal adenocarcinoma. Cancer Prev Res (Phila Pa) 2008;1:308–11. doi: 10.1158/1940-6207.CAPR-08-0170. [DOI] [PubMed] [Google Scholar]
- 77.Engel LS, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer. 2003;95:1404–1413. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- 78.Kim JY, et al. Prevalence of Barrett’s esophagus in Korea. J Gastroenterol Hepatol. 2005;20:633–6. doi: 10.1111/j.1440-1746.2005.03749.x. [DOI] [PubMed] [Google Scholar]
- 79.Lim SL, Goh WT, Lee JM, Ng TP, Ho KY. Changing prevalence of gastroesophageal reflux with changing time: longitudinal study in an Asian population. J Gastroenterol Hepatol. 2005;20:995–1001. doi: 10.1111/j.1440-1746.2005.03887.x. [DOI] [PubMed] [Google Scholar]
- 80.Wu JC. Gastroesophageal reflux disease: an Asian perspective. J Gastroenterol Hepatol. 2008;23:1785–93. doi: 10.1111/j.1440-1746.2008.05684.x. [DOI] [PubMed] [Google Scholar]
- 81.Vaughan TL, et al. NSAID use, BMI, and anthropometry in relation to genetic and cell cycle abnormalities in Barrett’s Esophagus. Cancer Epidemiology, Biomarkers & Prevention. 2002;11:745–752. [PubMed] [Google Scholar]
- 82.Edelstein ZR, Farrow DC, Bronner MP, Rosen SN, Vaughan TL. Central adiposity and risk of Barrett’s esophagus. Gastroenterology. 2007;133:403–11. doi: 10.1053/j.gastro.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 83.Corley DA, et al. Abdominal obesity and body mass index as risk factors for Barrett’s esophagus. Gastroenterology. 2007;133:34–41. doi: 10.1053/j.gastro.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 84.El-Serag HB, Kvapil P, Hacken-Bitar J, Kramer JR. Abdominal obesity and the risk of Barrett’s esophagus. Am J Gastroenterol. 2005;100:2151–6. doi: 10.1111/j.1572-0241.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 85.Barlow WJ, Orlando RC. The pathogenesis of heartburn in nonerosive reflux disease: a unifying hypothesis. Gastroenterology. 2005;128:771–8. doi: 10.1053/j.gastro.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 86.El-Serag HB, Tran T, Richardson P, Ergun G. Anthropometric correlates of intragastric pressure. Scand J Gastroenterol. 2006;41:887–91. doi: 10.1080/00365520500535402. [DOI] [PubMed] [Google Scholar]
- 87.Corley DA, Kubo A. Body mass index and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Gastroenterol. 2006;101:2619–28. doi: 10.1111/j.1572-0241.2006.00849.x. [DOI] [PubMed] [Google Scholar]
- 88.Kendall BJ, et al. Leptin and the risk of Barrett’s oesophagus. Gut. 2008;57:448–54. doi: 10.1136/gut.2007.131243. [DOI] [PubMed] [Google Scholar]
- 89.Hursting SD, et al. Reducing the weight of cancer: mechanistic targets for breaking the obesity-carcinogenesis link. Best Pract Res Clin Endocrinol Metab. 2008;22:659–69. doi: 10.1016/j.beem.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 90.Edelstein ZR, Bronner MP, Rosen SN, Vaughan TL. Risk factors for Barrett’s esophagus among patients with gastroesophageal reflux disease: a community clinic-based case-control study. Am J Gastroenterol. 2009;104:834–42. doi: 10.1038/ajg.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith KJ, O’Brien SM, Green AC, Webb PM, Whiteman DC. Increased Risks of Barrett’s Esophagus With Smoking But not Self-Reported Body Mass Index. Clin Gastroenterol Hepatol. 2009 doi: 10.1016/j.cgh.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 92.Kubo A, et al. Cigarette smoking and the risk of Barrett’s esophagus. Cancer Causes Control. 2009;20:303–11. doi: 10.1007/s10552-008-9244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kubo A, et al. Dietary antioxidants, fruits, and vegetables and the risk of Barrett’s esophagus. Am J Gastroenterol. 2008;103:1614–23. doi: 10.1111/j.1572-0241.2008.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thompson OM, Beresford SA, Kirk EA, Vaughan TL. Vegetable and fruit intakes and risk of Barrett’s esophagus in men and women. Am J Clin Nutr. 2009;89:890–6. doi: 10.3945/ajcn.2008.26497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Risques RA, et al. Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16:2649–55. doi: 10.1158/1055-9965.EPI-07-0624. [DOI] [PubMed] [Google Scholar]
- 96.Valdes AM, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–4. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 97.Demissie S, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–30. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 98.Xing J, et al. Constitutive short telomere length of chromosome 17p and 12q but not 11q and 2p is associated with an increased risk for esophageal cancer. Cancer Prev Res (Phila Pa) 2009;2:459–65. doi: 10.1158/1940-6207.CAPR-08-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jenkins GJ, et al. The bile acid deoxycholic acid has a non-linear dose response for DNA damage and possibly NF-kappaB activation in oesophageal cells, with a mechanism of action involving ROS. Mutagenesis. 2008;23:399–405. doi: 10.1093/mutage/gen029. [DOI] [PubMed] [Google Scholar]
- 100.Grisham MB, Jourd’heuil D, Wink DA. Review article: chronic inflammation and reactive oxygen and nitrogen metabolism--implications in DNA damage and mutagenesis. Aliment Pharmacol Ther. 2000;14(Suppl 1):3–9. doi: 10.1046/j.1365-2036.2000.014s1003.x. [DOI] [PubMed] [Google Scholar]
- 101.Sihvo EI, et al. Oxidative stress has a role in malignant transformation in Barrett’s oesophagus. Int J Cancer. 2002;102:551–5. doi: 10.1002/ijc.10755. [DOI] [PubMed] [Google Scholar]
- 102.Trayhurn P, Bing C, Wood IS. Adipose tissue and adipokines--energy regulation from the human perspective. J Nutr. 2006;136:1935S–1939S. doi: 10.1093/jn/136.7.1935S. [DOI] [PubMed] [Google Scholar]
- 103.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–44. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 104.Turker MS, et al. A novel signature mutation for oxidative damage resembles a mutational pattern found commonly in human cancers. Cancer Res. 1999;59:1837–9. [PubMed] [Google Scholar]
- 105.Poynton AR, Walsh TN, O’Sullivan G, Hennessy TP. Carcinoma arising in familial Barrett’s esophagus. Am J Gastroenterol. 1996;91:1855–6. [PubMed] [Google Scholar]
- 106.Romero Y, et al. Familial aggregation of gastroesophageal reflux in patients with Barrett’s esophagus and esophageal adenocarcinoma. Gastroenterology. 1997;113:1449–56. doi: 10.1053/gast.1997.v113.pm9352846. [DOI] [PubMed] [Google Scholar]
- 107.Groves C, Jankowski J, Barker F, Holdstock G. A family history of Barrett’s oesophagus: another risk factor? Scand J Gastroenterol. 2005;40:1127–8. doi: 10.1080/00365520510023189. [DOI] [PubMed] [Google Scholar]
- 108.Munitiz V, et al. High risk of malignancy in familial Barrett’s esophagus: presentation of one family. J Clin Gastroenterol. 2008;42:806–9. doi: 10.1097/MCG.0b013e3180329015. [DOI] [PubMed] [Google Scholar]
- 109.Cameron AJ, et al. Gastroesophageal reflux disease in monozygotic and dizygotic twins. Gastroenterology. 2002;122:55–9. doi: 10.1053/gast.2002.30301. [DOI] [PubMed] [Google Scholar]
- 110.Mohammed I, Cherkas LF, Riley SA, Spector TD, Trudgill NJ. Genetic influences in gastro-oesophageal reflux disease: a twin study. Gut. 2003;52:1085–9. doi: 10.1136/gut.52.8.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chak A, et al. Identification of Barrett’s esophagus in relatives by endoscopic screening. Am J Gastroenterol. 2004;99:2107–14. doi: 10.1111/j.1572-0241.2004.40464.x. [DOI] [PubMed] [Google Scholar]
- 112.Fitzgerald RC. Complex diseases in gastroenterology and hepatology: GERD, Barrett’s, and esophageal adenocarcinoma. Clin Gastroenterol Hepatol. 2005;3:529–37. doi: 10.1016/s1542-3565(05)00248-x. [DOI] [PubMed] [Google Scholar]
- 113.Chak A, et al. Familiality in Barrett’s esophagus, adenocarcinoma of the esophagus, and adenocarcinoma of the gastroesophageal junction. Cancer Epidemiol Biomarkers Prev. 2006;15:1668–73. doi: 10.1158/1055-9965.EPI-06-0293. [DOI] [PubMed] [Google Scholar]
- 114.Chak A, et al. Familial aggregation of Barrett’s oesophagus, oesophageal adenocarcinoma, and oesophagogastric junctional adenocarcinoma in Caucasian adults. Gut. 2002;51:323–8. doi: 10.1136/gut.51.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ochs-Balcom HM, et al. Consortium approach to identifying genes for Barrett’s esophagus and esophageal adenocarcinoma. Transl Res. 2007;150:3–17. doi: 10.1016/j.trsl.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 116.Drovdlic CM, et al. Demographic and phenotypic features of 70 families segregating Barrett’s oesophagus and oesophageal adenocarcinoma. J Med Genet. 2003;40:651–6. doi: 10.1136/jmg.40.9.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Casson AG, et al. Polymorphisms in DNA repair genes in the molecular pathogenesis of esophageal (Barrett) adenocarcinoma. Carcinogenesis. 2005;26:1536–41. doi: 10.1093/carcin/bgi115. [DOI] [PubMed] [Google Scholar]
- 118.Terry MB, et al. Alcohol dehydrogenase 3 and risk of esophageal and gastric adenocarcinomas. Cancer Causes Control. 2007;18:1039–46. doi: 10.1007/s10552-007-9046-0. [DOI] [PubMed] [Google Scholar]
- 119.Wideroff L, et al. GST, NAT1, CYP1A1 polymorphisms and risk of esophageal and gastric adenocarcinomas. Cancer Detect Prev. 2007;31:233–6. doi: 10.1016/j.cdp.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121:1643–58. doi: 10.1002/ijc.23044. [DOI] [PubMed] [Google Scholar]
- 121.Murphy SJ, et al. A population-based association study of SNPs of GSTP1, MnSOD, GPX2 and Barrett’s esophagus and esophageal adenocarcinoma. Carcinogenesis. 2007;28:1323–8. doi: 10.1093/carcin/bgm007. [DOI] [PubMed] [Google Scholar]
- 122.Doecke J, et al. Polymorphisms in MGMT and DNA repair genes and the risk of esophageal adenocarcinoma. Int J Cancer. 2008;123:174–80. doi: 10.1002/ijc.23410. [DOI] [PubMed] [Google Scholar]
- 123.El-Omar EM, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124:1193–201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 124.Ye W, et al. The XPD 751Gln allele is associated with an increased risk for esophageal adenocarcinoma: a population-based case-control study in Sweden. Carcinogenesis. 2006;27:1835–41. doi: 10.1093/carcin/bgl017. [DOI] [PubMed] [Google Scholar]
- 125.Di Martino E, et al. IGFBP-3 and IGFBP-10 (CYR61) up-regulation during the development of Barrett’s oesophagus and associated oesophageal adenocarcinoma: potential biomarkers of disease risk. Biomarkers. 2006;11:547–61. doi: 10.1080/13547500600896791. [DOI] [PubMed] [Google Scholar]
- 126.di Martino E, et al. The NAD(P)H:quinone oxidoreductase I C609T polymorphism modifies the risk of Barrett esophagus and esophageal adenocarcinoma. Genet Med. 2007;9:341–7. doi: 10.1097/gim.0b013e3180654ccd. [DOI] [PubMed] [Google Scholar]
- 127.Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373:1301–9. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 128.Moons LM, et al. COX-2 CA-haplotype is a risk factor for the development of esophageal adenocarcinoma. Am J Gastroenterol. 2007;102:2373–9. doi: 10.1111/j.1572-0241.2007.01373.x. [DOI] [PubMed] [Google Scholar]
- 129.Ferguson HR, et al. Cyclooxygenase-2 and inducible nitric oxide synthase gene polymorphisms and risk of reflux esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:727–31. doi: 10.1158/1055-9965.EPI-07-2570. [DOI] [PubMed] [Google Scholar]
- 130.Galipeau PC, et al. NSAIDs modulate CDKN2A, TP53, and DNA content risk for future esophageal adenocarcinoma. PLoS Med. 2007;4:e67. doi: 10.1371/journal.pmed.0040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anderson LA, et al. Nonsteroidal anti-inflammatory drugs and the esophageal inflammation-metaplasia-adenocarcinoma sequence. Cancer Res. 2006;66:4975–82. doi: 10.1158/0008-5472.CAN-05-4253. [DOI] [PubMed] [Google Scholar]
- 132.Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003;124:47–56. doi: 10.1053/gast.2003.50008. [DOI] [PubMed] [Google Scholar]
- 133.Farrow DC, et al. Use of aspirin and other nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 1998;7:97–102. [PubMed] [Google Scholar]
- 134.Vaughan TL, et al. Non-steroidal anti-inflammatory drugs and risk of neoplastic progression in Barrett’s oesophagus: a prospective study. Lancet Oncol. 2005;6:945–52. doi: 10.1016/S1470-2045(05)70431-9. [DOI] [PubMed] [Google Scholar]
- 135.Chao DL, et al. Mutagen sensitivity and neoplastic progression in patients with Barrett’s esophagus: A prospective analysis. CEBP. 2006;15:1935–40. doi: 10.1158/1055-9965.EPI-06-0492. [DOI] [PubMed] [Google Scholar]
- 136.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–40. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 137.Fleming TR, Prentice RL, Pepe MS, Glidden D. Surrogate and auxiliary endpoints in clinical trials, with potential applications in cancer and AIDS research. Statistics in Medicine. 1994;13:955–68. doi: 10.1002/sim.4780130906. [DOI] [PubMed] [Google Scholar]
- 138.Reid BJ, et al. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum Pathol. 1988;19:166–78. doi: 10.1016/s0046-8177(88)80344-7. [DOI] [PubMed] [Google Scholar]
- 139.Montgomery E, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol. 2001;32:368–78. doi: 10.1053/hupa.2001.23510. [DOI] [PubMed] [Google Scholar]
- 140.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–97. doi: 10.1111/j.1572-0241.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 141.Cameron AJ, Carpenter HA. Barrett’s esophagus, high-grade dysplasia, and early adenocarcinoma: a pathological study. American Journal of Gastroenterology. 1997;92:586–91. [PubMed] [Google Scholar]
- 142.Ofman JJ, et al. The economic impact of the diagnosis of dysplasia in Barrett’s esophagus. Am J Gastroenterol. 2000;95:2946–52. doi: 10.1111/j.1572-0241.2000.03209.x. [DOI] [PubMed] [Google Scholar]
- 143.Weston AP, et al. p53 protein overexpression in low grade dysplasia (LGD) in Barrett’s esophagus: immunohistochemical marker predictive of progression. Am J Gastroenterol. 2001;96:1355–62. doi: 10.1111/j.1572-0241.2001.03851.x. [DOI] [PubMed] [Google Scholar]
- 144.Gatenby P, et al. Routinely diagnosed low-grade dysplasia in Barrett’s oesophagus: a population-based study of natural history. Histopathology. 2009;54:814–9. doi: 10.1111/j.1365-2559.2009.03316.x. [DOI] [PubMed] [Google Scholar]
- 145.Dulai GS, et al. Dysplasia and risk of further neoplastic progression in a regional Veterans Administration Barrett’s cohort. Am J Gastroenterol. 2005;100:775–83. doi: 10.1111/j.1572-0241.2005.41300.x. [DOI] [PubMed] [Google Scholar]
- 146.Jin Z, et al. A multicenter, double-blinded validation study of methylation biomarkers for progression prediction in Barrett’s esophagus. Cancer Res. 2009;69:4112–5. doi: 10.1158/0008-5472.CAN-09-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang JS, et al. DNA Promoter Hypermethylation of p16 and APC Predicts Neoplastic Progression in Barrett’s Esophagus. Am J Gastroenterol. 2009 doi: 10.1038/ajg.2009.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Overholt BF, et al. Photodynamic therapy with porfimer sodium for ablation of high-grade dysplasia in Barrett’s esophagus: international, partially blinded, randomized phase III trial. Gastrointest Endosc. 2005;62:488–98. doi: 10.1016/j.gie.2005.06.047. [DOI] [PubMed] [Google Scholar]
- 149.Overholt BF, et al. Five-year efficacy and safety of photodynamic therapy with Photofrin in Barrett’s high-grade dysplasia. Gastrointest Endosc. 2007;66:460–8. doi: 10.1016/j.gie.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 150.Buttar NS, et al. Extent of high-grade dysplasia in Barrett’s esophagus correlates with risk of adenocarcinoma. Gastroenterology. 2001;120:1630–9. doi: 10.1053/gast.2001.25111. [DOI] [PubMed] [Google Scholar]
- 151.Srivastava A, et al. Extent of low-grade dysplasia is a risk factor for the development of esophageal adenocarcinoma in Barrett’s esophagus. Am J Gastroenterol. 2007;102:483–93. doi: 10.1111/j.1572-0241.2007.01073.x. quiz 694. [DOI] [PubMed] [Google Scholar]
- 152.Kaye PV, et al. Barrett’s dysplasia and the Vienna classification: reproducibility, prediction of progression and impact of consensus reporting and p53 immunohistochemistry. Histopathology. 2009;54:699–712. doi: 10.1111/j.1365-2559.2009.03288.x. [DOI] [PubMed] [Google Scholar]
- 153.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–8. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 154.Nancarrow DJ, et al. Genome-wide copy number analysis in esophageal adenocarcinoma using high-density single-nucleotide polymorphism arrays. Cancer Res. 2008;68:4163–72. doi: 10.1158/0008-5472.CAN-07-6710. [DOI] [PubMed] [Google Scholar]
- 155.Li X, et al. Single nucleotide polymorphism-based genome-wide chromosome copy change, loss of heterozygosity, and aneuploidy in BE neoplastic progression. Can Prev Res. 2008;1:413–23. doi: 10.1158/1940-6207.CAPR-08-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jenkins GJ, et al. Genetic pathways involved in the progression of Barrett’s metaplasia to adenocarcinoma. Br J Surg. 2002;89:824–37. doi: 10.1046/j.1365-2168.2002.02107.x. [DOI] [PubMed] [Google Scholar]
- 157.van Baal JW, et al. A comparative analysis by SAGE of gene expression profiles of Barrett’s esophagus, normal squamous esophagus, and gastric cardia. Gastroenterology. 2005;129:1274–81. doi: 10.1053/j.gastro.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 158.van Baal JW, et al. A comparative analysis by SAGE of gene expression profiles of esophageal adenocarcinoma and esophageal squamous cell carcinoma. Cell Oncol. 2008;30:63–75. doi: 10.1155/2008/328529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Barrett MT, et al. Transcriptional analyses of Barrett’s metaplasia and normal upper GI mucosae. Neoplasia. 2002;4:121–8. doi: 10.1038/sj.neo.7900221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Helm J, et al. Dedifferentiation precedes invasion in the progression from Barrett’s metaplasia to esophageal adenocarcinoma. Clin Cancer Res. 2005;11:2478–85. doi: 10.1158/1078-0432.CCR-04-1280. [DOI] [PubMed] [Google Scholar]
- 161.Peng D, et al. Alterations in Barrett’s-related adenocarcinomas: a proteomic approach. Int J Cancer. 2008;122:1303–10. doi: 10.1002/ijc.23258. [DOI] [PubMed] [Google Scholar]
- 162.Zhao J, et al. Comparative proteomics analysis of Barrett metaplasia and esophageal adenocarcinoma using two-dimensional liquid mass mapping. Mol Cell Proteomics. 2007;6:987–99. doi: 10.1074/mcp.M600175-MCP200. [DOI] [PubMed] [Google Scholar]
- 163.Kraly JR, et al. Reproducible Two-Dimensional Capillary Electrophoresis Analysis of Barrett’s Esophagus Tissues. Anal Chem. 2006;78:5977–5986. doi: 10.1021/ac061029+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wijnhoven BP, Tilanus HW, Dinjens WN. Molecular biology of Barrett’s adenocarcinoma. Ann Surg. 2001;233:322–37. doi: 10.1097/00000658-200103000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Paulson TG, Reid BJ. Focus on Barrett’s esophagus and esophageal adenocarcinoma. Cancer Cell. 2004;6:11–6. doi: 10.1016/j.ccr.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 166.Lai LA, et al. Increasing genomic instability during premalignant neoplastic progression revealed through high resolution array-CGH. Genes Chromosomes Cancer. 2007;46:532–42. doi: 10.1002/gcc.20435. [DOI] [PubMed] [Google Scholar]
- 167.Smith E, et al. Similarity of aberrant DNA methylation in Barrett’s esophagus and esophageal adenocarcinoma. Mol Cancer. 2008;7:75. doi: 10.1186/1476-4598-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fitzgerald RC. Molecular basis of Barrett’s oesophagus and oesophageal adenocarcinoma. Gut. 2006;55:1810–20. doi: 10.1136/gut.2005.089144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rakyan VK, et al. An integrated resource for genome-wide identification and analysis of human tissue-specific differentially methylated regions (tDMRs) Genome Res. 2008;18:1518–29. doi: 10.1101/gr.077479.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bloushtain-Qimron N, Yao J, Shipitsin M, Maruyama R, Polyak K. Epigenetic patterns of embryonic and adult stem cells. Cell Cycle. 2009;8:809–17. doi: 10.4161/cc.8.6.7938. [DOI] [PubMed] [Google Scholar]
- 171.Irizarry RA, et al. The human colon cancer methylome shows similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet. 2009;41:178–86. doi: 10.1038/ng.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chao DL, et al. Cell proliferation, cell cycle abnormalities, and cancer outcome in patients with Barrett’s esophagus: a long-term prospective study. Clin Cancer Res. 2008;14:6988–95. doi: 10.1158/1078-0432.CCR-07-5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ouatu-Lascar R, Fitzgerald RC, Triadafilopoulos G. Differentiation and proliferation in Barrett’s esophagus and the effects of acid suppression. Gastroenterology. 1999;117:327–35. doi: 10.1053/gast.1999.0029900327. [DOI] [PubMed] [Google Scholar]
- 174.Sirieix PS, et al. Surface expression of minichromosome maintenance proteins provides a novel method for detecting patients at risk for developing adenocarcinoma in Barrett’s esophagus. Clin Cancer Res. 2003;9:2560–6. [PubMed] [Google Scholar]
- 175.Murray L, et al. TP53 and progression from Barrett’s metaplasia to oesophageal adenocarcinoma in a UK population cohort. Gut. 2006;55:1390–7. doi: 10.1136/gut.2005.083295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Maley CC, et al. Selectively advantageous mutations and hitchhikers in neoplasms: p16 lesions are selected in Barrett’s esophagus. Cancer Res. 2004;64:3414–27. doi: 10.1158/0008-5472.CAN-03-3249. [DOI] [PubMed] [Google Scholar]
- 177.Maley CC, et al. The combination of genetic instability and clonal expansion predicts progression to esophageal adenocarcinoma. Cancer Res. 2004;64:7629–33. doi: 10.1158/0008-5472.CAN-04-1738. [DOI] [PubMed] [Google Scholar]
- 178.Maley CC, et al. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat Genet. 2006;38:468–73. doi: 10.1038/ng1768. [DOI] [PubMed] [Google Scholar]
- 179.Leedham SJ, et al. Individual crypt genetic heterogeneity and the origin of metaplastic glandular epithelium in human Barrett’s oesophagus. Gut. 2008;57:1041–8. doi: 10.1136/gut.2007.143339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Haibe-Kains B, et al. Comparison of prognostic gene expression signatures for breast cancer. BMC Genomics. 2008;9:394. doi: 10.1186/1471-2164-9-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dumur CI, et al. Interlaboratory performance of a microarray-based gene expression test to determine tissue of origin in poorly differentiated and undifferentiated cancers. J Mol Diagn. 2008;10:67–77. doi: 10.2353/jmoldx.2008.070099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rajagopalan H, Nowak MA, Vogelstein B, Lengauer C. The significance of unstable chromosomes in colorectal cancer. Nature Reviews Cancer. 2003;3:695–701. doi: 10.1038/nrc1165. [DOI] [PubMed] [Google Scholar]
- 183.Barrett MT, et al. Evolution of neoplastic cell lineages in Barrett oesophagus. Nat Gen. 1999;22:106–9. doi: 10.1038/8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Fritcher EG, et al. A comparison of conventional cytology, DNA ploidy analysis, and fluorescence in situ hybridization for the detection of dysplasia and adenocarcinoma in patients with Barrett’s esophagus. Hum Pathol. 2008;39:1128–35. doi: 10.1016/j.humpath.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Paulson TG, et al. Chromosomal instability and copy number alterations in Barrett’s esophagus and esophageal adenocarcinoma. Clin Cancer Res. 2009;15:3305–14. doi: 10.1158/1078-0432.CCR-08-2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kissel HD, Galipeau PC, Li X, Reid BJ. Translation of an STR-based biomarker into a clinically compatible SNP-based platform for loss of heterozygosity. Cancer Biomarkers. 2009;5:143–58. doi: 10.3233/CBM-2009-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Breivik J. The evolutionary origin of genetic instability in cancer development. Semin Cancer Biol. 2005;15:51–60. doi: 10.1016/j.semcancer.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 188.Bani-Hani K, et al. Prospective study of cyclin D1 overexpression in Barrett’s esophagus: association with increased risk of adenocarcinoma. J Natl Cancer Inst. 2000;92:1316–21. doi: 10.1093/jnci/92.16.1316. [DOI] [PubMed] [Google Scholar]
- 189.Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9:701–13. doi: 10.1038/nrc2693. [DOI] [PubMed] [Google Scholar]
- 190.Shaheen N, Ransohoff DF. Gastroesophageal reflux, barrett esophagus, and esophageal cancer: scientific review. Jama. 2002;287:1972–81. doi: 10.1001/jama.287.15.1972. [DOI] [PubMed] [Google Scholar]
- 191.Kahrilas PJ, Shaheen NJ, Vaezi MF. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1392–1413. 1413 e1–5. doi: 10.1053/j.gastro.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 192.Kelloff GJ, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer--a plan to move forward. Clin Cancer Res. 2006;12:3661–97. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 193.Lao-Sirieix P, et al. Non-endoscopic screening biomarkers for Barrett’s oesophagus: From microarray analysis to the clinic. Gut. 2009 doi: 10.1136/gut.2009.180281. [DOI] [PubMed] [Google Scholar]
- 194.Ampt AJ, et al. Attitudes, norms and controls influencing lifestyle risk factor management in general practice. BMC Fam Pract. 2009;10:59. doi: 10.1186/1471-2296-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cook MB, et al. Risk of mortality and cancer incidence in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16:2090–6. doi: 10.1158/1055-9965.EPI-07-0432. [DOI] [PubMed] [Google Scholar]
- 196.Abnet CC, et al. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. Br J Cancer. 2009;100:551–7. doi: 10.1038/sj.bjc.6604880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Jankowski J, Barr H. Improving surveillance for Barrett’s oesophagus: AspECT and BOSS trials provide an evidence base. BMJ. 2006;332:1512. doi: 10.1136/bmj.332.7556.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shaheen NJ, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 199.Khoury MJ, et al. A Decade of Public Health Genomics in the United States: Centers for Disease Control and Prevention 1997-2007. Public Health Genomics. 2008 doi: 10.1159/000153427. [DOI] [PubMed] [Google Scholar]
- 200.Streitz JM, J., Andrews CW, J., Ellis FH. Endoscopic Surveillance of Barrett’s Esophagus. The Journal of Thoracic and Cardiovascular Surgery. 1993;105:383–388. [PubMed] [Google Scholar]
- 201.Peters JH, et al. Outcome of adenocarcinoma arising in Barrett’s esophagus in endoscopically surveyed and nonsurveyed patients. J Thorac Cardiovasc Surg. 1994;108:813–21. discussion 821-2. [PubMed] [Google Scholar]
- 202.van Sandick JW, et al. Impact of endoscopic biopsy surveillance of Barrett’s oesophagus on pathological stage and clinical outcome of Barrett’s carcinoma. Gut. 1998;43:216–22. doi: 10.1136/gut.43.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Incarbone R, Bonavina L, Saino G, Bona D, Peracchia A. Outcome of esophageal adenocarcinoma detected during endoscopic biopsy surveillance for Barrett’s esophagus. Surg Endosc. 2002;16:263–6. doi: 10.1007/s00464-001-8161-3. [DOI] [PubMed] [Google Scholar]
- 204.Ferguson MK, Durkin A. Long-term survival after esophagectomy for Barrett’s adenocarcinoma in endoscopically surveyed and nonsurveyed patients. J Gastrointest Surg. 2002;6:29–35. doi: 10.1016/s1091-255x(01)00052-x. discussion 36. [DOI] [PubMed] [Google Scholar]
- 205.Fountoulakis A, et al. Effect of surveillance of Barrett’s oesophagus on the clinical outcome of oesophageal cancer. Br J Surg. 2004;91:997–1003. doi: 10.1002/bjs.4591. [DOI] [PubMed] [Google Scholar]
- 206.Rubenstein JH, Sonnenberg A, Davis J, McMahon L, Inadomi JM. Effect of a prior endoscopy on outcomes of esophageal adenocarcinoma among United States veterans. Gastrointest Endosc. 2008;68:849–55. doi: 10.1016/j.gie.2008.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Buttar NS, et al. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett’s esophagus. Gastroenterology. 2002;122:1101–12. doi: 10.1053/gast.2002.32371. [DOI] [PubMed] [Google Scholar]
- 208.Duan L, Wu AH, Sullivan-Halley J, Bernstein L. Nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric adenocarcinomas in Los Angeles County. Cancer Epidemiol Biomarkers Prev. 2008;17:126–34. doi: 10.1158/1055-9965.EPI-07-0664. [DOI] [PubMed] [Google Scholar]
- 209.Sadeghi S, et al. Aspirin, nonsteroidal anti-inflammatory drugs, and the risks of cancers of the esophagus. Cancer Epidemiol Biomarkers Prev. 2008;17:1169–78. doi: 10.1158/1055-9965.EPI-07-2852. [DOI] [PubMed] [Google Scholar]
- 210.Heath EI, et al. Secondary chemoprevention of Barrett’s esophagus with celecoxib: results of a randomized trial. J Natl Cancer Inst. 2007;99:545–57. doi: 10.1093/jnci/djk112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shar AO, et al. Modeling using baseline characteristics in a small multicenter clinical trial for Barrett’s esophagus. Contemp Clin Trials. 2009;30:2–7. doi: 10.1016/j.cct.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nguyen DM, et al. Medication Usage and the Risk of Neoplasia in Patients With Barrett’s Esophagus. Clin Gastroenterol Hepatol. 2009 doi: 10.1016/j.cgh.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cooper BT, Chapman W, Neumann CS, Gearty JC. Continuous treatment of Barrett’s oesophagus patients with proton pump inhibitors up to 13 years: observations on regression and cancer incidence. Aliment Pharmacol Ther. 2006;23:727–33. doi: 10.1111/j.1365-2036.2006.02825.x. [DOI] [PubMed] [Google Scholar]
- 214.Feagins LA, et al. Acid has antiproliferative effects in nonneoplastic Barrett’s epithelial cells. Am J Gastroenterol. 2007;102:10–20. doi: 10.1111/j.1572-0241.2006.01005.x. [DOI] [PubMed] [Google Scholar]
- 215.Das D, et al. Management of Barrett’s esophagus in the UK: overtreated and underbiopsied but improved by the introduction of a national randomized trial. Am J Gastroenterol. 2008;103:1079–89. doi: 10.1111/j.1572-0241.2008.01790.x. [DOI] [PubMed] [Google Scholar]
- 216.Pouw RE, et al. Eradication of Barrett esophagus with early neoplasia by radiofrequency ablation, with or without endoscopic resection. J Gastrointest Surg. 2008;12:1627–36. doi: 10.1007/s11605-008-0629-1. discussion 1636-7. [DOI] [PubMed] [Google Scholar]
- 217.Mork H, Al-Taie O, Berlin F, Kraus MR, Scheurlen M. High recurrence rate of Barrett’s epithelium during long-term follow-up after argon plasma coagulation. Scand J Gastroenterol. 2007;42:23–7. doi: 10.1080/00365520600825125. [DOI] [PubMed] [Google Scholar]