Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes (original) (raw)
. Author manuscript; available in PMC: 2010 Jun 17.
Published in final edited form as: Nat Rev Mol Cell Biol. 2008 May;9(5):367–377. doi: 10.1038/nrm2391
Abstract
Acquired resistance to the action of insulin to stimulate glucose transport in skeletal muscle is associated with obesity and promotes the development of type 2 diabetes. In skeletal muscle, insulin resistance can result from high levels of circulating fatty acids that disrupt insulin signalling pathways. However, the severity of insulin resistance varies greatly among obese people. Here we postulate that this variability might reflect differences in levels of lipid-droplet proteins that promote the sequestration of fatty acids within adipocytes in the form of triglycerides, thereby lowering exposure of skeletal muscle to the inhibitory effects of fatty acids.
A perplexing byproduct of contemporary human behaviour related to feeding and physical activity is an increasing accumulation of body fat compared with lean mass. The incidence of obesity (defined as having a body mass index (BMI) of greater than 30 kg per m2) is increasing dramatically in virtually all societies of the world, and with it come important pathological consequences such as type 2 diabetes mellitus and cardiovascular disease1. The global incidence of type 2 diabetes is projected to double to 350 million cases by the year 2030, with expenditure attributed to diabetes estimated to reach $132 billion in the United States alone2,3. Clearly, there would be great benefits if research could achieve effective prevention and therapies for obesity and associated type 2 diabetes. Hampering these efforts are many complexities in studying metabolic disease, including a strong social influence on the incidence of obesity. This is reflected in the United States by the striking inverse correlation between obesity and income. Furthermore, it is difficult to determine the molecular mechanisms that underlie metabolic disease from studies on human subjects, and it is difficult to extrapolate data from studies on rodent metabolism, which differs substantially from human metabolism.
Although progression to type 2 diabetes occurs more frequently in obese rodents and humans compared with lean individuals, this association is highly dependent on genetic background. Inbred mouse strains vary widely in their metabolic response to high-fat diets and to the impact of obesity on insulin sensitivity and development of diabetes4. Likewise, despite an increased risk, many obese human subjects do not progress to the diabetic state, which suggests that genetic and/or environmental factors also play a part. Nonetheless, it is generally accepted that two features are particularly critical for obesity to elicit type 2 diabetes. First, impaired responsiveness of skeletal muscle to insulin is a primary condition in obesity and a precondition for the onset of type 2 diabetes. The association between obesity and skeletal muscle insulin resistance is probably a causal relationship, as studies in humans and animals indicate that weight loss and gain correlate with increasing and decreasing insulin sensitivity, respectively5,6. In insulin-resistant individuals that are not diabetic, glycaemic control can be maintained by compensatory increases in insulin secretion by pancreatic β-cells. Thus, a second defect required for progression from insulin resistance to type 2 diabetes is the failure of β-cells to secrete the required levels of insulin that maintain normal fasting blood glucose levels7–9.
Remarkably, work over the past several years has revealed that adipose tissue (BOX 1) has an important role in controlling whole-body glucose homeostasis in both normal and disease states. This Review focuses on our current understanding of the cellular and molecular mechanisms through which adipose tissue metabolism, which is altered by obesity, causes primary insulin resistance in skeletal muscle. The contribution of lipid overload and lipotoxicity in promoting obesity by perturbing insulin signalling pathways through fatty acids in the skeletal muscle10,11 have recently been highlighted. We emphasize the main role of the adipose tissue as a master regulatory tissue in controlling whole-body lipid flux, thereby modulating both glucose and lipid homeostasis in humans. We focus on specific molecules and pathways in human adipose tissue that are key switches that favour lipid storage in adipocytes over fatty acid release into the circulation. These metabolic pathways confer on adipocytes the role of gatekeepers for fatty acids that can circulate in the blood and enter skeletal muscle. High levels of circulating fatty acids are thought to cause insulin resistance in skeletal muscle. Thus discovery of new targets that regulate fatty acids in adipocytes might ultimately lead to therapeutic modalities that can prevent insulin resistance and type 2 diabetes mellitus.
Box 1. Functions of adipose tissue.
White adipose tissue has long been recognized as the main site of storage of excess energy derived from food intake30. White adipocytes (the predominant cell type in white adipose tissue) store dietary energy in a highly concentrated form as triglyceride, mostly in a single large lipid droplet. These structures are associated with a unique complement of proteins, which enable the sequestration or mobilization of lipids. In times of caloric need, these triglycerides can be rapidly hydrolysed by lipases (a process known as lipolysis) and the resulting fatty acids are transported to other tissues to be oxidized in mitochondria as an energy source.
By contrast, brown fat is specialized primarily for non-shivering thermogenesis, a cold climate adaptation in many homeotherms127. Brown adipocytes are characterized by multiple, smaller droplets of triglyceride, which are accessible for rapid hydrolysis and rapid oxidation of the fatty acids. A high content of mitochondria (which produce the brown colour) and the presence of the uncoupling protein-1 (UCP-1) allow the use of the energy derived from fatty acid oxidation for the generation of heat. Significant depots of brown fat are found in rodents and other animals throughout life. Brown fat depots are present in human infants and recent evidence suggests that dispersed brown adipocytes might persist in adults128.
The discovery of leptin production by adipocytes marked the first indication that adipose tissue also functions as an endocrine organ. Adipose tissue secretes a large number of peptide hormones and cytokines (known as adipokines) as well as non-peptide biologically active molecules such as activated lipids36. These molecules affect energy metabolism in other tissues such as the liver and muscle, as well as behaviours related to feeding through effects on neuroendocrine pathways. Direct neural connections between adipose tissue and the brain have also been implicated in the integration of whole-body energy control129,130.
Adipocytes and insulin resistance
Circulating fatty acids mediate insulin resistance
The key question of which factor or factors actually mediate insulin resistance in skeletal muscle has been difficult to solve, but at least one such important mediator is now identified — free fatty acids (FFAs). The hypothesis that FFAs mediate insulin resistance is consistent with data that show a strong association of obesity and insulin resistance with high circulating FFA levels12. This hypothesis was further supported by the demonstration that elevated levels of circulating FFAs can cause peripheral insulin resistance in both animals and humans13,14. By contrast, acute lowering of FFAs with the antilipolytic drug acipimox enhances insulin action on glucose uptake in the periphery15. In addition to the effects of circulating FFAs, deposition of fatty acids into non-adipose fat stores, including muscle, might contribute to insulin resistance in obesity; however, a similar increase in muscle triglyceride during exercise correlates with high insulin sensitivity16–20. These studies underscore the hypothesis that mobilization of FFAs into the circulation and high uptake into skeletal muscle promote insulin resistance rather than excess body fat per se.
The importance of adipose tissue in controlling whole-body metabolism by sequestering fat is reinforced by the observation that a lack of adipose tissue leads to elevated circulating concentrations of triglycerides and fatty acids and leads to insulin resistance in mice and humans21–24. The presence of adipose tissue is also required for normal secretion of adipokines such as leptin and adiponectin, which enhance insulin sensitivity. Human and mouse lipodystrophies cause impaired adipokine secretion25,26. Together, these observations are consistent with the notion that normal insulin sensitivity and glucose homeostasis require functional adipose tissue in proper proportion to body size. Two crucial roles of adipose tissue seem to contribute to this function: the secretion of appropriate levels of adipokines, which influence both whole-body metabolism and neuroendocrine control of behaviours that are related to feeding27,28; and the sequestration of lipids as adipose triglyceride stores, which attenuates the deleterious effects of both circulating FFAs and ectopic triglyceride stores10,11,29.
Progression of adipose tissue dysfunction in obesity
Adipocytes have a particularly large capacity to synthesize and store triglycerides during feeding, as well as to hydrolyse and release triglycerides as FFAs and glycerol during fasting30,31. In fasting states, there is a dynamic equilibrium between the release of fatty acids into the circulation and their uptake and oxidation by the peripheral tissues, mainly the skeletal muscle. Based on studies by many laboratories on rodents and humans with high caloric intakes, alterations in adipose tissue change the dynamics between fatty acid release and use (FIG. 1). In the lean state, fasting fatty acid concentrations in the circulation are approximately 0.4–0.8 mM. Inside cells, fatty acids are esterified with coenzyme A in order to reduce their detergent properties and toxicity. In the lean state, fatty acyl-CoA levels within muscle cells are low owing to their rapid oxidation in mitochondria (FIG. 1a). In most lean individuals, insulin sensitivity of skeletal muscle and glucose uptake is normal. In hyperphagia-induced obesity, as caloric intake increases, adipocytes enlarge (FIG. 1b) because of increased triglyceride deposition within these cells. At early stages of high caloric intake, adipocytes continue to actively store additional triglycerides and maintain near normal lipolytic rates during fasting. Humans and mice under these conditions show increased expression of enzymes that are involved in triglyceride synthesis in adipocytes, in keeping with a higher capacity to store triglyceride loads32,33. Fatty acid levels can rise slightly, but skeletal muscle maintains high insulin sensitivity in this early stage of high caloric intake (FIG. 1b).
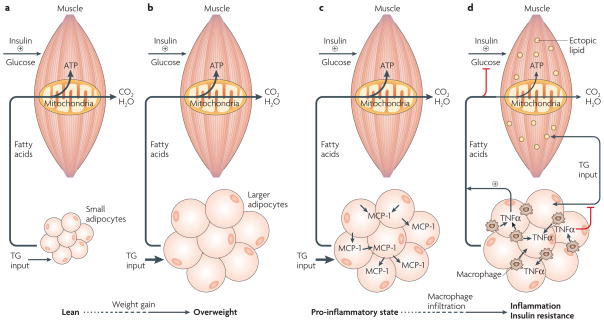
Figure 1. Chronic inflammation in adipose tissue triggers insulin resistance in skeletal muscle.
a | In the lean state, small adipocytes efficiently store fatty acids as triglyceride (TG input, arrow), which can be mobilized and used to generate ATP through the mitochondrial β-oxidation pathway in muscle during periods of caloric need. Insulin-stimulated glucose uptake under these conditions is normal. b | Excess caloric intake leads to metabolic overload, increased TG input and adipocyte enlargement. Nonetheless, in non-diabetic overweight individuals, TG storage by adipose cells and β-oxidation in muscle can often be maintained to prevent insulin resistance. c | On further overloading with TG, hypertrophy of adipocytes and increased secretion of macrophage chemoattractants occurs, including the secretion of monocyte chemoattractant protein-1 (MCP-1; arrows), which recruits additional macrophages. d | Macrophage recruitment in turn results in a pro-inflammatory state in obese adipose tissue. Infiltrating macrophages secrete large amounts of tumour-necrosis factor-α (TNFα), which results in a chronic inflammatory state with impaired TG deposition and increased lipolysis (arrow and plus signal). The excess of circulating TG and free fatty acids results in the accumulation of activated lipids in the muscle (yellow dots), disrupting functions such as mitochondrial oxidative phosphorylation and insulin-stimulated glucose transport, thus triggering insulin resistance.
As adiposity increases, the ability of adipocytes to function as endocrine cells and secrete multiple biologically active proteins is affected (for reviews on adipokines and their roles in appetite control, obesity and insulin sensitivity see REFS 34–36). In this section, we focus on peptides that are secreted by adipocytes and either directly or indirectly regulate metabolism of triglycerides and fatty acids within adipocytes themselves. Examples of such adipokines are monocyte chemoattractant protein-1/chemokine (C–C motif) ligand-2 (MCP-1/CCL2) and tumour-necrosis factor-α (TNFα), which modulates an inflammatory response in adipose tissue. Adipokine-mediated inflammatory response regulates adipocyte metabolism and the ability of adipocytes to store triglycerides. Specifically, hypertrophied adipocytes have been found to secrete large amounts of MCP-1 (REF. 37), which functions as a chemoattractant that enhances macrophage infiltration into adipose tissue in obese mice38 and humans39 (FIG. 1c). Consistent with this role of MCP-1, adipose tissue of lean subjects usually consists of approximately 5–10% macrophages, whereas in obese patients, macrophage content in adipose tissue can be as high as 50% of the total number of cells40 (FIG. 1d). Thus, the increased production of MCP-1 by larger adipocytes might contribute to a pro-inflammatory state (FIG. 1c). However, recent studies of a mouse MCP-1-knockout model suggest that other chemoattractant peptides can also promote macrophage infiltration41.
The development of the inflammatory state in adipose tissue is associated with insulin resistance in skeletal muscle (FIG. 1d). Adipocytes and macrophages secrete MCP-1 and other attractants for inflammatory cells, as well as large amounts of TNFα and other cytokines such as interleukin-1β (IL1β)42. The action of such cytokines has two dramatic effects on adipocyte function — an increase in lipolysis and a decrease in triglyceride synthesis. These actions in turn result in increased levels of circulating FFAs and in the availability of triglycerides to be taken up directly by skeletal muscle through the action of lipoprotein lipase, an enzyme that catalyses the hydrolysis of triglycerides in circulating lipoproteins, which carry fat through the blood. The excess circulating FFAs cause accumulation of triglycerides and activated lipids in the form of long-chain fatty acyl-CoA esters in the skeletal muscle (FIG. 1d), the liver and β-cells. Long-chain fatty acyl-CoA esters and other fatty acid derivatives seem to disrupt normal metabolic and secretory functions of these tissues10,11. Taken together, the scenario that is depicted in FIG. 1 suggests that adipocyte function during prolonged caloric overload causes an inflammatory response, which in turn causes adipocyte dysfunction through the actions of cytokines such as TNFα on adipocyte metabolism. Hyperphagia also leads to increased fatty acid synthesis, very-low-density lipoprotein synthesis in the liver and increased activity of lipoprotein lipase in skeletal muscle. These also contribute to lipid overload and the flow of fatty acids into the muscle.
In agreement with the scheme depicted in FIG. 1, high MCP-1 and TNFα levels are observed during obesity, and their genetic disruption ameliorates insulin resistance in mice that are fed a high-fat diet43,44. The TNFα produced by macrophages within adipose tissue of obese subjects probably requires both the IKKβ–NF-κB (inhibitor of nuclear factor (NF)-κB (IκB) kinase-β–NF-κB) and the JNK–MAP4K4–AP1 (Jun N-terminal kinase–mitogen-activated protein kinase kinase kinase kinase-4– activator protein-1) signalling pathways (FIG. 2). Deletion of JNK1 from macrophages and other haematopoietic cells confers protection against high-fat, diet-induced insulin resistance by decreasing obesity-induced inflammation45. Furthermore, JNK1 is required for FFA-mediated induction of proinflammatory cytokines in macrophages45. These studies, and those reporting that human omental adipose tissue from obese patients shows extensive macrophage infiltration46–48, support the hypothesis that the inflammatory response contributes to metabolic dysfunction in obesity.
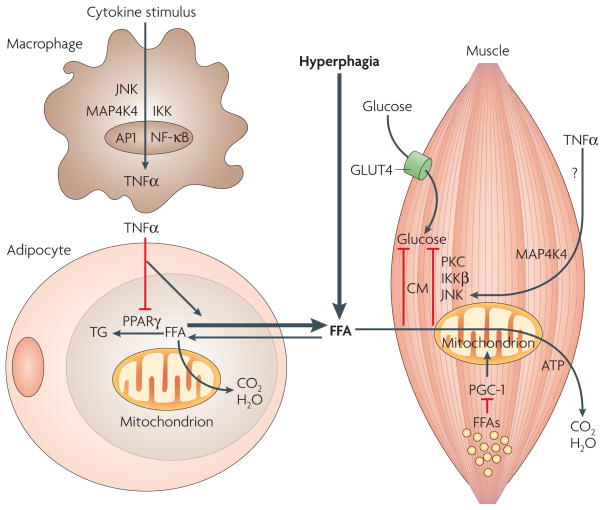
Figure 2. Chronic inflammation impairs triglyceride deposition in adipose tissue.
The chronic inflammatory state induced by lipid overload in adipose cells triggers macrophage recruitment within the adipose tissue. Secreted cytokines stimulate macrophages to produce large amounts of tumour-necrosis factor-α (TNFα) through the IKKβ–NF-κB (inhibitor of nuclear factor (NF)-κB (IκB) kinase-β–NF-κB) and the JNK–AP1 (Jun N-terminal kinase–activator protein-1) signalling pathways. MAP4K4 (mitogen-activated protein kinase kinase kinase kinase-4) might also be required for TNFα production in activated macrophages. The macrophage-secreted TNFα enhances lipolysis and downregulates peroxisome proliferator-activated receptor-γ (PPARγ)-mediated triglyceride (TG) biosynthesis and storage in adipocytes. Hyperphagia, combined with enhanced lipolysis and impaired TG sequestration triggered by TNFα, results in increased levels of circulating free fatty acids (FFAs) and TG deposition in the muscle. Ectopic lipid and FFAs (yellow dots) attenuate expresssion of genes that are involved in mitochondrial function, such as PPARγ co-activator-1 (PGC-1); enhance ceramide (CM) biosynthesis and inhibit insulin-stimulated glucose transport through activation of the protein kinases protein kinase C (PKC), IKKβ and JNK. TNFα can also inhibit insulin-stimulated glucose transporter type-4 (GLUT4) glucose transport in muscle through activation of MAP4K4 and JNK kinases.
Inflammation could also cause insulin resistance by a direct action of TNFα on muscle insulin signalling. Similar to FFA, exogenously administered TNFα in humans attenuates both insulin signalling and glucose transport in skeletal muscle, at least in part by triggering Ser/Thr phosphorylation of insulin receptor substrate (IRS) proteins49, key mediators of the metabolic actions of insulin (FIG. 2). This effect of TNFα requires the protein kinases JNK1 and MAP4K4 to cause insulin resistance in muscle49. Small interfering (si)RNA-mediated silencing of MAP4K4 completely restores insulin sensitivity in isolated skeletal-muscle tissue from diabetics49, in part by downregulating TNFα-mediated activation of JNK1/2 and extracellular signal-regulated kinase (ERK)1/2. Based on results by our group50,51 and others49, MAP4K4 seems to function in the signalling pathways that mediate some inhibitory effects of TNFα on insulin-regulated glucose transport in adipose tissue and skeletal muscle. Insulin resistance in type 2 diabetes might therefore occur in part through these direct actions of TNFα52,53. However, this hypothesis is tenuous because administration of anti-TNFα antibody to humans, which ameliorates arthritis54, or treatment with a TNFα antagonist55, had little or no effect on insulin resistance. Thus, in humans it is not clear whether TNFα exerts significant actions directly on skeletal muscle cells to inhibit insulin signalling.
Impaired insulin signalling in muscle
The activities of several protein Ser/Thr kinases have been implicated as mediators of fatty acid-induced insulin insensitivity in skeletal muscle. FFAs inhibit proximal insulin signalling steps, such as Tyr phosphorylation of insulin receptor and IRS proteins56. Several protein kinases, including JNK, IKKβ, protein kinase Cθ (PKCθ), mammalian target of rapamycin complex-1 (mTORC1) and p70 ribosomal S6 kinase (p70S6K), phosphorylate various Ser residues of IRS proteins57–60 and, in general, Ser phosphorylation negatively regulates IRS function.
Consistent with the hypothesis that these Ser/Thr kinases mediate the deleterious effects of fatty acids, increases in intracellular lipid metabolites, such as fatty acyl-CoA and diacylglycerol, activate PKC, which in turn phosphorylates and inhibits IRS signalling in the skeletal muscle of rodents and humans60–63. Conversely, insulin resistance induced by high-fat feeding is ameliorated by pharmacological inhibitors of these protein kinases. For example, high doses of salicylates, which inhibit IKKβ activity, promote insulin sensitivity and improve glucose tolerance in obese mice64 and in diabetic human patients65. Furthermore, mouse knockout models of PKCθ, JNK, IKKβ and p70S6K1 have resistance to high-fat diet-induced defects in insulin signalling58,62,64,66. Excess circulating fatty acids might also contribute to insulin resistance through the activation of Toll-like receptors (TLRs). Both TLR2 and TLR4 are required for FFA-induced insulin resistance in myotubes and in adipocytes67–69. It has been suggested that FFAs function through TLR4 on adipose cells and macrophages to induce inflammatory signalling and suppress insulin signalling70. A loss-of-function point mutation in TLR4 prevents diet-induced obesity and insulin resistance in mice fed on a high-fat diet, as well as saturated fatty acidinduced insulin resistance in isolated muscle from those mice71. Together these results suggest that activation of TLRs by FFAs and stimulation of protein Ser/Thr protein kinase cascades by derivatives, such as fatty acyl-CoA and diacylglycerol, lead to attenuation of insulin signalling and glucose transport in skeletal muscle (FIG. 2). Several studies have focused on skeletal muscle mitochondria defects as a potential cause of insulin resistance, perhaps through decreased elimination of intracellular fatty acids or their derivatives (BOX 2).
Box 2. Mitochondrial dysfunction and muscle insulin resistance.
As the site of the machinery for β-oxidation of fatty acids as well as the complete oxidation of fat and carbohydrate metabolites in the tricarboxylic acid (TCA) cycle and respiratory chain, mitochondria have a central role in fuel use. Impairment of the expression of genes that are required for mitochondrial function and reduced oxidative phosphorylation in pre-diabetic and age-related insulin resistance indicates a connection between mitochondrial dysfunction and metabolic disease131–133. Existence of such defects in healthy relatives of type 2 diabetics suggests that such defects might precede development of disease and could signal susceptibility134. In addition, some reports have suggested downregulation of mitochondrial function and decreased expression of genes, which encode proteins that catalyse oxidative phosphorylation, in response to high-fat feeding or lipid infusion135,136 and conversely, improvement in these parameters by decreased intracellular lipids137.138. One consequence of reduced mitochondrial function would be an impaired capacity to consume fatty acids through oxidative metabolism, which could in turn further exacerbate lipotoxicity and accumulation of intramuscular triglyceride139. However, several recent studies have indicated increased rather than reduced fatty acid oxidation capacity in rodent models of lipid-induced insulin resistance140,141. Such observations have led to the suggestion that lipid overload of mitochondria and increased fatty acid oxidation, rather than excess intracellular lipid per se, mediates insulin resistance in muscle141. Thus, although the precise role of mitochondrial function in lipid-induced insulin resistance remains unresolved, the importance of mitochondrial fuel use in muscle and its impact on metabolic disease is assured, and will probably be a fertile area of future investigation.
Another class of lipid metabolite that might exacerbate insulin resistance observed in obesity and type 2 diabetes is the sphingolipid ceramide. Ceramide biosynthesis is dependent on the availability of long-chain saturated fats, which participate in the initial and rate-limiting reaction that involves the condensation of palmitoyl-CoA and serine72,73. Increased ceramide biosynthesis induced by TNFα, glucocorticoids or saturated FFAs (FIG. 2) seems to impair insulin action on glucose uptake and glycogen synthesis through inhibition of insulin-stimulated AKT/protein kinase B (PKB) activation74–76. Pharmacological inhibitors and genetic changes that inhibit ceramide biosynthesis in rodents showed that decreased levels of ceramide ameliorate insulin resistance induced by glucocorticoids, saturated fat and obesity77. However, the increases in the levels of ceramide observed in obese rodents and humans are quite small, raising the question of whether the intracellular accumulation of this sphingolipid significantly contributes to insulin resistance that is associated with adiposity78,79.
Inflammation and adipocyte function
PPARγ downregulation by TNFα at multiple levels
What might be the main target of the inflammatory process observed in adipose tissue that drives adipose dysfunction? The chronic inflammatory state in adipose tissue is accompanied by the overproduction of cytokines such as TNFα, mostly by macrophages but also by adipocytes (FIG. 1). Many studies have established that TNFα affects adipocytes profoundly and results in the attenuation of insulin signalling and inhibition of adipogenesis. Thus, TNFα functions to compromise normal adipocyte functions, including optimal storage of triglycerides. We emphasize here the negative regulation of the nuclear hormone receptor peroxisome proliferator-activated receptor-γ (PPARγ) as a key element in mediating these effects of inflammatory cytokines. Given that PPARγ is an essential transcriptional regulator of adipogenesis and is required for maintenance of mature adipocyte function80,81, including triglyceride synthesis and storage, we propose that downregulation of PPARγ could strongly contribute to the effects of inflammatory cytokines on adipocytes.
Emerging evidence suggests that TNFα can affect PPARγ at multiple levels, including the transcription, translation and turnover of PPARγ mRNA and protein (see sites of TNFα action in FIG. 3). At the transcriptional level, TNFα influences cellular responses by altering gene expression through activation of the NF-κB and AP1 transcription factors; TNFα treatment of adipocytes activates these transcription factors through stimulation of IKKβ (for NF-κB) and MAP4K4 (for AP1) cascades (FIG. 3). Early studies revealed that TNFα potently decreases expression of numerous adipocyte specific genes and adipogenic transcription factors including C/EBPα and PPARγ82,83. Evidence in cultured adipocytes suggests that NF-κB signalling is required for transcriptional downregulation of PPARγ84. Although the details of transcriptional control of PPARγ have yet to be fully worked out, the rapid decrease in _PPAR_γ mRNA following TNFα addition to adipocytes is striking.
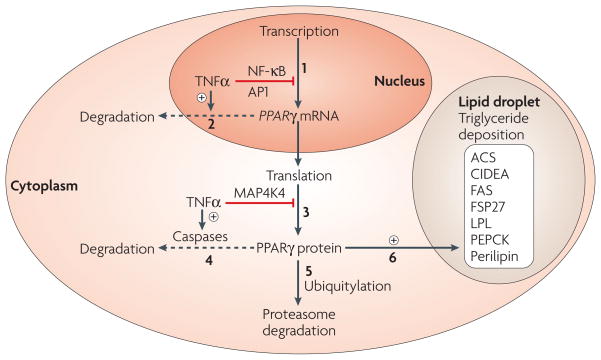
Figure 3. PPARγ downregulation by TNFα impairs triglyceride storage in adipose cells.
Peroxisome proliferator-activated receptor-γ (_PPAR_γ) expression can be regulated at the transcriptional level by tumour-necrosis factor-α (TNFα) (arrow 1) through the activation of nuclear factor-κB (NF-κB) and activator protein-1 (AP1), which negatively regulate _PPAR_γ expression82–84. Recent data indicate rapid turnover of _PPAR_γ mRNA in adipocytes50 and treatment of cultured adipocytes with TNFα might enhance _PPAR_γ mRNA degradation (dashed arrow 2). Translational control of PPARγ that is mediated by MAP4K4 (mitogen-activated protein kinase kinase kinase kinase-4), a protein kinase that is upregulated by TNFα, can also occur (arrow 3). Furthermore, activation of caspases by TNFα signalling might trigger PPARγ protein degradation in adipocytes (dashed arrow 4). Regulation of PPARγ activity and stability are also negatively regulated by kinase-mediated phosphorylation88 and ubiquitylation89, which promote PPARγ protein degradation through a proteasome-dependent pathway (arrow 5). TNFα action at multiple levels might therefore result in decreased PPARγ activity. Precise regulation of PPARγ expression and function can contribute to the control of triglyceride biosynthesis, hydrolysis and deposition in the lipid droplet — the lipid storage organelle of adipocytes. This can occur through the regulation of the expression of triglyceride metabolism enzymes such as phosphoenolpyruvate carboxykinase (PEPCK), fatty acid synthase (FAS), Acyl-CoA synthetase (ACS), lipoprotein lipase (LPL) and lipid-droplet proteins including CIDEA, FSP27 and perilipin (arrow 6). Dashed arrows that emanate from _PPAR_γ mRNA (step 2) and protein (step 4) indicate hypotheses that await definitive testing.
Recent studies have indicated that TNFα also exerts post-transcriptional regulation of PPARγ. We found that _PPAR_γ mRNA is rapidly degraded in adipocytes32 and that this turnover rate can be further enhanced by TNFα treatment. TNFα-mediated signalling pathways can also influence PPARγ levels at the level of protein translation. Our group recently discovered that MAP4K4 is a negative regulator of PPARγ protein expression and adipogenesis50. RNA interference (RNAi)-mediated silencing of MAP4K4 resulted in a twofold to threefold increase in the levels of both PPARγ1 and PPARγ2 proteins in 3T3-L1 adipocytes, without affecting mRNA levels85, which suggests that MAP4K4 regulates PPARγ at a post-transcriptional stage85. Although the degradation rates of PPARγ are remarkably rapid (t1/2 = 2 hours)32,86, abrogation of MAP4K4 had no effect on PPARγ protein degradation85. MAP4K4 expression itself is upregulated by TNFα treatment of adipocytes, through TNFα receptor-1 (TNFR1)50,51. Based on these preliminary results, we speculate that TNFα signals through MAP4K4 to downregulate PPARγ function by inhibiting PPARγ protein translation in cultured adipocytes (FIG. 3). Thus, the overall data suggest concerted actions of TNFα on transcription, mRNA stability and translation that control PPARγ protein levels (FIG. 3).
It is also noteworthy that activation of the caspase cascade by TNFα might downregulate PPARγ protein levels in adipose cells (FIG. 3, dashed arrow 4), as TNFα triggers a caspase-dependent signalling protein cleavage in cultured adipocytes87. Confirmation of this caspase-dependent pathway in adipocytes under physiological conditions is required to determine if this mechanism operates in vivo. PPARγ controls genes that encode enzymes involved in fatty acid esterification and triglyceride synthesis and sequestration, (FIG. 3), and therefore its disruption decreases triglyceride storage in adipocytes and increases lipid distribution to skeletal muscle and liver, which in turn leads to insulin resistance and type 2 diabetes.
TNFα-activated protein kinases might also directly target PPARγ protein, influencing PPARγ activity and/or stability by phosphorylation (reviewed in REF. 88). It has been proposed that phosphorylation of PPARγ promotes its degradation through a ubiquitin/proteasome dependent pathway89 (FIG. 3, arrow 5). PPARγ phosphorylation has also been proposed to decrease ligand-binding affinity of PPARγ, controlling its interaction with its coregulators90. Growth factors, such as epidermal growth factor (EGF) and platelet-derived growth factor (PDGF), decrease ligand-dependent transcriptional activity of PPARγ via increased phosphorylation through MAPK signalling in adipocytes91,92. Regulation of PPARγ function through phosphorylation might be functionally relevant in human subjects. A polymorphism observed in morbidly obese humans (Gln115–Pro115) prevents Ser112 phosphorylation of PPARγ and increases its activity93. These subjects seem to have greater than expected insulin sensitivity, consistent with the hypothesis that PPARγ-mediated increases in adipogenesis or adipocyte function sequester fatty acids away from skeletal muscle. Mice homozygous for the non-phosphorylated PPARγ protein with the Ser112 mutation maintained insulin sensitivity when fed a high-fat diet. This was associated with decreased adipocyte size, reduced plasma FFAs and increased secretion of adiponectins94. Together, these observations demonstrate that post-translational modification of PPARγ activity, through kinase-mediated phosphorylation, is physiologically active in the regulation of human metabolism.
Coregulators of PPARγ and other nuclear receptors
Yet another means whereby PPARγ function might be modulated by inflammatory signalling is through its interactions with co-activators and corepressors. Numerous such activities have been found in association with PPARγ and other nuclear receptors (reviewed in REF. 95). The net effect of PPARγ on energy storage (as triglyceride) compared with energy use (through oxidative pathways) depends in part on the balance among coregulators, which favour distinct pathways. Co-activators of PPARγ include steroid receptor co-activator-2 (SRC-2) and C-terminal-binding protein (CBP), which promote adipogenesis and energy storage95. The nuclear hormone corepressor receptor-interacting protein-140 (RIP140), however, negatively regulates oxidative pathways in adipocytes at least in part through interaction with PPARγ96,97. The importance of such coregulators in regulating energy balance is illustrated by the observation that a mouse knockout of the corepressor RIP140 was found to be resistant to diet-induced obesity and diabetes96. Conversely, PPARγ activity can be enhanced by the PPARγ co-activator-1α (PGC-1α), initially identified through its interaction with PPARγ in brown fat98,99. An important role for PGC-1 family members in regulating mitochondrial biogenesis and fatty acid oxidation in brown fat adaptive thermogenesis has been demonstrated, but their functions in white fat are less clear95.
Adipocyte lipolysis is enhanced by TNFα
Hormonal regulation of lipolysis in adipocytes provides a main switch between lipid storage versus lipid mobilization in response to dietary needs. In the fed state, insulin receptor stimulation in adipose cells results in activation of the phosphatidylinositol 3-kinase (PI3K)–AKT/PKB–phosphodiesterase-3 (PDE3) pathway with a consequent decrease in intracellular cyclic AMP (cAMP) (FIG. 4a). Conversely, in fasting conditions, activation of adrenoreceptors by catecholamines activates adenyl cyclase, thereby increasing cAMP levels. Increases in cAMP regulate the protein kinase A (PKA)-dependent phosphorylation of hormone-sensitive lipase (HSL)100–103. The phosphorylated, active HSL hydrolyses triglycerides in adipocyte fat droplets, which results in the release of fatty acids and glycerol. A second enzyme, adipose triglyceride lipase (ATGL), also participates in fat mobilization104. However, recent studies in human adipocytes suggest that ATGL is important for basal but not catecholamine-stimulated lipolysis that occurs during fasting105. The perilipin family of lipid-droplet proteins is involved in packaging of fat droplets and control of lipolysis106,107.
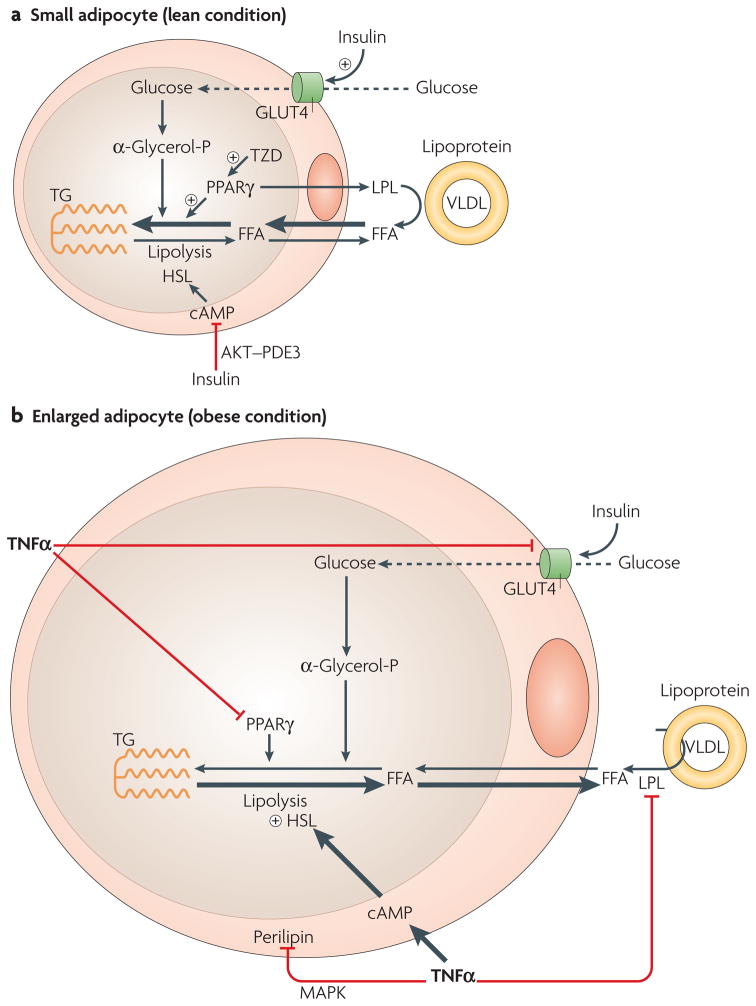
Figure 4. TNFα decreases triglyceride deposition and increases lipolysis in adipose cells.
a | In small adipocytes (lean condition), insulin promotes free fatty acid (FFA) esterification into triglycerides (TG, schematically represented as the three-carbon glycerol backbone with three acyl fatty acid chains (wavy lines)) through stimulation of glucose transporter type-4 (GLUT4)-mediated glucose uptake. Glucose can be converted to α-glycerol phosphate, the main source of the glycerol backbone of TG. Peroxisome proliferator-activated receptor-γ (PPARγ) activates lipoprotein lipase (LPL) expression and the TG biosynthetic pathway. Secreted LPL hydrolyses TG from circulating very low-density lipoprotein (VLDL), releasing FFAs to be re-esterified. Several thiazolidinediones (TZDs) can activate PPARγ. Insulin signalling also downregulates TG lipolysis through hormone-sensitive lipase (HSL). Insulin stimulation of the phosphatidylinositol 3-kinase (PI3K)–AKT/protein kinase B (PKB) pathway leads to activation of the enzyme phosphodiesterase-3 (PDE3). This enzyme catalyses the breakdown of cyclic AMP (cAMP) which in turn reduces activation of HSL. b | In enlarged adipocytes from inflamed fat tissue, high levels of tumour necrosis factor-α (TNFα) result in decreased fatty acid esterification and enhanced lipolysis. GLUT4, LPL and PPARγ protein levels are attenuated by TNFα, resulting in decreased glucose transport and fatty acid esterification. TNFα also has a stimulatory effect on lipolysis by increasing the levels of cAMP and activation (plus signal) of HSL, combined with the downregulation of perilipin through activation of the mitogen-activated protein kinase (MAPK) pathway.
Among the factors that contribute to enhanced lipolysis associated with obesity, TNFα and adipocyte size seem to be the most relevant. Secreted TNFα by macrophages and adipocytes within the adipose tissue of obese humans and animals108 chronically stimulates lipolysis109. TNFα has also been implicated in mediating insulin resistance in adipocytes through the suppression of insulin signalling110. It has been proposed that the mechanisms by which TNFα stimulates lipolysis in adipocytes involve attenuation of the antilipolytic action of insulin by reducing insulin signalling; enhancement of cAMP levels by downregulation of inhibitory G-proteincoupled receptors; and downregulation of function and expression of perilipin (FIG. 4b) (reviewed in REF. 109). Suppression of perilipin function by TNFα might facilitate association of HSL with triglyceride in the lipid droplet, thus increasing the basal lipolytic rate and enhancing fatty acid concentrations in the circulation.
Increased adipocyte size associated with obesity might also contribute to increased lipolytic activity101, based on a correlation between the rate of lipolysis and the size of adipocytes111. Levels of cAMP are elevated in larger adipocytes, resulting in increased PKA–HSL activation and lipolysis101. Larger adipocytes are known to be less insulin-sensitive112, enhancing the lipolytic rate through the impaired antilipolytic action of insulin. There is also a greater correlation between excess visceral fat and insulin resistance, compared with excess subcutaneous fat. Consistent with this, the lipolytic effect of catecholamines is enhanced in visceral fat tissue101. Mobilization of visceral fat increases the transport of FFAs to the liver through the portal vein and inhibits insulin signalling. Thus dysfunction of this adipose depot might be a particularly strong cause of hepatic insulin resistance and increased glucose output by the liver, as well as insulin resistance in skeletal muscle. In summary, TNFα-stimulated lipolysis, combined with TNFα-mediated inhibition of FFA esterification to triglyceride, caused by the downregulation of PPARγ noted above, further enhances circulating FFA levels (FIG. 4b).
Lipid droplets: targets in dysfunction?
In addition to triglyceride synthesis through PPARγ-mediated regulation of proteins (such as lipoprotein lipase (LPL)), phosphoenolpyruvate carboxykinase (PEPCK) and fatty acid synthase (FAS) enzymes promote the synthesis and deposition of adipocyte triglyceride80,84,113. PPARγ regulates a subset of proteins of the lipid droplet, a specialized organelle of adipocytes. Perilipin, the best-studied member of the PAT (perilipin, ADRP and TIP47-related protein)-domain family has well-characterized roles in both lipid-droplet formation and lipid-droplet-regulated lipolysis106. Overexpression and knockout studies suggest that perilipin stabilizes lipid droplets and shields triglyceride from active lipases. Like perilipin, the lipid-droplet protein S3–12 is adipocyte-specific and regulated by PPARγ114, although its functions are not well established. Another PAT family member, myocardial lipid-droplet protein (MLDP; also known as OXPAT or LSDP5), which is expressed in highly oxidative tissues, is a target of PPARγ in mouse and human white adipose tissue115. Fat-inducing transcript-1 (FIT1) and FIT2, members of an evolutionarily conserved family that is distinct from the PAT-domain group, are also required for the formation of lipid droplets116.
CIDE-domain-containing proteins
We have recently identified two members of a newly characterized family of lipid-droplet-associated proteins, which promote enlargement of adipocyte lipid droplets. Using siRNA screening techniques followed by functional analysis, we found that a highly expressed protein in adipocytes, FSP27 (also known as CIDEC), is localized with lipid storage droplets in cultured adipocytes117. FSP27 belongs to a family of proteins that contain CIDE domains, previously associated with regulating apoptosis118,119. Three CIDE family proteins are known in mammals (CIDEA, CIDEB and FSP27 in mice and CIDEA, CIDEB and CIDEC in humans)118. Analysis of CIDEA and FSP27 protein sequences revealed regions of low, but significant, similarity to the fat-droplet targeting and anchoring domains of perilipin120. A previous study noted the presence of FSP27 among many proteins that were co-isolated with lipid droplets from adipocytes121. Taken together, these observations suggested a novel role for CIDEA and FSP27 in lipid-droplet structure or function. Indeed, we found that these proteins greatly enhance lipid-droplet size when expressed in non-adipose cells117,120. By contrast, siRNA-based depletion of FSP27 causes fragmentation of lipid droplets and increases lipolysis in adipocytes117,120, similar to what is observed upon loss of perilipin122. A role of CIDEA in restraining lipolysis in human and mouse adipocytes has also been previously noted123. Thus, these CIDE-domain-containing proteins join a growing list of lipid droplet-associated proteins that function in the biogenesis, maturation or regulation of the triglyceride storage organelles and influence the ability of adipocytes to sequester lipids124.
Of special importance with respect to CIDEA and FSP27 is their expression in adipocytes under the influence of PPARγ. FSP27 expression increases over 50-fold during adipogenesis in cultured mouse 3T3-L1 cells, and is highly expressed in primary adipocytes from white adipose tissue117. Although Cidea mRNA levels are low in mouse white adipocytes, they rise almost an order of magnitude in response to rosiglitazone in these cells. Furthermore, Fsp27 mRNA measured by micro-arrays decreases more than fourfold following RNAi-mediated depletion of PPARγ in differentiated 3T3-L1 adipocytes32. Thus, based on their ability to markedly enhance triglyceride content and lipid-droplet size when overexpressed in adipocytes, CIDEA and FSP27 are potential important mediators of PPARγ to promote triglyceride deposition.
The role of lipid-droplet proteins in diabetes
New results have revealed a potential key role for lipid-droplet proteins in the development of insulin resistance and human diabetes. In these studies, lipid-droplet proteins in the adipose tissue of obese humans were analysed as a function of insulin sensitivity independent of BMI. Recent studies have shown that patients with high BMI values undergoing gastric bypass operations segregate into high and low insulin sensitivity, based on homeostatic model of insulin resistance (HOMA-IR) calculations125. Surprisingly, a significant population of these patients exhibit HOMA-IR values that are within the range of insulin-sensitive lean patients. This allowed us to compare CIDEA, FSP27 and perilipin expression levels in human subcutaneous and omental adipose tissues from obese subjects that are matched for BMI, but differ in HOMA-IR values. These experiments revealed that the levels of mRNAs that encode these lipid droplet-associated proteins inversely correlate with insulin sensitivity in the BMI-matched subjects120.
CIDEA in particular shows a highly significant sixfold increase in its expression in both omental and subcutaneous adipose tissues in obese patients with a low HOMA-IR index (<2.3; high insulin sensitivity) compared with the insulin resistant group. Perilipin expression is also elevated in omental and subcutaneous adipose tissue from the low HOMA-IR group, whereas FSP27 was elevated in omental adipose tissue from this group120. Consistent with these findings, an inverse correlation between CIDEA expression in adipose tissue samples and whole-body insulin resistance was observed in lean insulin-sensitive compared with obese insulin-resistant human subjects123. Although further studies will be required to rigorously test whether these proteins directly influence insulin sensitivity in humans, the data suggest important roles for CIDEA, FSP27 and potentially other lipid-droplet proteins in maintaining triglyceride stores in lean and obese individuals.
Based on the above considerations, we propose the hypothesis that elevated expression of CIDEA and other lipid-droplet proteins in adipose cells of a subset of obese individuals improves their ability to sequester triglyceride and other lipids, and decreases their release of fatty acids into the circulation (FIG. 5). This in turn hypothetically attenuates the deleterious consequences of excess circulating FFAs, preserving insulin sensitivity in skeletal muscle. Furthermore, as targets of PPARγ in adipose tissue, decreased lipid-droplet protein expression might reflect downregulation of PPARγ function by inflammatory cytokines such as TNFα, causing excess mobilization of fatty acids from adipocyte stores. Upregulation of FSP27 and perilipin expression by rosiglitazone might also be associated with fat sequestration and the insulin-sensitizing effects of the drug in humans. Indeed, a recent study shows increased perilipin expression in subcutaneous fat of rosiglitazone-treated fatty rats126, consistent with this hypothesis and our data from mice.
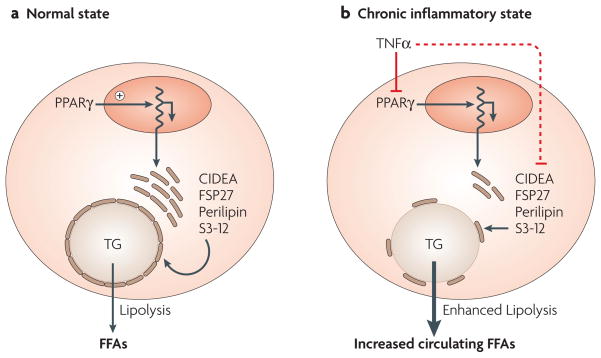
Figure 5. TNFα downregulates lipid-droplet proteins and enhances lipolysis in adipose cells.
a | In the normal state, peroxisome proliferator-activated receptor-γ (PPARγ) function drives the expression of lipid-droplet proteins, such as FSP27, CIDEA, perilipin, ADRP and S3–12 in adipose cells. Their presence on lipid droplets inhibits basal lipolysis and free fatty acid (FFA) release, thereby promoting net triglyceride (TG) storage. b | We propose that in chronic inflammatory states, tumour-necrosis factor-α (TNFα) downregulates PPARγ function, attenuating the expression of these lipid-droplet proteins. With reduced levels of these proteins, their ability to shield and protect the lipid droplet from active lipases might contribute to enhanced TG lipolysis and circulating FFAs. Additionally, post-transcriptional regulation of lipid-droplet proteins by TNFα can occur (dashed inhibitory red line).
Concluding remarks
Work over the past decade has provided a framework for our understanding of the functions of adipose tissue in metabolic disease. The role of adipose tissue as a dominant regulator of whole-body lipid and glucose homeostasis is now well established, based on extensive experimental evidence, which shows that dysfunctions in adipose tissue metabolism have a direct impact on lipid and glucose homeostasis. Indeed, the combination of hyperphagia and adipose dysfunction seems to underlie important metabolic pathologies, such as insulin resistance, type 2 diabetes and cardiovascular diseases. Many observations reinforce the concept that normal lipid and glucose homeostasis as well as normal insulin sensitivity requires fully functional adipose tissue. Adipose dysfunctions in obesity include secretions of abnormal levels of cytokines linked to insulin resistance, impairments in triglyceride storage and increases in lipolysis. These abnormalities in turn can contribute to increased fatty acids in the circulation and lead to an overload of fatty acids in the skeletal muscle and the liver. Such increases in fatty acids in these compartments are likely to cause decreased responsiveness to insulin in these tissues in obesity.
Research in the field has also established the important concept that obesity can lead to a chronic inflammatory state within adipose tissue depots, which at least in part causes the adipocyte dysfunctions described above. Hypertrophied adipocytes in obese subjects secrete large amounts of the macrophage chemoattractant MCP-1, perhaps contributing to macrophage infiltration into adipose tissue. Other inflammatory cytokines probably have important roles and others remain to be discovered. Importantly, significant progress in understanding the mechanisms whereby cytokines act on adipose tissue to disrupt triglyceride storage and increase lipolysis has been made. These studies highlight the negative regulation by cytokines like TNFα of gene products that are crucial for lipid storage, such as the transcriptional factor PPARγ and its downstream target genes that control fatty acid synthesis, esterification and sequestration as triglyceride in lipid droplets. Intriguingly, recent data implicate perilipin and the novel lipid-droplet-associated proteins FSP27 and CIDEA as key regulated proteins in human obesity. Expression levels of these proteins seem to inversely correlate with insulin resistance in multiple studies. These new data lead us to present the hypothesis that lipid-droplet proteins function to strongly sequester triglyceride within lipid droplets of adipocytes, which might represent a contributing factor in the varying degrees of insulin resistance observed among obese human subjects.
Acknowledgments
We thank the members of our laboratory group for excellent discussions on the issues addressed here. We acknowledge funding for our studies covered here by the National Institutes of Health (grants DK030648 and DK030898), including the University of Massachusetts Medical School Diabetes and Endocrinology Research Center (DK32520).
White adipose tissue
The predominant fat storage tissue in animals, consisting mostly of adipocytes but also other cell types such as mast cells and macrophages. It is distributed in a number of subcutaneous and visceral depots
Free fatty acid
A carboxylic acid with aliphatic chains of 4–28 carbons, which can be esterified with glycerol to form triglycerides, the main stored form of lipid
Acipimox
A nicotinic-acid analogue that reduces plasma and intracellular fatty acid levels by suppressing lipolysis
Adipokine
A cytokine or hormone that is secreted by adipose tissue
Lipodystrophy
Abnormality of the adipose tissue that is associated with total or partial loss of body fat. This might have a genetic origin in humans or occur as a result of other diseases
MAP4K4
(Mitogen-activated protein kinase kinase kinase kinase-4). A mammalian S er/Thr protein kinase related to Saccharomyces cerevisiae Sterile-20 (STE20)
Omental adipose tissue
The fat depot found within the peritoneum, in close association with the stomach and other internal organs
Diacylglycerol
A molecule that consists of two fatty acid chains esterified with glycerol, produced by the cleavage of membrane phosphatidylinositol 4,5-bisphosphate. Diacylglycerol functions as a signalling molecule by activating protein kinase C
Salicylates
A group of derivatives of salicylic acid, including aspirin and acetylsalicylic acid, which are widely used as analgesics and anti-inflammatory medications
Toll-like receptors
Cell-surface receptors that recognize a wide variety of molecules, primarily markers of foreign organisms including bacteria. Main functions include the activation of the innate immune response in infection
Sphingolipids
Lipids that consist of the aliphatic alcohol sphingosine linked to a fatty acid chain and a variety of head groups. Sphingolipids include ceramides, sphingomyelin and glycosphingolipids
Glucocorticoids
Steroid hormones, including cortisol, that are produced in the adrenal gland. These hormones have potent effects on energy metabolism in the liver, fat and elsewhere
C/EBPα
(CCAAT/enhancer-binding protein-α). A transcription factor that has a key role in the differentiation of adipocytes
Adrenoreceptors
A class of G-protein-coupled receptors activated by catecholamines. Adrenoreceptors in white fat stimulate lipolysis in response to catecholamines
Catecholamines
A group of amine hormones including adrenaline and noradrenaline. These are produced by the adrenal gland in response to starvation and other stresses
Perilipin
A main protein component of adipocyte lipid droplets, which surrounds the lipids and participates in the regulation of lipolysis
Rosiglitazone
An antidiabetic drug, one of several thiazolidinediones (TZDs) used therapeutically. Many TZDs are known to be high-affinity ligand activators of PPARγ
HOMA-IR
(Homeostatic model assessment of insulin resistance). An estimation of the degree of insulin resistance that is calculated from clinical measurement of fasting blood glucose and insulin levels
Footnotes
References
- 1.Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116:337–350. doi: 10.1016/s0092-8674(03)01081-x. [DOI] [PubMed] [Google Scholar]
- 2.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 3.Alberti KG. The costs of non-insulin-dependent diabetes mellitus. Diabet Med. 1997;14:7–9. doi: 10.1002/(SICI)1096-9136(199701)14:1<7::AID-DIA321>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Clee SM, Attie AD. The genetic landscape of type 2 diabetes in mice. Endocr Rev. 2007;28:48–83. doi: 10.1210/er.2006-0035. [DOI] [PubMed] [Google Scholar]
- 5.Sims EA, et al. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res. 1973;29:457–496. doi: 10.1016/b978-0-12-571129-6.50016-6. [DOI] [PubMed] [Google Scholar]
- 6.Freidenberg GR, Reichart D, Olefsky JM, Henry RR. Reversibility of defective adipocyte insulin receptor kinase activity in non-insulin-dependent diabetes mellitus. Effect of weight loss. J Clin Invest. 1988;82:1398–1406. doi: 10.1172/JCI113744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn SE. The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 8.Butler AE, et al. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 9.Rhodes CJ. Type 2 diabetes—a matter of β-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 10.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 11.Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 12.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. A recent review focusing on studies of lipid effects on insulin resistance in human subjects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. Demonstrates that infusion of free fatty acids acutely induces insulin resistance in human subjects. [PubMed] [Google Scholar]
- 14.Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest. 1993;92:91–98. doi: 10.1172/JCI116603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santomauro AT, et al. Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48:1836–1841. doi: 10.2337/diabetes.48.9.1836. [DOI] [PubMed] [Google Scholar]
- 16.Oakes ND, et al. Diet-induced muscle insulin resistance in rats is ameliorated by acute dietary lipid withdrawal or a single bout of exercise: parallel relationship between insulin stimulation of glucose uptake and suppression of long-chain fatty acyl-CoA. Diabetes. 1997;46:2022–2028. doi: 10.2337/diab.46.12.2022. [DOI] [PubMed] [Google Scholar]
- 17.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 18.Perseghin G, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48:1600–1606. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 19.Krssak M, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 20.Szczepaniak LS, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–E989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 21.Sovik O, Vestergaard H, Trygstad O, Pedersen O. Studies of insulin resistance in congenital generalized lipodystrophy. Acta Paediatr Suppl. 1996;413:29–37. doi: 10.1111/j.1651-2227.1996.tb14263.x. [DOI] [PubMed] [Google Scholar]
- 22.Moitra J, et al. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laustsen PG, et al. Lipoatrophic diabetes in Irs1−/−/Irs3−/− double knockout mice. Genes Dev. 2002;16:3213–3222. doi: 10.1101/gad.1034802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimomura I, et al. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998;12:3182–3194. doi: 10.1101/gad.12.20.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arioglu E, Rother KI, Reitman ML, Premkumar A, Taylor SI. Lipoatrophy syndromes: when ‘too little fat’ is a clinical problem. Pediatr Diabetes. 2000;1:155–168. doi: 10.1034/j.1399-5448.2000.010307.x. [DOI] [PubMed] [Google Scholar]
- 26.Oral EA, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 27.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 28.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 29.Unger RH, Orci L. Diseases of liporegulation: new perspective on obesity and related disorders. FASEB J. 2001;15:312–321. doi: 10.1096/fj.00-0590. [DOI] [PubMed] [Google Scholar]
- 30.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalderon B, Mayorek N, Berry E, Zevit N, Bar-Tana J. Fatty acid cycling in the fasting rat. Am J Physiol Endocrinol Metab. 2000;279:E221–E227. doi: 10.1152/ajpendo.2000.279.1.E221. [DOI] [PubMed] [Google Scholar]
- 32.Christianson JL, Nicoloro S, Straubhaar J, Czech MP. Stearoyl CoA desaturase 2 is required for PPARγ expression and adipogenesis in cultured 3T3-L1 cells. J Biol Chem. 2007;283:2906–2916. doi: 10.1074/jbc.M705656200. [DOI] [PubMed] [Google Scholar]
- 33.Frayn KN, et al. Regulation of fatty acid movement in human adipose tissue in the postabsorptive-to-postprandial transition. Am J Physiol. 1994;266:E308–E317. doi: 10.1152/ajpendo.1994.266.3.E308. [DOI] [PubMed] [Google Scholar]
- 34.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21:1443–1455. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 35.Rajala MW, Scherer PE. Minireview: the adipocyte-at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 36.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 37.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curat CA, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 40.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inouye KE, et al. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes. 2007;56:2242–2250. doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- 42.Lagathu C, et al. Long-term treatment with interleukin-1β induces insulin resistance in murine and human adipocytes. Diabetologia. 2006;49:2162–2173. doi: 10.1007/s00125-006-0335-z. [DOI] [PubMed] [Google Scholar]
- 43.Kanda H, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature. 1997;389:610–614. doi: 10.1038/39335. Strongly suggests a role for an inflammatory cytokine in mediating insulin resistance. [DOI] [PubMed] [Google Scholar]
- 45.Solinas G, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 46.Harman-Boehm I, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92:2240–2247. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- 47.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290:E961–E967. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 48.Cancello R, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 49.Bouzakri K, Zierath JR. MAP4K4 gene silencing in human skeletal muscle prevents tumor necrosis factor-α-induced insulin resistance. J Biol Chem. 2007;282:7783–7789. doi: 10.1074/jbc.M608602200. [DOI] [PubMed] [Google Scholar]
- 50.Tang X, et al. An RNA interference-based screen identifies MAP4K4/NIK as a negative regulator of PPARγ, adipogenesis, and insulin-responsive hexose transport. Proc Natl Acad Sci USA. 2006;103:2087–2092. doi: 10.1073/pnas.0507660103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tesz GJ, et al. Tumor necrosis factor α (TNFα) stimulates Map4k4 expression through TNFα receptor 1 signaling to c-Jun and activating transcription factor 2. J Biol Chem. 2007;282:19302–19312. doi: 10.1074/jbc.M700665200. [DOI] [PubMed] [Google Scholar]
- 52.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 54.Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R. Effects of an engineered human anti-TNF-α antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes. 1996;45:881–885. doi: 10.2337/diab.45.7.881. [DOI] [PubMed] [Google Scholar]
- 55.Paquot N, Castillo MJ, Lefebvre PJ, Scheen AJ. No increased insulin sensitivity after a single intravenous administration of a recombinant human tumor necrosis factor receptor: Fc fusion protein in obese insulin-resistant patients. J Clin Endocrinol Metab. 2000;85:1316–1319. doi: 10.1210/jcem.85.3.6417. [DOI] [PubMed] [Google Scholar]
- 56.Kraegen EW, Cooney GJ, Ye JM, Thompson AL, Furler SM. The role of lipids in the pathogenesis of muscle insulin resistance and β cell failure in type II diabetes and obesity. Exp Clin Endocrinol Diabetes. 2001;109 (Suppl 2):S189–S201. doi: 10.1055/s-2001-18581. [DOI] [PubMed] [Google Scholar]
- 57.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH2-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser307. J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 58.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 59.Gao Z, et al. Serine phosphorylation of insulin receptor substrate 1 by inhibitor κB kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 60.Griffin ME, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C θ and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 61.Yu C, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 62.Kim JK, et al. PKC-θ knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004;114:823–827. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dresner A, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkβ. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 65.Hundal RS, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Um SH, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431:200–205. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 67.Senn JJ. Toll-like receptor-2 is essential for the development of palmitate-induced insulin resistance in myotubes. J Biol Chem. 2006;281:26865–26875. doi: 10.1074/jbc.M513304200. [DOI] [PubMed] [Google Scholar]
- 68.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 2006;346:739–745. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- 69.Suganami T, et al. Role of the Toll-like receptor 4/NF-κB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 70.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsukumo DM, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 72.Hannun YA. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- 73.Shimabukuro M, et al. Lipoapoptosis in β-cells of obese prediabetic fa/fa rats. Role of serine palmitoyltransferase overexpression. J Biol Chem. 1998;273:32487–32490. doi: 10.1074/jbc.273.49.32487. [DOI] [PubMed] [Google Scholar]
- 74.Summers SA, Garza LA, Zhou H, Birnbaum MJ. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol Cell Biol. 1998;18:5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS. Intracellular ceramide synthesis and protein kinase Cζ activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J. 2004;382:619–629. doi: 10.1042/BJ20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Summers SA, Nelson DH. A role for sphingolipids in producing the common features of type 2 diabetes, metabolic syndrome X, and Cushing’s syndrome. Diabetes. 2005;54:591–602. doi: 10.2337/diabetes.54.3.591. [DOI] [PubMed] [Google Scholar]
- 77.Holland WL, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 78.Turinsky J, O’Sullivan DM, Bayly BP. 1, 2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem. 1990;265:16880–16885. [PubMed] [Google Scholar]
- 79.Adams JM, et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 80.Tamori Y, Masugi J, Nishino N, Kasuga M. Role of peroxisome proliferator-activated receptor-γ in maintenance of the characteristics of mature 3T3-L1 adipocytes. Diabetes. 2002;51:2045–2055. doi: 10.2337/diabetes.51.7.2045. [DOI] [PubMed] [Google Scholar]
- 81.Imai T, et al. Peroxisome proliferator-activated receptor γ is required in mature white and brown adipocytes for their survival in the mouse. Proc Natl Acad Sci USA. 2004;101:4543–4547. doi: 10.1073/pnas.0400356101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang B, et al. Negative regulation of peroxisome proliferator-activated receptor-γ gene expression contributes to the antiadipogenic effects of tumor necrosis factor-α. Mol Endocrinol. 1996;10:1457–1466. doi: 10.1210/mend.10.11.8923470. [DOI] [PubMed] [Google Scholar]
- 83.Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-α-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem. 1997;272:971–976. doi: 10.1074/jbc.272.2.971. [DOI] [PubMed] [Google Scholar]
- 84.Ruan H, Hacohen N, Golub TR, Van Parijs L, Lodish HF. Tumor necrosis factor-α suppresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes: nuclear factor-κB activation by TNF-α is obligatory. Diabetes. 2002;51:1319–1336. doi: 10.2337/diabetes.51.5.1319. [DOI] [PubMed] [Google Scholar]
- 85.Puri V, Virbasius JV, Guilherme A, Czech MP. RNAi screens reveal novel metabolic regulators: RIP140, MAP4k4 and the lipid droplet associated fat specific protein (FSP) 27. Acta Physiol (Oxf) 2008;192:103–115. doi: 10.1111/j.1748-1716.2007.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Floyd ZE, Stephens JM. Interferon-γ-mediated activation and ubiquitin-proteasome-dependent degradation of PPARγ in adipocytes. J Biol Chem. 2002;277:4062–4068. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- 87.Medina EA, et al. Tumour necrosis factor-α decreases Akt protein levels in 3T3-L1 adipocytes via the caspase-dependent ubiquitination of Akt. Endocrinology. 2005;146:2726–2735. doi: 10.1210/en.2004-1074. [DOI] [PubMed] [Google Scholar]
- 88.Diradourian C, Girard J, Pegorier JP. Phosphorylation of PPARs: from molecular characterization to physiological relevance. Biochimie. 2005;87:33–38. doi: 10.1016/j.biochi.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 89.Hauser S, et al. Degradation of the peroxisome proliferator-activated receptor γ is linked to ligand-dependent activation. J Biol Chem. 2000;275:18527–18533. doi: 10.1074/jbc.M001297200. [DOI] [PubMed] [Google Scholar]
- 90.Shao D, et al. Interdomain communication regulating ligand binding by PPAR-γ. Nature. 1998;396:377–380. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- 91.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinasemediated phosphorylation of PPARγ. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 92.Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor γ activity by mitogen-activated protein kinase. J Biol Chem. 1997;272:10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 93.Ristow M, Muller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med. 1998;339:953–959. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- 94.Rangwala SM, et al. Genetic modulation of PPARγ phosphorylation regulates insulin sensitivity. Dev Cell. 2003;5:657–663. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- 95.Feige JN, Auwerx J. Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol. 2007;17:292–301. doi: 10.1016/j.tcb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 96.Leonardsson G, et al. Nuclear receptor corepressor RIP140 regulates fat accumulation. Proc Natl Acad Sci USA. 2004;101:8437–8442. doi: 10.1073/pnas.0401013101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Powelka AM, et al. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J Clin Invest. 2006;116:125–136. doi: 10.1172/JCI26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu Z, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 99.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Large V, Peroni O, Letexier D, Ray H, Beylot M. Metabolism of lipids in human white adipocyte. Diabetes Metab. 2004;30:294–309. doi: 10.1016/s1262-3636(07)70121-0. [DOI] [PubMed] [Google Scholar]
- 101.Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005;19:471–482. doi: 10.1016/j.beem.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 102.Degerman E, Resjo S, Landstrom TR, Manganiello V. Methods to study phosphorylation and activation of the hormone-sensitive adipocyte phosphodiesterase type 3B in rat adipocytes. Methods Mol Biol. 2001;155:167–180. doi: 10.1385/1-59259-231-7:167. [DOI] [PubMed] [Google Scholar]
- 103.Granneman JG, Moore HP. Location, location: protein trafficking and lipolysis in adipocytes. Trends Endocrinol Metab. 2008;19:3–9. doi: 10.1016/j.tem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 104.Zimmermann R, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 105.Ryden M, et al. Comparative studies of the role of hormone-sensitive lipase and adipose triglyceride lipase in human fat cell lipolysis. Am J Physiol Endocrinol Metab. 2007;292:E1847–E1855. doi: 10.1152/ajpendo.00040.2007. [DOI] [PubMed] [Google Scholar]
- 106.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 107.Ducharme NA, Bickel PE. Minireview: lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. doi: 10.1210/en.2007-1713. Summarizes recent developments in the field of lipid-droplet structure and function. [DOI] [PubMed] [Google Scholar]
- 108.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Langin D, Arner P. Importance of TNFα and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol Metab. 2006;17:314–320. doi: 10.1016/j.tem.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 110.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-α in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Engfeldt P, Arner P. Lipolysis in human adipocytes, effects of cell size, age and of regional differences. Horm Metab Res Suppl. 1988;19:26–29. [PubMed] [Google Scholar]
- 112.Czech MP. Cellular basis of insulin insensitivity in large rat adipocytes. J Clin Invest. 1976;57:1523–1532. doi: 10.1172/JCI108422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tontonoz P, Hu E, Devine J, Beale EG, Spiegelman BM. PPAR γ 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1995;15:351–357. doi: 10.1128/mcb.15.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dalen KT, et al. Adipose tissue expression of the lipid droplet-associating proteins S3–12 and perilipin is controlled by peroxisome proliferator-activated receptor-γ. Diabetes. 2004;53:1243–1252. doi: 10.2337/diabetes.53.5.1243. [DOI] [PubMed] [Google Scholar]
- 115.Wolins NE, et al. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55:3418–3428. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 116.Kadereit B, et al. Evolutionarily conserved gene family important for fat storage. Proc Natl Acad Sci USA. 2008;105:94–99. doi: 10.1073/pnas.0708579105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Puri V, et al. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem. 2007;282:34213–34218. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 118.Liang L, Zhao M, Xu Z, Yokoyama KK, Li T. Molecular cloning and characterization of CIDE-3, a novel member of the cell-death-inducing DNA-fragmentation-factor (DFF45)-like effector family. Biochem J. 2003;370:195–203. doi: 10.1042/BJ20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Inohara N, Koseki T, Chen S, Wu X, Nunez G. CIDE, a novel family of cell death activators with homology to the 45 kDa subunit of the DNA fragmentation factor. EMBO J. 1998;17:2526–2533. doi: 10.1093/emboj/17.9.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Puri V, et al. Cidea: a novel lipid droplet protein associated with insulin sensitivity in humans. Proc Natl Acad Sci USA. (in the press) [Google Scholar]
- 121.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 122.Martinez-Botas J, et al. Absence of perilipin results in leanness and reverses obesity in Leprdb/db mice. Nature Genet. 2000;26:474–479. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- 123.Nordstrom EA, et al. A human-specific role of cell death-inducing DFFA (DNA fragmentation factor-α)-like effector A (CIDEA) in adipocyte lipolysis and obesity. Diabetes. 2005;54:1726–1734. doi: 10.2337/diabetes.54.6.1726. [DOI] [PubMed] [Google Scholar]
- 124.Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 125.Perugini RA, et al. Metabolic characterization of nondiabetic severely obese patients undergoing Roux-en-Y gastric bypass: preoperative classification predicts the effects of gastric bypass on insulinglucose homeostasis. J Gastrointest Surg. 2007;11:1083–1090. doi: 10.1007/s11605-007-0158-3. [DOI] [PubMed] [Google Scholar]
- 126.Kim HJ, et al. Depot-specific regulation of perilipin by rosiglitazone in a diabetic animal model. Metabolism. 2007;56:676–685. doi: 10.1016/j.metabol.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 127.Smith RE, Horwitz BA. Brown fat and thermogenesis. Physiol Rev. 1969;49:330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- 128.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 129.Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals. Circ Res. 2007;101:27–39. doi: 10.1161/CIRCRESAHA.107.151621. [DOI] [PubMed] [Google Scholar]
- 130.Bartness TJ, Song CK. Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res. 2007;48:1655–1672. doi: 10.1194/jlr.R700006-JLR200. [DOI] [PubMed] [Google Scholar]
- 131.Mootha VK, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 132.Patti ME, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Petersen KF, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Richardson DK, et al. Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J Biol Chem. 2005;280:10290–10297. doi: 10.1074/jbc.M408985200. [DOI] [PubMed] [Google Scholar]
- 136.Sparks LM, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 137.Petersen KF, et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Reznick RM, Shulman GI. The role of AMP-activated protein kinase in mitochondrial biogenesis. J Physiol. 2006;574:33–39. doi: 10.1113/jphysiol.2006.109512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55 (Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Turner N, et al. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 2007;56:2085–2092. doi: 10.2337/db07-0093. [DOI] [PubMed] [Google Scholar]
- 141.Koves TR, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]