Long Term Results of the Children’s Cancer Group Studies for Childhood Acute Lymphoblastic Leukemia 1983–2002: a Children’s Oncology Group Report (original) (raw)
. Author manuscript; available in PMC: 2010 Aug 1.
Published in final edited form as: Leukemia. 2009 Dec 17;24(2):285–297. doi: 10.1038/leu.2009.262
Abstract
The Children’s Cancer Group enrolled 13,298 young people age < 21 years on one of 16 protocols between 1983 and 2002. Outcomes were examined in three time periods, 1983–1988, 1989–1995, 1996–2002. Over the three intervals, 10-year event-free survival (EFS) for Rome/NCI standard risk and higher risk B-precursor patients was 68% and 58%, 77% and 63%, and 78% and 67%, respectively; while for standard risk and higher risk T-cell patients, EFS was 65% and 56%, 78% and 68%, and 70% and 72%, respectively. Five-year EFS for infants was 36%, 38%, and 43%, respectively. Seminal randomized studies led to a number of important findings. Stronger post induction intensification improved outcome for both standard and higher risk patients. With improved systemic therapy, additional IT methotrexate effectively replaced cranial radiation. For standard risk patients receiving three-drug induction, iso-toxic substitution of dexamethasone for prednisone improved EFS. Pegylated asparaginase safely and effectively replaced native asparaginase. Thus, rational therapy modifications yielded better outcomes for both standard and higher risk patients. These trials provide the platforms for current Children’s Oncology Group trials.
Keywords: Acute lymphoblastic leukemia, children, randomized clinical trials
Introduction
Children’s Oncology Group (COG) member institutions care for the majority of infants, children, and adolescents with Acute Lymphoblastic Leukemia in North America and Oceania. Work by the legacy Children’s Cancer Group (CCG), dating back more than 40 years, serves as the foundation for many current COG trials. Studies prior to 1983 built on the pioneering work of Donald Pinkel and his colleagues at St. Jude Children’s Research Hospital,(1) and introduced Berlin Frankfurt Münster (BFM)-based post induction intensification (Protocol Ib or Consolidation and Protocol II or Delayed Intensification (DI),(2) and a widely-used age-based dosing schedule for IT (IT) therapy.(3) The prognostic significance of early marrow response, assessed by marrow blast percentage 7 and 14 days into induction, was defined. (4) Event-free survival (EFS) improved with vincristine and prednisone pulses as the sole post-induction intensification and extended maintenance IT methotrexate replaced pre-symptomatic whole brain irradiation for lowest risk patients.(5)
Thirteen trials, conducted from 1983 through 1995 were summarized in the December 2000 issue of Leukemia. (6) Two 1983–1988 studies (100 series), namely, CCG-106 (7) and CCG-123, (8) proved the advantage of early BFM-based strategies, prior to the introduction of BFM protocol M or methotrexate 5 g/m2, over previous CCG efforts for higher risk (HR) children. A third study, CCG-105, showed that more effective systemic therapy and extended IT methotrexate could spare all CNS negative standard risk (SR) children from whole brain irradiation and proved the value of post induction intensification.(9, 10) Induction anthracycline, higher dose induction prednisone, and intensive consolidation added no further benefit for standard risk patients receiving DI and vincristine-prednisone pulses in maintenance. The 1989–1995 studies (1800 series) further restricted whole brain irradiation (11) and showed the value of longer and stronger post-induction intensification, the so-called “Augmented BFM” regimen for HR patients (12) and dexamethasone for SR patients. (13) Patients received monthly vincristine and prednisone or dexamethasone pulses through maintenance in all of these trials, unlike contemporary BFM practice.
This report provides further follow-up on past 1983–1995 studies, and adds 4 additional trials and 3482 additional patients from 1996–2002 (1900 series). Replacement of 6-mercaptopurine (6MP) with 6-thioguanine (6TG) provided an EFS advantage but with unacceptable liver toxicity for SR patients on CCG-1952.(14) IT triple therapy, i.e., cytarabine, methotrexate, and hydrocortisone, halved CNS relapse rates compared to IT methotrexate alone but allowed excess marrow and testes relapses on a methotrexate-poor platform, resulting in an inferior survival.(15) CCG-1962 showed that pegylated asparaginase safely and effectively replaced native asparaginase.(16) CCG-1961 explored the components of the Augmented “BFM” regimen for higher risk patients with a rapid Day 7 response and showed the advantage for stronger, not longer post induction intensification.(17) This report excludes the final CCG trial, CCG-1991, which completed accrual only in 2005.(18, 19)
Materials and Methods
The Clinical Trials Evaluation Panel of the National Cancer Institute of the United States approved all protocols. Local Institutional Review Board approval and written individual/parental informed consent were required. Details of all studies have been published.
Between 1983 and 2002, 13,298 infants, children, and adolescents, age < 21 years at diagnosis enrolled on one of 16 treatment protocol. The diagnosis of ALL was based on morphology,(20) histochemistry, and increasingly on flow cytometry. Patients with FAB L3 morphology and myeloperoxidase positivity were excluded.
Between 1983 and 1988, a total of 3713 eligible, evaluable patients were entered on the CCG-100 series studies. Patients were stratified by age, white blood cell count (WBC), gender, platelet count, FAB classification,(21) and lymphomatous features.(22) The ‘lowest risk’ patients received vincristine, prednisone, and L-asparaginase during induction, IT methotrexate in induction, consolidation, and maintenance, and daily oral 6MP, weekly oral methotrexate, and monthly vincristine/prednisone pulses in maintenance on CCG-104. With a 2×2×2 factorial design, ‘intermediate risk’ patients were randomly allocated to receive standard or intensive induction/consolidation, DI or no intensification, and 18 Gy cranial irradiation or every 12 week IT methotrexate on CCG-105.(9, 10) A small number of intermediate risk patients were enrolled on CCG-139 and received either intermediate dose methotrexate 0.5 g/m2 with leucovorin rescue or oral methotrexate. No patient received DI.(23) ‘Higher risk’ patients with lymphomatous features were randomly allocated to LSA2L2 with or without cranial irradiation, the New York (NY) I regimen, or the CCG modified BFM regimen on CCG-123.(8) ‘Higher risk’ patients without lymphomatous features were randomly allocated to the standard CCG regimen, NY I regimen, or the CCG-modified BFM regimen on CCG-106.(7) Infants were treated on CCG-107, which employed very high-dose methotrexate (33.6 g/m2) with leucovorin rescue. (24)
Between 1989 and 1995, a total of 5121 eligible, evaluable patients were entered on the CCG-1800 series studies. Age, WBC, gender, platelet count, and lymphomatous features stratified patients. ‘Lowest risk’ patients were randomly allocated to receive DI or not on CCG-1881. (25) ‘Intermediate risk patients,’ now excluding anyone 10 years of age or older, all received prednisone in induction and a single DI phase and were randomly allocated to receive or not a second DI phase and vincristine/prednisone pulses every 3 or 4 weeks on CCG-1891.(26) Upon completion of these initial studies in 1992 and 1993, subsequent SR patients(27) were enrolled on CCG-1922, which compared oral vs parenteral 6MP and dexamethasone vs prednisone in induction and maintenance. All patients received dexamethasone during a single DI phase and IT methotrexate every 12 weeks in maintenance. (13) Cranial irradiation was reserved for those with overt CNS disease at diagnosis. ‘Higher risk’ patients with lymphomatous features were randomly allocated to NYI or NYII therapy on CCG-1901. All received cranial irradiation. (28) ‘Higher risk’ patients with WBC ≥50,000/μl or age ≥10 years who lacked lymphomatous features were assigned to CCG-1882. On CCG-1882, patients with no CNS disease at diagnosis (<5 leukocytes/μl or no blasts in the cerebrospinal fluid) and <25% marrow blasts on day 7 of an induction phase consisting of vincristine, prednisone, L-asparaginase, and daunorubicin (rapid early responders, RER) were randomly allocated to receive 18 Gy cranial irradiation or additional IT methotrexate.(11) Patients on CCG-1882 with >25% marrow blasts on day 7 of induction (slow early responders, SER) were initially treated on a pilot study of longer and stronger post induction intensification, the “Augmented BFM regimen.” After an initial cohort demonstrated the safety of this regimen, SER patients were randomly allocated to our standard CCG-modified BFM regimen or to the Augmented BFM regimen.(12) Infants <1 year of age, were treated on CCG-1883 and received intensive induction, consolidation including very high-dose methotrexate (33.6 g/m2), and intensive post-consolidation therapy without cranial irradiation.(24) Classification as B-precursor and T-lineage was determined centrally. FAB L2 morphology no longer contributed to treatment allocation. Cytogenetic diagnosis was obtained locally but reviewed centrally.
Between 1996 and 2002, 4464 eligible, evaluable patients were enrolled on the CCG-1900 series studies. Treatment was allocated by age, WBC, and Day 7 or 14 marrow response. Specifically, T-cell patients who met SR age and WBC criteria were now classified as SR. On CCG-1952, SR patients received three drug – vincristine, prednisone, and native e. coli asparaginase induction, two 2-month DI phases, and daily oral 6MP, weekly oral methotrexate, every 4 week vincristine/prednisone pulses, and every 12 week IT therapy. (14, 15) All patients received IT cytarabine at the start of treatment, IT methotrexate in induction and 6TG in DI. Patients were randomly assigned to receive IT methotrexate or IT triple therapy after induction and to receive either 6TG or 6MP in consolidation, interim maintenance, and maintenance. SR patients with marrow blasts > 25% on Day 14 of induction received the Augmented BFM regimen after induction. A small number of SR patients were enrolled on CCG-1962. Patients were randomized to receive native (21 doses) or pegylated (3 doses) asparaginase. All received three-drug induction (with prednisone) and two DI phases. (16) On CCG-1961, HR RER patients were randomly assigned to receive standard or longer duration and standard or stronger intensity post induction intensification. HR SER patients received the Augmented BFM regimen and were randomly assigned to either weekly doxorubicin or sequential idarubicin/cyclophosphamide in each of two DI phases. (17) At the start of the study, B-precursor SER patients were randomly assigned to receive or not to receive B43-PAP, an anti-CD19 pokeweed antiviral protein immunotoxin. However, the manufacturer withdrew the drug from study. (29) Infants, <1 year of age, were treated on CCG-1953 (30) with an intensive “triple induction” strategy shared with POG 9407 (31, 32) and received intensive induction, consolidation including high-dose methotrexate (5 g/m2), and intensive post-consolidation therapy with no cranial irradiation. Classification as B-precursor and T-lineage was determined at a central reference laboratory. Cytogenetic diagnosis was obtained locally but reviewed centrally.
Statistical Considerations
EFS time was defined as the time from diagnosis to first event (induction failure, relapse, death, or second malignant neoplasm) or last contact for those who did not have an event. Overall survival (OS) time was defined as time from diagnosis to death or last contact. Event-free survival and OS rates were computed by the method of Kaplan-Meier (33) and were compared using the log-rank test. Cox proportional hazards regression was used to identify independent prognostic factors for EFS. For patients who achieved complete remission, cumulative incidence rates of isolated CNS or any (isolated plus combined) CNS relapse, therapy-related second malignancies, and remission deaths, were computed and compared using Gray’s method (34) adjusting for competing events. Data for the various studies frozen as of 03/28/2008 were used in the analyses.
Results
Table 1 summarizes the 21 randomized questions posed in the twelve studies that posed a randomized question.
Table 1.
Randomized Questions by study
| Study | Population | Question | Result |
|---|---|---|---|
| CCG-105 | Intermediate Risk | ±Intensive Induction/Consolidation | Intensive induction/consolidation improves EFS but adds adds nothing to Delayed Intensification |
| ±Delayed Intensification | Better EFS and survival with stronger intensification | ||
| WB XRT vs extended IT Mtx | IT Mtx adequate with intensified systemic therapy | ||
| CCG-123 | Lymphomatous features | LSA2L2 ± WB XRTBFMNYI | Better EFS and survival with BFM and NY I |
| CCG-106 | Higher risk | “CCG”BFMNYI | Better EFS and survival with BFM and NY I |
| CCG-139 | Standard risk | ± Intermediate dose methotrexate (500 mg/m2) | No difference |
| CCG-1881 | Lower risk | ±Delayed Intensification | Better EFS with Delayed Intensification |
| CCG-1891 | Intermediate risk (prednisone in induction) | Delayed Intensification x 1 or x 2 | Better EFS with double Delayed Intensification |
| q3 vs q4 weeks Vcr/Pred pulses | No difference | ||
| CCG-1882 | Higher risk Rapid early response | WB XRT vs additional IT Mtx | Additional IT Mtx adequate |
| Higher risk Slow early response | Longer and stronger post induction intensification | Better EFS and survival with longer and stronger post induction intensification | |
| CCG-1901 | Lymphomatous features | NY I vs NY II (extended versus briefer intensification) | NY II less toxic but similarly effective |
| CCG-1922 | Standard risk | Dexamethasone vs prednisone | Better EFS with Dexamethasone |
| ± IV 6MP | Similar EFS and inferior survival with IV 6MP | ||
| CCG-1952 | Standard risk | IT triples vs IT Mtx | Fewer CNS relapses but more frequent marrow relapses and inferior survival with IT triples |
| Oral 6TG vs Oral 6MP | 6TG better, especially in boys with unacceptable liver toxicity | ||
| CCG-1961 | Higher Risk Rapid early response | Longer versus standard duration intensification | No difference |
| Stronger versus standard strength intensification | Better EFS and survival with stronger intensification | ||
| Higher Risk Slow early response | ±B43-PAP immunotoxin | Study aborted with loss of drug supply | |
| Sequential idarubicin/cyclophosphamide versus weekly doxorubicin | No difference | ||
| CCG-1962 | Standard Risk | Pegylated vs native asparaginase | Pegylated asparaginase similarly effective and less immunogenic |
In all three periods, patients with marrow blasts ≥ 25% at the end of induction were removed from protocol therapy as induction failures (an event) and may have later undergone allogeneic stem cell transplantation. The CCG-100 series (1983–1988) and CCG-1800 series (1989–1995) studies made no specific allowance for first remission transplantation. The CCG-1921 study, 1993–1996, captured 29 patients receiving first remission transplant. Patients with t(4;11), t(9;22), hypodiploidy (chromosomes ≤ 44) or induction failure were eligible. In addition, infants (2–12 months) with CD10 negativity, presenting WBC ≥ 100,000/μl or Day 14 marrow blasts > 5% and older children, age ≥ 10 years, with presenting WBC ≥ 200,000/μl were also eligible. (35) On the CCG-1900 series (1996–2002), patients with t(4;11), t(9;22), hypodiploidy < 44 chromosomes, or marrow blasts between 5% and 25% at the end of induction were eligible for allogeneic transplant, if a suitable donor might be found. Any transplanted patients are included in all analyses.
Over time, the percentage of patients receiving cranial irradiation decreased substantially with 65%, 35%, and 15% of patients receiving 18 Gy pre-symptomatic whole brain irradiation therapy in the 2nd month of therapy on the CCG-100 series (1983–1988), CCG-1800 series (1989–1995), and CCG-1900 series (1996–2002), respectively.
Table 2 summarizes the data on induction failures, induction deaths, relapses, secondary malignant neoplasm and remission deaths for the three series. The data are presented separately for B-precursor SR and HR, infant, and T-cell ALL. Induction failure rates for the B-precursor SR patients ranged from 0% to 0.4% across the three series and between 0.9% and 1.3% for the HR patients. Induction death rates fell from 1.1% to 0.2% for SR patients; and from 2.5% to 1.4% for the HR patients. Induction death rates for T-cell ALL fell from 2.2% to 1.3% across series. Induction failure rates for infants dropped from 3.1% to 0.9% over time. However the induction death rates increased significantly (3.1%, 1.5%, and 13.0%, respectively) in the last time period.
Table 2.
Event Summary by series
| CCG 100 series 1983–1988 | CCG 1800 series 1989–1995 | CCG 1900 series 1996–2002 | Total | |
|---|---|---|---|---|
| B-Precursor NCI Standard Risk | 560 | 1461 | 1242 | 3263 |
| First Event | ||||
| No Event | ||||
| Induction Failure | 0 (0%) | 7 (0.4%) | 2 (0.1%) | 9 (0.2%) |
| Induction Death | 9 (1.1%) | 13 (0.7%) | 3 (0.2%) | 25 (0.6%) |
| Relapse | 223 (27.5%) | 344 (18.4%) | 270 (17.5%) | 837 (19.8%) |
| Isolated Marrow | 101 (12.4%) | 171 (9.2%) | 131 (8.5%) | 403 (9.5%) |
| Isolated CNS | 64 (7.9%) | 103 (5.5%) | 65 (4.2%) | 232 (5.5%) |
| Combined and Other | 58 (7.2%) | 70 (3.7%) | 74 (4.8%) | 202 (4.8%) |
| Second Malignancy | 3 (0.4%) | 8 (0.4%) | 8 (0.5%) | 19 (0.4%) |
| Remission Death | 16 (2.0%) | 33 (1.8%) | 19 (1.2%) | 68 (1.6%) |
| Total | 811 | 1866 | 1544 | 4221 |
| B-Precursor NCI High Risk | 257 | 608 | 787 | 1652 |
| No Event | ||||
| Induction Failure | 4 (0.9%) | 12 (1.3%) | 11 (1.0%) | 27 (1.1%) |
| Induction Death | 11 (2.5%) | 14 (1.5%) | 16 (1.4%) | 41 (1.6%) |
| Relapse | 152 (34.2%) | 244 (26.2%) | 277 (24.3%) | 673 (26.8%) |
| Isolated Marrow | 110 (24.8%) | 191 (20.5%) | 163 (14.3%) | 464 (18.5%) |
| Isolated CNS | 13 (2.9%) | 22 (2.4%) | 52 (4.6%) | 87 (3.5%) |
| Combined and Other | 29 (6.5%) | 31 (3.3%) | 62 (5.4%) | 122 (4.8%) |
| Second Malignancy | 8 (1.8%) | 10 (1.1%) | 11 (1.0%) | 29 (1.2%) |
| Remission Death | 12 (2.7%) | 43 (4.6%) | 37 (3.2%) | 92 (3.7%) |
| Total | 444 | 931 | 1139 | 2514 |
| Infants | 31 | 50 | 49 | 130 |
| No Event | ||||
| Induction Failure | 3 (3.1%) | 2 (1.5%) | 1 (0.9%) | 6 (1.7%) |
| Induction Death | 3 (3.1%) | 2 (1.5%) | 15 (13.0%) | 20 (5.7%) |
| Relapse | 58 (59.2%) | 75 (55.6%) | 24 (20.9%) | 157 (45.1%) |
| Isolated Marrow | 35 (35.7%) | 55 (40.7%) | 20 (17.4%) | 110 (31.6%) |
| Isolated CNS | 8 (8.2%) | 4 (3.0%) | 0 (0%) | 12 (3.4%) |
| Combined and Other | 15 (15.3%) | 16 (11.9%) | 4 (3.5%) | 35 (10.1%) |
| Second Malignancy | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Remission Death | 3 (3.1%) | 6 (4.4%) | 26 (22.6%) | 35 (10.0%) |
| Total | 98 | 135 | 115 | 348 |
| T-Cell | 187 | 312 | 376 | 875 |
| No Event | ||||
| Induction Failure | 5 (1.6%) | 9 (2.1%) | 5 (1.0%) | 19 (1.5%) |
| Induction Death | 7 (2.2%) | 7 (1.6%) | 7 (1.3%) | 21 (1.6%) |
| Relapse | 109 (34.2%) | 90 (20.9%) | 114 (21.8%) | 313 (24.6%) |
| Isolated Marrow | 60 (18.8%) | 47 (10.9%) | 55 (10.5%) | 162 (12.7%) |
| Isolated CNS | 21 (6.6%) | 15 (3.5%) | 32 (6.1%) | 68 (5.4%) |
| Combined and Other | 28 (8.8%) | 28 (6.5%) | 27 (5.2%) | 83 (6.5%) |
| Second Malignancy | 2 (0.6%) | 1 (0.2%) | 6 (1.1%) | 9 (0.7%) |
| Remission Death | 9 (2.8%) | 12 (2.8%) | 14 (2.7%) | 35 (2.8%) |
| Total | 319 | 431 | 522 | 1272 |
Relapses are broken down by site, namely, isolated marrow, isolated CNS, and combined or other sites. Isolated marrow relapse comprised about one-half of all relapses across the three series for B-precursor SR patients. Isolated marrow relapse among B-precursor HR patients comprised a similar proportions in the 100 and 1800 series, namely 72% and 78%, but decreased significantly to 59% in the most recent 1900 series. For infants, the proportion of isolated marrow relapses increased (60% vs 73% vs 83%) while the proportion of CNS relapse fell across series. For T-cells, the proportion of isolated marrow relapses remained the same across series (48% to 55%).
Outcomes for various patient subsets are presented in Tables 3–5. Analyses include estimation of outcomes by lineage and NCI risk classification and by study series. Univariate analyses include a variety of presenting features as detailed. Gender, age, WBC, and early marrow response maintained prognostic significance over all three time periods. The prognostic significance of CNS disease at diagnosis increased over the three time intervals as outcomes did not improve for this challenging subset while impropving substantially for patients without CNS disease at diagnosis. Ethnicity lost significance. At 10-years, EFS improved from 51% for black patients diagnosed between 1983 and 1988 (100 series) to 67% for patients diagnosed between 1996 and 2002 (1900 series), while EFS for white patients improved from 63% to 73%. The 5-year EFS for t(1;19), t(4;11), and t(9;22) also improved from 69%, 24%, and 30%, respectively, for the 1800 series to 78%, 44% and 37%, respectively, for the 1900 series. Hypodiploid (<45 chromosomes) and hyperdiploid (> 50 chromosomes) patients went from 35% and 80% to 54% and 83%, respectively, over the same time periods.
Table 3.
CCG-100 series 1983–1988
| # of patients | Event-free survival ± standard error | p-value | Overall survival ± standard error | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 5-year (%) | 10-year (%) | 15-year (%) | 5-year (%) | 10-year (%) | 15-year (%) | ||||
| All patients | 3713 | 65.5 ± 0.8 | 62.0 ± 0.9 | 60.6 ± 1.6 | 78.7 ± 0.7 | 73.3 ± 0.9 | 71.31.4 | ||
| Infants | 98 | 32.6 ± 4.9 | 31.5 ± 5.4 | 31.5 ± 8.3 | <0.0001 | 42.8 ± 5.1 | 38.2 ± 5.5 | 38.2 ± 8.7 | <0.0001 |
| NCI Higher Risk | 1390 | 58.3 ± 1.4 | 54.9 ± 1.6 | 52.9 ± 3.0 | 68.0 ± 1.3 | 62.1 ± 1.6 | 61.5 ± 2.8 | ||
| NCI Standard Risk | 2225 | 71.5 ± 1.0 | 67.8 ± 1.2 | 66.5 ± 1.9 | 86.8 ± 0.8 | 81.8 ± 0.9 | 79.0 ± 1.6 | ||
| B-Lineage | 1280 | 67.9 ± 1.4 | 63.8 ± 1.6 | 62.3 ± 2.6 | 0.003* | 81.4 ± 1.1 | 76.2 ± 1.4 | 74.4 ± 2.3 | <0.0001* |
| Infants | 25 | 36.0 ± 10.2 | 36.0 ± 12.9 | 36.0 ± 16.6 | <0.0001 | 43.6 ± 10.4 | 43.6 ± 12.4 | 43.6 ± 18.9 | <0.0001 |
| NCI Higher Risk | 444 | 61.6 ± 2.4 | 57.8 ± 2.8 | 55.8 ± 4.7 | 72.3 ± 2.2 | 65.9 ± 2.7 | 65.5 ± 4.5 | ||
| NCI Standard Risk | 811 | 72.3 ± 1.7 | 68.0 ± 1.9 | 66.7 ± 3.1 | 87.7 ± 1.2 | 82.9 ± 1.5 | 80.3 ± 2.6 | ||
| T-Lineage | 319 | 60.4 ± 2.9 | 58.1 ± 3.5 | 56.3 ± 7.2 | 69.5 ± 2.8 | 64.7 ± 3.3 | 63.5 ± 6.6 | ||
| Infants | 5 | 20.0 ± 17.9 | 20.0 ± 17.9 | 20.0 ± 17.9 | 0.004 | 20.0 ± 17.9 | 20.0 ± 17.9 | 20.0 ± 17.9 | 0.004 |
| NCI Higher Risk | 214 | 58.0 ± 3.7 | 55.6 ± 4.5 | 52.7 ± 8.3 | 65.5 ± 3.5 | 61.9 ± 4.4 | 61.9 ± 7.8 | ||
| NCI Standard Risk | 100 | 67.6 ± 4.9 | 65.3 ± 5.4 | 65.3 ± 13.6 | 79.7 ± 4.3 | 72.6 ± 5.0 | 69.7 ± 12.1 | ||
| Gender | |||||||||
| Male | 2194 | 63.0 ± 1.1 | 58.8 ± 1.2 | 57.1 ± 2.2 | <0.0001 | 76.9 ± 0.9 | 71.7 ± 1.1 | 69.4 ± 1.9 | 0.002 |
| Female | 1519 | 69.2 ± 1.2 | 66.6 ± 1.4 | 65.5 ± 2.3 | 81.2 ± 1.1 | 75.6 ± 1.3 | 74.1 ± 2.1 | ||
| Age | |||||||||
| 1–9 Years | 2788 | 69.5 ± 0.9 | 65.9 ± 1.0 | 64.5 ± 1.7 | 83.5 ± 0.7 | 78.6 ± 0.9 | 76.3 ± 1.5 | ||
| ≥ 10 Years | 827 | 56.1 ± 1.8 | 52.3 ± 2.2 | 50.4 ± 4.1 | 66.6 ± 1.7 | 59.6 ± 2.1 | 58.4 ± 4.0 | ||
| Ethnicity | |||||||||
| White | 2872 | 66.6 ± 0.9 | 63.1 ± 1.0 | 61.6 ± 1.7 | 0.0006 | 79.7 ± 0.8 | 74.0 ± 0.9 | 72.1 ± 1.5 | 0.009 |
| Black | 212 | 56.6 ± 3.6 | 50.8 ± 4.9 | 46.9 ± 9.9 | 69.7 ± 3.4 | 66.7 ± 4.1 | 64.8 ± 8.2 | ||
| Hispanic | 382 | 61.1 ± 2.7 | 58.7 ± 3.2 | 58.0 ± 6.2 | 75.3 ± 2.4 | 70.3 ± 2.9 | 66.1 ± 5.6 | ||
| Other | 181 | 64.8 ± 3.7 | 59.9 ± 4.6 | 59.9 ± 7.7 | 80.2 ± 3.1 | 77.1 ± 3.9 | 74.8 ± 6.3 | ||
| WBC | |||||||||
| <10,000/μl | 1640 | 70.5 ± 1.1 | 66.0 ± 1.4 | 64.7 ± 2.2 | <0.0001 | 84.8 ± 0.9 | 79.2 ± 1.2 | 76.2 ± 1.9 | <0.0001 |
| 10,000–50,000/μl | 1226 | 65.8 ± 1.4 | 62.7 ± 1.6 | 61.1 ± 2.9 | 80.6 ± 1.2 | 74.7 ± 1.5 | 72.9 ± 2.6 | ||
| 50,000–100,000/μl | 358 | 60.4 ± 2.8 | 58.7 ± 3.1 | 58.7 ± 5.5 | 70.0 ± 2.6 | 65.3 ± 3.0 | 65.3 ± 5.2 | ||
| >100,000/μl | 488 | 51.6 ± 2.4 | 49.0 ± 2.7 | 46.5 ± 4.8 | 59.3 ± 2.4 | 55.9 ± 2.7 | 55.9 ± 4.7 | ||
| CNS at Diagnosis | |||||||||
| Yes | 88 | 59.5 ± 5.6 | 59.5 ± 6.2 | 54.0 ± 9.5 | 0.16 | 69.7 ± 5.3 | 66.7 ± 5.9 | 64.4 ± 9.6 | 0.08 |
| No | 3624 | 65.6 ± 0.8 | 62.1 ± 1.0 | 60.7 ± 1.6 | 78.9 ± 0.7 | 73.5 ± 0.9 | 71.5 ± 1.4 | ||
| Day 7 Marrow | |||||||||
| M1 | 136 | 75.5 ± 3.8 | 73.6 ± 4.4 | 72.1 ± 17.0 | <0.0001 | 79.2 ± 3.6 | 77.6 ± 4.2 | 77.6 ± 15.0 | 0.0008 |
| M2 | 34 | 50.0 ± 9.1 | 50.0 ± 10.2 | NA | 58.8 ± 8.9 | 55.2 ± 9.9 | NA | ||
| M3 | 55 | 42.4 ± 7.2 | 42.4 ± 9.3 | NA | 51.5 ± 7.2 | 51.5 ± 9.3 | 51.5 ± 25.4 | ||
| Day 14 Marrow | |||||||||
| M1 | 1598 | 69.8 ± 1.2 | 66.0 ± 1.4 | 64.7 ± 2.5 | <0.0001 | 83.7 ± 1.0 | 78.5 ± 1.2 | 76.5 ± 2.2 | <0.0001 |
| M2 | 131 | 56.0 ± 4.6 | 51.1 ± 5.4 | 51.1 ± 11.3 | 73.3 ± 4.1 | 64.7 ± 5.0 | 62.2 ± 8.8 | ||
| M3 | 63 | 28.6 ± 5.9 | 25.2 ± 6.1 | 22.4 ± 11.4 | 44.8 ± 6.5 | 39.4 ± 7.0 | 36.8 ± 14.6 |
Table 5.
CCG-1900 series: 1996–2002
| # of patients | Event-free survival ± standard error | p-value | Overall survival ± standard error | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 5-year (%) | 10-year (%) | 15-year (%) | 5-year (%) | 10-year (%) | 15-year (%) | ||||
| All patients | 4464 | 76.0 ± 0.7 | 72.6 ± 2.9 | NA | 86.3 ± 0.6 | 82.1 ± 2.5 | NA | ||
| Infants | 115 | 43.2 ± 4.8 | NA | NA | <0.0001 | 46.8 ± 4.9 | NA | NA | <0.0001 |
| NCI Higher Risk | 2054 | 71.8 ± 1.1 | 68.5 ± 4.6 | NA | 81.0 ± 1.0 | 75.7 ± 4.3 | NA | ||
| NCI Standard Risk | 2295 | 81.4 ± 0.9 | 77.8 ± 3.7 | NA | 92.9 ± 0.6 | 89.3 ± 2.8 | NA | ||
| B-Lineage | 2764 | 76.0 ± 0.9 | 72.3 ± 3.7 | NA | 86.8 ± 0.7 | 82.1 ± 3.2 | NA | ||
| Infants | 81 | 45.3 ± 5.8 | NA | NA | <0.0001 | 49.7 ± 6.0 | NA | NA | <0.0001 |
| NCI Higher Risk | 1139 | 70.1 ± 1.5 | 67.0 ± 6.6 | NA | 81.0 ± 1.3 | 74.2 ± 6.3 | NA | ||
| NCI Standard Risk | 1544 | 81.9 ± 1.0 | 77.8 ± 4.3 | NA | 92.9 ± 0.7 | 89.2 ± 3.3 | NA | ||
| T-Lineage | 522 | 72.8 ± 2.1 | 70.7 ± 8.4 | NA | 80.3 ± 1.9 | 77.3 ± 8.0 | NA | ||
| Infants | 1 | NA | NA | NA | 0.02 | NA | NA | NA | 0.001 |
| NCI Higher Risk | 412 | 72.9 ± 2.4 | 71.5 ± 9.3 | NA | 79.4 ± 2.2 | 77.0 ± 8.7 | NA | ||
| NCI Standard Risk | 109 | 73.1 ± 4.4 | 69.6 ± 19.2 | NA | 84.6 ± 3.6 | 80.0 ± 17.9 | NA | ||
| Gender | |||||||||
| Male | 2557 | 73.8 ± 0.9 | 70.3 ± 3.8 | NA | <0.0001 | 85.6 ± 0.7 | 80.8 ± 3.3 | NA | 0.05 |
| Female | 1907 | 78.9 ± 1.0 | 75.7 ± 4.6 | NA | 87.1 ± 0.8 | 84.0 ± 3.9 | NA | ||
| Age | |||||||||
| 1–9 Years | 3061 | 79.4 ± 0.8 | 75.8 ± 3.3 | NA | 90.7 ± 0.6 | 86.1 ± 2.6 | NA | ||
| ≥ 10 Years | 1288 | 70.9 ± 1.4 | 67.6 ± 6.3 | NA | 79.1 ± 1.3 | 75.9 ± 5.9 | NA | ||
| Ethnicity | |||||||||
| White | 2996 | 76.9 ± 0.8 | 73.1 ± 3.4 | NA | 0.10 | 87.2 ± 0.7 | 83.2 ± 2.9 | NA | 0.16 |
| Black | 216 | 70.7 ± 3.3 | 66.7 ± 12.2 | NA | 82.9 ± 2.8 | 74.8 ± 11.3 | NA | ||
| Hispanic | 945 | 74.9 ± 1.5 | 73.4 ± 7.2 | NA | 84.5 ± 1.3 | 81.4 ± 6.6 | NA | ||
| Other | 271 | 74.4 ± 2.8 | 70.7 ±13.5 | NA | 85.2 ± 2.3 | 81.0 ± 12.5 | NA | ||
| WBC | |||||||||
| <10,000/μl | 2088 | 80.6 ± 0.9 | 78.1 ± 3.9 | NA | <0.0001 | 90.3 ± 0.7 | 87.2 ± 3.2 | NA | <0.0001 |
| 10,000–50,000/μl | 1212 | 77.0 ± 1.3 | 71.9 ± 6.0 | NA | 88.2 ± 1.0 | 84.1 ± 4.9 | NA | ||
| 50,000–100,000/μl | 531 | 70.6 ± 2.1 | 66.6 ± 9.3 | NA | 81.4 ± 1.8 | 74.7 ± 8.6 | NA | ||
| >100,000/μl | 629 | 63.6 ± 2.1 | 60.7 ± 7.8 | NA | 73.1 ± 2.0 | 67.0 ± 7.4 | NA | ||
| CNS at Diagnosis | |||||||||
| CNS Disease | 133 | 57.1 ± 4.7 | 51.5 ± 17.9 | NA | <0.0001 | 66.1 ± 4.5 | 53.3 ± 16.3 | NA | <0.0001 |
| CNS-2 | 345 | 63.5 ± 2.8 | 61.9 ± 9.3 | NA | 77.5 ± 2.4 | 74.2 ± 8.0 | NA | ||
| No CNS Disease | 3830 | 77.8 ± 0.7 | 74.3 ± 3.2 | NA | 87.9 ± 0.6 | 84.1 ± 2.7 | |||
| Day 7 Marrow | |||||||||
| M1 | 2005 | 81.1 ± 0.9 | 78.8 ± 4.2 | NA | <0.0001 | 89.1 ± 0.8 | 85.8 ± 3.5 | NA | <0.0001 |
| M2 | 1169 | 74.6 ± 1.3 | 72.0 ± 5.3 | NA | 86.7 ± 1.1 | 82.7 ± 4.6 | NA | ||
| M3 | 1186 | 69.6 ± 1.4 | 64.1 ± 5.9 | NA | 81.9 ± 1.2 | 76.2 ± 5.4 | |||
| Ploidy | |||||||||
| Normal | 663 | 73.8 ± 1.8 | 71.6 ± 8.1 | NA | <0.0001 | 87.6 ± 1.4 | 83.4 ± 6.8 | NA | <0.0001 |
| Hypo <46C | 165 | 59.9 ± 4.0 | 56.8 ± 26.4 | NA | 75.3 ± 3.6 | 67.2 ± 22.2 | NA | ||
| 45C | 145 | 60.7 ± 4.3 | 58.0 ± 21.7 | NA | 0.35* | 76.9 ± 3.7 | 73.0 ± 19.0 | NA | 0.15* |
| <45C | 20 | 54.2 ± 12.2 | 47.4 ± 34.4 | NA | 63.8 ± 11.6 | 42.6 ± 32.3 | NA | ||
| Pseudo | 627 | 69.4 ± 2.0 | 67.4 ± 9.9 | NA | 76.8 ± 1.8 | 74.6 ± 9.1 | NA | ||
| 47–50C | 229 | 73.6 ± 3.1 | 67.3 ± 15.7 | NA | 84.3 ± 2.6 | 81.2 ± 14.4 | NA | ||
| >50C | 505 | 83.3 ± 1.8 | 80.2 ± 8.0 | NA | 94.3 ± 1.1 | 92.6 ± 5.3 | NA | ||
| Translocations | |||||||||
| Normal | 2008 | 76.2 ± 1.0 | 72.8 ± 4.8 | NA | <0.0001 | 86.9 ± 0.8 | 83.7 ± 4.0 | NA | <0.0001 |
| t(4;11) | 51 | 44.1 ± 7.6 | 39.4 ± 30.7 | NA | 47.9 ± 7.6 | 47.9 ± 34.6 | NA | ||
| t(9;22) | 63 | 36.7 ± 6.7 | NA | NA | 45.5 ± 7.2 | NA | NA | ||
| t(1;19) | 67 | 77.6 ± 5.5 | 70.5 ± 38.3 | NA | 85.0 ± 4.8 | 85.0 ± 23.3 | NA |
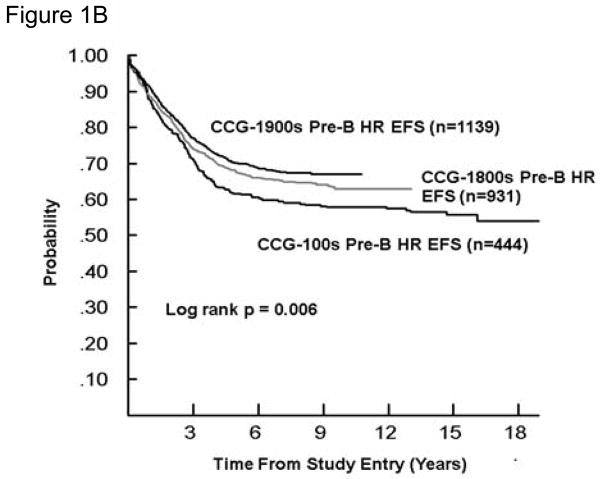
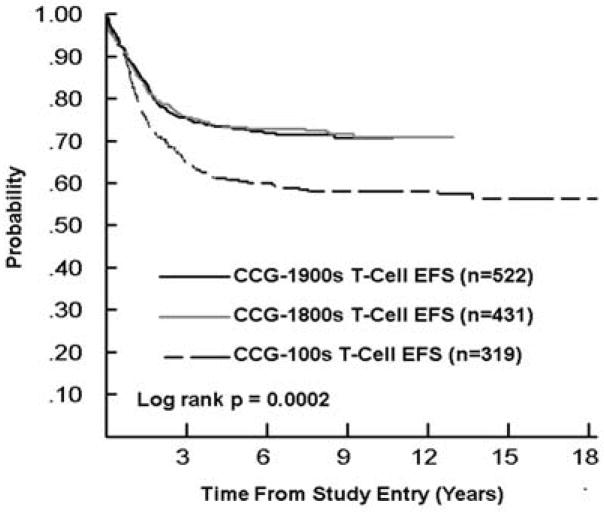
As the mix of infants and higher and lower risk patients differed over time, Figures 1–3 display EFS by study series for SR and HR B-precursor, T-cell, and infants, respectively, in order to facilitate cross series comparisons. The EFS and OS improved significantly overtime for SR B-precursor patients (p<0.0001 and p=0.0001, respectively) and for HR B-precursor patients. For HR T cell patients, 5-year EFS was 58% and 73% in 1983–1988 and 1996–2002 (Tables 3 and 5). For SR T cell patients, 5-year EFS was 68% and 73% in 1983–1988 and 1996–2002 (Tables 3 and 5) with gains to 80% in 1989–1995 (Table 4), which were subsequently lost when SR T-cell patients were assigned to less intensive therapy. The change in outcome for infants was not statistically significant.
Figure 1. Event-Free Survival for B-precursor ALL by Study Series.
- Standard risk [CCG-100 series (1983–1988), CCG-1800 series (1989–1995), and CCG-1900 series (1996–2002)]; SR, Standard risk; EFS, event-free survival
- Higher risk [CCG-100 series (1983–1988), CCG-1800 series (1989–1995), and CCG-1900 series (1996–2002)]; HR, high risk; EFS, event free survival
Figure 3.
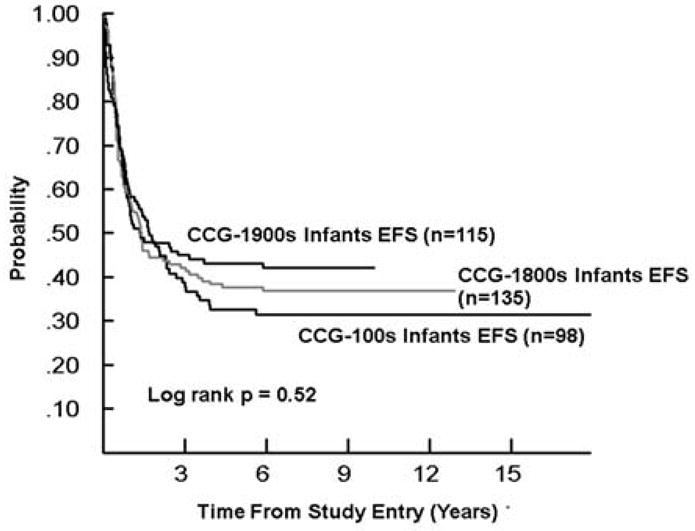
Event-Free Survival for Infant ALL by Study Series CCG-100 series (1983–1988), CCG-1800 series (1989–1995), and CCG-1900 series (1996–2002)]; EFS, event-free survival
Table 4.
CCG-1800 series: 1989–1995
| # of patients | Event-free survival ± standard error | p-value | Overall survival ± standard error | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| 5-year (%) | 10-year (%) | 15-year (%) | 5-year (%) | 10-year (%) | 15-year (%) | ||||
| All patients | 5121 | 75.2 ± 0.6 | 72.1 ± 1.3 | NA | 85.0 ± 0.5 | 81.1 ± 1.2 | NA | ||
| Infants | 135 | 37.6 ± 4.3 | 36.8 ± 6.5 | NA | <0.0001 | 50.2 ± 4.4 | 49.4 ± 7.2 | NA | <0.0001 |
| NCI Higher Risk | 1841 | 68.5 ± 1.1 | 65.1 ± 2.6 | NA | 76.1 ± 1.1 | 71.4 ± 2.4 | NA | ||
| NCI Standard Risk | 3145 | 80.8 ± 0.7 | 77.8 ± 1.6 | NA | 91.7 ± 0.5 | 88.0 ± 1.2 | NA | ||
| B-Lineage | 2883 | 74.6 ± 0.8 | 71.4 ± 1.7 | NA | 74.6 ± 0.8 | 71.4 ± 1.7 | NA | ||
| Infants | 86 | 38.0 ± 5.6 | 38.0 ± 8.0 | NA | <0.0001 | 52.1 ± 5.6 | 50.8 ± 9.2 | NA | <0.0001 |
| NCI Higher Risk | 931 | 67.3 ± 1.6 | 63.0 ± 3.6 | NA | 76.0 ± 1.5 | 69.9 ± 3.4 | NA | ||
| NCI Standard Risk | 1866 | 80.0 ± 1.0 | 77.1 ± 1.9 | NA | 91.3 ± 0.7 | 87.1 ± 1.5 | NA | ||
| T-Lineage | 431 | 73.2 ± 2.2 | 71.0 ± 4.6 | NA | 73.2 ± 2.2 | 71.0 ± 4.6 | NA | ||
| Infants | 2 | 50.0 ± 35.4 | NA | NA | 0.089 | 50.0 ± 35.4 | NA | NA | 0.04 |
| NCI Higher Risk | 300 | 70.3 ± 2.8 | 68.3 ± 5.6 | NA | 75.9 ± 2.6 | 73.9 ± 5.3 | NA | ||
| NCI Standard Risk | 129 | 80.2 ± 3.7 | 77.8 ± 7.6 | NA | 87.4 ± 3.1 | 84.7 ± 6.3 | NA | ||
| Gender | |||||||||
| Male | 2817 | 74.2 ± 0.9 | 71.2 ± 1.9 | NA | 0.1 | 84.0 ± 0.7 | 80.0 ± 1.7 | NA | 0.03 |
| Female | 2304 | 76.5 ± 0.9 | 73.4 ± 1.9 | NA | 86.1 ± 0.8 | 82.5 ± 1.6 | NA | ||
| Age | |||||||||
| 1–9 Years | 3879 | 79.1 ± 0.7 | 76.0 ± 1.5 | NA | 89.6 ± 0.5 | 85.8 ± 1.2 | NA | ||
| ≥ 10 Years | 1107 | 66.3 ± 1.5 | 62.9 ± 3.4 | NA | 72.8 ± 1.4 | 68.3 ± 3.3 | NA | ||
| Ethnicity | |||||||||
| White | 3834 | 77.2 ± 0.7 | 74.4 ± 1.5 | NA | <0.0001 | 86.3 ± 0.6 | 82.8 ± 1.3 | NA | <0.0001 |
| Black | 291 | 65.7 ± 3.1 | 60.9 ± 9.0 | NA | 78.0 ± 2.7 | 71.5 ± 8.0 | NA | ||
| Hispanic | 691 | 69.1 ± 1.9 | 65.0 ± 4.3 | NA | 80.5 ± 1.7 | 76.2 ± 3.9 | NA | ||
| Other | 301 | 73.1 ± 2.7 | 69.7 ± 5.5 | NA | 84.3 ± 2.2 | 80.6 ± 4.8 | NA | ||
| WBC | |||||||||
| <10,000/μl | 2530 | 79.0 ± 0.9 | 75.8 ± 1.8 | NA | <0.0001 | 89.1 ± 0.7 | 85.4 ± 1.4 | NA | <0.0001 |
| 10,000–50,000/μl | 1514 | 76.4 ± 1.1 | 73.5 ± 2.6 | NA | 86.2 ± 0.9 | 82.2 ± 2.2 | NA | ||
| 50,000–100,000/μl | 478 | 70.4 ± 2.2 | 67.8 ± 4.6 | NA | 80.3 ± 1.9 | 75.9 ± 4.3 | NA | ||
| >100,000/μl | 599 | 60.1 ± 2.1 | 56.9 ± 4.5 | NA | 68.4 ± 2.0 | 64.4 ± 4.3 | NA | ||
| CNS at Diagnosis | |||||||||
| Yes | 168 | 60.2 ± 3.9 | 56.0 ± 8.5 | NA | <0.0001 | 66.1 ± 3.8 | 61.3 ± 8.7 | NA | <0.0001 |
| No | 4903 | 75.8 ± 0.6 | 72.8 ± 1.4 | NA | 85.7 ± 0.5 | 81.8 ± 1.2 | NA | ||
| Day 7 Marrow | |||||||||
| M1 | 2075 | 79.7 ± 0.9 | 77.2 ± 2.3 | NA | <0.0001 | 87.2 ± 0.8 | 84.4 ± 2.0 | NA | <0.0001 |
| M2 | 926 | 73.7 ± 1.5 | 70.8 ± 3.5 | NA | 85.1 ± 1.2 | 80.6 ± 3.0 | NA | ||
| M3 | 1025 | 65.0 ± 1.6 | 60.4 ± 3.8 | NA | 76.0 ± 1.4 | 69.8 ± 3.5 | NA | ||
| Ploidy | |||||||||
| Normal | 596 | 81.4 ± 1.7 | 79.4 ± 3.5 | NA | <0.0001 | 88.8 ± 1.4 | 85.4 ± 3.0 | NA | <0.0001 |
| Hypo <46C | 114 | 57.7 ± 5.0 | 54.3 ± 11.1 | NA | 70.3 ± 4.6 | 65.4 ± 9.9 | NA | ||
| 45C | 91 | 63.7 ± 5.4 | 60.9 ± 11.0 | NA | 0.003* | 76.3 ± 4.8 | 72.3 ± 10.2 | NA | 0.004* |
| <45C | 23 | 34.8 ± 9.9 | 29.0 ± 24.4 | NA | 47.1 ± 10.8 | 37.7 ± 29.7 | NA | ||
| Pseudo | 536 | 67.5 ± 2.1 | 63.9 ± 4.2 | NA | 76.2 ± 1.9 | 72.3 ± 3.9 | NA | ||
| 47–50C | 206 | 65.1 ± 3.4 | 59.9 ± 7.4 | NA | 79.4 ± 2.9 | 69.3 ± 6.7 | NA | ||
| >50C | 494 | 80.4 ± 1.9 | 77.6 ± 3.9 | NA | 90.3 ± 1.4 | 87.8 ± 3.0 | NA | ||
| Translocations | |||||||||
| Normal | 1793 | 76.7 ± 1.0 | 73.7 ± 2.2 | NA | <0.0001 | 86.1 ± 0.9 | 82.2 ± 1.9 | NA | <0.0001 |
| t(4;11) | 42 | 23.8 ± 6.9 | 23.8 ± 12.0 | NA | 30.8 ± 7.4 | 30.8 ± 14.8 | NA | ||
| t(9;22) | 44 | 29.6 ± 7.5 | NA | NA | 45.1 ± 8.1 | 22.4 ± 19.7 | NA | ||
| t(1;19) | 67 | 68.6 ± 5.8 | 65.3 ± 10.7 | NA | 77.6 ± 5.2 | 71.3 ± 10.2 | NA |
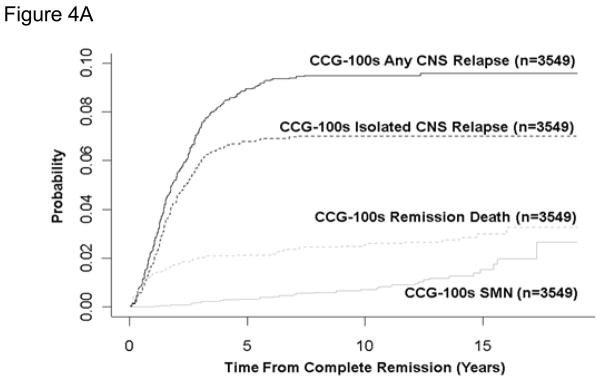
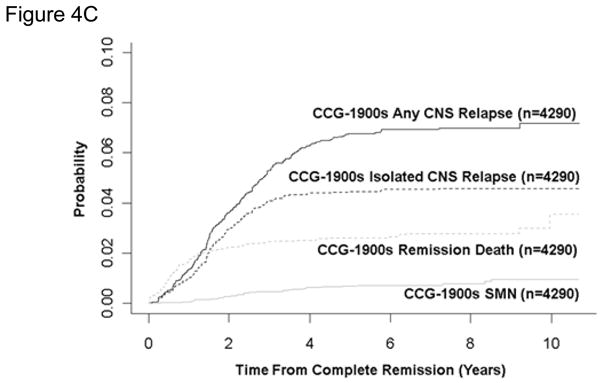
For 100 series, 1800 series, and 1900 series patients, the 10-year cumulative incidence rates for death in remission were 2.6±0.3%, 3.0±0.3%, and 3.6±0.7%, respectively (Table 7, figure 4A, B, C). Rates were highest in the infant studies with 5-year remission death rates of 7.0±2.9% and 31.3±5.5% on CCG-1883 and CCG-1953. Ten-year rates were between 1% and 1.5% in the SR studies. On the HR study CCG-1961, the remission death rate was 3.2±0.4% at 5 years and increased to 5.0±1.5% at 10 years. Two of the 4 late deaths are attributed to the late complications of bone marrow transplantation; 1 death was accidental and 1 was unknown.
Table 7.
Post Induction Event Cumulative incidence rates (± standard error)
| CCG-100 series (1983–1988) | CCG-1800 series (1989–1995) | CCG-1900 series (1996–2002) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | 3549 | 4940 | 4290 | ||||||
| 5-year (%) | 10-year (%) | 15-year (%) | 5-year (%) | 10-year (%) | 15-year (%) | 5-year (%) | 10-year (%) | 15-year (%) | |
| Remission deaths | 2.1± 0.3 | 2.6± 0.3 | 3.0 ± 0.4 | 2.5± 0.2 | 3.0±0.3 | NA | 2.6±0.2 | 3.6±0.7 | NA |
| Isolated CNS relapse | 6.8±0.4 | 7.0±0.5 | 7.0 ± 0.5 | 4.7±0.3 | 4.8±0.3 | NA | 4.5±0.3 | 4.6±0.3 | NA |
| CNS relapse Isolated or Combined | 8.9±0.5 | 9.5±0.5 | 9.6 ± 0.5 | 6.6±0.4 | 7.0±0.4 | NA | 6.8±0.4 | 7.2±0.5 | NA |
| Second malignant neoplasm | 0.3±0.1 | 0.7±0.2 | 1.6±0.3 | 0.4±0.1 | 1.1±0.2 | NA | 0.7±0.1 | 1.0±0.2 | NA |
Figure 4. Cumulative Incidence of Remission Death, Isolated Central Nervous System (CNS) Relapse, Any CNS Relapse, and Secondary Malignant Neoplasm (SMN).

- CCG-100 Series (1983–1988)
- CCG-1800 Series (1989–1995)
- CCG-1900 Series (1996–2002)
For 100 series and 1900 series patients, the 10-year cumulative incidence of isolated and combined CNS relapse (Table 7, figure 4A, B, C) decreased from 7.0±0.5% to 4.6±0.3% and 9.5±0.5% to 7.2±0.5%, despite less use of brain irradiation.
For 100 series, 1800 series, and 1900 series patients, the 10-year cumulative incidence of second malignant neoplasm (Table 7, figure 4A, B, C) was 0.7±0.2%, 1.1±0.2%, and 1.0±0.2%, respectively. For SR patients, rates remained between 0.4% and 1.4%.
Discussion
In this report, we review the outcome of 13,298 children with ALL and enrolled in one of sixteen CCG trials between 1983 and 2002. During this period, EFS and OS increased significantly for all groups except infants <1 year of age, who had only a 4-percentage point improvement in 5-year OS. The smallest gains were attained for T-ALL patients with SR features for whom outcomes actually deteriorated between 1989–1995 and 1996–2002, likely due to allocation to less aggressive SR regimens on the CCG-1900 series studies as opposed to treatment on HR regimens in earlier eras. Overall, patients in first remission at 5 years had a consistent 4% risk for an adverse event between 5 and 10 years from diagnosis. This risk was similar for boys and girls.
The results of the randomized questions of these trials have shaped contemporary COG ALL therapy. Vincristine and prednisone pulses, shown to be effective for SR patients as the sole post induction intensification on CCG-161, were the first effective post induction intensification introduced in CCG (5) and remain a part of current COG regimens. Subsequently, every three-week pulses had no advantage over four-weekly pulses on CCG 1891. (26) Recent IBFM data show no advantage for vincristine/dexamethasone pulses in the context of intensive BFM-based therapy (36) but yet more recent EORTC data differ (37) for uncertain reasons. Nonetheless, maintenance vincristine and steroid pulses may now be redundant in the context of more aggressive current BFM-based therapy.
The increased toxicity with combined dexamethasone and anthracycline use in induction is well documented. (38, 39) CCG-105 showed that induction anthracycline added nothing to a three-drug, vincristine, l-asparaginase, and prednisone induction for SR patients who received DI. (9, 10) On CCG-1922, omission of induction anthracycline facilitated near iso- toxic substitution of induction and maintenance dexamethasone for prednisone at a dose ratio of 1 to 6.7 (13) Recent MRC (40) and BFM (41) data support this advantage at ratios of 1 to 6.1 and 1 to 6, with no advantage evident in Japanese (42) and EORTC (43) trials with ratios of 1 to 7.5 and 1 to 10. The CCG-1922 results were not available when CCG 1952 opened and CCG 1952 patients received induction prednisone but subsequent CCG and COG SR ALL trials have used dexamethasone in three-drug induction to good effect. BFM reports excessive dexamethasone morbidity in the context of 4-drug induction that includes daunorubicin. (41).
The Augmented “BFM” regimen, employing longer and stronger post induction intensification, was found superior for HR SER patients on CCG 1882 and has become the mainstay of current COG therapy. (12) The successor trial, CCG 1961, trial found that stronger intensification, derived from the Augmented “BFM” regimen improved outcome for HR RER patients also, but that longer intensification did not. Six months of intensification was as effective as 10 months. (17) Longer intensification also added nothing for SR patients who received induction dexamethasone on CCG-1991. (18) These findings focus attention on improving the quality of the first six months of post induction therapy.
Administration of the second block of therapy, termed Protocol Ib by the BFM Group and Consolidation by COG, requires approximately two months. Together with similar cyclophosphamide, cytarabine, and 6TG block of DI, this element occupies 3 of the first 7 months of treatment. Despite its long-standing place in treatment, neither its rationale nor its specific contribution is well established. Augmented Consolidation introduced vincristine and asparaginase during the neutropenic periods that follow administration of cyclophosphamide, cytarabine and 6MP. The MRC (UK) reports that the addition of vincristine and asparaginase in Consolidation increases the clearance of minimal residual disease for patients who are still positive at the end of the first month of therapy. (44) COG is now testing Augmented Consolidation for SR B-precursor patients on AALL0331.
The two months of therapy following Ib (BFM) or Consolidation (COG) and preceding Protocol II (BFM) or DI (COG), termed Interim Maintenance (IM) by COG and Consolidation by the BFM Group, have diverged over the years. Earliest BFM and CCG trials employed daily oral 6 MP and weekly oral methotrexate. BFM ALL 86 replaced weekly oral methotrexate with 4 courses of parenteral methotrexate 5 gram/m2 with leucovorin rescue in protocol M. (45) CCG introduced five courses of vincristine and escalating-dose intravenous methotrexate given every 10–11 days without leucovorin rescue followed by asparaginase during this IM phase on CCG 1882. (12) This Augmented IM phase is now compared to 4 courses of parenteral methotrexate 5 gram/m2 with leucovorin rescue in the current COG HR B-precursor (AALL0232) and T-cell (AALL0434) trials. Of interest, CCG 5971 found no advantage for every 2 week 5 gram/m2 methotrexate and leucovorin over weekly oral 20 mg/m2 methotrexate for patients with lymphoblastic lymphoma. (46) CCG 1991 found better EFS with five courses of vincristine and escalating-dose intravenous methotrexate given every 10–11 days without leucovorin given before and after DI versus of oral methotrexate and 6MP. Thus after 60 years, investigators are still exploring the best ways to administer methotrexate. (19)
CCG 1962 showed that 3 intramuscular (IM) doses of pegylated asparaginase can safely replace 21 IM doses of native asparaginase. The pegylated product provided a superior Day 14 marrow response and lower rates of antibody development. (16) CCG 1961 employed pegylated asparaginase after induction for the augmented arms. (17) POG 9900 changed from 6 doses of native asparaginase 10,000 u/m2 administered three times a week to one dose of pegylated asparaginase in induction for SR patients. The incidence of end induction minimum residual disease (MRD) positivity (> 10−4) went from 18.9% to 14.3%. (47) Following this, all COG ALL trials now use pegylated rather than native asparaginase, thus sparing children unneeded intramuscular injections.
Several other critical observations emerge from these twenty years of CCG ALL trials. Prevention of relapse is the most effective means to prevent mortality from childhood ALL. Gains were achieved despite no improvement in outcome for patients who relapsed. (48) Freyer et al examined survival after relapse for HR patients on CCG-1961 randomized to more or less effective post induction intensification. (49) Contrary to intuition but in agreement with other observations excluding intravenous 6MP, post relapse survival was identical for patients relapsing from more and less effective regimens.
As relapse rates decrease over time with improved therapy, remission deaths become larger contributors to overall death rates. The rate of remission deaths is about 1% for SR studies and 3–4% for HR studies, with adolescent patients being at higher risk. Among patients older than 15 years, remission deaths comprise 25% of adverse events.(50) Remission deaths after 5 years may be increasing as more patients receive hematopoietic stem cell transplant in first remission. Bhatia et al comment that about 16% of ALL patients alive and in remission at two years after allogeneic bone marrow will expire in the next 8 years.(51) Transplantation accounts for the increased remission death rate among adolescents and young adults treated according to adult versus pediatric protocols.(52)
Unfortunately, improvements in therapy have been accompanied by increases in toxicity. The most striking has been the increase in avascular necrosis of bone (AVN), which was rarely recognized in patients diagnosed before 1986, but became more common, especially in adolescents and young adults, with the CCG 1800 era trials.(53,54) This complication can lead to significant life-long morbidity with many patients requiring joint replacement surgery during adolescence or early adulthood. AVN has continued to be a problem on subsequent CCG and COG trials. Altered dosing of dexamethasone during DI, i.e., days 1–7 and days 15–21, rather than days 1–21, (55) provide some decrease in the incidence but excessive AVN led to a suspension of the randomization to induction dexamethasone for adolescents on AALL0232.(56) Screening for exceedingly rare anthracycline cardiotoxicity is standard while screening for AVN in older populations with a risk that exceeds 10% has not been adapted. While identification of lesions prior to collapse seems desirable (57), the significance of early MRI findings remains in doubt.(58)
Another lesson learned from these twenty years of trials is the need for adequate sample size to answer critical questions. Statistical power depends on the magnitude of the impact of an intervention and the number of captured events – not patients. As trial planning is based on prior data and outcomes tend to improve over time, baseline event rates are often overestimated. Investigators tend to overestimate the impact of experimental interventions. In the CCG trials, successful experimental regimens provided a 25–40% reduction in risk of failure. If the trials had been designed to detect a 50% or greater reduction in risk of failure, effective interventions, such as dexamethasone for SR ALL, or augmented BFM for HR ALL, may have been missed. Marginal sample size limits opportunity for exploration of potential interactions and generation of novel hypotheses that will support future trials. Sample size estimates should be based on most recent event rates and moderate treatment impact.
With improved outcomes, a geometrically increasing number of patients must be treated to prevent one event (“number needed to treat”). When EFS went from 40% to 60% on CCG-106, (7) a one-third reduction in failures, only five patients had to be exposed to a novel therapy to benefit one patient. Contemporary COG ALL trials require a much larger number to treat. For example, increasing EFS from 88% to 92%, a one third reduction in failure, requires that 25 patients receive the experimental intervention to benefit one patient. On CCG 1991, 300 doses of parenteral methotrexate prevented one relapse. (19) Increasing EFS through better primary treatment can obviate the need for salvage treatment of prevented relapses, usually morbid and too often ineffective, and provide a net decrease in the use of medical services. (59)
For the future, better ascertainment of patients at higher and lower risk of relapse is critical, and new therapies must be developed that are targeted at the molecular abnormalities that cause leukemia and/or treatment failure. Several important strategies to accomplish these goals are being explored at this time. Most cooperative treatment groups have incorporated minimal residual disease (MRD) testing to identify patients at higher or lower risk of relapse. Patients with an MRD burden greater than 0.01% at end induction have an increased risk of relapse. However in contemporary COG trials, half of relapses still arise among patients with end-induction MRD < 0.01%. (60) Adding a second MRD time point earlier (60) or later (61) in therapy can help to refine MRD-based risk assessment. Minimal residual disease is prognostic in T-cell as well as B-precursor leukemia. (62) The newer genomic technologies including gene expression profiles (63) and arrays to detect genomic copy number alterations (64) may lead to better insight into the molecular basis of leukemogenesis (65) and identify new potential therapeutic targets like JAK2. (66) The roles of pharmacogenomics (67) and patient/family treatment adherence (68) are under study. In Philadelphia chromosome positive chronic myelogenous leukemia(69) and ALL (70), imatinib has made a major impact on outcome. (70) Understanding the mechanism(s) of imatinib resistance has led to novel, effective treatments. (71) One might reasonably hope that understanding of the mechanism(s) of treatment failure in childhood ALL holds similar promise.
Over the past 40 years, cure of this once incurable disease has become commonplace. With deeper insight into leukemia biology, one may only expect that the next twenty years holds similar or greater promise
Figure 2.
Event-Free Survival for T-cell ALL by Study Series [CCG-100 series (1983–1988), CCG-1800 series (1989–1995), and CCG-1900 series (1996–2002)] EFS, event-free survival
Table 6.
Comparison of EFS and OS by Series
| A. B-precursor Standard Risk | |||||||
|---|---|---|---|---|---|---|---|
| CCG-100 series (1983–1988) | CCG-1800 series (1989–1995) | CCG-1900 series (1996–2002) | |||||
| n | 811 | 1866 | 1544 | ||||
| 5-year (%) | 10-year (%) | 15-year (%) | 5-year (%) | 10-year (%) | 5-year (%) | 10-year (%) | |
| EFS ± SE | 72.3 ± 1.7 | 68.0 ± 1.9 | 66.7 ± 3.1 | 79.9 ± 1.0 | 77.1 ± 1.9 | 81.9 ± 1.0 | 77.8 ± 4.4 |
| OS ± SE | 87.7 ± 1.2 | 82.9 ± 1.5 | 80.3 ± 2.5 | 91.3 ± 0.7 | 87.1 ± 1.5 | 92.9 ± 0.7 | 89.2 ± 3.3 |
| B. B-precursor Higher Risk | |||||||
|---|---|---|---|---|---|---|---|
| CCG-100 series (1983–1988) | CCG-1800 series (1989–1995) | CCG-1900 series (1996–2002) | |||||
| n | 444 | 931 | 1139 | ||||
| 5-year (%) | 10-year (%) | 15-year (%) | 5-year (%) | 10-year (%) | 5-year (%) | 10-year (%) | |
| EFS ± SE | 61.6 ± 2.4 | 57.8 ±2.8 | 55.8 ± 4.7 | 67.3 ± 1.6 | 63.0 ± 3.6 | 70.1 ± 1.5 | 67.0 ± 6.6 |
| OS ± SE | 72.3 ± 2.2 | 65.9 ± 27 | 65.5 ± 4.5 | 76.0 ± 1.5 | 69.9 ± 3.4 | 81.0 ± 1.3 | 74.2 ± 6.3 |
| C. T-Cell | |||||||
|---|---|---|---|---|---|---|---|
| CCG-100 series (1983–1988) | CCG-1800 series (1989–1995) | CCG-1900 series (1996–2002) | |||||
| n | 319 | 431 | 522 | ||||
| 5-year (%) | 10-year (%) | 15-year (%) | 5-year (%) | 10-year (%) | 5-year (%) | 10-year (%) | |
| EFS ± SE | 60.4 ± 2.9 | 58.1 ± 3.5 | 56.3 ± 7.0 | 73.2 ± 2.3 | 71.0 ± 4.6 | 72.8 ± 2.1 | 70.7 ± 8.6 |
| OS ± SE | 69.5 ± 2.8 | 64.7 ± 3.4 | 63.5 ± 6.5 | 79.2 ± 2.1 | 77.0 ± 4.2 | 80.3 ± 1.9 | 77.3 ± 8.0 |
| D. Infants | |||||||
|---|---|---|---|---|---|---|---|
| CCG-100 series (1983–1988) | CCG-1800 series (1989–1995) | CCG-1900 series (1996–2002) | |||||
| n | 98 | 135 | 115 | ||||
| 5-year (%) | 10-year (%) | 15-year (%) | 5-year (%) | 10-year (%) | 5-year (%) | 10-year (%) | |
| EFS ± SE | 32.6 ± 4.9 | 31.5 ± 5.4 | 31.5 ± 7.9 | 37.6 ± 4.3 | 36.8 ± 6.4 | 43.2 ± 4.8 | NA |
| OS ± SE | 42.8 ± 5.1 | 38.2 ± 5.5 | 38.2 ± 8.3 | 50.2 ± 4.4 | 49.4 ± 7.0 | 46.8 ± 4.9 | NA |
Acknowledgments
Research is supported by the Chair’s Grant U10 CA98543 and the Statistics and Data Center Grant U10 CA98413 of the Children’s Oncology Group from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
References
- 1.Pinkel D, Simone J, Hustu HO, Aur RJ. Nine years’ experience with “total therapy” of childhood acute lymphocytic leukemia. Pediatr. 1972;50(2):246–251. [PubMed] [Google Scholar]
- 2.Henze G, Langermann HJ, Bramswig J, Breu H, Gadner H, Schellong G, Welte K, Riehm H. The BFM 76/79 acute lymphoblastic leukemia therapy study (author’s transl) Klin Padiatr. 1981;193(3):145–154. doi: 10.1055/s-2008-1034450. [DOI] [PubMed] [Google Scholar]
- 3.Bleyer WA, Coccia PF, Sather HN, Level C, Lukens J, Niebrugge DJ, Siegel S, Littman PS, Leikin SL, Miller DR, et al. Reduction in central nervous system leukemia with a pharmacokinetically derived IT methotrexate dosage regimen. J Clin Oncol. 1983;1(5):317–325. doi: 10.1200/JCO.1983.1.5.317. [DOI] [PubMed] [Google Scholar]
- 4.Miller DR, Leikin S, Albo V, Sather H, Karon M, Hammond D. Prognostic factors and therapy in acute lymphoblastic leukemia of childhood: CCG-141. A report from the Children’s Cancer Study Group. Cancer. 1983;51(6):1041–1049. doi: 10.1002/1097-0142(19830315)51:6<1041::aid-cncr2820510612>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Bleyer WA, Sather HN, Nickerson HJ, Coccia PF, Finklestein JZ, Miller DR, et al. Monthly pulses of vincristine and prednisone prevent bone marrow and testicular relapse in low-risk childhood acute lymphoblastic leukemia: a report of the CCG-161 study by the Childrens Cancer Study Group. J Clin Oncol. 1991;9(6):1012–1021. doi: 10.1200/JCO.1991.9.6.1012. [DOI] [PubMed] [Google Scholar]
- 6.Gaynon PS, Trigg ME, Heerema NA, Sensel MG, Sather HN, Hammond GD, et al. Children’s Cancer Group trials in childhood acute lymphoblastic leukemia: 1983–1995. Leukemia. 2000;14(12):2223–2233. doi: 10.1038/sj.leu.2401939. [DOI] [PubMed] [Google Scholar]
- 7.Gaynon PS, Steinherz PG, Bleyer WA, Ablin AR, Albo VC, Finklestein JZ, et al. Improved therapy for children with acute lymphoblastic leukemia and unfavorable presenting features: a follow-up report of the Childrens Cancer Group Study CCG-106. J Clin Oncol. 1993;11(11):2234–2242. doi: 10.1200/JCO.1993.11.11.2234. [DOI] [PubMed] [Google Scholar]
- 8.Steinherz PG, Gaynon PS, Breneman JC, Cherlow JM, Grossman NJ, Kersey JH, et al. Treatment of patients with acute lymphoblastic leukemia with bulky extramedullary disease and T-cell phenotype or other poor prognostic features: randomized controlled trial from the Children’s Cancer Group. Cancer. 1998;82(3):600–612. doi: 10.1002/(sici)1097-0142(19980201)82:3<600::aid-cncr24>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Tubergen DG, Gilchrist GS, O’Brien RT, Coccia PF, Sather HN, Waskerwitz MJ, et al. Prevention of CNS disease in intermediate-risk acute lymphoblastic leukemia: comparison of cranial radiation and IT methotrexate and the importance of systemic therapy: a Childrens Cancer Group report. J Clin Oncol. 1993;11(3):520–526. doi: 10.1200/JCO.1993.11.3.520. [DOI] [PubMed] [Google Scholar]
- 10.Tubergen DG, Gilchrist GS, O’Brien RT, Coccia PF, Sather HN, Waskerwitz MJ, et al. Improved outcome with delayed intensification for children with acute lymphoblastic leukemia and intermediate presenting features: a Childrens Cancer Group phase III trial. J Clin Oncol. 1993;11(3):527–537. doi: 10.1200/JCO.1993.11.3.527. [DOI] [PubMed] [Google Scholar]
- 11.Nachman J, Sather HN, Cherlow JM, Sensel MG, Gaynon PS, Lukens JN, et al. Response of children with high-risk acute lymphoblastic leukemia treated with and without cranial irradiation: a report from the Children’s Cancer Group. J Clin Oncol. 1998;16(3):920–930. doi: 10.1200/JCO.1998.16.3.920. [DOI] [PubMed] [Google Scholar]
- 12.Nachman JB, Sather HN, Sensel MG, Trigg ME, Cherlow JM, Lukens JN, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338(23):1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 13.Bostrom BC, Sensel MR, Sather HN, Gaynon PS, La MK, Johnston K, et al. Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2003;15;101(10):3809–3817. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- 14.Stork L, Sather H, Hutchinson R, Broxson E, Matloub Y, Yanofsky R, et al. Comparison of mercaptopurine (MP) with thioguanine (TG) and IT methotrexate (ITM) with IT “Triples”(ITT) in children with SR-ALL: results of CCG-1952. Blood. 2002;100(11):585a. [Google Scholar]
- 15.Matloub Y, Lindemulder S, Gaynon PS, Sather H, La M, Broxson E, Yanofsky R, et al. IT triple therapy decreases central nervous system relapse but fails to improve event-free survival when compared with IT methotrexate: results of the Children’s Cancer Group (CCG) 1952 study for standard-risk acute lymphoblastic leukemia, reported by the Children’s Oncology Group. Blood. 2006;108(4):1165–1173. doi: 10.1182/blood-2005-12-011809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avramis VI, Sencer S, Periclou AP, Sather H, Bostrom BC, Cohen LJ, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood. 2002;99(6):1986–1994. doi: 10.1182/blood.v99.6.1986. [DOI] [PubMed] [Google Scholar]
- 17.Seibel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008;111(5):2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matloub Y, Angiolillo A, Bostrom B, Hunger SP, Nachman J, Sather H, et al. Double Delayed Intensification (DDI) Is Equivalent to Single DI (SDI) in Children with National Cancer Institute (NCI) Standard-risk Acute Lymphoblatic Leukemia (SR-ALL) Treated on Children’s Cancer Group (CCG) Clinical Trial 1991 (CCG-1991) Blood. 2006;108(11):47a. [Google Scholar]
- 19.Matloub Y, Bostrom BC, Hunger SP, Angiolillo AL, Cole C, Thomson B, et al. Escalating Dose Intravenous Methotrexate without Leukovorin Rescue during Interim Maintenance Is Superior to Oral Methotrexate for Children with Standard Risk Acute Lymphoblastic Leukemia (SR-ALL): a Children’s Concology Group Study 1991. Blood. 2008;112(11):9–10a. [Google Scholar]
- 20.Bennett J, Catovsky D, Daniel M, Flandrin G, Galton D, Gralnick H, et al. Proposals for the Classification of the Acute Leukaemias French-American-British (FAB) Co-operative Group. British Journal of Haematology. 1976;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 21.Miller DR, Krailo M, Bleyer WA, Lukens JN, Siegel SE, Coccia PR, et al. Prognostic implications of blast cell morphology in childhood acute lymphoblastic leukemia: a report from the Childrens Cancer Study Group. Cancer Treat Rep. 1985;69(10):1211–1221. [PubMed] [Google Scholar]
- 22.Steinherz PG, Siegel SE, Bleyer WA, Kersey J, Chard R, Jr, Coccia P, et al. Lymphomatous presentation of childhood acute lymphoblastic leukemia. A subgroup at high risk of early treatment failure. Cancer. 1991;68(4):751–758. doi: 10.1002/1097-0142(19910815)68:4<751::aid-cncr2820680416>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 23.Lange BJ, Blatt J, Sather HN, Meadows AT. Randomized comparison of moderate-dose methotrexate infusions to oral methotrexate in children with intermediate risk acute lymphoblastic leukemia: a Childrens Cancer Group study. Med Pediatr Oncol. 1996;27(1):15–20. doi: 10.1002/(SICI)1096-911X(199607)27:1<15::AID-MPO4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 24.Reaman GH, Sposto R, Sensel MG, Lange BJ, Feusner JH, Heerema NA, et al. Treatment outcome and prognostic factors for infants with acute lymphoblastic leukemia treated on two consecutive trials of the Children’s Cancer Group. J Clin Oncol. 1999;17(2):445–455. doi: 10.1200/JCO.1999.17.2.445. [DOI] [PubMed] [Google Scholar]
- 25.Hutchinson RJ, Gaynon PS, Sather H, Bertolone SJ, Cooper HA, Tannous R, et al. Intensification of therapy for children with lower-risk acute lymphoblastic leukemia: long-term follow-up of patients treated on Children’s Cancer Group Trial 1881. J Clin Oncol. 2003;21(9):1790–1797. doi: 10.1200/JCO.2003.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Lange BJ, Bostrom BC, Cherlow JM, Sensel MG, La MK, Rackoff W, et al. Double-delayed intensification improves event-free survival for children with intermediate-risk acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 2002;99(3):825–833. doi: 10.1182/blood.v99.3.825. [DOI] [PubMed] [Google Scholar]
- 27.Smith M, Arthur D, Camitta B, Carroll A, Crist W, Gaynon P, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. Journal of Clinical Oncology. 1996;14(1):18–24. doi: 10.1200/JCO.1996.14.1.18. [DOI] [PubMed] [Google Scholar]
- 28.Heath JA, Steinherz PG, Altman A, Sather H, Jhanwar S, Halpern S, et al. Human granulocyte colony-stimulating factor in children with high-risk acute lymphoblastic leukemia: a Children’s Cancer Group Study. J Clin Oncol. 2003;21(8):1612–1617. doi: 10.1200/JCO.2003.07.129. [DOI] [PubMed] [Google Scholar]
- 29.Seibel N, Sather H, Steinherz P, De Laat C, Ettinger L, Freyer D. Upfront treatment with B43 PAP immunotoxin in newly diagnosed children with higher risk ALL–Children’s Cancer Group 1961. Blood. 2000;96:3115a. [Google Scholar]
- 30.Hilden JM, Dinndorf PA, Meerbaum SO, Sather H, Villaluna D, Heerema NA, et al. Analysis of prognostic factors of acute lymphoblastic leukemia in infants: report on CCG 1953 from the Children’s Oncology Group. Blood. 2006;108(2):441–451. doi: 10.1182/blood-2005-07-3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dreyer Z, Steuber C, Bowman W, Murray J, Dinndorf P, Camitta B. Induction intensification for infant acute lymphoid leukemia (ALL) Proc Am Soc Clin Oncol. 1996;15:369a. [Google Scholar]
- 32.Dreyer Z, Dinndorf P, Sather H, Hilden J, Devidas M, Heerema N, et al. Hematopoietic stem cell transplant (HSCT) versus intensive chemotherapy in infant acute lymphoblastic leukemia (ALL) Proc Am Soc Clin Oncol. 2007;25:9514a. [Google Scholar]
- 33.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]
- 34.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist. 1988:1141–1154. [Google Scholar]
- 35.Satwani P, Sather H, Ozkaynak F, Heerema NA, Schultz KR, Sanders J, et al. Allogeneic bone marrow transplantation in first remission for children with ultra-high-risk features of acute lymphoblastic leukemia: A Children’s Oncology Group study report. Biol Blood Marrow Transplant. 2007;13(2):218–227. doi: 10.1016/j.bbmt.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conter V, Valsecchi MG, Silvestri D, Campbell M, Dibar E, Magyarosy E, et al. Pulses of vincristine and dexamethasone in addition to intensive chemotherapy for children with intermediate-risk acute lymphoblastic leukaemia: a multicentre randomised trial. Lancet. 2007;369(9556):123–131. doi: 10.1016/S0140-6736(07)60073-7. [DOI] [PubMed] [Google Scholar]
- 37.De Moerloose B, Suciu S, Bertrand Y, Mazingue F, Robert A, Uyttebroeck A, Yacouben K, et al. Improved outcome with pulses of vincristine and dexamethasone in continuation therapy of children with average risk acute lymphoblastic leukemia (ALL) and non Hodgkin lymphoma (NHL): final report of the EORTC randomized phase III trial 58951. Blood. 2008;112(11):10a. doi: 10.1182/blood-2009-10-247965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurwitz CA, Silverman LB, Schorin MA, Clavell LA, Dalton VK, Glick KM, et al. Substituting dexamethasone for prednisone complicates remission induction in children with acute lymphoblastic leukemia. Cancer. 2000;88(8):1964–1969. [PubMed] [Google Scholar]
- 39.Belgaumi AF, Al-Bakrah M, Al-Mahr M, Al-Jefri A, Al-Musa A, Saleh M, et al. Dexamethasone-associated toxicity during induction chemotherapy for childhood acute lymphoblastic leukemia is augmented by concurrent use of daunomycin. Cancer. 2003;97(11):2898–2903. doi: 10.1002/cncr.11390. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell CD, Richards SM, Kinsey SE, Lilleyman J, Vora A, Eden TO. Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL97 randomized trial. Br J Haematol. 2005;129(6):734–745. doi: 10.1111/j.1365-2141.2005.05509.x. [DOI] [PubMed] [Google Scholar]
- 41.Schrappe M, Zimmermann M, Moricke A, Mann G, Valsecchi MG, Bartram CR, et al. Dexamethasone in induction can eliminate one third of all relapses in childhood acute lymphoblastic leukemia (ALL): results of an international trial in 3655 Patients (Trial AIEOP-BFM ALL 2000) Blood. 2008;112(11):9a. [Google Scholar]
- 42.Igarashi S, Manabe A, Ohara A, Kumagai M, Saito T, Okimoto Y, et al. No advantage of dexamethasone over prednisolone for the outcome of standard- and intermediate-risk childhood acute lymphoblastic leukemia in the Tokyo Children’s Cancer Study Group L95-14 protocol. J Clin Oncol. 2005;23(27):6489–6498. doi: 10.1200/JCO.2005.01.982. [DOI] [PubMed] [Google Scholar]
- 43.Bertrand Y, Suciu S, Benoit Y, Robert A, Nelken B, Uyttebroeck L, et al. Dexamethasone (DEX) (6 mg/sm/d) and prednisolone (PRED) (60 mg/sm/d) in induction therapy of childhood ALL are equally effective: results of the second interim analysis of EORTC trial 58951. Blood. 2008;112(11):9a. [Google Scholar]
- 44.Cutting RJ, Richards S, Hancock J, Goulden N, Mitchell CD, Vora AJ. Post-remission MRD kinetics in children with acute lymphoblastic leukemia receiving augmented BFM consolidation compared with other regimens: results of the Medical Research Council trial UKALL 2003. Blood. 2008;112:539a. [Google Scholar]
- 45.Schrappe M, Reiter A, Zimmermann M, Harbott J, Ludwig W-D, Henze G, et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group form 1981 to 19195. Leukemia. 2000;14(12):2205–2222. doi: 10.1038/sj.leu.2401973. [DOI] [PubMed] [Google Scholar]
- 46.Abromowitch M, Termuhlen A, Chang M, Perkins SL, Gross T, Weinstein HJ, et al. High-dose methotrexate and early intensification of therapy do not improve 3-year EFS in children and adolescents with disseminated lymphoblastic lymphoma. results of the randomized arms of COG A5971. Blood. 2008;112(11):1235a. [Google Scholar]
- 47.Winick N, Borowitz MJ, Devidas M, Martin PL, Pullen J, Hunger SP, et al. Changes in delivery of standard chemotherapeutic agents during induction affect early measures of minimal residual disease (MRD): POG 9900 for patients with B-precursor low and standard risk ALL. Blood. 2006;108(11):643a. [Google Scholar]
- 48.Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22:2142–2150. doi: 10.1038/leu.2008.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freyer DR, Seibel NL, La MK, Devidas M, Carroll WL, Hunger SP, et al. Survival after relapse in higher risk acute lymphoblastic leukemia (ALL) in children and adolescents Is independent of prior treatment intensity: a report from the Children’s Oncology Group (COG) Blood. 2008;112(11):340a. doi: 10.1182/blood-2010-07-294678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nachman J, Siebel N, Sather H, Steinherz P, DeLaat C, Fryer D, et al. Outcome for adolescent and young adults 16–21 years of age (AYA) with acute lymphoblastic leukemia (ALL) treated on the Children’s Cancer Group (CCG) 1961 study. Blood. 2004;104:683a. [Google Scholar]
- 51.Bhatia S, Francisco L, Carter A, Sun C, Baker K, Gurney J, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110(10):3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramanujachar R, Richards S, Hann I, Goldstone A, Mitchell C, Vora A, et al. Adolescents with acute lymphoblastic leukaemia: outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatr Blood Cancer. 2007;48(3):254–261. doi: 10.1002/pbc.20749. [DOI] [PubMed] [Google Scholar]
- 53.Kadan-Lottick N, Dinu I, Wasilewski-Masker K, Kaste S, Meacham L, Mahajan A, et al. Osteonecrosis in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26(18):3038–3045. doi: 10.1200/JCO.2007.14.9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mattano L, Jr, Sather H, Trigg M, Nachman J. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. J Clin Oncol. 2000;18(18):3262–3272. doi: 10.1200/JCO.2000.18.18.3262. [DOI] [PubMed] [Google Scholar]
- 55.Mattano L, Sather H, La M, Nachman J, Seibel N. Modified dexamethasone (DXM) reduces the incidence of treatment-related osteonecrosis (ON) in children and adolescents with higher risk acute lymphoblastic leukemia (HR ALL): a report of CCG-1961. Blood. 2003;102:221a. [Google Scholar]
- 56.Mattano LA, Jr, Nachman JB, Devidas M, Winick N, Raetz E, Carroll WL, et al. Increased incidence of osteonecrosis (ON) with a dexamethasone (DEX) induction for high risk acute lymphoblastic leukemia (HR-ALL): a report from the Children’s Oncology Group (COG) Blood. 2008;112(11):898a. [Google Scholar]
- 57.Mont M, Jones L, Seyler T, Marulanda G, Saleh K, Delanois R. New treatment approaches for osteonecrosis of the femoral head: an overview. Instr Course Lect. 2007;56:197–212. [PubMed] [Google Scholar]
- 58.Ribeiro R, Fletcher B, Kennedy W, Harrison P, Neel M, Kaste S, et al. Magnetic resonance imaging detection of avascular necrosis of the bone in children receiving intensive prednisone therapy for acute lymphoblastic leukemia or non-Hodgkin lymphoma. Leukemia. 2001;15(6):891–897. doi: 10.1038/sj.leu.2402139. [DOI] [PubMed] [Google Scholar]
- 59.Gaynon PS, Bostrom BC, Hutchinson RJ, Lange BJ, Nachman JB, Steinherz PG, et al. Duration of hospitalization as a measure of cost on Children’s Cancer Group acute lymphoblastic leukemia studies. J Clin Oncol. 2001;19(7):1916–1925. doi: 10.1200/JCO.2001.19.7.1916. [DOI] [PubMed] [Google Scholar]
- 60.Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111(12):5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Flohr T, Schrauder A, Cazzaniga G, Panzer-Grűmayer R, Van der Velden V, Fischer S, et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-receptor gene rerearragnements in the international multicenter trialAIEOP BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia. 2008;22:771–782. doi: 10.1038/leu.2008.5. [DOI] [PubMed] [Google Scholar]
- 62.Willemse MJ, Seriu T, Hettinger K, d’Aneillo E, Hop WCJ, Panzer-Grűmayer ER, et al. Detection of minimal residual disease identifies differences in treatment response between T-ALL and precursor BALL. Blood. 2002;99(12):4386–4393. doi: 10.1182/blood.v99.12.4386. [DOI] [PubMed] [Google Scholar]
- 63.Bhojwani D, Kang H, Menezes RX, Yang W, Sather H, Moskowitz NP, et al. Gene expression signatures predictive of early response and outcome in high-risk childhood acute lymphoblastic leukemia: A Children’s Oncology Group Study [corrected] J Clin Oncol. 2008;26(27):4376–4384. doi: 10.1200/JCO.2007.14.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mullighan CG, Phillips LA, Su X, Ma J, Miller CB, Shurtleff SA, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322(5906):1377–1380. doi: 10.1126/science.1164266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mullighan C, Su X, Zhang J, Radtke I, Phillips L, Miller C, et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J of Med. 2009;360(5):470–480. doi: 10.1056/NEJMoa0808253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mullighan CG, Zhang J, Harvey RC, Collins-Underwood JR, Schulman BA, Phillips LA, et al. JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2009;106:9414–9418. doi: 10.1073/pnas.0811761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheok M, Evans W. Acute lymphoblastic leukaemia: a model for the pharmacogenomics of cancer therapy. Nature Reviews Cancer. 2006;6(2):117–129. doi: 10.1038/nrc1800. [DOI] [PubMed] [Google Scholar]
- 68.Pritchard M, Butow P, Stevens M, Duley J. Understanding medication adherence in pediatric acute lymphoblastic leukemia: a review. J Pediatr Hematol Oncol. 2006;28(12):816–823. doi: 10.1097/01.mph.0000243666.79303.45. [DOI] [PubMed] [Google Scholar]
- 69.O’Brien S, Guilhot F, Larson R, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J of Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 70.Schultz KR, Bowman WP, Slayton W, Aledo A, Devidas M, Sather H, et al. Improved early event free survival (EFS) in children with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) with imatinib in combination with high dose chemotherapy: Children’s Oncology Group (COG) study AALL0031. J Clin Oncol. 2009 doi: 10.1200/JCO.2008.21.2514. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hochhaus A. Management of Bcr-Abl-positive leukemias with dasatinib. Expert Rev Anticancer Ther. 2007;7(11):1529–1536. doi: 10.1586/14737140.7.11.1529. [DOI] [PubMed] [Google Scholar]