The Blind Leading the Obese: The Molecular Pathophysiology of a Human Obesity Syndrome (original) (raw)
Abstract
Bardet-Biedl syndrome (BBS) is a genetically heterogeneous disorder affecting multiple organ systems and resulting in blindness, obesity, cognitive impairment, and congenital defects. Interest in the etiology of this disorder stems, in part, from the fact that patients with BBS develop common clinical problems, including obesity, diabetes and hypertension. Twelve genes independently causing BBS have been identified. The heterogeneity is explained by the existence of two BBS complexes, the BBSome consisting of seven known BBS proteins, and the BBS chaperone complex consisting of three known BBS proteins. The formation of the BBSome requires the function of the BBS chaperone complex. Both mouse and zebrafish data support a role for BBS genes in cilia function, and in intracellular and intraflagellar trafficking. From the work described here, a common primary function of BBS proteins has emerged, specifically the mediation and regulation of microtubule-based intracellular transport.
In 1991, I saw a young boy in my medical genetics clinic. The patient's mother had noted that he had vision loss and had taken him to an ophthalmologist, who diagnosed retinitis pigmentosa (RP). RP is a disorder of retinal degeneration resulting in blindness which occurs due to mutations in dozens of different genes encoding proteins with highly diverse functions. RP displays different forms of inheritance, including X-linked recessive, autosomal recessive, autosomal dominant and digenic. In addition, it can occur in isolation or along with other clinical features as part of a genetic syndrome. Due to the fact that the patient had other clinical features in association with the RP, the ophthalmologist referred him to me to evaluate him for a possible genetic syndrome. I noted that the patient was obese, had some cognitive impairment and had a history of having been born with extra fingers and toes (removed by surgery). This boy had a disorder known as Bardet-Biedl syndrome (BBS). At the time, the only thing known about the etiology of BBS was that it was an autosomal recessive disorder. Since that time, my laboratory has studied BBS, which leads to degeneration of the photoreceptor cells and is associated with non-vision abnormalities.
In 1866, Laurence and Moon described a syndrome consisting of retinal degeneration, hypogonadism, mental retardation and spastic paraplegia (1). Years later, Bardet and Biedl independently described a similar syndrome with the additional features of polydactyly and obesity (2, 3). Subsequently, Solis-Cohen and Weiss referred to the association of these phenotypes as Laurence-Moon-Biedl syndrome, because of a single family that had features of both syndromes (4). The disorder was subsequently referred to in the scientific and medical literature as Laurence-Moon-Bardet-Biedl syndrome. Currently, the Laurence-Moon-Bardet-Biedl syndrome is considered to be two distinct disorders: polydactyly and obesity are not components of Laurence-Moon syndrome, but are major components of BBS (5). BBS is a genetically heterogeneous autosomal recessive disorder with the primary features of obesity, polydactyly, pigmentary retinopathy, hypogonadism, renal anomalies and mental retardation (6). Secondary features of BBS include diabetes, hypertension and congenital heart defects (7). Obesity generally begins in infancy and eventually becomes severe. The retinal degeneration component of the phenotype usually results in legal blindness by age 20. Patients have learning disabilities, and some have mental retardation.
Although BBS is rare, considerable interest in the identification of genes causing BBS exists, because components of the phenotype are common (i.e., obesity and diabetes). It is hypothesized that genes involved in BBS may provide clues to the identification of biochemical and developmental pathways involved in these more common disorders as well as to provide additional understanding of normal developmental processes.
As suggested by Sir Archibald Garrod, rare autosomal recessive disorders are more commonly found in consanguineous families (8). We began studying BBS in isolated, consanguineous, Bedouin populations in the Negev region of Israel at a time when it was thought that a single autosomal recessive locus would account for this disorder. The high rate of consanguinity in these populations results in an increase in the frequency of recessive genetic disorders, and three separate Bedouin populations with BBS were identified by our collaborators Drs. Rivka Carmi and Khalil Elbedour, in Israel (9). Previous efforts to map this disorder using multiple small nuclear families from diverse populations had failed to reveal a disease locus. Historical data indicated that the three Bedouin groups with BBS were unrelated to each other; thus each kindred was considered to be of a separate population and genetic mapping studies were performed independently for each of them. To our initial surprise, the disorder was shown to be linked to a different region of the human genome in each population (10–12). This was the first demonstration that BBS is a genetically heterogeneous disorder and demonstrated that at least three separate genes independently cause BBS. Subsequent work by us and others using genetic mapping, positional cloning and comparative genomic analysis focused on identification of additional loci and the genes that cause BBS. This effort resulted in the identification of twelve genes that independently cause autosomal recessive BBS (13–24).
The twelve BBS genes were identified using genetic methods without providing consistent clues to the overall cause of BBS based on predicted protein similarities. Protein-protein interaction domains were found in five of the proteins (beta-propeller repeats in BBS1, BBS2 and BBS7; tetratricopeptide repeats in BBS4 and BBS8). BBS3 encodes an ADP-ribosylation-like factor GTPase; BBS11 is the E3 ubiquitin-ligase TRIM32; and BBS6, BBS10 and BBS12 have similarity to the ATP-dependent chaperonin TRiC/CCT (13–24). While these data did not point to a single unifying hypothesis regarding the function of BBS proteins, two general hypotheses were generated based on the extensive genetic heterogeneity of BBS and the extensive pleiotropic effect of the disorder: [1] The BBS proteins formed one or more protein complexes, and [2] BBS proteins were involved in a fundamental process common to many tissue/cell types.
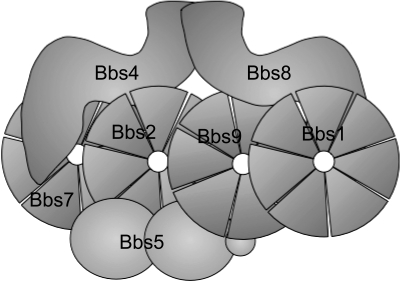
In order to pursue the hypothesis that BBS proteins formed a multi-subunit complex, methods to identify BBS protein interactions were utilized including yeast 2-hybrid, co-immunoprecipitation, and tandem affinity purification. In collaboration with the laboratory of Peter Jackson, tandem affinity purification identified a protein complex, called the BBSome, consisting of seven BBS proteins (BBS1, BBS2, BBS4, BBS5, BBS7, BBS8 and BBS9), as well as a novel protein (Bardet-Biedl interacting protein 10; BBIP10) (25, 26) (Figure 1). Specific protein-protein interactions were confirmed using co-immunoprecipitation. In more recent experiments, our laboratory has shown that the three BBS proteins with chaperone homology (BBS6, BBS10 and BBS12) form a second complex which is required for formation or stability of the BBSome (27).
Fig. 1.
Schematic drawing of the BBSome complex showing protein-protein interactions. BBS1, BBS2, BBS7 and BBS9 contain Beta-propeller repeats. BBS4 and BBS8 contain TPR repeats.
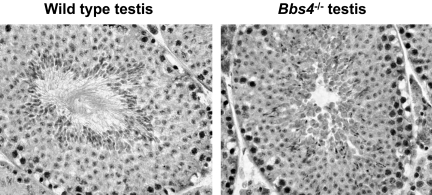
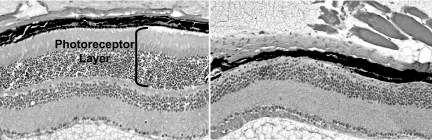
In order to gain further insight into the functional role of BBS genes/proteins, we elected to develop BBS animal models. To date, we have developed several BBS knockout mouse models and one knockin mouse line containing the single most common BBS mutation, BBS1 M390R (28–31). Early analysis of these mice revealed that mice homozygous for a single BBS gene or mice homozygous for the BBS1 M390R mutation completely lack spermatozoa flagella (Figure 2). In addition, these mice undergo photoreceptor degeneration (Figure 3). It should be noted that photoreceptor cells of the retina contain a cilia structure known as the connecting cilium. The connecting cilium functions in the trafficking of proteins and membrane vesicles from the cell body to the outer segment of photoreceptors by a process known as intraflagellar transport (IFT) (32). IFT uses molecular motors to traffic molecules both in an antegrade and retrograde direction. The early mouse data provided evidence that BBS genes/proteins play a role in cilia function. In subsequent work, the BBSome was shown to localize to non-membranous centriolar satellites in the cytoplasm, as well as to the membrane of cilia. The BBSome was also shown to play a role in ciliogenesis, and this role was demonstrated to be mediated by the Rab8 GDP/GTP exchange factor, rabin8 (25). The identification of two BBS complexes explains the genetic heterogeneity of BBS, and the fundamental role of these complexes explains, in part, the pleiotropic nature of BBS. It is not known whether some defects in BBS mice result from abnormal cilia motility or abnormal sensing through cilia. In ongoing work, we have evaluated motile cilia in the airway epithelium in BBS mouse models (33). This work indicates that mutation of BBS genes does not result in defects in cilia motility, leading to the hypothesis that motile cilia may also have a sensory function. BBS mouse models generated in our laboratory are a valuable resource to answer this question in future studies. These mice are also valuable for studies to further investigate the role of individual BBS proteins and complexes in specific tissues and organ systems, and to investigate additive gene-gene interactions in a mammal system.
Fig. 2.
Histological samples from control (left) and BBS knockout (right) mouse testes. Spermatozoa from BBS mice (Bbs4 knockout mouse shown) lack flagella.
Fig. 3.
Histological slides of retina from eight month old wild-type (left) and Bbs4 knockout (right) mice. Note that Bbs4-/- mice lack the photoreceptor layer below the retinal pigmented epithelium (RPE). The photoreceptor layer has completely degenerated in these mice.
In order to further evaluate the role of BBS proteins we utilized zebrafish. One can use antisense oligonucleotides, known as morpholinos (MOs), to knock down the function of specific genes during development. We used MOs against each of the twelve individual zebrafish BBS genes (34, 35). Two interesting phenotypes were observed. First, knockdown of each BBS gene results in failure of development of an early ciliated structure known as Kupffer's vesicle. Second, embryos lacking BBS genes have delayed retrograde melanosome transport. Melanosomes are pigmented vesicles found in melanocytes on the surface of zebrafish that are trafficked to and from the periphery of the cell resulting in lightening and darkening of the zebrafish. These zebrafish phenotypes demonstrate that BBS genes are required for normal cilia function and normal vesicle trafficking, indicating a role for BBS genes in intraflagellar trafficking.
To understand the pathophysiology of BBS, we have further investigated BBS mouse models. Of note, BBS mice become obese (Figure 4). The obesity is due to increased appetite and decreased locomotor activity. Further investigation of the obesity reveals that BBS mutant mice have increased leptin levels. Leptin is a protein produced by adipocytes which have an anorexigenic affect by binding to the leptin receptor (LepR) in proopiomelanocortin (POMC) neurons within the hypothalamus to increase the levels of POMC. The signaling through POMC occurs when leptin binds to the leptin receptor resulting in the activation of Jak2 kinase leading to phosphorylation of Stat3. In mutant BBS mice, Stat3 phosphorylation is attenuated in the presence of elevated leptin, levels indicating leptin resistance. Our data indicate that the likely reason for leptin resistance is abnormal handling or trafficking of the LepR (36, 37).
Fig. 4.
BBS knockout mouse showing the obesity phenotype.
In order to demonstrate a role for the BBSome in LepR function, we performed co-immunoprecipitation experiments to determine whether the BBSome directly interacts with the receptor. The results of these experiments demonstrated that BBS1 protein directly interacts with LepR. However, the M390R BBS1 mutation disrupts this interaction with LepR interaction. These data demonstrate a novel mechanism of leptin resistance (37).
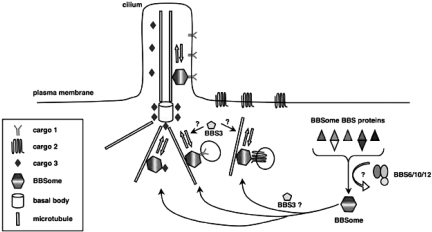
In summary, our results demonstrate that BBS is a genetically heterogeneous disorder affecting multiple organ systems. The heterogeneity is explained by the existence of two BBS complexes, the BBSome consisting of seven known BBS proteins, and the BBS chaperone complex consisting of three known BBS proteins. The formation of the BBSome requires the function of the BBS chaperone complex. Both mouse and zebrafish data support a role for BBS genes in cilia function, and in intracellular and intraflagellar trafficking. Loss of vision in BBS patients is likely due to abnormal trafficking across the connecting cilium in photoreceptor cells. Obesity in BBS patients appears to be due to leptin resistance resulting from an abnormality in LepR handling. A common primary function of BBS proteins has emerged, specifically the mediation and regulation of microtubule-based intracellular transport (Figure 5). These BBS studies are an important example of how the investigation of a rare human disorder can lead to disease gene discovery and novel insights into basic biology and pathophysiology.
Fig. 5.
BBS working model. Seven BBS proteins form a stable complex (BBSome) which localizes to centriolar satellites and to the membrane of cilia. BBSome components play a role in regulation of protein and vesicle trafficking to cilia, and perhaps to the plasma membrane. Chaperone-like BBS proteins (BBS6, BBS10 and BBS12) form a second BBS complex that is required for BBSome assembly.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health, the Carver Endowment for Molecular Ophthalmology, and Research to Prevent Blindness. VCS is an investigator of the Howard Hughes Medical Institute.
Footnotes
Potential Conflicts of Interest: None disclosed.
DISCUSSION
Crystal, New York: We've noticed the BBS genes expressed in the airway epithelium, and so, did the mice and/or the humans have lung disease?
Sheffield, Iowa City: Yes. We've look at that pretty extensively, actually, with Mike Welsh's lab; and you're right, they are expressed in the airway epithelium. If you look closely, there are some defects in the airway epithelial cilia. There is some bulging at the tips of the cilia, which would be consistent with a retrograde transport defect. However this is in a minority of the cilia, and we don't really see a functional consequence of this so far in the mice.
Boyer, New Haven: Another disease of the cilia are polycystic diseases of the kidney and liver. Do these children, or mice, develop renal or liver cysts, and which of these proteins is involved with the structure of the cilia per se?
Sheffield, Iowa City: So in response to the first question, do the humans or mice get cystic kidneys or cystic livers? The humans do get cystic kidneys, but not all humans. Cystic kidneys is a defect that is part of BBS. The mice do not seem to get cystic kidneys, and we don't know why. Regarding which proteins are involved cystic kidneys, have been found in all the different BBS types that has been reported, but again, it is not in all of the patients.
Czeisler, Boston: Thank you for the interesting presentation. I am wondering, is the blindness—are they completely blind, lacking in all conscious light perception?
Sheffield, Iowa City: No. So, as I maybe didn't indicate very well, the blindness is actually due to a photoreceptor degeneration. So initially, they can see okay, but subsequently they lose vision. Remember I reported that we had three different Bedouin kindreds and the disease is quite rare except in these Bedouin populations? With the three Bedouin kindreds we had the opportunity of examining multiple different patients, and so we actually sent an ophthalmology fellow over to Israel to study these patients. What we found out was that the BBS3 patients had a very mild retinopathy, but they have quite a severe myopia; and people assumed that they were blind because of the retinopathy. They weren't giving the children glasses. So, we found this out by studying the children. We got them glasses, and now they are going around seeing just fine; but eventually their photoreceptors do die, and then they have a retinitis-pigmentosa-type phenotype. So, they can still have some light perception, but they gradually lose vision.
Howley, Boston: I have a question about one of the components of the BBSome that you had there, which was the E3 ubiquitin ligase. I am wondering what its function is?
Sheffield, Iowa City: Regarding the E3 ubiquitin ligase, we also refer to it as BBS11, and we identified it by positional cloning. It is not part of the BBSome complex. Neither is it part of the chaperone complex. It is an independent one. We don't know what the targets of the ubiquitin ligase are at this point. In fact, there is a student that is starting to work on that particular molecule.
Sacher, Cincinnati: If I recall, another genetic obesity syndrome is the Prader-Willi Syndrome. Have you looked at BBS in that phenotype?
Sheffield, Iowa City: Prader-Willi is due to a defect on chromosome 15, where there's a loss of the paternal copy of part of chromosome 15, and those patients have really varacious appetites. The BBS patients that I know do eat quite a bit, but they don't have the same voracious appetite as the Prader-Willi Syndrome. There are no BBS genes that map to the Prader-Willi locus.
Mann, St. Louis: There is a Laurence-Moon-Biedl Syndrome, I think, that also has six digits and is associated with obesity. BBS related to that at all?
Sheffield, Iowa City: Yes. BBS was formerly known as Laurence-Moon-Biedl syndrome. There are some patients which have a disorder known as Laurence-Moon syndrome that is likely related, but the details are not known.
REFERENCES
- 1.Laurence JZ, Moon RC. Four cases of retinitis pigmentosa occurring in the same family, and accompanied by general imperfections of development. Ophthal Rev. 1866;2:32–41. doi: 10.1002/j.1550-8528.1995.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 2.Bardet G. Thesis. Paris: University of Paris; 1920. Sur un syndrome d'obesite' congenitale avec polydactylie et retinite pigmentaire (contribution a l'etude des formes cliniques de l'obesite hypophysaire) [Google Scholar]
- 3.Biedl A. Ein Geschwisterpaar mir adipose-genitaler Dystrophie. Dtsch Med Wochenschr. 1922;48:1630. [Google Scholar]
- 4.Solis-Cohens S, Weiss E. The Laurence Biedl syndrome: a report of four cases in one family. Am J Med Sci. 1925;169:489. [Google Scholar]
- 5.Hrynchak PK. Bardet-Biedl syndrome. Optom Vis Sci. 2000;77:236–43. doi: 10.1097/00006324-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Sheffield VC, et al. BBS genes and the Bardet-Biedl syndrome. In: Epstein C.J., Erickson R.P., Wynshaw-Boris A., editors. Inborn Errors of Development. New York, New York: Oxford University Press; 2004. pp. 1044–50. [Google Scholar]
- 7.Green JS, Parfrey PS, Harnett JD, Farid NR, Cramer BC, Johnson G, Heath O, McManamon PJ, O'Leary E, Pryse-Phillips W. The cardinal manifestations of Bardet-Biedl syndrome, a form of Laurence-Moon-Biedl syndrome. N Engl J Med. 1989;321:1002–9. doi: 10.1056/NEJM198910123211503. [DOI] [PubMed] [Google Scholar]
- 8.Garrod AE. The Croonian Lectures of Inborn Errors of Metabolism. Delivered before the Royal College of Physicians on June 18th, 23rd, 25th and 30th. Lancet. 1908;172(4427):1–7. 172(4428); 73–9; 172(4429); 142–8; 172(4430); 214–30. [Google Scholar]
- 9.Sheffield VC, Stone EM, Carmi R. Use of isolated inbred human populations for identification of disease genes. Trends in Genetics. 1998;14:391–6. doi: 10.1016/s0168-9525(98)01556-x. [DOI] [PubMed] [Google Scholar]
- 10.Carmi R, Rokhlina T, Kwitek-Black AE, Elbedour K, Nishimura D, Stone EM, Sheffield VC. Use of a DNA pooling strategy to identify a human obesity syndrome locus on chromosome 15. Hum Mol Genet. 1995;4:9–13. doi: 10.1093/hmg/4.1.9. [DOI] [PubMed] [Google Scholar]
- 11.Kwitek-Black AE, Carmi R, Duyk GM, Buetow KH, Elbedour K, Parvari R, Yandava CN, Stone EM, Sheffield VC. Linkage of Bardet-Biedl syndrome to chromosome 16q and evidence for non-allelic genetic heterogeneity. Nat Genet. 1993;5:392–6. doi: 10.1038/ng1293-392. [DOI] [PubMed] [Google Scholar]
- 12.Sheffield VC, Carmi R, Kwitek-Black A, Rokhlina T, Nishimura D, Duyk GM, Elbedour K, Sunden SL, Stone EM. Identification of a Bardet-Biedl syndrome locus on chromosome 3 and evaluation of an efficient approach to homozygosity mapping. Hum Mol Genet. 1994;3:1331–5. doi: 10.1093/hmg/3.8.1331. [DOI] [PubMed] [Google Scholar]
- 13.Slavotinek AM, Stone EM, Mykytyn K, Heckenlively JR, Green JS, Heon E, Musarella MA, Parfrey PS, Sheffield VC, Biesecker LG. Mutations in MKKS cause Bardet-Biedl syndrome. Nat Genet. 2000;26:15–6. doi: 10.1038/79116. [DOI] [PubMed] [Google Scholar]
- 14.Mykytyn K, Braun T, Carmi R, Haider NB, Searby CC, Shastri M, Beck G, Wright AF, Iannaccone A, Elbedour K, Riise R, Baldi A, Raas-Rothschild A, Gorman SW, Duhl DM, Jacobson SG, Casavant T, Stone EM, Sheffield VC. Identification of the gene that, when mutated, causes the human obesity syndrome BBS4. Nat Genet. 2001;28:188–91. doi: 10.1038/88925. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura DY, Searby CC, Carmi R, Elbedour K, Van Maldergem L, Fulton AB, Lam BL, Powell BR, Swiderski RE, Bugge KE, Haider NB, Kwitek-Black AE, Ying L, Duhl DM, Gorman SW, Heon E, Iannaccone A, Bonneau D, Biesecker LG, Jacobson SG, Stone EM, Sheffield VC. Positional-cloning of a novel gene on chromosome 16q causing Bardet-Biedl syndrome (BBS2) Hum Mol Genet. 2001;10:865–74. doi: 10.1093/hmg/10.8.865. [DOI] [PubMed] [Google Scholar]
- 16.Chiang AP, Nishimura D, Searby C, Elbedour K, Carmi R, Ferguson AL, Secrist J, Braun T, Casavant T, Stone EM, Sheffield VC. Comparative genomic analysis identifies an ADP-ribosylation factor-like gene as the cause of Bardet-Biedl syndrome (BBS3) Am J Hum Genet. 2004;75:475–84. doi: 10.1086/423903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mykytyn K, Nishimura DY, Searby CC, Shastri M, Yen HJ, Beck JS, Braun T, Streb LM, Cornier AS, Cox GF, Fulton AB, Carmi R, Luleci G, Chandrasekharappa SC, Collins FS, Jacobson SG, Heckenlively JR, Weleber RG, Stone EM, Sheffield VC. Identification of the gene (BBS1) most commonly involved in Bardet-Biedl syndrome, a complex human obesity syndrome. Nat Genet. 2002;31:435–8. doi: 10.1038/ng935. [DOI] [PubMed] [Google Scholar]
- 18.Badano JL, Ansley SJ, Leitch CC, Lewis RA, Lupski JR, Katsanis N. Identification of a novel Bardet-Biedl syndrome protein, BBS7, that shares structural features with BBS1 and BBS2. Am J Hum Genet. 2003;72:650–8. doi: 10.1086/368204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–33. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura DY, Swiderski RE, Searby CC, Berg EM, Ferguson AL, Hennekam R, Merin S, Weleber RG, Biesecker LG, Stone EM, Sheffield VC. Comparative genomics and gene expression analysis identifies BBS9, a new Bardet-Biedl syndrome gene. Am J Hum Genet. 2005;77:1021–33. doi: 10.1086/498323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoetzel C, Laurier V, Davis EE, Muller J, Rix S, Badano JL, Leitch CC, Salem N, Chouery E, et al. BBS10 encodes a vertebrate-specific chaperonin-like protein and is a major BBS locus. Nat Genet. 2006;38:521–4. doi: 10.1038/ng1771. [DOI] [PubMed] [Google Scholar]
- 22.Chiang AP, Beck JS, Yen HJ, Tayeh MK, Scheetz TE, Swiderski RE, Nishimura DY, Braun TA, Kim KY, Huang J, Elbedour K, Carmi R, Slusarski DC, Casavant TL, Stone EM, Sheffield VC. Homozygosity mapping with SNP arrays identifies TRIM32 an E3 ubiquitin ligase, as a Bardet-Biedl syndrome gene (BBS11) Proc Natl Acad Sci U S A. 2006;103:6287–92. doi: 10.1073/pnas.0600158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoetzel C, Muller J, Laurier V, Davis EE, Zaghloul NA, Vicaire S, Jacquelin C, Plewnlak F, Leitch CC, Sarda P, et al. Identification of a novel BBS gene (BBS12) highlights the major role of a vertebrate-specific branch of chaperonin-related proteins in Bardet-Biedl syndrome. Am J Hum Genet. 2007;80:1–11. doi: 10.1086/510256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang AP, Nishimura D, Searby C, Elbedour K, Carmi R, Ferguson AL, Secrist J, Braun T, Casavant T, Stone EM, Sheffield VC. Comparative genomic analysis identifies an ADP-ribosylation factor-like gene as the cause of Bardet-Biedl syndrome (BBS3) Am J Hum Genet. 2004;78:475–584. doi: 10.1086/423903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–13. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 26.Loktev AV, Zhang Q, Beck JS, Searby CC, Scheetz TE, Bazan JF, Slusarski DC, Sheffield VC, Jackson PK, Nachury MV. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell. 2008;15:854–65. doi: 10.1016/j.devcel.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Seo S, Baye L, Schulz NP, Beck JS, Zhang Q, Slusarski DC, Sheffield VC. BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0910268107. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, Andorf JL, Mykytyn K, Swiderski RE, Yang B, Carmi R, Stone EM, Sheffield VC. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci U S A. 2004;101:16588–93. doi: 10.1073/pnas.0405496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mykytyn K, Mullins RF, Andrews M, Chiang AP, Swiderski RE, Yang B, Braun T, Casavant T, Stone EM, Sheffield VC. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci U S A. 2004;101:8664–9. doi: 10.1073/pnas.0402354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fath MA, Mullins RF, Searby C, Nishimura DY, Wei J, Rahmouni K, Davis RE, Tayeh MK, Andrews M, Yang B, Sigmund CD, Stone EM, Sheffield VC. Mkks-null mice have a phenotype resembling Bardet-Biedl syndrome. Hum Mol Genet. 2005;14:1109–18. doi: 10.1093/hmg/ddi123. [DOI] [PubMed] [Google Scholar]
- 31.Davis RE, Swiderski RE, Rahmouni K, Nishimura DY, Mullins RF, Agassandian K, Philp AR, Searby CC, Andrews MP, Thompson S, Berry CJ, Thedens DR, Yan B, Weiss RM, Cassell MD, Stone EM, Sheffield VC. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci U S A. 2007;104:19422–7. doi: 10.1073/pnas.0708571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen LB, Rosenbaum JL. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signaling. Current Topics in Devel Biol. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 33.Shah AS, Farmen SL, Moninger TO, Businga TR, Andrews MP, Bugge K, Searby CC, Nishimura D, Brogden KA, Kline JN, Sheffield VC, Welsh MJ. Loss of Bardet-Biedl syndrome proteins alters the morphology and function of motile cilia in airway epithelia. Proc Natl Acad Sci U S A. 2008;105:3380–5. doi: 10.1073/pnas.0712327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yen H-J, Tayeh MK, Mullins RF, Stone EM, Sheffield VC, Slusarski DC. Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer's vesicle cilia function. Hum Mol Genet. 2006;15:667–77. doi: 10.1093/hmg/ddi468. [DOI] [PubMed] [Google Scholar]
- 35.Tayeh MK, Yen HJ, Beck JS, Searby CC, Westfall TA, Griesbach H, Sheffiedl VC, Slusarski DC. Genetic interaction between Bardet-Biedl syndrome genes and implications for limb patterning. Hum Mol Genet. 2008;17:1956–67. doi: 10.1093/hmg/ddn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet-Biedl syndrome. J Clin Invest. 2008;118:1458–67. doi: 10.1172/JCI32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18:1323–31. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]