NOVEL REGULATORS OF CHAPERONE-MEDIATED AUTOPHAGY (original) (raw)
. Author manuscript; available in PMC: 2011 Aug 27.
Summary
Chaperone-mediated autophagy (CMA) is a selective mechanism for the degradation of cytosolic proteins in lysosomes that contributes to cellular quality control and becomes an additional source of amino acids when nutrients are scarce. A chaperone complex delivers CMA substrates to a receptor protein at the lysosomal membrane that assembles into multimeric translocation complexes. However, the mechanisms regulating this process remain, for the most part, unknown. In this work, we have identified two regulatory proteins, GFAP and EF1α, that mediate a previously unknown inhibitory effect of GTP on CMA. GFAP stabilizes the multimeric translocation complex against chaperone-mediated disassembly, whereas GTP-mediated release of EF1α from the lysosomal membrane promotes self-association of GFAP, disassembly of the CMA translocation complex and the consequent decrease in CMA. The dynamic interactions of these two proteins at the lysosomal membrane unveil now a role for GTP as negative regulator of CMA.
Introduction
The degradation of intracellular proteins in lysosomes, or autophagy, takes place in mammalian cells through different mechanisms, namely macroautophagy, microautophagy and chaperone-mediated autophagy (CMA) (Mizushima et al., 2008). Cargo proteins are segregated “in bulk” from the rest of cytoplasmic components by a limiting membrane that seals to form a double membrane vesicle – in macroautophagy – or by invaginations in the surface of the lysosomal membrane that pinch off into the lysosomal lumen – in microautophagy (Mizushima et al., 2008). In contrast, the selective pool of cytosolic proteins degraded by CMA are directly translocated across the lysosomal membrane (Cuervo, 2010; Dice, 2007).
CMA is activated as part of the cellular response to oxidative stress to target oxidized proteins to lysosomes without perturbing neighboring unaffected proteins (Kiffin et al., 2004). Also, during prolonged starvation, the selectivity of CMA provides cells amino acids through selective degradation of expendable proteins. CMA has proven to be important for maintenance of cellular homeostasis, in the cellular response to different stressors (oxidative stress, nutritional stress, etc) and in antigen presentation. Alterations of CMA have been linked to different human pathologies such as Parkinson’s disease, the hypertrophy associated with the diabetic kidney and several lysosomal storage disorders (Cuervo, 2010; Dice, 2007). CMA is active in almost all cells, although basal and inducible levels of CMA activity vary depending on the cell type and cellular conditions (Cuervo, 2010). CMA takes place through relatively well characterized steps. Cargo is first selected for CMA through the interaction of a cytosolic chaperone, the heat shock cognate member of the hsp70 family (hsc70), with a pentapeptide motif present in the amino acid sequence of all CMA substrates (biochemically related to the pentapeptide KFERQ). The complex hsc70/substrate protein is then targeted to the lysosomal membrane where it interacts with the lysosome-associated membrane protein type 2A (LAMP-2A), one of the three splice variants of the single gene lamp2 (Cuervo and Dice, 1996).
LAMP-2A and a lysosome resident variant of hsc70 (lys-hsc70) are the only components of the translocation complex identified so far (Cuervo, 2010; Dice, 2007). We have recently shown that the CMA translocation complex is dynamic (Bandyopadhyay et al., 2008). In fact, LAMP-2A undergoes cycles of rapid assembly/disassembly into a 700kDa protein complex at the lysosomal membrane (Bandyopadhyay et al., 2008). CMA substrates bind to LAMP-2A only in its monomeric form and this binding drives LAMP-2A multimerization into the 700kDa protein complex necessary to attain substrate translocation (Bandyopadhyay et al., 2008). Two lysosomal membrane chaperones, lys-hsc70 and lys-hsp90, participate in the LAMP-2A dynamics at the lysosomal membrane. Lys-hsc70 induces disassembly of LAMP-2A from the 700kDa complex once the substrate has crossed the membrane, and lys-hsp90 stabilizes LAMP-2A during its transition from monomeric to multimeric forms (Bandyopadhyay et al., 2008).
Despite these recent findings on the LAMP-2A dynamics at the lysosomal membrane and their effect on CMA, the mechanisms regulating protein translocation across the lysosomal membrane via CMA are still poorly characterized. In this work, we have identified a LAMP-2A-interacting protein at the lysosomal membrane that regulates the transport of substrate proteins via CMA in a nucleotide-dependent manner, revealing a previously unknown role for GTP in the regulation of CMA. Our studies support the existence of a fine-tuned regulatory mechanism for substrate binding and translocation via CMA based on the assembly of LAMP-2A into a multimeric complex and on the stability of this translocation complex at the lysosomal membrane.
Results
Identification of LAMP-2A-interacting proteins at the lysosomal membrane
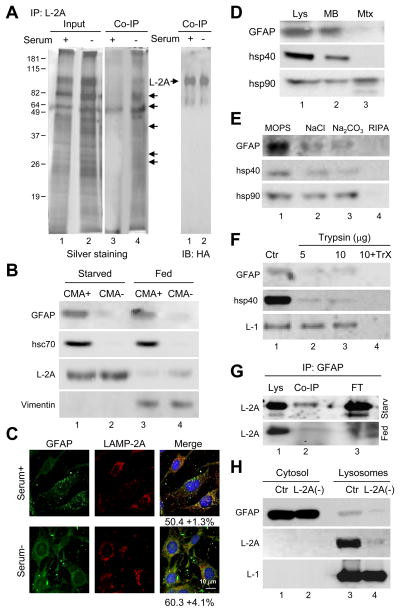
To identify protein components that may associate with the CMA translocation complex to modulate substrate transport, we first analyzed proteins that interact with LAMP-2A at the lysosomal membrane preferentially when CMA is activated. We used lysosomes isolated from mouse fibroblasts stably expressing a previously described an HA (hemagglutinin)-tagged form of LAMP-2A (Cuervo and Dice, 2000), which allows retrieval of only this LAMP-2 variant by immunoprecipitation with an antibody against HA. We displaced endogenous substrates bound to LAMP-2A by incubation with an excess of ribonuclease A (RNase A), a CMA substrate, to enhance the probability of recovering CMA regulators rather than CMA substrates. Analysis of the proteins co-immunoprecipitated with similar amounts of HA-LAMP-2A from lysosomes of cells maintained in the presence (low CMA activity) or absence (high CMA activity) of serum revealed that more proteins co-purified with LAMP-2A in lysosomes from cells maintained in serum-deprived conditions (Fig. 1A, middle panel). Immunoblot analysis of the co-immunoprecipitates confirmed that the band at about 70kDa corresponded to hsc70 (data not shown). Tandem mass spectrometry analysis of the protein band at 50kDa identified it as glial fibrillary acidic protein (GFAP), a cytoplasmic intermediate filament protein involved in the formation of the intracellular framework. Despite its name, GFAP has been identified in multiple cell types and tissues (Morini et al., 2005). Immunofluorescence for GFAP in hepatocytes in culture (RALA cells) or in mouse fibroblasts (3T3 cells) revealed a reticular/punctate pattern for this protein, rather than the characteristic filamentous pattern of intermediate filaments (Fig. S1A). In fact, co-staining for vimentin, another intermediate filament protein, showed only partial colocalization of both proteins, as vimentin was mainly enriched in filamentous structures compatible with intermediate filaments (Fig S1B). Immunoblot of rat liver lysosomes with high activity for CMA (CMA+; enriched in hsc70) and lysosomes incompetent for CMA (CMA-; lacking hsc70 in their lumen) (Cuervo et al., 1997), revealed the preferential association of GFAP with CMA+ lysosomes (Fig. 1B). Lysosomal GFAP was not mere contamination by intermediate filaments, as vimentin presented a rather different pattern of association to lysosomes (detected in both CMA+ and CMA- lysosomes but only under fed conditions) (Figure 1B). Although more GFAP interacted with LAMP-2A during CMA activation (Fig. 1A), the total lysosomal content of GFAP did not change significantly upon CMA upregulation by starvation in rat liver (Fig. 1B) or serum removal in cultured cells (Fig. 1C). These results support that whereas an almost constant fraction of intracellular GFAP resides in lysosomes, activation of CMA promotes the interaction of lysosomal GFAP with LAMP-2A.
Figure 1. GFAP interacts with LAMP-2A in CMA-active lysosomes.
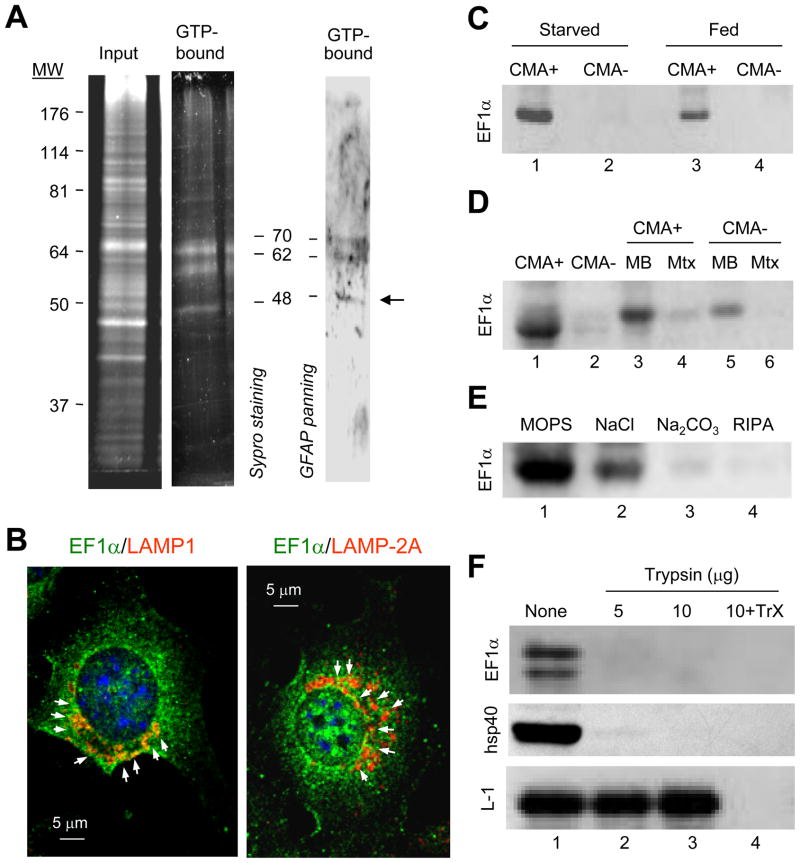
(A) Lysosomes from mouse fibroblasts expressing HA-LAMP-2A and maintained with or without serum (20 h) were incubated with an excess of RNase A and subjected to co-immunoprecipitation (Co-IP) for HA. Silver staining (left) and immunoblot for HA (right). Arrows: bands detectable by silver staining. (B) Immunoblot for the indicated proteins of liver lysosomes active (CMA+) and inactive (CMA-) for CMA from fed and 48 h starved rats. Percentages of cellular protein recovered in CMA+ fed liver lysosomes: 1% GFAP, 0.65% hsc70 and 1.7% vimentin. (C) Immunofluorescence for GFAP and LAMP-2A of mouse fibroblasts. Percentage of colocalization calculated in 20 cells is indicated. (D–F) Immunoblot for the indicated proteins of lysosomes from 48h starved rat liver (Lys) fractionated into membranes (MB) and matrices (Mtx) (D), washed with buffers of increasing stringency (E) or treated with trypsin with or without Triton X-100 (TrX) (F). (G) Co-IP for GFAP and immunoblot for LAMP-2A of lysosomes from fed or 48h starved rat livers. FT: flow through. (H) GFAP in lysosomes from control (CTR) or LAMP-2A RNAi mouse fibroblasts (L2A (-)). LAMP-1 and LAMP-2A are shown as lysosomal markers.
To further characterize the association of GFAP with lysosomes and determine its topology in this compartment, we first separated the lysosomal membrane and matrix by hypotonic shock and high speed centrifugation and found most of the lysosomal GFAP (about 85%) in the lysosomal membrane fraction (Fig. 1D, top panel; hsp40 is used as an example of membrane associated peripheral protein and hsp90 of protein present both in the membrane and lumenal fractions). Close to 80% of lysosomal GFAP was released from the lysosomal membrane by high salt or alkaline wash (Fig. 1E; NaCl and Na2CO3 released 72+12% and 78+13% of the membrane associate GFAP, respectively). GFAP was accessible to degradation by exogenous proteases (trypsin shown in Fig. 1F), supporting its peripheral association with the cytosolic side of the lysosomal membrane. The resistance of the lumenal region of LAMP-1 to the protease treatment and the susceptibility of hsp40 were used as control (Fig. 1F).
A percentage of LAMP-2A can be co-immunoprecipitated with GFAP from rat liver lysosomal membranes and this interaction increases with starvation (Fig. 1G). We confirmed that association of GFAP to the lysosomal membrane requires, at least in part, the presence of LAMP-2A, because lysosomes lacking LAMP-2A (from mouse fibroblasts RNAi for LAMP-2A (Massey et al., 2006)) contained about 25% of the GFAP detected in lysosomes isolated from control mouse fibroblasts (Fig. 1H).
Binding to LAMP-2A and the fact that GFAP bears in its sequence three KFERQ-like motifs makes it a putative CMA substrate. However, in contrast to the rapid degradation of CMA substrates in lysosomes (GAPDH is shown in Fig. S1C), GFAP remained stably associated with the lysosomal membrane. To directly analyze lysosomal uptake of GFAP by CMA, we used a well established in vitro assay with isolated lysosomes. Although both GAPDH and GFAP bound to the lysosomal membrane in a concentration-dependent manner, uptake, which usually correlates well with binding, was only detectable for GAPDH (Fig. S1D). Furthermore, lysosomal levels of GFAP remained unchanged in mice injected with leupeptine to inhibit lysosomal protein degradation, in contrast with the increase observed for bona fide CMA substrates such as IκBα (Fig. S1E compare lanes 3 and 4). Despite the preferential association of GFAP with CMA+ lysosomes, our results do not support GFAP being a substrate for degradation via CMA, at least under normal conditions, which led us to investigate a possible functional role for GFAP in CMA.
GFAP modulates CMA activity in a GTP-dependent manner
To further characterize the lysosomal association of GFAP, we used purified GFAP and first determined its oligomeric status before and after presenting it to lysosomes. Blue native electrophoresis (BNE) revealed that almost 95% of purified GFAP resolved as a monomer of approximately 50kDa (Fig. S2A) and this was still the predominant form after incubation with lysosomes (Fig. S2B). At this concentration of lysosomes, only the exogenously added GFAP was readily detectable in lysosomes. However, upon prolonged exposure (middle panel) or loading higher amount of lysosomes (right) we detect the endogenous lysosome-associated GFAP at 100kDa, 250 and 700kDa (Fig. S2B, right). We did not observe very high order multimerization of GFAP (filaments) in the conditions of our assay, although purified GFAP self-assembled when incubated with the adequate buffer and in the absence of lysosomes (Fig. S2A lane 2 and Fig. S2C lanes 3–4 >900kDa). Purified GFAP efficiently bound to isolated lysosomes in a LAMP-2A-dependent manner, as binding was markedly reduced in lysosomes lacking LAMP-2A (Fig. S2D).
Using the purified GFAP we further characterized its association to lysosomes. Rho-GTP is known to regulate GFAP in intermediate filaments (Boran and Garcia, 2007), prompting us to analyze the effect of different nucleotides on lysosomal GFAP. As shown in Fig. 2A, ATP or its non-hydrolyzable analogue (γ-ATP) did not significantly change the amount of GFAP that binds to the lysosomal membrane, whereas both GTP and its non-hydrolyzable analogue (γ-GTP) markedly enhanced binding of GFAP to lysosomes. At the concentrations used in this study none of the nucleotides affected the stability of the lysosomal membrane (Fig. S2E). BNE revealed that GTP resulted in a shift of a percentage of the exogenously added GFAP to the 100kDa region (Fig. S2B).
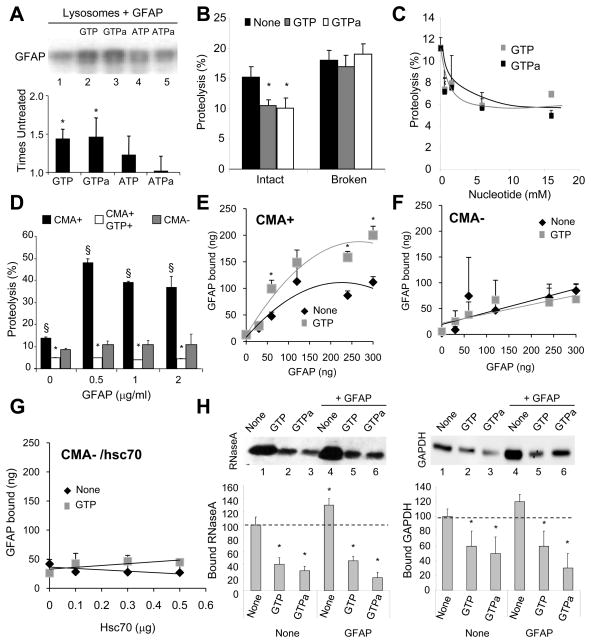
Figure 2. GFAP modulates CMA activity in a GTP-dependent manner.
(A) Binding of GFAP to intact rat liver lysosomes. GTPa and ATPa: non-hydrolyzable analogues. Bottom: densitometric analysis of immunoblots (n= 5). (B) Degradation of a pool of radiolabeled cytosolic proteins by intact or broken rat liver lysosomes alone or with 1mM nucleotides (n= 4). (C) Effect of increasing concentrations of the indicated nucleotides on the proteolysis of a pool of cytosolic proteins by intact rat liver lysosomes (n= 3). (D) Effect of GFAP and/or GTP (1mM) on the proteolysis of the pool of cytosolic proteins by lysosomes active (CMA+) or inactive (CMA-) for CMA (n= 4). (E–F) Binding of GFAP to the same lysosomes as in D. (n= 4–5). (G) Effect of hsc70 and/or GTP (1mM) on the binding of GFAP (120 ng) to CMA incompetent lysosomes (n =3). (H) Binding of RNase A (left) and GAPDH (right) to intact lysosomes incubated with GTP, GTPa and GFAP, as labeled. Values are as percentage of protein bound without additions (None) (n = 6). All values are mean +S.E. p < 0.05 with control samples (*) or for GFAP samples with or without GTP (§).
We found that similar amounts of GTP reduced CMA activity. The amount of radiolabeled cytosolic proteins taken up and degraded by intact isolated lysosomes in the presence of GTP or γ-GTP was significantly reduced when compared with untreated samples (Fig. 2B). This effect was no longer evident when the lysosomal membrane was previously disrupted, discarding a possible effect of GTP on the proteases (Fig. 2B). The inhibitory effect of GTP on CMA was dose-dependent and reached a plateau of 57% inhibition at concentrations of 5mM (Fig. 2C). Incubation of intact lysosomes with increasing concentrations of GFAP in the absence of GTP increased substrate uptake and degradation but only by CMA+ lysosomes (Fig. 2D). Addition of GTP neutralized this stimulatory effect of GFAP on CMA at all concentrations tested (Fig. 2D). Lysosomal binding of GFAP increased proportionally to the amount added in the media, both in the presence and absence of GTP, but was saturable in both cases (Fig. 2E). In contrast, binding of GFAP to CMA- lysosomes was markedly lower than for CMA+ lysosomes, not saturable (likely due to non-selective binding) and insensitive to GTP (Fig. 2F) even upon addition of hsc70 (Fig. 2G). In summary, our results support the existence of unique components/properties at the membrane of CMA+ lysosomes that differently modulate GFAP binding and that binding of GFAP to the lysosomal membrane has different consequences on CMA depending on whether it occurs in the presence or absence of GTP.
To determine the CMA step(s) modulated by GTP and GFAP we incubated lysosomes with substrate proteins at low temperatures, which allows dissociating substrate binding from uptake (Fig. 2H). Binding of two well-characterized CMA substrate proteins, RNase A (left) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (right), was significantly reduced in the presence of GTP, accounting for the reduced uptake observed with the previous assay. The small but consistent stimulatory effect of GFAP on the levels of substrate binding was also neutralized in the presence of GTP (Fig. 2H).
Lentivirus-mediated RNAi against GFAP in rat liver hepatocytes (Fig. 3A; RNAi against two different GFAP regions are shown) did not alter the general organization of the intermediate filament network, reinforcing the minimal participation of GFAP in the formation of intermediate filaments in hepatocytes (Fig. S3A), but eliminated the 50kDa band in lysosomes, confirming that the protein detected in lysosomes was indeed GFAP (Fig. 3A, bottom panels). Contrary to our initial prediction, based on the stimulatory effect of GFAP in vitro, we found increased degradation of radiolabeled substrate by lysosomes in the knock-down cells (Fig. 3B). A similar increase (about 20%) was observed in the binding of substrate protein (Fig. 3C) and in the proteolysis of a pool of radiolabeled cytosolic proteins (data not shown) by intact lysosomes isolated from livers of GFAP knock-out mice, when compared to lysosomes from wild-type littermates. In agreement with their higher CMA activity, lysosomes from GFAP knock-out mice also displayed higher levels of both LAMP-2A and hsc70 (Fig 3D). This observation suggests a compensatory mechanism only detected in cells knocked-out for GFAP (chronic) but not in knock-down (acute) cells until they were maintained in culture for several weeks (Fig. S3B). Therefore, all the functional experiments were performed at 2 weeks of knock-down when LAMP-2A compensation is still not evident (i.e. see LAMP-2A in Fig. 3E). Changes in proteolysis were mainly due to increased substrate binding to lysosomes from knock-down cells (RNase A shown in Fig. 3E). Interestingly, the inhibitory effect of GTP on substrate binding was significantly lower in the absence of GFAP (Fig. 3E), supporting that GTP inhibits CMA, at least partially, through its effect on GFAP.
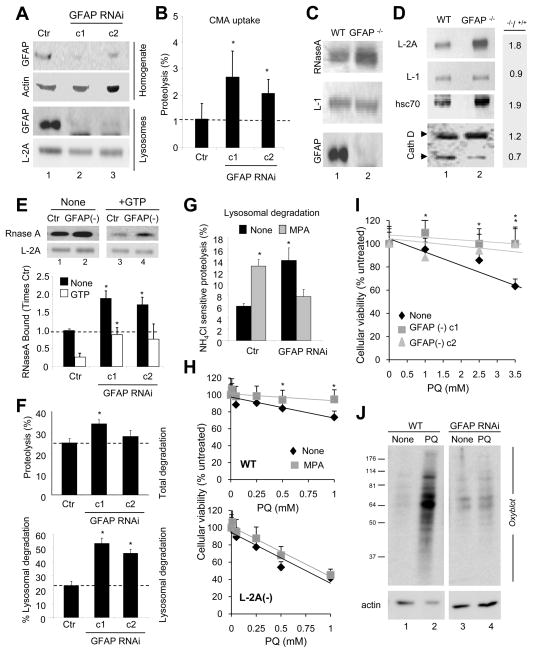
Figure 3. Changes in CMA in cells deficient for GFAP.
(A) Immunobloted for the indicated proteins in fractions from RALA cells control (Ctr) or stably RNAi for GFAP (clone 1 and 2 are against two different regions). (B) Degradation of a pool of radiolabeled cytosolic proteins by intact lysosomes show in (A). Proteolysis in control was given an arbitrary value of 1(n = 4). (C) Binding of RNase A to lysosomes from wild-type (WT) and GFAP knock-out mice (GFAP−/−). L-1: LAMP-1. (D) Immunoblot of the same lysosomes described in (C) for the indicated proteins. Right: folds-change in GFAP−/− mice compared to control. (E) Binding of RNase A to lysosomes isolated from control (Ctr) and GFAP RNAi mouse fibroblasts without (none) or with GTP (1mM). Bottom: densitometric analysis. Binding in untreated control lysosomes was given an arbitrary value of 1 (n= 4). (F) Degradation of long-lived proteins in control (Ctr) or GFAP RNAi cells. Top: Total protein degradation. Bottom: Lysosomal degradation (sensitive to ammonium chloride) (n =4). (G) Percentage of lysosomal degradation in control (Ctr) and GFAP RNAi cells untreated (None) or not with mycophenolic acid (MPA) (n= 3). (H) Viability of wild-type (WT) (top) or RNAi LAMP-2A cells (L2A(-)) (bottom) untreated (None) or treated with MPA after exposure to the indicated concentrations of paraquat (PQ) (n =4). (I) Viability of control (Ctr) and GFAP RNAi cells upon exposure to PQ (n =3). (J) Oxiblot of cells in I. All values are mean +S.E. * p<0.05 with control samples.
Similar effects for GFAP and GTP were observed when we measured protein degradation in intact cells. Although cells knocked-down for GFAP display only slightly higher rates of degradation of long-half life proteins compared to control cells (Fig. 3F, top), these differences become significant when the percentage of degradation contributed by the lysosomal system (sensitive to inhibition by ammonium chloride) is determined (Fig. 3F, bottom). Depletion of intracellular levels of GTP with mycophenolic acid (MPA), a potent selective inhibitor of GTP synthesis shown to reduce intracellular levels of this nucleotide by up to 80% (Nguyen et al., 1983), led to a significant increase in rates of protein degradation (Fig. 3G). This effect can be completely eliminated when lysosomal proteolysis is inhibited (Fig. S3C) supporting its lysosomal origin. Although different types of autophagy contribute to lysosomal degradation, the fact that depletion of GTP did not have any effect on the rates of lysosomal degradation in cells incompetent for CMA (knocked-down for LAMP-2A) (Fig. S3D) strongly supports that GTP was mainly acting on CMA. As further support that the inhibitory effect of GTP is exerted, at least in part, through GFAP, the increase in lysosomal protein degradation observed upon GTP depletion in control cells was no longer evident in GFAP knock-down cells (Fig. 3G). In fact, depletion of GTP abolished their enhanced lysosomal proteolysis.
To gain further insight on the physiological relevance of the GTP-dependent regulation of CMA by GFAP, we analyzed the response to oxidative stress of control and GFAP knock-down cells. CMA is upregulated during mild oxidative stress (Kiffin et al., 2004) and failure to activate CMA under these conditions compromises cell viability (Massey et al., 2006). Depletion of GTP increased resistance of cells to the pro-oxidant paraquat but only in CMA competent cells (Fig. 3H). In support of the net positive outcome on CMA upon knocking-down GFAP, GFAP-deficient cells displayed higher resistance (Fig. 3I) and lower levels of oxidized proteins (Fig. 3J) after exposure to the pro-oxidant.
Our results both in vitro and in intact cultured cells support that GFAP contributes to modulate CMA in response to both starvation and oxidative stress, two well characterized CMA activators, and that the effect of GFAP on CMA is regulated by GTP.
GTP and GFAP modify the dynamics of LAMP-2A at the lysosomal membrane
To explore the mechanism by which GFAP and GTP modulate CMA activity, we first analyzed the interaction of GFAP with different components of the CMA translocation machinery. In addition to LAMP-2A, a fraction of GFAP interacted with hsc70 at the lysosomal membrane. Although GFAP failed to pull-down hsc70 from the lysosomal membrane (Fig.4A), part of the lysosome-associated GFAP was recovered in lysosomal hsc70 pull-downs (Fig. 4B). Hsc70 and LAMP-2A participate in substrate targeting and binding to the membrane, however the fact that GFAP did not interact with entering substrates (GAPDH shown in Fig. S4A) suggested that GFAP may not be involved in these CMA steps. The other functional interaction between hsc70 and LAMP-2A pertains to the assembly/disassembly of LAMP-2A in a 700kDa multimeric complex at the lysosomal membrane required for substrate translocation (Bandyopadhyay et al., 2008). We analyzed the effect of GTP and GFAP on the formation of this translocation complex using BNE. Treatment with GFAP increased the amount of LAMP-2A present in the high molecular weight complex (Fig. 4C), whereas GTP reduced the amount of LAMP-2A in this complex even when lysosomes were supplemented with exogenous GFAP (Fig. 4C). Inversely, lysosomes isolated from GTP-depleted cells showed a higher percentage of LAMP-2A in the multimeric complex, but this effect was no longer observed in cells knock-down for GFAP (Fig. 4D), supporting that the regulatory effect of GTP on CMA is mediated through GFAP. Interestingly, lysosomes from cells knocked-down for GFAP were still able to form the 700kDa translocation complex (Fig. 4D), suggesting that although GFAP enhances the amount of LAMP-2A present in this complex, it is not required for its assembly but it may be important to maintain its stability. We have previously demonstrated a disassembly function for hsc70 over this complex (Bandyopadhyay et al., 2008) and have now found that hsc70-mediated disassembly of the LAMP-2A complex was, to a large extent, neutralized in the presence of GFAP (Fig. 4E).
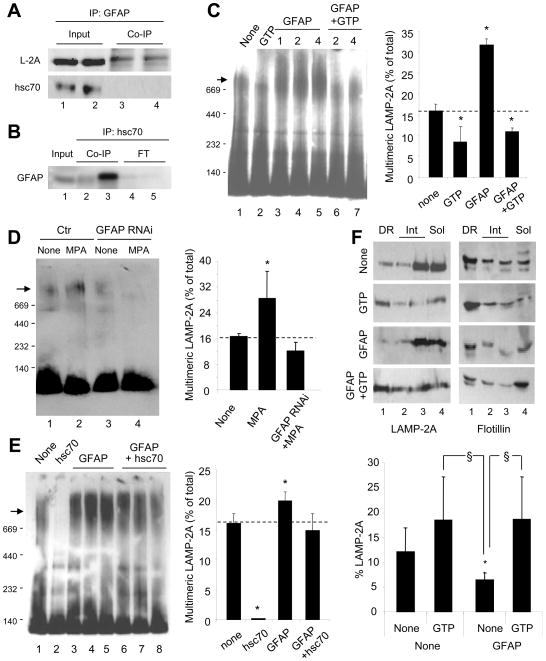
Figure 4. GFAP modifies the dynamics of the CMA receptor at the lysosomal membrane.
(AB) Co-immunoprecipitation with GFAP (duplicate) (A) or with two different antibodies against hsc70 (lanes 2 and 3) (B) of lysosomes from 48h starved rat livers. (C) Blue native electrophoresis (BNE) and LAMP-2A immunoblot of rat liver lysosomes incubated alone (None) and/or with 1 mM GTP and GFAP at the indicated concentrations (mg/ml). Arrow: 700kDa translocation complex. Right: Percentage of LAMP-2A in the 700kDa complex (n= 4). (D–E) BNE for LAMP-2A of lysosomes from control (Ctr) or GFAP RNAi cells (D) or from rat liver (E) after the indicated treatments. Right: Percentage of LAMP-2A in the 700kDa complex (n= 3). (F) Immunoblot for LAMP-2A and flotillin of rat liver lysosomes incubated alone (None) or with GFAP and/or GTP and subjected to Triton X-114 extraction and sucrose density gradient floatation. Detergent-resistant (DR), intermediate (Int) or detergent-soluble (Sol) fractions are shown. Bottom: Percentage of LAMP-2A in DR (n= 5). All values are mean +S.E. p<0.05 with untreated (*) or with GTP treated (§).
The changes in LAMP-2A multimerization are in agreement with the differences that we observed in the association of LAMP-2A to lipid microdomains at the lysosomal membrane. A percentage of LAMP-2A associates in a monomeric state with specific lipid microdomains at the lysosomal membrane, but upon CMA activation, LAMP-2A is excluded from these regions and organizes into multimeric complexes (Kaushik et al., 2006). We have now found higher levels of LAMP-2A in lipid microdomains in the presence of GTP whereas GFAP reduced the levels of LAMP-2A in these regions (Fig. 4F). As observed for substrate binding and LAMP-2A multimerization, combined addition of GFAP and GTP neutralized the effect of GFAP alone (Fig. 4F). GFAP did not mobilize to the microdomains under these conditions (Fig. S4B). We confirmed the GTP-induced mobilization of LAMP-2A toward the microdomains by measuring the degradation rates of LAMP-2A in lysosomes supplemented with Ca+2 and incubated in the absence of protease inhibitors. We have previously shown that LAMP-2A is degraded in a Ca+2-dependent manner in the microdomains (Kaushik et al., 2006). Lysosomes treated with GTP displayed higher rates of LAMP-2A degradation than control lysosomes (Fig. S4C). Our studies support that the regulatory effect of GFAP and GTP on CMA occurs at the level of the organization of the translocation complex at the lysosomal membrane.
A GTP-binding protein at the lysosomal membrane modulates CMA activity
To elucidate how the GTP-dependent effect of GFAP on CMA is modulated, we first analyzed changes on the lysosome-associated GFAP. Immunoblot for GFAP phosphorylation, which modulates GFAP self-assembly and stability (Noetzel, 1990; Takemura et al., 2002), revealed that about 1.5% of cellular PGFAP was recovered in CMA+ lysosomes, versus the 0.5–1% of total GFAP, or 0.5% of hsc70 (a well-established CMA regulator) (Fig. S5B). The total lysosomal content of PGFAP did not change when CMA was activated by prolonged starvation (Fig. S5A) or mild oxidative stress (Fig. S5C). Although phosphorylation of GFAP is not required for lysosomal binding (the exogenously added GFAP was not phosphorylated) the endogenous GFAP in the 100kDa region was phosphorylated (Fig. S5D). GFAP phosphorylation may contribute to stabilizing GFAP once at the lysosomal membrane and prevent further self-assembly in this compartment (compare with the high order multimeration of GFAP in a purified intermediate filament enriched fraction (Fig. S5F).
The interdependent effect of GTP and GFAP on CMA activity, the GTP-dependent enhanced binding of GFAP to the lysosomal membrane and the inability of GFAP to directly bind GTP led us to hypothesize the possible participation of a GTP-binding protein in the regulatory effect of GFAP on CMA. GTP-affinity chromatography of solubilized membranes from CMA+ lysosomes revealed a subset of lysosomal proteins that bound to the immobilized GTP (Fig. 5A). To determine which of the GTP-binding proteins could be functionally related to GFAP and consequently contribute to its GTP-dependent binding to the lysosomal membrane, we subjected the eluted lysosomal GTP-binding proteins to panning with GFAP (Fig. 5A, right panel). Out of the three proteins able to bind both GTP and GFAP, we obtained positive identification by MS/MS of the one of approximately 50kDa to be Elongation Factor 1 alpha (EF1α).
Figure 5. The GTP-binding protein EF1α interacts with GFAP at the lysosomal membrane.
(A) GTP-affinity chromatography of 48h starved rat liver lysosomal membranes. Left: SyproRuby staining. Right: Panning for GFAP of the eluted fraction. Arrow: elongation factor 1α (EF1α). (B) Immunofluorescence for LAMP-1 (left) or LAMP-2A (right) and EF1α in mouse fibroblasts maintained in the absence of serum. (C–D) Immunoblot for EF1α of starved rat liver lysosomes with high (CMA+) and low (CMA-) CMA activity (C) and their corresponding membranes (MB) and matrices (Mtx) (D). (E–F) Immunoblot of CMA active lysosomes washed with buffers of increasing stringency (E) or incubated with increasing concentrations of trypsin in presence or absence of Triton X-100 (TrX) (F).
A portion of intracellular EF1α colocalized with LAMP-1 or LAMP-2A (Fig. 5B) by immunofluorescence in fibroblasts in culture. Immunoblot for EF1α in lysosomes isolated from rat liver confirmed that EF1α was more abundant in CMA+ lysososomes (Fig. 5C). Topological analysis similar to the one described for GFAP revealed that lysosomal EF1α is preferentially found in the membrane fraction (Fig. 5D). High salt concentration washes and alkali extraction removed about 65+15% and 87+8% of the lysosomal EF1α, respectively (Fig. 5E). Susceptibility of EF1α to treatment with an exogenous protease (trypsin) confirmed its association with the cytosolic side of the lysosome membrane (Fig. 5F).
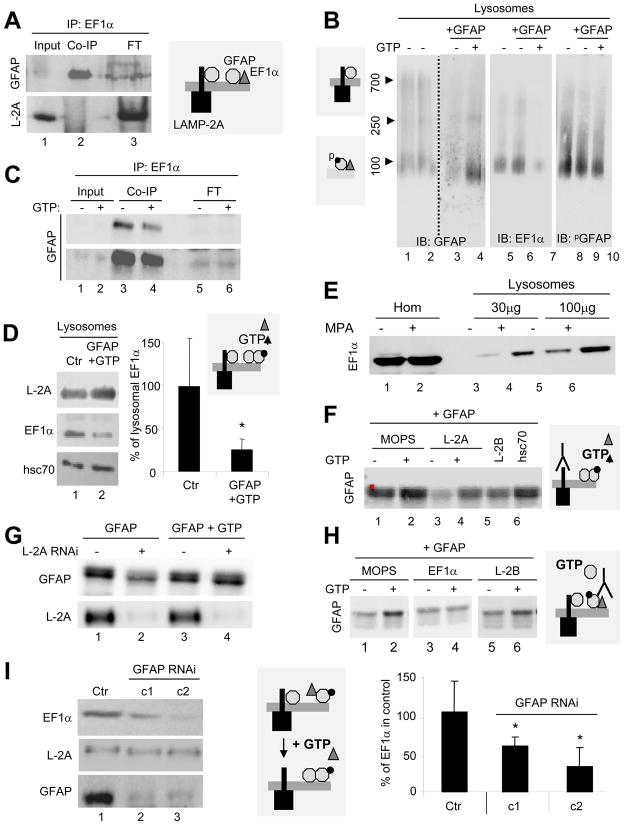
GFAP (Fig. 6A, top) but not LAMP-2A (Fig. 6A, bottom) can be co-immunoprecipitated with EF1α from lysosomes supporting that, contrary to GFAP, EF1α did not interact directly with LAMP-2A. Endogenous GFAP and EF1α were both be detected in the 100KDa complex by BNE (Fig. 6B), but only GFAP was detected in the 700kDa region. Immunoblot for PGFAP revealed that most of the GFAP in the 100kDa was phosphorylated. Exogenous GFAP (not detected by the PGFAP antibody), when incubated with lysosomes did not change levels of PGFAP in the 100kDa complex but markedly reduced levels of EF1α in this complex. Since both proteins have a molecular weight of 50KDa, it is difficult to differentiate homodimers from heterodimers of both proteins. However, different independent evidence supports that GFAP and EF1α form heterodimers at the membrane which are disrupted in the presence of GTP. First, incubation of lysosomes with exogenous GFAP and GTP increased the amount of GFAP in the 100kDa complex while markedly reducing the amount of EF1α present in this complex (Fig. 6B). In addition, the amount of GFAP co-immunoprecipitated with EF1α also decreased upon addition of GTP (Fig. 6C; 47.2% decrease). Lastly, incubation of lysosomes with exogenous GFAP in the presence of GTP significantly reduced levels of endogenous EF1α in lysosomes (Fig. 6D). This reduction was not due to EF1α proteolysis, because all the experiments were performed in the presence of protease inhibitors, suggesting instead that the decrease in lysosomal levels of EF1α originates from its release from the lysosomal membrane. Conversely, we found markedly higher levels of EF1α in lysosomes isolated from GTP-depleted cells (Fig. 6E).
Figure 6. The dynamic interaction of EF1α, GFAP and LAMP-2A at the lysosomal membrane is modulated by GTP.
(A) Co-immunoprecipitation for EF1α of starved rat liver lysosomes. FT: flow through. (B) Blue native electrophoresis and immunoblot for the indicated proteins of lysosomes as in (A) incubated or not with 1mM GTP. Left: Molecular weights. (C) CO-IP for EF1α of the same lysosomes as in (B). Bottom: higher exposure. FT: flow through. (D) Immunoblot of rat liver lysosomes untreated (Ctr) or incubated with GTP and GFAP. Right: Percentage of EF1α remaining after the treatments (n= 3). (E) Immunoblot for EF1α of cell homogenates (Hom) and lysosomes from cells treated or not with mycophenolic acid (MPA). (F) Immunoblot for GFAP of rat liver lysosomes were pre-incubated in MOPS buffer alone or with antibodies against LAMP-2A (L-2A), LAMP-2B (L-2B) or hsc70, followed by GFAP with or without 1mM GTP. (G) Immunoblot of lysosomes from control or LAMP-2A RNAi cells incubated with GFAP with or without 1mM GTP. (H) Immunoblot for GFAP of rat liver lysosomes pre-incubated alone (MOPS) or with antibodies against EF1α or LAMP-2B (L-2B), followed by GFAP. (I) Immunoblot of lysosomes from control and GFAP RNAi cells (two clones shown). Right: EF1α at the lysosomal as percentage of EF1α in control (n = 3). Values are all mean +S.E. *p<0.05.
Our results support the presence of at least two different pools of GFAP at the lysosomal membrane, because part of GFAP was associated to LAMP-2A and part to EF1α, but LAMP-2A and EF1α were never recovered in the same complex. In fact, blockage of the cytosolic tail of LAMP-2A at the lysosomal membrane by pre-incubation with antibodies against this tail, reduced binding of GFAP to the lysosomal membrane (Fig. 6F), but did not affect its GTP-dependent binding (Fig. 6F; a 3.5 fold increase in membrane levels of GFAP was still evident even when LAMP-2A was blocked). Pre-incubation of lysosomes with antibodies against LAMP-2B or hsc70 did not reduce GFAP binding, supporting the selectivity for LAMP-2A binding (Fig. 6F). We further confirmed the existence of this LAMP-2A-independent binding of GFAP to the lysosomal membrane in the presence of GTP using cells knocked-down for LAMP-2A (Fig. 6G). To further investigate the requirements for EF1α in this process, we first attempted to knockdown EF1α, but probably because of the many other functions reported for this protein, cells presented marked alterations in the lysosomal compartment (leaky lysosomes and severe reduction of LAMPs; data not shown) that prevented us from analyzing CMA activity in them. As an alternative approach, we used antibodies to block lysosome-associated EF1α and found that its blockage preferentially reduced the GTP-dependent binding of GFAP (Fig. 6H), supporting that the additional binding of GFAP to the lysosomal membrane in the presence of GTP was attained by exchange/release of the membrane-associated EF1α. Interestingly, binding of EF1α to the lysosomal membrane required GFAP. In fact, levels of EF1α were markedly reduced in lysosomes isolated form cells knocked-down for GFAP (Fig. 6I), although total cellular levels of EF1α were higher in those cells (Fig. S6).
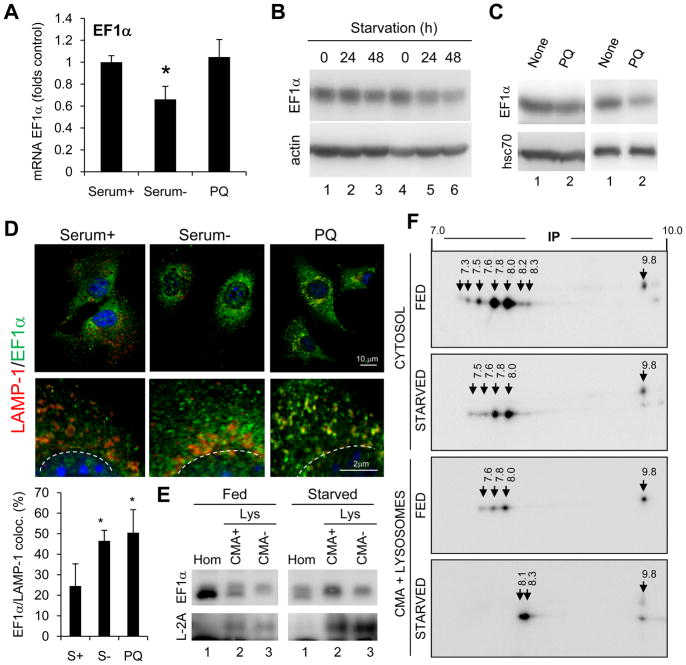
Lysosomal levels of EF1α, rather than total intracellular levels of this protein, contribute to modulate CMA activity. We did not observe transcriptional activation of EF1α (Fig. 7A) or an increase in total cellular EF1α (Fig. 7B, C) in two conditions known to activate CMA – starvation and mild oxidative stress. However, immunofluorescence of cultured cells (Fig. 7D) and immunoblot of isolated liver lysosomes (Fig. 7E), revealed a significant increase in association of EF1α to lysosomes in these conditions. Blockage of lysosomal proteolysis did not affect cellular levels of EF1α discarding degradation in this compartment (Fig. S6B). Bidimensional electrophoretic analysis revealed that only particular isoforms of EF1α associate to lysosomes (Fig. 7F), and that lysosomal EF1α, but not the cytosolic form, undergoes charge changes during CMA activation (Fig. 7F). Our results support that the effect of EF1α on CMA is likely exerted by a particular isoform of this protein.
Figure 7. Regulation of the association of EF1α with lysosomes.
(A) EF1α mRNA levels in fibroblasts maintained in the presence or absence (48h) of serum or treated with paraquat (PQ) for 6 h. Values are fold control after normalization for actin (n= 3–4). (B–C) Immunoblot of fibroblasts maintained in the absence of serum for the indicated times (B) or of livers of rats untreated (None) or treated with PQ (C). Two different sets are shown. (D) Immunofluorescence of fibroblasts maintained in presence (Serum+) or absence of serum (Serum-) for 24h or treated with PQ for 6h. Bottom: Higher magnification insets and quantification of colocalization in >25 cells (n=2). (E) Immunoblot of fed or starved (48h) rat liver homogenates (Hom) and lysosomes (Lys) active (CMA+) or inactive (CMA-) for CMA. (F) Bidimensional electrophoresis and immunoblot for EF1α of cytosol or CMA+ lysosomes isolated from fed or 48h starved rats. Arrows: Isoelectric point values.
Overall, our results support that EF1α binds to the lysosomal membrane through the pool of GFAP not interacting with LAMP-2A (see model in Fig. S7); upon addition of GTP, EF1α is released from this GFAP, allowing binding of other GFAP molecules (likely those previously interacting with LAMP-2A).
Discussion
This study provides evidence of the complexity and fine tuning of the regulation of the degradation of cytosolic proteins via CMA. We have shown that cytosolic levels of GTP exert a previously unknown regulatory effect on CMA through the intricate dynamics of two proteins, GFAP and EF1α, with the CMA receptor protein LAMP-2A, at the lysosomal membrane (Fig. S7). GFAP binding to LAMP-2A stabilizes the LAMP-2A multimeric complex by preventing the disassembling effect of hsc70. In the presence of GTP, EF1α is released from the GFAP at the lysosomal membrane, allowing its self-assembly with the GFAP molecules released from LAMP-2A, which is then mobilized to the lipid microdomains for degradation.
The presence of two different pools of GFAP at the lysosomal membrane provides an explanation for the initial apparent dichotomy between our results in isolated lysosomes, where addition of GFAP enhanced CMA activity, and in cultured cells lacking GFAP, where CMA activity was also enhanced. The pool of GFAP bound to LAMP-2A prevents the disassembly of the CMA translocation complex, having a stimulatory effect on CMA. In contrast, the portion of GFAP at the lysosomal membrane that is not bound to LAMP-2A contributes to the GTP-inhibitory effect of CMA by binding the GFAP previously associated to the LAMP-2A complexes. The amount of EF1α at the lysosomal membrane may be the limiting factor that prevents binding of GFAP to the LAMP-2A unbound fraction in the in vitro system. In cultured cells, knock-down of GFAP decreases both pools, the LAMP-2A bound and unbound. The enhanced CMA activity detected in these cells suggests that the net outcome of GFAP activity at the lysosomal membrane in the presence of normal cytosolic levels of GTP is mainly inhibitory, and also reinforces that GFAP is not required for formation of the CMA translocation complex, but rather it prevents or slows down its disassembly by hsc70. Regulation of CMA by GFAP could reach an additional level of complexity through the degradation of GFAP by this pathway. Although our results do not support GFAP being a CMA substrate, the existence of three KFERQ-like motifs in its amino acid sequence could allow GFAP to undergo CMA in yet to be determined conditions.
An intriguing aspect of our findings is that the GTP-inhibitory effect was still observed even in the absence of exogenously added GFAP in vitro. This finding suggests that GTP modifies or redistributes the GFAP already present at the lysosomal membrane in order to attain the inhibitory effect. We have found that this redistribution is associated with the release of EF1α, a GTP-binding protein, from the lysosomal membrane. Although further investigation is required, it is possible that upon addition of GTP, release of EF1α from the LAMP-2A unbound GFAP, results in a conformational change in this GFAP, that enhances its affinity for other GFAP molecules (those bound to LAMP-2A) and favors its self assembly at the lysosomal membrane. Furthermore, because phosphorylation of lysosomal GFAP seems to be restricted to the protein present in the 100kDa region but not interacting with the LAMP-2A complex, it is possible that changes in phosphorylation of GFAP may modulate its distribution between these two pools.
The GTP-dependent effect of GFAP on LAMP-2A dynamics provides a regulatory mechanism for the previously described association of LAMP-2A to lysosomal lipid microdomains (Kaushik et al., 2006). Movement of LAMP-2A toward these regions decreases the amount of LAMP-2A multimeric complexes and reduces CMA activity (Kaushik et al., 2006). However, recruitment of LAMP-2A to microdomains is not only a mechanism for CMA down-regulation but also serves to ensure the normal turnover of LAMP-2A. In fact, we have recently reported that alterations in the ability of LAMP-2A to reach the lysosomal microdomains, such as those observed in aging, result in destabilization of this protein and its abnormal random degradation in the lysosomal compartment (Cuervo, 2010; Dice, 2007). From this perspective, the GTP-modulated effect of GFAP may contribute to normal homeostasis of LAMP-2A at the lysosomal membrane by favoring its association to microdomains. The dynamic balance between the LAMP-2A bound and unbound pools of GFAP at the lysosomal membrane becomes in this way an important modulator of the distribution of LAMP-2A between the CMA translocation complex and the regions responsible for its turnover.
Neither GFAP nor EF1α have been described before as lysosome-associated proteins, although localization of GFAP in compartments other than intermediate filaments has been reported (Morini et al., 2005). Here, we have found association of GFAP with lysosomes in hepatocytes and mouse and rat fibroblasts, cells in which only a small percentage of GFAP seems to associate into intermediate filaments. To the best of our knowledge, the only connection between GFAP and the lysosomal system reported so far, is the upregulation of macroautophagy in astrocytes expressing mutant forms of GFAP known to produce Alexander disease (Tang et al., 2008). Blockage of CMA has been shown to upregulate macroautophagy (Massey et al., 2006). Consequently it is possible that the high levels of GFAP in Alexander-affected cells result in CMA inhibition and this contributes, at least in part, to the observed increase in macroautophagy activity in these cells.
The presence of a portion of cellular EF1α associated to lysosomes, comes as less of a surprise, because multiple functions and diverse cellular locations have already been described for this protein. A role in protein translation (Dreher et al., 1999), cytoskeleton rearrangement and protein deubiquitination and chaperoning (Xia et al., 2008) have been proposed for EF1α. However, it is still not clear how the participation of EF1α in these different functions is modulated. Recently, two competent EF1α genes, which are differentially expressed, have been identified in mammals. Although the significance of the two different protein products (92% identical) remains elusive, it is possible that the different isoforms have different intracellular locations/functions, and that the lysosome-associated form of EF1α could correspond to only one of these variants. In fact, our results support preferential association of particular EF1α isoforms to lysosomes and further changes with CMA activation. The electrophoretic differences observed for the lysosomal EF1α may originate from alternative splicing or post-translational modifications. The absence of transcriptional changes in EF1α during CMA activation makes us favor the second possibility. EF1α has been shown to be phosphorylated (Izawa et al., 2000), methylated and covalently bind ethanolamine (Coppard et al., 1983). Whether changes in these post-translational modifications could modulate its interaction with GFAP at the lysosomal membrane requires further investigation.
GTP has been connected to autophagy as it modulates some of the Rab proteins (Rab7, 32, 33) known to participate in macroautophagy. Our findings reveal a connection between GTP and the degradation of cytosolic proteins by CMA through its effect on EF1α and GFAP. These components could represent possible new targets for interventions aimed at upregulating CMA activity in conditions where declined CMA activity has been reported, such as aging or familial forms of Parkinson’s disease (Cuervo, 2010).
Experimental Procedures
Animals and cells
We used adult male Wistar rats (200–250 g). Mouse fibroblasts (NIH3T3) were from the American Type Culture Collection and clones stably RNAi for LAMP-2A were described previously (Massey et al., 2006). The rat hepatocyte line RALA255-10G was cultured as described (Bandyopadhyay et al., 2008).
Chemicals
Sources of antibodies and reagents are described in Supplementary Data.
Isolation of subcellular fractions
Lysosomes from rat liver and cultured cells were isolated from a light mitochondrial-lysosomal fraction by centrifugation through a discontinuous metrizamide (Cuervo et al., 1997) or metrizamide/percoll discontinuous density gradient (Storrie and Madden, 1990), respectively. Only preparations with more than 95% intact lysosomes were used. Lysosomal matrices and membranes were separated by centrifugation after hypotonic shock (Storrie and Madden, 1990).
Measurement of CMA activity
CMA was measured in vitro as the uptake and degradation of substrate proteins by isolated lysosomes, and in cells in culture, as the rates of degradation of long-lived protein sensitive to ammonium chloride and insensitive to 3-methyl adenine as described before (Kaushik and Cuervo, 2009) and detailed in Supplementary data.
Protein co-immunoprecipitation
Co-immunoprecipitation was done from lysosomal membranes solubilized in 25 mM Tris, pH 7.2, 150 mM NaCl, 5 mM MgCl2, 0.5% NP-40, 1 mM DTT, 5% glycerol and protease inhibitors (CoIP buffer) for 15 min on ice and then centrifuged for 15 min at 16,000 g.
GTP-affinity chromatography
Guanosine-5′ triphosphate agarose was used for GTP-affinity chromatography. Lysosomal membranes solubilized as above were passed through the GTP-affinity column and the bound fraction was eluted using an excess of GTP.
Immunofluorescence staining
Cells were grown on coverslips until confluent and kept in the presence or absence of serum for 20 h were fixed in methanol kept at −20°C, blocked (1% BSA, 2% New born calf serum, 0.01% Triton X-100), and then incubated with the primary and corresponding fluorescent-conjugated secondary antibodies as described (Kaushik et al., 2006). Images were acquired with an Axiovert 200 fluorescence microscope (Carl Zeiss) and subjected to deconvolution with the manufacturer’s software. Colocalization was determined using MetaMorph (Universal Imaging). Confocal images were acquired using a Leica SP5II AOBS laser-scanning confocal microscope. Colocalization was determined using ImageJ (JCOP plug-In).
RNAi
shRNAs against GFAP were from the Sigma Mission Library. Sequence is described in the Supplemental Data.
Statistical analysis
The statistical significance of the difference between experimental groups was determined by two-tailed unpaired Student’s t-test.
Highlights.
- GTP inhibits CMA activity by modulating GFAP and EF1α interactions in lysosomes
- GFAP prevents disassembly by hsc70 of LAMP-2A from the CMA translocation complex
- GTP induces dissociation of GFAP from LAMP-2A and promotes its self-association
- EF1α is a lysosomal GTP-binding protein that prevents GFAP self-association
Supplementary Material
01
Acknowledgments
We are grateful to Samantha J. Orenstein and Dr. Fernando Macian for their insights and critical revision of this manuscript. This work was supported by NIH grants AG031782, AG021904. S.K. is supported by TG32AG023475 and R.K. by a Ruth L. Kirschstein National Service Award F31 AG034040.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bandyopadhyay U, Kaushik S, Vartikovsky L, Cuervo AM. Dynamic organization of the receptor for chaperone-mediated autophagy at the lysosomal membrane. Mol Cell Biol. 2008;28:5747–5763. doi: 10.1128/MCB.02070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boran MS, Garcia A. The cyclic GMP-protein kinase G pathway regulates cytoskeleton dynamics and motility in astrocytes. J Neurochem. 2007;102:216–230. doi: 10.1111/j.1471-4159.2007.04464.x. [DOI] [PubMed] [Google Scholar]
- Coppard NJ, Clark BF, Cramer F. Methylation of elongation factor 1 alpha in mouse 3T3B and 3T3B/SV40 cells. FEBS Lett. 1983;164:330–334. doi: 10.1016/0014-5793(83)80311-1. [DOI] [PubMed] [Google Scholar]
- Cuervo A, Dice J. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- Cuervo A, Dice J. Unique properties of lamp2a compared to other lamp2 isoforms. J Cell Sci. 2000;113:4441–4450. doi: 10.1242/jcs.113.24.4441. [DOI] [PubMed] [Google Scholar]
- Cuervo AM. Chaperone-mediated autophagy: selectivity pays off. Trends Endocrinol Metab. 2010;21:142–150. doi: 10.1016/j.tem.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF, Knecht E. A population of rat liver lysosomes responsible for the selective uptake and degradation of cytosolic proteins. J Biol Chem. 1997;272:5606–5615. doi: 10.1074/jbc.272.9.5606. [DOI] [PubMed] [Google Scholar]
- Dice J. Chaperone-mediated autophagy. Autophagy. 2007;3:295–299. doi: 10.4161/auto.4144. [DOI] [PubMed] [Google Scholar]
- Dreher TW, Uhlenbeck OC, Browning KS. Quantitative assessment of EF-1alpha.GTP binding to aminoacyl-tRNAs, aminoacyl-viral RNA, and tRNA shows close correspondence to the RNA binding properties of EF-Tu. J Biol Chem. 1999;274:666–672. doi: 10.1074/jbc.274.2.666. [DOI] [PubMed] [Google Scholar]
- Izawa T, Fukata Y, Kimura T, Iwamatsu A, Dohi K, Kaibuchi K. Elongation factor-1 alpha is a novel substrate of rho-associated kinase. Biochem Biophys Res Commun. 2000;278:72–78. doi: 10.1006/bbrc.2000.3772. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM. Methods to monitor chaperone-mediated autophagy. Methods Enzymol. 2009;452:297–324. doi: 10.1016/S0076-6879(08)03619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Massey AC, Cuervo AM. Lysosome membrane lipid microdomains: novel regulators of chaperone-mediated autophagy. EMBO J. 2006;25:3921–3933. doi: 10.1038/sj.emboj.7601283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiffin R, Christian C, Knecht E, Cuervo A. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell. 2004;15:4829–4840. doi: 10.1091/mbc.E04-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Nat Acad Sci USA. 2006;103:5905–5910. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morini S, Carotti S, Carpino G, Franchitto A, Corradini SG, Merli M, Gaudio E. GFAP expression in the liver as an early marker of stellate cells activation. Ital J Anat Embryol. 2005;110:193–207. [PubMed] [Google Scholar]
- Nguyen BT, Cohen MB, Sadee W. Guanine ribonucleotide depletion in mammalian cells. A target of purine antimetabolites. Cancer Chemother Pharmacol. 1983;11:117–119. doi: 10.1007/BF00254259. [DOI] [PubMed] [Google Scholar]
- Noetzel MJ. Phosphorylation of the glial fibrillary acidic protein. J Neurosci Res. 1990;27:184–192. doi: 10.1002/jnr.490270208. [DOI] [PubMed] [Google Scholar]
- Storrie B, Madden EA. Isolation of subcellular organelles. Meth Enzymol. 1990;182:203–225. doi: 10.1016/0076-6879(90)82018-w. [DOI] [PubMed] [Google Scholar]
- Takemura M, Gomi H, Colucci-Guyon E, Itohara S. Protective role of phosphorylation in turnover of glial fibrillary acidic protein in mice. J Neurosci. 2002;22:6972–6979. doi: 10.1523/JNEUROSCI.22-16-06972.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Yue Z, Talloczy Z, Hagemann T, Cho W, Messing A, Sulzer DL, Goldman JE. Autophagy induced by Alexander disease-mutant GFAP accumulation is regulated by p38/MAPK and mTOR signaling pathways. Hum Mol Genet. 2008;17:1540–1555. doi: 10.1093/hmg/ddn042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Webster A, Du F, Piatkov K, Ghislain M, Varshavsky A. Substrate-binding sites of UBR1, the ubiquitin ligase of the N-end rule pathway. J Biol Chem. 2008;283:24011–24028. doi: 10.1074/jbc.M802583200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01