A Smac Mimetic Reduces TNF Related Apoptosis Inducing Ligand (TRAIL)-Induced Invasion and Metastasis of Cholangiocarcinoma Cells (original) (raw)
. Author manuscript; available in PMC: 2010 Oct 19.
Published in final edited form as: Hepatology. 2010 Aug;52(2):550–561. doi: 10.1002/hep.23729
Abstract
Cholangiocarcinoma (CCA) cells paradoxically express tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), a death ligand that, failing to kill CCA cells, instead promotes their tumorigenicity and especially the metastatic behaviors of cell migration and invasion. Second mitochondria-derived activator of caspase (smac) mimetics are promising cancer therapeutic agents that enhance proapoptotic death receptor signaling by causing cellular degradation of inhibitor of apoptosis (IAP) proteins. Our aim was to examine the in vitro and in vivo effects of the smac mimetic JP1584 in CCA. Despite JP1584-mediated loss of cellular inhibitor of apoptosis-1 (cIAP-1) and cIAP-2, TRAIL failed to induce apoptosis in KMCH-1, TFK-1, and BDEneu CCA cells; a finding consistent with a downstream block in death signaling. Because cIAP-1 and cIAP-2 also promote nuclear factor kappa B (NF-κB) activation by the canonical pathway, the effect of JP1584 on this signaling pathway was examined. Treatment with JP1584 inhibited TRAIL-induced NF-κB activation as well as TRAIL-mediated up-regulation of the NF-κB target gene, matrix metalloproteinase 7 (MMP7). JP1584 also reduced TRAIL-mediated CCA cell migration and invasion in vitro. Finally, in a syngeneic rat orthotopic CCA model, JP1584 administration reduced MMP7 messenger RNA levels and extrahepatic metastases.
Conclusion
Although the smac mimetic JP1584 does not sensitize cells to apoptosis, it reduces TRAIL-induced CCA cell metastatic behavior. These data support the emerging concept that IAPs are prometastatic and represent targets for antimetastatic therapies.
Cholangiocarcinoma (CCA) is a highly malignant neoplasm originating from the bile duct system with markers of cholangiocyte differentiation.1,2 It is the second most common primary hepatic malignancy, and epidemiological studies have suggested that its incidence is increasing in Western countries.3–8 Most patients present with advanced metastatic disease and are not amenable to surgical extirpation or liver transplantation.3,9,10 Indeed, this tumor has an early propensity to metastasize to regional lymph nodes, the peritoneum, and the omentum. Although our understanding of the molecular pathogenesis of CCA has increased in recent years and includes the recognition that it is a highly apoptosis-resistant neoplasm,3 viable therapeutic strategies for advanced CCA are limited.3,11
Human CCA paradoxically expresses the death ligand tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) and its cognate death receptors.12 TRAIL actually promotes tumorigenesis in CCA cells by facilitating metastatic cell behavior, especially cell migration and invasion.12 TRAIL also promotes metastasis in an in vivo xenograft model of pancreatic cancer.13 In both studies, the prometastatic behavior of TRAIL was, in part, due to nuclear factor kappa B (NF-κB)–dependent pathways initiated by TRAIL signaling cascades. CCA abundantly expresses matrix metalloproteinase 7 (MMP7), which likely contributes to the invasive properties.14–16 MMP7 is an established NF-κB target gene providing a mechanism by which TRAIL-mediated NF-κB activation promotes invasion of these cancers.17 Unlike signaling by the death ligand tumor necrosis factor-α (TNF-α) after binding to its death receptor,18,19 inhibition of NF-κB does not convert TRAIL signaling from a prosurvival stimulus to an apoptotic stimulus but rather reduces the effects of TRAIL on metastatic behavior.12 Thus, approaches to sensitize cells to TRAIL cytotoxicity and/or inhibit its NF-κB signaling pathways may be salutary in the treatment of CCA.
Small-molecule second mitochondria-derived activator of caspase (smac) mimetics are promising anticancer therapeutics that have been reported to induce apoptosis as single agents or act as sensitizers to TRAIL-induced apoptosis in various cancer cell lines.20–24 These therapeutic agents are designed to resemble the smac N-terminal alanine-valine-proline-isoleucine sequence and mimic the proapoptotic function of smac by binding to inhibitor of apoptosis (IAP) proteins.21,23 Interestingly, the two IAP proteins involved in death receptor signaling, cellular inhibitor of apoptosis-1 (cIAP-1) and cIAP-2, undergo rapid cellular elimination after binding to smac mimetics via autoubiquitination and subsequent proteasome-mediated degradation.25,26 These smac mimetics promote apoptosis by TNF-α23,25–28 and TRAIL20–24 and also inhibit canonical NF-κB activation upon death ligand signaling.22,29–31 In addition, IAPs have been implicated as potential prometastatic genes, especially by promoting cell motility.32 Thus, targeting IAPs such as cIAP-1 and cIAP-2 by a smac mimetic may potentially limit the tumorigenicity of TRAIL in CAA.
The objective of this study was to examine the in vitro and in vivo effects of the smac mimetic JP1584 in CCA cells and especially their effect on TRAIL-potentiated metastatic behavior. The results of this study suggest that in vitro JP1584 reduces TRAIL-induced migration, invasion, and matrix degradation. Furthermore, in a syngeneic rat orthotopic CCA model, JP1584 achieved significant metastasis suppression as a single agent. These results have implications for the employment of JP1584 and other smac mimetics in the treatment of human CCA.
Materials and Methods
Materials
JP1584 was supplied by Joyant Pharmaceuticals (Dallas, TX). For in vitro use, JP1584 was dissolved at 500 µM in dimethyl sulfoxide and aliquoted. Aliquots were further diluted in a cell culture medium prior to each experiment. The maximal dimethyl sulfoxide concentration was 0.1% (vol/vol), and this was also used as a vehicle for all in vitro control experiments. For in vivo experiments, JP1584 was prepared according to the supplier’s protocol. Recombinant human TRAIL (rhTRAIL) and recombinant mouse TRAIL (rmTRAIL) were obtained from R&D Systems (Minneapolis, MN) and dissolved according to the manufacturer’s recommendations. rmTRAIL has a higher amino acid identity with rat TRAIL than rhTRAIL (85% versus 70%) and was therefore used for the treatment of rat BDEneu cells. BDEneu cells also displayed the highest TRAIL resistance and were therefore treated with higher TRAIL dosages than the other CCA cell lines employed in this study (20 versus 2.5 ng/mL).
Cell Lines and Culture
The ErbB-2/neu transformed malignant rat cholangiocyte cell line BDEneu, normal rat cholangiocytes, the human CCA cell lines KMCH-1 and TFK-1, the nonmalignant human cholangiocyte cell line H69, isolated human hepatocytes, and the human hepatocellular carcinoma (HCC) cell line Huh-7 were cultured as previously described.33–38 Written, informed consent was obtained from all patients before the hepatocytes were obtained.
Quantitation of Apoptosis
Apoptosis in cell culture was quantified by the assessment of the characteristic nuclear changes of apoptosis after staining with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma, St. Louis, MO) with fluorescence microscopy.39 Caspase-3/caspase-7 activity was quantitated with the Apo-ONE homogeneous caspase-3/caspase-7 assay (Promega, Madison, WI) according to the manufacturer’s recommendations.39
MMP7 Silencing
Rat MMP7 was knocked down with commercially available small interfering RNA [siRNA; sc-108053 (MMP7) and sc-37007 (Scramble), Santa Cruz Biotechnology, Santa Cruz, CA]. Reverse transfection of BDEneu cells was performed with the siPORT NeoFX transfection agent (Ambion, Austin, TX). Cells were transfected in Opti-MEM with 30 nM siRNA with the siPORT NeoFX transfection agent for 24 hours according to the manufacturer’s instructions. Knockdown of MMP7 was confirmed by immunoblot analysis.
Animal Experiments
All animal studies were performed in accordance with and were approved by the institutional animal care and use committee. In vivo intrahepatic cell implantation was carried out in adult male Fischer 344 rats (Harlan, Indianapolis, IN) with initial body weights between 180 and 200 g as previously described (the syngeneic rat orthotopic CCA model).34,37 In the nonhepatic studies, 4 × 106 BDEneu cells, suspended in 0.05 mL of sterile phosphate-buffered saline, were injected directly into the greater omentum in order to mimic abdominal tumor growth derived from metastatic cells (the abdominal CCA cell implantation model). JP1584 (2 mg/kg, 0.1 mL) or vehicle was given intravenously every other day six times (first injection on postoperative day 7 and sixth injection on postoperative day 17). Twenty-four hours after the last injection, the rats were euthanized. In the CCA studies, the livers were removed for further analysis, which included histopathology, messenger RNA (mRNA), and protein extraction, as previously described.34 To assess the numbers of metastasis-free and metastasis-bearing rats, the abdominal cavities, the retroperitoneal spaces, and the thoracic cavities were thoroughly examined by visual inspection and palpation for the presence of extrahepatic tumors. In all experiments, metastatic tumors were completely removed, and the malignant nature of the lesions was confirmed by histopathology.
Supporting Methods
The immunoblot analysis, quantitative real-time polymerase chain reaction (RT-PCR), immunocytochemistry for (phospho-)NF-κB (p65), electrophoretic mobility shift assay (EMSA), quantitation of interleukin-6 (IL-6) secretion, migration/invasion/fluorescent gelatin degradation/cell proliferation assays, immunohistochemistry for cytokeratin 7/TRAIL, and statistical analysis are described in the online supporting information.
Results
JP1584 Does Not Sensitize CCA Cells to TRAIL-Induced Apoptosis
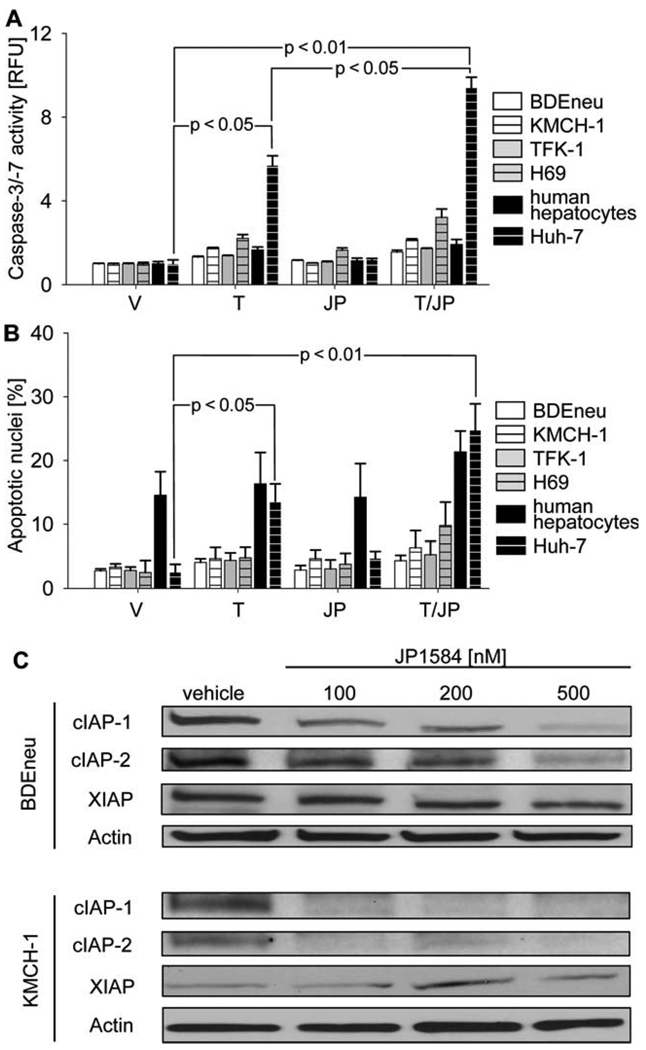
Initially, we explored the potential proapoptotic effect of JP1584 treatment or JP1584 and TRAIL treatment on rat (BDEneu) and human (KMCH-1 and TFK-1) CCA cell lines and on a human nonmalignant cholangiocyte cell line (H69) and primary human hepatocytes (Fig. 1A,B). Unexpectedly, the administration of JP1584, TRAIL, and JP1584 plus TRAIL did not induce significant apoptosis in any of the CCA cell lines. In contrast, treatment with TRAIL (P < 0.05) and especially JP1584 plus TRAIL (P < 0.01) induced significant apoptosis in the human HCC cell line (Huh-7 cells; Fig. 1A,B). The failure of JP1584 to promote apoptosis in CCA cells was not due to a lack of pharmacological efficacy because JP1584 induced cellular elimination of both cIAP-1 and cIAP-2 in these cell lines (Fig. 1C). Thus, despite cIAP-1 and cIAP-2 cellular elimination by JP1584, CCA cell lines remained resistant to TRAIL-mediated apoptosis.
Fig. 1.
CCA cells are not sensitized to TRAIL-induced apoptosis by JP1584 despite cellular loss of cIAP-1 and cIAP-2. (A,B) Cells were treated for 6 hours with vehicle, TRAIL [BDEneu: 20 ng/mL rmTRAIL; KMCH-1, TFK-1, H69, isolated human hepatocytes, and Huh-7 (positive control): 2.5 ng/mL rhTRAIL], JP1584 (500 nM), or TRAIL plus JP1584. Cell treatment was followed by (A) fluorescent analysis of caspase-3/caspase-7 activity or (B) DAPI staining with quantitation of apoptotic nuclei by fluorescence microscopy. Note the significant increases in caspase-3/caspase-7 activity as well as apoptotic cell nuclei upon the treatment of Huh-7 HCC cells with TRAIL alone and especially with the combination of TRAIL and JP1584. In contrast, BDEneu, KMCH-1, H69, isolated human hepatocytes, and TFK-1 cells displayed no significant response to any treatment. Means and standard errors of the mean are shown (n = 3). (C) Immunoblot analysis for cIAP-1, c-IAP-2, and XIAP in BDEneu and KMCH-1 cells treated with JP1584 for 6 hours at the indicated concentrations. Abbreviations: JP, JP1584; RFU, relative fluorescence unit; T, TRAIL; T/JP, TRAIL plus JP1584; V, vehicle; XIAP, X-linked inhibitor of apoptosis.
JP1584 Inhibits TRAIL-Mediated NF-κB Activation
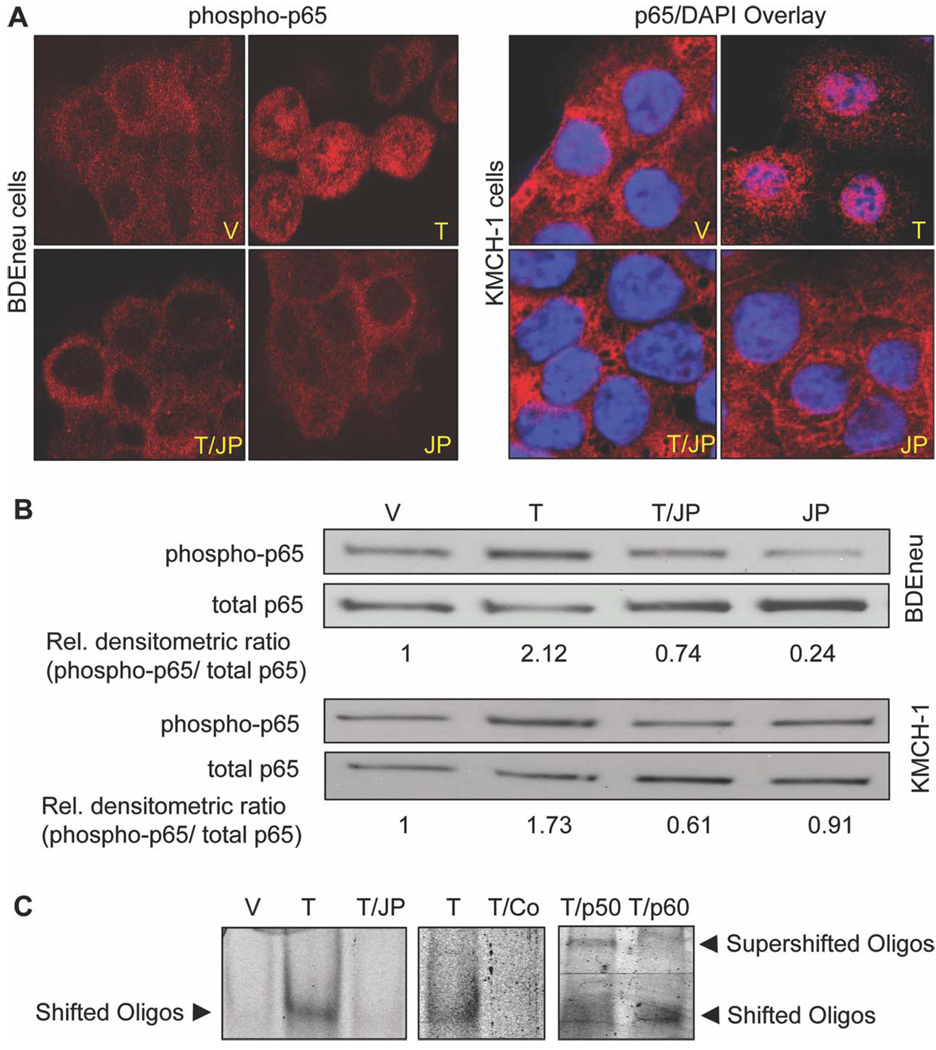
Because cellular elimination of cIAP-1 and cIAP-2 reduces NF-κB activation via the canonical pathway after death receptor stimulation,29–31 we examined the effect of JP1584 on NF-κB activation. Canonical NF-κB activation was assessed by immunocytochemistry, phospho-immunoblot analysis, and EMSA (Fig. 2A–C). Immunocytochemistry for phospho-p65 (BDEneu) or p65 (KMCH-1) demonstrated phosphorylation and/or nuclear accumulation of (phospho-)p65 only in TRAIL-treated cells; this effect was reduced in cells treated with TRAIL plus JP1584 (Fig. 2A). Immunoblot analysis for phospho-p65 revealed increased phosphorylation of p65 in both BDEneu and KMCH-1 cells upon TRAIL treatment. Again, JP1584 prevented the TRAIL-mediated increase of p65 phosphorylation (Fig. 2B). Consistent with these results, EMSA analysis identified a protein/oligonucleotide complex when nuclear protein extracts were employed from TRAIL-treated KMCH-1 cells (Fig. 2C); this complex was reduced when nuclear protein extracts were obtained from cells treated with JP1584 plus TRAIL (Fig. 2C). The specificity of the complex for NF-κB binding to a consensus oligonucleotide binding motif was confirmed by competition with an excess of an unlabeled probe and characteristic supershifts with anti-p50 and p65 antisera (Fig. 2C). Thus, cellular elimination of cIAP-1 and cIAP-2 by JP1584 inhibits TRAIL-mediated NF-κB activation by the canonical pathway in CCA cells.
Fig. 2.
JP1584 inhibits TRAIL-mediated NF-κB activation. Tumor cells were treated with vehicle, TRAIL (BDEneu: 20 ng/mL rmTRAIL; KMCH-1: 2.5 ng/mL rhTRAIL; 40 minutes), JP1584 (500 nM and 2-hour pretreatment), or TRAIL plus JP1584, and this was followed by analysis via immunocytochemistry, phospho-immunoblotting, and EMSA. (A) Tumor cells were subjected to immunocytochemistry for phospho-p65 (BDEneu) or p65 (KMCH-1) and analyzed with confocal microscopy. The phospho-p65/DAPI overlay demonstrates phosphorylation and nuclear accumulation of p65 only in TRAIL-treated rat BDEneu cells. Similarly, in human KMCH-1 cells, TRAIL induced nuclear accumulation of p65. This effect was inhibited by cotreatment with JP1584. (B) Immunoblot analysis for phospho-p65 and p65 protein expression. Consistent with immunocytochemistry, increased phosphorylation of p65 in BDEneu and KMCH-1 cells occurred solely upon treatment with TRAIL. JP1584 inhibited the TRAIL-mediated increase in p65 phosphorylation. (C) EMSA analysis identified a protein/target oligonucleotide complex when nuclear protein extracts were employed from TRAIL-treated KMCH-1 cells (left); this complex was reduced in the presence of JP1584 (left). This TRAIL-associated protein/oligonucleotide complex was inhibited by an excess of unlabeled NF-κB consensus oligonucleotide (middle) and was supershifted with anti-p50 and p65 antisera (right). Abbreviations: JP, JP1584; T, TRAIL; T/Co, TRAIL plus consensus oligonucleotide; T/JP, TRAIL plus JP1584; T/p50, TRAIL plus p50; T/p60, TRAIL plus p60; V, vehicle.
JP1584 Reduces TRAIL-Induced Tumor Cell Migration, Invasion, and Matrix Degradation
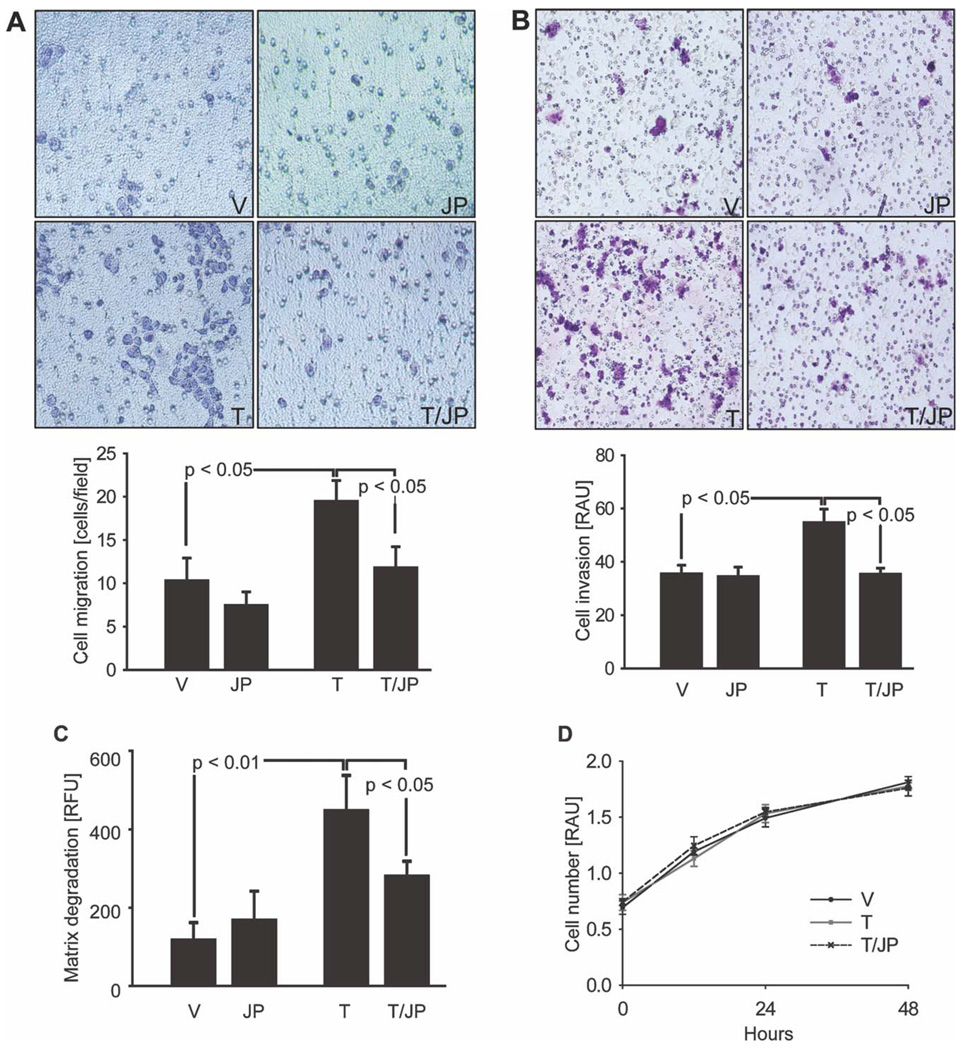
Because TRAIL promotes NF-κB–mediated cell invasion and migration in apoptosis-resistant CCA cells and pancreatic ductal adenocarcinoma cells,12,13 we examined tumor cell migration, invasion, and matrix degradation in response to the administration of TRAIL, JP1584, and TRAIL plus JP1584. TRAIL significantly increased in vitro BDEneu tumor cell migration through a porous filter (Fig. 3A). This effect was efficiently blocked by JP1584. In addition, tumor cell invasion through an artificial extracellular matrix was enhanced by TRAIL and blocked by JP1584 (Fig. 3B). Finally, TRAIL also induced degradation of the extracellular matrix, as measured with fluorescent gelatin as a substrate (Fig. 3C). Similarly to its effect on TRAIL-induced NF-κB activation, cell migration, and invasion, JP1584 also decreased TRAIL-induced matrix degradation (Fig. 3C). Because TRAIL administration or cotreatment with TRAIL plus JP1584 could potentially alter cell proliferation and confound the interpretation of these cellular migration and invasion assays, we ascertained whether cellular proliferation was altered in any of these paradigms. However, exposure to neither TRAIL nor TRAIL plus JP1584 had an effect on cell proliferation in CCA cells (Fig. 3D) or nonmalignant cholangiocytes (Supporting Fig. 1).
Fig. 3.
JP1584 blocks TRAIL-induced tumor cell migration, invasion, and matrix degradation. BDEneu cells were (A,B) cultured for 48 hours onto the upper compartments of migration/invasion assay inserts or (C) plated onto fluorescent gelatin matrix-coated cover slips in the presence of vehicle, TRAIL (20 ng/mL rmTRAIL), JP1584 (500 nM and 2-hour pretreatment), or TRAIL plus JP1584. (A) The number of cells that had migrated to the lower surface of the 8-µm-pore filter membrane was counted in six random fields under a light microscope (×200). Means and standard errors of the mean are shown. (B) Shown are invasive cells that had reached the lower surface of an 8-µm-pore filter membrane additionally equipped with an extracellular matrix coat. Means and standard errors of the mean are shown. (C) Areas of degraded gelatin matrix were measured after digital image inversion with iVision software (BioVision, Exton, PA). Similarly to cell migration and invasion, TRAIL significantly increased matrix degradation by tumor cells; this effect was blocked by cotreatment with JP1584. Means and standard errors of the mean are shown. (D) BDEneu cells were treated as indicated, and cell proliferation was assessed with the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2_H_-tetrazolium assay (n = 3). Exposure to TRAIL (20 ng/mL rmTRAIL) with and without JP1584 (500 nM and 2-hour pretreatment) did not significantly alter cell proliferation in comparison with the vehicle treatment. Means and standard errors of the mean are shown. Abbreviations: JP, JP1584; RAU, relative absorbance unit; RFU, relative fluorescence unit; T, TRAIL; T/JP, TRAIL plus JP1584; V, vehicle.
To further investigate if the administration of TRAIL or TRAIL plus JP1584 has an effect on selected markers of the epithelial-mesenchymal transition (EMT), which plays an emerging role in cancer invasion and metastasis,40–42 we measured by quantitative RT-PCR the mRNA expression of the following four EMT markers: S100 calcium binding protein A4 (S100A4), SRY (sex determining region Y)-box 9 (SOX9), Snail1, and Twist1. No significant effect of the TRAIL or TRAIL plus JP1584 treatment on mRNA expression of S100A4, SOX9, Snail1, and Twist1 was observed (Supporting Fig. 2A–D). Taken together, these observations suggest that disturbances in cIAP-1 and cIAP-2 cellular levels by a smac mimetic are sufficient to alter TRAIL-mediated prometastatic behavior in CCA cells but not proliferation or an EMT.
TRAIL-Induced Tumor Cell Invasion Is Mediated by Up-Regulation of the NF-κB Target Gene MMP7
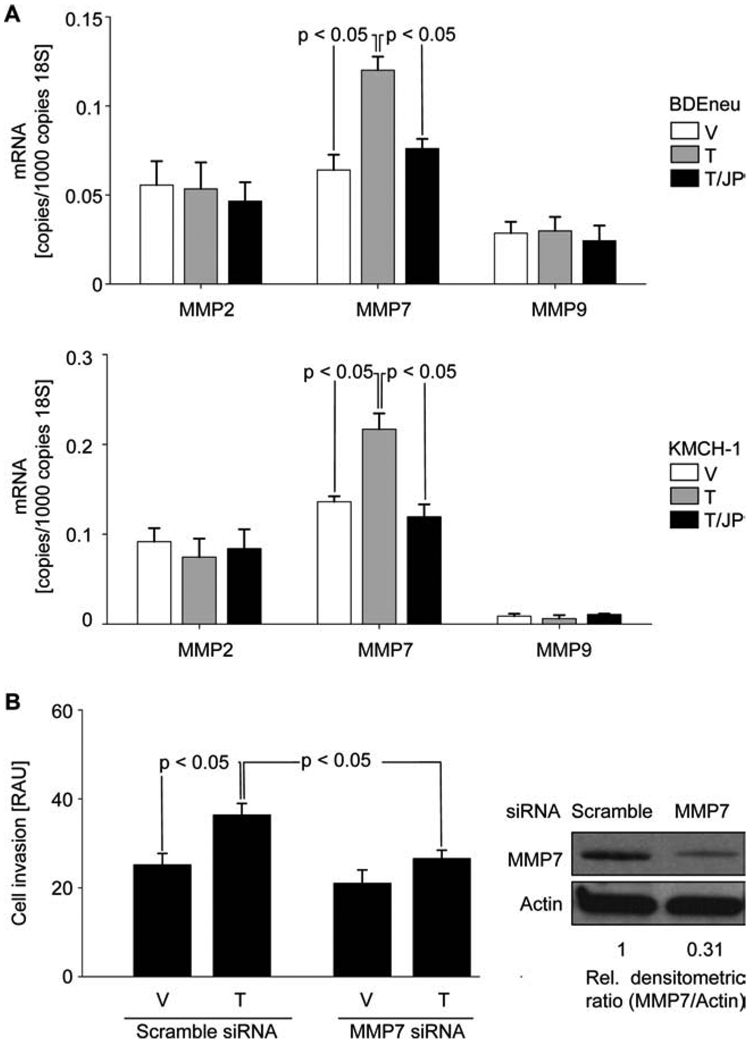
Because MMP2, MMP7, and MMP9 as well as vascular endothelial growth factor (VEGF) and IL-6 are among the group of relevant cancer-related NF-κB target genes,17,43–47 we measured mRNA expression of MMP2, MMP7, MMP9, and VEGF after administration of TRAIL or TRAIL plus JP1584. The effect of TRAIL or TRAIL plus JP1584 on IL-6 secretion was examined by enzyme-linked immunosorbent assay. Administration of TRAIL significantly increased MMP7 mRNA expression in both BDEneu and KMCH-1 cells, and cotreatment with JP1584 reduced this TRAIL-mediated up-regulation of MMP7 (Fig. 4A). In contrast, no significant effect of treatment with TRAIL or TRAIL plus JP1584 on MMP2, MMP9 (Fig. 4A), VEGF, or secreted IL-6 (Supporting Fig. 2E,F) was observed. To determine if JP1584-mediated inhibition of TRAIL-induced tumor cell invasion acts by blocking TRAIL-mediated MMP7 expression, the effect of MMP7 silencing on tumor cell invasion was examined. Similarly to JP1584 cotreatment, validated siRNA knockdown of MMP7 significantly inhibited TRAIL-induced tumor cell invasion (Fig. 4B). Taken together, these data suggest that regulation of MMP7 expression modulates the invasive properties of CCA cells.
Fig. 4.
TRAIL-induced tumor cell invasion is mediated by up-regulation of NF-κB target gene MMP7. (A) BDEneu cells (top) and KMCH-1 cells (bottom) were treated for 3 hours with vehicle or TRAIL (BDEneu: 20 ng/mL rmTRAIL; KMCH-1: 2.5 ng/mL rhTRAIL) with and without JP1584 (500 nM and 2-hour pretreatment), and this was followed by quantitative RT-PCR analysis for mRNA of MMP2, MMP7, and MMP9 (n = 3). MMP7 mRNA expression was significantly increased upon TRAIL treatment in BDEneu and KMCH-1 cells. Cotreatment with JP1584 reduced TRAIL-mediated up-regulation of MMP7. Means and standard errors of the mean are shown. (B) BDEneu cells transfected with MMP7 siRNA or scrambled siRNA were cultured for 48 hours onto the upper compartments of invasion assay inserts in the presence of vehicle, TRAIL (20 ng/mL rmTRAIL), JP1584 (500 nM and 2-hour pretreatment), or TRAIL plus JP1584. Invasive cells were extracted and analyzed photometrically (n = 3). MMP7 silencing (confirmed by immunoblot analysis) inhibited TRAIL-induced tumor cell invasion. Means and standard errors of the mean are shown. Abbreviations: RAU, relative absorbance unit; T, TRAIL; T/JP, TRAIL plus JP1584; V, vehicle.
JP1584 Displays Antimetastatic Single-Agent Activity In Vivo
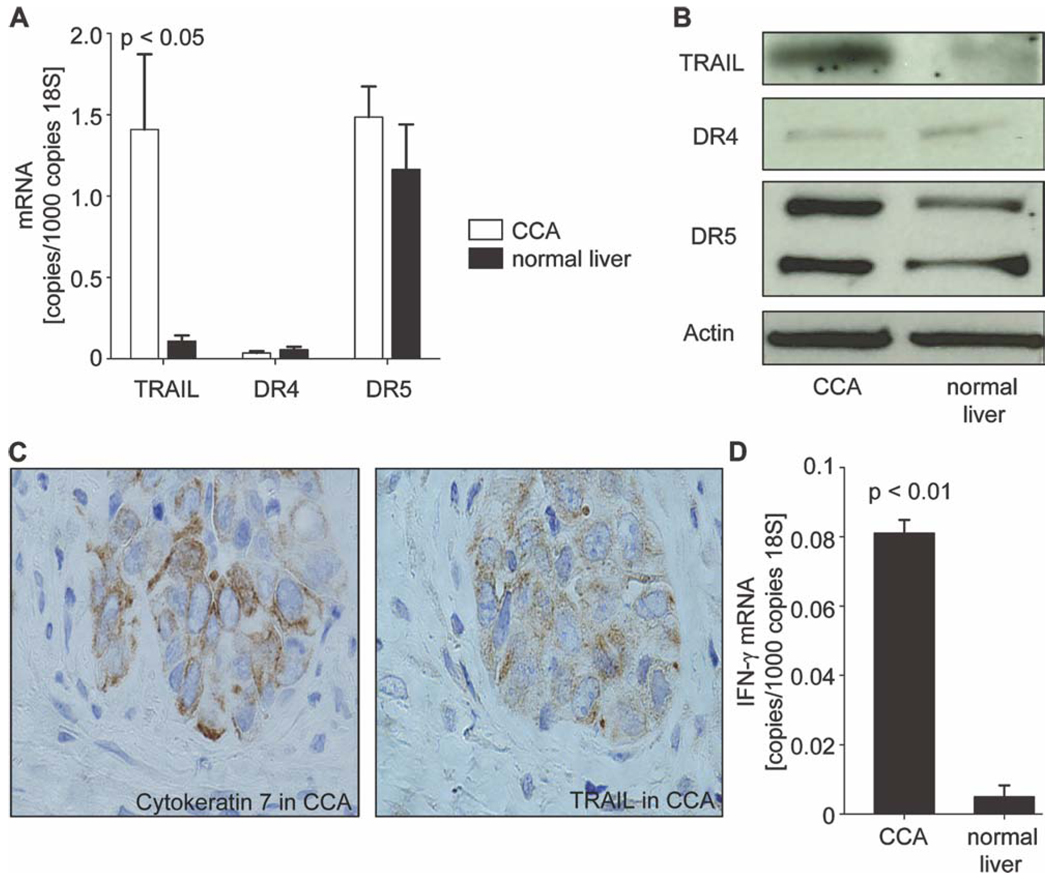
To determine if the anti-invasive effects of JP1584 on CCA observed in vitro are translatable to an in vivo model, we employed a syngeneic rat orthotopic CCA model (BDEneu cells and male Fischer 344 rats).34,37 First, we sought to further validate this model by determining if these CCAs in vivo express TRAIL analogously to human tumors.12 CCA and normal liver specimens of tumor-bearing rats (18 days after tumor cell implantation into the left lateral liver lobe) were examined for expression of TRAIL and its receptors by quantitative RT-PCR and by immunoblotting (Fig. 5A,B). Indeed, TRAIL mRNA (Fig. 5A) and protein (Fig. 5B) were expressed in this rodent model of CCA. In addition, TRAIL protein expression by immunohistochemistry colocalized to glands also expressing cytokeratin 7, a biliary epithelial cell marker expressed by CCA cells (Fig. 5C); thus, tumor-infiltrating cells were excluded as the source of TRAIL. Although TRAIL is expressed in tumor tissues, TRAIL expression is not an inherent characteristic of cultured CCA cells but can be stimulated by administration of exogenous interferon-γ (IFN-γ) in vitro.12 To investigate if TRAIL expression in vivo is associated with endogenous IFN-γ expression by inflammatory cells within the tumor stroma, quantitative RT-PCR for IFN-γ mRNA was performed in CCA and normal liver specimens of tumor-bearing rats. IFN-γ mRNA levels were significantly increased in CCA in comparison with normal liver tissue (Fig. 5D). These elevated in vivo IFN-γ mRNA levels are not likely to result from cancer cell secretion because IFN-γ mRNA was not detectable in CCA cells (BDEneu) or normal rat cholangiocytes when it was measured by RT-PCR in vitro (data not shown). Thus, analogous to human CCA, this rodent model of CCA expresses TRAIL in vivo, likely in response to IFN-γ–secreting cells within the tumor stroma.
Fig. 5.
Orthotopic BDEneu cells express TRAIL in vivo similarly to human cancers. A syngeneic rat orthotopic model of CCA (BDEneu cells and Fischer 344 rats) was employed for this examination. CCA and normal liver specimens of untreated tumor-bearing rats (18 days after tumor cell implantation into the left lateral liver lobe) were analyzed for mRNA and protein expression of TRAIL and its cognate receptors DR4 and DR5 by quantitative RT-PCR and immunoblot analysis. (A,B) TRAIL mRNA and protein levels in tumors were expressed similarly to those in human cancers in this rodent model of CCA. No differences with respect to mRNA or protein expression of DR4 and DR5 were observable (n = 4 livers). Means and standard errors of the mean are shown. (C) TRAIL protein expression by immunohistochemistry (right) colocalized to glands also expressing cytokeratin 7 as a marker of CCA (left). Selectively, the tumorous gland was positive for TRAIL and cytokeratin 7 staining. (D) IFN-γ, a known stimulator of TRAIL, showed significantly up-regulated mRNA levels in CCA in vivo in comparison with normal liver tissue (n = 4 livers). Means and standard errors of the mean are shown.
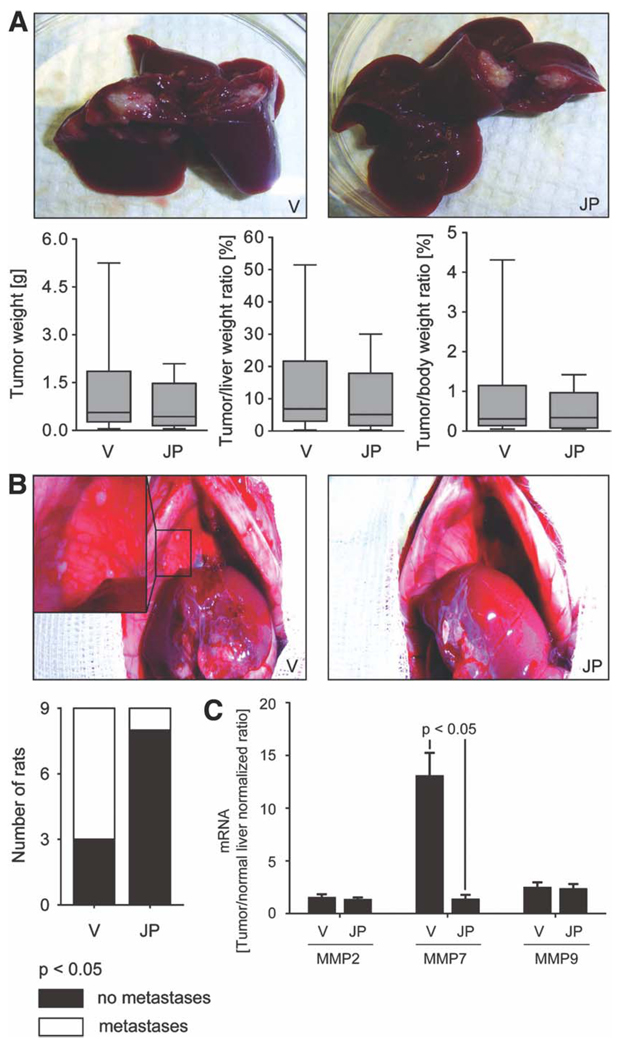
Having demonstrated TRAIL expression by this rodent model of CCA, we next examined the potential antimetastatic effect of JP1584 administration. In JP1584-treated rats and vehicle-treated rats, tumor/liver/body weights and metastases were assessed 18 days after tumor cell implantation into the left lateral liver lobe (11 days after treatment initiation). No significant decreases in tumor weights or tumor/liver or tumor/body weight ratios were observed in JP1584-treated rats (Fig. 6A). However, animals treated with JP1584 had minimal extrahepatic metastases in comparison with vehicle-treated animals (11% of the rats had metastases in the JP1584-treated group versus 67% in the vehicle-treated group, P < 0.05; Fig. 6B). Extrahepatic metastases predominantly occurred in the greater omentum and peritoneum.
Fig. 6.
JP1584 has no significant effect on intrahepatic tumor growth but shows antimetastatic single-agent activity in vivo. A syngeneic rat orthotopic model of CCA (BDEneu cells and Fischer 344 rats) was employed for this examination. In JP1584-treated (2 mg/kg intravenously every other day for six times starting on day 7 after surgery) and vehicle-treated tumor-bearing rats (n = 9 rats per group), the tumor/liver/body weights and extrahepatic metastases were assessed 18 days after tumor cell implantation into the left lateral liver lobe. (A) Representative explanted livers of JP1584-treated and vehicle-treated tumor-bearing rats are depicted. No significant decreases in tumor weights or tumor/liver or tumor/body weight ratios were observed in JP1584-treated rats. Box and whisker plots show the minimum, 25th percentile, median, 75th percentile, and maximum. (B) Representative abdominal cavities of JP1584-treated and vehicle-treated tumor-bearing rats are depicted. JP1584 treatment significantly reduced the number of rats with extrahepatic metastases. Extrahepatic metastases predominantly occurred in the greater omentum and peritoneum. The stacked column plot indicates the numbers of animals with and without metastases. P < 0.05 by the χ2 test. (C) MMP7 mRNA levels but not MMP2 or MMP9 mRNA levels (quantitative RT-PCR) were significantly up-regulated in CCA in comparison with nonmalignant liver tissue. This up-regulation of MMP7 was reduced in CCA samples of JP1584-treated rats versus vehicle-treated rats (mRNA expression in CCA samples was normalized to mRNA expression in normal liver tissue). Means and standard errors of the mean are shown. Abbreviations: JP, JP1584; V, vehicle.
To further assess if the suppression of metastasis observed in the syngeneic rat orthotopic CCA model was related to the blocking of a metastatic pathway or rather the suppression of metastatic tumor growth, we additionally employed a new abdominal CCA cell implantation model (BDEneu cells and Fischer 344 rats). In this model, tumor cells were injected directly into the greater omentum without affecting the liver in order to mimic abdominal tumor growth derived from metastatic cells. Similarly to the previous in vivo study, the abdominal/retroperitoneal tumor burden and animal body weight were assessed 18 days after tumor cell implantation into the greater omentum (11 days after treatment initiation) in JP1584-treated rats and vehicle-treated rats. No significant decreases in the abdominal/retroperitoneal tumor weight or tumor/body weight ratio were observed in rats treated with JP1584 (Supporting Fig. 3). Thus, JP1584 as a single agent reduces metastasis but not intrahepatic or extrahepatic tumor growth in this rodent model of CCA. To ascertain if JP1584 inhibits TRAIL-mediated up-regulation of the NF-κB target gene MMP7 in vivo, quantitative RT-PCR for MMP7, MMP2, and MMP9 mRNA was performed in CCA and normal liver specimens of JP1584-treated and vehicle-treated tumor-bearing rats (the syngeneic rat orthotopic CCA model). In agreement with the in vitro studies, MMP7 mRNA levels, but not MMP2 or MMP9 mRNA levels (normalized to the mRNA expression in the normal liver), were up-regulated in tumor specimens; this up-regulation of MMP7 was significantly reduced in CCA samples of rats treated with JP1584 in comparison with vehicle-treated animals (Fig. 6C). In aggregate, these data suggest that a smac mimetic is capable of reducing MMP7 expression and the metastatic behavior in an in vivo rodent model of CCA.
Discussion
The results of this study provide new mechanistic insights regarding the use of the smac mimetic JP1584 for the treatment of CCA. These data indicate that JP1584 does not promote TRAIL cytotoxicity but rather (1) attenuates TRAIL-induced migration, invasion, and matrix degradation in vitro, (2) reduces TRAIL-induced NF-κB activation and thereby inhibits the up-regulation of the NF-κB target gene MMP7, and (3) displays significant antimetastatic single-agent activity in an in vivo rodent model of CCA. Each of these findings is discussed in greater detail next.
The death ligand TRAIL has attracted considerable attention for its potential use as an anticancer agent.48,49 However, many cancer cells are resistant to TRAIL cytotoxicity. Expression of cIAP-1 and cIAP-2 has been linked to TRAIL resistance, and enhanced degradation of these proteins by smac mimetics sensitizes numerous cancer cells to TRAIL cytotoxicity.20–24 Despite causing cellular loss of cIAP-1 and cIAP-2, the smac mimetic JP1584 did not sensitize CCA cells to TRAIL cytotoxicity. This observation, albeit disappointing from a therapeutic perspective, agrees with previous studies suggesting that TRAIL must activate the mitochondrial pathway of apoptosis in these cells for efficient killing; this pathway is blocked in CCA cells because of abundant expression of myeloid cell leukemia-1, a potent antiapoptotic B cell lymphoma 2 family protein whose expression is unaffected by smac mimetics.50,51 Thus, although more robust death receptor signaling due to cIAP-1 and cIAP-2 elimination is sufficient to bypass or overcome apoptosis resistance in other cancer cell types, it is not in CCA.
After receptor binding by their cognate death ligands, cIAP-1 and cIAP-2 are recruited to the death receptor complex.52 Via their E3 ligase activities, cIAP-1 and IAP-2 promote the nondegradative Lys-63–linked polyubiquitination of receptor interacting protein 1 (RIP1), which in turn induces NF-κB activation.29–31 Cellular elimination of cIAP-1 and cIAP-2 by smac mimetics such as JP1584 prevents this ubiquitination-dependent function of RIP1 and inhibits death receptor activation of NF-κB by the canonical pathway.22,31 Although NFκB inhibition by a smac mimetic has been reported to sensitize prostate cancer cells to TRAIL cytotoxicity,22 this did not occur with CCA cells. Instead, NF-κB inhibition reduced the prometastatic activity of TRAIL in these malignant cells.
Inhibition of cell invasion by suppressing NF-κB activation is consistent with other studies linking NF-κB signaling to prometastatic activity.53 Although NF-κB activation has been associated with EMT, a phenotypic cellular alteration thought to favor metastatic behavior,40–42 we did not observe TRAIL induction of key genes responsible for EMT. Instead, the NF-κB target gene MMP7 (or matrilysin) was determined to be up-regulated by TRAIL. This MMP has previously been reported to play a predominant role in CCA progression.14–16 JP1584 inhibited TRAIL-mediated MMP7 up-regulation and additionally attenuated TRAIL-induced cell invasion, with the latter effect also being achieved by siRNA knockdown of MMP7. Similar to these findings, a TRAIL/NF-κB/MMP7 pathway promoting invasive behavior of pancreatic cancer cells has been reported.17 Our current studies are also consistent with the emerging concept that IAPs are prometastatic and represent antimetastatic therapeutic targets.32 Finally, we note that smac mimetics also have been reported to activate the noncanonical NF-κB pathway by inhibiting cIAP-1/cIAP-2–mediated degradative Lys-48–linked polyubiquitination of NF-κB–inducing kinase25,28; if this pathway occurs in CCA, it does not affect TRAIL-mediated metastatic behavior in these cancer cells.
In accordance with our in vitro observations, JP1584 in vivo reduced MMP7 mRNA levels in tumors and achieved significant CCA metastasis suppression without decreasing primary tumor growth. This effect of JP1584 likely represents TRAIL/NF-κB antagonism as exogenously administered TRAIL has been reported to increase metastasis but not primary tumor growth in another orthotopic rodent in vivo model employing pancreatic cancer cells.13 As a potential metastasis suppressor, JP1584 in a clinical setting could be a promising therapeutic agent when administered perioperatively or in an adjuvant fashion in patients undergoing surgery for CCA or in combination with targeted local regional CCA therapy (e.g., radiotherapy). Its use in CCA patients awaiting liver transplantation (possibly in addition to established bridging techniques) is also conceivable. However, smac mimetics should be used with caution in CCA patients who additionally have primary sclerosing cholangitis because there is evidence of up-regulation of TRAIL expression in these cholestatic liver diseases.12,54 If a smac mimetic potentiates TRAIL-mediated cytotoxicity of normal cholangiocytes in vitro, it could potentiate these disease processes. However, we did not observe significant JP1584-mediated sensitization of nonmalignant cholangiocytes to TRAIL-induced apoptosis in vitro.
In conclusion, the smac mimetic JP1584 potently reduces TRAIL-induced CCA cell invasion in vitro and shows significant metastasis suppression as a single agent in an in vivo rodent model of CCA that recapitulates key features of the human cancer. These effects are, at least in part, mediated by inhibition of TRAIL-induced NF-κB activation resulting in reduced MMP7 expression. Thus, in CCA, the smac mimetic JP1584 does not sensitize cells to TRAIL-induced cytotoxicity but rather antagonizes prometastatic signaling cascades.
Supplementary Material
Supplemental table 1
Acknowledgment
The assistance of P. L. Splinter (with the normal rat cholangiocytes), A. Krishnan (with the human hepatocytes), and E. W. Krueger (with the fluorescent gelatin degradation assay) and the superb secretarial service of E. M. Nystuen-Bungum are gratefully acknowledged.
This work was supported by the National Institutes of Health [grants DK59427 (to Gregory J. Gores), R01 CA 83650, R01 CA 39225 (to Alphonse E. Sirica), and DK84567 (the optical microscopy core)] and by the Mayo Foundation. Christian D. Fingas is a German Research Foundation fellow (grant FI 1630/1-1).
Abbreviations
CCA
cholangiocarcinoma
cIAP
cellular inhibitor of apoptosis
DAPI
4′,6-diamidino-2-phenylindole dihydrochloride
EMSA
electrophoretic mobility shift assay
EMT
epithelial-mesenchymal transition
HCC
hepatocellular carcinoma
IAP
inhibitor of apoptosis
IFN-γ
interferon-γ
IL-6
interleukin-6
MMP
matrix metalloproteinase
mRNA
messenger RNA
NF-κB
nuclear factor kappa B
RAU
relative absorbance unit
RFU
relative fluorescence unit
rhTRAIL
recombinant human tumor necrosis factor–related apoptosis-inducing ligand
rmTRAIL
recombinant mouse tumor necrosis factor–related apoptosisinducing ligand
RIP1
receptor interacting protein 1
RT-PCR
real-time polymerase chain reaction
S100A4
S100 calcium binding protein A4
siRNA
small interfering RNA
SMAC
second mitochondria-derived activator of caspase
SOX9
SRY (sex determining region Y)-box 9
TRAIL
tumor necrosis factor–related apoptosis-inducing ligand
TNF-α
tumor necrosis factor-α
VEGF
vascular endothelial growth factor
XIAP
X-linked inhibitor of apoptosis
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999;341:1368–1378. doi: 10.1056/NEJM199910283411807. [DOI] [PubMed] [Google Scholar]
- 2.Roberts SK, Ludwig J, Larusso NF. The pathobiology of biliary epithelia. Gastroenterology. 1997;112:269–279. doi: 10.1016/s0016-5085(97)70244-0. [DOI] [PubMed] [Google Scholar]
- 3.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. HEPATOLOGY. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. HEPATOLOGY. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 5.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 7.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Welzel TM, McGlynn KA, Hsing AW, O’Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873–875. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 9.Blechacz BR, Gores GJ. Cholangiocarcinoma. Clin Liver Dis. 2008;12:131–150. doi: 10.1016/j.cld.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Farley DR, Weaver AL, Nagorney DM. “Natural history” of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc. 1995;70:425–429. doi: 10.4065/70.5.425. [DOI] [PubMed] [Google Scholar]
- 11.Hejna M, Pruckmayer M, Raderer M. The role of chemotherapy and radiation in the management of biliary cancer: a review of the literature. Eur J Cancer. 1998;34:977–986. doi: 10.1016/s0959-8049(97)10166-6. [DOI] [PubMed] [Google Scholar]
- 12.Ishimura N, Isomoto H, Bronk SF, Gores GJ. Trail induces cell migration and invasion in apoptosis-resistant cholangiocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G129–G136. doi: 10.1152/ajpgi.00242.2005. [DOI] [PubMed] [Google Scholar]
- 13.Trauzold A, Siegmund D, Schniewind B, Sipos B, Egberts J, Zorenkov D, et al. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene. 2006;25:7434–7439. doi: 10.1038/sj.onc.1209719. [DOI] [PubMed] [Google Scholar]
- 14.Leelawat K, Sakchinabut S, Narong S, Wannaprasert J. Detection of serum MMP-7 and MMP-9 in cholangiocarcinoma patients: evaluation of diagnostic accuracy. BMC Gastroenterol. 2009;9:30. doi: 10.1186/1471-230X-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miwa S, Miyagawa S, Soeda J, Kawasaki S. Matrix metalloproteinase-7 expression and biologic aggressiveness of cholangiocellular carcinoma. Cancer. 2002;94:428–434. doi: 10.1002/cncr.10235. [DOI] [PubMed] [Google Scholar]
- 16.Sirica AE, Dumur CI, Campbell DJ, Almenara JA, Ogunwobi OO, Dewitt JL. Intrahepatic cholangiocarcinoma progression: prognostic factors and basic mechanisms. Clin Gastroenterol Hepatol. 2009;7:S68–S78. doi: 10.1016/j.cgh.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou DH, Trauzold A, Roder C, Pan G, Zheng C, Kalthoff H. The potential molecular mechanism of overexpression of uPA, IL-8, MMP-7 and MMP-9 induced by TRAIL in pancreatic cancer cell. Hepatobiliary Pancreat Dis Int. 2008;7:201–209. [PubMed] [Google Scholar]
- 18.Guicciardi ME, Gores GJ. Life and death by death receptors. FASEB J. 2009;23:1625–1637. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 20.Bockbrader KM, Tan M, Sun Y. A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells. Oncogene. 2005;24:7381–7388. doi: 10.1038/sj.onc.1208888. [DOI] [PubMed] [Google Scholar]
- 21.Chen DJ, Huerta S. Smac mimetics as new cancer therapeutics. Anticancer Drugs. 2009;20:646–658. doi: 10.1097/CAD.0b013e32832ced78. [DOI] [PubMed] [Google Scholar]
- 22.Dai Y, Liu M, Tang W, Li Y, Lian J, Lawrence TS, et al. A Smacmimetic sensitizes prostate cancer cells to TRAIL-induced apoptosis via modulating both IAPs and NF-kappaB. BMC Cancer. 2009;9:392. doi: 10.1186/1471-2407-9-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 24.Petrucci E, Pasquini L, Petronelli A, Saulle E, Mariani G, Riccioni R, et al. A small molecule Smac mimic potentiates TRAIL-mediated cell death of ovarian cancer cells. Gynecol Oncol. 2007;105:481–492. doi: 10.1016/j.ygyno.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 26.Wu H, Tschopp J, Lin SC. Smac mimetics and TNFalpha: a dangerous liaison? Cell. 2007;131:655–658. doi: 10.1016/j.cell.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci U S A. 2008;105:11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J Biol Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehrotra S, Languino LR, Raskett CM, Mercurio AM, Dohi T, Altieri DC. IAP regulation of metastasis. Cancer Cell. 2009;17:53–64. doi: 10.1016/j.ccr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akazawa Y, Mott JL, Bronk SF, Werneburg NW, Kahraman A, Guicciardi ME, et al. Death receptor 5 internalization is required for lysosomal permeabilization by TRAIL in malignant liver cell lines. Gastroenterology. 2009;136:2365–2376. doi: 10.1053/j.gastro.2009.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blechacz BR, Smoot RL, Bronk SF, Werneburg NW, Sirica AE, Gores GJ. Sorafenib inhibits signal transducer and activator of transcription-3 signaling in cholangiocarcinoma cells by activating the phosphatase shatterproof 2. HEPATOLOGY. 2009;50:1861–1870. doi: 10.1002/hep.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnan A, Viker K, Rietema H, Telgenkamp M, Knudsen B, Charlton M. Prolonged engraftment of human hepatocytes in mice transgenic for the deleted form of human hepatocyte growth factor. Hepatol Res. 2007;37:854–862. doi: 10.1111/j.1872-034X.2007.00139.x. [DOI] [PubMed] [Google Scholar]
- 36.Lai GH, Zhang Z, Shen XN, Ward DJ, Dewitt JL, Holt SE, et al. erbB-2/neu transformed rat cholangiocytes recapitulate key cellular and molecular features of human bile duct cancer. Gastroenterology. 2005;129:2047–2057. doi: 10.1053/j.gastro.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Sirica AE, Zhang Z, Lai GH, Asano T, Shen XN, Ward DJ, et al. A novel “patient-like” model of cholangiocarcinoma progression based on bile duct inoculation of tumorigenic rat cholangiocyte cell lines. HEPATOLOGY. 2008;47:1178–1190. doi: 10.1002/hep.22088. [DOI] [PubMed] [Google Scholar]
- 38.Vroman B, LaRusso NF. Development and characterization of polarized primary cultures of rat intrahepatic bile duct epithelial cells. Lab Invest. 1996;74:303–313. [PubMed] [Google Scholar]
- 39.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–1131. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 42.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–6830. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 45.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 47.Wroblewski LE, Noble PJ, Pagliocca A, Pritchard DM, Hart CA, Campbell F, et al. Stimulation of MMP-7 (matrilysin) by Helicobacter pylori in human gastric epithelial cells: role in epithelial cell migration. J Cell Sci. 2003;116:3017–3026. doi: 10.1242/jcs.00518. [DOI] [PubMed] [Google Scholar]
- 48.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 49.Ganten TM, Koschny R, Sykora J, Schulze-Bergkamen H, Buchler P, Haas TL, et al. Preclinical differentiation between apparently safe and potentially hepatotoxic applications of TRAIL either alone or in combination with chemotherapeutic drugs. Clin Cancer Res. 2006;12:2640–2646. doi: 10.1158/1078-0432.CCR-05-2635. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128:2054–2065. doi: 10.1053/j.gastro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 51.Taniai M, Grambihler A, Higuchi H, Werneburg N, Bronk SF, Farrugia DJ, et al. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res. 2004;64:3517–3524. doi: 10.1158/0008-5472.CAN-03-2770. [DOI] [PubMed] [Google Scholar]
- 52.Rothe M, Pan MG, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 53.Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26. doi: 10.1016/j.gde.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeda K, Kojima Y, Ikejima K, Harada K, Yamashina S, Okumura K, et al. Death receptor 5 mediated-apoptosis contributes to cholestatic liver disease. Proc Natl Acad Sci U S A. 2008;105:10895–10900. doi: 10.1073/pnas.0802702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental table 1