XIAP AS A UBIQUITIN LIGASE IN CELLULAR SIGNALING (original) (raw)
. Author manuscript; available in PMC: 2011 Jan 1.
Published in final edited form as: Cell Death Differ. 2010 Jan;17(1):54–60. doi: 10.1038/cdd.2009.81
Abstract
The ability of the vertebrate X-linked inhibitor of apoptosis (XIAP) protein to directly suppress apoptotic cell death pathways has been the subject of much research. Studies of this broadly expressed protein have largely focused on the unique interactions between XIAP and caspases – proteases that conduct and participate in the ordered disassembly of the cell during apoptosis. However, relatively less attention has been given to the RING domain of XIAP, which functions as an E3 ligase to catalyze the ubiquitination of substrate proteins. Here we discuss the evidence implicating the RING domain of XIAP in the ubiquitin-mediated regulation of three somewhat arbitrarily divided categories of substrate: XIAP itself, XIAP-interacting proteins involved in apoptosis, and other targets whose physiologic roles likely extend beyond cell death. Collectively, these multiple activities of XIAP demonstrate that this enigmatic protein participates in a range of cellular activities beyond apoptotic suppression.
Despite the immeasurable value to the biomedical research community of readily accessible, easily searchable DNA sequence databases, a major challenge created by this explosion in the identification of new genes is to understand the physiological functions of their products. The X-linked Inhibitor of Apoptosis (XIAP) is a good example of this: originally identified by its homology to the Iap genes contained within the genomes of baculoviruses1–3, which themselves were discovered in genetic screens for suppressors of cell death4, 5 (see accompanying article by Rollie Clem and colleagues), XIAP has been shown to participate in a range of cellular activities which include, but are not limited to apoptotic regulation6. This has created a challenge in understanding how XIAP can be such a versatile, multifunctional protein, and how these apparently unrelated cellular roles might be reconciled.
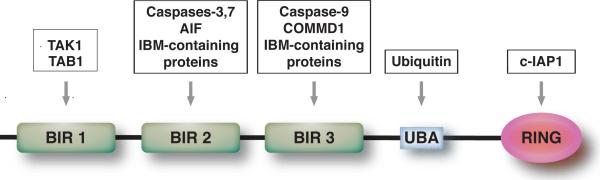
Cellular IAP proteins contain between one and three baculovirus IAP repeats (BIRs), ~70-residue zinc-binding domains that are named from their original discovery in baculoviruses and which resemble a classical zinc finger configuration7–9. XIAP contains three BIRs (Figure 1), which, together with flanking residues, can bind directly to caspases-3, -7 and -9, thereby inhibiting their proteolytic activity. The structural features of these interactions, together with the mechanisms by which XIAP can inhibit the enzymatic activities of these caspases, have been studied in detail10, yet despite the abundance of biochemical data supporting an extremely high affinity of XIAP for caspases, there are surprisingly few published reports describing endogenous XIAP:caspase interactions11. Nevertheless, based on these structural studies XIAP is considered to be the only mammalian IAP protein that can function as a direct, competitive inhibitor of the enzymatic activity of caspases through binding to their catalytically active sites10.
Figure 1. Structural representation of XIAP.
XIAP contains 3 BIRs, a ubiquitin-binding (UBA) domain and a RING finger domain. TAB1 binds the BIR1 domain, whereas activated caspase-3 and -7 bind in an indistinguishable manner to a groove in BIR2 that also requires amino terminal flanking residues which can competed by IAP-binding motif (IBM) containing proteins. The RING domain has been shown to be important for heterodimerization with c-IAP138.
Although the presence of a BIR is often considered the defining feature of the IAPs, many cellular IAPs contain a second class of domain also found in the baculovirus proteins, a C3HC4 RING finger motif12 (Figure 1) whose functions were unknown when first discovered, but which are now widely accepted as participating in the ubiquitin-proteasome pathway through their roles as E3 ubiquitin ligases13 (see article by Catherine Day and colleagues). The RINGs of the IAPs are much more closely related to each other than they are to the RINGs found in other proteins, and are also located at the extreme carboxyl termini of IAPs in a remarkably conserved manner. In earlier groundbreaking studies, the RINGs of the antiapoptotic baculovirus IAPs were found to be essential for cytoprotection5, 14, at least in the context of a virus infection, and the inability of IAPs from other baculoviruses to suppress apoptosis was traced, in part, to the RING. Subsequent work revealed, however, that high-level, ectopic expression of isolated BIRs from baculovirus IAPs can confer protection15, 16, together indicating that the RINGs of these proteins might augment, but are not solely responsible for, the antiapoptotic effects of the IAP (see accompanying article by Rollie Clem and colleagues). As discussed below, the E3 ligase activity inherent to the RING of XIAP plays a variety of roles that include, but are not limited to apoptotic inhibition (Figure 3).
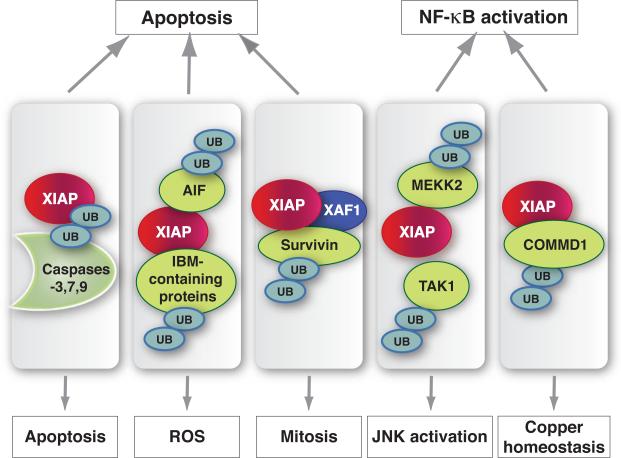
Figure 3. E3 ubiquitin-ligase substrates of XIAP.
Binding proteins interact with XIAP through distinct domains, and ubiquitination has been demonstrated for the depicted proteins. K48-linked ubiquitination and subsequent proteasomal degradation has been reported for COMMD1, AIF, TAK1 and MEKK2.
Regulation of XIAP itself by ubiquitination
The realization that XIAP can be degraded through the ubiquitin-proteasome pathway emerged from a pivotal study by Yang and coworkers17 in which thymocytes that were induced to die by dexamethasone treatment could be rescued with proteasome inhibitors. From this earlier work, it became apparent that both XIAP, as well as another RING-containing IAP, c-IAP1, can be stabilized by proteasome inhibition, and that XIAP is capable of autoubiquitination in a manner dependent on its RING. The general assumption has been that the autoubiquitination of XIAP is a K48 ubiquitin conjugation process leading to proteasomal degradation (see accompanying article by Ciechanover and colleagues), although the types and degree of complexity of the ubiquitin chains formed have not been examined in detail18. Nevertheless, the Yang study raised two major questions: firstly, does this occur because the cell is dying, or does the cell die as a consequence of the loss of XIAP? Secondly, at the molecular and structural level, how is XIAP ubiquitination regulated? The answers to these questions have remained elusive. Although it has been known for some time that _Xiap_-deficient mice appear healthy, are born at normal Mendelian ratios, and exhibit no obvious defects related to apoptosis19, 20, and dexamethasone-induced apoptosis of thymocytes from these and littermate control mice appear equally sensitive to apoptosis (J. Wilkinson and C.S.D., unpublished data), more recent studies have revealed a more subtle modulatory role for XIAP in establishing the apoptotic threshold, which has been revealed by the analysis of murine and human _XIAP_-deficient cells using lower doses of apoptotic stimuli21–23. Nevertheless, an obligate role for XIAP degradation in the induction of apoptosis can be excluded, and while cells from _Xiap_-deficient mice have been reported to express higher levels of other IAP proteins19, the different physiologic roles of these IAPs likely preclude redundancy or compensation as alternative interpretations.
The second question raised above, regarding the mechanisms by which XIAP is ubiquitinated, is still unresolved. In a recombinant, cell-free system, Yang and coworkers originally demonstrated that XIAP can function as an E3 ubiquitin ligase to catalyze its autoubiquitination24, but it has been a challenge to confirm this endogenously in living cells. An attractive model might be that the induced binding of XIAP-interacting proteins might confer a structural change in XIAP that permits autoubiquitination. The best-characterized XIAP-interacting proteins, which were identified using affinity purification approaches25, 26, are diverse in sequence and structure but related by a tetrapeptide domain known as an IAP-binding motif (IBM), and are located in the mitochondria of non-apoptotic cells (Figure 2). In mammalian cells, the prototype of these IBM-containing proteins is Smac27 (also termed DIABLO28), and is released from mitochondria in response to apoptotic signals coincident with the release of cytochrome c (Figure 2). Through steric occlusion of the domains utilized by XIAP to bind caspases, IBM-containing proteins such as Smac, as well as synthetic, small-molecule IBM mimetics, can neutralize the caspase-inhibitory properties of XIAP. Several studies have demonstrated that in addition to reversing the caspase-inhibitory effects of XIAP, IBM-bearing antagonists can, under some circumstances, trigger the degradation of XIAP in a RING-dependent manner23, 29, 30, 31. Although these studies are suggestive of autoubiquitination, not all reports reached the same conclusion32, 33, and others have revealed the ability of other RING-bearing IAPs, notably c-IAP1, to cause the ubiquitination of XIAP34, 35. Since the RING of XIAP is also required for oligomerization34, as well as for interaction with c-IAP1, it has been difficult to formally demonstrate that the ubiquitination of XIAP is caused by its own E3 activity, rather than that of another E3 ligase, such as c-IAP1. For example, in a recent study utilizing a murine model in which gene targeting was used to generate a truncated Xiap protein lacking the RING, higher basal levels of the truncated protein were observed, and this protein appeared more stable after apoptotic stimulation36. While it is very possible that the RING is required for autoubiquitination, a formal alternative explanation is that the loss of the RING results in a version of XIAP unable to be ubiquitinated by c-IAP1. In either case, the ubiquitination of XIAP has been shown to occur primarily at lysine residues outside the RING domain itself37, implying that the docking site of c-IAP1 and its target lysines on XIAP are separable domains. Additional studies, for example using mutants of the RING that lack the ability to autoubiquitinate but retain other functions, such as oligomerization38 and perhaps c-IAP1 binding, will be helpful to further understand the mechanisms by which XIAP is ubiquitinated.
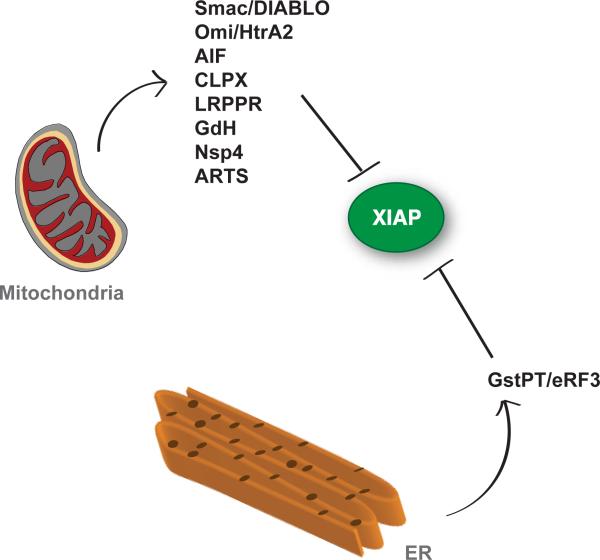
Figure 2. Mitochondrial and ER associated proteins antagonizing XIAP function.
Mitochondrial proteins Smac/DIABLO, Omi/HtrA2, CLPX, LRPPR, GdH, Nsp4 or ER associated protein GstPT/eRF3 have all been described to bind and potentially antagonize XIAP via their IAP binding motifs (IBMs)26. AIF and ARTS are thought to bind IAPs through distinct mechanisms independent of IBMs42, 78.
Ubiquitination of XIAP-interacting proteins involved in apoptosis
Given the remarkable degree of conservation of the IAP RINGs, yet the paucity of in vivo data regarding the ability of XIAP to autoubiquitinate as discussed above, several studies have addressed the question of whether XIAP can ubiquitinate caspases. Suzuki and coworkers described the ability of ectopically expressed XIAP to target the active, but not the pro-form, of caspase-3 for ubiquitination and proteasomal degradation39. However, _Xiap_-deficient mice were not found to express any higher levels of caspase-3 protein compared to control animals19, 36, although interestingly, higher levels of caspase-3 activity were observed in the RING-targeted mouse despite no obvious differences in basal apoptotic sensitivity in the cell types examined36. One implication of these data is that the ubiquitination of XIAP-associated caspases might not induce proteasomal degradation but could nevertheless inactivate the enzymatic activity of the caspase, as has been described for the Drosophila IAP protein DIAP140.
As discussed above, a number of reports have described the ability of IBM-containing proteins to cause the ubiquitination of XIAP upon binding, even if the issue of whether this process is due to autoubiquitination or is induced by another E3 ligase may not have been definitively resolved (Figure 3). Evidence also exists to support the converse: ubiquitination of XIAP-associated proteins including Smac41 and apoptosis-inducing factor (AIF42) has been demonstrated to occur through a process that requires the RING of XIAP. XIAP has also been reported to induce the downregulation of another IAP family member, Survivin, by a mechanism requiring the associated factor, XAF1, and an intact XIAP RING43. If these findings are substantiated with endogenous levels of protein physiological conditions, it will be of great interest to identify the factors, other than mere cellular compartmentalization as is the case for mitochondrially located IAP binding proteins, that regulate what appears to be a dynamic equilibrium between ubiquitinated XIAP and its associated proteins. Another provocative implication of this model is that proteins such as Smac, which are traditionally considered to participate solely in the apoptotic pathway, might participate in other cellular signaling cascades regulating, for example, NF-κB activation, and by targeting these proteins for ubiquitination XIAP might exert effects on the cell through processes not directly related to apoptotic cell death16, 44. If this turns out to be the case, clearly the distinction between targets of XIAP involved in apoptosis and non-apoptotic signaling becomes somewhat arbitrary, as discussed below.
XIAP: a signaling intermediate looking for a pathway?
The ordered hierarchy of events in which caspases are sequentially activated is itself a specialized signal transduction pathway, similar to the paradigm of the tissue factor pathway that regulates blood coagulation45. Additionally, it is becoming increasingly clear that caspases have diverse substrates which are themselves key components of signaling pathways46; for example, caspase-3 has been shown to proteolytically cleave IκB, the inhibitor of NF-κB47, and presupposing that caspase-3 can be enzymatically active under conditions in which the cell is not destined to die, XIAP might be expected to regulate NF-κB activity through its caspase-3 inhibitory activity. In this light, XIAP in principle can be thought of as a signaling molecule simply through its ability to regulate caspases, without invoking any additional intracellular targets. However, experimental evidence is emerging to indicate that XIAP can also function as a signal transduction intermediate independent of its caspase-suppressing properties.
The means by which c-IAP1 and c-IAP2 were first isolated48 was highly anticipatory of the kinds of cellular roles played by IAPs. These proteins were originally identified as components of a cytoplasmic signaling complex containing members of the TNF receptor associated factors TRAF1 and TRAF2 (see article by John Silke and colleagues), and these types of complexes have been shown to be recruited to the cytoplasmic domains of numerous members of the TNF receptor (TNFR) superfamily. TRAFs and c-IAPs are now known to participate in the activation of several conserved intracellular signaling pathways, notably the pleiotropic transcription factor NF-κB and c-Jun N-terminal kinase (JNK) cascades6. Although XIAP is not thought to be a TRAF-binding protein49, 50, it has been implicated in the activation of NF-κB51, 52 and JNK53, and so a candidate approach has largely been taken in an effort to identify an analogous cellular signaling pathway in which XIAP might participate. Evidence exists to suggest possible roles for XIAP in the innate immune response54 (e.g. through TLR and NOD signaling) as well as the bone morphogenetic/transforming growth factor-β (BMP/TGF-β) receptor signaling pathways51, 55, 56, however, there are less data to support roles for XIAP in these pathways compared to the well-established involvement of the c-IAPs in TNFR superfamily signaling. This is largely because endogenous c-IAP proteins have been readily identified as components of intracellular signaling complexes (particularly those activated by TNFR superfamily members), whereas to date XIAP has not.
The idea that certain IAPs might be involved in the TGF-β/BMP signaling was first raised over a decade ago. The Drosophila IAP proteins, DIAP1 and DIAP2, were shown to associate with Thickveins, a Drosophila receptor for the BMP-related ligand, Decapentaplegic57. Subsequently, Yamaguchi and colleagues identified human XIAP, also through a yeast two-hybrid screen, by virtue of its interaction with the TGF-β/BMP receptor-associated protein, TAB1 (Figure 1), and the same study revealed a functional role for XIAP as a component of a TAB1:TAK1 signaling complex in BMP-dependent Xenopus model of embryonic patterning55. In ectopic expression studies, XIAP was later shown to coassociate with the TGF-β type I receptor (TβR/I) and to augment TGF-β-dependent gene expression51. A specific element in the kinase domain of TβRI known as the L45 loop was shown to be necessary for XIAP binding56; since this loop is also required for Smad-dependent signaling and JNK activation, these correlative data lend support to a model in which XIAP can modulate TGF-β-dependent signaling in a manner which appears distinct from its caspase inhibitory role. Although deletion analyses in overexpression systems suggest that the E3 activity of XIAP is not required for TGF-β-mediated activation of either JNK or Smad-dependent signaling58, differences in TGF-β/BMP-dependent signaling in XIAP-deficient cells and animals have not been described. Therefore, despite the existence of some evidence supporting a role for XIAP in TGF-β/BMP signaling, in mechanistic and physiologic terms this role remains to be fully characterized.
XIAP and NF-κB: a chicken-and-egg story
Many studies have described the ability of NF-κB to transcriptionally activate the expression of XIAP. Paradoxically, however, a growing body of evidence also exists to support a modulatory role for XIAP in NF-κB activation. A number of earlier studies revealed the ability of ectopically expressed XIAP to activate NF-κB-responsive reporter activity51, 52, 59. XIAP was shown to require an intact RING domain for this effect51, suggesting a requirement for its ubiquitin ligase activity. However, the targets of XIAP in this process are less clear. An attractive candidate is the serine threonine kinase, TAK1, a MAPKKK originally identified as a signaling intermediate in the TGF-β pathway that has been shown to form a complex with XIAP and TAB1. TAK1 is a major participant in NF-κB-inducing signals60, and indeed studies have suggested that XIAP can trigger the ubiquitination of TAK161 through a mechanism that presumably requires an association with TAB155, 62, and which is mediated by the most amino terminal BIR of XIAP (BIR163; Figure 1). An intriguing recent study also implicated a requirement for maximal NF-κB activation of a conserved ubiquitin-binding domain (UBA), located between the BIR and RING domains38. Conflicting data, however, have been described regarding the question of whether TAK1 is required for NF-κB dependent activation by XIAP51, 52, and additional studies have suggested alternative pathways.
The originally described model of NF-κB regulation, in which a preformed p50:RelA heterodimeric complex is sequestered in the cytosol prior to activation of the appropriate signaling cascade, has stood the test of time. However, it has become clear that much of the NF-κB response is regulated at the level of nuclear NF-κB, for example by controlling the half-life of NF-κB complexes on chromatin-associated promoters64. One factor found to regulate the stability of NF-κB subunits is COMMD1, a protein that appears to play a pivotal role in the targeted ubiquitination of chromatin-associated NF-κB proteins65, which was identified in an XIAP interaction screen (Figure 1)66 and is itself a target of the ubiquitin ligase activity of XIAP67. So an additional mechanism by which XIAP might modulate NF-κB-dependent transcription is to function as a negative regulator of the NF-κB suppressor, COMMD1 (Figure 3). The realization that COMMD1 itself is also involved in a variety of other cellular functions, including copper homeostasis68 and hypoxia69, leads to the interesting possibility that XIAP might also participate in these activities, and evidence exists to support this idea67, 70.
While the activation of NF-κB by XIAP overexpression has frequently been observed as described above, until recently relatively few studies have examined the contribution to NF-κB signaling in vivo. XIAP-deficient cells do not demonstrate dramatic differences in NF-κB signaling, compared to control cells (reference 19 and J. Rumble and C.S.D., unpublished observations). NF-κB activation is generally considered to support tumor development, but _Xiap_-targeted mice have been examined in the context of the TRAMP transgenic murine model of prostate cancer and found to trend towards more aggressive disease71, which would not appear to support this model. In contrast, the RING-deficient _Xiap_-targeted mouse has been crossed to the Eμ-Myc transgenic lymphoma model and found to skew towards enhanced survival, although it is important to note that the degree of NF-κB activation is clearly one of a large number of factors that determine tumor burden and disease progression. Additionally, the expression of NF-κB-responsive genes in both of these models has not been described to date.
Two recent studies have implicated XIAP as a regulator of NF-κB in vivo. XIAP was found to play a pivotal role in the innate immune response to the cytosolic bacterial pathogen Listeria monocytogenes. _Xiap_-deficient mice were observed to be highly sensitive to this pathogen, and the activation of both NF-κB and JNK was markedly impaired in macrophages, a primary target of L. monocytogenes, following infection54. This suppression did not appear to lead to enhanced cellular apoptotic sensitivity, but rather seems to reflect an alteration in the secondary induction of proinflammatory cytokines, presumably as a consequence of impaired NF-κB/JNK-dependent signaling. Additionally, Winsauer and coworkers examined the kinetics of NF-κB activation by TNF in _Xiap_-deficient cells, and found a marked impairment, compared to control cells, of nuclear RelA(p65) in a second wave of activation which was found to occur around 60 minutes after stimulation72. These interesting findings do not preclude a role for an XIAP:COMMD1 regulatory mechanism, although the same study also demonstrated the ability of overexpressed XIAP to coassociate with, and direct the ubiquitination of the NF-κB-activating kinase MEKK2 (Figure 3), through a mechanism that appears to involve both K48 and K63 conjugated ubiquitin chains. This may become a more central issue for XIAP, as more is known about the physiological significance of distinct patterns of ubiquitination to proteasomal targeting, intracellular trafficking and functional regulation of the target protein73.
XIAP, ubiquitination and cellular signaling: the road ahead
As discussed above, a growing body of evidence supports the notion that XIAP participates in cellular signaling cascades, and that the E3 ubiquitin ligase activity of this protein plays a key role in these processes. However, despite intensive studies, XIAP cannot yet definitively be placed in a physiologically confirmed `wiring' diagram describing any specific receptor-initiated signaling cascade analogous to the positions of the c-IAPs in TNF receptor signaling. A quantum leap for the field would be to define such a role for XIAP, and currently the lead candidate pathways might appear to be those of the TGF-β/BMP receptors and the pattern recognition receptors of the innate immune system.
A potentially informative avenue into the physiological roles of XIAP may come from the recent identification of loss of function mutations in a subset of patients with X-linked lymphoproliferative disease (XLP)74, a primary immunodeficiency resulting in an inappropriate response to Epstein-Barr virus (EBV) infection that frequently presents as a fatal hemophagocytic lymphohistiocytosis (HLH)75. Profiling of the hematopoietic repertoire and responsiveness of patients with mutations in XIAP to those with the primary cause of XLP, SAP, may help to shed light into the functional roles of XIAP, and as more mutations in XIAP are uncovered76, this type of characterization may be highly illuminative.
Along with the search for specific cellular signaling pathways in which XIAP participates is the question of how its ubiquitin ligase properties are activated and regulated. It is most likely that the intrinsic biological activity of XIAP is controlled by cellular processes such as induced dimerization, transubiquitination, phosphorylation, proteolytic cleavage or subcellular compartmentalization; this is certain to be a highly fertile area of study in the future. Focus in the IAP field is currently moving towards the c-IAPs, due to the realization that synthetic IBM mimetics efficiently target the c-IAPs and represent an exciting new therapeutic approach to the treatment of disorders associated with cellular proliferation77. It will be key to remember that the design of these mimetics was based, in almost every case, on the structural features of the XIAP:Smac interaction6. While it is a happy accident that these compounds exhibit an apparently higher efficacy for the c-IAPs than they do XIAP, as attention is understandably turned to an examination of the physiological roles of the c-IAPs, XIAP clearly has yet to reveal many of its secrets.
ACKNOWLEDGEMENTS
We thank our many colleagues and collaborators for their insightful comments and suggestions and we apologize to those scientists whose contributions, due to space limitations, we were unable to discuss. This work was supported by the NIH R01 GM067827 and an award from the American Asthma Foundation to C.S.D, and Immunopathology Training Grant T32HL07517) to S.G.
LIST OF ABBREVIATIONS
XIAP
X-linked inhibitor of apoptosis
BIR
baculovirus IAP repeat
IAP
inhibitor of apoptosis protein
c-IAP
cellular IAP
IBM
IAP-binding motif
Smac/DIABLO
second mitochondria-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI
DIAP1 & 2
Drosophila IAP protein 1 & 2
AIF
apoptosis-inducing factor
XAF1
XIAP associated factor
NF-κB
nuclear factor κ-light-chain-enhancer of activated B cells
TNF
tumor necrosis factor
TRAFs
TNF receptor associated factors
TNFR
TNF receptor
JNK
c-Jun N-terminal kinase
TLR
toll-like receptor
NOD
nucleotide-binding oligomerization domain containing protein
BMP
bone morphogenetic protein
TGF-β
transforming growth factor-β
TAK1
TGF-β activated kinase 1
TAB1
TAK1-binding protein 1
UBA
ubiquitin-binding domain
COMMD1
copper metabolism (MURR1) domain containing 1
TRAMP
transgenic murine model of prostate cancer
MEKK2
MEK kinase 2
XLP
X-linked lymphoproliferative disease
EBV
Epstein-Barr virus
SAP
SLAM-associated protein
Omi/HtrA2
high temperature requirement protein A2
CLPX
a regulatory component of mitochondrial Clp protease
LRPPR
leucine-rich pentatricopeptide repeat motif-containing protein
GdH
glutamate dehydrogenase
Nsp4
Nipsnap 4
ER
endoplasmatic reticulum
REFERENCES
- 1.Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat G, Farahani R, McLean M, Ikeda J-E, MacKenzie A, Korneluk RG. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature. 1996;379:349–353. doi: 10.1038/379349a0. [DOI] [PubMed] [Google Scholar]
- 2.Duckett CS, Nava VE, Gedrich RW, Clem RJ, Van Dongen JL, Gilfillan MC, Shiels H, Hardwick JM, Thompson CB. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. EMBO J. 1996;15:2685–2694. [PMC free article] [PubMed] [Google Scholar]
- 3.Uren A, Pakusch M, Hawkins C, Puls KL, Vaux DL. Cloning and expression of apoptosis inhibitory proteins homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc. Natl. Acad. Sci. USA. 1996;93:4974–4978. doi: 10.1073/pnas.93.10.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnbaum MJ, Clem RJ, Miller LK. An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifs. J Virol. 1994;68:2521–2528. doi: 10.1128/jvi.68.4.2521-2528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crook NE, Clem RJ, Miller LK. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J. Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srinivasula SM, Ashwell JD. IAPs: What's in a name? Mol Cell. 2008;30:123–135. doi: 10.1016/j.molcel.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun C, Cai M, Meadows RP, Xu N, Gunasekera AH, Herrmann J, Wu JC, Fesik SW. NMR structure and mutagenesis of the third Bir domain of the inhibitor of apoptosis protein XIAP. J Biol Chem. 2000;275:33777–33781. doi: 10.1074/jbc.M006226200. [DOI] [PubMed] [Google Scholar]
- 8.Sun C, Cai M, Gunasekera AH, Meadows RP, Wang H, Chen J, Zhang H, Wu W, Xu N, Ng SC, Fesik SW. NMR structure and mutagenesis of the inhibitor-of-apoptosis protein XIAP. Nature. 1999;401:818–822. doi: 10.1038/44617. [DOI] [PubMed] [Google Scholar]
- 9.Hinds MG, Norton RS, Vaux DL, Day CL. Solution structure of a baculoviral inhibitor of apoptosis (IAP) repeat. Nat Struct Biol. 1999;6:648–651. doi: 10.1038/10701. [DOI] [PubMed] [Google Scholar]
- 10.Eckelman BP, Salvesen GS, Scott FL. Human inhibitor of apoptosis proteins: why XIAP is the black sheep of the family. EMBO Rep. 2006;7:988–994. doi: 10.1038/sj.embor.7400795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vaux DL, Silke J. IAPs, RINGs and ubiquitylation. Nat Rev Mol Cell Biol. 2005;6:287–297. doi: 10.1038/nrm1621. [DOI] [PubMed] [Google Scholar]
- 13.Joazeiro CA, Weissman AM. RING finger proteins: mediators of ubiquitin ligase activity. Cell. 2000;102:549–552. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 14.Clem RJ, Miller LK. Control of programmed cell death by the baculovirus genes p35 and iap. Mol. Cell. Biol. 1994;14:5212–5222. doi: 10.1128/mcb.14.8.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vucic D, Kaiser WJ, Miller LK. A mutational analysis of the baculovirus inhibitor of apoptosis Op-IAP. J Biol Chem. 1998;273:33915–33921. doi: 10.1074/jbc.273.51.33915. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson JC, Wilkinson AS, Scott FL, Csomos RA, Salvesen GS, Duckett CS. Neutralization of Smac/Diablo by IAPs: a caspase-independent mechanism for apoptotic inhibition. J Biol Chem. 2004:51082–51090. doi: 10.1074/jbc.M408655200. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science. 2000;288:874–877. doi: 10.1126/science.288.5467.874. [DOI] [PubMed] [Google Scholar]
- 18.Lotocki G, Alonso OF, Frydel B, Dietrich WD, Keane RW. Monoubiquitination and cellular distribution of XIAP in neurons after traumatic brain injury. J Cereb Blood Flow Metab. 2003;23:1129–1136. doi: 10.1097/01.WCB.0000086938.68719.E0. [DOI] [PubMed] [Google Scholar]
- 19.Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB. Characterization of XIAP-deficient mice. Mol Cell Biol. 2001;21:3604–3608. doi: 10.1128/MCB.21.10.3604-3608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olayioye MA, Kaufmann H, Pakusch M, Vaux DL, Lindeman GJ, Visvader JE. XIAP-deficiency leads to delayed lobuloalveolar development in the mammary gland. Cell Death Differ. 2005;12:87–90. doi: 10.1038/sj.cdd.4401524. [DOI] [PubMed] [Google Scholar]
- 21.Cummins JM, Kohli M, Rago C, Kinzler KW, Vogelstein B, Bunz F. X-linked inhibitor of apoptosis protein (XIAP) is a nonredundant modulator of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis in human cancer cells. Cancer Res. 2004;64:3006–3008. doi: 10.1158/0008-5472.can-04-0046. [DOI] [PubMed] [Google Scholar]
- 22.Rumble JM, Bertrand MJ, Csomos RA, Wright CW, Albert L, Mak TW, Barker PA, Duckett CS. Apoptotic sensitivity of murine IAP-deficient cells. Biochem J. 2008;415:21–25. doi: 10.1042/BJ20081188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galban S, Hwang C, Rumble JM, Oetjen KA, Wright CW, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Duckett CS. Cytoprotective effects of IAPs revealed by a small molecule antagonist. Biochem J. 2009;417:765–771. doi: 10.1042/BJ20081677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science. 2000;288:874–877. doi: 10.1126/science.288.5467.874. [DOI] [PubMed] [Google Scholar]
- 25.Vaux DL, Silke J. Mammalian mitochondrial IAP binding proteins. Biochem Biophys Res Commun. 2003;304:499–504. doi: 10.1016/s0006-291x(03)00622-3. [DOI] [PubMed] [Google Scholar]
- 26.Verhagen AM, Kratina TK, Hawkins CJ, Silke J, Ekert PG, Vaux DL. Identification of mammalian mitochondrial proteins that interact with IAPs via N-terminal IAP binding motifs. Cell Death Differ. 2007;14:348–357. doi: 10.1038/sj.cdd.4402001. [DOI] [PubMed] [Google Scholar]
- 27.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 28.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102:43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 29.Fu J, Jin Y, Arend LJ. Smac3, a novel Smac/DIABLO splicing variant, attenuates the stability and apoptosis-inhibiting activity of X-linked inhibitor of apoptosis protein. J Biol Chem. 2003;278:52660–52672. doi: 10.1074/jbc.M308036200. [DOI] [PubMed] [Google Scholar]
- 30.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 Facilitate Cancer Cell Survival by Functioning as E3 Ligases that Promote RIP1 Ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Silke J, Kratina T, Ekert PG, Pakusch M, Vaux DL. Unlike Diablo/smac, Grim promotes global ubiquitination and specific degradation of X chromosome-linked inhibitor of apoptosis (XIAP) and neither cause apoptosis. J Biol Chem. 2004;279:4313–4321. doi: 10.1074/jbc.M305661200. [DOI] [PubMed] [Google Scholar]
- 32.Yang QH, Du C. Smac/DIABLO selectively reduces the levels of c-IAP1 and c-IAP2 but not that of XIAP and livin in HeLa cells. J Biol Chem. 2004;279:16963–16970. doi: 10.1074/jbc.M401253200. [DOI] [PubMed] [Google Scholar]
- 33.Creagh EM, Murphy BM, Duriez PJ, Duckett CS, Martin SJ. Smac/Diablo antagonizes Ubiquitin ligase activity of inhibitor of apoptosis proteins. J Biol Chem. 2004 doi: 10.1074/jbc.M313859200. [DOI] [PubMed] [Google Scholar]
- 34.Silke J, Kratina T, Chu D, Ekert PG, Day CL, Pakusch M, Huang DC, Vaux DL. Determination of cell survival by RING-mediated regulation of inhibitor of apoptosis (IAP) protein abundance. Proc Natl Acad Sci U S A. 2005;102:16182–16187. doi: 10.1073/pnas.0502828102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung HH, Plenchette S, Kern CJ, Mahoney DJ, Korneluk RG. The RING domain of cIAP1 mediates the degradation of RING-bearing inhibitor of apoptosis proteins by distinct pathways. Mol Biol Cell. 2008;19:2729–2740. doi: 10.1091/mbc.E08-01-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schile AJ, Garcia-Fernandez M, Steller H. Regulation of apoptosis by XIAP ubiquitin-ligase activity. Genes Dev. 2008;22:2256–2266. doi: 10.1101/gad.1663108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin H, Okada K, Wilkinson JC, Solomon KM, Duckett CS, Reed JC, Salvesen GS. Identification of ubiquitination sites on the X-linked inhibitor of apoptosis protein. Biochem J. 2003;373:965–971. doi: 10.1042/BJ20030583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gyrd-Hansen M, Darding M, Miasari M, Santoro MM, Zender L, Xue W, Tenev T, da Fonseca PC, Zvelebil M, Bujnicki JM, Lowe S, Silke J, Meier P. IAPs contain an evolutionarily conserved ubiquitin-binding domain that regulates NF-κB as well as cell survival and oncogenesis. Nat Cell Biol. 2008;10:1309–1317. doi: 10.1038/ncb1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci U S A. 2001;98:8662–8667. doi: 10.1073/pnas.161506698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ditzel M, Broemer M, Tenev T, Bolduc C, Lee TV, Rigbolt KT, Elliott R, Zvelebil M, Blagoev B, Bergmann A, Meier P. Inactivation of effector caspases through nondegradative polyubiquitylation. Mol Cell. 2008;32:540–553. doi: 10.1016/j.molcel.2008.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MacFarlane M, Merrison W, Bratton SB, Cohen GM. Proteasome-mediated degradation of Smac during apoptosis: XIAP promotes Smac ubiquitination in vitro. J Biol Chem. 2002;277:36611–36616. doi: 10.1074/jbc.M200317200. [DOI] [PubMed] [Google Scholar]
- 42.Wilkinson JC, Wilkinson AS, Galban S, Csomos RA, Duckett CS. Apoptosis-inducing factor is a target for ubiquitination through interaction with XIAP. Mol Cell Biol. 2008;28:237–247. doi: 10.1128/MCB.01065-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arora V, Cheung HH, Plenchette S, Micali OC, Liston P, Korneluk RG. Degradation of survivin by the X-linked inhibitor of apoptosis (XIAP)-XAF1 complex. J Biol Chem. 2007;282:26202–26209. doi: 10.1074/jbc.M700776200. [DOI] [PubMed] [Google Scholar]
- 44.Csomos RA, Wright CW, Galbán S, Oetjen KA, Duckett CS. Two distinct signalling cascades target the NF-κB regulatory factor c-IAP1 for degradation. Biochem J. 2009 doi: 10.1042/BJ20082140. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 46.Kim HS, Chang I, Kim JY, Choi KH, Lee MS. Caspase-mediated p65 cleavage promotes TRAIL-induced apoptosis. Cancer Res. 2005;65:6111–6119. doi: 10.1158/0008-5472.CAN-05-0472. [DOI] [PubMed] [Google Scholar]
- 47.Barkett M, Xue D, Horvitz HR, Gilmore TD. Phosphorylation of IkB-a inhibits its cleavage by caspase CPP32 in vitro. J. Biol. Chem. 1997;272:29419–29422. doi: 10.1074/jbc.272.47.29419. [DOI] [PubMed] [Google Scholar]
- 48.Rothe M, Pan M-G, Henzel WJ, Ayres TM, Goeddel DV. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 49.Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duckett CS, Li F, Wang Y, Tomaselli KJ, Thompson CB, Armstrong RC. Human IAP-like protein regulates programmed cell death downstream of Bcl-xL and cytochrome c. Mol. Cell. Biol. 1998;18:608–615. doi: 10.1128/mcb.18.1.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Birkey Reffey S, Wurthner JU, Parks WT, Roberts AB, Duckett CS. X-linked inhibitor of apoptosis protein functions as a cofactor in transforming growth factor-b signaling. J Biol.Chem. 2001;276:26542–26549. doi: 10.1074/jbc.M100331200. [DOI] [PubMed] [Google Scholar]
- 52.Hofer-Warbinek R, Schmid JA, Stehlik C, Binder BR, Lipp J, de Martin R. Activation of NF-κB by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1. J Biol Chem. 2000;275:22064–22068. doi: 10.1074/jbc.M910346199. [DOI] [PubMed] [Google Scholar]
- 53.Sanna MG, Duckett CS, Richter BWM, Thompson CB, Ulevitch RJ. Selective activation of JNK1 is necessary for the anti-apoptotic activity of hILP. Proc. Natl. Acad. Sci. USA. 1998;95:6015–6020. doi: 10.1073/pnas.95.11.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bauler LD, Duckett CS, O'Riordan MX. XIAP regulates cytosol-specific innate immunity to Listeria infection. PLoS Pathog. 2008;4:e1000142. doi: 10.1371/journal.ppat.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi K, Nagai S, Ninomiya-Tsuji J, Nishita M, Tamai K, Irie K, Ueno N, Nishida E, Shibuya H, Matsumoto K. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 1999;18:179–187. doi: 10.1093/emboj/18.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Itoh S, Thorikay M, Kowanetz M, Moustakas A, Itoh F, Heldin CH, ten Dijke P. Elucidation of Smad requirement in transforming growth factor-β type I receptor-induced responses. J Biol Chem. 2003;278:3751–3761. doi: 10.1074/jbc.M208258200. [DOI] [PubMed] [Google Scholar]
- 57.Oeda E, Oka Y, Miyazono K, Kawabata M. Interaction of Drosophila inhibitors of apoptosis with thick veins, a type I serine/threonine kinase receptor for decapentaplegic. J Biol Chem. 1998;273:9353–9356. doi: 10.1074/jbc.273.16.9353. [DOI] [PubMed] [Google Scholar]
- 58.Lewis J, Burstein E, Birkey Reffey S, Bratton SB, Roberts AB, Duckett CS. Uncoupling of the signaling and caspase-inhibitory properties of XIAP. J Biol Chem. 2004;279:9023–9029. doi: 10.1074/jbc.M312891200. [DOI] [PubMed] [Google Scholar]
- 59.Levkau B, Garton KJ, Ferri N, Kloke K, Nofer JR, Baba HA, Raines EW, Breithardt G. XIAP induces cell-cycle arrest and activates nuclear factor-κB: new survival pathways disabled by caspase-mediated cleavage during apoptosis of human endothelial cells. Circ Res. 2001;88:282–290. doi: 10.1161/01.res.88.3.282. [DOI] [PubMed] [Google Scholar]
- 60.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaur S, Wang F, Venkatraman M, Arsura M. X-linked inhibitor of apoptosis (XIAP) inhibits c-Jun N-terminal kinase 1 (JNK1) activation by transforming growth factor β1 (TGF-β1) through ubiquitin-mediated proteasomal degradation of the TGF-β1-activated kinase 1 (TAK1) J Biol Chem. 2005;280:38599–38608. doi: 10.1074/jbc.M505671200. [DOI] [PubMed] [Google Scholar]
- 62.Sanna MG, Da Silva Correia J, Luo Y, Chuang B, Paulson LM, Nguyen B, Deveraux QL, Ulevitch RJ. ILPIP, a novel anti-apoptotic protein that enhances XIAP-mediated activation of JNK1 and protection against apoptosis. J Biol Chem. 2002;277:30454–30462. doi: 10.1074/jbc.M203312200. [DOI] [PubMed] [Google Scholar]
- 63.Lu M, Lin SC, Huang Y, Kang YJ, Rich R, Lo YC, Myszka D, Han J, Wu H. XIAP induces NF-κB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol Cell. 2007;26:689–702. doi: 10.1016/j.molcel.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 65.Burstein E, Hoberg JE, Wilkinson AS, Rumble JM, Csomos RA, Komarck CM, Maine GN, Wilkinson JC, Mayo MW, Duckett CS. COMMD proteins, a novel family of structural and functional homologs of MURR1. J Biol Chem. 2005;280:22222–22232. doi: 10.1074/jbc.M501928200. [DOI] [PubMed] [Google Scholar]
- 66.Ganesh L, Burstein E, Guha-Niyogi A, Louder MK, Mascola JR, Klomp LW, Wijmenga C, Duckett CS, Nabel GJ. The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature. 2003;426:853–857. doi: 10.1038/nature02171. [DOI] [PubMed] [Google Scholar]
- 67.Burstein E, Ganesh L, Dick RD, van De Sluis B, Wilkinson JC, Lewis J, Klomp LWJ, Wijmenga C, Brewer GJ, Nabel GJ, Duckett CS. A novel role for XIAP in copper homeostasis through regulation of MURR1. EMBO J. 2004;23:244–254. doi: 10.1038/sj.emboj.7600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van De Sluis B, Rothuizen J, Pearson PL, van Oost BA, Wijmenga C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet. 2002;11:165–173. doi: 10.1093/hmg/11.2.165. [DOI] [PubMed] [Google Scholar]
- 69.van de Sluis B, Muller P, Duran K, Chen A, Groot AJ, Klomp LW, Liu PP, Wijmenga C. Increased activity of hypoxia-inducible factor 1 is associated with early embryonic lethality in Commd1 null mice. Mol Cell Biol. 2007;27:4142–4156. doi: 10.1128/MCB.01932-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mufti AR, Burstein E, Csomos RA, Graf PC, Wilkinson JC, Dick RD, Challa M, Son JK, Bratton SB, Su GL, Brewer GJ, Jakob U, Duckett CS. XIAP Is a copper binding protein deregulated in Wilson's disease and other copper toxicosis disorders. Mol Cell. 2006;21:775–785. doi: 10.1016/j.molcel.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 71.Hwang C, Oetjen KA, Kosoff D, Wojno KJ, Albertelli MA, Dunn RL, Robins DM, Cooney KA, Duckett CS. X-linked inhibitor of apoptosis deficiency in the TRAMP mouse prostate cancer model. Cell Death Differ. 2008;15:831–840. doi: 10.1038/cdd.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winsauer G, Resch U, Hofer-Warbinek R, Schichl YM, de Martin R. XIAP regulates biphasic NF-κB induction involving physical interaction and ubiquitination of MEKK2. Cell Signal. 2008;20:2107–2112. doi: 10.1016/j.cellsig.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 73.O'Riordan MXD, Bauler, LD, Scott, FL, Duckett, CS. Inhibitor of apoptosis proteins in eukaryotic evolution and development: a model of thematic conservation. Dev Cell. 2008;15:497–508. doi: 10.1016/j.devcel.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nichols KE, Ma CS, Cannons JL, Schwartzberg PL, Tangye SG. Molecular and cellular pathogenesis of X-linked lymphoproliferative disease. Immunol Rev. 2005;203:180–199. doi: 10.1111/j.0105-2896.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 75.Rigaud S, Fondaneche MC, Lambert N, Pasquier B, Mateo V, Soulas P, Galicier L, Le Deist F, Rieux-Laucat F, Revy P, Fischer A, de Saint Basile G, Latour S. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 76.Marsh RA, Villanueva J, Zhang K, Snow AL, Su HC, Madden L, Mody R, Kitchen B, Marmer D, Jordan MB, Risma KA, Filipovich AH, Bleesing JJ. A rapid flow cytometric screening test for X-linked lymphoproliferative disease due to XIAP deficiency. Cytometry B Clin Cytom. 2009 doi: 10.1002/cyto.b.20473. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wu H, Tschopp J, Lin SC. Smac mimetics and TNFα: a dangerous liaison? Cell. 2007;131:655–658. doi: 10.1016/j.cell.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gottfried Y, Rotem A, Lotan R, Steller H, Larisch S. The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J. 2004;23:1627–1635. doi: 10.1038/sj.emboj.7600155. [DOI] [PMC free article] [PubMed] [Google Scholar]