Permanent reorganization of Ia afferent synapses on motoneurons after peripheral nerve injuries (original) (raw)
. Author manuscript; available in PMC: 2011 Jun 1.
Abstract
After peripheral nerve injuries to a motor nerve the axons of motoneurons and proprioceptors are disconnected from the periphery and monosynaptic connections from group I afferents and motoneurons become diminished in the spinal cord. Following successful reinnervation in the periphery, motor strength, proprioceptive sensory encoding, and Ia afferent synaptic transmission on motoneurons partially recover. Muscle stretch reflexes, however, never recover and motor behaviors remain uncoordinated. In this review, we summarize recent findings that suggest that lingering motor dysfunction might be in part related to decreased connectivity of Ia afferents centrally. First, sensory afferent synapses retract from lamina IX causing a permanent relocation of the inputs to more distal locations and significant disconnection from motoneurons. Second, peripheral reconnection between proprioceptive afferents and muscle spindles is imperfect. As a result, a proportion of sensory afferents that retain central connections with motoneurons might not reconnect appropriately in the periphery. A hypothetical model is proposed in which the combined effect of peripheral and central reconnection deficits might explain the failure of muscle stretch to initiate or modulate firing of many homonymous motoneurons.
Keywords: stretch reflex, spinal cord, plasticity, motor control, adult
Introduction
Recovery of normal neural function after injury requires the regeneration of damaged axons and the reestablishment of synaptic connections between neurons. Although some compensation can be achieved as the result of redundancy and plasticity in the adult nervous system,1, 2 behavior cannot be normal if a significant number of connections are lost. This lends strong justification for the research effort to promote axonal regeneration, in particular after spinal cord injuries.3, 4, 5 Axonal regrowth is only one step, however, in the recuperation of normal circuitry and unfortunately axonal regeneration is not synonymous with functional recovery. Failure to reestablish synapses of adequate strength together with non-adaptive secondary plasticity in surviving circuits could greatly interfere with normal function. This is best illustrated by the incomplete recovery of motor function that occurs after peripheral nerve injuries. Here we summarize recent findings on the plasticity of central synaptic connections between sensory afferents and spinal motoneurons that are triggered by injury to a peripheral nerve. These alterations are rather permanent and therefore likely contributors to the motor deficits that remain after normal regeneration in the periphery.
Peripheral regeneration does not fully restore motor function
In contrast to central axons, axons injured in the periphery readily regenerate and reconnect with dennervated target tissues.6 As a consequence, voluntary control over muscle contraction is restored and muscle strength is recovered.7 Similarly the flow of sensory information into the central nervous system from reinnervated skin or muscle is reestablished.8 These gains assist functional recovery, which nonetheless remains incomplete. The most relevant data comes from experiments on muscles reinnervated by their own cut nerve (self-reinnervated) in which axon navigating errors are minimized and target reinnervation, at a macro level, is most successful. In this situation, muscle activity patterns and interjoint coordination after reinnervation are normal during certain kinds of locomotion.9,10 Still, there is unmistakable disability outside the bounds of movements restricted to treadmill locomotion at low speed, over level ground, and in the absence of perturbations. Specifically, animals with reinnervated ankle extensor muscles exhibit abnormal ankle yield, lose coordination between ankle and the knee joints, and stumble during downhill walking.10 In addition, changing the speed of locomotion brings out abnormalities in ankle-knee-hip coordination.11 These global deficits in limb movement are made all the more impressive by the fact that experimental nerve damage in these cases involved only one or two muscles and that deficits persist long after peripheral reinnervation is completed.
Proprioceptive deficits after peripheral nerve regeneration
Ataxia expressed in limbs with reinnervated muscles prompted analysis of the proprioceptive system and, more specifically, the stretch reflex pathway after peripheral nerve injuries and surgical reunion. Surprisingly, after complete nerve transections, self-reinnervated muscles are absolutely unresponsive to stretch,12, 13 even though the reinnervated muscle is perfectly capable of contracting in response to other uninjured inputs, for example from cutaneous afferents. Muscle stretch superimposed on ongoing reflex activity yielded no additional force production highlighting two important conclusions. First, regenerated motoneurons are capable of responding to synaptic input. Second, stretch signals carried centrally by regenerated afferents are largely ineffective. Even a smaller than usual stretch synaptic input that is not strong enough to bring motoneurons from rest to firing threshold, should nevertheless exert some modulation of ongoing motoneuron firing.14 Lack of modulation therefore suggests a complete failure of stretch signals to reach the motoneuron.
Lack of stretch reflexes is not due to compromised motoneuron or proprioceptive sensory neuron survival or peripheral reinnervation
One common explanation for lingering motor dysfunction after large peripheral nerve injuries is that the reconnection of regenerating sensory and motor axons with their targets is incomplete or non-specific.2, 15, 16 Incomplete regeneration in self-reinnervated muscles could result from injury induced cell death of the axotomized sensory or motor neurons. After injuries to major nerve branches, e.g. sciatic nerve, approximately 10 to 50% of dorsal root ganglia (DRG) neurons are lost, but large DRG neurons, including proprioceptive sensory neurons, are relatively spared and die only after long delays (several months) if regeneration is prevented.17 Regarding motoneurons, their viability is not impaired after axotomy, even when regeneration is prevented,18 as long as the injury does not happen in early life19, 20 (neonates) or occur very proximally to the motoneuron soma (for example, after ventral root avulsion21).
Physiological and anatomical evidence further suggests that after self-reinnervation of the medial gastrocnemius (MG) muscle there is a significant reinnervation of muscle spindle receptors (~75-84%13, 22). Moreover, stretch-activated regenerated afferents display dynamic and static responses to stretch that are comparable to normal Ia afferents.13, 23 Similarly, motor unit properties recover after self-reinnervation quite successfully and only 10% of MG motoneurons fail to elicit a contraction.24, 25
Despite this impressive recovery of peripheral connections, there are important questions about their specificity. Afferent and motor axons have the capacity to regenerate into wrong tissue and reinnervate wrong targets, for example muscle afferents can innervate cutaneous territories with some recovery of afferent firing properties and central connectivity with motoneurons.26, 27 Moreover, after lesions of major nerve trunks, many motoneurons indeed fail to reinnervate their parent muscle.6, 15 When muscles are self-reinnervated, i.e. reinnervated by their original nerve, failure of motor axons to reform neuromuscular junctions with their original muscle fibers is offset by re-specification of muscle fiber properties, thereby restoring the normal proportion of motor unit types and sizes.24, 25 Thus, few differences are observed in force generation and motor unit recruitment is similar in self-reinnervated and normal muscle.28
More complex, however, is the situation involving sensory afferent peripheral reinnervation. Proprioceptive afferents in muscle can reinnervate the wrong receptor type (non-specific reinnervation), but cannot respecify receptor structure. Accordingly, Ib afferent fibers can successfully innervate muscle spindle receptors and replace the original Ia afferents with little difference in the resulting structure of the annulospiral endings or encoding properties to muscle stretch.22, 29 Therefore, group I afferents can change their peripheral apparatus and responses depending on the receptor type they innervate, but this afferent receptor switch seems not to be matched by re-specification of their central projections. In addition, a proportion of afferents fail to reinnervate any peripheral end organ and become unresponsive to natural stimuli. Thus, there are indeed significant targeting failures during sensory reconnection in the periphery. Nevertheless, there is also substantial specific reinnervation, whereby regenerating spindle afferents reconnect with spindle receptors, making it difficult to envision how non-specific reinnervation alone could be responsible for the total collapse of the stretch reflex. One possibility is that peripheral errors are accompanied by major reorganizations of central connectivity. It is well known that peripheral nerve injuries frequently result in profound structural and functional reorganizations of central synaptic circuits that can spread as far rostral as the cerebral cortex.2, 30 An obvious place to look for a functional deficit is then the central synapses established on spinal motoneurons by Ia afferent fibers.
Monosynaptic sensory inputs on motoneurons are altered following peripheral nerve injury and recover after regeneration, however stretch synaptic potentials are diminished or absent
Monosynaptic transmission between sensory afferents and motoneurons undergoes complex changes following peripheral nerve injuries. Within the first week and peaking at 3 days after nerve cut or crush, electrically-evoked group I EPSPs increase substantially in size.31-33 A few days later, however, there is a general decline in EPSP amplitude and an increase in time course.13, 34-39 There is also enhanced synaptic depression at high firing frequencies27 that lasts for several weeks. These changes revert toward normal values following peripheral regeneration and target reinnervation, such that electrically-evoked group I EPSPs regain the ability to sustain nearly normal amplitudes during low or high-frequency stimulation.13, 34, 38 Figure 1 shows no evidence of enhanced depression in regenerated afferents using frequency-modulated electrical stimulation patterns that replicate the normal Ia firing in response to a ramp and hold stretch paradigm of the same muscle.40 In other words, the regenerated group I afferent-motoneuron circuit operates normally when activated electrically. While consistent with reports of full recovery of the electrically-evoked H-reflex,41 these observations cannot explain the failure of muscle stretch to elicit any reflex activity or firing modulation of motoneurons.
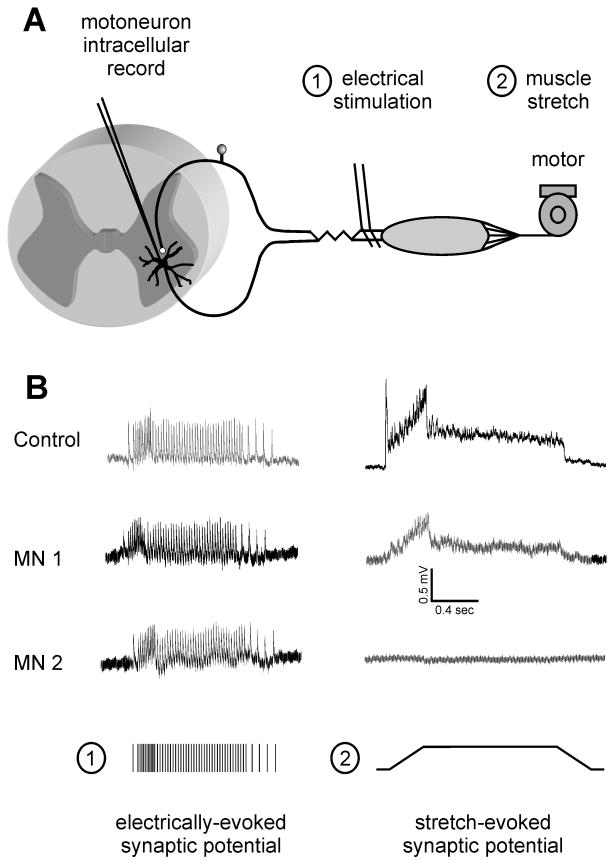
Figure 1. Synaptic responses in the stretch reflex circuit following peripheral nerve injuries.
(A) Diagram depicts the monosynaptic stretch reflex circuit and elements of the experimental paradigm. Adult rats with muscles normally innervated or reinnervated by a cut nerve were anesthetized for in vivo study wherein motoneuron membrane potential was recorded intracellularly during stimulation of afferents by either (1) electrical stimulation at group I strength or (2) ramp-hold-release stretch of the muscle by a motor. (B) Synaptic responses to afferent firing produced either mechanically (stretch-evoked synaptic potentials, SSP; right column) or electrically by trains designed to simulate the responses of single Ia afferents to the same ramp-hold-release stretch (train of group I EPSPs; left column), both recorded from each of 3 motoneurons: top traces from one control rat; middle traces from 2 motoneurons in one rat with the MG muscle reinnervated 1 year after nerve section and surgical reunion. Note the absence of a SSP in MN2 despite the presence of an electrically-evoked EPSP and the presence of a smaller than normal SSP in MN1. Electrically evoked EPSPs were present in all control and in all reinnervating motoneurons, and showed no signs of amplitude depression when firing at high-frequencies. (Modified from data in references # 13, 40 & 43).
Intriguingly, recuperation of electrically-evoked EPSPs is not accompanied by the recovery of muscle stretch-evoked synaptic excitation of motoneurons. In normal animals, muscle stretch causes a motoneuron membrane depolarization that we call a stretch synaptic potential (SSP).42 SSPs faithfully follow the time course and intensity of stretch-responsive spindle afferent firing. After peripheral regeneration in a rat model of MG self-reinnervation, SSPs are smaller than normal or completely absent in many motoneurons. This abnormality is observed even though normal electrically-evoked group I EPSPs occur in every motoneuron13, 40 (Fig. 1). These observations raise the question of whether after peripheral injuries electrically-evoked group I motoneuron EPSPs are evoked centrally by the same afferents that respond to stretch following reinnervation.
Spike triggered-averaging (STA) was used to test directly the central synaptic actions of individual regenerated afferents that responded with spindle-like firing to muscle stretch. From dorsal root filaments we discriminated action potentials of single regenerated afferents that fired identically to normal spindle afferents and tested their synaptic actions in homonymous regenerated motoneurons in control rats or in animals with regenerated medial gastrocnemius nerves.43 In control rats we found that single group I spindle afferents activated by stretch produced STA EPSPs in nearly all homonymous motoneurons, just as is observed for control cats.44 In contrast, after nerve injury and successful regeneration in the periphery, sensory afferents displaying normal responses to muscle stretch failed to generate STA EPSPs in nearly all homonymous motoneurons tested. More surprisingly, these same motoneurons displayed relatively normal sensory afferent EPSPs after electrical stimulation of the peripheral nerve.43 Two potential explanations can be put forward. First, that after regeneration the population of afferent fibers activated by stretch is different from the afferent inputs responsible for generating monosynaptic electrically-evoked EPSPs on motoneurons. Second, that stretch-evoked impulses entering the spinal cord lead to central suppression of the afferent synaptic input in a manner that does not occur after electrical stimulation. The following studies on the central structural remodeling of Ia afferent connections provide some support for the first explanation; however these data do not exclude the alternative possibility.
Central reorganization of synaptic connections after peripheral injury
One possible explanation for the reduction of SSPs is profound and permanent reorganization of central synapses as a consequence of peripheral nerve injury. This reorganization could weaken or suppress Ia synapses and/or change the balance between inhibition and excitation during the arrival of stretch-evoked signals into the spinal cord. There is indeed evidence for such possibilities. Synapses become detached (“stripped”) from somata and proximal dendrites of motoneurons that are axotomized after nerve injuries.45-49 This appears to be a relatively general phenomenon that is reproduced on other central and peripheral neurons after they become disconnected from their targets by either axotomy or excitotoxic lesions.50-52 The purpose of synaptic stripping has been frequently explained as a switch in the axotomized postsynaptic cell from “signaling” to “regenerative” function. Unfortunately, this hypothesis has never been experimentally tested nor does it explain why motoneurons need to lose proximal synapses to switch on a “regeneration” mode. Therefore, it is an open question whether synaptic stripping is a well-adapted response necessary for neurons to regenerate their axons or an unfortunate outcome of target disconnection that is a possible source of functional abnormalities after peripheral regeneration is completed.
In early studies, it was argued that synaptic stripping explained the changes in amplitude and time course of sensory afferent-evoked EPSPs in motoneurons after nerve injury.39, 53, 54 Indeed, the functional recovery of electrically-evoked group I EPSPs coincides temporally with the recovery of central synapses after motoneurons reinnervate peripheral targets.55-58 Later studies, however, discredited the early view of synaptic detachment as a relatively homogenous phenomenon that similarly affects all synapses. In fact, the degree of synaptic depletion after injury and recovery after regeneration depends on the type of synaptic input. For example, inhibitory terminals are more resistant to stripping55, 59-61 and recover even in the absence of peripheral regeneration.60, 62 One possible reason for their differential behaviors is that each synaptic input seems preferentially dependent on different trophic factor signals that can originate in the target or other sources. Brain-Derived Neurotrophic Factor (BDNF) is more effective in maintaining inhibitory and tonic inputs, while neurotrophin-3 (NT3) is best related to excitatory and phasic inputs including Ia afferent synapses.61, 63, 64 Major sources of NT3 and BDNF are (in addition to the motoneuron), respectively, the muscle spindle and spinal glia, which exhibit distinct regulation and relationships to regenerating afferents and central synapses after injury. As a result, imbalances in the excitatory/inhibitory ratio or synaptic composition are usually created during recovery of central synapses on motoneurons that have reconnected with muscle.55, 56, 60, 61 In summary, despite significant recovery of central synapses after peripheral reconnection there seems to be also important changes in synaptic composition that have not yet been studied in enough detail. What is then the exact behavior of Ia afferent synapses during synaptic stripping and recovery?
Ia afferents display distinctive dynamics during synaptic stripping and their lost central synapses are never recovered
Despite earlier assumptions, it is difficult to extrapolate data gathered on stripping of excitatory synapses in general to the Ia synapses in particular. Ia synapses represent a small proportion (~2%) of all synapses on the motoneuron surface65, 66 and their contributions during central synaptic remodeling are likely diluted in overall quantitative analyses of all synapses. Moreover, Ia synapses mostly target the dendritic arbor (5-10% of Ia synapses target motoneuron somata65, 66), while most studies on synaptic stripping have concentrated the analysis on the proximal somatodendritic region. Finally, a different behavior for Ia synapses should not be too surprising. This is because of all motoneuron inputs, they alone belong to presynaptic axons that are directly injured in the periphery.
Until recently, the main obstacle to uncover the fate of Ia afferent synapses after peripheral nerve injuries has been the lack of appropriate markers. Fortunately, the vesicular glutamate transporter isoform 1 (VGLUT1) was recently found enriched in the central terminals of proprioceptive primary afferents including the central synapses of Ia afferents.67, 68, 69 After peripheral nerve injuries, VGLUT1 content in these central synapses is rapidly reduced.70 We therefore investigated the extent of recovery following reinnervation. To our surprise, depletions of VGLUT1 varicosities in lamina IX and around motoneuron cell bodies (Fig. 2A) were rather permanent and similar in intensity and time course after nerve injuries in which peripheral nerve regeneration was allowed or prevented. In both situations, VGLUT1 synapses that were lost in lamina IX were not recovered.69 VGLUT1 contacts on motoneuron cell bodies remained depleted by more than 90% six months after the injury and following successful regeneration (Fig. 2B,C).69 Depletions on dendrites were also permanent and concentrated to the proximal regions (viewed in a transverse plane). VGLUT1 contacts were significantly depleted by 40-50% in the first 100 μm of dendrite, but no significant differences were detected beyond this point (Fig. 2D, unpublished results). It is possible that these data underestimated the real amount of VGLUT1 detachment from motoneuron dendrites because rostrocaudally oriented dendrites receive the maximum percentage of Ia afferent inputs (60% of all Ia synapses received by single motoneurons65) and these dendritic branches distribute within lamina IX, the region showing the maximal depletion in VGLUT1 varicosities. Unfortunately, rostrocaudally oriented dendrites could not be analyzed in the transverse plane of sectioning used. Independent of the exact amount of depletion, it is evident that a significant number of Ia afferent central synapses are lost as a consequence of peripheral injuries and these are not recovered even long after regeneration has been completed in the periphery.
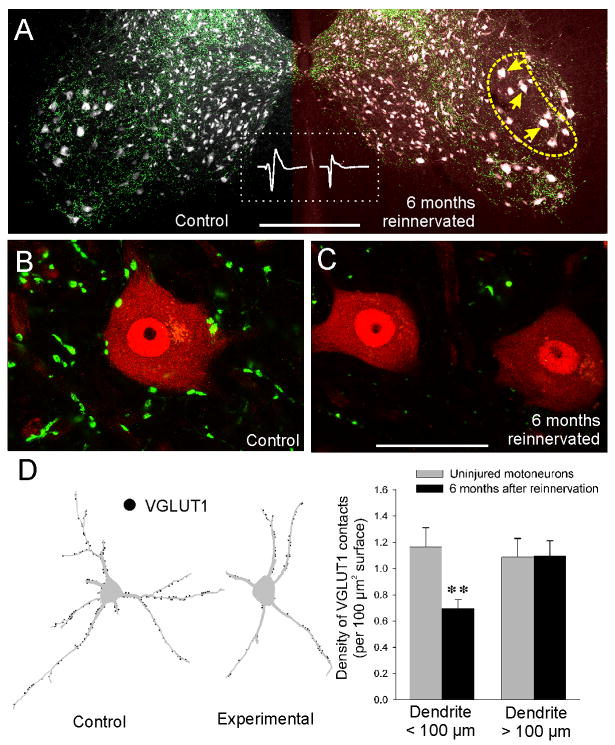
Figure 2. Permanent depletion of VGLUT1 contacts after peripheral nerve injuries.
(A) Low magnification confocal image of control and experimental ventral horns in an animal in which the left tibial nerve was completely resectioned and immediately surgically rejoined and then allowed to regenerate for 6 months. Middle inset (inside the dashed rectangle) shows electrically-evoked EMGs from the control and recovered MG muscles demonstrating peripheral reconnection. The section was dual immunostained for VGLUT1 (FITC, green) and NeuN (a general neuronal marker, Cy3, grey). In this section from the lumbar 5 segment most motoneurons are distributed in lateral pools (large NeuN-labeled cell bodies). In the ventral horn and particularly in lamina IX VGLUT1 punctae preferentially label presynaptic vesicle clusters of proprioceptive afferent synapses. The yellow dashed outline indicates the location of motor pools sending axons through the tibial nerve in the injured side. Compared to the contralateral side there is diminished density of VGLUT1 punctae in the experimental side. As a result many cell bodies (yellow arrows) are VGLUT1 denervated. (B,C) High magnification confocal images of one control (B) and two experimental motoneurons (C) (4 or 5 confocal planes at 1 μm z-steps and through the mid-region of each motoneuron are shown). Even 6 months after the injury and following successful reinnervation in the periphery, motoneurons in the injured side do not recover their somatic VGLUT1 contacts. Similarly the surrounding lamina IX neuropil shows diminished density of VGLUT1 punctae and these are usually of smaller size than in the control side. (D) VGLUT1 losses preferentially occur in the proximal somatodendritic regions. Neurolucida reconstructions of two MG motoneurons, one in the control and one in the injured side, that were retrogradely labeled from the MG muscle with cholera-toxin b subunit coupled to Alexa-555 (Ctb-555) one week before the analysis and 5.5 months after the injury (thus demonstrating peripheral connectivity in both cases). The distribution of VGLUT1 contacts was then plotted on their dendritic trees (black filled circles). A large depletion in VGLUT1 contacts is apparent in the cell body and proximal dendrite of MG motoneurons in the regenerated side compared to the uninjured side. Histograms show quantification of VGLUT1 contact density in dendritic segments within 100 μm distance from the cell body or beyond that point. Ten Ctb-555 labeled and reconstructed MG motoneurons were analyzed in the control and injured side. A significant 41% average depletion in VGLUT1 density was found in proximal dendrites (asterisks, p=0.01, t-test; error bars indicate SEM) while no significant differences were detected in more distal dendrites. Scale bars, 500 μm in A; 50 μm in C (B at the same magnification and C). (Panels A, B and C are modified from reference #69; Panel D include data from unpublished materials from FJA & TC).
Overall, the data strongly suggest a significant removal of Ia afferent synapses from lamina IX. This possibility was further confirmed by intraaxonal recording and labeling of individual regenerated afferents that were activated by stretch and showed responses typical of normal Ia afferents. In good agreement with the VGLUT1 data, of five identified stretch-activated regenerated afferents recorded intraaxonally and filled with neurobiotin to trace their central terminations, only one displayed a terminal collateral in lamina IX with a few varicosities.69 In contrast, all control afferents (n=14) profusely send branches into lamina IX that were studded with many varicosities. Synaptic varicosities on regenerated afferents were mainly found in branches terminating in lamina V and to a lesser extent in lamina VII.
The data lead us to propose the working model depicted in Figure 3 to explain the lack of stretch-evoked afferent information reaching some motoneurons. After peripheral nerve injury and disconnection of Ia afferents from muscle spindles, there is a significant retraction of central Ia afferent collaterals projecting into lamina IX. These central branches are not regrown following peripheral reconnection. As a result, a large number of Ia synapses are removed from the proximal region of motoneurons. The retention of synapses on distal dendritic branches might explain why all regenerated motoneurons display electrically-evoked compound monosynaptic EPSPs. But normally intact uninjured individual Ia afferents establish relatively few synapses on single motoneurons. For homonymous connections, each single Ia afferent establishes, on average, only 10 contacts per motoneuron. Moreover, the exact number is highly variable depending on the exact location of motoneurons within the pool and with respect to the entry rootlet of the Ia afferent.65, 66 Therefore, a significant reduction of proximal synapses, as observed using VGLUT1 as a marker, might result in a large number of disconnections of Ia afferent-motoneuron pairs. On the other hand, a proportion of Ia afferents that remain connected to motoneurons might incorrectly regenerate in the periphery. In the self-reinnervated medial gastrocnemious muscle of the cat, approximately 50% of Ia afferents are unsuccessful reconnecting with muscle spindle receptors, some of which were reinnervated by other afferents including Ib afferents.22
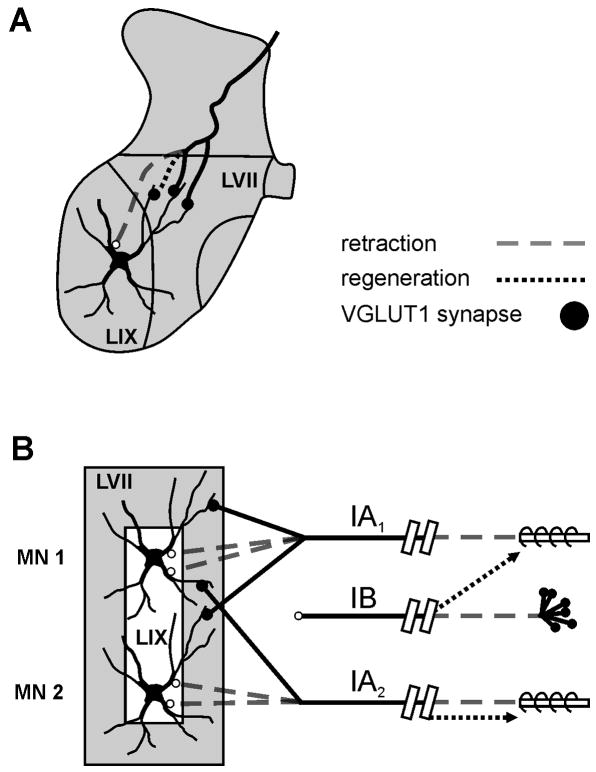
Figure 3. Working model consistent with available data including those described here for spinal motoneurons and their synaptic associations with muscle proprioceptors after muscle nerve cut and regeneration.
(A) Depicts retraction of afferent axon (dashed line) and the loss of afferent synapses from proximal somatodendritic regions located in lamina IX while contacts on more distal dendrites located in LVII are retained. It is possible that local sprouting (regeneration) in this region might add some synapses (dotted black line), however these never reach the more proximal somatodendritic regions. (B) Model: severing peripheral nerve cuts both motor and sensory axons, which undergo Wallerian degeneration peripherally and synaptic stripping (decreased VGLUT1) and presynaptic axon retraction centrally (dashed grey lines). With peripheral regeneration there is: (a) non-specific reinnervation of some spindle receptors (Ib afferents, top dotted black arrow), (b) specific reinnervation of some spindle receptors by Ia afferents (Ia2, bottom dotted black arrow), and (c) no reinnervation of spindle receptors by some Ia afferents (Ia1). Centrally Ia afferents fail to regain axonal projections into LIX on motoneuron soma and proximal dendrites; Ib afferent projections remain remote to LIX and not monosynaptically connected with motoneurons. The functional results are that stretch evoked synaptic potentials (SSPs) are lost in some motoneurons (MN 2) or and reduced in others (MN 1), yet group I electrical stimulation of the whole nerve evokes EPSPs in every motoneuron. (Modified from reference #43).
The combined effect of fewer Ia afferents connected peripherally to muscle spindle receptors after regeneration (some of which could be Ib afferents not monosynaptically connected to motoneurons, Fig. 3) compounded with a significant reduction in the number of central synapses and central Ia-motoneuron connectivity might explain the lack of stretch-evoked responses in a significant proportion of motoneurons despite the presence of enough connectivity from dorsal root afferents to elicit monosynaptic EPSPs on most of the regenerated motoneurons that reconnect with muscle.
If the above proposed hypothesis is correct, restorative actions could be taken to favor Ia afferent regrowth centrally in order to ensure that motoneurons retain innervation from at least a few afferents correctly innervating peripherally muscle spindles. We predict that manipulations intended to facilitate the proliferation of central afferent arborizations could contribute to the recovery of the lost stretch reflex and diminish the coordination deficits that impair motor function after peripheral nerve injuries and regeneration. Presently it is unknown if the failure of central axons from sensory afferents to repopulate lamina IX with VGLUT1 varicosities is due to lack or diminished peripheral signals that promote or maintain these central connections (for example spindle-derived NT364, 71, 72) or if alternatively, this is because the presence of negative factors in the adult spinal cord that impair regrowth of central sensory afferent synaptic arborizations.5, 73
Acknowledgments
This work was supported by NIH grant P01NS057228. We also wish to thank Mrs. Eileen Kantanmeni (formerly Fitzsimons) for her efforts during the early stages of this project and Mrs. Lori L. Goss, RVT for her invaluable help during animal surgeries, handling and preparation.
References
- 1.Stein DG, Hoffman SW. Concepts of CNS plasticity in the context of brain damage and repair. J Head Trauma Rehabil. 2003;18:317–41. doi: 10.1097/00001199-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Navarro X, Vivo M, Valero-Cabre A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol. 2007;82:163–201. doi: 10.1016/j.pneurobio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Buchli AD, Schwab ME. Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann Med. 2005;37:556–67. doi: 10.1080/07853890500407520. [DOI] [PubMed] [Google Scholar]
- 4.Deumens R, Koopmans GC, Joosten EA. Regeneration of descending axon tracts after spinal cord injury. Prog Neurobiol. 2005;77:57–89. doi: 10.1016/j.pneurobio.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Fawcett JW. Overcoming inhibition in the damaged spinal cord. J Neurotrauma. 2006;23:371–83. doi: 10.1089/neu.2006.23.371. [DOI] [PubMed] [Google Scholar]
- 6.Bisby M. Regeneration of peripheral nervous system axons. In: Waxman SG, K J, Stys PK, editors. The Axon: Structure, Function and Pathophsyiology. Oxford University Press; Oxford: 1995. pp. 579–589. [Google Scholar]
- 7.Thomas CK, et al. Patterns of reinnervation and motor unit recruitment in human hand muscles after complete ulnar and median nerve section and resuture. J Neurol Neurosurg Psychiatry. 1987;50:259–68. doi: 10.1136/jnnp.50.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackel R, et al. Reinnervation of mechanoreceptors in the human glabrous skin following peripheral nerve repair. Brain Res. 1983;268:49–65. doi: 10.1016/0006-8993(83)90389-x. [DOI] [PubMed] [Google Scholar]
- 9.O’Donovan MJ, et al. Kinesiological studies of self- and cross-reinnervated FDL and soleus muscles in freely moving cats. J Neurophysiol. 1985;54:852–66. doi: 10.1152/jn.1985.54.4.852. [DOI] [PubMed] [Google Scholar]
- 10.Abelew TA, et al. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. J Neurophysiol. 2000;84:2709–14. doi: 10.1152/jn.2000.84.5.2709. [DOI] [PubMed] [Google Scholar]
- 11.Chang YH, Scholtz JP, Nichols TR. Hindlimb control during cat locomotion after loss of stretch reflexes. Integrative and Comparative Biology. 2003;43:987. [Google Scholar]
- 12.Cope TC, Bonasera SJ, Nichols TR. Reinnervated muscles fail to produce stretch reflexes. J Neurophysiol. 1994;71:817–20. doi: 10.1152/jn.1994.71.2.817. [DOI] [PubMed] [Google Scholar]
- 13.Haftel VK, et al. Central suppression of regenerated proprioceptive afferents. J Neurosci. 2005;25:4733–42. doi: 10.1523/JNEUROSCI.4895-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cope TC, Fetz EE, Matsumura M. Cross-correlation assessment of synaptic strength of single Ia fibre connections with triceps surae motoneurones in cats. J Physiol. 1987;390:161–88. doi: 10.1113/jphysiol.1987.sp016692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumner AJ. Aberrant reinnervation. Muscle Nerve. 1990;13:801–3. doi: 10.1002/mus.880130905. [DOI] [PubMed] [Google Scholar]
- 16.Jonhson RD, Munson JB. Specificity of regenerating sensory neurons in adult mammals. In: Scott SS, editor. Sensory neurons: diversity, development and plasticity. Oxford University Press; Oxford: 1992. pp. 384–403. [Google Scholar]
- 17.Tandrup T, Woolf CJ, Coggeshall RE. Delayed loss of small dorsal root ganglion cells after transection of the rat sciatic nerve. J Comp Neurol. 2000;422:172–80. doi: 10.1002/(sici)1096-9861(20000626)422:2<172::aid-cne2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Vanden Noven S, et al. Adult spinal motoneurons remain viable despite prolonged absence of functional synaptic contact with muscle. Exp Neurol. 1993;123:147–56. doi: 10.1006/exnr.1993.1147. [DOI] [PubMed] [Google Scholar]
- 19.Kashihara Y, Kuno M, Miyata Y. Cell death of axotomized motoneurones in neonatal rats, and its prevention by peripheral reinnervation. J Physiol. 1987;386:135–48. doi: 10.1113/jphysiol.1987.sp016526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmalbruch H. Motoneuron death after sciatic nerve section in newborn rats. J Comp Neurol. 1984;224:252–8. doi: 10.1002/cne.902240206. [DOI] [PubMed] [Google Scholar]
- 21.Koliatsos VE, et al. Ventral root avulsion: an experimental model of death of adult motor neurons. J Comp Neurol. 1994;342:35–44. doi: 10.1002/cne.903420105. [DOI] [PubMed] [Google Scholar]
- 22.Collins WF, 3rd, Mendell LM, Munson JB. On the specificity of sensory reinnervation of cat skeletal muscle. J Physiol. 1986;375:587–609. doi: 10.1113/jphysiol.1986.sp016135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown MC, Butler RG. Regeneration of afferent and efferent fibres to muscle spindles after nerve injury in adults cats. J Physiol. 1976;260:253–66. doi: 10.1113/jphysiol.1976.sp011514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foehring RC, Sypert GW, Munson JB. Properties of self-reinnervated motor units of medial gastrocnemius of cat. II. Axotomized motoneurons and time course of recovery. J Neurophysiol. 1986;55:947–65. doi: 10.1152/jn.1986.55.5.947. [DOI] [PubMed] [Google Scholar]
- 25.Foehring RC, Sypert GW, Munson JB. Properties of self-reinnervated motor units of medial gastrocnemius of cat. I. Long-term reinnervation. J Neurophysiol. 1986;55:931–46. doi: 10.1152/jn.1986.55.5.931. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RD, et al. Rescue of motoneuron and muscle afferent function in cats by regeneration into skin. I. Properties of afferents. J Neurophysiol. 1995;73:651–61. doi: 10.1152/jn.1995.73.2.651. [DOI] [PubMed] [Google Scholar]
- 27.Mendell LM, et al. Rescue of motoneuron and muscle afferent function in cats by regeneration into skin. II. Ia-motoneuron synapse. J Neurophysiol. 1995;73:662–73. doi: 10.1152/jn.1995.73.2.662. [DOI] [PubMed] [Google Scholar]
- 28.Cope TC, Clark BD. Motor-unit recruitment in self-reinnervated muscle. J Neurophysiol. 1993;70:1787–96. doi: 10.1152/jn.1993.70.5.1787. [DOI] [PubMed] [Google Scholar]
- 29.Banks RW, Barker D. Specificities of afferents reinnervating cat muscle spindles after nerve section. J Physiol. 1989;408:345–72. doi: 10.1113/jphysiol.1989.sp017463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundborg G. Richard P. Bunge memorial lecture. Nerve injury and repair--a challenge to the plastic brain. J Peripher Nerv Syst. 2003;8:209–26. doi: 10.1111/j.1085-9489.2003.03027.x. [DOI] [PubMed] [Google Scholar]
- 31.Bichler EK, et al. Enhanced transmission at a spinal synapse triggered in vivo by an injury signal independent of altered synaptic activity. J Neurosci. 2007;27:12851–9. doi: 10.1523/JNEUROSCI.1997-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manabe T, Kaneko S, Kuno M. Disuse-induced enhancement of Ia synaptic transmission in spinal motoneurons of the rat. J Neurosci. 1989;9:2455–61. doi: 10.1523/JNEUROSCI.09-07-02455.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyata Y, Yasuda H. Enhancement of Ia synaptic transmission following muscle nerve section: dependence upon protein synthesis. Neurosci Res. 1988;5:338–46. doi: 10.1016/0168-0102(88)90035-1. [DOI] [PubMed] [Google Scholar]
- 34.Eccles JC, et al. Experiments utilizing monosynaptic excitatory action on motoneurons for testing hypotheses relating to specificity of neuronal connections. J Neurophysiol. 1962;25:559–80. doi: 10.1152/jn.1962.25.4.559. [DOI] [PubMed] [Google Scholar]
- 35.Eccles JC, Krnjevic K, Miledi R. Delayed effects of peripheral severance of afferent nerve fibres on the efficacy of their central synapses. J Physiol. 1959;145:204–20. doi: 10.1113/jphysiol.1959.sp006136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallego R, et al. Disuse enhances synaptic efficacy in spinal mononeurones. J Physiol. 1979;291:191–205. doi: 10.1113/jphysiol.1979.sp012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallego R, et al. Enhancement of synaptic function in cat motoneurones during peripheral sensory regeneration. J Physiol. 1980;306:205–18. doi: 10.1113/jphysiol.1980.sp013392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldring JM, et al. Reaction of synapses on motoneurones to section and restoration of peripheral sensory connexions in the cat. J Physiol. 1980;309:185–98. doi: 10.1113/jphysiol.1980.sp013503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendell LM. Physiological aspects of synaptic plasticity: the Ia/motoneuron connection as a model. Adv Neurol. 1988;47:337–60. [PubMed] [Google Scholar]
- 40.Cope TC, Bullinger KL. Synaptic responses to high frequency activation of regenerated Ia afferents. Society for Neuroscience. 2008;74:12. [Google Scholar]
- 41.Valero-Cabre A, Navarro X. H reflex restitution and facilitation after different types of peripheral nerve injury and repair. Brain Res. 2001;919:302–12. doi: 10.1016/s0006-8993(01)03052-9. [DOI] [PubMed] [Google Scholar]
- 42.Westbury DR. A study of stretch and vibration reflexes of the cat by intracellular recording from motoneurones. J Physiol. 1972;226:37–56. doi: 10.1113/jphysiol.1972.sp009972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bullinger KL, Cope TC. Connectivity of individual Ia-motoneuron synapses after peripheral nerve regeneration. Society for Neuroscience. 2009;442:7. [Google Scholar]
- 44.Mendell LM, Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971;34:171–87. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- 45.Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85:145–57. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- 46.Chen DH. Qualitative and quantitative study of synaptic displacement in chromatolyzed spinal motoneurons of the cat. J Comp Neurol. 1978;177:635–64. doi: 10.1002/cne.901770407. [DOI] [PubMed] [Google Scholar]
- 47.Kerns JM, Hinsman EJ. Neuroglial response to sciatic neurectomy. II. Electron microscopy. J Comp Neurol. 1973;151:255–80. doi: 10.1002/cne.901510304. [DOI] [PubMed] [Google Scholar]
- 48.Sumner BE. A quantitative analysis of the response of presynaptic boutons to postsynaptic motor neuron axotomy. Exp Neurol. 1975;46:605–15. doi: 10.1016/0014-4886(75)90129-6. [DOI] [PubMed] [Google Scholar]
- 49.Sumner BE, Sutherland FI. Quantitative electron microscopy on the injured hypoglossal nucleus in the rat. J Neurocytol. 1973;2:315–28. doi: 10.1007/BF01104033. [DOI] [PubMed] [Google Scholar]
- 50.Chen DH, Chambers WW, Liu CN. Synaptic displacement in intracentral neurons of Clarke’s nucleus following axotomy in the cat. Exp Neurol. 1977;57:1026–41. doi: 10.1016/0014-4886(77)90125-x. [DOI] [PubMed] [Google Scholar]
- 51.de la Cruz RR, Pastor AM, Delgado-Garcia JM. Effects of target depletion on adult mammalian central neurons: morphological correlates. Neuroscience. 1994;58:59–79. doi: 10.1016/0306-4522(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 52.Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975;252:429–63. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuno M, Llinas R. Alterations of synaptic action in chromatolysed motoneurones of the cat. J Physiol. 1970;210:823–38. doi: 10.1113/jphysiol.1970.sp009244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Titmus MJ, Faber DS. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog Neurobiol. 1990;35:1–51. doi: 10.1016/0301-0082(90)90039-j. [DOI] [PubMed] [Google Scholar]
- 55.Brannstrom T, Kellerth JO. Changes in synaptology of adult cat spinal alpha-motoneurons after axotomy. Exp Brain Res. 1998;118:1–13. doi: 10.1007/s002210050249. [DOI] [PubMed] [Google Scholar]
- 56.Brannstrom T, Kellerth JO. Recovery of synapses in axotomized adult cat spinal motoneurons after reinnervation into muscle. Exp Brain Res. 1999;125:19–27. doi: 10.1007/s002210050653. [DOI] [PubMed] [Google Scholar]
- 57.Cull RE. Role of nerve-muscle contact in maintaining synaptic connections. Exp Brain Res. 1974;20:307–10. doi: 10.1007/BF00238321. [DOI] [PubMed] [Google Scholar]
- 58.Sumner BE. Quantitative ultrastructural observations on the inhibited recovery of the hypoglossal nucleus from the axotomy response when regeneration of the hypoglossal nerve is prevented. Exp Brain Res. 1976;26:141–50. doi: 10.1007/BF00238278. [DOI] [PubMed] [Google Scholar]
- 59.Linda H, Cullheim S, Risling M. A light and electron microscopic study of intracellularly HRP-labeled lumbar motoneurons after intramedullary axotomy in the adult cat. J Comp Neurol. 1992;318:188–208. doi: 10.1002/cne.903180205. [DOI] [PubMed] [Google Scholar]
- 60.Linda H, et al. Ultrastructural evidence for a preferential elimination of glutamate-immunoreactive synaptic terminals from spinal motoneurons after intramedullary axotomy. J Comp Neurol. 2000;425:10–23. [PubMed] [Google Scholar]
- 61.Novikov LN, et al. Exogenous brain-derived neurotrophic factor regulates the synaptic composition of axonally lesioned and normal adult rat motoneurons. Neuroscience. 2000;100:171–81. doi: 10.1016/s0306-4522(00)00256-6. [DOI] [PubMed] [Google Scholar]
- 62.Guntinas-Lichius O, et al. Differences in glial, synaptic and motoneuron responses in the facial nucleus of the rat brainstem following facial nerve resection and nerve suture reanastomosis. Eur Arch Otorhinolaryngol. 1994;251:410–7. doi: 10.1007/BF00181967. [DOI] [PubMed] [Google Scholar]
- 63.Davis-Lopez de Carrizosa MA, et al. Complementary actions of BDNF and neurotrophin-3 on the firing patterns and synaptic composition of motoneurons. J Neurosci. 2009;29:575–87. doi: 10.1523/JNEUROSCI.5312-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mendell LM, Johnson RD, Munson JB. Neurotrophin modulation of the monosynaptic reflex after peripheral nerve transection. J Neurosci. 1999;19:3162–70. doi: 10.1523/JNEUROSCI.19-08-03162.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burke RE, Glenn LL. Horseradish peroxidase study of the spatial and electrotonic distribution of group Ia synapses on type-identified ankle extensor motoneurons in the cat. J Comp Neurol. 1996;372:465–85. doi: 10.1002/(SICI)1096-9861(19960826)372:3<465::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 66.Fyffe REW. Spinal motoneurons: synaptic inputs and receptor organization. In: Cope TC, editor. Motor neurobiology of the spinal cord. Boca Raton, FL: 2001. pp. 21–46. [Google Scholar]
- 67.Alvarez FJ, et al. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–80. doi: 10.1002/cne.20012. [DOI] [PubMed] [Google Scholar]
- 68.Todd AJ, et al. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- 69.Alvarez FJ, et al. VGLUT1 content in central synapses of normal and regenerated Ia afferents. Society for Neuroscience. 2008;74:1. [Google Scholar]
- 70.Hughes DI, et al. Peripheral axotomy induces depletion of the vesicular glutamate transporter VGLUT1 in central terminals of myelinated afferent fibres in the rat spinal cord. Brain Res. 2004;1017:69–76. doi: 10.1016/j.brainres.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 71.Li LY, et al. Neurotrophin-3 ameliorates sensory-motor deficits in Er81-deficient mice. Dev Dyn. 2006;235:3039–50. doi: 10.1002/dvdy.20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z, et al. Prenatal exposure to elevated NT3 disrupts synaptic selectivity in the spinal cord. J Neurosci. 2007;27:3686–94. doi: 10.1523/JNEUROSCI.0197-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harvey PA, et al. Blockade of Nogo receptor ligands promotes functional regeneration of sensory axons after dorsal root crush. J Neurosci. 2009;29:6285–95. doi: 10.1523/JNEUROSCI.5885-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]