Is ecstasy an ‘empathogen’? Effects of MDMA on prosocial feelings and identification of emotional states in others (original) (raw)
. Author manuscript; available in PMC: 2011 Dec 15.
Abstract
Background
MDMA (±3,4-methylenedioxymethamphetamine, ‘ecstasy’) users report that the drug produces unusual psychological effects, including increased empathy and prosocial feelings. These ‘empathogenic’ effects are cited as reasons for recreational ecstasy use, and also form the basis for the proposed use of MDMA in psychotherapy. However, they have yet to be characterized in controlled studies. Here, we investigate effects of MDMA on an important social cognitive capacity, the identification of emotional expression in others, and on socially-relevant mood states.
Methods
Over four sessions, healthy ecstasy-using volunteers (_N_=21) received MDMA (0.75mg/kg; 1.5mg/kg), methamphetamine (20mg; METH), and placebo (PBO) under double-blind, randomized conditions. They completed self-report ratings of relevant affective states, and undertook tasks in which they identified emotions from images of faces, pictures of eyes, and vocal cues.
Results
MDMA (1.5mg/kg) significantly increased ratings of feeling ‘loving’ and ‘friendly’, and MDMA (0.75mg/kg) increased ‘loneliness’. Both MDMA (1.5mg/kg) and METH increased ‘playfulness’; only METH increased ‘sociability’. MDMA (1.5mg/kg) robustly decreased accuracy of facial fear recognition relative to PBO.
Conclusions
MDMA increased ‘empathogenic’ feelings, but it reduced accurate identification of threat-related facial emotional signals in others, findings consistent with increased social approach behavior, rather than empathy. This effect of MDMA on social cognition has implications for both recreational and therapeutic use. In recreational users, acute drug effects may alter social risk-taking while intoxicated. Socioemotional processing alterations such as those documented here may underlie possible psychotherapeutic benefits of this drug; further investigation of such mechanisms may inform treatment design to maximize active components of MDMA-assisted psychotherapy.
Keywords: ecstasy, MDMA, methamphetamine, social cognition, emotion identification, empathy
INTRODUCTION
MDMA (±3,4-methylenedioxymethamphetamine, ‘ecstasy’) is reported to have unusual, so-called ‘empathogenic’ effects, such as increased empathy and prosocial feelings [e.g. see 1]. Such effects are cited as a motivation to use ecstasy recreationally [2–3] and may contribute to its reinforcing capacity [4]. MDMA’s apparent empathogenic effects are also central to the rationale for the use of the drug as a psychotherapeutic adjunct [1, 5–6]. Thus, the social behavioral profile of MDMA is relevant to understanding both recreational use and possible therapeutic effects of this drug.
To date, only a small number of controlled laboratory studies have assessed relevant subjective experiences after MDMA. These studies indicate that MDMA increases feelings related to empathy and sociability, including self-rated friendliness [7–8], extroversion [9–10], closeness to others [11], sociability [7, 12], talkativeness [7–8], amicability, and gregariousness [13], although some of these effects are inconsistent [14–16]. Despite these reports on subjective effects of the drug, there have been no published reports in humans of the effect of controlled administration of MDMA on behaviors related to empathy and sociability. Using fMRI, we recently reported effects of MDMA on neural processing of social material [12], but in that study the drug did not alter the behavioral response to the stimuli (i.e. accuracy of identification of emotions from pictures of facial effect). Identification of others’ emotions based on facial, vocal or postural cues is a critical social cognitive capacity and an important ‘first step’ in empathy [17], and thus may be an information processing domain that is useful for studying a purportedly empathogenic drug. The altered brain response in our imaging study suggested that emotion recognition may play a role in the drug’s effects, but the methods we used were not optimal to detect a behavioral effect. The emotional expressions shown were not subtle [18], they were presented for a long period (4 seconds) [17], and we selected a relatively narrow range of basic emotions (e.g. fear, anger and happiness [19]), excluding more complex, culturally-determined affective states, such as shame and jealousy [20].
The present study was designed to further investigate the behavioral effects of MDMA on social cognition using emotional identification paradigms. We employed more sensitive methods than in our imaging study [12] to investigate the effect of MDMA on identification of basic emotions from facial cues. Other behavioral measures included a test of recognition of complex emotions based on cues from the eye region [21], and a test of emotion identification from vocal cues [22]. We also assessed a range of relevant subjective effects of MDMA, and included a comparison drug, methamphetamine, to determine the specificity of these effects to MDMA.
We hypothesized that, in addition to increasing feelings related to sociability and empathy, MDMA would alter emotion recognition based on pictures of faces, pictures of eyes, and voices. Notably, either increases or decreases in emotional identification could result in apparent empathogenic effects. Improved identification of emotions would be consistent with increased empathy [17], whereas decreased sensitivity to threat-related emotions such as fear and anger, may reduce social avoidance [19] and hence increase social approach behavior (sociability). We further hypothesized that effects of MDMA on social cognition and relevant affective states would be specific to MDMA, rather than generalizing to a prototypic psychostimulant, methamphetamine.
METHODS AND MATERIALS
Participants
Healthy volunteers, aged 18–38, who reported using MDMA or ecstasy on at least two occasions were recruited with internet advertisements and word-of-mouth. Candidates underwent extensive screening and were excluded based on: psychiatric disorder [DSM-IV Axis 1 diagnosis including substance dependence; 23]; signs of medical or neurological illness assessed with medical examination, electrocardiogram and structured clinical interview; Body Mass Index outside healthy range (18.5–30); cardiovascular illness in first-degree relative; prior adverse response to ecstasy; and pregnancy or lactation. All participants provided written informed consent after receiving protocol descriptions, and were fully debriefed at study completion as approved by the University of Chicago Institutional Review Board.
Experimental Protocol
A 4-session, within-participants, double-blind design was employed. At each session participants received a capsule containing: MDMA (0.75mg/kg [MDMA0.75] or 1.5 mg/kg [MDMA1.5]), methamphetamine (20 mg [METH]) or placebo [PBO]; capsules were administered in randomized order. Sessions were scheduled at least 5 days apart to allow for drug elimination. Participants were asked not to eat for 2 hours before sessions. They were required to abstain from cannabis for 7 days, alcohol and medications for 24 hours, and all other recreational drugs (e.g. ecstasy) for 48 hours prior to sessions. Compliance was verified with urine (QuickTox Drug Screen Dipcard, Branan Medical Corporation, Irvine, CA), saliva (Oratect III, Branan Medical Corporation) and breathalyzer (Alco-sensor III, Intoximeters, St. Louis, MO) screens. Female participants were required to test negative for pregnancy at each session (Aimstrip, Craig Medical, Vista, CA).
Drug doses were selected based on previous research; MDMA doses in this low-to-moderate range have been safely administered to humans previously, and produce modest to robust alterations in mood state [24]. We have previously shown MDMA (1.5mg/kg) to increase sociability [12]; it is also within recreational dose ranges [25]. Three prior studies have employed d-amphetamine as a reference compound for MDMA [7, 15, 26]. Tancer et al. (2003) used d-amphetamine 10mg and 20mg, and reported overlapping reinforcing and subjective effects of MDMA (2mg/kg) and 20mg amphetamine, although in many cases MDMA (2mg) produced higher ratings. Johanson et al. (2006) employed 20mg d-amphetamine in a discriminative stimulus procedure and reported that 50% of participants discriminated MDMA (1mg/kg;1.5mg/kg) as d-amphetamine (20mg). Cami et al. (2000) used a higher dose of amphetamine (40 mg), but only tested subjects with histories of amphetamine use. For the current study, we selected a methamphetamine dose (20mg) that is safe, and induces robust stimulant and euphoric effects, in amphetamine-naive volunteers [27].
Drug sessions commenced at noon. After baseline measures (cardiovascular and subjective state; see below), participants ingested an opaque gelatin capsule (size 00) containing MDMA or methamphetamine with lactose or dextrose, or placebo (filler only). For at least 4.5 hours after capsule ingestion, participants remained in a comfortable laboratory environment undergoing regular cardiovascular checks and subjective state questionnaires. Participants were alone in testing rooms; their only social contact was with a research assistant, who was instructed not to interact with participants outside of the requirements of the protocol. Participants did not have access to telephones or the internet. They remained in the laboratory until they no longer showed noticeable drug effects. Participants started cognitive tasks (e.g. emotional recognition) 65 minutes after capsule administration, to coincide with peak drug effect onset [26].
Assessment Measures
Subjective measures included Visual Analogue Scales [VAS; 28] and the Profile of Mood States [POMS; 29]. The VAS comprised the following adjectives: stimulated, bored, sedated, anxious, insightful, nauseated, loving, dizzy, sociable, confused, lonely, elated, playful, blank, and restless. The POMS is a 72-item adjective checklist rated on a 5-point Likert scale from ‘not at all’ (0) to ‘extremely’ (4). The POMS yields 8 subscores, including a friendliness scale. The VAS was administered at 0, 30, 60, 90, 120, 150, 210 and 240 minutes post capsule. The POMS was administered at 0, 90 and 240 minutes post capsule, with the middle time point designed to occur during peak drug effects [26]. Based on the study hypotheses, subjective outcome measures were: 1) VAS Sociable, Playful, Loving, and Lonely; and 2) POMS Friendliness. VAS outcome measures were single items. The POMS Friendliness subscale is the mean of scores for the following items: ‘friendly’, ’agreeable’, ‘helpful’, ‘forgiving’, ‘good-natured’, ‘warm-hearted’, ‘good-tempered’, and ‘kindly’.
Cognitive measures included two facial affect identification tasks and a vocal affect task. Each cognitive task was completed once in each session, during the period of anticipated peak drug effects [26]. The Facial Emotional Recognition task (FER) is sensitive to serotonin and norepinephrine reuptake inhibition [30]. Stimuli consist of facial pictures from the Ekman and Friesen series [31]. The version of the task employed includes four basic emotions (anger, fear, happiness and sadness), with pictures morphed between neutral (0%) and prototype emotion (100%) in 10% increments [32]. For each emotion, four different actors (two of each sex) were employed, resulting in 40 stimuli for each of four emotions (10 incrementally morphed pictures per actor per emotion). In addition, 10 neutral stimuli were added, for a total of 170 pictures. Faces were presented in randomized order for 500msecs and replaced by a rating screen. Participants rated each face by selecting the emotion depicted, from the four emotions and neutral. The main outcome measure was accuracy (proportion correct).
Identification of complex emotions used the Reading the Mind in the Eyes - Revised test (Eyes test) [21]. This task consists of 36 images of the eye region of males and females presented in randomized order. Each pair of eyes is taken from a face expressing a complex emotional state, such as ‘reflective’ or ‘ashamed’. Participants choose between four options to describe the emotion depicted; after selection, the next image is presented. This task employs only the eye region because this area carries subtle yet critical information about emotional state [21]. The Eyes test is sensitive to oxytocin administration [33]. The outcome measure was accuracy.
The vocal affect recognition task was the Diagnostic Analysis of Nonverbal Accuracy [DANVA-2; 22] Adult Paralanguage test. This task comprises 24 audio clips of professional actors saying a single neutral phrase (“I’m going out of the room now and I’ll be back later”) in a happy, sad, angry, or fearful tone. Within each emotion category, there are three high emotional intensity and three low emotional intensity items. After hearing each sentence, participants choose which emotion was expressed from the four emotions above. The outcome measure was accuracy.
Statistical Analyses
For subjective outcomes, repeated measures Analyses of Variance (ANOVA) were used to assess the effects of drug on peak change from baseline scores. Significant omnibus F tests were followed with post-hoc pairwise comparisons with full Bonferroni adjustment of the significance threshold. For the FER and DANVA-2 scores, two-way repeated measures ANOVAs assessed for main effects of drug and interactions between drug and emotion type. In the case that a significant interaction was identified, simple main effects analysis assessed for effects of drug on identification of each emotion. The significance threshold was set at 0.05 for the omnibus ANOVA, with Bonferroni-adjusted thresholds employed for each of the simple main effects analyses (p = 0.05/number of emotions). Significant simple main effects were followed with post-hoc pairwise comparisons with full Bonferroni adjustment for the total number of analyses undertaken (i.e. 0.05 corrected for simple main effects analyses as well as pairwise comparisons). An ANOVA assessed the effect of drug on total Eyes score. Alpha was set at p < .05 (adjusted as described above). In the case that Mauchley’s test of sphericity indicated significant departure from sphericity (p < .05), Greenhouse-Geisser corrected degrees of freedom and significance levels were interpreted. Where the assumption of sphericity was met, we conservatively interpreted Huynh-Feldt corrected significance levels [34]. Session order did not have a significant effect in any of the analyses, and was therefore not retained in the models. We assessed for normality of the data and univariate outliers prior to analysis. Data for four outcome measures were non-normal (VAS Loving, VAS Lonely, POMS Friendly, and DANVA scores). In these cases, we conducted non-parametric analysis (Friedman’s ANOVA followed by Wilcoxon signed rank tests). In all cases except VAS Lonely, the non-parametric approach yielded the same results; therefore parametric statistics are reported. For VAS Lonely, non-parametric statistics yielded slight alterations to the outcome; therefore non-parametric statistics are reported. Truncation of two outlying data points (both in DANVA scores) did not alter results and original data were retained. Effect sizes are presented as η2 for parametric analyses and r values for non-parametric analysis. All analyses were conducted using SPSS 17.0.
RESULTS
Participants’ mean age was 24.4 years (S.D.= 4.9) and 9 of the 21 were female. Seventeen participants identified as Caucasian, two were Asian, one was African-American, and one was of mixed race. Participants reported first use of ecstasy at a mean age of 19.8 (S.D. = 2.7) years and lifetime ecstasy use on a mean of 15.0 (S.D. = 23.1) occasions; 13 participants had used the drug less than 10 times. In the month prior to participation, 12 reported smoking cigarettes at least weekly, all had consumed alcohol, and 16 had consumed cannabis. All participants had used cannabis at some time in their lives, all but two reported lifetime use of stimulants such as cocaine, and all but one had used hallucinogens.
The drugs increased ratings on all five subjective state measures. MDMA1.5 significantly increased loving and friendliness ratings, whereas MDMA0.75 increased loneliness. Both MDMA1.5 and METH increased playfulness, whereas only METH significantly increased sociability.
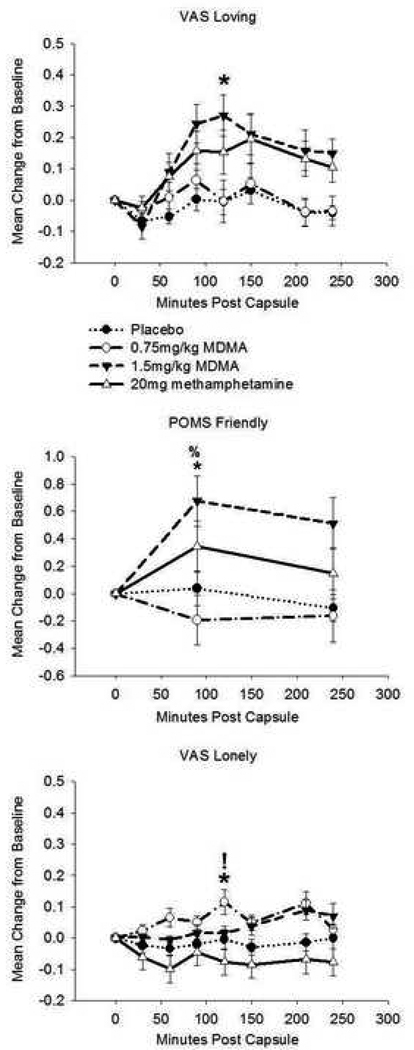
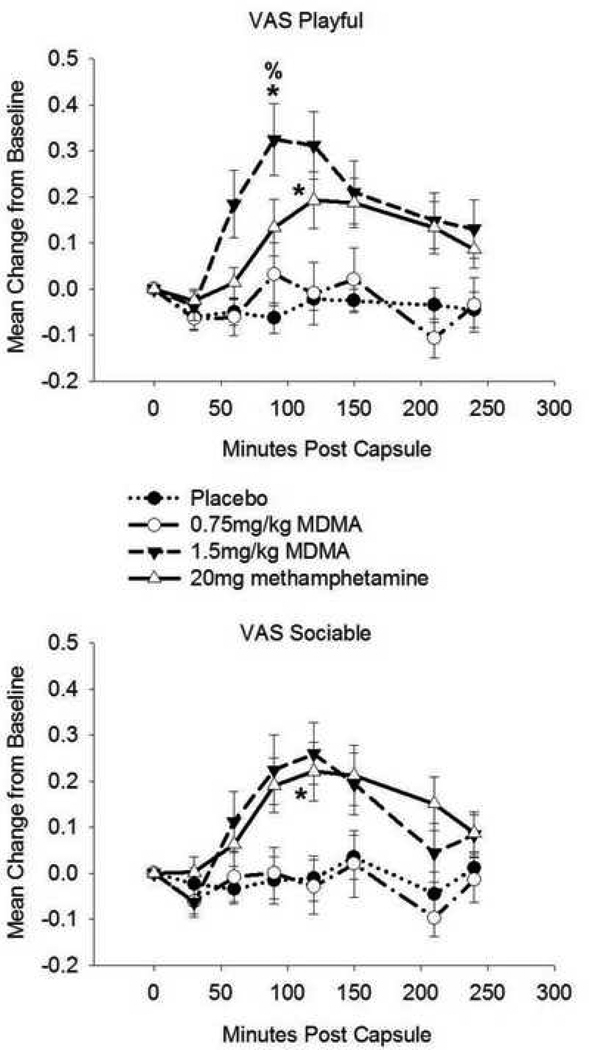
Drug condition affected ratings on the VAS Loving scale (F(3,57) = 5.04, p = 0.004; η2 = 0.21). Follow-up analyses indicated that MDMA1.5 increased loving feelings relative to PBO. There was an effect of drug on POMS Friendliness (F(2.7,51) = 6.81, p = 0.001; η2 = 0.26). MDMA1.5 increased friendliness ratings compared to both PBO and MDMA0.75. Drug condition affected ratings on VAS Loneliness (χ2(3) = 11.94, p = 0.008; r = 0.54). Post-hoc analysis showed that MDMA0.75 increased loneliness ratings relative to METH and PBO (see Figure 1). Drug condition affected VAS Playfulness (F(3,57) = 8.57, p < 0.001, η2 = 0.31), with MDMA1.5 increasing playfulness relative to PBO and MDMA0.75 and METH increasing playfulness compared to PBO. There was a drug effect on VAS Sociability (F(2.5, 47.7) = 3.06, p < 0.05, η2 = 0.14). METH increased sociability relative to PBO (see Figure 2).
Figure 1.
Drug effects on self-reported loving, friendly, and lonely feelings. Top: Visual Analogue Scale (VAS) Loving. Middle: Profile of Mood states (POMS) Friendly. Bottom: VAS Lonely. Data are mean change from pre-drug baseline (± S.E.M.) as a function of minutes post-capsule. Asterisk denotes difference (peak change from baseline) from placebo (p< 0.05, with Bonferroni correction); % denotes difference from MDMA (0.75mg/kg; p< 0.05, with Bonferroni correction); ! denotes difference from methamphetamine (20mg; p< 0.05, with Bonferroni correction). N = 20 due to missing data.
Figure 2.
Drug effects on self-reported playfulness and sociability. Top: Visual Analogue Scale (VAS) Playful. Bottom: VAS Sociable. Data are mean change from pre-drug baseline (± S.E.M.) as a function of minutes post-capsule. Asterisk denotes difference (peak change from baseline) from placebo (p< 0.05, with Bonferroni correction); % denotes difference from MDMA (0.75mg/kg; p< 0.05, with Bonferroni correction). N = 20 due to missing data.
There was a significant interaction between drug condition and emotion on the FER accuracy of emotion identification (F(5.2, 104.6) = 3.23, p = 0.008). After Bonferroni correction of the Type 1 error rate, simple main effects analysis did not yield significant effects of drug on identification of angry, happy, neutral, or sad faces. However, drug condition affected identification of fearful facial expressions (F(2.8, 56.0) = 4.45, p = 0.008, η2 = 0.18), indicating that MDMA1.5 decreased accuracy of fear recognition relative to placebo (Table 1).
Table 1.
Emotional Identification
| PBO | MDMA0.75 | MDMA1.5 | METH | Overall | 1 vs 2 | 1 vs 3 | 1 vs 4 | 2 vs 3 | 2 vs 4 | 3 vs 4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Mean | Mean | Mean | F | t | t | t | t | t | t | |
| (S.D.) | (S.D.) | (S.D.) | (S.D.) | (df) | (df) | (df) | (df) | (df) | (df) | (df) | |
| FER | 0.74 | 0.71 | 0.71 | 0.73 | 0.96 | - | - | - | - | - | - |
| Happiness a | (0.07) | (0.09) | (0.11) | (0.10) | (3,60) | ||||||
| FER | 0.51 | 0.54 | 0.47 | 0.52 | 3.60 | - | - | - | - | - | - |
| Sadnessa | (0.14) | (0.16) | (0.14) | (0.16) | (3,60) | ||||||
| FER | 0.63 | 0.59 | 0.55 | 0.59 | 4.45* | 2.27 | 3.94* | 2.59 | 1.41 | 0.12 | 1.31 |
| Feara | (0.07) | (0.12) | (0.10) | (0.10) | (3,60) | (20) | (20) | (20) | (20) | (20) | (20) |
| FER | 0.64 | 0.63 | 0.59 | 0.63 | 2.0 | - | - | - | - | - | - |
| Angera | (0.07) | (0.09) | (0.11) | (0.06) | (3,60) | ||||||
| FER | 0.69 | 0.68 | 0.77 | 0.67 | 2.84 | - | - | - | - | - | - |
| Neutrala | (0.17) | (0.15) | (0.12) | (0.20) | (3,60) | ||||||
| FER Neutral | 44.90 | 46.52 | 51.71 | 46.48 | 3.25% | 0.79 | 2.52% | 0.84 | 2.02 | 0.02 | 1.95 |
| Errorsb | (11.31) | (12.79) | (10.58) | (12.14) | (3,60) | (20) | (20) | (20) | (20) | (20) | (20) |
| Eyes Total | 26.81 | 26.67 | 26.10 | 27.24 | 0.58 | - | - | - | - | - | - |
| (5.14) | (3.73) | (2.70) | (3.08) | (3, 60) | |||||||
| DANVA2 | 18.05 | 18.00 | 17.20 | 18.70 | 3.01 | - | - | - | - | - | - |
| Total c | (1.70) | (1.97) | (2.19) | (2.11) | (3, 57) |
MDMA did not affect accuracy on the Eyes or the DANVA-2 tests (Table 1). There was no interaction between drug condition and emotion type on DANVA-2 scores.
To explore possible mechanisms of the effect of MDMA1.5 on facial fear recognition, we conducted exploratory analysis on incorrectly identified emotional expressions. MDMA1.5 increased the tendency to misclassify emotional expressions as neutral, compared to PBO (p < 0.05, uncorrected; Table 1). We also attempted to determine whether subjects’ apparent expectancies of receiving MDMA affected their responses during sessions. Data were reanalysed according to the subjects’ classification of what drug they thought they had received in each session. There was no significant relationship between drug identification and any of the measures that were sensitive to drug effects.
DISCUSSION
We found that MDMA (1.5mg/kg only) altered a behavioral indicator of social cognition. Specifically, it robustly reduced recognition of fearful faces, without changing recognition of other emotions from facial or vocal cues. Although previous studies have confirmed that the drug induces subjective feelings related to sociability and empathy, this is the first published demonstration of an overt behavioral effect of MDMA in humans.
The pattern of findings in our study may be more consistent with increased social approach behavior (i.e. sociability) than increased empathy. Anecdotal reports indicate that ecstasy increases interpersonal connection [3], which may suggest increased sensitivity to emotions in others. In the present study, MDMA produced self-reports of loving feelings and friendliness, but it decreased participants’ accuracy in identifying fear in others. A decreased ability to identify negative emotions, particularly threat-related signals such as fear, may facilitate social approach behavior [12, 35]. Thus, MDMA may facilitate social interactions because it reduces the impact of others’ negative emotions, rather than by enhancing recognition of, and sensitivity to, others’ emotions.
Unexpectedly, some subjective effects measured were increased by methamphetamine as well as MDMA. Indeed, only methamphetamine significantly increased self-rated sociability. Moreover, whereas MDMA (1.5mg/kg) significantly increased loving feelings and friendliness compared to placebo, these ratings were not significantly different from ratings on methamphetamine. These similarities were unexpected based on the different pharmacological profile and anecdotal reports of the effects of amphetamine and MDMA [36–37]. It is possible that the expectation of receiving MDMA influenced subjects’ reports of empathogenic feelings. Although expectancies were minimized using double-blind conditions, and a range of possible drugs to be administered, it was difficult to completely control expectancies. Indeed, 6 participants of 21 guessed that they had consumed ecstasy when they had received methamphetamine. However, follow-up analyses revealed that participants’ guesses about the drug received were not significantly related to outcome measures, suggesting that expectancies play a minor role in these results. A more likely alternative is that methamphetamine may have a more prosocial profile than previously thought. Psychostimulants have been shown to enhance the rewarding value of social interactions in rodents [38] and humans [39], and methamphetamine is associated with social, and particularly sexual, enhancement in some subcultures [40]. Thus, the prosocial effects of MDMA may be less selective than believed.
The effect of MDMA on social cognition was limited to identification of emotions from facial expressions, with no apparent effect on recognition of affective cues from the eye region or from voices. Thus, it appears that performance alterations arising from MDMA are specific to processing of whole-face visual affective cues. However, whereas the facial task required participants to identify emotions based on subtle, briefly presented pictures, there were no time limits on responding to items in the Eyes task. Moreover, whereas the vocal stimuli presented contained two levels of emotion intensity (high versus low), the facial task included 10 levels (from neutral to 100% intensity, in 10% increments). Thus, it may be that MDMA affects identification of subtle emotional cues, rather than having modality-specific effects. Future research requiring affect recognition from subtle, briefly presented vocal and eye-region stimuli could clarify this issue.
Some limitations should be noted. First, as noted above, the behavioral tasks we used may not have been sensitive to all of the unusual social effects attributed to MDMA. For example, the static photos of facial affect we employed may be less ecologically valid than dynamic stimuli [e.g. see 41]. At the time of study initiation, no suitable dynamic stimuli were available. Second, we are not aware of tasks that include both social and non-social stimuli, making it difficult to determine the specificity of these findings to social stimuli. A third possible limitation was that we adjusted the dose to the body weight for MDMA but not for methamphetamine. This is unlikely to be a major factor because participants were within a narrow weight range (BMI 18.5 – 30), and post-hoc analysis indicated that only one measure of methamphetamine’s effects (playfulness) correlated with body weight (r = − 0.45, p = 0.04). A final limitation is that, for ethical reasons, all participants had prior experience with ecstasy or MDMA, so the findings may not generalize to MDMA-naive individuals [see 42]. However, we intentionally recruited candidates with limited previous exposure to minimize possible effects of prior use.
There are a number of directions for future enquiry. In rodents, MDMA increases prosocial behavior [36, 43] and this effect is attenuated by an oxytocin receptor antagonist [2]. Oxytocin is a neuropeptide known to be a critical modulator of social behavior in mammals [44–45]. In humans, MDMA increases plasma oxytocin levels [13, 46], and plasma oxytocin levels also vary positively with enhanced subjective sociability after MDMA [13]. This suggests that a fruitful area of future research will be to examine the role of oxytocin in the social cognitive, affective and behavioral effects of MDMA in humans. Second, there is a need for studies of the cognitive mechanisms underlying alterations to social cognition. For example, we found that MDMA (1.5mg/kg) increased the number of non-neutral faces erroneously identified as neutral, apparently reducing the capacity to detect subtle emotional signals. This phenomenon, which may underlie the reduced fear identification demonstrated in this study, requires further investigation. Finally, it is possible that certain social predispositions, such as high or low levels of trait sociability, affect the degree to which MDMA alters social processing in humans, potentially contributing to individual preferences for MDMA.
The present findings have implications for both recreational and therapeutic MDMA use. Recreational ecstasy users may benefit from knowledge that ecstasy, while increasing feelings of interpersonal connection, can subtly impair interpersonal competence. For instance, compromised social cognition may increase social risk-taking while under the influence of the drug. In addition, considering that social expectancies predict use of other drugs such as alcohol [47], modifying users’ expectations about positive social effects of MDMA may alter ecstasy-use behavior [see 48]. Regarding therapeutic use of MDMA, ongoing research on MDMA-assisted therapy should investigate possible mechanisms of any therapeutic effects [1, 6]. A recent placebo-controlled pilot study has indicated that MDMA-assisted psychotherapy may be useful in reducing symptoms in patients with treatment-resistant post-traumatic stress disorder (PTSD). Although mechanisms of this apparent effect remain unclear, reductions in the intensity of fear perception, including but not limited to those reported here, might facilitate engagement with traumatic material during therapy [49]. Moreover, reduced sensitivity to subtle signs of negative emotions in others (e.g., the therapist) may enable a patient to express thoughts or feelings that were previously inhibited because of perceived negative responses in the listener. In addition to further controlled research to investigate the effectiveness of MDMA in psychotherapy, an understanding of the socioemotional cognitive mechanisms underlying such effects will help clinical researchers design treatments that optimize the drug’s therapeutic potential.
Acknowledgements
Thanks to Kate Cederbaum for technical assistance, to Royce Lee for medical support, and Emmanuel Semmes for pharmaceutical support. Thanks to Lisa Jerome for comments on an earlier version of this manuscript. Thanks also to participants. This research was supported by NIDA (DA02812).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parrott A. The psychotherapeutic potential of MDMA (3,4- methylenedioxymethamphetamine): An evidence-based review. Psychopharmacology. 2007;191:181–193. doi: 10.1007/s00213-007-0703-5. [DOI] [PubMed] [Google Scholar]
- 2.Thompson MR, Callaghan PD, Hunt GE, Cornish JL, McGreor IS. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine ("ecstasy") Neuroscience. 2007;146:509–514. doi: 10.1016/j.neuroscience.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 3.Sumnall HR, Cole JC, Jerome L. The varieties of ecstasy experience: An exploration of the subjective experiences of ecstasy. J Psychopharmacol. 2006;20:670–682. doi: 10.1177/0269881106060764. [DOI] [PubMed] [Google Scholar]
- 4.McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: A role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol. 2008;154:358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holland J. Ecstasy: The complete guide: A comprehensive look at the risks and benefits of MDMA. Rochester, VT, US: Park Street Press; 2001. [Google Scholar]
- 6.Johansen PO, Krebs TS. How could ecstasy help anxiety disorders? A neurobiological rationale. J Psychopharmacol. 2009;23:389–391. doi: 10.1177/0269881109102787. [DOI] [PubMed] [Google Scholar]
- 7.Tancer ME, Johanson CE. Reinforcing, subjective, and physiological effects of MDMA in humans: A comparison with d-amphetamine and mCPP. Drug Alcohol Depend. 2003;72:33–44. doi: 10.1016/s0376-8716(03)00172-8. [DOI] [PubMed] [Google Scholar]
- 8.Tancer ME, Johanson CE. The effects of fluoxetine on the subjective and physiological effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2007;189:565–573. doi: 10.1007/s00213-006-0576-z. [DOI] [PubMed] [Google Scholar]
- 9.Vollenweider FX, Remensberger S, Hell D, Geyer MA. Opposite effects of 3,4- methylenedioxymethamphetamine (MDMA) on sensorimotor gating in rats versus healthy humans. Psychopharmacology. 1999;143:365–372. doi: 10.1007/s002130050960. [DOI] [PubMed] [Google Scholar]
- 10.Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX. Psychological and physiological effects of MDMA ("Ecstasy") after pretreatment with the 5-HT-sub-2 antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000;23:396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- 11.Kolbrich EA, Goodwin RS, Gorelick DA, Hayes RJ, Stein EA, Huestis MA. Physiological and subjective responses to controlled oral 3,4- methylenedioxymethamphetamine administration. J Clin Psychopharmacol. 2008;28:432–440. doi: 10.1097/JCP.0b013e31817ef470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedi G, Phan KL, Angstadt M, de Wit H. Effects of MDMA on sociability and neural response to social threat and social reward. Psychopharmacology. 2009;207:73–83. doi: 10.1007/s00213-009-1635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumont GJH, Sweep FCJG, van der Steen R, Hermsen R, Donders ART, Touw DJ, van Gerven JMA, Buitelaar JK, Verkes RJ. Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc Neurosci. 2009;4:359–366. doi: 10.1080/17470910802649470. [DOI] [PubMed] [Google Scholar]
- 14.Liechti ME, Baumann C, Gamma A, Vollenweider FX. Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, "Ecstasy") are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology. 2000;22:513–521. doi: 10.1016/S0893-133X(99)00148-7. [DOI] [PubMed] [Google Scholar]
- 15.Johanson CE, Kilbey M, Gatchalian K, Tancer M. Discriminative stimulus effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans trained to discriminate among d-amphetamine, meta-chlorophenylpiperazine and placebo. Drug Alcohol Depend. 2006;81:27–36. doi: 10.1016/j.drugalcdep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Harris DS, Baggott M, Mendelson JH, Mendelson JE, Jones RT. Subjective and hormonal effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2002;162:396–405. doi: 10.1007/s00213-002-1131-1. [DOI] [PubMed] [Google Scholar]
- 17.Clark TF, Winkielman P, McIntosh DN. Autism and the extraction of emotion from briefly presented facial expressions: Stumbling at the first step of empathy. Emotion. 2008;8:803–809. doi: 10.1037/a0014124. [DOI] [PubMed] [Google Scholar]
- 18.Philippot P, Kornreich C, Blairy S. Nonverbal deficits and interpersonal regulation in alcoholics. In: Coates EK, Feldman RS, Philippot P, editors. Nonverbal Behavior in Clinical Setting. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 19.Ekman P. Emotions Revealed: Recognizing Faces and Feelings to Improve Communication and Emotional Life. New York, NY: Times Books/Henry Holt and Co; 2003. [Google Scholar]
- 20.Golan O, Baron-Cohen A, Hill J. The Cambridge Mindreading (CAM) face-voice battery: Testing complex emotion recognition in adults with and without Asperger Syndrome. J Autism Dev Disorders. 2006;36:169–183. doi: 10.1007/s10803-005-0057-y. [DOI] [PubMed] [Google Scholar]
- 21.Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The "Reading the Mind in the Eyes" Test Revised version: A study with normal adults, and adults with Asperger Syndrome or high-functioning Autism. J Child Psychol Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- 22.Baum KM, Nowicki S., Jr Perception of emotion: Measuring decoding accuracy of adult prosodic cues of varying intensity. J Nonverbal Behav. 1998;22:89–107. [Google Scholar]
- 23.APA. DSM-IV: Diagnostic and Statistical Manual of Mental Disorders. MD, US: American Psychiatric Association; 1994. [Google Scholar]
- 24.Dumont GJH, Verkes RJ. A review of acute effects of 3,4-methylenedioxymethamphetmine in healthy volunteers. J Psychopharmacol. 2006;20:176–187. doi: 10.1177/0269881106063271. [DOI] [PubMed] [Google Scholar]
- 25.Cole JC, Bailey M, Sumnall HR, Wagstaff GF, King LA. The content of ecstasy tablets: Implications for the study of their long-term effects. Addiction. 2002;97:1531–1536. doi: 10.1046/j.1360-0443.2002.00222.x. [DOI] [PubMed] [Google Scholar]
- 26.Cami J, Farre M, Mas M, Roset PN, Poudevida S, Mas A, San L, de le Torre R. Human pharmacology of 3,4-methylenedioxymethamphetamine ("Ecstasy"): Psychomotor performance and subjective effects. J Clin Psychopharmacol. 2000;20:455–466. doi: 10.1097/00004714-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Wachtel SR, Ortengren A, de Wit H. The effects of acute haloperidol or risperidone on subjective responses to methamphetamine in healthy volunteers. Drug Alcohol Depend. 2002;68:23–33. doi: 10.1016/s0376-8716(02)00104-7. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3:479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- 29.McNair DLM, Droppleman L. Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 30.Harmer CJ, Shelley NC, Cowen PJ, Goodwin GM. Increased positive versus negative affective perception and memory in healthy volunteers following selective serotonin and norepinephrine reuptake inhibition. Am J Psychiatry. 2004;161:1256–1263. doi: 10.1176/appi.ajp.161.7.1256. [DOI] [PubMed] [Google Scholar]
- 31.Ekman P, Friesen WV. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- 32.Young AW, Rowland D, Calder AJ, Etcoff NL, Seth A, Perrett DI. Facial expression megamix: Tests of dimensional and category accounts of emotional recognition. Cognition. 1997;63:271–313. doi: 10.1016/s0010-0277(97)00003-6. [DOI] [PubMed] [Google Scholar]
- 33.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves 'mind-reading' in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Field A. Discovering Statistics Using SPSS. 3rd ed. London: Sage Publications Ltd; 2009. [Google Scholar]
- 35.Porter MA, Coltheart M, Langdon R. The neuropsychological basis of hypersociability in Williams and Down syndrome. Neuropsychologia. 2007;45:2839–2849. doi: 10.1016/j.neuropsychologia.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Morley KC, McGregor IS. (±)-3,4,-methylenedioxymethamphetamine (MDMA, 'Ecstasy') increases social interaction in rats. Eur J Pharmacol. 2000;408:41–49. doi: 10.1016/s0014-2999(00)00749-4. [DOI] [PubMed] [Google Scholar]
- 37.Clemens KJ, McGregor IS, Hunt GE, Cornish JL. MDMA, methamphetamine and their combination: Possible lessons for party drug users. Drug Alcohol Rev. 2007;26:9–15. doi: 10.1080/09595230601036945. [DOI] [PubMed] [Google Scholar]
- 38.Thiele KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: A model revealing an interaction between cocaine and social context rewards in rats. Drug Alcohol Depend. 2008;96:202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heishman SJ, Stitzer ML. Effect of d-amphetamine, secobarbital and marijuana on choice behavior: Social versus non-social options. Psychopharmacology. 1989;99:156–162. doi: 10.1007/BF00442801. [DOI] [PubMed] [Google Scholar]
- 40.Meyer JS, Grande M, Johnson K, Ali SF. Neurotoxic effects of MDMA ("ecstasy") administration to neonatal rats. Int J Dev Neurosci. 2004;22:261–271. doi: 10.1016/j.ijdevneu.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Zaki J, Bolger N, Ochsner K. It takes two: The interpersonal nature of empathic accuracy. Psychol Sci. 2008;19:399–404. doi: 10.1111/j.1467-9280.2008.02099.x. [DOI] [PubMed] [Google Scholar]
- 42.Thompson MR, Callaghan PD, Hunt GE, McGregor IS. Reduced sensitivity to MDMA-induced facilitation of social behavior in MDMA pre-exposed rats. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1013–1021. doi: 10.1016/j.pnpbp.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 43.Morley KC, Arnold JC, McGregor IS. Serotonin (1A) receptor involvement in acute 3,4-methylenedioxymethamphetamine (MDMA) facilitation of social interaction in the rat. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:648–657. doi: 10.1016/j.pnpbp.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Heinrichs M, Domes G. Neuropeptides and social behavior: Effects of oxytocin and vasopressin in humans. Prog Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- 45.Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Tr Cogn Sci. 2008;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Wolff K, Tsapakis EM, Winstock AR, Hartley D, Holt D, Forsling ML, Aitchison KJ. Vasopressin and oxytocin secretion in response to the consumption of ecstasy in a clubbing population. J Psychopharmacol. 2006;20:400–410. doi: 10.1177/0269881106061514. [DOI] [PubMed] [Google Scholar]
- 47.Darkes J, Greenbaum P, Goldman M. Alcohol expectancy mediation of biopsychosocial risk: Complex patterns of mediation. Exp Clin Psychopharmacol. 2004;12:27–38. doi: 10.1037/1064-1297.12.1.27. [DOI] [PubMed] [Google Scholar]
- 48.Lau-Barraco C, Dunn D. Evaluation of a single-session expectancy challenge intervention to reduce alcohol use among college students. Psychol Addict Behav. 2008;22:168–175. doi: 10.1037/0893-164X.22.2.168. [DOI] [PubMed] [Google Scholar]
- 49.Mithoefer M, Wagner MT, Mithoefer AT, Jerome L, Doblin R. The safety and efficacy of ± 3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistent posttraumatic stress disorder: The first randomized, controlled pilot study. J Psychopharmacol. doi: 10.1177/0269881110378371. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]