Improved Glycemic Control Without Hypoglycemia in Elderly Diabetic Patients Using the Ubiquitous Healthcare Service, a New Medical Information System (original) (raw)
Abstract
OBJECTIVE
To improve quality and efficiency of care for elderly patients with type 2 diabetes, we introduced elderly-friendly strategies to the clinical decision support system (CDSS)-based ubiquitous healthcare (u-healthcare**)** service, which is an individualized health management system using advanced medical information technology.
RESEARCH DESIGN AND METHODS
We conducted a 6-month randomized, controlled clinical trial involving 144 patients aged >60 years. Participants were randomly assigned to receive routine care (control, n = 48), to the self-monitored blood glucose (SMBG, n = 47) group, or to the u-healthcare group (n = 49). The primary end point was the proportion of patients achieving A1C <7% without hypoglycemia at 6 months. U-healthcare system refers to an individualized medical service in which medical instructions are given through the patient’s mobile phone. Patients receive a glucometer with a public switched telephone network-connected cradle that automatically transfers test results to a hospital-based server. Once the data are transferred to the server, an automated system, the CDSS rule engine, generates and sends patient-specific messages by mobile phone.
RESULTS
After 6 months of follow-up, the mean A1C level was significantly decreased from 7.8 ± 1.3% to 7.4 ± 1.0% (P < 0.001) in the u-healthcare group and from 7.9 ± 1.0% to 7.7 ± 1.0% (P = 0.020) in the SMBG group, compared with 7.9 ± 0.8% to 7.8 ± 1.0% (P = 0.274) in the control group. The proportion of patients with A1C <7% without hypoglycemia was 30.6% in the u-healthcare group, 23.4% in the SMBG group (23.4%), and 14.0% in the control group (P < 0.05).
CONCLUSIONS
The CDSS-based u-healthcare service achieved better glycemic control with less hypoglycemia than SMBG and routine care and may provide effective and safe diabetes management in the elderly diabetic patients.
According to recent data from large clinical trials, approaches to adequate glycemic control focused on less hypoglycemia and less weight gain need to be used to reduce complication or mortality rates of diabetes (1,2). For this, close and consistent monitoring of glucose levels and individual specific interventions are required; however, this type of individualized approach has been difficult to obtain before advances in technology.
Advances in information technologies have enabled medicine to overcome time and location barriers by developing a system that provides real-time individualized medical treatments that are easily accessible using Internet and wireless technology (3–6). This system, referred to as ubiquitous healthcare (u-healthcare) (also known elsewhere as telemedicine, telehealth, or connected health), has been the center of attention for its revolutionary approach. The u-healthcare system can potentially provide disease prevention, early diagnosis, and early treatment, as well as continuous follow-up that are available whenever and wherever they are needed and requested. Such personalized health care services are import to diabetic patients whose disease management is depends primarily on time and frequency. Consequently, the innovative application of information technology for u-healthcare is being multilaterally researched in hopes of managing the enormous health care costs associated with increasing diabetes prevalence.
Appropriate self-care, including a healthy lifestyle, is essential in diabetes care but is difficult to monitor. Many diabetic patients lack physical exercise and have unhealthy dietary habits that bring about poor glycemic controls (7). As a result, a new health care delivery model with the u-healthcare system has been introduced to induce effective glucose control. A glucometer with a mobile system and Zigbee communication protocol, which is a specification for a suite of high-level communication protocols using small, low-power digital radios for wireless home area networks, showed that diabetic patients could be more autonomous in controlling their glucose levels (8). This system also demonstrated that health education in conjunction with personalized health care service was effective in improving self-care in diabetes management, which would also be an effective way to reduce diabetes complications and continue a healthy life (9).
A survey from a representative sample of the U.S. population using health information or consultation through the Internet or e-mail showed that 67% of respondents were more aware of their health conditions than nonusers. About 50% of participants answered that they were influenced by this system to change their lifestyle and choose an alternative route of medical service (10).
Recently, the application of an Internet-based glucose control system showed better long-term glucose control compared with the conventional treatment (11). The combined application of the mobile device and the Web-based monitoring system also showed improvement in various metabolic indicators in obese patients with diabetes and hypertension (12). Thus, the application of u-healthcare based on advanced information technology should be helpful in diabetes management (13). However, the beneficial effect of u-healthcare has not been investigated on glucose control without hypoglycemia in elderly diabetic patients. More importantly, these studies were not based on the automated clinical decision support system (CDSS), without direct involvement of health care professionals.
In this study, we provided an individualized interactive u-healthcare service using the advanced information technology of the CDSS rule engine that enabled more effective glucose control. This medical care service was designed to prevent significant hypoglycemia and to achieve better glycemic control in an elderly population aged ≥60 years.
RESEARCH DESIGN AND METHODS
Study participants
Patients aged ≥60 years were recruited from the outpatient clinic of the Seoul National University Bundang Hospital (SNUBH) from May to June 2009. Block randomization was used to assign each patient to one of three groups: routine care (control group), self-monitored blood glucose (SMBG) group, or u-healthcare group with wired telephone-connected glucometer plus mobile phone, in which the glucometer data were technically transmitted by wired telephone through public switched telephone network (PSTN).
All enrolled participants had been diagnosed with type 2 diabetes for at least 1 year, and their A1C level was 6.5–10.5%. The study excluded patients with severe diabetes complications (e.g., diabetic foot or severe diabetic retinopathy), liver dysfunction (aspartate aminotransferase or alanine aminotransferase >2.5 times the reference level), or renal dysfunction (serum creatinine >132 μmol/L [1.7 mg/dL]), or other medical problems that could affect study results or trial participation.
The study screened 180 patients and 26 were excluded: 19 because of the exclusion criteria, and 5 refused to participate after being informed of the study protocol, which required frequent self-blood glucose monitoring or the u-healthcare service. The study finally enrolled 154 individuals, comprising 51 in u-healthcare, 51 in SMBG, and 52 in control, and 144 (93.5%) completed the study. The SNUBH Institutional Review Board approved the study, and all patients gave their written, informed consent.
Study design and intervention
We provided pertinent diabetes education, including a therapeutic lifestyle change program, to standardize every patient’s education level and practice of diabetes management. After the education, individuals in the control group did not receive an intervention and were advised to follow-up according to their current medical care. The SMBG group was advised to measure their blood glucose level at least 8 times a week (≥3 at fasting, ≥3 postprandial, and ≥2 bedtimes). The u-healthcare group was educated to use PSTN-connected glucometer to measure their blood glucose level at the same frequency as the SMBG group and to start short message service (SMS) on their mobile phone to receive messages from the CDSS rule engine server. The PSTN-connected glucometer was designed to be elderly-friendly and thus was appropriate for use in this study: the control buttons on glucometer and the screen size of liquid-crystal display (LCD) displaying the glucose results were bigger than those of other commercially available glucometers. Also included in the PSTN-connected glucometer was a step-by-step video instruction for use. An average of 2 to 3 h was required to educate each patient for the device and system use.
A specialized diabetes management team consisting of well-trained professionals, including diabetologists, nurses, dietitians, and exercise trainers, organized and directed patient education. For participants who did not fully understand device and system use even after training, more time with simpler instructions were given by the trainers until all participants were comfortable with the glucometer and service delivery system. We also allowed the participants to have a lead-in period to ensure that they fully understood the system and were able to apply it. The study enrollment excluded patients without a text message function on their cellular phone or who were unable to use text messages for any reason.
The primary end point of the study was the proportion of patients achieving an A1C level of <7% without hypoglycemia at 6 months. For the intervention groups, the method and frequency of SMBG was informed to ensure compliance of multiple home glucose testing. For those who were randomized to the u-healthcare group, additional education was provided to help patients with its usage and message interpretation. Patients were also instructed to telephone the research center if technical difficulties arose at home and were given help directly over the telephone or through a home visit by a technician. All patients visited the outpatient clinic every 3 months for an interview conducted by their physician and provided a blood sample.
Device
Blood glucose monitoring system
This study used glucometers (GlucoDr Supersensor, AGM-2200, Allmedicus, Korea) that were specifically devised for u-healthcare. Various testing conditions, such as fasting, postprandial, and bedtime, or with control solution, could be set to the meter at the measurement time. Thus, more detailed information was provided to the CDSS rule engine. In addition, the measurement system adopted flavine-adenine dinucleotide–glucose dehydrogenase enzyme technology so the results to be highly glucose-specific without being affected by the oxygen concentration in the blood.
Data transfer
After glucose levels were measured, the GlucoDr Supersensor glucometer was placed onto its own cradle, after which all of the tested data were automatically transferred through the PSTN and stored in the database of the remote data collection server. These data were evaluated by the CDSS to generate patient-specific messages. CDSS-generated messages were sent to the patient’s mobile phone within 2 minutes of data transfer.
CDSS-based u-healthcare service
The u-healthcare service used in this study was based on a CDSS (Supplementary Fig. 1). The patient’s anthropometry, blood pressure, current blood glucose and A1C levels, and current medication were simultaneously uploaded from the hospital’s electronic medical record (EMR) server to the u-healthcare server. Personal information, including diet and exercise, was also collected and stored on the server to provide appropriate individualized service (Supplementary Fig. 1_A_).
Information from the patient’s glucometer was automatically sent to the server by the PSTN, after which instructions that were appropriate and specific for each patient were generated by the CDSS rule engine. The CDSS rule engine is based on the clinical practice recommendations of the American Diabetes Association and the Korean Diabetes Association (14,15) (Supplementary Fig. 1_B_). In addition to providing messages as a response to the patient’s glucose testing, the CDSS rule engine also generated evaluation messages on each patient’s the weekly and monthly average glucose levels. These messages were sent on Mondays and Tuesdays, respectively (Supplementary Fig. 1_C_). To ensure compliance with frequent glucose testing (at least 8 times/week), evaluation messages on the total number of weekly glucose measurements were also sent on Wednesdays as a reminder.
The CDSS-generated messages were patient-specific, for example:
- If the patient’s fasting glucose level was <4 mmol/L (72 mg/dL) twice in a week, a message to change the patient’s current diabetes medications (50% reduction of sulfonylurea or 2-unit reduction of insulin) was sent to the patient.
- If the fasting glucose level was >8 mmol/L (144 mg/dL) twice in a week, a message to change the patient’s lifestyle or increase current diabetes therapy (intensification of lifestyle or 2-unit increase of insulin) was sent to the patient.
- If the glucose level fell <2.8 mmol/L (50 mg/dL), a family member whose information was given to researchers by the patient before the study initiation was also notified to ensure appropriate action to treat hypoglycemia (Supplementary Fig. 1_B_).
Patients were fully educated on the type of messages and their implications to make sure patients knew exactly what to do when they received an automated message.
Definition of hypoglycemia
Events of hypoglycemia were considered of special interest in this study. Patients were counseled regarding the symptoms of hypoglycemia (e.g., weakness, dizziness, shakiness, increased sweating, palpitations, or confusion) and requested to immediately perform a finger stick glucose measurement if any symptoms occurred that might have been related to hypoglycemia. Events of symptomatic hypoglycemia were analyzed as follows: minor hypoglycemia, which was defined as symptoms coexisting with capillary blood glucose levels <3.5 mmol/L (63 mg/dL), and major hypoglycemia, which was defined as blood glucose levels <2.8 mmol/L (50 mg/dL) and an episode requiring medical intervention or exhibiting markedly depressed level of consciousness or seizure. Nocturnal hypoglycemia was defined as a hypoglycemic event occurring while asleep.
Statistical analysis
All results are expressed as means (SD). ANOVA was used to compare among the three groups. Paired t tests to analyze changes of parameters before and after intervention were used. Repeated measure of ANOVA was used to determine whether A1C differed significantly over time or among the three groups. For all tests, P < 0.05 was accepted as significant. The analysis was done using SPSS 11.0 software (SPSS Inc, Chicago, IL).
RESULTS
Baseline characteristics of participants
The clinical characteristics and baseline biochemical data of the 154 participants are summarized in Table 1. No significant differences were noted in anthropometry or biochemical parameters, including fasting glucose or A1C, and prescriptions of antidiabetic agents among the three groups.
Table 1.
Baseline characteristics of participants by group
| Characteristics | U-healthcare group (n = 51) | SMBG group (n = 51) | Control group (n = 52) | P |
|---|---|---|---|---|
| Age, years | 67.2 (4.1) | 67.2 (4.4) | 68.1 (5.5) | 0.542 |
| Sex | 0.706 | |||
| Male | 23 | 22 | 19 | |
| Female | 27 | 28 | 31 | |
| Diabetes duration, years | 14.1 (10.1) | 15.4 (8.3) | 15.8 (10.7) | 0.695 |
| Height, cm | 161.0 (8.2) | 161.6 (10.4) | 158.5 (8.4) | 0.191 |
| Weight, kg | 64.0 (8.5) | 65.3 (11.5) | 63.8 (9.5) | 0.739 |
| BMI, kg/m2 | 24.7 (2.3) | 24.9 (3.0) | 25.4 (3.3) | 0.408 |
| Systolic blood pressure, mmHg | 129.8 (18.2) | 127.9 (16.1) | 129.2 (17.1) | 0.856 |
| Diastolic blood pressure, mmHg | 73.2 (10.3) | 72.7 (10.3) | 74.2 (11.1) | 0.778 |
| Fasting plasma glucose, mg/dL | 137.3 (34.4) | 137.8 (40.1) | 141.6 (43.0) | 0.828 |
| Postprandial 2-h glucose, mg/dL | 242.5 (64.7) | 242.6 (50.1) | 246.3 (55.7) | 0.982 |
| A1C, % | 7.8 (1.0) | 7.9 (0.9) | 7.9 (0.8) | 0.884 |
| Total cholesterol, mg/dL | 173.7 (34.7) | 175.3 (28.2) | 169.1 (30.0) | 0.602 |
| Triglyceride, mg/dL | 144.4 (53.0) | 151.5 (66.2) | 164.2 (84.6) | 0.685 |
| HDL cholesterol, mg/dL | 49.1 (9.9) | 48.0 (10.4) | 51.9 (16.4) | 0.640 |
| LDL cholesterol, mg/dL | 110.4 (28.6) | 92.9 (22.9) | 101.5 (25.3) | 0.104 |
| Aspartate aminotransferase, IU/L | 20.9 (6.8) | 22.3 (9.1) | 22.3 (8.5) | 0.644 |
| Alanine aminotransferase, IU/L | 22.3 (9.9) | 26.7 (20.9) | 24.9 (15.4) | 0.425 |
| Creatinine, mg/dL | 1.06 (0.19) | 1.11 (0.34) | 1.16 (0.26) | 0.211 |
| Medication for glucose control | ||||
| Sulfonylurea, n (%) | 29 (58.0) | 24 (56.0) | 28 (48.0) | 0.317 |
| Metformin, n (%) | 34 (68.0) | 30 (65.2) | 28 (56.0) | 0.216 |
| Thiazolidinedione, n (%) | 4 (8.0) | 8 (16.0) | 3 (6.0) | 0.740 |
| Dipeptidyl peptidase 4, n (%) | 6 (12.0) | 11 (22.0) | 6 (12.0) | 0.999 |
| α-Glucosidase inhibitor, n (%) | 9 (18.0) | 13 (26.0) | 12 (22.7) | 0.475 |
| Insulin, n (%) | 12 (24) | 12 (24) | 19 (38) | 0.123 |
| Education level | 0.689 | |||
| None, n (%) | 2 (3.9) | 3 (5.8) | 1 (1.9) | |
| Primary school, n (%) | 10 (19.6) | 8 (15.4) | 11 (21.2) | |
| Junior high school, n (%) | 20 (39.2) | 21 (40.4) | 19 (36.5) | |
| ≥High school, n (%) | 19 (37.3) | 20 (38.5) | 21 (40.4) |
Follow-up of study participants
At the conclusion of the study, 49 (96.1%), 47 (92.2%), and 48 (92.3%) participants of the u-healthcare, SMBG, and control group, respectively, completed the study. The dropout rates were not different among the groups; two in the u-healthcare group and four in the SMBG group wanted to withdraw because of inconvenience of frequent testing. Four patients in control group were withdrawn because of missing laboratory follow-up tests.
Effects of intervention
Anthropometric and biochemical parameters after 6 months of follow-up are summarized in Table 2. Fasting and postprandial glucose concentrations were significantly decreased in the u-healthcare group, but no significant changes were observed in the SMBG or control groups. Body weight, BMI, and LDL-cholesterol levels were significantly reduced in the u-healthcare group compared with SMBG and control groups. The frequency of self-glucose monitoring was significantly increased in the u-healthcare and SMBG groups compared with the control group. The proportion of patients reaching target frequency of glucose testing (≥8 times/week) was 81.2%, 68.5%, and 31.2% in the u-healthcare, SMBG, and control groups, respectively (P < 0.01).
Table 2.
Changes of anthropometrics, biochemical parameters, and frequency of self-monitoring blood glucose by the groups after 6 months
| Variable | U-healthcare group (n = 49) | SMBG group (n = 47) | Control group (n = 48) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | P | Baseline | 6 months | P | Baseline | 6 months | P | |
| Weight, kg | 64.3 (8.5) | 63.5 (8.5) | 0.001 | 66.8 (11.5) | 66.4 (11.6) | 0.310 | 63.6 (9.9) | 64.2 (9.4) | 0.074 |
| BMI, kg/m2 | 24.7 (2.4) | 24.4 (2.5) | 0.009 | 25.1 (2.9) | 25.0 (3.2) | 0.303 | 25.5 (3.5) | 25.8 (3.4) | 0.005 |
| Fasting glucose, mg/dL | 137.3 (32.7) | 124.3 (29.7) | 0.047 | 137.6 (40.5) | 132.2 (15.6) | 0.403 | 146.8 (48.8) | 152.6 (58.0) | 0.388 |
| Postprandial glucose, mg/dL | 250.1 (68.0) | 210.1 (49.0) | 0.007 | 239.3 (42.5) | 229.80 (65.2) | 0.592 | 259.1 (64.5) | 291.1 (77.9) | 0.212 |
| Total cholesterol, mg/dL | 174.8 (36.0) | 171.8 (34.0) | 0.490 | 177.2 (27.1) | 183.4 (28.7) | 0.242 | 169.1 (30.0) | 174.1 (30.0) | 0.168 |
| Triglyceride, mg/dL | 150.1 (58.2) | 138.8 (56.5) | 0.278 | 175.8 (71.7) | 149.9 (85.0) | 0.275 | 135.2 (45.5) | 130.1 (69.5) | 0.911 |
| HDL cholesterol, mg/dL | 51.6 (11.8) | 49.7 (8.1) | 0.243 | 43.8 (9.2) | 46.2 (10.2) | 0.421 | 43.8 (10.9) | 45.0 (9.4) | 0.750 |
| LDL cholesterol, mg/dL | 115.1 (27.8) | 95.6 (26.4) | 0.038 | 92.8 (23.7) | 100.8 (31.3) | 0.302 | 109.8 (20.5) | 93.2 (15.0) | 0.099 |
| Frequency of SMBG, _n/_week | 3.2 (3.5) | 10.5 (5.1) | <0.001 | 3.1 (2.7) | 8.2 (4.2) | <0.001 | 2.7 (4.4) | 2.4 (3.3) | 0.664 |
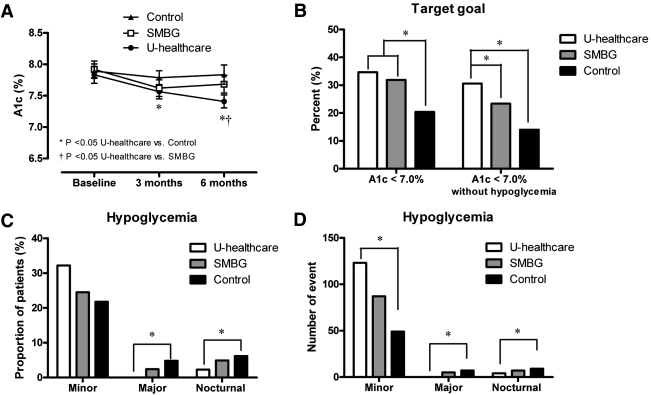
Figure 1_A_ shows the 6-month trends of A1C levels in the three groups. The A1C level of the control group did not change, but levels in the SMBG and u-healthcare groups significantly decreased at the 3-month follow-up (P < 0.05). The u-healthcare group showed a continuous decreased level of A1C at 6 months (P < 0.05). Thus, the mean A1C level of the u-healthcare group was lower than that of control at the 3-month follow-up, and was lower than that of both the SMBG or control groups at 6 months.
Figure 1.
A: Changes of A1C level over 6 months of study in the u-healthcare, SMBG, and control groups. B: Proportion of patients who achieved A1C <7.0% without hypoglycemia at 6 months. C: Proportion of patients experiencing minor, major, and nocturnal hypoglycemic event. D: Number of hypoglycemia incidences study period among the u-healthcare, SMBG, and control groups (*P < 0.05).
Achievement of primary target goal (A1C <7.0% without hypoglycemia)
The proportion of patients with A1C <7.0% after 6 months of follow-up was not significantly different among the three groups, although there was a trend showing a greater proportion of the u-healthcare group achieved A1C <7.0% than the control group. The proportion of patients that achieved A1C <7.0% without hypoglycemia, the primary end point of this study, was 30.6% in the u-healthcare group, which was significantly higher than in the SMBG (23.4%) or control groups (14.0%; vs. SMBG, P = 0.027 and vs. control P = 0.019, respectively; Fig. 1_B_).
Hypoglycemia
Before study enrollment, the participants in the three groups had experienced a similar number of hypoglycemic events. During the 6 months of this study, the proportion of patients experiencing minor hypoglycemia seemed to be higher in u-healthcare group (32.2%) than in the SMBG (24.5%) or control groups (21.8%; Fig. 1_C_), but statistical significance was not found. In contrast, major and nocturnal hypoglycemia was smaller in the u-healthcare group than in the SMBG or control groups (P < 0.05). Similar results were obtained with number of hypoglycemic events (Fig. 1_D_).
CONCLUSIONS
The CDSS-based u-healthcare service achieved better glycemic control with less hypoglycemia than SMBG and routine care, and may provide effective and safe diabetes management in elderly diabetic patients. The CDSS-based u-healthcare system generates instant feedback on patients’ glucose levels by providing appropriate recommendations, including lifestyle changes and drug adjustments.
In this study, we focused on the target goal of glycemic control (A1C <7%) without hypoglycemia. Because the participants were all elderly (>60 years), hypoglycemia may be critical in aggravating chronic complications as well as worsening glycemic control. Recent large-scale studies showed that intensive glucose control had not benefited patients; in fact, tighter control caused more harm than the conventional treatment (16,17). This lack of benefit may be explained by the occurrence of hypoglycemia, which is particularly important in elderly individuals, who are less aware of hypoglycemic symptoms (18,19).
The mean A1C value during the study decreased significantly in the u-healthcare group compared with the control group. There were more participants with a mean value of A1C <7.0% in the u-healthcare (34%) than in the SMBG (31.9%) or control (20.4%) group, although this was not statistically significant. Compared with the change of fasting glucose level, the decrease in the postprandial 2-h glucose level was more prominent in the u-healthcare group (by 40 mg/dL, P = 0.007). These data indicate that the CDSS-based u-healthcare service was effective in decreasing postprandial glucose surge by immediately alerting patients with lifestyle change recommendations. According to previous study results, postprandial glucose concentration is regarded as a risk factor for developing long-term complications (20–22). Therefore, less fluctuation of glucose levels would lead to better prevention of long-term diabetes complications.
In this study, glycemic control also improved in the SMBG group, but this effect was attenuated over time. This implies that self-management of diabetes is difficult without close and consistent supervision. Thus, prompt follow-up involving appropriate recommendations and consistent reminder messages that motivate patients may be more important than the frequency of glucose self-measurement per se. Although, the frequency of SMBG was directly related to the mean A1C levels, it is noteworthy to recognize its attenuating relationship at the end of the study.
In addition, u-healthcare service group showed a decrease in BMI and LDL-cholesterol level. These results can be interpreted as unintended benefits resulting from adhering to lifestyle change recommendations that were generated by the CDSS rule engine. Thus, u-healthcare service has many other beneficial effects pertaining to healthy lifestyle changes encouraged by automated messages.
Several studies have used a different telehealth system in different settings. A previous study from our group confirmed the use of the glucometer with a mobile system and Zigbee communication protocol and showed improved self-care in diabetes management in elderly diabetic patients (8). An Internet-based glucose control system used in the middle-aged type 2 diabetic patients recently showed a significant reduction of the A1C level (11). More recently, another group from South Korea reported that the combined application of a mobile device and a Web-based monitoring system for 12 weeks improved various metabolic parameters in obese patients with diabetes and hypertension (12). Thus, various applications of advanced information technology in different settings and populations would be helpful in diabetes management, and as a result, a great number of studies are ongoing in this field (13,23–25).
Patients in this study were allowed to change their therapeutic regimen according to the text messages generated by the CDSS rule engine. Generally, every change in the drug regimen in Korea must be certified by a doctor. This study, however, was conducted under controlled and special circumstances where the participants were educated intensively and the dose of self-adjustment was limited to a very narrow range. In addition, the investigators frequently monitored the text messages, and an active alert system also sent timely notification of adverse events. Nevertheless, the issue of self-changing therapy by patients is an area of uncertainty and concern, and more clinical studies are required to support its validity.
This study has some limitations. First, the study population size was relatively small. Second, the overall follow-up period was only 6 months. In addition, study participants were limited to elderly individuals, and only blood glucose levels were involved. Further development of the CDSS rule engine for patient’s blood pressure or other medical condition is warranted. Thus, a large-scale, long-term clinical trial using the advanced CDSS rule engine for type 2 diabetic patients is required in the near future.
Despite more frequent testing, 96.1% of the u-healthcare group completed the study. This could be attributed to education, reminder messages generated by the glucometer, and patient satisfaction with the glucose results associated with frequent testing. The high proportion of elderly patients who completed the study is important for several reasons. The successful completion serves as an indication that even technologically challenged elderly individuals can adopt a new and advanced system. It is critical, however, to provide sufficient education and training before such system implementation, as was done in this study. Another significance of this adapted population is its implication on the use of a new system in the general public. Successful adoption of new technology by the general public when the u-healthcare system is widely implemented is anticipated from the results of our study.
In conclusion, we have demonstrated that the 6-month application of the u-healthcare system, which is characterized by a proactive automated communication system using the CDSS rule engine, helped diabetic patients achieve target glycemic control with less hypoglycemia. In the near future, we hope that the individualized u-healthcare system will contribute to diabetes management by reducing complications and improving quality of life and self-care in patients with diabetes.
Acknowledgments
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A090001), a research grant (02-2008-036) from the SNUBH, and the Korea Science and Engineering Foundation grants funded by the Ministry of Science and Technology (M10642140004-06N4214-00410).
No potential conflicts of interest relevant to this article were reported.
S.L. and S.M.K. wrote the manuscript, researched data, and contributed to the discussion; H.S., H.J.L., S.Y.Y., J.W.Y., S.H.Y., S.-Y.K., J.O.R., H.S.J., and K.S.P. contributed to the discussion; and H.C.J. researched data, contributed to the discussion, and reviewed and edited the manuscript.
Footnotes
Clinical trial reg. no. NCT01137058, clinicaltrials.gov.
References
- 1.Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet 2009;373:1765–1772 [DOI] [PubMed] [Google Scholar]
- 2.Eldor R, Raz I. The individualized target HbA1c: a new method for improving macrovascular risk and glycemia without hypoglycemia and weight gain. Rev Diabet Stud 2009;6:6–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naseer A, Stergioulas LK. Web-services-based resource discovery model and service deployment on healthgrids. IEEE Trans Inf Technol Biomed 2010;14:838–845 [DOI] [PubMed] [Google Scholar]
- 4.Alasaarela E, Oliver NS. Wireless solutions for managing diabetes: a review and future prospects. Technol Health Care 2009;17:353–367 [DOI] [PubMed] [Google Scholar]
- 5.Pérez-Ferre N, Galindo M, Fernández MD, et al. A telemedicine system based on internet and short message service as a new approach in the follow-up of patients with gestational diabetes. Diabetes Res Clin Pract 2010;87:e15–e17 [DOI] [PubMed] [Google Scholar]
- 6.Mulvaney SA, Rothman RL, Wallston KA, Lybarger C, Dietrich MS. An internet-based program to improve self-management in adolescents with type 1 diabetes. Diabetes Care 2010;33:602–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolb H, Mandrup-Poulsen T. The global diabetes epidemic as a consequence of lifestyle-induced low-grade inflammation. Diabetologia 2010;53:10–20 [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ, Lee SH, Ha KS, et al. Ubiquitous healthcare service using Zigbee and mobile phone for elderly patients. Int J Med Inform 2009;78:193–198 [DOI] [PubMed] [Google Scholar]
- 9.Yu SH, Kim SH, Kim SY, et al. Effects of ‘ubiquitous healthcare’ on the ability of self-management in elderly diabetes patients. Korea Diabetes J 2009;33:58–64 [Google Scholar]
- 10.Baker L, Wagner TH, Singer S, Bundorf MK. Use of the internet and e-mail for health care information: results from a national survey. JAMA 2003;289:2400–2406 [DOI] [PubMed] [Google Scholar]
- 11.Cho JH, Chang SA, Kwon HS, et al. Long-term effect of the internet-based glucose monitoring system on HbA1c reduction and glucose stability: a 30-month follow-up study for diabetes management with a ubiquitous medical care system. Diabetes Care 2006;29:2625–2631 [DOI] [PubMed] [Google Scholar]
- 12.Yoo HJ, Park MS, Kim TN, et al. A ubiquitous chronic disease care system using cellular phones and the internet. Diabet Med 2009;26:628–635 [DOI] [PubMed] [Google Scholar]
- 13.Gómez EJ, del Pozo F, Hernando ME. Telemedicine for diabetes care: the DIABTel approach towards diabetes telecare. Med Inform (Lond) 1996;21:283–295 [DOI] [PubMed] [Google Scholar]
- 14.Korean Diabetes Association Treatment guideline for diabetes. Seoul, Korea, Korean Diabetes Association, Seoul, Korea, 2007 [Google Scholar]
- 15.American Diabetes Association Standards of medical care in diabetes—2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerstein HC, Miller ME, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 18.Bremer JP, Jauch-Chara K, Hallschmid M, Schmid S, Schultes B. Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care 2009;32:1513–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meneilly GS, Cheung E, Tuokko H. Altered responses to hypoglycemia of healthy elderly people. J Clin Endocrinol Metab 1994;78:1341–1348 [DOI] [PubMed] [Google Scholar]
- 20.Nalysnyk L, Hernandez-Medina M, Krishnarajah G. Glycaemic variability and complications in patients with diabetes mellitus: evidence from a systematic review of the literature. Diabetes Obes Metab 2010;12:288–298 [DOI] [PubMed] [Google Scholar]
- 21.Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability; does it matter? Endocr Rev 2010;31:171–182 [DOI] [PubMed] [Google Scholar]
- 22.Weber C, Schnell O. The assessment of glycemic variability and its impact on diabetes-related complications: an overview. Diabetes Technol Ther 2009;11:623–633 [DOI] [PubMed] [Google Scholar]
- 23.Larsen ME, Turner J, Farmer A, Neil A, Tarassenko L. Telemedicine-supported insulin optimisation in primary care. J Telemed Telecare 14 September 2010 [Epub ahead of print] [DOI] [PubMed]
- 24.Young D, Furler J, Vale M, et al. Patient Engagement and Coaching for Health: The PEACH study—a cluster randomised controlled trial using the telephone to coach people with type 2 diabetes to engage with their GPs to improve diabetes care: a study protocol. BMC Fam Pract 2007;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kollmann A, Riedl M, Kastner P, Schreier G, Ludvik B. Feasibility of a mobile phone-based data service for functional insulin treatment of type 1 diabetes mellitus patients. J Med Internet Res 2007;9:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]