Hyperbaric oxygen – its mechanisms and efficacy (original) (raw)
. Author manuscript; available in PMC: 2012 Jan 1.
Published in final edited form as: Plast Reconstr Surg. 2011 Jan;127(Suppl 1):131S–141S. doi: 10.1097/PRS.0b013e3181fbe2bf
Abstract
Background
This paper outlines therapeutic mechanisms of hyperbaric oxygen therapy (HBO2) and reviews data on its efficacy for clinical problems seen by plastic and reconstructive surgeons.
Methods
The information in this review was obtained from the peer-reviewed medical literature.
Results
Principal mechanisms of HBO2 are based on intracellular generation of reactive species of oxygen and nitrogen. Reactive species are recognized to play a central role in cell signal transduction cascades and the discussion will focus on these pathways. Systematic reviews and randomized clinical trials support clinical use of HBO2 for refractory diabetic wound healing and radiation injuries; treatment of compromised flaps and grafts and ischemia-reperfusion disorders is supported by animal studies and a small number of clinical trials, but further studies are warranted.
Conclusions
Clinical and mechanistic data support use of hyperbaric oxygen for a variety of disorders. Further work is needed to clarify clinical utility for some disorders and to hone patient selection criteria to improve cost-efficacy.
Introduction
Hyperbaric oxygen (HBO2) therapy is a treatment modality in which a person breathes 100% O2 while exposed to increased atmospheric pressure. HBO2 treatment is carried out in either a mono- (single person) or multi-place (typically 2 to 14 patients) chamber. Pressures applied while in the chamber are usually 2 to 3 atmospheres absolute (ATA), the sum of the atmospheric pressure (1 ATA) plus additional hydrostatic pressure equivalent to one or two atmospheres (1 atmosphere = a pressure of 14.7 pounds per square inch or 101 kPa). Treatments are usually about 1.5 to 2 hours long, depending on the indication and may be performed one to three times daily. Monoplace chambers are usually compressed with pure O2. Multiplace chambers are pressurized with air and patients breathe pure O2 through a tight-fitting face mask, a hood, or an endotracheal tube. During treatment, the arterial O2 tension often exceeds 2000 mmHg and levels of 200 to 400 mmHg occur in tissues. (2)
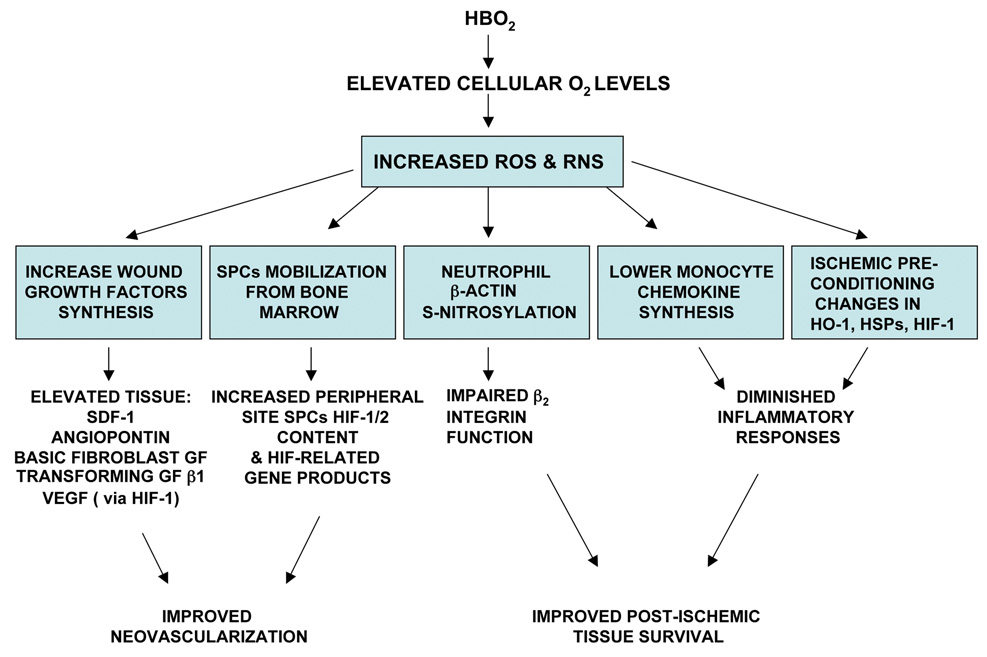
The initial effect of pressurizing the human body is intuitively obvious - elevating hydrostatic pressure increases partial pressure of gases and causes a reduction in the volume of gas-filled spaces according to Boyle's law. Gas volume reduction has direct relevance to treating pathological conditions in which gas bubbles are present in the body, such as arterial gas embolism and decompression sickness. The majority of patients who undergo HBO2 therapy are not treated for bubble-induced injuries hence therapeutic mechanisms are related to an elevated O2 partial pressure. A summary of these mechanisms is shown in Figure 1.
Figure 1.
Overview on therapeutic mechanisms of HBO2 related to elevations of tissue oxygen tensions. The figure outlines initial effects (denoted by boxes) that occur due to increased production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and their consequences. Other abbreviations: GF=growth factor, VEGF=vascular endothelial growth factor, HIF= hypoxia inducible factor, SPCs=stem/progenitor cells, HO-1 =heme oxygenase-1, HSPs=heat shock proteins.
It is well accepted that breathing greater than 1 ATA O2 will increase production of reactive oxygen species (ROS). (2) This is critically important as it is the molecular basis for a number of therapeutic mechanisms. ROS and also reactive nitrogen species (RNS) serve as signaling molecules in transduction cascades, or pathways, for a variety of growth factors, cytokines and hormones. (3–5) ROS is a collective term for O2-derived free radicals as well as O2-derived non-radical species such as hydrogen peroxide and hypochlorous acid. ROS are generated as part of normal metabolism by mitochondria, endoplasmic reticulum, peroxisomes, various oxidase enzymes and phospholipid metabolism. ROS act in conjunction with several redox systems involving glutathione, thioredoxin and pyridine nucleotides, and play central roles in coordinating cell signaling and also anti-oxidant, protective pathways. (3, 4, 6) (5) This point is central to the ensuing discussion – oxidative stress is not synonymous with oxygen toxicity.
RNS include nitric oxide (·NO) and agents generated by reactions between ·NO, or its oxidation products, and ROS. Peroxide-dependent enzymes such as myeloperoxidase can catalyze reactions between nitrite, a major oxidation product of ·NO, and hydrogen peroxide or hypochlorous acid to generate oxidants such as nitryl chloride and nitrogen dioxide that are capable of nitration and S-nitrosylation reactions. (11–13)There are three nitric oxide synthase. The effect of hyperoxia on catalytic activity is reflected by values for the apparent Michaelis-Menten constant for O2 and it differs among the three NOS isoforms. In part this is because enzyme activity is constrained by ferric-ferrous conversion at the active site. As a general statement, however, hyperoxia augments RNS production. (14–18)
Discussion in this review will focus on those HBO2 indications most pertinent to Plastic and Reconstructive Surgery. General discussions of HBO2 indications can be found in recent texts and for the general plastic surgeon, it is important to mention that consultation and advice on HBO2 may be sought through the Undersea and Hyperbaric Medical Society and more locally with board-certified physicians. (19–21) That is, Undersea and Hyperbaric Medicine sub-specialty certification is obtainable through the American Board of Medical Specialists.
Wound healing
HBO2 is used to treat refractory diabetic lower extremity wounds and delayed radiation injuries. Clearly, the pathophysiology of these disorders differs but they share several elements include depletion of epithelial and stromal cells, chronic inflammation, fibrosis, an imbalance or abnormalities in extracellular matrix components and remodeling processes, and impaired keratinocyte functions.(22–27) Diabetic wound healing is also impaired by decreased growth factor production, while in post-radiation tissues there appears to be an imbalance between factors mediating fibrosis versus normal tissue healing. (22, 23, 27) The reader is referred to several recent reviews for general discussions on pathophysiology. (28–30)
Clinical efficacy of HBO2
Wound healing HBO2 protocols involve daily treatments of 1.5 to 2 hours for 20 to 40 days. The effectiveness of HBO2 as an adjuvant therapy for diabetic lower extremity ulcerations can be examined from the perspective of hastened healing and also reduced risk of major amputations. Clearly related, these two vantage points are not synonymous as current diabetic wound care often includes a ray or partial foot amputation as an acceptable approach to obtain wound closure and prompt rehabilitation.
This is the era of meta-analysis and despite drawbacks with these evaluations they are used regularly as a final judgment on efficacy of an intervention. According to the most recent evaluation, employing HBO2 as a component to refractory diabetic wound management decreases risk of a major amputation with an odds ratio of 0.236 [95% confidence interval (CI) 0.133 – 0.418]. Adjunctive use of HBO2 as a component to diabetic wound care improves healing with an odds ratio of 11.64 [95% CI 3.457 – 39.196] (31) This analysis was based on clinical trials conducted through 2007.(32–40) The results continue to demonstrate that HBO2 markedly improves outcome. Another meta-analysis concluded that only four patients needed to be treated with HBO2 to prevent one amputation.(41) Since this publication, two additional groups have reported benefits to use of HBO2; one was a double-blinded randomized trial. (42, 43) The results continue to demonstrate that HBO2 improves outcome. The double blinded trial was a single-center study that enrolled individuals with diabetes foot ulcers. Individuals were randomized to receive either HBO2 (100% oxygen, 2.5 ATA for 85 minutes five days per week for 8-weeks) or control (room air, 2.5 ATA for 85 minutes five days per week for 8-weeks) and good wound care. The outcome was a healed wound by 12 months after the commencement of therapy. A total of 99 individuals were evaluated, 38 received HBO and 37 received control therapy. By one year of follow up 52% of those receiving HBO2 healed and 29% of those receiving control (p=0.03).
The benefit of HBO2 for radiation injury also has been shown in randomized trials and its utilization supported by independent evidence-based reviews.(44–46) It is important to state that for both diabetic wounds and radiation injuries, HBO2 is used in conjunction with standard wound care management techniques. That was the format followed in clinical trials and it is fully understandable based on mechanisms of action. If used only in a post-operative period, or in the absence of appropriate surgical care, one should expect HBO2 to be ineffective treatment. (47, 48)
Mechanisms of action
Animal trials have documented wound healing benefits of HBO2.(49–52) The basis for its efficacy continues to be investigated and appears to be a combination of systemic events as well as local alterations within the wound margin (see Fig. 1). Neovascularization occurs by two processes. Regional angiogenic stimuli influence the efficiency of new blood vessel growth by local endothelial cells (termed angiogenesis) and they stimulate the recruitment and differentiation of circulating stem/progenitor cells (SPCs) to form vessels de novo in a process termed vasculogenesis. (53–55) HBO2 has effects on both these processes.
Bone marrow eNOS activity is required for SPCs mobilization and this is compromised by diabetes (56–60) Radiation, chemotherapy and several other factors also diminish SPCs mobilization, although mechanisms for these effects are unclear. (61–64) By stimulating ·NO synthesis in bone marrow, HBO2 mobilizes SPCs in normal humans, patients previously exposed to radiation and in diabetics.(65–67) Importantly, in contrast to SPCs mobilization stimulated by infusion of growth factors; HBO2 does not concomitantly elevate the circulating leukocyte count which may be thrombogenic. (68) In animal models, SPCs mobilized by HBO2 home to wounds and accelerate healing. (50, 52, 69)
Separate from its effect on SPCs mobilization, HBO2-mediated oxidative stress at sites of neovascularization will stimulate SPCs growth factor production. (70, 71) This is due at least in part to augmented synthesis and stabilization of hypoxia inducible factors (HIF). (72–74) These transcription factors are heterodimers of HIF-α and a constitutively expressed HIF-β. It is well recognized that expression and activation of HIF-α subunits are tightly regulated and their degradation by the ubiquitin-proteasome pathway typically occurs when cells are replete with O2. (75, 76) However, whether hypoxic or normoxic conditions prevail, free radicals are required for HIF expression. (76–78) HBO2 elevates HIF-1 and −2 levels in vasculogenic SPCs because of increases in ROS. One consequence of ROS-mediated stress is augmented production of the antioxidant thioredoxin and one of its regulatory enzymes, thioredoxin reductase. (74) Thioredoxin can act as a transcription factor and in SPCs appears to be the proximal species responsible for promoting the expression and activity of HIFs.(79–81) HIF-1 and −2 then stimulate transcription of many genes involved with neovascularization. A physiological oxidative stress that triggers the same pathway is lactate metabolism. (71)
Pluripotent mesenchymal stem cells were shown in vitro to be stimulated by HBO2 to synthesize placental growth factor. This too is an ROS-dependent phenomenon and will significantly increase cell migratory and tube formation functions. (82) Microvascular endothelial cells exposed to HBO2 in ex vivo studies up-regulate a variety of protein damage-control pathways that lead to enhanced oxidative stress resistance, cell proliferation and tube formation. (83) HBO2 does not alter circulating levels of insulin, insulin-like growth factors, or pro-inflammatory cytokines [e.g. tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8] in normal healthy humans. (84, 85) Vascular endothelial growth factor (VEGF) and angiopoietin, as well as stromal derived factor (SDF-1) influence SPCs homing to wounds and SPCs differentiation to endothelial cells. (86) (87) Synthesis of VEGF has been shown to be increased in wounds by HBO2, and it is the most specific growth factor for neovascularization.(72) HBO2 also stimulates synthesis of basic fibroblast growth factor (bFGF) and transforming growth factor β1 by human dermal fibroblasts, (88) angiopoietin-2 by human umbilical vein endothelial cells, (89) bFGF and hepatocyte growth factor in ischemic limbs, (90) and it up-regulates platelet derived growth factor (PDGF) receptor in wounds.(91) Extracellular matrix formation is closely linked to neovascularization and it is another O2-dependent process. (92) Enhanced collagen synthesis and cross-linking by HBO2 have been described, but whether changes are linked to the O2-dependence of fibroblast hydroxylases, alteration in balance of wound growth factors, metalloproteinases and/or inhibitors of metalloproteases, is as yet unclear. (92, 93)
Before leaving the subject of wound healing, mention should be made of conflicting data and where further work is needed. The influence HBO2 has on HIF isoform expression appears to vary with different tissues and possibly with chronology [e.g. looking early or late after wounding or an ischemic insult]. One recent model showing accelerated wound healing by HBO2 reported lower HIF-1 levels at wound margins, along with reduced inflammation and fewer apoptotic cells. (51) In contrast, higher levels of HIF-1 have been linked to elevated VEGF in wounds in response to hyperoxia. (72, 94) With regard to diabetes, there is a complex interplay present between ROS and RNS. (22, 59, 60) Impairments in eNOS function are related to hyperglycemia, insulin resistance, impaired enzyme synthesis, disordered caveolin associations and enhanced protein kinase C activity.(59, 60, 95) Production of O2· is augmented in diabetes and this will reduce bioavailability of ·NO because the two radicals react rapidly to generate alternative RNS. (96, 97) Disordered balance between O2· and ·NO is reflected by elevated levels of nitrotyrosine in plasma of type II diabetics. (98) The reason for outlining these issues with regard to HBO2 is because there is a need for more investigations. Data from diabetic animals and humans indicate that HBO2 can overcome some aspects of eNOS inhibition but it is doubtful that all issues have been resolved. (66, 67, 99, 100)
To summarize, HBO2 can stimulate healing in refractory wounds and irradiated tissues. Therapy for refractory diabetic wounds is likely to reduce the risk of lower extremity amputation by 2 to 3 times, with an absolute rate of major amputation reductions of about 20% (e.g., 11% versus 32%) and a number needed to treat of about 4. With respect to cost-effectiveness, a study from Canada indicated that over a 12-year period, the use of HBO2 should save about $9,000 in overall costs to the care of a patient with diabetes.(101, 102) It is likely that these estimates can be honed further with improved patient selection criteria and the benefits in radiation injury are not well elucidated. The common mechanistic theme for both indications is oxidative stress responses improve neovascularization events. Cells within the wound exhibit increased collagen synthesis, growth factors production, improved cell migration and tube-formation functions. A separate free radical-based mechanism for augmentation of neovascularization by HBO2 is through SPCs. Hyperoxia stimulates bone marrow SPCs mobilization and also improves their functions once they home to peripheral sites.
Compromised Flaps and Grafts
HBO2 is used on occasion to treat compromised flaps and grafts, a practice supported by Guidelines from the Undersea and Hyperbaric Medical Society. (21) This discussion was placed between wound healing and reperfusion injuries because, depending on the situation, graft/flap treatment may be more or less related to supporting tissues through either of these two main mechanistic categories. For example, in clinical practice a wound may not be ready for coverage by a graft and neovascularization/granulation tissue formation can be hastened according to mechanisms outlined above. This was the focus for a recent clinical series. (103) Alternatively, a major flap may suffer an ischemic insult in the process of its creation and thus mechanisms described in the next section are pertinent.
A comprehensive review of HBO2 use for flaps and grafts was recently published. (104) There is no need to recapitulate the information except to say that there is one prospective, blinded clinical trial. Administration of HBO2 prior to and for three days following skin grafting led to a significant 29% improvement in graft survival. (105) A problem with this trial, however, is that the success in the control arm of the study was markedly less that one would expect in current practice. As was emphasized in the review, support for use of HBO2 in flap/graft compromise comes from a very large number of animal studies. (104, 106) Comparative clinical trials support its use but more work is needed. (107, 108)
Reperfusion injuries and HBO2
Clinical studies have documented significant survival enhancement with HBO2 for extremity re-implantation and free tissue transfer, and following crush injury. (109, 110) As is the case with flaps and grafts, however, the amount of controlled clinical data is small and insufficient to perform an evidence-based assessment of HBO2 efficacy. None-the-less, the breadth of clinical experience across a variety of disorders should spur closer assessment of its use. Clinical trials have shown that HBO2 can reduce coronary artery re-stenosis after balloon angioplasty/stenting, (111, 112) decrease muscle loss after thrombolytic treatment for myocardial infarction, (113–115) improve hepatic survival after transplantation and lead to more rapid return of donor liver function (116, 117) and reduced the incidence of encephalopathy seen after cardiopulmonary bypass and following carbon monoxide poisoning. (118, 119) In contrast to protocols for wound healing, HBO2 treatments for reperfusion injuries are done for just a few days rather than weeks; they are performed at higher O2 partial pressures (~2.5 to 3.0 ATA) and may occur multiple times in the same day.
An early event associated with post-ischemic tissue reperfusion is adherence of circulating neutrophils to vascular endothelium by β2 integrins. When animals or humans are exposed to HBO2 at 2.8 to 3.0 ATA for at least 45 minutes, the ability of circulating neutrophils to adhere to target tissues is temporarily inhibited. (120–123) In animal models, HBO2-mediated inhibition of neutrophil β2 integrin adhesion has been shown to ameliorate reperfusion injuries of brain, heart, lung, liver, skeletal muscle and intestine, as well as smoke-induced lung injury and encephalopathy due to carbon monoxide poisoning. (123–131) It also appears that benefits of HBO2 in decompression sickness are related to the temporary inhibition of neutrophil β2 integrins, in addition to the Boyle’s Law-mediated reduction in bubble volume as discussed in the introduction. (132)
Exposure to HBO2 inhibits neutrophil β2 integrin function because hyperoxia increases synthesis of reactive species derived from iNOS and myeloperoxidase, leading to excessive S-nitrosylation of cytoskeletal β actin. (133). This modification increases the concentration of short, non-cross-linked filamentous (F)-actin which alters F-actin distribution within the cell. HBO2 does not reduce neutrophil viability and functions such as degranulation, phagocytosis and oxidative burst in response to chemoattractants remain intact. (134, 135) Inhibiting β2 integrins with monoclonal antibodies will also ameliorate ischemia-reperfusion injuries but in contrast to HBO2, antibody therapy causes profound immunocompromise. (136, 137) HBO2 does not inhibit neutrophil antibacterial functions because the G-protein coupled ‘inside-out’ pathway for activation (such as that triggered by endotoxin) remains intact, and actin S-nitrosylation is reversed as a component of this activation process. (133) (138) Probably the most compelling evidence that HBO2 does not cause immunocompromise comes from studies in sepsis models, where HBO2 has a beneficial effect. (139–141)
A separate anti-inflammatory pathway for HBO2 involves impaired pro-inflammatory cytokine production by monocyte-macrophages. This action has been shown in animal models and human beings. (142–144) The effect on monocyte/macrophages may be the basis for reduced levels of circulating pro-inflammatory cytokines under stress conditions. (84) The molecular mechanism is unknown, but could be related to HBO2-mediated enhancement of heme oxygenase-1 and heat shock proteins (HSP) [e.g. HSP 70]. (7, 10) Hence, once again, an oxidative stress response seems to occur.
Finally, HBO2 has been shown in numerous models to augment ischemic tolerance of brain, spinal cord, liver, heart and skeletal muscle by mechanisms involving induction of antioxidant enzymes and anti-inflammatory proteins. (15, 145–149) A common theme among some studies is alterations in HIF-1 production but, as was the case in wound healing models, timing of HBO2 application appears to influence cellular responses. In several models, exposure to HBO2 ameliorates post-ischemic injuries by decreasing HIF-1 expression. (150, 151) When HBO2 is used in a prophylactic manner to induce ischemic tolerance, however, its mechanism appears related to up-regulation of HIF-1 and at least one of its target genes, erythropoietin. (152)
In review, oxidative stress responses triggered by HBO2 improve outcome from a wide variety of post-ischemic/inflammatory insults. HBO2 also improves ischemic tolerance when used in a prophylactic manner. Augmented synthesis of reactive species temporarily inhibits adherence/sequestration of neutrophils by inhibiting β2 integrin function and in many tissues HBO2 will induce antioxidant enzymes and anti-inflammatory proteins. More trials to assess clinical efficacy are needed.
Treatment risks
This review has emphasized the positive aspects of HBO2-induced reactive species, but there is clearly a potential for negative effects. The risks for O2 toxicity depend on the concentration and intracellular localization of reactive species. Because exposure to hyperoxia in clinical HBO2 protocols is rather brief, studies show that antioxidant defenses are adequate so that biochemical stresses related to increases in reactive species are reversible (8, 10, 153, 154). Treatments often include so-called air breaks, where a patient breathes just air for 5 minutes once or twice through the course of a treatment. This intervention has been demonstrated to enhance pulmonary O2 tolerance (1). CNS O2 toxicity is manifested as a grand mal seizure. This occurs at an incidence of approximately 1 to 4 in 10,000 patient treatments. (155–157) Pathological changes in association with isolated O2-mediated seizures have not been found in studies with guinea pigs, rabbits and humans. (158) Progressive myopia has been reported in patients who undergo prolonged daily therapy, but this typically reverses within 6 weeks after termination of treatments. (159) Development of nuclear cataracts has been reported with excessive treatments that exceed a total of 150 to 200 hours, and the change does not spontaneously reverse. (160)
Summary
This brief review has highlighted some of the beneficial actions of HBO2 and the data that indicate oxidative stress brought about by hyperoxia can have therapeutic effects. Figure 1 provides a summary of mechanisms, all of which appear to stem from elevations in reactive species. While there has been substantial advancement of the field in recent years, more work is required to establish the place of HBO2 in 21st century medicine. Investigations of fundamental mechanisms are still needed, and better patient selection criteria would improve cost-efficacy. An extended discussion on other indications for HBO2 can be found in recent texts. (19–21)
Acknowledgement
This work was supported by grants from the Office of Naval Research and from the NIH DK080376.
Funding sources: Grants from NIH and Office of Naval Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author has no financial interest in any products, devices or drugs mentioned in this manuscript.
References
- 1.Hendricks P, Hall D, Hunter W, et al. Extension of pulmonary O2 tolerance in man at 2 ATA by intermittent O2 exposure. J Appl Physiol. 1977;42:593–599. doi: 10.1152/jappl.1977.42.4.593. [DOI] [PubMed] [Google Scholar]
- 2.Thom SR. Hyperbaric oxygen therapy. J. Intensive Care Med. 1989;4:58–74. [Google Scholar]
- 3.Kemp M, Go YM, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems in biology. Fr. Radic. Biol. Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Circu ML, Aw TY. Glutathione and apoptosis. Free Rad Res. 2008;42:689–706. doi: 10.1080/10715760802317663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Piston DW, Goodman RH. Regulation of corepressor function by nuclear NADH. Science. 2002;295:1895–1897. doi: 10.1126/science.1069300. [DOI] [PubMed] [Google Scholar]
- 7.Dennog C, Radermacher P, Barnett YA, et al. Antioxidant status in humans after exposure to hyperbaric oxygen. Mutation.Res. 1999;428:83–89. doi: 10.1016/s1383-5742(99)00034-4. [DOI] [PubMed] [Google Scholar]
- 8.Dennog C, Hartmann A, Frey G, et al. Detection of DNA damage after hyperbaric oxygen (HBO) therapy. Mutagenesis. 1996;11:605–609. doi: 10.1093/mutage/11.6.605. [DOI] [PubMed] [Google Scholar]
- 9.Markowicz S, Mehta A. The effect of human dendritic cells on the lectin-induced responsiveness of CD4+T cells to IL-2 and IL-4. Adv Exp Med Biol. 1993;329:75–80. doi: 10.1007/978-1-4615-2930-9_13. [DOI] [PubMed] [Google Scholar]
- 10.Rothfuss A, Radermacher P, Speit G. Involvement of heme oxygenase-1 (HO-1) in the adaptive protection of human lymphocytes after hyperbaric oxygen (HBO) treatment. Carcinogenesis. 2001;22:1979–1985. doi: 10.1093/carcin/22.12.1979. [DOI] [PubMed] [Google Scholar]
- 11.Brennan ML, Wu W, Fu X, et al. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 12.Lakshmi V, Nauseef W, Zenser T. Myeloperoxidase potentiates nitric oxide-mediated nitrosation. J Biol Chem. 2005;280:1746–1753. doi: 10.1074/jbc.M411263200. [DOI] [PubMed] [Google Scholar]
- 13.Sampson J, Ye Y, Rosen H, et al. Myeloperoxidase and horseradish peroxidase catalyze tyrosine nitration in proteins from nitrite and hydrogen peroxide. Arch Biochem Biophys. 1998;356:207–213. doi: 10.1006/abbi.1998.0772. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Soud HM, Rousseau DL, Stuehr DJ. Nitric oxide binding to the heme of neuronal nitric-oxide synthase links its activity to changes in oxygen tension. J.Biol.Chem. 1996;271:32515–32518. doi: 10.1074/jbc.271.51.32515. [DOI] [PubMed] [Google Scholar]
- 15.Cabigas BP, Su J, Hutchins W, et al. Hyperoxic and hyperbaric-induced cardioprotection: role of nitric oxide synthase 3. Cardiovasc Res. 2006;72:143–151. doi: 10.1016/j.cardiores.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 16.Hink J, Thom SR, Simonsen U, et al. Vascular reactivity and endothelial NOS activity in rat thoracic aorta during and after hyperbaric oxygen exposure. Am J Physiol Heart Circ Physiol. 2006;291:H1988–H1998. doi: 10.1152/ajpheart.00145.2006. [DOI] [PubMed] [Google Scholar]
- 17.Thom SR, Bhopale V, Fisher D, et al. Stimulation of nitric oxide synthase in cerebral cortex due to elevated partial pressures of oxygen: an oxidative stress response. J Neurobiol. 2002;51:85–100. doi: 10.1002/neu.10044. [DOI] [PubMed] [Google Scholar]
- 18.Thom SR, Fisher D, Zhang J, et al. Stimulation of perivascular nitric oxide synthesis by oxygen. Am J Physiol Heart Circ Physiol. 2003;284:H1230–H1239. doi: 10.1152/ajpheart.01043.2002. [DOI] [PubMed] [Google Scholar]
- 19.Mathieu De. Handbook on Hyperbaric Medicine. Netherlands: Springer, Dordrecht; 2006. [Google Scholar]
- 20.Neuman TS, Thom SRe. Physiology and Medicine of Hyperbaric Oxygen Therapy. Philadelphia, PA: Saunders-Elsevier; 2008. [Google Scholar]
- 21.Gesell Le. Hyperbaric oxygen therapy indications. 12th ed. Durham, NC: Undersea and Hyperbaric Medical Society; 2008. [Google Scholar]
- 22.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denham JW, Hauer-Jensen M. The radiotherapeutic injury--a complex ‘wound’. Radiother Oncol. 2002;63:129–145. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 24.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 25.Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: A master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277–290. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 26.Stojadinovic O, Brem H, Vouthounis C, et al. Molecular pathogenesis of chronic wounds: the role of beta-catenin and c-myc in the inhibition of epithelialization and wound healing. Am J Pathol. 2005;167:59–69. doi: 10.1016/s0002-9440(10)62953-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueno H, Ohya T, Ito H, et al. Chitosan application to X-ray irradiated wound in dogs. J Plast Reconstr Asthetic Surg. 2007;60:304–310. doi: 10.1016/j.bjps.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Denham J, Hauer-Jensen M. The radiotherapeutic injury-a complex ‘wound’. Radiother Oncol. 2002;63:129–145. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 29.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 30.Peppa M, Stavroulakis P, Raptis SA. Advanced glucoxidation products and impaired diabetic wound healing. Wound Rep.Reg. 2009;17:461–472. doi: 10.1111/j.1524-475X.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 31.Goldman RJ. Hyperbaric oxygen therapy for wound healing and limb salvage: a systematic review. Physical Med. and Rehabilitation. 2009;1:471–489. doi: 10.1016/j.pmrj.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Abidia A, Laden G, Kuhan G, et al. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomised-controlled trial. Eur J Vasc Endovasc Surg. 2003;25:513–518. doi: 10.1053/ejvs.2002.1911. [DOI] [PubMed] [Google Scholar]
- 33.Kessler L, Bilbault P, Ortega F, et al. Hyperbaric oxygenation accelerates the healing rate of nonischemic chronic diabetic foot ulcers: a prospective randomized study. Diabetes Care. 2003;26:2378–2382. doi: 10.2337/diacare.26.8.2378. [DOI] [PubMed] [Google Scholar]
- 34.Faglia E, Favales F, Aldeghi A, et al. Adjunctive systemic hyperbaric oxygen therapy in treatment of severe prevalently ischemic diabetic foot ulcer. Diabetes Care. 1996;19:1338–1343. doi: 10.2337/diacare.19.12.1338. [DOI] [PubMed] [Google Scholar]
- 35.Faglia E, Favales F, Aldeghi A, et al. Change in major amputation rate in a center dedicated to diabetic foot care during the 19080s: prognosis determinants for major amputation. J. Diabetes Complications. 1998;12:96. doi: 10.1016/s1056-8727(97)98004-1. [DOI] [PubMed] [Google Scholar]
- 36.Doctor N, Pandya S, Supe A. Hyperbaric oxygen therapy in diabetic foot. J. Postgraduate Medicine. 1991;38:112–114. [PubMed] [Google Scholar]
- 37.Kalani M, Jorneskog G, Naderi N, et al. Hyperbaric oxygen therapy in treatment of diabetic foot ulcers. J. Diabetes Complications. 2002;16:153–158. doi: 10.1016/s1056-8727(01)00182-9. [DOI] [PubMed] [Google Scholar]
- 38.Baroni G, Porro T, Faglia E, et al. Hyperbaric oxygen in diabetic gangrene treatment. Diabetes Care. 1987;10:81–86. doi: 10.2337/diacare.10.1.81. [DOI] [PubMed] [Google Scholar]
- 39.Oriani G, Meazza D, Favales F, et al. Hyperbaric oxygen therapy in diabetic gangrene. J.Hyper.Med. 1990;5:171–175. [Google Scholar]
- 40.Fife CE, Buyukcakir C, Otto G, et al. Factors influencing the outcome of lower-extremity diabetic ulcers treated with hyperbaric oxygen therapy. Wound Repair Regen. 2007;15:322–331. doi: 10.1111/j.1524-475X.2007.00234.x. [DOI] [PubMed] [Google Scholar]
- 41.Kranke P, Bennett M, Roeckl-Wiedmann I, et al. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD004123.pub2. CD004123. [DOI] [PubMed] [Google Scholar]
- 42.Duzgun AP, Satir AZ, Ozozan O, et al. Effect of hyperbaric oxygen therapy on healing of diabetic foot ulcers. J. Foot & Ankle Surg. 2008;47:515–519. doi: 10.1053/j.jfas.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Londahl M, Katzman P, Nilsson A, et al. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diab Care. 2010;33:998–1003. doi: 10.2337/dc09-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bennett M, Feldmeier J, Hampson N, et al. Hyperbaric oxygen therapy for late radiation tisue injury (Cochrane review) The Cochrane Library. 2008;(Issue 1) doi: 10.1002/14651858.CD005005.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Clarke R, Tenorio C, Hussey J, et al. Hyperbaric oxygen treatment of chronic radiation proctitis: A randomized and controlled doouble blind crossover trial with long-term follow-up. Int J Rad Oncol Biol Phys. 2008;72:134–143. doi: 10.1016/j.ijrobp.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 46.Marx RE, Johnson RP, Kline SN. Prevention of osteoradionecrosis: a randomized prospective clinical trial of hyperbaric oxygen versus penicillin. JADA. 1985;111:49–54. doi: 10.14219/jada.archive.1985.0074. [DOI] [PubMed] [Google Scholar]
- 47.Annane D, Depondt J, Aubert P, et al. Hyperbaric oxygen therapy for radionecrosis of the jaw: a randomized, placebo-controlled, double-blind trial from the ORN96 study group. J Clin Oncol. 2004;22:4893–4900. doi: 10.1200/JCO.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Maier A, Gaggl A, Klemen H, et al. Review of severe osteoradionecrosis treated by surgery alone or surgery with postoperative hyperbaric oxygenation. Br J Oral Maxillofac Surg. 2000;38:173–176. doi: 10.1054/bjom.1999.0285. [DOI] [PubMed] [Google Scholar]
- 49.Marx RE, Ehler WJ, Tayapongsak P, et al. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am.J.Surg. 1990;160:519–524. doi: 10.1016/s0002-9610(05)81019-0. [DOI] [PubMed] [Google Scholar]
- 50.Gallagher KA, Liu ZJ, Xiao M, et al. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Q, Chang Q, Cox RA, et al. Hyperbaric oxygen attenuates apoptosis and decreases inflammation in an ischemic wound model. J Invest Dermatol. 2008;128:2102–2112. doi: 10.1038/jid.2008.53. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein LJ, Gallagher KA, Bauer SM, et al. Endothelial progenitor cell release into circulation is triggered by hyperoxia-induced increases in bone marrow nitric oxide. Stem Cells. 2006;24:2309–2318. doi: 10.1634/stemcells.2006-0010. [DOI] [PubMed] [Google Scholar]
- 53.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 54.Hattori K, Dias S, Heissig B, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J. Exp. Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tepper OM, Capla JM, Galiano RD, et al. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood. 2005;105:1068–1077. doi: 10.1182/blood-2004-03-1051. [DOI] [PubMed] [Google Scholar]
- 56.Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 57.Bivalacqua TJ, Champion HC, Usta MF, et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2004;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bucci M, Roviezzo F, Brancaleone V, et al. Diabetic mouse angiopathy is linked to progressive sympathetic receptor deletion coupled to an enhanced caveolin-1 expression. Arterioscler Thromb Vasc Biol. 2004;24:721–726. doi: 10.1161/01.ATV.0000122362.44628.09. [DOI] [PubMed] [Google Scholar]
- 59.Du XL, Edelstein D, Dimmeler S, et al. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du XL, Edelstein D, Obici S, et al. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J Clin Invest. 2006;116:1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 62.Seggewiss R, Buss EC, Herrmann D, et al. Kinetics of peripheral blood stem cell mobilization following G-CSF-supported chemotherapy. Stem Cells. 2003;21:568–574. doi: 10.1634/stemcells.21-5-568. [DOI] [PubMed] [Google Scholar]
- 63.Platzbecker U, Bornhauser M, Zimmer K, et al. Second donation of granulocyte-colony-stimulating factor-mobilized peripheral blood progenitor cells: risk factors associated with a low yield of CD34+ cells. Transfusion. 2005;45:11–15. doi: 10.1111/j.1537-2995.2005.04107.x. [DOI] [PubMed] [Google Scholar]
- 64.Ikeda KTKMH. Factors for PBPC collection efficiency and collection predictors. Transfus Apher Sci. 2004;31:245–259. doi: 10.1016/j.transci.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 65.Thom SR, Bhopale VM, Velazquez OC, et al. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol. 2006;290:H1378–H1386. doi: 10.1152/ajpheart.00888.2005. [DOI] [PubMed] [Google Scholar]
- 66.Thom SR, Bhopale VM, Milovanova TN. Nitric oxide synthase activation and CD34+ stem cell mobilization by hyperbaric oxygen in diabetic patients. Undersea and Hyperbaric Med. 2010 Suppl in press. [Google Scholar]
- 67.Yang B, Milovanova T, Hardy K, et al. Stem cell mobilization in diabetics - Responses to hyperbaric oxygen. Undersea and Hyperbaric Medicine. 2007;34 Suppl:235–236. [Google Scholar]
- 68.Powell TM, Paul JD, Hill JM, et al. Granulocyte colony-stimulating factor mobilizes functional endothelial progenitor cells in patients with coronary artery disease. Arter.Thromb. Vasc. Biol. 2005;25:296–301. doi: 10.1161/01.ATV.0000151690.43777.e4. [DOI] [PubMed] [Google Scholar]
- 69.Gu GJ, Li YP, Peng ZY, et al. Mechanism of ischemic tolerance induced by hyperbaric oxygen preconditioning involves upregulation of hypoxia-inducible factor-1alpha and erythropoietin in rats. J Appl Physiol. 2008;104:1185–1191. doi: 10.1152/japplphysiol.00323.2007. [DOI] [PubMed] [Google Scholar]
- 70.Hunt T, Aslam R, Beckert S, et al. Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid Redox Signal. 2007;9:1115–1124. doi: 10.1089/ars.2007.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milovanova T, Bhopale VM, Sorokina EM, et al. Lactate stimulates vasculogenic stem cells via the thioredoxin system and engages an autocrine activation loop involving hypoxia inducible factor-1. Mol Biol Cell. 2008;28:6248–6261. doi: 10.1128/MCB.00795-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheikh AY, Gibson JJ, Rollins MD, et al. Effect of hyperoxia on vascular endothelial growth factor levels in a wound model. Arch Surg. 2000;135:1293–1297. doi: 10.1001/archsurg.135.11.1293. [DOI] [PubMed] [Google Scholar]
- 73.Milovanova TN, Bhopale VM, Sorokina EM, et al. Lactate stimulates vasculogenic stem cells via the thioredoxin system and engages an autocrine activation loop involving hypoxia-inducible factor 1. Mol Cell Biol. 2008;28:6248–6261. doi: 10.1128/MCB.00795-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Milovanova T, Bhopale VM, Sorokina EM, et al. Hyperbaric oxygen stimulates vasculogenic stem cell growth and differentiation in vivo. Journal of Applied Physiology. 2009;106:711–728. doi: 10.1152/japplphysiol.91054.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salceda S, Caro J. Hypoxia-inducible factor 1 alpha protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions: its stabilization by hypoxia depends upon redox-induced changes. J Biol Chem. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 76.Semenza GL. HIF-1 and mechanisms of hypoxia sensing. Current Opinion in Cell Biology. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 77.Dulak J, Jozkowicz A. Regulation of vascular endothelial growth factor synthesis by nitric oxide: facts and controversies. Antioxid Redox Signal. 2003;5:123–132. doi: 10.1089/152308603321223612. [DOI] [PubMed] [Google Scholar]
- 78.Schroedl C, McClintock DS, Budinger GR, et al. Hypoxic but not anoxic stabilization of HIF-1alpha requires mitochondrial reactive oxygen species. Am J Physiol Lung Cell Mol Physiol. 2002;283:L922–L931. doi: 10.1152/ajplung.00014.2002. [DOI] [PubMed] [Google Scholar]
- 79.Ema M, Hirota K, Mimura J, et al. Molecular mechanisms of transcription activation by HLF and HIF1alpha in response to hypoxia: Their stabilization and redox signal-induced interaction with CBP/p300. Embo J. 1999;18:1905–1914. doi: 10.1093/emboj/18.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaga S, Zhan L, Matusmoto M, et al. Resveratrol enhances neovascularization in the infarcted rat myocardium through the induction of thioredoxin-1, heme oxygenase-1 and vascular endothelial growth factor. J Mol Cell Cardiol. 2005;38:813–822. doi: 10.1016/j.yjmcc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 81.Welsh S, Bellamy W, Briehl M, et al. The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor-1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res. 2002;62:5089–5095. [PubMed] [Google Scholar]
- 82.Shyu KG, Hung HF, Wang BW, et al. Hyperbaric oxygen induces placental growth factor expression in bone marrow-derived mesenchymal stem cells. Life Sci. 2008;83:65–73. doi: 10.1016/j.lfs.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 83.Godman CA, Chheda KP, Hightower LE, et al. Hyperbaric oxygen induces a cytoprotective and angiogenic response in human microvascular endothelial cells. Cell Stress and Chaperones. 2009:12190–12192. doi: 10.1007/s12192-009-0159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fildissis G, Venetsanou K, Myrianthefs P, et al. Whole blood pro-inflammatory cytokines and adhesion molecules post-lipopolysaccharides exposure in hyperbaric conditions. Eur Cytokine Netw. 2004;15:217–221. [PubMed] [Google Scholar]
- 85.Chen SJ, Yu CT, Cheng YL, et al. Effects of hyperbaric oxygen therapy on circulating interleukin-8, nitric oxide, and insulin-like growth factors in patients with type 2 diabetes mellitus. Clin Biochem. 2007;40:30–36. doi: 10.1016/j.clinbiochem.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 86.Hildbrand P, Cirulli V, Prinsen RC, et al. The role of angiopoietins in the development of endothelial cells from cord blood CD34+ progenitors. Blood. 2004;104:2010–2019. doi: 10.1182/blood-2003-12-4219. [DOI] [PubMed] [Google Scholar]
- 87.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 88.Kang TS, Gorti GK, Quan SY, et al. Effect of hyperbaric oxygen on the growth factor profile of fibroblasts. Arch Facial Plast Surg. 2004;6:31–35. doi: 10.1001/archfaci.6.1.31. [DOI] [PubMed] [Google Scholar]
- 89.Lin S, Shyu KG, Lee CC, et al. Hyperbaric oxygen selectively induces angiopoietin-2 in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2002;296:710–715. doi: 10.1016/s0006-291x(02)00924-5. [DOI] [PubMed] [Google Scholar]
- 90.Asano T, Kaneko E, Shinozaki S, et al. Hyperbaric oxygen induces basic fibroblast growth factor and hepatocyte growth factor expression and enhances blood perfusion and muscle regeneration in mouse ischemic hind limbs. Circ J. 2007;71:405–411. doi: 10.1253/circj.71.405. [DOI] [PubMed] [Google Scholar]
- 91.Bonomo SR, Davidson JD, Yu Y, et al. Hyperbaric oxygen as a signal transducer: upregulation of platelet derived growth factor-beta receptor in the presence of HBO2 and PDGF. Undersea Hyperb Med. 1998;25:211–216. [PubMed] [Google Scholar]
- 92.Hopf HW, Gibson JJ, Angeles AP, et al. Hyperoxia and angiogenesis. Wound Repair Regen. 2005;13:558–564. doi: 10.1111/j.1524-475X.2005.00078.x. [DOI] [PubMed] [Google Scholar]
- 93.Dinar S, Agir H, Sen C, et al. Effects of hyperbaric oxygen therapy on fibrovascular ingrowth in porous polyethylene blocks implanted under burn scar tissue: an experimental study. Burns. 2008;34:467–473. doi: 10.1016/j.burns.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 94.Hunt TK, Aslam RS, Beckert S, et al. Aerobically derived lactate stimulates revascularization and tissue repair via redox mechanisms. Antioxid Redox Signal. 2007;9:1115–1124. doi: 10.1089/ars.2007.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peppa M, Stavroulakis P, Raptis SA. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen. 2009;17:461–472. doi: 10.1111/j.1524-475X.2009.00518.x. [DOI] [PubMed] [Google Scholar]
- 96.Koppenol WH, Moreno JJ, Pryor WA, et al. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem.Res.Toxicol. 1992;5:834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- 97.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 98.Ceriello A, Mercuri F, Quagliaro L, et al. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress Diabetologia. 2001;44:834–838. doi: 10.1007/s001250100529. [DOI] [PubMed] [Google Scholar]
- 99.Gallagher KA, Goldstein LJ, Buerk DG, et al. Diabetic impairments in NO-mediated endothelial progenitor-cell mobilization and homing are reversed by hyperoxia and SDF-1a. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thom S, Milovanova T. Hyperbaric oxygen therapy increases stem cell number and HIF-1 content in diabetics. Undersea and Hyperbaric Med. 2008;35:280. (abstract) [Google Scholar]
- 101.Hailey D, Jacobs P, Perry D, et al. Technology Report: Adjunctive hyperbaric oxygen therapy for diabetic foot ulcer: An economic analysis. Canadian Agency for Drugs and Technologies in Health. Report No. 2007;75:1–32. [Google Scholar]
- 102.Chuck AW, Haily D, Jacobs P, et al. Cost-effectiveness and budget impact of adjunctive hyperbaric oxygen therapy for diabetic foot ulcers. International J Technol Assess in Hlth Care. 2008;24:176–183. doi: 10.1017/S0266462308080252. [DOI] [PubMed] [Google Scholar]
- 103.Snyder SM, Beshlian KM, Hampson NB. Hyperbaric oxygen and reduction mammaplasty in the previously irradiated breast. Plast Reconstr Surg. 2010;125:255e–257e. doi: 10.1097/PRS.0b013e3181cb67d0. [DOI] [PubMed] [Google Scholar]
- 104.Friedman HIF, Fitzmaurice M, Lefaivre JF, et al. An evidence-based appraisal of the use of hyperbaric oxygen on flaps and grafts. Plast Reconstr Surg. 2006;117 Suppl:175S–190S. doi: 10.1097/01.prs.0000222555.84962.86. [DOI] [PubMed] [Google Scholar]
- 105.Perrins DJD, Cantab MB. Influence of hyperbaric oxygen on the survival of split skin grafts. Lancet. 1967;II:868–871. doi: 10.1016/s0140-6736(67)91428-6. [DOI] [PubMed] [Google Scholar]
- 106.Kindwall E, Gottlieb L, Larson D. Hyperbaric oxygen therapy in plastic surgery: a review article. Plast Reconstr Surg. 1991;88:898–908. doi: 10.1097/00006534-199111000-00029. [DOI] [PubMed] [Google Scholar]
- 107.Roje Z, Roje Z, Eterovic D, et al. Influence of adjuvant hyperbaric oxygen therapy on short-term complications during surgical reconstruction of upper and lower extremity war injuries: retrospective cohort study. Croat Med J. 2008;49:224–232. doi: 10.3325/cmj.2008.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bowersox J, Strauss M, Hart G. Clinical experience with hyperbaric oxygen therapy in the salvage of ischemic skin flaps and grafts. J Hyperbaric Med. 1986;1:141–149. [Google Scholar]
- 109.Bouachour G, Cronier P, Gouello JP, et al. Hyperbaric oxygen therapy in the management of crush injuries: a randomized double-blind placebo-controlled clinical trial. J Trauma. 1996;41:333–339. doi: 10.1097/00005373-199608000-00023. [DOI] [PubMed] [Google Scholar]
- 110.Waterhouse M, Zamboni W, Brown R, et al. The use of HBO in compromised free tissue transfer and replantation: a clinical review. Undersea and Hyperbaric Med. 1993;20 Suppl:64. [Google Scholar]
- 111.Sharifi M, Fares W, Abdel-Karim I, et al. Usefulness of hyperbaric oxygen therapy to inhibit restenosis after percutaneous coronary intervention for acute myocardial infarction or unstable angina pectoris. Am J Cardiol. 2004;93:1533–1535. doi: 10.1016/j.amjcard.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 112.Sharifi M, Fares W, Abdel-Karim I, et al. Inhibition of restenosis by hyperbaric oxygen: a novel indication for an old modality. Cardiovasc Radiat Med. 2002;3:124–126. doi: 10.1016/s1522-1865(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 113.Dekleva M, Neskovic A, Vlahovic A, et al. Adjunctive effect of hyperbaric oxygen treatment after thrombolysis on left ventricular function in patients with acute myocardial infarction. Am Heart J. 2004;148:E14. doi: 10.1016/j.ahj.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 114.Shandling AH, Ellestad MH, Hart GB, et al. Hyperbaric oxygen and thrombolysis in myocardial infarction: The HOT MI pilot study. Am.Heart J. 1997;134:544–550. doi: 10.1016/s0002-8703(97)70093-0. [DOI] [PubMed] [Google Scholar]
- 115.Stavitsky Y, Shandling AH, Ellestad MH, et al. Hyperbaric oxygen and thrombolysis in myocardial infarction: the "HOT MI" randomized multicenter study. Cardiology. 1998;90:131–136. doi: 10.1159/000006832. [DOI] [PubMed] [Google Scholar]
- 116.Mazariegos G, O’Toole K, Mieles L, et al. Hyperbaric oxygen therapy for hepatic artery thrombosis after liver transplantation in children. Liver Transpl Surg. 1999;5:429–436. doi: 10.1002/lt.500050518. [DOI] [PubMed] [Google Scholar]
- 117.Suehiro T, Shimura T, Okamura K, et al. The effect of hyperbaric oxygen treatment on postoperative morbidity of left lobe donor in living donor adult liver transplantation. Hepato.Gastroenterology. 2008;55:1014–1019. [PubMed] [Google Scholar]
- 118.Alex J, Laden G, Cale A, et al. Pretreatment with hyperbaric oxygen and its effect on neuropsychometric dysfunction and systemic inflammatory response after cardiopulmonary bypass: a prospective randomized double-blind trial. J Thorac Cardiovasc Surg. 2005;130:1623–1630. doi: 10.1016/j.jtcvs.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 119.Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002;347:1057–1067. doi: 10.1056/NEJMoa013121. [DOI] [PubMed] [Google Scholar]
- 120.Kalns J, Lane J, Delgado A, et al. Hyperbaric oxygen exposure temporarily reduces Mac-1 mediated functions of human neutrophils. Immunol Lett. 2002;83:125–131. doi: 10.1016/s0165-2478(02)00068-8. [DOI] [PubMed] [Google Scholar]
- 121.Labrouche S, Javorschi S, Leroy D, et al. Influence of hyperbaric oxygen on leukocyte functions and haemostasis in normal volunteer divers. Thromb Res. 1999;96:309–315. doi: 10.1016/s0049-3848(99)00107-3. [DOI] [PubMed] [Google Scholar]
- 122.Thom SR. Leukocytes in carbon monoxide-mediated brain oxidative injury. Toxicol Appl Pharmacol. 1993;123:234–247. doi: 10.1006/taap.1993.1242. [DOI] [PubMed] [Google Scholar]
- 123.Zamboni WA, Roth AC, Russell RC, et al. Morphologic analysis of the microcirculation during reperfusion of ischemic skeletal muscle and the effect of hyperbaric oxygen. Plast. Reconstr. Surg. 1993:91. doi: 10.1097/00006534-199305000-00022. [DOI] [PubMed] [Google Scholar]
- 124.Atochin D, Fisher D, Demchenko I, et al. Neutrophil sequestration and the effect of hyperbaric oxygen in a rat model of temporary middle cerebral artery occlusion. Undersea and Hyperbaric Med. 2000;27:185–190. [PubMed] [Google Scholar]
- 125.Kihara K, Ueno S, Sakoda M, et al. Effects of hyperbaric oxygen exposure on experimental hepatic ischemia reperfusion injury: relationship between its timing and neutrophil sequestration. Liver Transpl. 2005;11:1574–1580. doi: 10.1002/lt.20533. [DOI] [PubMed] [Google Scholar]
- 126.Tahepold P, Valen G, Starkopf J, et al. Pretreating rats with hyperoxia attenuates inschemia-reperfusion injury of the heart. Life Sci. 2001;68:1629–1640. doi: 10.1016/s0024-3205(01)00964-x. [DOI] [PubMed] [Google Scholar]
- 127.Thom S, Mendiguren I, Fisher D. Smoke inhalation-induced alveolar lung injury is inhibited by hyperbaric oxygen. Undersea and Hyperbaric Med. 2002;28:175–180. [PubMed] [Google Scholar]
- 128.Ueno S, Tanabe G, Kihara K, et al. Early post-operative hyperbaric oxygen therapy modifies neutrophile activation. Hepato. Gastroenterology. 1999;46:1798–1799. [PubMed] [Google Scholar]
- 129.Wong HP, Zamboni WA, Stephenson LL. Effect of hyperbaric oxygen on skeletal muscle necrosis following primary and secondary ischemia in a rat model. Surgical. Forum. 1996:705–707. [Google Scholar]
- 130.Yang ZJ, Bosco G, Montante A, et al. Hyperbaric O2 reduces intestinal ischemia-reperfusion-induced TNF-alpha production and lung neutrophil sequestration. Eur J Appl Physiol. 2001;85:96–103. doi: 10.1007/s004210100391. [DOI] [PubMed] [Google Scholar]
- 131.Thom SR. Functional inhibition of leukocyte B2 integrins by hyperbaric oxygen in carbon monoxide-mediated brain injury in rats. Toxicol Appl Pharmacol. 1993;123:248–256. doi: 10.1006/taap.1993.1243. [DOI] [PubMed] [Google Scholar]
- 132.Martin JD, Thom SR. Vascular leukocyte sequestration in decompression sickness and prophylactic hyperbaric oxygen therapy in rats. Aviat Space Environ Med. 2002;73:565–569. [PubMed] [Google Scholar]
- 133.Thom SR, Bhopale VM, Mancini JD, et al. Actin S-nitrosylation inhibits neutrophil beta-2 integrin function. J Biol Chem. 2008;283:10822–10834. doi: 10.1074/jbc.M709200200. [DOI] [PubMed] [Google Scholar]
- 134.Juttner B, Scheinichen D, Bartsch S, et al. Lack of toxic side effects in neutrophils following hyperbaric oxygen. Undersea and Hyperbaric Med. 2003;30:305–311. [PubMed] [Google Scholar]
- 135.Thom SR, Mendiguren I, Hardy K, et al. Inhibition of human neutrophil beta2-integrin-dependent adherence by hyperbaric O2. Am J Physiol. 1997;272:C770–C777. doi: 10.1152/ajpcell.1997.272.3.C770. [DOI] [PubMed] [Google Scholar]
- 136.Mileski WJ, Sikes P, Atiles L, et al. Inhibition of leukocyte adherence and susceptibility to infection. J. Surg. Res. 1993;54:349–354. doi: 10.1006/jsre.1993.1056. [DOI] [PubMed] [Google Scholar]
- 137.Mileski WJ, Winn RK, Vedder NB, et al. Inhibition of CD18-dependent neutrophil adherence reduces organ injury after hemorrhagic shock in primates. Surgery. 1990;108:206–212. [PubMed] [Google Scholar]
- 138.Thom SR, Bhopale VM, Milovanova TN. Neutrophil beta-2 integrin inhibition by hyperoxia is due to actin S-nitrosylation and delocalized thioredoxin reductase. Undersea and Hyperbaric Med. 2010 Suppl in press. [Google Scholar]
- 139.Buras J, Holt D, Orlow D, et al. Hyperbaric oxygen protects from sepsis mortality via an interleukin-10-dependent mechanism. Crit Care Med. 2006;34:2624–2629. doi: 10.1097/01.CCM.0000239438.22758.E0. [DOI] [PubMed] [Google Scholar]
- 140.Ross RM, McAllister TA. Protective action of hyperbaric oxygen in mice with pneumococcal sipticaemia. Lancet. 1965:579–581. doi: 10.1016/s0140-6736(65)91149-9. [DOI] [PubMed] [Google Scholar]
- 141.Thom SR, Lauermann MW, Hart GB. Intermittent hyperbaric oxygen therapy for reduction of mortality in experimental polymicrobial sepsis. J Infect Dis. 1986;154:504–510. doi: 10.1093/infdis/154.3.504. [DOI] [PubMed] [Google Scholar]
- 142.Benson RM, Minter LM, Osborne BA, et al. Hyperbaric oxygen inhibits stimulus-induced proinflammatory cytokine synthesis by human blood-derived monocyte-macrophages. Clin Exp Immunol. 2003;134:57–62. doi: 10.1046/j.1365-2249.2003.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lahat N, Bitterman H, Yaniv N, Kinarty A, Bitterman N. Exposure to hyperbaric oxygen induces tumor necrosis factor-alpha (TNF-alpha) secretion from rat macrophages. Clin Exp Immunol. 1995;102:655–659. doi: 10.1111/j.1365-2249.1995.tb03867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Weisz G, Lavy A, Adir Y, et al. Modification of in vivo and in vitro TNF-alpha, IL-1, and IL-6 secretion by circulating monocytes during hyperbaric oxygen treatment in patients with perianal Crohn's disease. J Clin Immunol. 1997;17:154–159. doi: 10.1023/a:1027378532003. [DOI] [PubMed] [Google Scholar]
- 145.Gregorevic P, Lynch GS, Williams DA. Hyperbaric oxygen modulates antioxidant enzyme activity in rat skeletal muscles. Eur J Appl Physiol. 2001;86:24–27. doi: 10.1007/s004210100503. [DOI] [PubMed] [Google Scholar]
- 146.Hirata T, Cui Y, Funakoshi T, et al. The temporal profile of genomic responses and protein synthesis in ischemic tolerance of the rat brain induced by repeated hyperbaric oxygen. Brain Res. 2007;1130:214–222. doi: 10.1016/j.brainres.2006.10.077. [DOI] [PubMed] [Google Scholar]
- 147.Kim C, Choi H, Chun Y, et al. Hyperbaric oxygenation pretreatment induces catalase and reduces infarct size in ischemic rat myocardium. Pflugers Arch. 2001;442:519–525. doi: 10.1007/s004240100571. [DOI] [PubMed] [Google Scholar]
- 148.Nie H, Xiong L, Lao N, et al. Hyperbaric oxygen preconditioning induces tolerance against spinal cord ischemia by upregulation of antioxidant enzymes in rabbits. J Cereb Blood Flow Metab. 2006;26:666–674. doi: 10.1038/sj.jcbfm.9600221. [DOI] [PubMed] [Google Scholar]
- 149.Yu S, Chiu J, Yang S, et al. Preconditioned hyperbaric oxygenation protects the liver against ischemia-reperfusion injury in rats. J Surg Res. 2005;128:28–36. doi: 10.1016/j.jss.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 150.Calvert J, Cahill J, Yamaguchi-Okada M, et al. Oxygen treatment after experimental hypoxia-ischemia in neonatal rats alters the expression of HIF-1a and its downstream target genes. J Appl Physiol. 2006;101:853–865. doi: 10.1152/japplphysiol.00268.2006. [DOI] [PubMed] [Google Scholar]
- 151.Li Y, Zhou C, Calvert J, et al. Multiple effects of hyperbaric oxygen on the expression of HIF-1a and apoptotic genes in a global ischemia-hypotension rat model. Exp Neurol. 2005;191:198–210. doi: 10.1016/j.expneurol.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 152.Gu G-J, Li Y-P, Peng Z-Y, et al. Mechanism of ischemic tolerance induced by hyperbaric oxygen preconditioning involves upregulation of hypoxia-inducible factor-1a and erythropoietin in rats. J Appl Physiol. 2008;104:1185–1191. doi: 10.1152/japplphysiol.00323.2007. [DOI] [PubMed] [Google Scholar]
- 153.Narkowicz CK, Vial JH, McCartney PW. Hyperbaric oxygen therapy increases free radical levels in the blood of humans. Free Radic Res Commun. 1993;19:71–80. doi: 10.3109/10715769309056501. [DOI] [PubMed] [Google Scholar]
- 154.Dennog C, Gedik C, Wood S, et al. Analysis of oxidative DNA damage and HPRT mutations in humans after hyperbaric oxygen treatment. Mutation Res. 1999;431:351–359. doi: 10.1016/s0027-5107(99)00178-5. [DOI] [PubMed] [Google Scholar]
- 155.Hart GB, Strauss MB. Central nervous system oxygen toxicity in a clinical setting. In: Bove AA, Bachrack AJ, Greenbaum LJ, editors. Undersea and Hyperbaric Physiology IX. Bethesda: Undersea and Hyperbaric Med. Soc; 1987. pp. 695–699. [Google Scholar]
- 156.Davis JC, Dunn JM, Heimbach RD. Hyperbaric medicine: patient selection, treatment procedures, and side-effects. In: Davis JC, Hunt TK, editors. Problem Wounds. New York: Elsevier; 1988. pp. 225–235. [Google Scholar]
- 157.Plafki C, Peters P, Almeling M, et al. Complications and side effects of hyperbaric oxygen therapy. Aviat. Space Environ. Med. 2000;71:119–124. [PubMed] [Google Scholar]
- 158.Clark J. Oxygen toxicity. In: Neuman TS, Thom SR, editors. Physiology and Medicine of Hyperbaric Oxygen Therapy. Philadelphia: Saunders; 2008. pp. 527–563. [Google Scholar]
- 159.Lyne AJ. Ocular effects of hyperbaric oxygen. Trans. Ophthalmol. Soc. U.K. 1978;98:66–68. [PubMed] [Google Scholar]
- 160.Palmquist BM, Philipson BO, Barr PO. Nuclear cataract and myopia during hyperbaric oxygen therapy. Br. J. Ophthalmol. 1984;68:113–117. doi: 10.1136/bjo.68.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]