Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria (original) (raw)
Abstract
Ozonide OZ439 is a synthetic peroxide antimalarial drug candidate designed to provide a single-dose oral cure in humans. OZ439 has successfully completed Phase I clinical trials, where it was shown to be safe at doses up to 1,600 mg and is currently undergoing Phase IIa trials in malaria patients. Herein, we describe the discovery of OZ439 and the exceptional antimalarial and pharmacokinetic properties that led to its selection as a clinical drug development candidate. In vitro, OZ439 is fast-acting against all asexual erythrocytic Plasmodium falciparum stages with IC50 values comparable to those for the clinically used artemisinin derivatives. Unlike all other synthetic peroxides and semisynthetic artemisinin derivatives, OZ439 completely cures _Plasmodium berghei_-infected mice with a single oral dose of 20 mg/kg and exhibits prophylactic activity superior to that of the benchmark chemoprophylactic agent, mefloquine. Compared with other peroxide-containing antimalarial agents, such as the artemisinin derivatives and the first-generation ozonide OZ277, OZ439 exhibits a substantial increase in the pharmacokinetic half-life and blood concentration versus time profile in three preclinical species. The outstanding efficacy and prolonged blood concentrations of OZ439 are the result of a design strategy that stabilizes the intrinsically unstable pharmacophoric peroxide bond, thereby reducing clearance yet maintaining the necessary Fe(II)-reactivity to elicit parasite death.
Keywords: 1,2,4-trioxolane; antimalarial drug discovery
The need for new, fast acting, and effective antimalarial drugs has never been greater; drug resistance continues to threaten conventional therapy (1), there are new reports of resistance to the artemisinin derivatives (2, 3), and the global aspiration of malaria eradication has been rekindled (4) and is supported by many of the world's most influential organizations (5). The semisynthetic artemisinin (ART) derivatives (Fig. 1_A_), considered an essential component of malaria chemotherapy (6), are the only class of drug effective against multidrug-resistant forms of the parasite, and artemisinin combination therapies (ACT) are the recommended first-line treatment for uncomplicated Plasmodium falciparum malaria in all endemic regions (7).
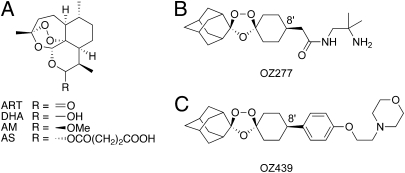
Fig. 1.
Structures of artemisinin derivatives and synthetic ozonides. (A) Artemisinin (ART), dihydroartemisinin (DHA), artemether (AM), and artesunate (AS); (B) OZ277; and (C) OZ439.
The clinical effectiveness of the ART derivatives stems from their rapid onset of action and activity against all erythrocytic stages of the parasite, a feature unique among currently available antimalarial drugs (6). Despite their clinical utility, the current ART derivatives suffer from notable disadvantages. First, they have short in vivo half-lives, and require 3-d treatment regimens in combination with longer-acting antimalarials to maximize cure rates (8, 9). Second, isolation from the plant source is still the only practical means of accessing ART as a starting material, and with harvest and extraction costs being highly variable and often subsidy-driven, the supply of ART fluctuates widely (10). Finally, alarming reports of ACT treatment failures along the Thai-Cambodian border, and more recently, increased parasite clearance times with artesunate (AS) monotherapy, have raised significant concerns that resistance to these agents may be emerging (2, 3).
Over the past several years, we have been working in conjunction with the Medicines for Malaria Venture (www.mmv.org) to design and optimize a completely synthetic ozonide antimalarial based upon the 1,2,4-trioxolane pharmacophore, which exhibits a rapid onset of action, potent activity against all blood stages of P. falciparum and Plasmodium vivax, high oral bioavailability, a good safety profile, low projected cost of goods, and most importantly, the potential for a single-dose oral cure. The availability of such a drug would have a profound and positive impact on patient access, treatment, and compliance, and would contribute to the goal of malaria eradication. Importantly, as the ozonides are structurally dissimilar to the ART derivatives, they may offer an alternative if ART resistance becomes more widespread (3). Currently, sulfadoxine-pyrimethamine is the only available antimalarial effective when given as a single dose; however, its clinical utility is threatened by resistance in many parts of the world (1).
Ozonide OZ277 (11) (Fig. 1_B_), also known as RBx11160 or arterolane maleate, was the first synthetic ozonide to be evaluated clinically and is now in Phase III clinical trials as a combination product with piperaquine phosphate (12). Like the artemisinins, OZ277 contains a pharmacophoric peroxide bond, which is essential for activity (13, 14), exhibits antimalarial activity against all asexual blood stages of P. falciparum (15, 16) and has a rapid onset of action in an established murine model of malaria (11). However, in Phase I clinical trials, the half-life in healthy volunteers was only about two- to threefold longer than that of dihydroartemisinin (DHA) (17), the active metabolite of clinically used semisynthetic ART derivatives. Furthermore, when administered to malaria patients as monotherapy (18), OZ277 displayed reduced plasma exposure compared with that in volunteers (12). To achieve a single-dose oral cure, we therefore considered that the primary challenge was to substantially increase the in vivo half-life and blood exposure profile of a next-generation synthetic ozonide drug candidate compared with that of the ART derivatives and OZ277. Herein we describe the exceptional pharmacokinetic and antimalarial properties of OZ439 (Fig. 1_C_) and the studies that led to its selection as a clinical development candidate (3, 5, 12).
Results and Discussion
The critical design criteria that led to the selection of OZ439 as a clinical candidate was the relationship between Fe(II)-mediated activation and antimalarial activity, and the competing mechanisms of in vivo degradation and clearance. The focus of initial investigations was to define the mechanistic basis for clearance of the first generation ozonide, OZ277, and identify synthetic strategies to reduce clearance, and subsequently increase the half-life and overall exposure profile. Previous studies indicated a role of cytochrome P450 (CYP450)-mediated metabolism in the clearance of OZ277, resulting in the formation of inactive adamantane-hydroxylated metabolites (19). In vivo studies with OZ277 in rats (Fig. S1) resulted in a high blood clearance of 61 mL/min/kg and a half-life of only 1.3 h. Following predosing of rats with the irreversible CYP450 inhibitor, 1-aminobenzotriazole, the clearance reduced to 31 mL/min/kg. Although these results confirmed a contribution of CYP-metabolism to the clearance of OZ277, they also indicated a role for non-CYP mechanisms, as the reduction in clearance with 1-aminobenzotriazole was considerably less than what would be expected for a compound predominantly cleared through CYP450 metabolism (20, 21).
Subsequent investigations pointed to reaction with endogenous sources of Fe(II) in blood and tissues as a probable mechanism for the rapid clearance of OZ277 and other first-generation ozonides. When incubated with healthy rat blood at 37 °C, OZ277 and structurally similar analogs degraded with a half-life of ~1 h, with formation of inactive adamantane lactone and cyclohexanone cleavage products. A synthetic strategy to stabilize the peroxide bond was therefore a potential route to increase the in vivo half-life and in vivo exposure profile, provided that we could maintain the necessary Fe(II)-reactivity to ensure antimalarial activity. Although the precise mechanism of action of peroxide antimalarials remains elusive (11, 22–28), the most widely accepted view is that the pharmacophoric peroxide bond undergoes reductive activation by ferrous iron and heme released during hemoglobin digestion to produce carbon-centered radicals that alkylate heme and other critical parasite proteins. Studies from our laboratories with OZ277 and structural analogs are consistent with this hypothesis (29), and have suggested that reactivity with Fe(II)-containing species is a necessary, yet insufficient, requirement for antiplasmodial activity (30, 31). In other words, ozonides that are essentially unreactive with Fe(II) in vitro are virtually devoid of antimalarial activity, although evidence of Fe(II)-reactivity on its own does not ensure antimalarial activity. We have also previously demonstrated that for a series of early synthetic ozonides, there is a good correlation between the extent of in vitro heme alkylation (which occurs subsequent to heme electron-transfer to the peroxide bond) and the in vitro IC50 against P. falciparum, suggesting a potential link to antimalarial activity (32).
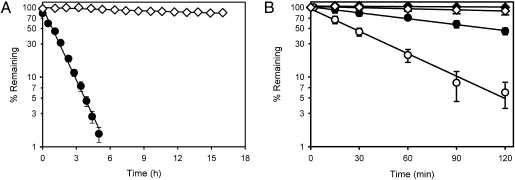
We then conducted investigations to assess the reactivity of selected next-generation ozonides with Fe(II) and heme and compared results with those for first-generation ozonides, such as OZ277. Next-generation ozonides (Fig. S2), such as OZ439 (Fig. 1_C_), containing a _cis_-8′-phenyl substituent, were >50-fold more stable to Fe(II)-mediated degradation (Fig. 2_A_) compared with first-generation ozonides, such as OZ277, which contain a _cis_-8′-alkyl group. The apparent first-order degradation rate constant for OZ439 in the presence of ferrous sulfate was 0.011 ± 0.001 h−1 (mean ± SD, n = 3) compared with 0.76 ± 0.03 h−1 for OZ277 (30). Previous quantum mechanical studies with OZ27 (Fig. S2_B_), a structural precursor of the next-generation ozonides, attributed this enhanced stability to greater steric interactions between the bound iron complex and the _cis_-8′-phenyl substituent compared with the _cis_-8′-alkyl group (33). Importantly, some Fe(II)-reactivity could still be detected for the next-generation ozonides. The _cis_-8′-phenyl-containing ozonides effectively and rapidly reacted with heme (with complete disappearance of the ozonide within minutes), with the extent of heme alkylation being comparable to that observed with the _cis_-8′-alkyl–containing compounds [e.g., 84 ± 1.5% heme alkylation for OZ439 and 83 ±1.5% for OZ277 (32), mean n = 3 ± SD]. Given that OZ439 and structurally related ozonides are potent antimalarials both in vitro against P. falciparum and in vivo against Plasmodium berghei (see below), these results strongly suggest that even low levels of Fe(II)-reactivity are sufficient to maintain antimalarial efficacy.
Fig. 2.
Fe(II) and blood stability profiles for OZ277 and OZ439. (A) Stability of OZ277 (●) and OZ439 (◇) in the presence of aqueous ferrous sulfate at 37 °C (mean ± SD, n = 3). (B) Stability of OZ277 (●) and OZ439 (◆) in noninfected and _P. falciparum_-infected (OZ277 ○, OZ439 ◇) human RBCs suspended in plasma and incubated at 37 °C (mean ± SD, n = 3–5).
Consistent with the trends observed for Fe(II)-reactivity, OZ439 was more than 15-fold more stable than OZ277 when incubated in healthy rat blood at 37 °C (Fig. S3). A similar trend was also observed upon incubation with noninfected human blood where OZ439 was more than 20-fold more stable than OZ277 (Fig. 2_B_). In human blood containing a low level of parasitemia (1% parasitemia, 45% hematocrit), there was an increase in the rate of degradation of OZ277 of approximately fivefold compared with that in the absence of parasites (Fig. 2_B_). These observations led to the hypothesis that the increased rate of degradation in infected blood was the basis for the lower exposure of OZ277 in malaria patients compared with that in volunteers (12). The increased rate of degradation in infected blood is consistent with cleavage of the peroxide bond by Fe(II) species liberated during hemoglobin digestion in the parasite food vacuole (23, 25, 26, 28), and provides further support for the heme-mediated activation hypothesis for OZ277 and other ozonides. In contrast, although the rate of degradation of OZ439 also increased marginally in the presence of parasites, the infected blood stability was still more than 20-fold greater than that of OZ277 under these test conditions. For both OZ277 and OZ439, the measured blood to plasma ratio (~1.5 for OZ277 and 0.8 for OZ439) using a specific LC-MS assay was constant throughout the incubation period for both noninfected and infected human blood, suggesting the absence of significant accumulation in erythrocytes. It is important to note that the blood stability differences do not affect the in vitro potency (IC50) measurements because of the rapid onset of action of the peroxides and insignificant degradation under the standard in vitro assay conditions (1.25% hematocrit and 0.3% parasitemia). Importantly, _cis_-8′-phenyl substituted ozonides, such as OZ439, have improved stability to blood-mediated degradation, yet maintain sufficient Fe(II)-reactivity to rapidly kill parasites (vide infra).
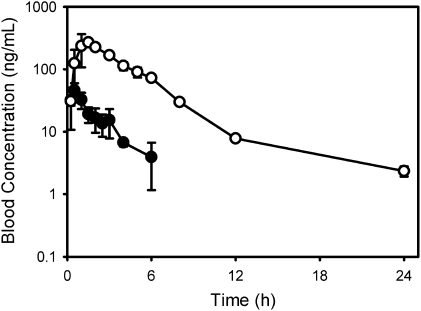
The outstanding blood stability characteristics of OZ439 can be further appreciated by comparing the in vivo blood concentration versus time profiles for OZ439 and OZ277 following oral administration to rats (Fig. 3). At an oral dose of 3 mg/kg, there was a 10-fold increase in the area under the concentration versus time profile of OZ439 compared with that of OZ277 as a result of a significant reduction in clearance [at least in part because of a decrease in Fe(II)-mediated degradation], as well as an overall improvement in bioavailability. OZ439 also exhibited an increased volume of distribution as a result of an increase in Log D, and together with reduced clearance, this contributed to a long half-life of greater than 20 h following oral dosing to rats (Table S1). This finding compares to a half-life of ~0.5 h for DHA and 1 h for OZ277 (11) following oral dosing to rats. Similar trends for prolonged blood concentration profiles were also seen with other next-generation ozonides (Fig. S4_A_). Importantly, plasma concentration versus time profiles for OZ439 increased proportionally across the dose range of 3 to 30 mg/kg in rats (Fig. S4_B_), and there was a high degree of consistency in oral exposure and half-life (~30 h) across the tested preclinical species (Fig. S4_C_).
Fig. 3.
Blood concentration versus time profiles for OZ439 (○) and OZ277 (●) following a single 3-mg/kg oral dose to rats (mean ± SD, n = 3–4). For OZ277, concentrations were below the analytical limit of quantitation (~1 ng/mL) by 7.5 h.
Even though this half-life represents a substantial increase relative to other peroxide antimalarials, including OZ277, the half-life is still relatively modest compared with drugs for which resistance is evident [e.g., chloroquine (CQ) or mefloquine (MF), which have half-lives of weeks]. Because OZ439 would be administered clinically with another antimalarial agent, most likely having a longer half-life (note that studies are still underway to identify a suitable partner drug), parasites would not be exposed to OZ439 in the absence of the partner drug. Thus, combined with a rapid onset of action and activity against all asexual blood stages [and potentially gametocytes based on the activity of the artemisinin derivatives (34)], this would suggest a low risk of developing resistance as long as efficacious treatment regimens are adhered to (35).
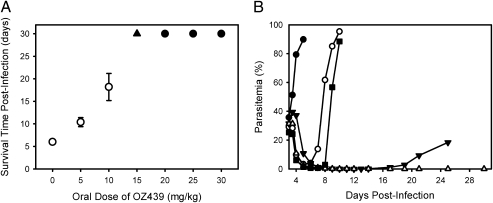
The promising iron-reactivity and pharmacokinetic profile of the next-generation ozonides led to a series of extensive investigations to characterize antimalarial efficacy. Antimalarial activity was assessed on the basis of in vitro activity against P. falciparum and in vivo efficacy following a single oral dose of 30 mg/kg to _P. berghei_-infected mice. Each of the ozonides, including OZ439, displayed potent in vitro activity, with IC50 values (concentration required for 50% parasite growth inhibition relative to a control) ranging from 0.43 to 3.3 ng/mL (Table S2). This range is comparable to that for other semisynthetic artemisinin derivatives and synthetic peroxides. OZ439 was also equally active against all asexual erythrocytic stages with IC50 values of 1.9, 3.0, and 2.8 ng/mL for rings, trophozoites, and schizonts, respectively (mean, n = 3). In vivo, the antimalarial efficacy of OZ439 (Table 1) and other next-generation ozonides (Table S3) surpassed that observed with comparator compounds (Table 1), including CQ, MF, sulfadoxine, pyrimethamine, AS, and OZ277. Specifically, a single oral dose of 20 mg/kg (Fig. 4_A_) or three daily oral doses of 5 mg/kg OZ439 were completely curative in the P. berghei model, a level of efficacy superior to all reported synthetic peroxides and semisynthetic artemisinin derivatives (Table S4). Partial cures (four of five animals parasite-free at day 30) were also achieved at a single oral dose of 15 mg/kg OZ439 (Fig. 4_A_). To put these results in perspective, attainment of cures in the P. berghei model with AS and CQ required four daily oral doses of 100 mg/kg, and for MF, four daily oral doses of 30 mg/kg. Neither CQ, MF, nor OZ277 were curative in this model at single doses up to 100 (OZ277) or 200 (MF and CQ) mg/kg.
Table 1.
In vivo efficacy of comparator antimalarial drugs, OZ277 and OZ439, against murine P. berghei
| In vivo efficacy 1 × 30 mg/kg (oral)* | In vivo prophylaxis 1 × 30 mg/kg (oral) given 24 h before infection* | |||||
|---|---|---|---|---|---|---|
| Compound | Activity (%)† | Survival (d) | Cure (%)‡ | Activity (%)† | Survival (d) | Protected (%)‡ |
| Control | 0 | 6–7 | 0 | 0 | 6–7 | 0 |
| AS§ | 92 | 9 | 0 | 21 | 7 | 0 |
| CQ§ | 99.9 | 10 | 0 | 37 | 7 | 0 |
| MF§ | 99.6 | 22 | 0 | 99.9 | 27 | 60 |
| Sulfadoxine | 97 | 15 | 0 | Not tested | ||
| Pyrimethamine | 99.8 | 9 | 0 | Not tested | ||
| OZ277§ | 99.9 | 11 | 0 | 0 | 7 | 0 |
| OZ439 | >99.9 | >30 | 100 | 99.8 | >30 | 100 |
Fig. 4.
In vivo efficacy of OZ439 in P. berghei infected mice. (A) Average survival time postinfection (mean ± SEM, n = 5 at each dose level) following single oral doses of OZ439 on day 1 postinfection. The filled circles represent doses for which no parasites could be detected at day 30 postinfection in any mice; at 15 mg/kg (▲), four of five mice were parasite-free at day 30. (B) Onset of action and recrudescence for control (●), AS (○), OZ277 (■), MF (▼), and OZ439 (△) following a single oral dose of 100 mg/kg on day 3 postinfection (n = 5 for each).
One of the most important and somewhat unique features of the peroxide antimalarials is their ability to rapidly reduce parasitemia by virtue of their activity against young ring forms (9). This ability is illustrated in an onset-of-action and parasite-recrudescence experiment (Fig. 4_B_), in which single oral doses of 100 mg/kg AS, OZ277, MF, or OZ439 were administered to _P. berghei_-infected mice on day 3 postinfection. In this model, the initial parasitemia is considerably higher compared with the standard testing protocol in which compounds are administered on day 1 postinfection, allowing the onset of action to be assessed. In vivo, OZ439 had a rapid onset of action against P. berghei comparable to that seen for AS and OZ277, whereas MF was slower to reduce parasite burden (Fig. 4_B_). In the case of OZ277 and AS, parasites could be detected throughout the postdosing interval and recrudescence was evident by days 6 and 8 postinfection, respectively. Both OZ439 and MF reduced parasitemia to levels below the detection limit (<0.1%) within 7 to 8 d after treatment. For MF, recrudescence was evident by day 14 postinfection, whereas animals treated with OZ439 had no detectable parasites at day 30 postinfection.
A further compelling characteristic of OZ439 and its analogs is their significant prophylactic activity, which for several compounds exceeded that of the benchmark chemoprophylactic drug, MF (Table 1 and Table S3). In parallel studies, a single 30 mg/kg oral dose of OZ439 administered 48 h before parasite inoculation was also completely protective, whereas MF at the same dose increased survival time by only 5 d. Notably, neither AS nor OZ277 demonstrate any measurable prophylactic activity.
On the basis of the results described, we propose that Fe(II) reactivity is central to both potent antimalarial activity and in vivo clearance, and that structural modifications to optimize the degree of Fe(II) reactivity provided a means for balancing these two opposing mechanisms. As a result, the outstanding single-dose curative efficacy and prophylactic activity of OZ439 is a direct consequence of the greatly enhanced in vivo exposure profile and prolonged half-life, which in turn is linked to an optimized Fe(II) reactivity profile.
Before the selection of OZ439 as a development candidate, exploratory (non-GLP) toxicological studies were conducted in rats, where multiple oral doses as high as 300 mg/kg resulted in only minimal adverse effects, which were completely reversible. There were no drug-related effects on body weight or general condition, no changes in urinary parameters, and no drug-related necropsy findings over the dosing or recovery periods. At 300 mg/kg, there was a minimal increase in the number of white blood cells and minimally reduced triglyceride levels, but changes were fully reversible. Histopathological examination indicated minimal gastric irritation, but findings were no longer evident after the recovery period. Importantly, under these dosing conditions, high plasma concentrations were also achieved. OZ439 was also found to be nongenotoxic. OZ439 completed a full GLP preclinical safety program, and recently completed Phase I clinical trials, where it was shown to be safe and well-tolerated at doses up to 1,600 mg. Phase IIa trials in malaria patients are currently underway (http://www.mmv.org/research-development/project-portfolio/phase-iia).
The discovery of OZ439 and the research that ultimately led to its selection as a clinical development candidate were conducted by an integrated multidisciplinary project team formed by a collaboration between academic research groups, industry affiliates, and the Medicines for Malaria Venture, a Product Development Partnership dedicated to the development of new drugs for malaria. Critical to the success of this project was strict adherence to a target product profile and established candidate selection criteria to guide the work program. This work highlights the potential impact of drug-discovery research conducted through the Product Development Partnership model and further emphasizes the role of these relationships in the research and development landscape for neglected diseases (36).
Materials and Methods
Ozonide Synthesis.
Ozonides were synthesized using Griesbaum coozonolysis (37) and postozonolysis reactions, as previously described (13, 38), and were isolated as mesylate or tosylate salts (SI Text). X-ray crystallographic analysis (38) of structural precursors confirmed an axial peroxide bond and an equatorial phenyl substituent, establishing the stereochemistry of the target ozonides as cis.
Animal Experiments.
In vivo efficacy studies in mice were conducted at the Swiss Tropical and Public Health Institute (Basel), and toxicology studies were conducted by Basilea Pharmaceutica Ltd. (Basel). Both were approved by the Veterinary Office of Canton Basel-Stadt. Rat and mouse pharmacokinetic studies were conducted at Monash University and were approved by the Monash Institute of Pharmaceutical Sciences Animal Ethics Committee. Dog pharmacokinetic studies were conducted by RCC Ltd. (Itingen, Switzerland) and were approved by the Veterinary Office of Canton Basel-Land.
Antimalarial Activity.
Ozonides were screened against chloroquine-resistant (K1) and chloroquine-sensitive (NF54) strains of P. falciparum in vitro (11). For studies with synchronized parasites, stages were incubated for 24 h with OZ439, washed, and then incubated with hypoxanthine, as previously described (15). In vivo efficacy was conducted as previously described (11), with the modification that mice (n = 5) were infected with a GFP-transfected P. berghei ANKA strain (donated by A. P. Waters and C. J. Janse, Leiden University, The Netherlands), and parasitemia determined using standard flow cytometry techniques. Ozonides and comparator drugs were dissolved or suspended in a suspending vehicle consisting of 0.5% wt/vol hydroxymethyl cellulose, 0.5% vol/vol benzyl alcohol, 0.4% vol/vol Tween 80, and 0.9% wt/vol sodium chloride in water, and administered orally on day 1 postinfection. Antimalarial activity was measured as a percent reduction in parasitemia on day 3 postinfection (11). Animals were considered cured if there were no detectable parasites on day 30 postinfection.
The onset of action was determined after a single oral dose of compounds to mice (n = 5) on day 3 postinfection, resulting in a high initial parasitemia to allow the onset of action to be assessed. The reduction in parasitemia was initially monitored at 12 h posttreatment, and the time of recrudescence was assessed by daily blood smears for 14 d followed by intermittent assessment up to 30 d. Prophylactic activities were assessed after administering a single oral dose of 30 mg/kg to mice (n = 5) at various times before infection. All groups, including an untreated control group, were infected simultaneously with P. berghei. Parasitemia was determined on day 3 postinfection, and compared with values in control animals.
Reactivity in the Presence of Ferrous Iron, Heme, and Blood.
Reactivity of the ozonides with Fe(II) (30) and the extent of heme alkylation (32) were assessed, as previously described. Blood stability was measured by incubating ozonides (50 ng/mL) in rat blood or noninfected or infected (K1 strain of P. falciparum) human RBCs, which had been washed and resuspended in plasma (45% hematocrit, ±1% parasitemia) for 2 to 4 h at 37 °C. Aliquots of blood were periodically centrifuged to obtain plasma which was stored at −20 °C until analysis (within 24 h). All blood samples were stabilized by the addition of potassium dichromate (final concentration of 36 μM) before centrifugation. For analysis, plasma samples were thawed and internal standard (either diazepam or OZ209) added, proteins were precipitated with acetonitrile, and an aliquot of supernatant analyzed by LC/MS using a Waters 2795 HPLC and a Micromass ZQ single quadrupole mass spectrometer. Chromatographic and MS conditions were adapted from previously published methods (11). Concentrations were determined by comparison with calibration standards prepared in rat or human plasma.
Pharmacokinetic Properties.
In vivo pharmacokinetic studies were conducted in fasted male Sprague-Dawley rats, as previously described (11). Intravenous formulations were administered as 10-min infusions and consisted of 10% vol/vol DMSO/0.4% vol/vol Tween 80 in either 5% wt/vol glucose, 0.9% wt/vol normal saline, or 20 mM phosphate buffer, pH 3 (OZ439, OZ466, OZ539); 7.5% vol/vol DMSO in either 5% wt/vol glucose with or without 15 mM citrate buffer pH 3.0 (OZ401, OZ429, OZ277); 20% vol/vol propylene glycol/20% vol/vol ethanol/0.1% vol/vol Tween 80 in 5% dextrose containing 15 mM citrate buffer, pH 3.0 (OZ323); or 40% vol/vol propylene glycol/10% vol/vol ethanol/ 50% vol/vol water (OZ482). Selected studies with OZ277 were also conducted following oral predosing of rats with 1-aminobenzotriazole (100 mg/kg prepared in water) ~15 h before administration of OZ277. Oral ozonide formulations were prepared in suspending vehicle and administered by gavage. Blood samples were collected using a Culex automated blood sampler, and immediately centrifuged at 4 °C. Plasma was separated, stored at −20 °C until processing as described for the blood stability studies, and assayed by LC/MS using either a Waters Micromass Quattro Ultima PT triple quadrupole instrument coupled to a Waters 2795 HPLC or a Waters Micromass Quattro Premier triple quadrupole instrument coupled to a Waters Acquity UPLC. Plasma pharmacokinetic parameters were calculated using noncompartmental methods (WinNonlin ver. 5.2, Pharsight Corp.) and converted to the corresponding blood parameters using the measured in vitro blood to plasma ratios.
Exploratory Toxicology.
OZ439 or vehicle was administered orally to male rats (100 or 300 mg/kg) every third day for five doses. Satellite groups were included for the collection of blood samples for kinetic examinations and for a 2-wk recovery phase. Toxicity was assessed by clinical observations, body-weight development, and clinical laboratory investigations (hematology, clinical chemistry, and urine analysis). At the end of the study periods, all rats were killed, necropsied, and selected organs examined histopathologically.
Supplementary Material
Supporting Information
Acknowledgments
The authors thank Drs. Win Gutteridge, Tim Wells, Joerg Moehrle, Daniel Hunziker, Arnulf Dorn, and Marcel Tanner for ongoing support and advice throughout the program and Josefina Santo-Tomas, Christopher Snyder, Chantal Thouvay, Maria Koltun, Alison Gregg, Jason Green, Quoc Wu, Kasiram Katneni, and Jessica Steuten for expert scientific and technical assistance. This work was supported in part by Medicines for Malaria Venture.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.World Health Organization. Global Report on Antimalarial Drug Efficacy and Drug Resistance: 2000–2010. Geneva: World Health Organization; 2010. [Google Scholar]
- 2.Dondorp AM, et al. Artemisinin resistance: Current status and scenarios for containment. Nat Rev Microbiol. 2010;8:272–280. doi: 10.1038/nrmicro2331. [DOI] [PubMed] [Google Scholar]
- 3.Enserink M. Malaria's drug miracle in danger. Science. 2010;328:844–846. doi: 10.1126/science.328.5980.844. [DOI] [PubMed] [Google Scholar]
- 4.Roberts L, Enserink M. Malaria. Did they really say … eradication? Science. 2007;318:1544–1545. doi: 10.1126/science.318.5856.1544. [DOI] [PubMed] [Google Scholar]
- 5.Wells TNC, Alonso PL, Gutteridge WE. New medicines to improve control and contribute to the eradication of malaria. Nat Rev Drug Discov. 2009;8:879–891. doi: 10.1038/nrd2972. [DOI] [PubMed] [Google Scholar]
- 6.White NJ. Qinghaosu (artemisinin): The price of success. Science. 2008;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Guidelines for the Treatment of Malaria. Geneva: World Health Organization; 2006. [Google Scholar]
- 8.Ridley RG. Medical need, scientific opportunity and the drive for antimalarial drugs. Nature. 2002;415:686–693. doi: 10.1038/415686a. [DOI] [PubMed] [Google Scholar]
- 9.White NJ. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kindermans JM, Pilloy J, Olliaro P, Gomes M. Ensuring sustained ACT production and reliable artemisinin supply. Malar J. 2007;6:125. doi: 10.1186/1475-2875-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vennerstrom JL, et al. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature. 2004;430:900–904. doi: 10.1038/nature02779. [DOI] [PubMed] [Google Scholar]
- 12.Olliaro P, Wells TNC. The global portfolio of new antimalarial medicines under development. Clin Pharmacol Ther. 2009;85:584–595. doi: 10.1038/clpt.2009.51. [DOI] [PubMed] [Google Scholar]
- 13.Dong Y, et al. Spiro and dispiro-1,2,4-trioxolanes as antimalarial peroxides: Charting a workable structure-activity relationship using simple prototypes. J Med Chem. 2005;48:4953–4961. doi: 10.1021/jm049040u. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser M, et al. Peroxide bond-dependent antiplasmodial specificity of artemisinin and OZ277 (RBx11160) Antimicrob Agents Chemother. 2007;51:2991–2993. doi: 10.1128/AAC.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofer S, et al. In vitro assessment of the pharmacodynamic properties of DB75, piperaquine, OZ277 and OZ401 in cultures of Plasmodium falciparum. J Antimicrob Chemother. 2008;62:1061–1064. doi: 10.1093/jac/dkn315. [DOI] [PubMed] [Google Scholar]
- 16.Maerki S, et al. In vitro assessment of the pharmacodynamic properties and the partitioning of OZ277/RBx-11160 in cultures of Plasmodium falciparum. J Antimicrob Chemother. 2006;58:52–58. doi: 10.1093/jac/dkl209. [DOI] [PubMed] [Google Scholar]
- 17.Orrell C, et al. Pharmacokinetics and tolerability of artesunate and amodiaquine alone and in combination in healthy volunteers. Eur J Clin Pharmacol. 2008;64:683–690. doi: 10.1007/s00228-007-0452-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valecha N, et al. Arterolane, a new synthetic trioxolane for treatment of uncomplicated Plasmodium falciparum malaria: A phase II, multicenter, randomized, dose-finding clinical trial. Clin Infect Dis. 2010;51:684–691. doi: 10.1086/655831. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, et al. Characterization of the two major CYP450 metabolites of ozonide (1,2,4-trioxolane) OZ277. Bioorg Med Chem Lett. 2008;18:1555–1558. doi: 10.1016/j.bmcl.2008.01.087. [DOI] [PubMed] [Google Scholar]
- 20.Balani SK, et al. Effective dosing regimen of 1-aminobenzotriazole for inhibition of antipyrine clearance in rats, dogs, and monkeys. Drug Metab Dispos. 2002;30:1059–1062. doi: 10.1124/dmd.30.10.1059. [DOI] [PubMed] [Google Scholar]
- 21.Strelevitz TJ, Foti RS, Fisher MB. In vivo use of the P450 inactivator 1-aminobenzotriazole in the rat: varied dosing route to elucidate gut and liver contributions to first-pass and systemic clearance. J Pharm Sci. 2006;95:1334–1341. doi: 10.1002/jps.20538. [DOI] [PubMed] [Google Scholar]
- 22.Eckstein-Ludwig U, et al. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 23.Jefford CW. Why artemisinin and certain synthetic peroxides are potent antimalarials. Implications for the mode of action. Curr Med Chem. 2001;8:1803–1826. doi: 10.2174/0929867013371608. [DOI] [PubMed] [Google Scholar]
- 24.Krishna S, Woodrow CJ, Staines HM, Haynes RK, Mercereau-Puijalon O. Re-evaluation of how artemisinins work in light of emerging evidence of in vitro resistance. Trends Mol Med. 2006;12:200–205. doi: 10.1016/j.molmed.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meshnick SR. Artemisinin: Mechanisms of action, resistance and toxicity. Int J Parasitol. 2002;32:1655–1660. doi: 10.1016/s0020-7519(02)00194-7. [DOI] [PubMed] [Google Scholar]
- 26.O'Neill PM, Posner GH. A medicinal chemistry perspective on artemisinin and related endoperoxides. J Med Chem. 2004;47:2945–2964. doi: 10.1021/jm030571c. [DOI] [PubMed] [Google Scholar]
- 27.O'Neill PM, Barton VE, Ward SA. The molecular mechanism of action of artemisinin—The debate continues. Molecules. 2010;15:1705–1721. doi: 10.3390/molecules15031705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robert A, Benoit-Vical F, Claparols C, Meunier B. The antimalarial drug artemisinin alkylates heme in infected mice. Proc Natl Acad Sci USA. 2005;102:13676–13680. doi: 10.1073/pnas.0500972102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fügi MA, Wittlin S, Dong Y, Vennerstrom JL. Probing the antimalarial mechanism of artemisinin and OZ277 (arterolane) with nonperoxidic isosteres and nitroxyl radicals. Antimicrob Agents Chemother. 2010;54:1042–1046. doi: 10.1128/AAC.01305-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Creek DJ, et al. Iron-mediated degradation kinetics of substituted dispiro-1,2,4-trioxolane antimalarials. J Pharm Sci. 2007;96:2945–2956. doi: 10.1002/jps.20958. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, et al. Spiro- and dispiro-1,2-dioxolanes: Contribution of iron(II)-mediated one-electron vs two-electron reduction to the activity of antimalarial peroxides. J Med Chem. 2007;50:5840–5847. doi: 10.1021/jm0707673. [DOI] [PubMed] [Google Scholar]
- 32.Creek DJ, et al. Relationship between antimalarial activity and heme alkylation for spiro- and dispiro-1,2,4-trioxolane antimalarials. Antimicrob Agents Chemother. 2008;52:1291–1296. doi: 10.1128/AAC.01033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Creek DJ, Chalmers DK, Charman WN, Duke BJ. Quantum chemical study of the intermediate complex required for iron-mediated reactivity and antimalarial activity of dispiro-1,2,4-trioxolanes. J Mol Graph Model. 2008;27:394–400. doi: 10.1016/j.jmgm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Chen PQ, et al. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chin Med J (Engl) 1994;107:709–711. [PubMed] [Google Scholar]
- 35.White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran M, Guzman J, Ropars AL, Illmer A. The role of Product Development Partnerships in research and development for neglected diseases. In Health. 2010;2:114–122. doi: 10.1016/j.inhe.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 37.Griesbaum K, Liu XJ, Kassiaris A, Scherer M. Ozonolyses of O-alkylated ketoximes in the presence of carbonyl groups: A facile access to ozonides. Liebigs Annalen-Recueil. 1997;1997:1381–1390. [Google Scholar]
- 38.Tang Y, Dong Y, Vennerstrom JL. Synthetic peroxides as antimalarials. Med Res Rev. 2004;24:425–448. doi: 10.1002/med.10066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information